Abstract
Myelosuppression is a major and frequently dose-limiting side effect of anticancer therapy and is responsible for most treatment-related morbidity and mortality. In addition, repeated cycles of DNA damage and cell death of hematopoietic stem and progenitor cells, followed by compensatory proliferation and selection pressure, lead to genomic instability and pave the way for therapy-related myelodysplastic syndromes and secondary acute myeloid leukemia. Protection of hematopoietic stem and progenitor cells from chemo- and radiotherapy in patients with solid tumors would reduce both immediate complications and long-term sequelae. Epidermal growth factor (EGF) and prostaglandin E2 (PGE2) were reported to prevent chemo- or radiotherapy-induced myelosuppression in mice. We tested both molecules for potentially protective effects on human CD34+ cells in vitro and established a xenograft mouse model to analyze stress resistance and regeneration of human hematopoiesis in vivo. EGF was neither able to protect human stem and progenitor cells in vitro nor to promote hematopoietic regeneration following sublethal irradiation in vivo. PGE2 significantly reduced in vitro apoptotic susceptibility of human CD34+ cells to taxol and etoposide. This could, however, be ascribed to reduced proliferation rather than to a change in apoptosis signaling and BCL-2 protein regulation. Accordingly, 16,16-dimethyl-PGE2 (dmPGE2) did not accelerate regeneration of the human hematopoietic system in vivo. Repeated treatment of sublethally irradiated xenograft mice with known antiapoptotic substances, such as human FLT3L and thrombopoietin (TPO), which suppress transcription of the proapoptotic BCL-2 proteins BIM and BMF, also only marginally promoted human hematopoietic regeneration in vivo.
Introduction
Myelosuppression occurs transiently after intensive chemotherapy and radiotherapy, and is characterized by anemia, bleeding tendency and susceptibility to infection. It is a major and frequently dose-limiting side effect of anticancer therapy and is responsible for most treatment-related morbidity and mortality.1 In addition, repeated cycles of DNA damage, cell attrition and subsequent compensatory proliferation of hematopoietic stem and progenitor cells (HSPCs) lead to genomic instability and pave the way for late complications such as therapy-related myelodysplastic syndromes (t-MDS) and secondary acute myeloid leukemia (AML).2 Protection of HSPCs from chemo- and radiotherapy in patients with solid tumors would reduce both immediate complications and long-term sequelae.
Numerous endogenous pathways and chemical compounds have been reported to prevent chemo- or radiotherapy-induced myelosuppression in mice, either by protecting HSPCs from damage and preventing their cell death or by fostering subsequent hematopoietic regeneration.3–10 In contrast, only limited preclinical data on substances protective for the human hematopoietic system in vivo are available.11,12
The aim of this study was to identify substances that protect human HSPCs from irradiation-induced apoptosis in vivo and to delineate their effects on the BCL-2 protein family. BCL-2 proteins are the master regulators of the intrinsic apoptosis pathway and have either pro- or anti-apoptotic function. Anti-apoptotic BCL-2 proteins (i.e. BCL-2, BCL-XL, MCL-1 and A1/BFL) protect cells from apoptotic stimuli by binding and inactivating their pro-apoptotic antagonists. The pro-apoptotic family members can be subdivided into the downstream ‘effector’ proteins, BAK and BAX, and the BH3-only proteins (e.g. BIM, PUMA, BMF, BAD and others) that act upstream as cell stress sensors. Upon activation, BH3-only proteins activate BAX and BAK either directly or indirectly through inhibition of the anti-apoptotic BCL-2 proteins. BAX/BAK activation leads to outer mitochondrial membrane permeabilization, caspase activation and cell death.13 Radiotherapy as well as most conventional chemotherapeutic drugs converge at the level of BCL-2 proteins and engage the intrinsic apoptosis pathway.2
A particularly attractive candidate for our study was the epidermal growth factor (EGF) that was recently described to prevent irradiation-induced apoptosis of murine HSPCs in vivo.10 Mechanistically, EGF receptor was up-regulated on bone marrow HSPCs subjected to irradiation, and binding of EGF resulted in suppression of p53-mediated transcriptional activation of the pro-apoptotic BCL-2 protein PUMA,10 the p53 target responsible for most DNA damage-induced apoptosis in hematopoietic cells.14,15
A second candidate was prostaglandin E2 (PGE2), which was shown to have multiple beneficial effects on both murine and human HSPCs, including increased survival, self-renewal and homing, together resulting in increased long-term repopulation potential.5,16,17 In a mouse model of sublethal total body irradiation (TBI), treatment with the long-acting PGE2 analog, 16,16-dimethyl-PGE2 (dmPGE2), resulted in increased HSPC survival and accelerated hematopoietic regeneration.9 The increase in apoptosis resistance was explained by upregulation of BCL-2 and BCL-XL and reduced expression of BAX.17
Finally, the cytokines FLT3L, stem cell factor (SCF) and thrombopoietin (TPO) have protective effects on murine and human HSPCs and drive their proliferation in vitro.3 Together, these cytokines are frequently used for culture and ex vivo expansion of human CD34+ cells. We have shown earlier that their pro-survival activity can be attributed to reduced transcription of BIM and BMF mRNA.18
None of these molecules have been tested yet for possible protective effects on human hematopoiesis in vivo. We focused on EGF, PGE2, FLT3L and TPO but excluded SCF from our studies since it had been shown earlier to induce extensive proliferation and premature exhaustion in murine HSPCs.19 We analyzed the effects of these substances on human HSPCs subjected to cell stress in vitro and, in addition, developed a xenograft model to analyze stress resistance and regeneration of human hematopoiesis in vivo.
Despite promising data obtained in the above-described mouse model, EGF was not able to protect human HSPCs in vitro nor to promote hematopoietic regeneration following sublethal irradiation in vivo. PGE2 significantly reduced in vitro apoptotic susceptibility of human HSPCs to taxol and etoposide. This could, however, be ascribed to reduced proliferation rather than to a change in BCL-2 protein regulation. Accordingly, PGE2 did not accelerate regeneration of the human hematopoietic system in vivo. Repeated treatment of sublethally irradiated xenograft mice with the combination of FLT3L and TPO also resulted in only minor beneficial effects during human hematopoietic regeneration.
Methods
Cell isolation and culture
Human umbilical cord blood was obtained after caesarean birth. Informed consent was obtained from the parents and the study was approved by the local ethics committee. CD34+ cells were enriched by MACS-technology (Miltenyi), cell purity was generally more than 90%. Purified cells were frozen in CryoStor CS10 (Stem Cell Technologies), stored in liquid nitrogen and used at later time points. Thawed cells were cultured in serum-free medium supplemented with 10% ES-FBS (Invitrogen), human TPO (50 ng/mL, Immunotools), FLT3L, SCF, IL3 (100 ng/mL each, Immunotools), human EGF (hEGF) (20 or 200 ng/mL Immunotools), PGE2 (10, 25 or 50 μM, Sigma-Aldrich) and/or cytotoxic drugs (etoposide, taxol, tunicamycin; Sigma-Aldrich). Alternatively, thawed cells were used for xenotransplantation.
Xenotransplantation
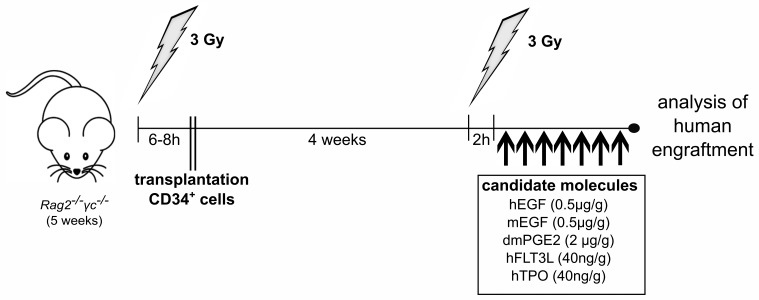
All experiments were performed according to the guidelines of the German “Tierversuchsgesetz” and approved by the local committee (RP Freiburg/Germany). Rag2−/−γc−/− mice were irradiated at five weeks of age with 3 Gy and 6–8 hours (h) later they were injected intravenously into the retrobulbar venous plexus with 3×105 human CD34+ cells. Four weeks later, animals were irradiated again. Subsequently, xenograft mice were treated once daily intraperitoneally (i.p.) with human EGF (0.5 μg/g body weight), murine EGF (0.5 µg/g), human dmPGE2 (2 μg/g), human FLT3L (40 ng/g), human TPO (40 ng/g), combinations thereof, or respective carrier solutions (Figure 1). At indicated time points, mice were sacrificed for analysis. Alternatively, mice were treated once daily for seven days with etoposide (20 mg/k, i.p.), and the anti-apoptotic substances were given simultaneously.
Figure 1.
Xenograft model for evaluation of radioprotective substances. Cord blood-derived human CD34+ cells were transplanted into sublethally irradiated 5-week old Rag2−/−γc−/− mice. Four weeks later, xenograft mice were again irradiated with 3 Gy in order to subject human hematopoiesis to sublethal stress. Subsequently, mice were treated intraperitoneally (i.p.) once daily with the indicated molecules. Control mice were treated with the respective carrier solution (saline or ethanol). At day 8 after second irradiation, mice were sacrificed for analysis. Single cell suspensions were obtained from bone marrow and spleen. h: hours; hu EGF: human epidermal growth factor; mu EGF: murine epidermal growth factor; hu dmPGE2: human 16,16-dimethyl-PGE2; hu FLT3L: human FLT3L; TPO: human thrombopoietin.
Proliferation, apoptosis and colony formation assays
Cell cycle status and proliferation were determined by double staining for Ki-67 (BioLegend) and DAPI (Sigma-Aldrich) or incubation with CFSE (1 μM; Sigma-Aldrich). Apoptosis was determined by combined staining with 7-AAD and Annexin-V. Specific apoptosis triggered by stress was calculated as follows: (induced apoptosis – spontaneous apoptosis)/(100 – spontaneous apoptosis). For colony forming assays, 150,000 human CD45+ cells isolated from murine bone marrow (BM) were plated for 11 days on a semi-solid medium containing insulin, transferrin, human SCF, IL-3, IL-6, EPO, G-CSF and GM-CSF (SF H4436 MethoCult).
Flow cytometric analysis
Single cell suspensions of hematopoietic organs were surface-stained with antibodies conjugated with FITC, PE, APC, PerCP/Cy5.5, PE/Cy7 or biotin. Antibodies for murine markers: anti-CD45 (30-F11). Antibodies for human markers: H13 and 2D1, anti-CD45; AC136, anti-CD34. Biotinylated antibodies were detected using streptavidin-APC. Flow cytometric analysis was performed using a FACS-Fortessa (BD).
RT-MLPA
RNA was isolated with Fast-Spin columns (ZymoResearch). For RT-MLPA (MRC Holland, R011-C1), specific mRNAs were reversely transcribed into cDNA and bound by two oligonucleotides consequently ligated. The generated amplification products of unique length were separated by capillary sequencer (Genescan). Analysis was performed with Sequence Pilot (JSI Medical Systems). The sum of all peak data was set to 100% to normalize for fluctuations between different samples, and single peaks were calculated relative to 100%.
Statistical analysis
Statistical analysis was performed using the Mann-Whitney-Test (Graphpad Prism). P<0.05 was considered statistically significant.
Results
Epidermal growth factor does not protect human hematopoietic stem cells from DNA damage-induced apoptosis in vitro and in vivo
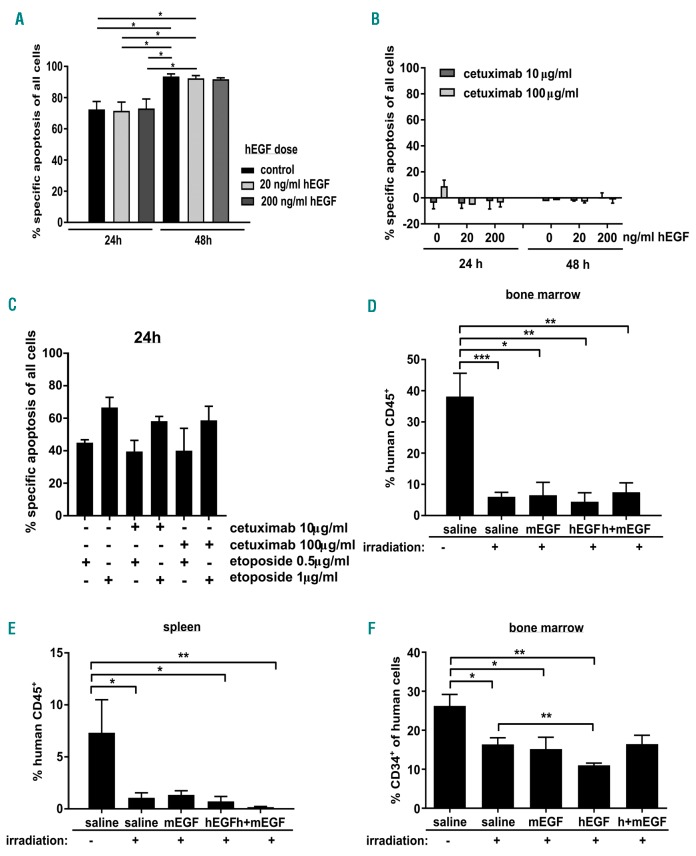
To induce DNA damage-induced apoptosis, CD34+ cells were treated with the topoisomerase inhibitor etoposide (0.5 or 1 μg/mL) for 24 and 48 h. Apoptosis could not be prevented by addition of 20 or 200 ng/mL human EGF, respectively (Figure 2A). To exclude the possibility that EGF contained in medium or serum or produced by the cells themselves was sufficient to reduce apoptotic susceptibility even in the absence of accessory EGF, cells were treated with neutralizing EGF-R-antibodies (cetuximab) for 24 and 48 h. Inhibition of EGF-R-signaling did not induce apoptosis by itself (Figure 2B) nor increase DNA damage-induced apoptosis of human HSPCs (Figure 2C).
Figure 2.
Epidermal growth factor (EGF) does not protect human CD34+ from apoptosis induced by sublethal irradiation. (A) Cord blood-derived human CD34+ cells were treated for 24 and 48 hours (h) with etoposide (1 µg/mL) and different doses of human EGF (hEGF). Apoptosis was measured using AnnexinV/7AAD and specific apoptosis was calculated. Bars represent means±Standard Error of Mean (SEM) of 5 independent experiments. (B) Cord blood-derived human CD34+ cells were cultured for 24 and 48 h in the presence of human EGF and/or cetuximab at indicated concentrations and specific apoptosis was determined. Bars represent means±SEM of 4 independent experiments. (C) Human CD34+ cells were treated with both etoposide and cetuximab, and specific apoptosis was determined 24 h later. Bars represent means±SEM of 3 independent experiments. (D-F) Human CD34+ cells were transplanted into sublethally irradiated Rag2−/−γc−/− mice. Four weeks later, mice were irradiated with 3 Gy or left untreated. Mice that were irradiated received daily injections of human and/or murine EGF. Eight days after irradiation, mice were sacrificed and % human CD45+ cells was determined in bone marrow (D) and spleen (E). In addition, the proportion of CD34+ immature cells was determined within the human cell population (F). Bars represent means±SEM of 4–7 animals from 5 independent experiments. Mann-Whitney test, *P≤0.05; **P≤0.01; ***P≤0.001).
Since the effects of EGF on murine hematopoiesis were exclusively shown in vivo,10 we performed analogous experiments in a xenograft model using immunodeficient Rag2−/−γ−/− recipient mice. Animals were xenotransplanted at five weeks of age with 3×105 cord blood-derived CD34+ cells. Upon successful human engraftment, mice were irradiated with 3 Gy to mimic myelosuppression occurring during therapeutic irradiation. Subsequently, mice were treated daily with human EGF (0.5 mg/g, i.p.) or saline (100 μl, i.p.) for seven days. Regeneration of human hematopoiesis, as defined by percentage and cell count of human CD45+ cells, was determined in BM and spleen seven days after irradiation (Figure 2D and E and Online Supplementary Figure S1). In addition, we determined the proportion of immature CD34+ cells within all human cells as a surrogate for their regenerative capacity (Figure 2F). While non-irradiated mice showed consistent human engraftment, irradiated mice lost most of their human hematopoietic cells irrespectively of whether they were treated with human EGF or not. To test for a possibly indirect, cell-extrinsic effect of EGF on HSPCs conferred by the murine microenvironment, we performed the same experiment using murine EGF. In addition, we combined treatment with human and murine EGF to test for synergies. None of these treatment regimens resulted in protection of the xenografted human hematopoietic system (Figure 2D and E and Online Supplementary Figure S1). As we observed no protective effects of EGF both in vitro and in vivo, we waived the analysis of BCL-2 protein regulation.
Prostaglandin E2 protects human hematopoietic stem cells short-term from apoptosis but has toxic long-term effects
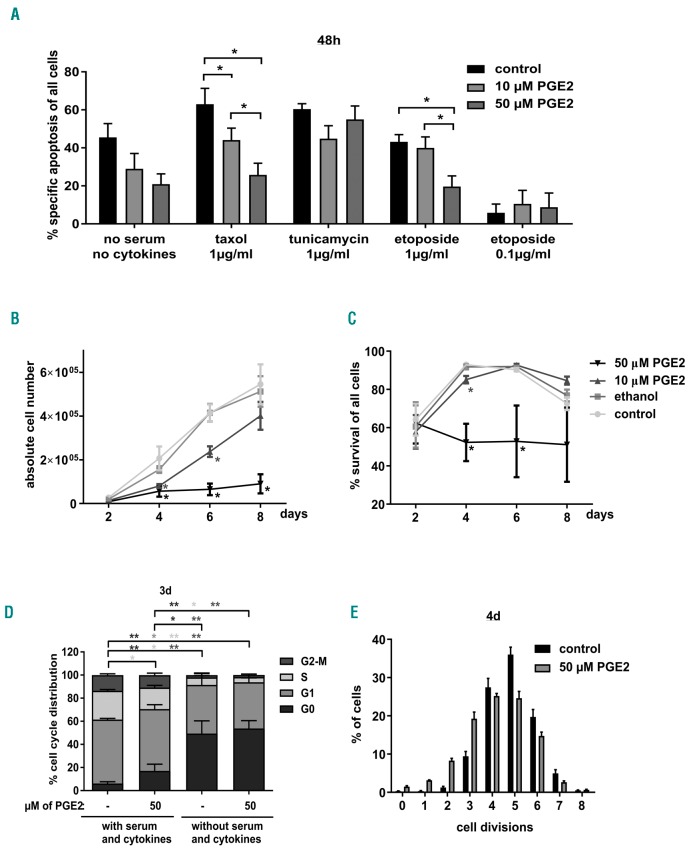
We subjected CD34+ cells for 48 h to various stress stimuli all known to induce intrinsic apoptosis and investigated whether PGE2 was able to reduce their apoptotic susceptibility. Apoptosis induced by the spindle drug taxol or the topoisomerase inhibitor etoposide was significantly reduced in a dose-dependent manner by PGE2. In addition, apoptosis induced by serum and cytokine deprivation was reduced when cells were cultured in the presence of PGE2, albeit not significantly. In contrast, apoptosis induced by the ER stressor tunicamycin could not be prevented by PGE2 (Figure 3A). Altogether, the data point towards a dose-dependent protective effect of PGE2 that is, however, restricted to certain forms of cell stress.
Figure 3.
Prostaglandin E2 (PGE2) has both protective and toxic effects on human CD34+ cells. (A) Cord blood-derived CD34+ cells were subjected to different cytotoxic agents or serum and cytokine withdrawal. Control cells were treated with serum, FLT3L, stem cell factor (SCF), thrombopoietin (TPO) and IL3. PGE2 was added at indicated concentration. After 48 hour (h), cells were stained with AnnexinV/7AAD and specific apoptosis was determined. Bars represent means±Standard Error of Mean (SEM) of 5–6 from 6 independent experiments. P-values were determined using the Mann-Whitney test. (B and C) CD34+ cells were cultured in serum, FLT3L, SCF, TPO and IL3 plus different concentrations of PGE2. Cell count (B) and viability (C) were determined every other day for eight days. Bars represent means±SEM of 4 from 3 independent experiments. P-values were determined using the Mann-Whitney test. (D) After three days of culture in the presence or absence of PGE2 (50 μM), cell cycle status was determined by Ki67 and DAPI staining and flow cytometric analysis. Bars represent means±SEM of 6 from 5 independent experiments. Mann-Whitney test. (E) PGE2-treated CD34+ cells were cultured in the presence of carboxy-fluorescein diacetate succinimidyl ester (CFSE), and CFSE content representing amount of cell divisions was determined by flow cytometry four days later. Bars represent means ± SEM of n=3 independent experiments.
Unexpectedly, long-term culture of CD34+ cells revealed a toxic effect of PGE2 itself when it was used at high concentrations for longer than two days. Numbers and viability of CD34+ cells were strongly reduced when they were cultured in the presence of 50 μM PGE2 for up to eight days (Figure 3B and C). To unravel the paradoxical finding that PGE2 can both protect from and induce apoptosis, we analyzed regulation of BCL-2 family members on mRNA level. After 4 h of PGE2 treatment, CD34+ cells showed increased mRNA levels of the anti-apoptotic protein MCL-1 and the pro-apoptotic protein BIM while BMF levels were reduced. mRNA levels returned to normal at 12 h of treatment. Other BCL-2 proteins were not transcriptionally regulated at either time point (Online Supplementary Figure S2A and B).
As we observed no HSPC expansion when cells were cultured in the presence of 50 μM PGE2 (Figure 3B), we suspected a proliferation repression in addition to apoptosis induction. Indeed, more cells were in G0 phase when cells cultured with FLT3L, SCF, TPO and IL3 were treated with PGE2 (50 mM) (Figure 3D and Online Supplementary Figure S3). Consistently, CFSE staining showed 4–5 divisions in four days in most untreated cells but fewer divisions in PGE2-treated cells (Figure 3E and Online Supplementary Figure S3B). Considering this, the reduced susceptibility of PGE2-treated HSPCs to taxol and etoposide might be rather due to the proliferation repression than to deregulated apoptosis signaling since both drugs primarily target proliferating cells. We hypothesized that PGE2-mediated inhibition of cell proliferation 24 h prior cytotoxic stress would further reduce susceptibility to apoptosis but noted no additional benefit (Online Supplementary Figure S2C).
To test whether the protective in vitro effect of PGE2 can be translated to an in vivo situation, we irradiated recipient mice sublethally four weeks after xenotransplantation. A second group of xenograft mice was subjected once daily to etoposide for one week to better mimic a chemotherapy cycle. Animals from both groups were treated daily with dmPGE2 at a dose that had been shown earlier to protect murine HSPCs from apoptosis induced by sublethal irradiation in vivo.9 Seven days after treatment, animals were sacrificed and analyzed. Sublethal irradiation cleared most of human hematopoietic cells and daily dmPGE2 administration did not result in their protection (Figure 4A and B). In contrast, etoposide treatment appeared more toxic for murine hematopoietic cells (Online Supplementary Figure S4A). When etoposide was combined with dmPGE2 treatment, we observed enrichment of human CD45+ cells indicating some survival advantages over murine cells (Figure 4C and E). However, this was not reflected by increased absolute numbers of human cells (Figure 4D and F). Interestingly, dmPGE2 administration together with total body irradiation or etoposide treatment provoked profuse and nearly fatal diarrhea making such an approach unusable.
Figure 4.
Prostaglandin E2 (PGE2) does not promote human hematopoietic regeneration. (A and B) Human CD34+ cells were transplanted into sublethally irradiated Rag2−/−γc−/− mice. Four weeks later, mice were irradiated with 3 Gy or left untreated. Mice that were irradiated received daily injections of dmPGE2. Eight days after irradiation, mice were sacrificed and % human CD45+ cells were determined in bone marrow (BM) (A) and spleen (B). Bars represent means±Standard Error of Mean (SEM) of n=4-7 animals from 5 independent experiments. Mann-Whitney test, *P≤0.05; **P≤0.01; ***P≤0.001. (C-F) Human CD34+ cells were transplanted into sublethally irradiated Rag2−/−γc−/− mice. Four weeks later, mice were treated daily with etoposide alone, or together with dmPGE2. Eight days after treatment start, mice were sacrificed and % human CD45+ cells was determined in BM (C) and spleen (E). Cell counts of human CD45+ cells were determined in BM (D) and spleen (F). Bars represent means±SEM of 2–7 animals from at least 2 independent experiments.
Minor in vivo effects of the anti-apoptotic cytokines FLT3L and thrombopoietin
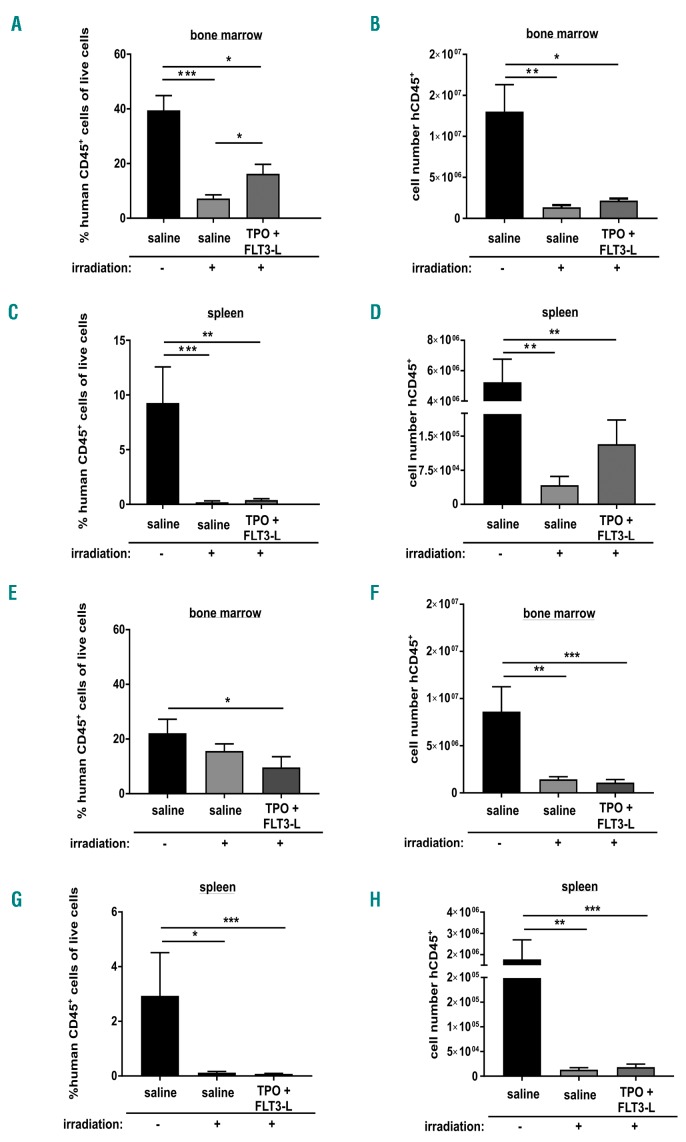
We next wondered whether substances known to inhibit apoptosis by interfering with the BCL-2 protein family would be able to protect human hematopoiesis from irradiation-induced injury in vivo. We focused on the cytokines FLT3L and TPO that suppress activation of the BH3-only proteins BIM and BMF in both murine and human HSPCs but also have proliferative effects.18 Sublethally irradiated mice were treated daily with FLT3L, TPO or their combination. While treatment of either FLT3L or TPO alone did not have any effects on regeneration of human hematopoiesis (data not shown), combined treatment for seven days resulted in a relative increase of human cells in BM and a mildly increased number of human cells in the spleen (Figure 5A-D and Online Supplementary Figure S5A). Both effects were transient, and no increase in human cell numbers was observed when cytokine combination was administered for 14 days (Figure 5E-H). To investigate the frequency of human HSPCs giving rise to colonies, we isolated 150,000 human CD45+ cells from BM of untreated and treated mice and seeded them into methylcellulose medium. There was no significant difference in colony numbers between mice treated with cytokines and control mice (Online Supplementary Figure S5B). Finally, we started TPO/FLT3L treatment 24 h prior to irradiation, but again did not see any protective effects on human hematopoiesis in vivo (data not shown).
Figure 5.
Mild beneficial effects of FLT3L and thrombopoietin (TPO) in vivo. (A-D) Human CD34+ cells were transplanted into sublethally irradiated Rag2−/−γc−/− mice. Four weeks later, mice were irradiated with 3 Gy or left untreated. Mice that were irradiated received daily injections of human TPO and/or FLT3L. Eight days after irradiation, mice were sacrificed and % human CD45+ cells were determined in bone marrow (BM) (A) and spleen (C). Human CD45+ cell numbers were determined in BM (B) and spleen (D). Bars represent means±Standard Error of Mean (SEM) of n=4-7 animals from 3 independent experiments. Mann-Whitney test, *P≤0.05; **P≤0.01; ***P≤0.001. (E-H) Treatment with human TPO and FLT3L was given for 14 days and % human CD45+ cells was determined in BM (E) and spleen (G). Cell counts of human CD45+ cells were determined (F and H). Bars represent means±SEM of 5–11 animals from 3 independent experiments. Mann-Whitney test, *P≤0.05; **P≤0.01; ***P≤0.001.
Discussion
Although there is a strong clinical need to reduce hematologic side effects in cancer patients treated with chemo- or radiotherapy, to date, only few therapeutic options are available.11,12 This is in contrast to the many chemical compounds and endogenous substances that were described to be radioprotective in vitro or in mouse models. A major limitation in translating preclinical findings to routine clinical practice is the lack of suitable model systems. While research on primates is laborious, expensive and not feasible everywhere, xenograft models that allow studies on human cells in vivo can be a good alternative.
The first aim of this study was to generate a xenograft mouse model of human hematopoiesis that can be used to analyze hematopoietic regeneration following sublethal stresses. Upon successful engraftment of human hematopoietic cells, recipient mice were subjected to total body irradiation (TBI) or daily etoposide treatment. Immediately after irradiation, or in parallel to etoposide administration, treatment with possibly protective substances was initiated and continued for one week before the human hematopoiesis was analyzed in detail. Although the substances tested in this work did not perform convincingly, we successfully demonstrated that our model can be used to investigate the presumed radioprotective effects of given molecules or chemical compounds on the human hematopoietic system treated with chemo- or radiotherapy in vivo. In addition, our model can be used to test novel cytotoxic drugs for their hematotoxicity by substituting TBI with repeated drug treatment. One shortcoming of our model is that the murine microenvironment does not provide optimal support to human hematopoietic cells. This should, however, be no major issue since radio- and intensive chemotherapy also harm the human hematopoietic niche, thereby imposing an additional layer of stress to HSPCs. A more relevant shortcoming is that irradiation and cytotoxic drugs result in depletion of both human and murine cells, implicating that human cells have to compete against the more dominant murine cells during the stage of subsequent regeneration. Relative increase in human cells within a murine tissue, as observed after TBI and 7-day treatment with TPO/FLT3, or when we applied etoposide together with dmPGE2, might thus indicate that human cells are favored even when absolute human cell numbers do not increase.
The substances used here were carefully selected on the basis of published data and own earlier work. However, despite the very promising data obtained in mouse models,9,10 neither EGF nor PGE2 were able to promote hematopoietic regeneration in our model system. In the case of EGF, non-conserved pathways seem to underlie this inconsistency. As for PGE2, both its antiproliferative and toxic effects might contribute to its lack of in vivo efficacy. Anyhow, the severe gastrointestinal side effects provoked by the combination of dmPGE2 and TBI/etoposide would render such a treatment unfeasible. FLT3L and TPO are both known to increase viability and promote proliferation of human HSPCs in vitro. Along that line, we observed some beneficial effects on human hematopoietic regeneration in vivo, even though these were marginal and transient.
One could speculate that the maximal beneficial effect on human hematopoietic regeneration can be achieved by the use of substances that foster both survival and proliferation of HSPCs. Certainly, short-term complications such as febrile neutropenia or transfusion-dependent anemia and thrombocytopenia could be reduced by increasing the proliferative capacity of HSPCs following chemo- or radiotherapy. Repeated cycles of forced proliferation, however, could result in premature stem cell exhaustion and BM failure in the long term; this has already been shown when mice were repeatedly treated with SCF.19 Forced proliferation of DNA-damaged HSPCs, as caused by chemo- or radiotherapy, together with selection pressure could also increase the risk of genetic instability and clonal evolution eventually leading to t-MDS and secondary AML.
Ideally, radioprotective substances should not stimulate proliferation but only inhibit apoptosis of healthy BM cells. Apoptosis resistance reduces therapy-induced myelosuppression, and at the same time lowers the risk of secondary leukemia by prolonging the time available for DNA repair and reducing compensatory proliferation and selection pressure.2,15,20,21 ROS scavengers, for example, are radioprotective by preventing both DNA damage and apoptosis.11
Direct inhibition of the pro-apoptotic effector proteins, BAX and BAK, could also keep hematopoietic cells alive without affecting proliferation. We recently showed that such transient apoptosis resistance can be achieved by short-term overexpression or protein transduction of the anti-apoptotic protein BCL-XL.22 Translation to clinical use will probably be pushed ahead by the development of specific BAX/BAK inhibitors.
Similar approaches will be useful to reduce risk of graft failure and shorten the time to full hematopoietic regeneration in the case of autologous or allogeneic hematopoietic stem cell transplantation (HSCT). We have already showed that inhibition of the intrinsic apoptosis pathway in donor cells, even when limited to a few days around transplantation, significantly improves outcome of HSCT, especially when only low donor cell numbers are available.18,22,23
While donor stem cells used for HSCT can be manipulated ex vivo, the major challenge in the field of cancer treatment will be to identify substances that exclusively protect hematopoietic cells without having any beneficial effect on cancer cells. EGF, PGE2 and SCF are known to have proliferative and pro-survival activities on certain types of cancer24–26 and FLT3L has not yet been systematically tested for its effects on solid tumors. As an alternative, radioprotective substances could be delivered only to healthy BM cells while sparing cancer cells to avoid therapy resistance. Novel approaches in targeted drug delivery will hopefully soon enable such therapies to be developed.
Supplementary Material
Acknowledgments
We are grateful to Nora Fischer for excellent technical assistance and to Natalie Krause for animal care. We also would like to thank our colleagues from FOR2036 for insightful discussion.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/104/4/669
Funding
This work was supported by grants from the German Research Foundation (DFG-FOR2036 to ME) and the European Research Council (ERC-StG-2014 – 638145 ApoptoMDS to ME).
References
- 1.Wang Y, Probin V, Zhou D. Cancer therapy-induced residual bone marrow injury-Mechanisms of induction and implication for therapy. Curr Cancer Ther Rev. 2006;2(3):271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Labi V, Erlacher M. How cell death shapes cancer. Cell Death Dis. 2015;6:e1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray LJ, Young JC, Osborne LJ, et al. Thrombopoietin, flt3, and kit ligands together suppress apoptosis of human mobilized CD34+ cells and recruit primitive CD34+ Thy-1+ cells into rapid division. Exp Hematol. 1999;27(6):1019–1028. [DOI] [PubMed] [Google Scholar]
- 4.Varnum-Finney B, Xu L, Brashem-Stein C, et al. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat Med. 2000;6(11):1278–1281. [DOI] [PubMed] [Google Scholar]
- 5.Hoggatt J, Singh P, Sampath J, Pelus LM. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113(22):5444–5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dygai AM, Khmelevskaya ES, Skurikhin EG, et al. Catecholamine regulation of stromal precursors and hemopoietic stem cells in cytostatic myelosuppression. Bull Exp Biol Med. 2012;15(6)2:723–727. [DOI] [PubMed] [Google Scholar]
- 7.Cheng CW, Adams GB, Perin L, et al. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell. 2014;14(6):810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burdelya LG, Krivokrysenko VI, Tallant TC, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008; 320(5873):226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porter RL, Georger MA, Bromberg O, et al. Prostaglandin E2 increases hematopoietic stem cell survival and accelerates hematopoietic recovery after radiation injury. Stem Cells. 2013;31(2):372–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doan PL, Himburg HA, Helms K, et al. Epidermal growth factor regulates hematopoietic regeneration after radiation injury. Nat Med. 2013;19(3):295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamran MZ, Ranjan A, Kaur N, Sur S, Tandon V. Radioprotective Agents: Strategies and Translational Advances. Med Res Rev. 2016;36(3):461–493. [DOI] [PubMed] [Google Scholar]
- 12.Johnke RM, Sattler JA, Allison RR. Radioprotective agents for radiation therapy: future trends. Future Oncol. 2014;10(15):2345–2357. [DOI] [PubMed] [Google Scholar]
- 13.Kollek M, Muller A, Egle A, Erlacher M. Bcl-2 proteins in development, health, and disease of the hematopoietic system. FEBS J. 2016;283(15):2779–2810. [DOI] [PubMed] [Google Scholar]
- 14.Erlacher M, Labi V, Manzl C, et al. Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. J Exp Med. 2006;203(13):2939–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erlacher M, Michalak EM, Coultas L, et al. The BH3-only proteins Puma/bbc3 and Bim are rate-limiting for g-radiation- and glucocorticoid-induced apoptosis of lymphoid cells in vivo. Blood. 2005; 106(13):4131–4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.North TE, Goessling W, Walkley CR, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447(7147):1007–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goessling W, Allen RS, Guan X, et al. Prostaglandin E2 enhances human cord blood stem cell xenotransplants and shows long-term safety in preclinical nonhuman primate transplant models. Cell Stem Cell. 2011;8(4):445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Labi V, Bertele D, Woess C, et al. Haematopoietic stem cell survival and transplantation efficacy is limited by the BH3-only proteins Bim and Bmf. EMBO Mol Med. 2013;5(1):122–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemoli RM, Gulati SC. Effect of stem cell factor (c-kit ligand), granulocyte-macrophage colony stimulating factor and interleukin 3 on hematopoietic progenitors in human long-term bone marrow cultures. Stem Cells. 1993;11(5):435–444. [DOI] [PubMed] [Google Scholar]
- 20.Labi V, Erlacher M, Krumschnabel G, et al. Apoptosis of leukocytes triggered by acute DNA damage promotes lymphoma formation. Genes Dev. 2010;24(15):1602–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michalak EM, Vandenberg CJ, Delbridge AR, et al. Apoptosis-promoted tumorigenesis: gamma-irradiation-induced thymic lymphomagenesis requires Puma-driven leukocyte death. Genes Dev. 2010; 24(15):1608–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kollek M, Voigt G, Molnar C, et al. Transient apoptosis inhibition in donor stem cells improves hematopoietic stem cell transplantation. J Exp Med. 2017; 214(10):2967–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Afreen S, Weiss JM, Strahm B, Erlacher M. Cheating death for a better transplant. Stem Cells. 2018. August 29 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19(56):6550–6565. [DOI] [PubMed] [Google Scholar]
- 25.Huang Q, Li F, Liu X, et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med. 2011;17(7):860–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cardoso HJ, Figueira MI, Socorro S. The stem cell factor (SCF)/c-KIT signalling in testis and prostate cancer. J Cell Commun Signal. 2017;11(4):297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.