Extramedullary (EM) disease is an unusual manifestation of de novo acute myeloid leukemia (AML) occurring in less than 10% of patients at diagnosis. EM AML may manifest as cellular infiltrates of soft tissues (myeloid sarcomas), lymph nodes, skin (leukemia cutis) and leptomeninges, and is generally associated with a poor prognosis.1–3 Therapeutic approaches are varied, but most include combination chemotherapy, radiation therapy and intrathecal therapy.4–6 To obtain a better understanding of EM AML we evaluated a subset of younger (<61 years) patients, presenting with clinical EM disease at the time of diagnosis, who were treated as part of the Eastern Cooperative Oncology Group – American College of Radiology Imaging Network (ECOG-ACRIN) Cancer Research Group E1900 trial. We describe the clinical, chromosomal, molecular and immunophenotypic characteristics, and the outcomes following standard-dose versus high-dose daunorubicin in induction.
All patients were enrolled in the National Cancer Institute-approved trial (NCT00049517) conducted by the ECOG-ACRIN Leukemia Committee. From December 2002 through November 2008, a total of 657 patients with de novo untreated AML ranging in age from 17 to 60 years were enrolled. We relied on physicians’ reporting of EM disease from clinical and diagnostic imaging findings. Disease in EM sites was not required to be biopsied nor were diagnostic imaging studies required to confirm involvement. Patients with isolated EM disease without bone marrow involvement were not eligible for this trial. The median follow-up of patients still alive is 6.7 years. Patients’ bone marrow and peripheral blood samples were collected and sent to ECOG-ACRIN’s leukemia reference laboratory for confirmation of the diagnosis of AML and immunophenotypic analysis by multiparameter flow cytometry. Internal tandem duplication (ITD) alterations in the Fms-like tyrosine kinase 3 (FLT3) gene and MLL partial tandem duplications (MLL-PTD) and mutational analysis profiling were performed on diagnostic samples from bone marrow and peripheral blood as previously described.7–9 Cytogenetic data were reviewed by ECOG-ACRIN’s Cytogenetics Committee. Data were collected and certified by the ECOG-ACRIN Data Coordinating Center and analyzed by the authors. The study was approved by the institutional review board at the National Cancer Institute and at each of the study centers. All patients provided written informed consent to participation in the study in accordance with the Declaration of Helsinki.
Eligible patients were randomly assigned to receive intravenous cytarabine 100 mg/m2/day infused continuously for 7 days plus intravenous daunorubicin daily at a dose of either 45 mg/m2 (standard dose) or 90 mg/m2 (high dose) for 3 days with risk-adapted consolidation.10
For the statistical analysis, patients’ baseline characteristics were compared using a Fisher exact test if the variables were categorical and Wilcoxon rank sum tests if they were continuous. Overall survival was defined as the time from randomization to death from any cause. For patients who achieved a complete response after induction, disease-free survival was defined as the time from documented complete response to relapse or death from any cause. Kaplan-Meier estimates were used to estimate the event-time distributions for overall survival and disease-free survival.
Logistic regression models were used to examine the association between EM disease and complete response. The univariate model included indicators of whether patients had EM disease involvement and induction treatment arms. The multivariable model was further controlled for age, gender, cytogenetic risk, ECOG Performance Status, white blood cell count, platelet count, percentage of marrow blasts, hemoglobin, peripheral blast percentage, and secondary AML. Univariate and multivariable Cox models stratified on induction treatment arms were performed on overall survival and disease-free survival. The stratified univariate models included indicators of whether patients had EM disease involvement. The stratified multivariable models were further adjusted for age, gender, cytogenetic risk, ECOG Performance Status, white blood cell count, platelet count, marrow blast percentage, hemoglobin, peripheral blast percentage, secondary AML, FLT-ITD, and MLL-PTD mutations. All P values were based on two-sided tests.
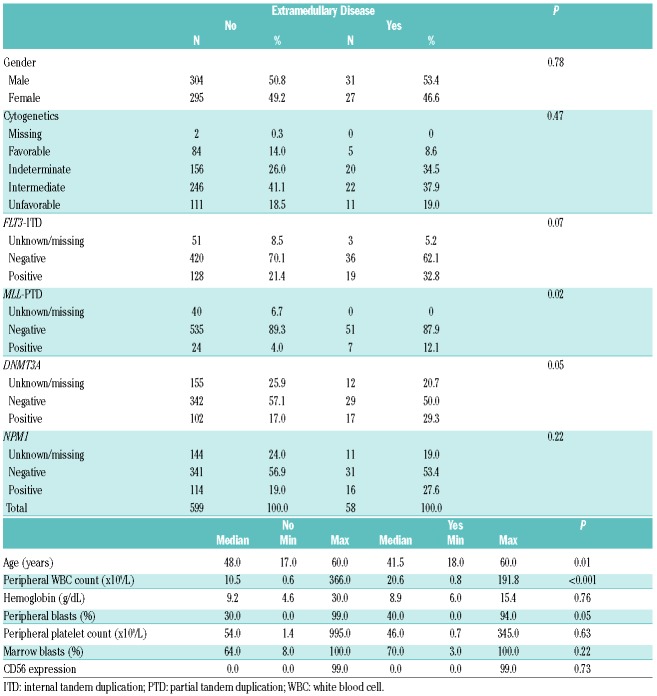
Fifty-eight patients had EM disease; four patients had more than one site involved. Six patients had central nervous system involvement; 32 had gingival hypertrophy; two had a mediastinal mass and seven had skin involvement. Fifteen had involvement of other EM sites, including lung, lymphadenopathy and splenomegaly. Table 1 presents the baseline characteristics of patients with and without EM disease. Cytogenetic risk in the subgroup with EM did not differ statistically from that of patients without EM disease. Patients with EM AML were younger (median 41.5 versus 48 years; P=0.01) and had higher peripheral white blood counts (P<0.001) and peripheral blasts (P=0.05). Patients with EM AML had a higher incidence of expression of MLL-PTD (12.1% versus 4.0%; P=0.02). In the EM cohort, among the patients with mutation data available, 34.6% expressed FLT3-ITD and 34.0% had NPM1 mutations. These data were not statistically different from those in patients without EM disease (P=0.07 and P=0.22, respectively). Patients with EM AML had a higher incidence of DNMT3A gene mutations (37.0% versus 23.0%; P=0.05). The complete response rate with induction therapy of all patients with EM disease was 63.8%. The complete response rates in patients treated with standard-dose and high-dose daunorubicin induction therapy were 61.3% and 66.7%, respectively. When the two induction arms were combined, EM disease involvement did not significantly affect the complete response rate in both univariate (P=0.93) and multivariable models when controlling for other variables (P=0.95) There was no interaction of complete response rate between EM-disease-positive or -negative patients and the induction treatment (P=0.58).
Table 1.
Baseline characteristics of patients with or without extramedullary disease.
For those patients who achieved a complete response after induction, EM involvement was not significantly associated with a worse disease-free survival in the univariate [hazard ratio (HR): 1.08, 95% confidence interval (95% CI): 0.73-1.61; P=0.70] or multivariable models (HR: 1.04, 95% CI: 0.65-1.64; P=0.88). There was no interaction of disease-free survival between whether or not patients had EM disease involvement and the induction treatment arms (P=0.83).
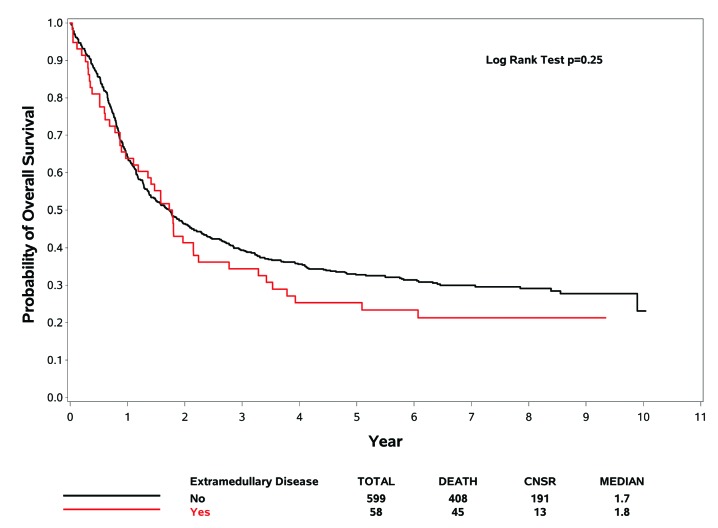
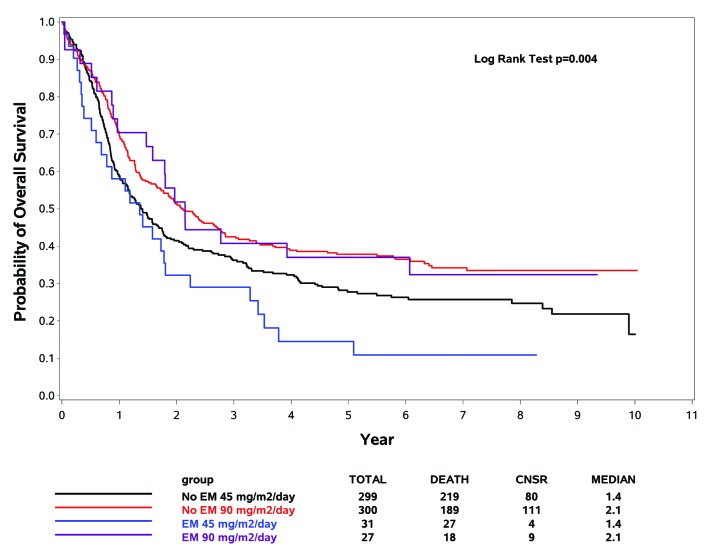
The probability curves of overall survival comparing the presence and absence of EM disease for the AML patients treated by induction arm (standard dose versus high dose) are given in Figures 1 and 2, respectively. When the two induction arms were combined, EM disease involvement was not significantly associated with a worse overall survival in comparison to that of patients without EM in univariate (HR: 1.20, 95% CI: 0.88-1.63; P=0.25) or multivariable models when controlling for other variables (HR: 1.21, 95% CI: 0.85-1.72; P=0.28). For patients randomized to receive 90 mg/m2/day daunorubicin, whether or not patients had EM AML had no effect on overall survival (univariate model: HR: 1.00, 95% CI: 0.61-1.61; P=0.98; multivariable model: HR: 0.90, 95% CI: 0.51-1.58; P=0.72). Patients with EM AML treated with high-dose daunorubicin had a better overall survival that that of patients treated with standard-dose daunorubicin (median 2.1 versus 1.4 years; univariate model: HR: 0.54, 95% CI: 0.29-0.98; P=0.04; multivariable model: HR: 0.40, 95% CI: 0.15-1.07; P=0.07) (Figure 2).
Figure 1.
Kaplan-Meier estimates of overall survival for patients with and without extramedullary disease. Data from the subset analysis are shown for overall survival of all patients with and without extramedullary disease treated in the trial. Data are from the time of randomization at the start of induction. CNSR indicates censored.
Figure 2.
Kaplan-Meier estimates of overall survival of patients with extramedullary disease based on induction therapy. Data from the subset analysis are shown for overall survival for patients with and without extramedullary disease who received protocol-prescribed induction therapy with a standard (45 mg/m2) or high (90 mg/m2) dose of daunorubicin. Data are from the time of randomization at the start of induction therapy. CNSR indicates censored.
In this subset analysis of EM AML patients from the E1900 study, higher doses of daunorubicin resulted in an improved overall survival compared to that in patients treated with standard-dose daunorubicin. These findings are consistent with our previously published results demonstrating that higher doses of anthracycline in induction therapy, followed by risk-adapted consolidation including hematopoietic cell transplantation, improves overall survival.10,11 Based on our results and the sensitivity of EM AML, we now propose high-dose anthracycline in induction for these patients as for all those with de novo AML.
We noted significant differences in the EM AML population: these patients were younger, had higher white blood cell counts and circulating blasts, a higher incidence of MLL-PTD, and mutations of NPM1 or DNMT3A. We noted a high incidence of the NPM1 mutation (34.0%), which may have contributed to the favorable results of this analysis. DNMT3A mutations, an unfavorable prognostic factor, appeared in 31.7% of the EM AML population. MLL-PTD expression has previously been associated with a poor complete response rate, disease-free survival and overall survival.12,13 Despite the presence of two adverse factors (DNMT3A and MLL-PTD) our patients’ outcomes were better than expected, probably because of the positive effect of high-dose anthracycline noted in patients with these specific mutations.9
This is one of the largest reports of patients presenting with EM involvement concurrent with the diagnosis of de novo AML. A major limitation of this analysis is that EM disease involvement had been noted clinically and/or detected by imaging studies and, therefore, there may have been other patients with undetected EM involvement who were not included in this analysis. Another limitation was this was not a planned subset analysis. A recently published, more encompassing retrospective analysis of prior ECOG-ACRIN trials demonstrated that AML with EM disease behaves similarly to that without EM involvement.14 Thus, all patients with EM manifestations should be included in future prospective AML trials, as their outcomes are similar to those of patients without EM involvement. Future studies should continue to collect data and tissues from patients with EM AML in order to refine the therapeutic approach based on the available prognostic markers.
Supplementary Material
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Byrd JC, Edenfield WJ, Shields DJ, Dawson NA. Extramedullary myeloid cell tumors in acute nonlymphocytic leukemia: a clinical review. J Clin Oncol. 1995;13(7):1800–1816. [DOI] [PubMed] [Google Scholar]
- 2.Paydas S, Zorludemir S, Ergin M. Granulocytic sarcoma: 32 cases and review of the literature. Leuk Lymphoma. 2006;47(12):2527–2541. [DOI] [PubMed] [Google Scholar]
- 3.Chang H, Brandwein J, Yi QL, et al. Extramedullary infiltrates of AML are associated with CD56 expression, 11q23 abnormalities and inferior clinical outcome. Leuk Res. 2004;28(10):1007–1011. [DOI] [PubMed] [Google Scholar]
- 4.Agis H, Weltermann A, Fonatsch C, et al. A comparative study on demographic, hematological, and cytogenetic findings and prognosis in acute myeloid leukemia with and without leukemia cutis. Ann Hematol. 2002;81(2):90–95. [DOI] [PubMed] [Google Scholar]
- 5.Pileri SA, Ascani S, Cox MC, et al. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia. 2007;21(2):340–350. [DOI] [PubMed] [Google Scholar]
- 6.Bakst RL, Tallman MS, Douer D, Yahalom J. How I treat extramedullary acute myeloid leukemia. Blood. 2011;118(14):3785–3793. [DOI] [PubMed] [Google Scholar]
- 7.Noguera NI, Breccia M, Divona M, et al. Alterations of the FLT3 gene in acute promyelocytic leukemia: association with diagnostic characteristics and analysis of clinical outcome in patients treated with the Italian AIDA protocol. Leukemia. 2002;16(11):2185–2189. [DOI] [PubMed] [Google Scholar]
- 8.Schnittger S, Kinkelin U, Schoch C, et al. Screening for MLL tandem duplication in 387 unselected patients with AML identify a prognostically unfavorable subset of AML. Leukemia. 2000;14(5):796–804. [DOI] [PubMed] [Google Scholar]
- 9.Patel JP, Gonen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366(12):1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361(13):1249–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luskin MR, Lee JW, Fernandez HF, et al. Benefit of high-dose daunorubicin in AML induction extends across cytogenetic and molecular groups. Blood. 2016;127(12):1551–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–474. [DOI] [PubMed] [Google Scholar]
- 13.Kong J, Zhao XS, Qin YZ, et al. The initial level of MLL-partial tandem duplication affects the clinical outcomes in patients with acute myeloid leukemia. Leuk Lymphoma. 2018;59(4):967–972. [DOI] [PubMed] [Google Scholar]
- 14.Ganzel C, Manola J, Douer D, et al. Extramedullary disease in adult acute myeloid leukemia is common but lacks independent significance: analysis of patients in ECOG-ACRIN Cancer Research Group trials, 1980-2008. J Clin Oncol. 2016;34(29):3544–3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.