Mantle cell lymphoma (MCL) is clinically and biologically heterogeneous, and while a subset of cases is observed initially, clinical and biological selection criteria to identify candidates for this strategy are not well defined. We hypothesized that the decision to monitor a patient should be made on the basis of clinical criteria, as is standard for chronic lymphocytic leukemia and other indolent B-cell non-Hodgkin lymphomas.1 Patients with leukemic non-nodal MCL are optimal candidates for initial observation, but those who meet other criteria, such as microscopic gastrointestinal (GI) tract involvement or low-volume nodal disease, are also potential candidates.2 Although biological criteria such as proliferation rate and TP53 mutation status have prognostic significance in MCL, we hypothesized that these unfavorable biological features do not disqualify asymptomatic patients from initial observation. In this retrospective analysis, we describe the clinical and biological characteristics of MCL patients who were initially observed versus immediately treated, and the associated outcomes.
Eligible adult patients had histologically confirmed MCL diagnosed between 2000 and 2014 and were managed at Memorial Sloan Kettering Cancer Center (MSK). Initial observation was defined as treatment deferral for at least three months after diagnosis with documentation of an ‘intent to observe’ by the treating oncologist.2,3 There were no predefined clinical or biological criteria for observation. The study was approved by the MSK Institutional Review Board. Fisher exact or Wilcoxon rank sum tests were used to compare the baseline characteristics of the immediate and deferred treatment groups. Cox regression modeling was used to analyze the time from tumor diagnosis to initiation of any treatment (TTI) in the observation group and overall survival (OS; defined as time from tumor diagnosis to death from any cause). Statistical significance was defined as P<0.05. All analyses were completed with R version 3.5.0. A data freeze was instituted on September 2, 2015.
We identified 404 untreated patients with histologically confirmed MCL who were managed at MSK: 90 were initially observed (OBS) and 314 were immediately treated (TX). Reasons for observation documented by the treating oncologist included: lack of symptoms, low tumor burden, perceived favorable biological features (low Ki-67 or SOX-11 negativity), leukemic phase disease without lymphadenopathy, or disease limited to GI tract. None of the OBS patients had significant constitutional symptoms, progressive marrow failure manifest by anemia and/or thrombocytopenia, symptomatic organomegaly, progressive or symptomatic nodal enlargement, or evidence of clinically significant organ compression or involvement. The baseline characteristics of the OBS versus TX patients are shown in Table 1.
Table 1.
Patients’ characteristics by treatment group.
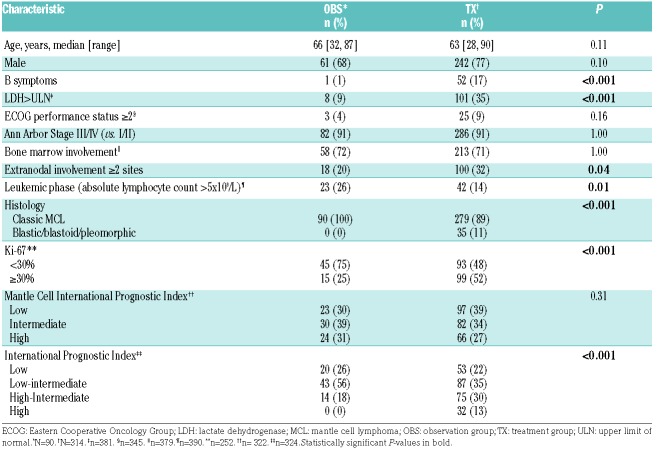
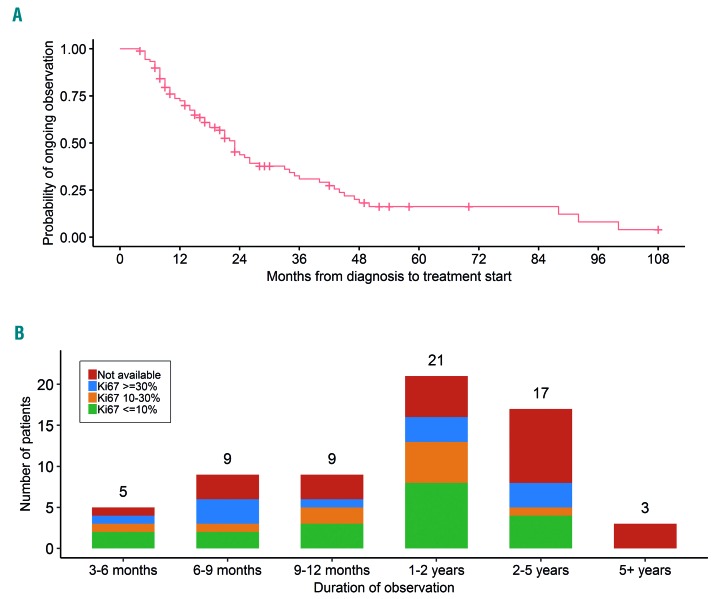
Among the 90 OBS patients, 64 were started on therapy after a period of observation while 26 were expectantly monitored. The median time of observation was 23 months (Figure 1A). Among the OBS patients who were subsequently treated, 23 (36%) were observed for 12 months or longer, 21 (33%) for 1-2 years, and 20 (31%) for 2 years or longer (Figure 1B). In univariate analysis, only the presence of lymphadenopathy [defined as any lymphoma-related lymph node(s) measuring >1.5 cm] was significantly associated with a shorter period of monitoring (HR 2.15; 95%CI: 1.18-3.92; P=0.01).
Figure 1.
Time from diagnosis to initiation of therapy and distribution of Ki-67 index among patients who were initially observed but subsequently treated. (A) Time from diagnosis to initiation of therapy among observed patients (n=90). The median time of observation is 23 months. (B) Distribution of Ki-67 index among patients of different duration of observation prior to initiation of therapy (n=64).
None of the OBS patients had blastic morphology. Ki-67 value was not significantly associated with length of observation (Figure 1B). Fifteen patients with a Ki-67 30% or over (range, 30-70%) were observed for a median of 22 months (95%CI: 7-33 months). Twenty-five OBS patients underwent tumor molecular profiling using the MSK-HEMEPACT assay to identify clinically relevant somatic mutations.4 Of these patients, 3 had a TP53 mutation (C242Y, G245C, and R280G) and these patients were observed for 4, 18, and 20 months.
To gain insight into the patients with the longest observation period, we reviewed the baseline characteristics of the 30 OBS patients for two years or more. All were asymptomatic and had essentially normal lactate dehydrogenase (LDH) at presentation (28 patients had normal LDH and 2 had LDH 248 U/L; upper limit of normal: 246 U/L) and a low burden of lymphadenopathy [defined as absence of either 3 lymph node sites ≥3 cm or any lymph node site ≥7 cm, per Groupe d’Etude des Lymphomas Folliculaires (GELF) criteria]. Most patients, 93% (28 out of 30), had a good Karnofsky Performance Score (KPS) (≥80%); 2 patients had poor baseline performance status as a result of frailty related to age and other medical comorbidities. Three dominant clinical categories of indolent MCL were successfully monitored for two years or longer: 1) leukemic phase disease with minimal nodal disease, with or without splenomegaly (n=9); 2) low-tumor-burden disease without leukemic phase disease (n=17); and 3) GI-tract-only disease (n=4). Three of 4 patients with GI-tract-only involvement underwent a colonoscopy for routine screening for colorectal cancer or for a history of polyps, and in one case a patient underwent a colonoscopy for evaluation of arthritis and iron deficiency (in absence of anemia).
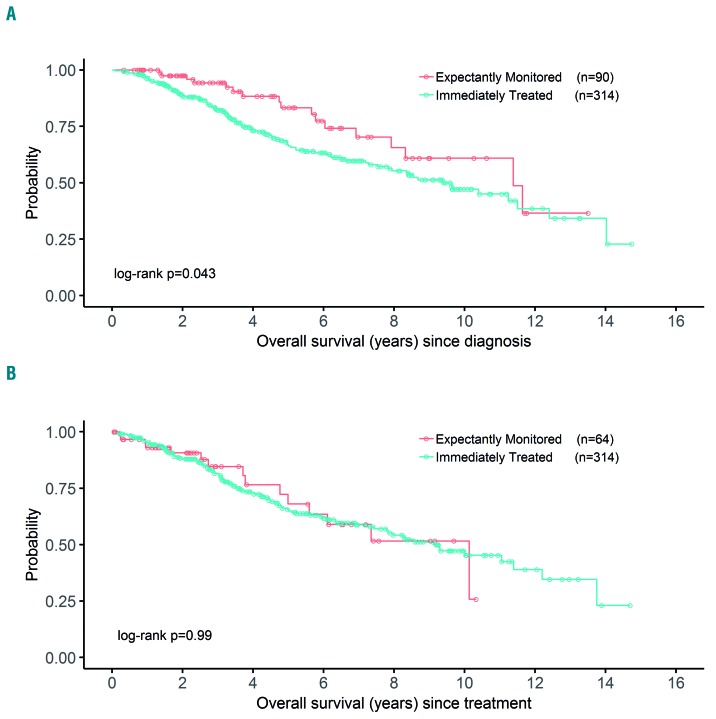
With a median follow up of 44 months (3.7 years) for survivors, OBS patients had superior OS compared to TX patients; OBS and TX patients had median OS of 11.4 years and 9.4 years, respectively (P=0.043) (Figure 2A). However, there was no difference in outcome between OBS and TX patients when comparing time from start of treatment to death (P=0.99) (Figure 2B), suggesting that the longer OS among OBS patients is not related to greater treatment sensitivity.
Figure 2.
Kaplan-Meier plots of overall survival (OS) from date of diagnosis and from start of therapy in patients who were initially observed or immediately treated. (A) OS since diagnosis. (B) OS since treatment.
Our study confirms that initial expectant monitoring is an appropriate management strategy for MCL patients and is not associated with inferior outcomes. We found the median duration of expectant monitoring in OBS patients was 23 months (95%CI: 17-28 months). In our cohort, 90 patients (22%) were identified to be candidates for expectant monitoring, and 84 of those patients (93%) were successfully monitored for six months or longer. Therefore, approximately 1 in 5 MCL patients can be initially observed. This proportion is concordant with published British Columbia Cancer Agency (BCCA) data.3 Martin et al. found that approximately one-third of MCL patients were monitored for six months or longer.5 In other population-based series, such as the National Cancer Database and Swedish and Danish Lymphoma Registries, the proportion of patients monitored for six months or longer is generally much lower, less than 10%.3,6 This suggests that more patients are selected for an initial watch-and-wait strategy at academic medical centers than in the general community.
The survival outcomes were excellent; median OS was 9.4 years among TX patients versus 11.4 years in OBS patients. The prolonged OS in OBS patients reflects their more favorable clinical presentation and disease biology.
The only clinical factor significantly correlated with longer TTI in our study was absence of lymphadenopathy. The BCCA report also found that a non-nodal presentation was associated with longer TTI and OS.3 These results reflect the indolence of non-nodal leukemic phase MCL that has biologically distinct features, including immunoglobulin heavy-chain variable region gene (IGHV) somatic hypermutation, SOX11 negativity, non- complex karyotype, and a unique gene-expression profile.7–10 Two other MCL clinical profiles described in our analysis and the BCCA series, and associated with observation for two years or longer, were non-bulky nodal disease and GI-tract-only disease, often incidentally discovered after routine colonoscopy.3
In MCL, alterations in TP53 have been shown to be associated with chemo-refractory disease and poor prognosis.11 TP53-mutant MCL does not appear to benefit from intensive induction chemotherapy followed by autologous stem cell transplant: median OS is only 1.8 years.11 The optimal therapy for MCL with TP53 alteration is not clear. In our analysis, 2 asymptomatic patients with low-volume lymphadenopathy and de novo TP53 mutation were successfully monitored for 18-20 months. Recently published data demonstrated that patients with leukemic non-nodal MCL and TP53 mutation had stable disease for 6-38 months without need for therapy.10 In the published BCCA series on observed MCL patients (n=75), although TP53 expression by immunohistochemistry (IHC) was associated with a significantly shorter OS, there was no significant difference between the observation periods for TP53-positive versus TP53-negative patients: median TTI was 18 months and 24 months, respectively.2 In CLL, the decision to initially monitor a patient is primarily made on the basis of clinical criteria.12 Deletion 17p CLL patients, particularly those who have favorable characteristics such as Rai stage 0 and a mutated IgVH gene, are candidates for initial observation. Our data suggest this paradigm should be similarly applied to MCL. In patients who harbor a genetic abnormality associated with resistance to cytotoxic anticancer drugs and shortened OS post-treatment, an initial phase of monitoring may critically prolong OS.13
An important prognostic marker in MCL is the Ki-67 proliferative index.14,15 In this study, MCL patients with Ki-67 of 30% or over were significantly more likely to receive immediate therapy versus observation, likely because elevated proliferative index is often associated with higher tumor burden and disease-related symptoms. This result may also reflect treating oncologists’ reluctance to initially observe a patient with a high-risk biological feature. However, OBS patients with Ki-67 of 30% or over had a median observation time of 22 months, and a subset of these patients were monitored for two years or longer. Again, in the BCCA series there was no difference in length of observation for patients with Ki-67 less than 30% or Ki-67 of 30% or more; the median observation period was 29 months versus 20 months, respectively. Collectively, these data reinforce the recommendation that the decision to initially monitor MCL patients should primarily be made based on clinical criteria, and even patients with high-risk biological factors, such as TP53 mutation or elevated proliferative index, may be appropriate candidates for initial monitoring.
Supplementary Material
Acknowledgments
Editorial support in the preparation of the manuscript was provided by Hannah Rice, ELS.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Solal-Celigny P, Lepage E, Brousse N, et al. Doxorubicin-containing regimen with or without interferon alfa-2b for advanced follicular lymphomas: final analysis of survival and toxicity in the Groupe d’Etude des Lymphomes Folliculaires 86 Trial. J Clin Oncol. 1998;16(7):2332–2338. [DOI] [PubMed] [Google Scholar]
- 2.Abrisqueta P, Scott DW, Slack GW, et al. Observation as the initial management strategy in patients with mantle cell lymphoma. Ann Oncol. 2017;28(10):2489–2495. [DOI] [PubMed] [Google Scholar]
- 3.Cohen JB, Han X, Jemal A, Ward EM, Flowers CR. Deferred therapy is associated with improved overall survival in patients with newly diagnosed mantle cell lymphoma. Cancer. 2016;122(15):2356–2363. [DOI] [PubMed] [Google Scholar]
- 4.Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23(6):703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin P, Chadburn A, Christos P, et al. Outcome of deferred initial therapy in mantle-cell lymphoma. J Clin Oncol. 2009;27(8):1209–1213. [DOI] [PubMed] [Google Scholar]
- 6.Abrahamsson A, Albertsson-Lindblad A, Brown PN, et al. Real world data on primary treatment for mantle cell lymphoma: a Nordic Lymphoma Group observational study. Blood. 2014;124(8):1288–1295. [DOI] [PubMed] [Google Scholar]
- 7.Kimura Y, Sato K, Imamura Y, et al. Small cell variant of mantle cell lymphoma is an indolent lymphoma characterized by bone marrow involvement, splenomegaly, and a low Ki-67 index. Cancer Sci. 2011;102(9):1734–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ondrejka SL, Lai R, Smith SD, Hsi ED. Indolent mantle cell leukemia: a clinicopathological variant characterized by isolated lymphocytosis, interstitial bone marrow involvement, kappa light chain restriction, and good prognosis. Haematologica. 2011;96(8):1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Royo C, Navarro A, Clot G, et al. Non-nodal type of mantle cell lymphoma is a specific biological and clinical subgroup of the disease. Leukemia. 2012;26(8):1895–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clot G, Jares P, Gine E. A new molecular assay and genomic complexity predict outcome in conventional and leukemic non-nodal mantle cell lymphoma. Blood. 2018;132(4):413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eskelund CW, Dahl C, Hansen JW, et al. TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood. 2017;130(17):1903–1910. [DOI] [PubMed] [Google Scholar]
- 12.Gribben JG. How I treat CLL up front. Blood. 2010;115(2):187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hientz K, Mohr A, Bhakta-Guha D, Efferth T. The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget. 2017;8(5):8921–8946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenwald A, Wright G, Wiestner A, et al. The proliferation gene expression signature is a quantitative integrator of oncogenic events that predicts survival in mantle cell lymphoma. Cancer Cell. 2003;3(2):185–197. [DOI] [PubMed] [Google Scholar]
- 15.Schaffel R, Hedvat CV, Teruya-Feldstein J, et al. Prognostic impact of proliferative index determined by quantitative image analysis and the International Prognostic Index in patients with mantle cell lymphoma. Ann Oncol. 2010;21(1):133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.