Abstract
We determined the incidence of disability related to chronic graft-versus-host disease (bronchiolitis obliterans, grade ≥2 keratoconjunctivitis sicca, sclerotic features or esophageal stricture) for three categories of alternative donor: cord blood, haplorelated marrow or peripheral blood with post-transplant cyclophosphamide, and unrelated single HLA-allele mismatched peripheral blood. Among 396 consecutive hematopoietic cell transplant recipients, 129 developed chronic graft-versus-host disease with 3-year cumulative incidences of 8% for cord blood, 24% for haplorelated grafts, and 55% for unrelated single HLA-allele mismatched peripheral blood. Disability rates were significantly lower for cord blood [hazard ratio (HR) 0.13; 95% confidence interval (CI): 0.1-0.4] and for the haplorelated group (HR 0.31; 95% CI: 0.1-0.7) compared to the rate in the group transplanted with unrelated single HLA-allele mismatched peripheral blood. Cord blood recipients were also >2-fold more likely to return to work/school within 3 years from the onset of chronic graft-versus-host disease (HR 2.54; 95% CI: 1.1-5.7, P=0.02), and the haplorelated group trended similarly (HR 2.38; 95% CI: 1.0-5.9, P=0.06). Cord blood recipients were more likely to discontinue immunosuppression than were recipients of unrelated single HLA-allele mismatched peripheral blood (HR 3.96; 95% CI: 1.9-8.4, P=0.0003), similarly to the haplorelated group (HR 4.93; 95% CI: 2.2-11.1, P=0.0001). Progression-free survival and non-relapse mortality did not differ between groups grafted from different types of donors. Our observations that, compared to recipients of unrelated single HLA-allele mismatched peripheral blood, recipients of cord blood and haplorelated grafts less often developed disability related to chronic graft-versus-host disease, and were more likely to resume work/school, should help better counseling of pre-hematopoietic cell transplant candidates.
Introduction
Hematopoietic cell transplantation (HCT) can be accomplished with grafts from alternative donors for patients lacking an HLA-matched related or unrelated donor. The optimal choice of an alternative donor stem cell source remains an open question and is influenced by several factors including chronic graft-versus-host disease (GvHD). Chronic GvHD is a heterogeneous syndrome associated with major morbidity and adverse effects on quality of life and functionality among long-term allogeneic HCT survivors.1–3 Because a diagnosis of chronic GvHD does not always indicate significant morbidity and poor quality of life, the frequency of severe chronic GvHD manifestations (e.g. severe keratoconjunctivitis sicca, bronchiolitis obliterans, cutaneous scleroderma, joint/fasciae features, and esophageal stricture requiring dilation), the duration of immunosuppressive therapy and resumption of pretransplant activities (e.g., work/school) may serve as better measures of outcome after HCT.
The aim of this study was to analyze chronic GvHD manifestations most likely to be associated with disability, requirement of secondary systemic treatment, discontinuation of systemic immunosuppressive therapy and functional outcomes among recipients of grafts from alternative HCT donors. Differences in these clinical outcomes could help inform patients about outcomes after HCT from alternative donors.
Methods
Patients and donors
This retrospective study included all consecutive adult patients who received a first alternative donor HCT for any underlying diagnosis at Fred Hutchinson Cancer Research Center/Seattle Cancer Care Alliance between 2006 and 2015 and subsequently developed chronic GvHD that required systemic treatment. The alternative grafts included unrelated 4–6/6-HLA-matched single or double umbilical cord blood units (UCB), related HLA-haploidentical bone marrow or mobilized peripheral blood stem cells plus post-transplant cyclophosphamide (Haplo/PTCY), and mobilized peripheral blood stem cells from unrelated donors with a single HLA allele mismatched at an A, B, C or DRB1 locus by high resolution typing (1-mMUD), regardless of whether the mismatch resulted in antigen disparity at the locus. Patients had given written consent allowing the use of medical records for research in accordance with the Declaration of Helsinki, and the institutional review board approved the study.
Clinical assessments and definitions
Involved sites and types of treatment at the onset of first systemic teraphy of chronic GvHD and treatment changes after initial teraphy were recorded prospectively via the Long-Term Follow-Up Program through medical records from our outpatient clinic and local clinics that provided primary care for patients. All patients were screened for evidence of chronic GvHD between days 80 and 100 after HCT, at 1 year after HCT, and whenever clinically indicated to establish the diagnosis of chronic GvHD or to determine treatment.
Acute GVHD was graded according to previously described criteria.4 Chronic GvHD was diagnosed using the 2014 National Institutes of Health consensus criteria.5 Disability related to chronic GvHD was defined as 2014 National Institutes of Health consensus grade 2 or 3 keratoconjunctivitis sicca, grade 2 or 3 scleroderma, any grade of bronchiolitis obliterans, grade 2 or 3 joint/fasciae involvement, or grade 3 esophageal stricture requiring dilation. While vulvovaginal chronic GvHD can result in fibrosis, this manifestation is under reported and unlikely to result in disability by itself, and thus not included in our study. National Institutes of Health score 2 or 3 gastrointestinal, oral or hepatic manifestations reflect GvHD activity but are less likely to cause irreversible damage and were also not included in our study. Return to work or school was considered only for patients who were working or in school before the HCT indication was diagnosed and had not resumed those activities before the onset of chronic GvHD. Treatment change was defined as any additional systemic treatment not used for the initial treatment of chronic GvHD. An increase in steroid dose in patients who were initially treated with steroid was not considered as a treatment change, because temporary increases in steroid doses or resumption of steroid treatment are often necessary during the initial treatment of chronic GvHD.6 Discontinuation of systemic immunosuppression was defined as cessation of treatment for at least 6 months after resolution of chronic GvHD.
Statistical analysis
The main endpoints of this study were chronic GvHD manifestations associated with disability and impaired functional outcomes (i.e., return to work/school after the diagnosis of chronic GvHD, discontinuation of systemic immunosuppressive therapy, and change in Karnovsky Performance Status). We also compared the overall severity of chronic GvHD at initial diagnosis and the incidences of avascular necrosis, new systemic immunosuppression or treatment after first-line therapy for chronic GvHD, non-relapse mortality and overall survival after chronic GvHD diagnosis.
The chronic GvHD characteristics between donor groups were compared using a χ2 test for categorical variables and Wilcoxon rank-sum test for continuous variables. Overall survival was estimated by the Kaplan-Meier method. Cumulative incidences of chronic GvHD and of events after the onset of chronic GvHD were estimated by methods for competing risks, as previously described.7 Death was a competing event for all risks except non-relapse mortality; relapse was a competing event for non-relapse mortality. All comparisons of time-to-event endpoints were performed using Cox regression. The analysis of return to work or school was restricted to patients who were working or in school before HCT, and had not returned to work or school before the onset of chronic GvHD. The analysis of high morbidity was based on the first defining complication. All P-values are two-sided and unadjusted for multiple comparisons.
Results
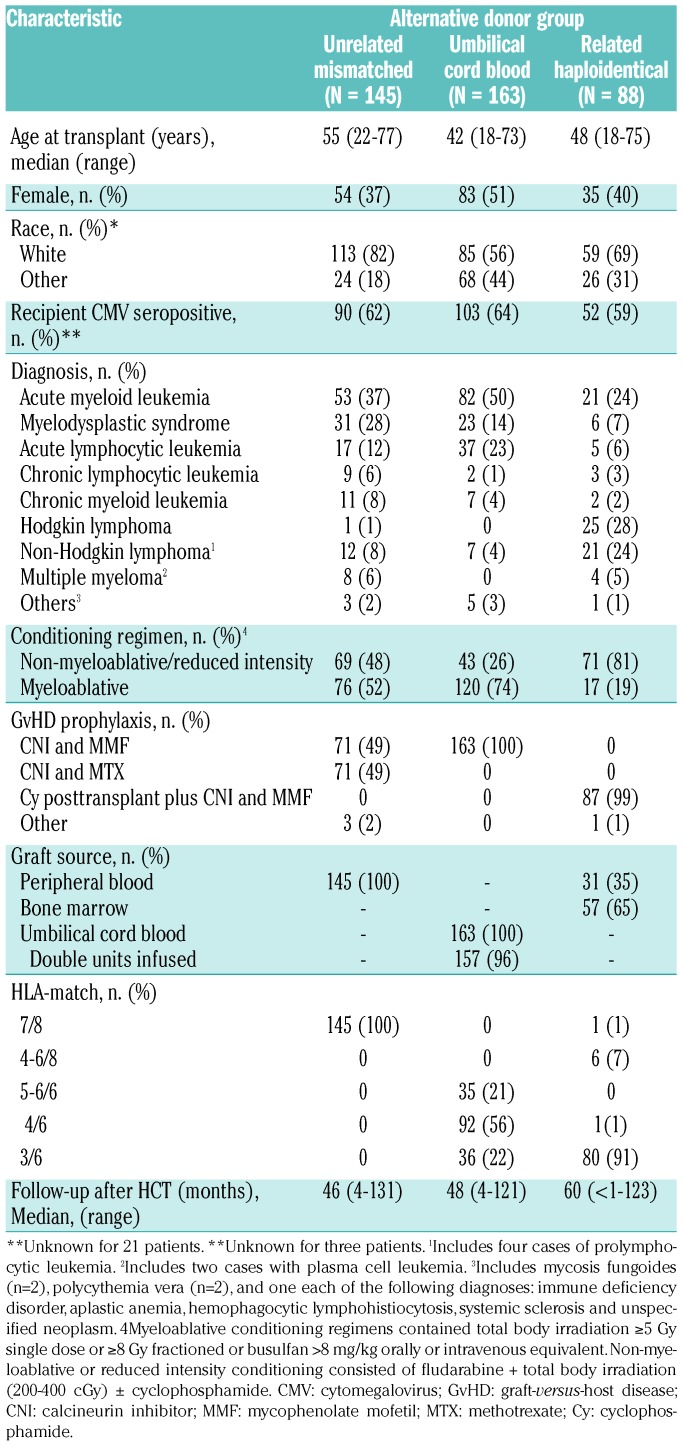
We identified 396 alternative donor HCT recipients who received a first allogeneic transplant for any disease between 2006 and 2015 at our center. The median age at HCT was 42 years (range, 18-73) for UCB, 48 years (range, 18-75) for Haplo/PTCY, and 55 years (range, 22-77) for 1-mMUD HCT recipients. Acute myeloid leukemia and myelodysplastic syndrome were the most common diagnoses at HCT, 64% and 65% among the UCB and 1-mMUD HCT recipients, respectively, while lymphomas were the most common diagnosis (52%) among the Haplo/PTCY HCT recipients. Other demographic characteristics are summarized in Table 1.
Table 1.
Characteristics of the study population according to alternative donor group.
Chronic graft-versus-host disease
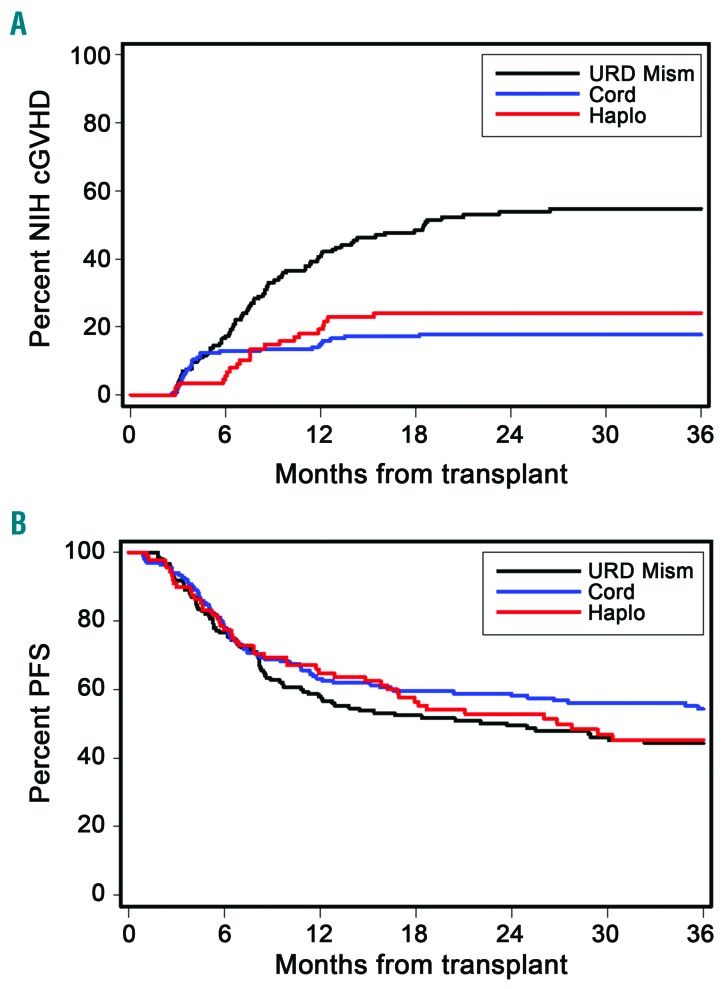
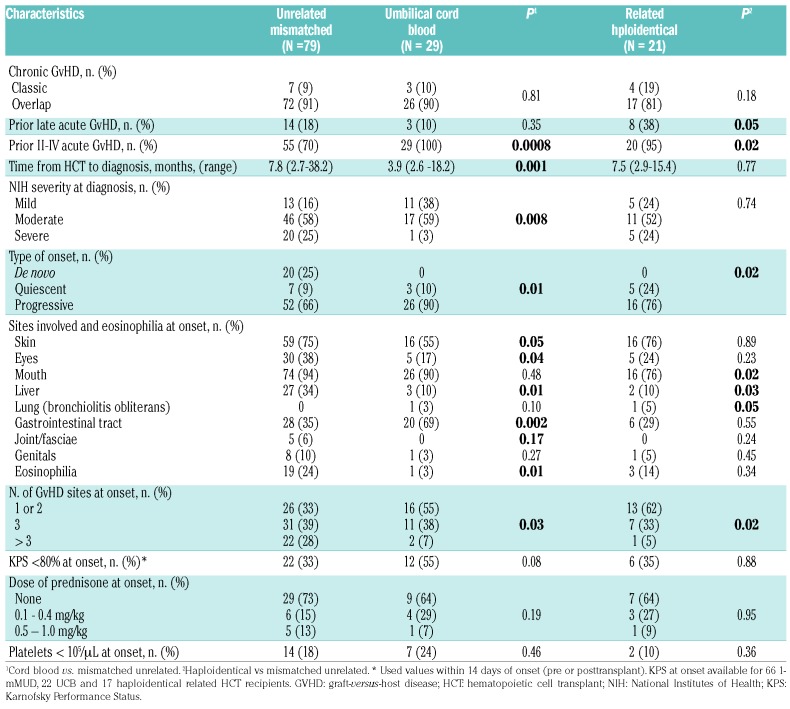
Of the 396 alternative donor HCT recipients, 129 developed chronic GvHD that required systemic treatment and were included in this study. The cases of treatment-requiring chronic GvHD were diagnosed in 29 of the 163 UCB HCT recipients, 21 of 88 Haplo/PTCY HCT recipients and 79 of 145 1-mMUD HCT recipients, for 3-year cumulative incidences of 18%, 24% and 55%, respectively. The rate of chronic GvHD was significantly lower in UCB and Haplo/PTCY recipients than in 1-mMURD recipients [hazard ratio (HR)=0.23; 95% confidence interval (95% CI): 0.2-0.4), P<0.0001, and HR=0.29 (95% CI: 0.2-0.5), P<0.0001, respectively] (Figure 1A). The incidence of chronic GvHD was comparable between patients who received 1-mMUD grafts with either allelic or antigenic mismatches (data not shown). Progression-free survival after HCT did not differ among the three donor groups (Figure 1B). The median follow-up times after the onset of chronic GvHD were 48 (range, 4–121) months for UCB, 60 (range, <1–123) for Haplo/PTCY, and 46 (range, 4–131) for 1-mMUD HCT. The median time from HCT to diagnosis of chronic GvHD was shorter in the UCB recipients than in 1-mMUD HCT recipients [3.9 (range, 2.6-18.2) versus 7.8 (range, 2.7-38.2) months, P=0.001]. As shown in Table 2, the incidence frequencies of overlap chronic GvHD (>80%) and of prior acute GvHD (>70%) were high in all three groups. These findings are consistent with the frequent diagnosis of upper gastrointestinal GvHD at our center.8 The severity of chronic GvHD at diagnosis was significantly lower in the UCB group than in the 1-mMUD group (P=0.008) (Table 2), but the severity of GvHD manifestations at the onset of chronic GvHD did not differ between the Haplo/PTCY and the 1-mMUD groups (P=0.74) (Table 2). According to the National Institutes of Health Global Severity scale, the incidence of moderate or severe chronic GvHD at diagnosis was 62% in the UCB group, 76% in the Haplo/PTCY group and 83% in the 1-mMUD group. Table 2 displays additional characteristics of the chronic GvHD according to the alternative donor HCT groups.
Figure 1.
The cumulative incidence of chronic graft-versus-host disease is higher in the mismatched unrelated donor group than in the cord blood or HLA-haploidentical groups, but survival does not differ between the groups. Cumulative incidence of (A) chronic graft-versus-host disease and (B) progression-free survival after transplant according to alternative donor hematopoietic cell transplant group. NIH: National Institutes of Health; cGVHD: chronic graft-versus-host disease; URD Mism: mismatched unrelated donor; Cord: umbilical cord blood; Haplo: haplorelated bone marrow or peripheral blood; PFS: progression-free survival.
Table 2.
Characteristics of chronic graft-versus-host disease according to alternative donor group.
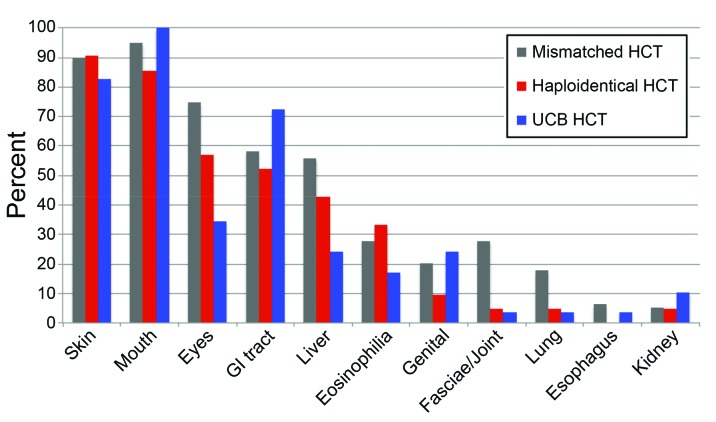
Sites of chronic GvHD and the presence of eosinophilia at any time during the course of chronic GvHD among the three alternative donor HCT groups are displayed in Figure 2. Mouth and skin were the most common sites of chronic GvHD in the three groups (>80%). Eyes were affected by chronic GvHD of any degree at any time in 75% of the 1-mMUD, 52% of the Haplo/PTCY, and 34% of the UCB HCT recipients. The same pattern was identified for hepatic involvement at any time (56% of 1-mmUD, 43% of Haplo/PTCY, and 24% of UCB HCT recipients). On the other hand, gastrointestinal tract involvement at any time developed in 72% of the UCB patients, but in 58% of the 1-mmUD and 52% of the Haplo/PTCY HCT recipients.
Figure 2.
Patterns of organ involvement differ according to the type of alternative donor. The figure shows the proportions of patients with involved sites or signs of chronic graft-versus-host disease at any time from initial diagnosis to last follow-up according to alternative donor HCT group. Patients could have more than one site involved. GI, gastrointestinal; HCT, hematopoietic cell transplant; UCB: umbilical cord blood.
Results of major outcomes according to the alternative donor HCT groups are shown in Table 3.
Table 3.
Outcomes analyzed according to alternative hematopoietic cell transplant donor group.
Chronic graft-versus-host disease manifestations of high morbidity
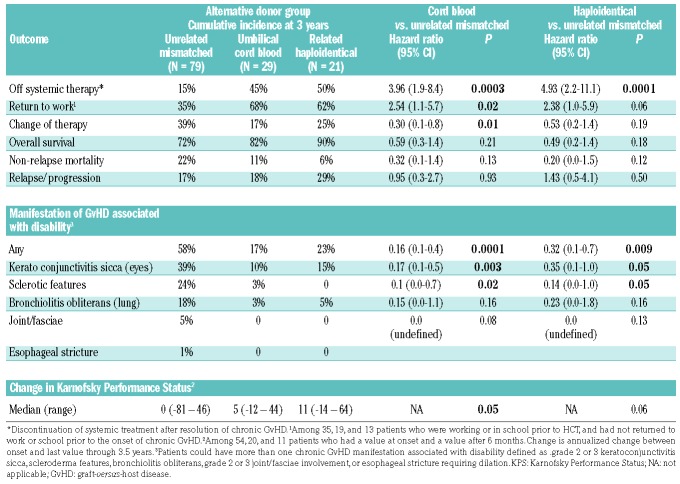
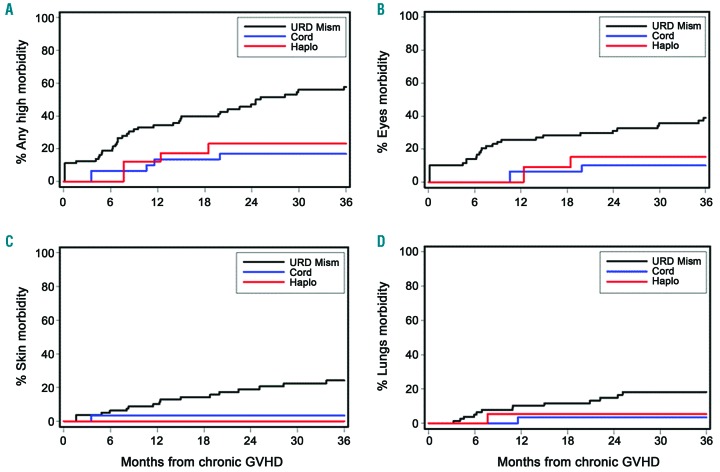
The cumulative incidence of any manifestation of high morbidity at 3 years after the diagnosis of chronic GvHD is shown in Figure 3A and was significantly lower in the UCB (17%) and Haplo/PTCY (23%) groups than in the 1-mMUD group (58%) [HR 0.16 (95% CI: 0.1-0.4); P=0.0001, and HR 0.32 (95% CI: 0.1-0.7), P=0.009, respectively], (Figure 3A). Table 3 shows the distribution of chronic GvHD manifestations of high morbidity according to the three HCT donor groups. The most frequent high morbidity was keratoconjunctivitis sicca followed by sclerosis and bronchiolitis obliterans (lungs). Moderate or severe joint/fasciae involvement and esophageal stricture requiring dilation were less frequent high morbidity manifestations. The cumulative incidence of keratoconjunctivitis sicca was significantly lower in the UCB group than in the 1-mMUD group (10% versus 39%, P=0.003) and was also lower in the Haplo/PTCY group (10%, P=0.05). The 3-year cumulative incidence of any high morbidity and the three most frequent high morbidity chronic GvHD manifestations according to the alternative donor groups are displayed in Figure 3.
Figure 3.
Patients in the group grafted from a mismatched unrelated donor had a high cumulative incidence of disability caused by morbidity involving the skin, eyes and lungs. Cumulative incidence of: (A) any manifestations of chronic GVHD associated with disability; (B) moderate or severe keratoconjunctivitis sicca, (C) skin sclerosis and (D) bronchiolitis obliterans according to alternative donor HCT group. URD Mism: mismatched unrelated donor; Cord: umbilical cord blood; Haplo: haplorelated bone marrow or peripheral blood; GVHD: graft-versus-host disease.
Duration of immunosuppressive therapy and change in systemic therapy
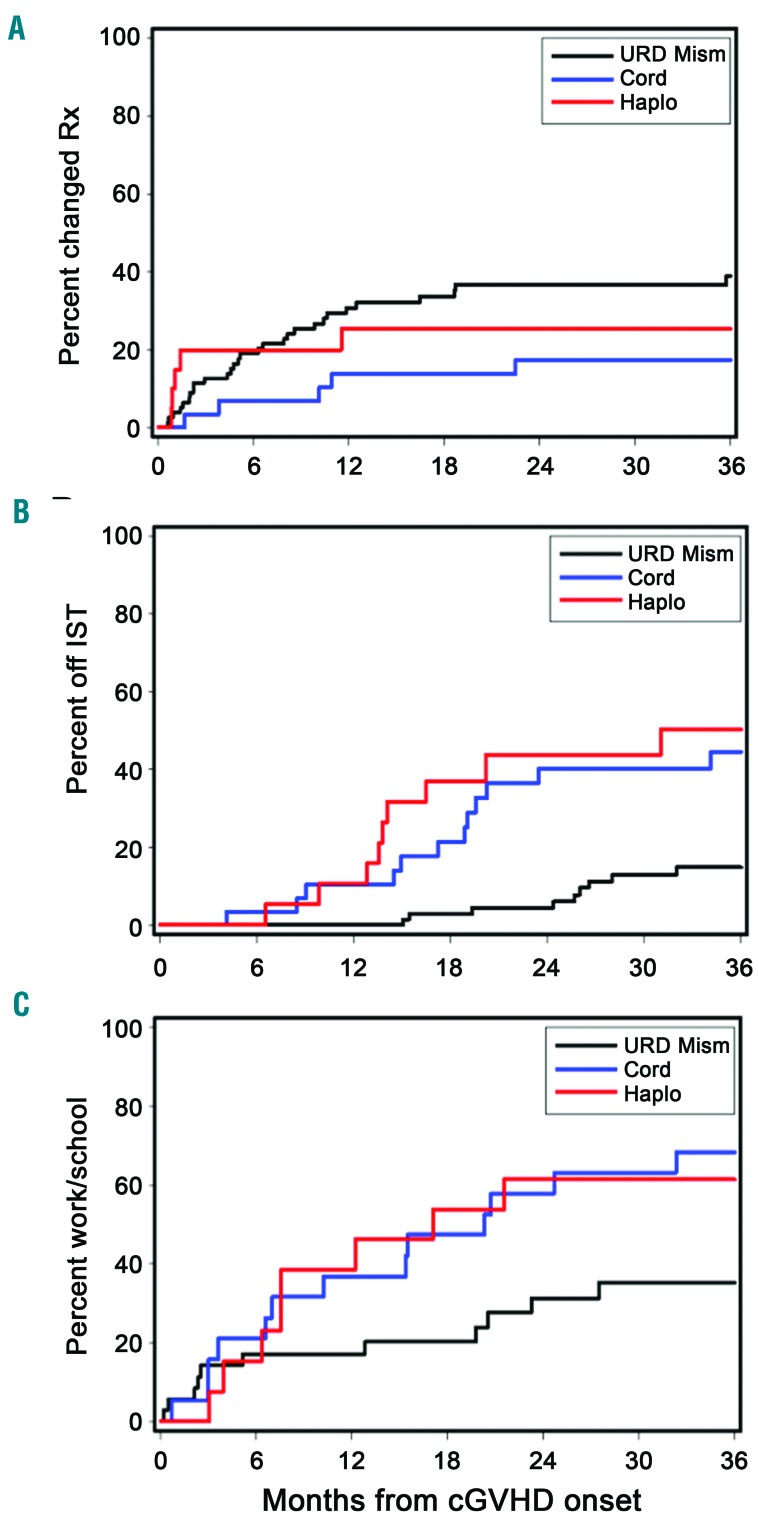
The proportion of patients in each group requiring changes in systemic therapy for control of chronic GvHD at 3 years after first-line treatment was 17% for the UCB group, 25% for the Haplo/PTCY groups and 39% for the 1-mMUD group, and was significantly lower for the UCB group than for the 1-mMUD group [HR 0.30 (95% CI: 0.1-0.8), P=0.01] (Figure 4A and Table 3).
Figure 4.
Patients in the mismatched unrelated donor group had a high cumulative incidence of second-line treatment, and withdrawal of immunosuppressive treatment and return to work or school were delayed. Cumulative incidence of (A) change of systemic therapy after first-line therapy for chronic GVHD; (B) discontinuation of systemic immunosuppressive therapy, and (C) return to work/school after the diagnosis of chronic GVHD according to the alternative HCT group. Rx: treatment; IST: immunosuppressive therapy; URD Mism, mismatched unrelated peripheral blood stem cell; Haplo, related haploidentical; cGVHD, chronic graft-versus-host disease.
The cumulative incidence of discontinued systemic immunosuppression at 3 years was significantly lower in the 1-mMUD (15%) group than in the UCB (45%) and Haplo/PTC (50%) groups [HR 3.96 (95% CI: 1.9–8.4), P=0.0003, and HR 4.93 (95% CI: 2.2–11.1), P=0.0001, respectively] (Figure 4B).
Functional endpoints
A higher proportion of UCB patients than 1-mMUD HCT recipients (68% versus 35%) returned to work or school within 3 years after the onset of chronic GvHD [HR 2.54 (95% CI: 1.1-5.7), P=0.02], and a similar trend was observed in the Haplo/PTCY group [62% versus 35%, HR 2.38 (95% CI: 1.0-5.9), P=0.06] (Figure 4C). Eighteen patients had returned to work or school before the onset of chronic GvHD (14 in the 1-mMUD and 4 in the Haplo/PTCY HCT groups). We also found trends suggesting improved annualized Karnovsky Performance Status change from the onset of chronic GvHD to 3.5 years afterwards in the UCB [+5 (range, −12 to +44)] and Haplo/PTCY [+11 (range, −14 to +64)] groups compared to the 1-mMUD group [0 (range, −81 to +46)] (P=0.05 and P=0.06, respectively). We found no difference in avascular necrosis at 3 years among the three alternative donor groups (12% in the 1-mMUD, 12% in the UCB, and 5% in the Haplo/PTCY HCT recipients).
Survival endpoints
The cumulative incidence of non-relapse mortality at 3 years among patients with chronic GvHD was 22% in 1-mMUD recipients, 11% in UCB recipients, and 6% in the Haplo/PTCY recipients, with no statistically significant differences between the three groups [1-mMUD versus UCB, HR 0.32 (95% CI: 0.1-1.4), P=0.13; 1-mMUD versus Haplo/PTCY, HR 0.20 (95% CI: 0.0-1.5), P=0.12] (Table 3). The cumulative incidence of overall survival at 3 years among patients with chronic GvHD was 72% in 1-mMUD recipients, 82% in UCB recipients, and 90% in Haplo/PTCY recipients, with no statistically significant differences between the three groups [1-mMUD versus UCB, HR 0.59 (95% CI: 0.3-1.4), P=0.21; 1-mMUD versus Haplo/PTCY, HR 0.49 (95% CI: 0.2-1.4), P=0.18] (Table 3). Progression-free survival after HCT did not differ between the three groups (Figure 1B).
Discussion
In parallel with the lower rates of overall chronic GvHD, we demonstrated that the cumulative incidence of chronic GvHD manifestations associated with disability was significantly lower after UCB and Haplo/PTCY HCT than that after 1-mMUD HCT.
The overall incidence of chronic GvHD in our study was lower in the UCB and Haplo/PTCY HCT cohorts than in the 1-mMUD HCT cohort, similar to results of previous studies.9–17 The overall incidence of chronic GvHD after 1-mMUD HCT was higher in our study than in previous reports.9,11,13,17–21 This difference may be explained by the higher proportion of bone marrow grafts and more frequent use of T-cell depletion in the previous studies.9,11,13,17,18,22 Older age could also be a contributor to the higher rates of chronic GvHD in the 1-mMUD group compared to the UCB and Haplo/PTCY groups.
Keratoconjunctivitis sicca was the most common chronic GvHD manifestation of high morbidity. Of note, the incidence of severe keratoconjunctivitis sicca at any time after the diagnosis of chronic GvHD was at least 4-fold higher in the 1-mMUD group than in the UCB group and at least 2-fold higher in the 1-mMUD group than in the Haplo/PTCY group. Prospective trials have shown that quality of life is impaired in patients with chronic GvHD involving the eyes.23,24
Sclerotic manifestations of chronic GvHD were the second most common severe morbidity in the three donor groups, but were seen in less than 5% of the UCB and Haplo/PTCY groups as compared to nearly a quarter of the 1-mMUD HCT group. The reported overall cumulative incidence of sclerotic chronic GvHD after HCT is approximately 20%, with lower rates in recipients of HLA-mismatched HCT than in recipients of HLA-matched HCT.25,26 The use of growth factor-mobilized blood cells is a known risk factor for the development of sclerotic GvHD after HCT. The frequent use of mobilized blood cells could explain the 25% incidence of sclerotic manifestations in our 1-mMUD HCT group. The remarkably low rate of sclerotic manifestations in the UCB cohort is consistent with prior reports of outcomes after UCB HCT.26,27
In order to assess protracted chronic GvHD, we evaluated the duration of systemic therapy used to control the disease manifestations and the impact on functional outcomes among the three alternative donor groups. A large proportion of UCB (45%) and Haplo/PTCY (59%) HCT recipients were able to discontinue all systemic treatment for chronic GvHD by 3 years after onset of the condition, in contrast to only 15% of the 1-mMUD group. The use of mobilized blood as the stem cell graft and the involvement of multiple sites at initial diagnosis are risk factors for prolonged immunosuppressive treatment.28–30 The significantly lower incidence of second-line systemic immunosuppressive treatment of chronic GvHD in the UCB and Haplo/PTCY groups than in the 1-mMUD group also supports the conclusion that the severity of chronic GvHD is greater after HCT with 1-mMUD donors than with UCB or HLA-haploidentical related donors.
The presence of chronic GvHD has been consistently associated with failure to return to work or school, limited resilience and poor quality of life among HCT survivors.1–3,31 In our study, a significantly higher proportion of the UCB group were more likely to return to work or school compared to the that of the 1-mMUD group, and a similar benefit trend was also evident for the Haplo/PTCY group, supporting the conclusion that GvHD manifestations associated with disability frequently impaired recovery of pre-transplant function in the 1-mMUD group.
Our study has several limitations. First, the three groups were heterogeneous with respect to the patients’ age, gender, race, underlying disease and conditioning regimen intensity. Among these factors, only the patients’ age has been associated with an increased incidence of chronic GvHD. An association of older patients’ age with higher risk of chronic GvHD has not been consistently observed after UCB HCT, and very few data addressing this issue are available for Haplo/PTCY recipients.32–37 A second limitation of the study is that the small numbers of patients with chronic GvHD in the UCB and Haplo/PTCY groups precluded a direct comparison of outcomes between these two groups. Third, we included both one-antigen and allele-mismatched unrelated donor peripheral blood stem cell recipients irrespective of the “direction” of mismatching. However, there was no significant difference in incidence of chronic GvHD between these mismatched unrelated donor groups (data not shown), consistent with the results of a large, previous study from the Center for International Blood and Marrow Transplant Research.38 Fourth, the incidence of chronic GvHD in the 1-mMUD group in our study may not be representative of results with T-cell depleted 1-mMUD grafts.26,34,39,40 Moreover, considering that mobilized blood stem cells were the graft source used for the 1-mMUD HCT group in our study, the risk and morbidity of chronic GvHD with unrelated donor bone marrow grafting might not differ from those for UCB or Haplo/PTCY HCT. Fifth, we were unable to assess quality of life in this study, because very few patients had outcomes based on validated measurement instruments. Sixth, the duration of immunosuppression may be driven ultimately by the selection of stem cell sources associated with a higher risk of severe chronic GvHD (i.e., unrelated mobilized blood cells) or with a lower risk of severe chronic GvHD (i.e., UCB or Haplo/PTCY). Seventh, some manifestations of chronic GvHD may have been underreported in this retrospective study (e.g., genital or lung involvement) Lastly, the analysis of functional endpoints at 3 years is limited by the small numbers.
In conclusion, our results show that compared to 1-mMUD recipients, UCB and Haplo/PTCY HCT recipients were less likely to develop chronic GvHD, were less likely to develop disability related to chronic GvHD, had a shorter duration of systemic treatment for chronic GvHD and returned to work or school earlier. While our data do not imply superiority of one alternative transplant strategy versus another (e.g. Haplo/PTCY versus 1-mMUD peripheral blood versus UCB), the current study provides detailed information regarding disability related to chronic GvHD when it occurs after alternative donor HCT. These findings should help transplant providers to counsel pre-HCT candidates better. Determination of disability associated with chronic GvHD is necessary in prospective studies testing alternative donor graft products such as Haplo/PTCY versus UCB to evaluate the overall efficacy of these strategies.
Supplementary Material
Acknowledgments
We thank Chris Davis, Kevin Bray and Aaron Johnson for their help in retrieving and assembling the data. We also thank Helen Crawford for assistance in submission of this paper.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/104/4/835
Funding
This work was supported in part by grants from the National Institutes of Health, National Cancer Institute (CA018029, CA118953, P30 CA015704) and in part by the National Heart, Lung and Blood Institute (HL122173). GF was supported by a grant from the Division of Hematology and Transfusion Medicine of the University of Sao Paulo, SP, Brazil.
References
- 1.Wong FL, Francisco L, Togawa K, et al. Long-term recovery after hematopoietic cell transplantation: predictors of quality-of-life concerns. Blood. 2010;115(12):2508–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg AR, Syrjala KL, Martin PJ, et al. Resilience, health, and quality of life among long-term survivors of hematopoietic cell transplantation. Cancer. 2015;121(23):4250–4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SJ, Logan B, Westervelt P, et al. Comparison of patient-reported outcomes in 5-year survivors who received bone marrow vs peripheral blood unrelated donor transplantation: long-term follow-up of a randomized clinical trial. JAMA Oncol. 2016;2(12):1583–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leisenring WM, Martin PJ, Petersdorf EW, et al. An acute graft-versus-host disease activity index to predict survival after hematopoietic cell transplantation with myeloablative conditioning regimens. Blood. 2006;108(2): 749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant. 2015;21(3):389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin PJ, Storer BE, Rowley SD, et al. Evaluation of mycophenolate mofetil for initial treatment of chronic graft-versus-host disease. Blood. 2009;113(21):5074–5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. [DOI] [PubMed] [Google Scholar]
- 8.Martin PJ, McDonald GB, Sanders JE, et al. Increasingly frequent diagnosis of acute gastrointestinal graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2004;10(5):320–327. [DOI] [PubMed] [Google Scholar]
- 9.Eapen M, Rocha V, Sanz G, et al. Effect of graft source on unrelated donor haemopoietic stem-cell tranpslantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol. 2010;11(7):653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunstein CG, Fuchs EJ, Carter SL, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated umbilical cord blood grafts. Blood. 2011;118(2):282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raiola AM, Dominietto A, di Grazia C, et al. Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts. Biol Blood Marrow Transplant. 2014;20(10):1573–1579. [DOI] [PubMed] [Google Scholar]
- 12.Di Stasi A, Milton DR, Poon LM, et al. Similar transplantation outcomes for acute myeloid leukemia and myelodysplastic syndrome patients with haploidentical versus 10/10 human leukocyte antigen-matched unrelated and related donors. Biol Blood Marrow Transplant. 2014;20(12):1975–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malard F, Milpied N, Blaise D, et al. Effect of graft source on unrelated donor hemopoietic stem cell transplantation in adults with acute myeloid leukemia after reduced-intensity or nonmyeloablative conditioning: a study from the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. Biol Blood Marrow Transplant. 2015;21(6):1059–1067. [DOI] [PubMed] [Google Scholar]
- 14.Bashey A, Zhang MJ, McCurdy SR, et al. Mobilized peripheral blood stem cells versus unstimulated bone marrow as a graft source for T-cell-replete haploidentical donor transplantation using post-transplant cyclophosphamide. J Clin Oncol. 2017;35(26):3002–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanate AS, Mussetti A, Kharfan-Dabaja MA, et al. Reduced-intensity transplantation for lymphomas using haploidentical related donors versus HLA-matched unrelated donors. Blood. 2016;127(7):938–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gauthier J, Castagna L, Garnier F, et al. Reduced-intensity and non-myeloablative allogeneic stem cell transplantation from alternative HLA-mismatched donors for Hodgkin lymphoma: a study by the French Society of Bone Marrow Transplantation and Cellular Therapy. Bone Marrow Transplant. 2017;52(5):689–696. [DOI] [PubMed] [Google Scholar]
- 17.Robin M, Ruggeri A, Labopin M, et al. Comparison of unrelated cord blood and peripheral blood stem cell transplantation in adults with myelodysplastic syndrome after reduced-intensity conditioning regimen: a collaborative study from Eurocord (Cord Blood Committee of Cellular Therapy & Immunobiology Working Party of EBMT) and Chronic Malignancies Working Party. Biol Blood Marrow Transplant. 2015;21(3): 489–495. [DOI] [PubMed] [Google Scholar]
- 18.Malard F, Furst S, Loirat M, et al. Effect of graft source on mismatched unrelated donor hemopoietic stem cell transplantation after reduced intensity conditioning. Leukemia. 2013;27(11):2113–2117. [DOI] [PubMed] [Google Scholar]
- 19.Marks DI, Woo KA, Zhong X, et al. Unrelated umbilical cord blood transplant for adult acute lymphoblastic leukemia in first and second complete remission: A comparison with allografts from adult unrelated donors. Haematologica. 2014;99(2):322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Granier C, Biard L, Masson E, et al. Impact of the source of hematopoietic stem cell in unrelated transplants: comparison between 10/10, 9/10-HLA matched donors and cord blood. Am J Hematol. 2015;90(10):897–903. [DOI] [PubMed] [Google Scholar]
- 21.Piemontese S, Ciceri F, Labopin M, et al. A comparison between allogeneic stem cell transplantation from unmanipulated haploidentical and unrelated donors in acute leukemia. J Hematol Oncol. 2017;10(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandes JF, Rocha V, Labopin M, et al. Transplantation in patients with SCID: mismatched related stem cells or unrelated cord blood? Blood. 2012;119(12):2949–2955. [DOI] [PubMed] [Google Scholar]
- 23.Sun YC, Chai X, Inamoto Y, et al. Impact of ocular chronic graft-versus-host disease on quality of life. Biol Blood Marrow Transplant. 2015;21(9):1687–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saboo US, Amparo F, Abud TB, Schaumberg DA, Dana R. Vision-related quality of life in patients with ocular graft-versus-host disease. Ophthalmology. 2015;122(8):1669–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inamoto Y, Storer BE, Petersdorf EW, et al. Incidence, risk factors and outcomes of sclerosis in patients with chronic graft-versus- host disease. Blood. 2013;121(25):5098–5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Detrait MY, Morisset S, Peffault de Latour R, et al. Pre-transplantation risk factors to develop sclerotic chronic GvHD after allogeneic HSCT: a multicenter retrospective study from the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire (SFGM-TC). Bone Marrow Transplant. 2015;50(2): 253–258. [DOI] [PubMed] [Google Scholar]
- 27.Newell LF, Flowers ME, Gooley TA, et al. Characteristics of chronic GVHD after cord blood transplantation. Bone Marrow Transplant. 2013;48(10):1285–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flowers MED, Parker PM, Johnston LJ, et al. Comparison of chronic graft-versus-host disease after transplantation of peripheral blood stem cells versus bone marrow in allogeneic recipients: long-term follow-up of a randomized trial. Blood. 2002;100(2):415–419. [DOI] [PubMed] [Google Scholar]
- 29.Stewart BL, Storer B, Storek J, et al. Duration of immunosuppressive treatment for chronic graft-versus-host disease. Blood. 2004;104(12):3501–3506. [DOI] [PubMed] [Google Scholar]
- 30.Vigorito AC, Campregher PV, Storer BE, et al. Evaluation of NIH consensus criteria for classification of late acute and chronic GVHD. Blood. 2009;114(3):702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamilton JG, Wu LM, Austin JE, et al. Economic survivorship stress is associated with poor health-related quality of life among distressed survivors of hematopoietic stem cell transplantation. Psychooncology. 2013;22(4):911–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Storb R, Prentice RL, Sullivan KM, et al. Predictive factors in chronic graft-versus-host disease in patients with aplastic anemia treated by marrow transplantation from HLA-identical siblings. Ann Intern Med. 1983;98(4):461–466. [DOI] [PubMed] [Google Scholar]
- 33.Carlens S, Ringden O, Remberger M, et al. Risk factors for chronic graft-versus-host disease after bone marrow transplantation: a retrospective single centre analysis. Bone Marrow Transplant. 1998;22(8):755–761. [DOI] [PubMed] [Google Scholar]
- 34.Atkinson K, Horowitz MM, Gale RP, et al. Risk factors for chronic graft-versus-host disease after HLA-identical sibling bone marrow transplantation. Blood. 1990;75(12): 2459–2464. [PubMed] [Google Scholar]
- 35.Flowers MED, Inamoto Y, Carpenter PA, et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood. 2011;117(11):3214–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanda J, Nakasone H, Atsuta Y, et al. Risk factors and organ involvement of chronic GVHD in Japan. Bone Marrow Transplant. 2014;49(2):228–235. [DOI] [PubMed] [Google Scholar]
- 37.Lazaryan A, Weisdorf DJ, DeFor T, et al. Risk factors for acute and chronic graft-versus-host disease after allogeneic hematopoietic cell transplantation with umbilical cord blood and matched sibling donors. Biol Blood Marrow Transplant. 2016;22(1):134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110(13):4576–4583. [DOI] [PubMed] [Google Scholar]
- 39.Socie G, Schmoor C, Bethge WA, et al. Chronic graft-versus-host disease: long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood. 2011;117(23):6375–6382. [DOI] [PubMed] [Google Scholar]
- 40.Uhm J, Hamad N, Shin EM, et al. Incidence, risk factors, and long-term outcomes of sclerotic graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20(11): 1751–1757. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.