Abstract
Thromboembolism is a serious complication of induction therapy for childhood acute lymphoblastic leukemia. We prospectively compared the efficacy and safety of antithrombotic interventions in the consecutive leukemia trials ALL-BFM 2000 and AIEOP-BFM ALL 2009. Patients with newly diagnosed acute lymphoblastic leukemia (n=949, age 1 to 18 years) were randomized to receive low-dose unfractionated heparin, prophylactic low molecular weight heparin (enoxaparin) or activity-adapted antithrombin throughout induction therapy. The primary objective of the study was to determine whether enoxaparin or antithrombin reduces the incidence of thromboembolism as compared to unfractionated heparin. The principal safety outcome was hemorrhage; leukemia outcome was a secondary endpoint. Thromboembolism occurred in 42 patients (4.4%). Patients assigned to unfractionated heparin had a higher risk of thromboembolism (8.0%) compared with those randomized to enoxaparin (3.5%; P=0.011) or antithrombin (1.9%; P<0.001). The proportion of patients who refused antithrombotic treatment as allocated was 3% in the unfractionated heparin or antithrombin arms, and 33% in the enoxaparin arm. Major hemorrhage occurred in eight patients (no differences between the groups). The 5-year event-free survival was 80.9±2.2% among patients assigned to antithrombin compared to 85.9±2.0% in the unfractionated heparin group (P=0.06), and 86.2±2.0% in the enoxaparin group (P=0.10). In conclusion, prophylactic use of antithrombin or enoxaparin significantly reduced thromboembolism. Despite the considerable number of patients rejecting the assigned treatment with subcutaneous injections, the result remains unambiguous. Thromboprophylaxis - for the present time primarily with enoxaparin - can be recommended for children and adolescents with acute lymphoblastic leukemia during induction therapy. Whether and how antithrombin may affect leukemia outcome remains to be determined.
Introduction
Thromboembolism is a serious complication of glucocorticoid and E. coli asparaginase-containing induction therapy for childhood acute lymphoblastic leukemia (ALL). Reported incidences vary between 1% and 37%, depending on the study design and definition of thrombosis, as well as diagnostic, supportive and therapeutic methods.1–6 Acquired antithrombin deficiency as a result of asparaginase-induced asparagine depletion is considered to be a crucial mechanism for the development of thromboembolism during ALL induction therapy. The presence of a central venous catheter (CVC) seems to be an additional – at least local – risk factor for thromboembolism as a significant proportion of thromboembolic events during ALL treatment is related to an indwelling CVC. Furthermore, the risk of thromboembolism has been shown to be associated with CVC location and insertion technique.1,5,7–12 Published data also provide good evidence that adolescent age is an important risk factor for thromboembolism whereas the additional impact of inherited thrombophilia in the context of childhood ALL treatment is controversial.5,13–16
Sufficiently powered randomized trials on thromboprophylaxis in children during ALL induction therapy have not been available,16–23 and evidence for the benefit of specific thromboprophylactic measures has therefore been lacking so far. In the absence of valid medical standards of care regarding thromboprophylaxis and the use of a CVC during ALL induction, various different approaches existed in the pediatric cancer centers in Switzerland and Germany in the early 2000s, each based on individual experiences and institutional standards. This unsatisfactory situation gave the impetus to initiate the THROMBOTECT trial, a prospective randomized study to evaluate the efficacy and safety of antithrombotic prophylaxis in children treated for ALL.
As drug administration through an indwelling CVC provides significant gain in comfort for the patients and increases the safety of therapy with tissue-toxic agents, the THROMBOTECT study was initially designed to include patients with implanted CVC from the initiation of the induction phase and was only later on also opened for patients without CVC. Two mechanisms of action to prevent thromboembolism were utilized in the two interventional arms of the trial: inhibition of thrombin through inactivation of coagulation factor X by treatment with the low molecular weight heparin enoxaparin (Clexane™) and replacement of antithrombin by the plasma-derived antithrombin preparation Kybernin™ to compensate for asparaginase-related aquired antithrombin deficiency. Being aware of the published data of Nowak-Göttl et al., which reported an almost 50% incidence of thromboembolism among ALL patients with a prothrombotic defect, and considering the additional risk factor of an indwelling CVC, a control arm without any intervention appeared difficult to justify.15 The third arm therefore included continuous infusion of low-dose unfractionated heparin (UFH) while the CVC was in use, with the aim of preventing local clot formation at the tip of the catheter, thereby preventing thrombotic occlusion of the indwelling CVC without causing relevant systemic anticoagulatory effects.7,24–27 Low-dose UFH was, therefore, considered the control arm.
The current report presents the clinical results of the THROMBOTECT study with respect to the incidence of symptomatic thromboembolism and hemorrhage as primary efficacy and safety outcomes as well as the secondary safety outcome of leukemia-related survival.
Methods
Study design
THROMBOTECT was an open-label, prospective, randomized, multicenter study to evaluate two different preventive antithrombotic measures during induction chemotherapy in children with ALL treated according to ALL-BFM 2000 (NCT 00430118) and AIEOP-BFM-ALL 2009 treatment protocols (NCT 01117441). THROMBOTECT was an add-on study to the ALL-BFM protocols and was approved by the leading ethics committees of the Medical School Hannover, Germany, and St. Gallen, Switzerland, and by the local ethics committees of each participating site. Written informed consent to participation in the study was obtained from guardians and/or patients before randomization. The detailed study protocol is available in the Online Supplementary Material.
Patients’ eligibility
Patients were eligible if treated on the ALL-BFM 2000 or AIEOP-BFM ALL 2009 protocol,28–30 if they had a CVC inserted by day 8 of induction and if the CVC remained in place until at least day 33. The choice of the CVC and decisions regarding its maintenance were made by the treating physicians according to institutional guidelines. In August 2004, the protocol was amended to allow participation of patients without a CVC. Exclusion criteria were known hemorrhagic disorders unrelated to leukemia, active gastrointestinal ulcer, previous cerebrovascular accident and/or known hypersensitivity to heparin.
Randomization and study treatment
After written informed consent had been given, randomization was performed by day 8 in a 1:1:1 ratio using permuted blocks of six patients and stratification by country and the glucocorticoid preparation (dexamethasone or prednisone) administered during induction.29 Randomization was performed centrally by the ALL-BFM study coordination center using computer-generated random number lists. This ensured that the participating centers had no access to the allocation sequence. The assigned arm was submitted to the center by fax.
Patients were randomly assigned to receive one of the two experimental thromboprophylactic treatments with either the low molecular weight heparin enoxaparin or with activity-adjusted antithrombin or to the control arm, i.e., low-dose UFH.
Thromboprophylaxis was started on day 8 and ended on day 33 of induction chemotherapy (Online Supplementary Figure S1). The observation period covered the induction and consolidation phases (Online Supplementary Figure S2) up to and including protocol day 64.
Patients in the enoxaparin group received Clexane™ at a dose of 80–100 IU/kg body weight once daily subcutaneously31–34 with a target anti-Xa level not exceeding 0.4 U/L, measured 4 h after the third or fourth injection. On days with lumbar puncture or other invasive procedures, enoxaparin was postponed until at least 4 h after the procedure. In the case of thrombocytopenia <30 × 109/L, platelet tranfusion was required or enoxaparin had to be withheld until platelet regeneration.
In the antithrombin group, antithrombin activity was measured every 3 days prior to each asparaginase administration. If antithrombin activity was below the lower limit of normal of 80%, the plasma-derived antithrombin preparation Kybernin™ was substituted calculating the dose according to the formula [antithrombintarget 100% – antithrombinactual] × kg body weight targeting at 100% AT activity.
Patients assigned to the control arm received UFH at a dose of 2 IU/kg body weight/h as long as an infusion drip was running to prevent local thrombotic occlusion of the indwelling CVC.24
Treatment with coagulation factors or anticoagulants beyond the interventions intended per protocol was not allowed unless clinically indicated. Management of thromboembolism was at the discretion of the treating physician.
Outcome measures
The diagnosis of thromboembolism was based on clinical suspicion and had to be confirmed by one or more suitable imaging methods within a routine diagnostic work-up (Online Supplementary Table S1). No systematic provision was made for blinding the attending physicians or radiologists to the randomization arm. Intermittent dysfunction of the CVC by a clot at the tip of the catheter was not considered a thrombotic event as long as CVC patency was restored. The principal safety outcome was absence of bleeding complications during the study period. The definition of major and minor hemorrhage met internationally defined standards (Online Supplementary Table S2).35–37 Secondary safety outcomes were event-free survival and overall survival. Event-free survival was defined as the time from diagnosis to the date of last follow-up or first event. Events were resistance to therapy, leukemia relapse, secondary neoplasm or death from any cause. Failure to achieve remission due to early death or resistance was considered as an event at time zero. Survival was defined as time from diagnosis to the date of last follow-up or death from any cause.
Statistical analysis
The primary objective was to test whether antithrombotic prophylaxis with enoxaparin or antithrombin was superior to that with UFH. The null hypothesis was that there was no difference between enoxaparin or antithrombin versus UFH tested with a one-tailed Fisher exact test at a significance level of P=0.025 each. The main analysis was by intention-to-treat. In order to reach a power of 85% with a significance level of 0.025, 315 patients had to be randomized per group, assuming an event rate of 9% within the UFH group and 3% in the two interventional groups. If both comparisons were significantly different, the thrombosis rates in the enoxaparin and antithrombin arm had to be tested for equivalence (secondary objective). Antithrombin replacement and enoxaparin therapy would be considered equivalent if the two-sided 95% confidence interval (95% CI) of the incidence difference did not exceed ±4%. For the equivalence test, patients were analyzed according to the treatment given (as treated).
The Kaplan-Meier method38 was used to estimate survival rates, and differences were compared with the log-rank test.39 A Cox proportional hazards model was used in univariate and multivariate survival analyses.40 Cumulative incidence functions for competing events were constructed by the method of Kalbfleisch and Prentice41 and compared with the Gray test.42 Odds ratios were calculated to compare the risks of thromboembolic events. Except for the confirmatory analyses of the primary study question, all other analyses were exploratory.
Results
Patients’ characteristics
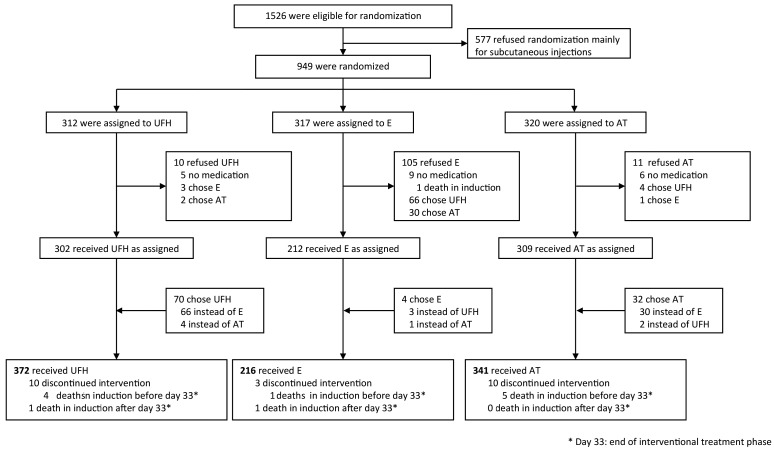
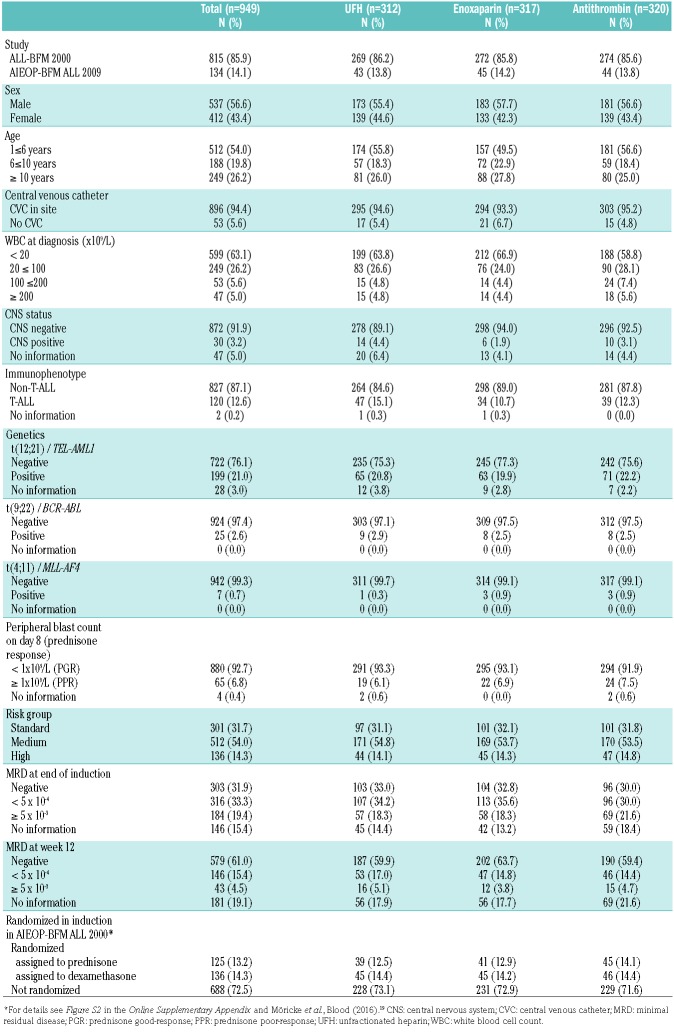
From December 1, 2002, to December 31, 2011, 1526 patients with ALL treated at one of the 26 study centers in Germany and Switzerland were eligible for randomization (Figure 1). Of these, 577 patients were not randomized, the vast majority because patients and/or parents refused consent to be randomized to the enoxaparin arm as they did not wish to accept a daily subcutaneous injection. Nine hundred and forty-nine patients (the population for the intention-to-treat analyses) were randomly assigned to receive either UFH (n=312), enoxaparin (n=317) or antithrombin (n=320). Randomized and non-randomized eligible patients did not differ with respect to their initial characteristics (Online Supplementary Table S3). The proportions of patients with a poor response to the prednisone prephase (prednisone poor-responders) and a slow treatment response as assessed by minimal residual disease were significantly higher in the group of non-randomized patients. In the intention-to-treat population, numbers and characteristics of patients were well-balanced between the three randomization arms except for a slight imbalance in the age distribution with fewer children below 6 years in the enoxaparin group (Table 1). Patients’ characteristics were evenly distributed between the randomization arms as treated except for a significantly lower proportion of patients below 6 years of age in the enoxaparin arm (details provided in Online Supplementary Table S4).
Figure 1.
Consolidated standards for reporting of trials (CONSORT) diagram. AT: antithrombin; E: enoxaparin; UFH: unfractionated heparin.
Table 1.
Patients’ characteristics by thromboprophylaxis group as assigned by randomization.
The proportion of patients who refused antithrombotic treatment as allocated was 3% in patients randomized to UFH (10/312) or antithrombin (11/320), and 33% (105/317) in those assigned to enoxaparin (Figure 1). Rejection of the enoxaparin arm was more frequent in patients below 6 years of age than in older patients [62/157 (39%) versus 42/160 (27%), respectively] with a preferential switch to UFH in the younger cohort (Online Supplementary Table S5). Based on this finding additional exploratory analyses with respect to thromboembolism rate and leukemia-related outcomes were performed, stratified by age and in the as-treated groups.
Thromboembolic events
Among the 949 randomized patients, 42 thromboembolic events were observed (4.4%; 95% CI: 3.2 to 5.9). Of these events, 20 (47.6%) occurred in the upper deep venous system, seven (16.7) in the lower deep venous system, and 13 (30.9%) in cerebral sinus veins; two patients (4.8%) had a cerebral arterial stroke. Eight of the 42 thromboembolic events (19%) were distant to the site of the CVC. Thirty-three events occurred between treatment day 9 and 36 during induction therapy, the other nine events occurred between treatment day 37 and 52 of induction consolidation.
Children below 6 years of age had a significantly lower risk of thromboembolism (14/512, 2.7%) than those aged 6 to 9 years (11/188, 5.9%) or 10 years and older (17/249, 6.8%; P=0.018). Other patients’ characteristics and features, such as gender, initial white blood cell count, immunophenotype or treatment response did not influence the incidence of thromboembolism (data not shown).
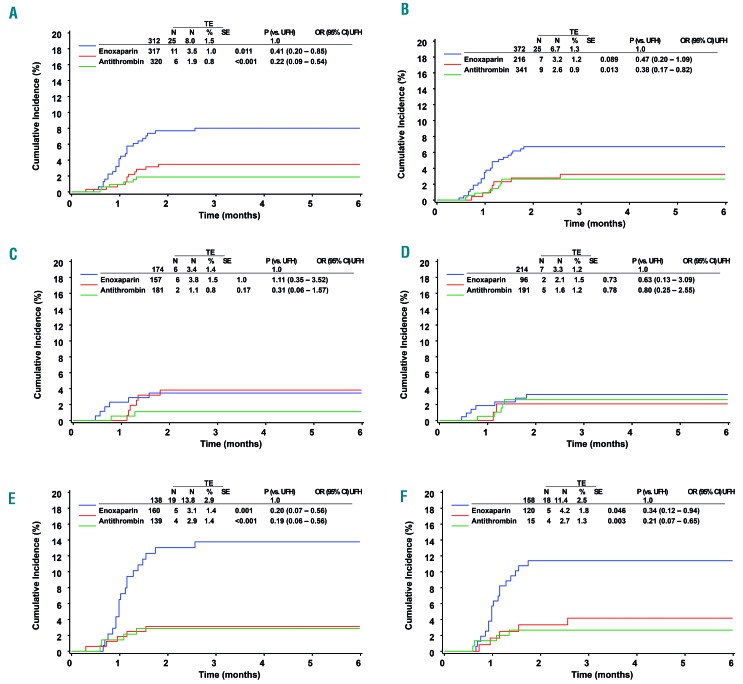
The incidence of thromboembolism was significantly higher among patients randomized to UFH (25/312; 8.0%) than in the enoxaparin (11/317; 3.5%; P=0.011) or antithrombin group (6/320; 1.9%; P<0.001). The as-treated analysis revealed an incidence of 6.7% in the UFH group (25/372) compared to 3.2% in the enoxaparin (7/216; P=0.089) and 2.6% in the antithrombin group (9/341; P=0.013). The respective cumulative incidences are depicted in Figure 2A,B. The difference between the incidence of thromboembolism in the enoxaparin and antithrombin groups as treated was -0.6%; the lower and upper limits of the 95% CI were -3.5% and +2.3%, respectively (P-values for the corresponding one-sided tests were P=0.01 and P=0.001). Thus, antithrombin and enoxaparin were equally effective.
Figure 2.
Thromboembolic events according to the randomization arms. Results are shown by intention to treat (A, C and E) and by treatment as given (B, D and F) for the total cohort (A and B) and stratified by age <6 years (C and D) and ≥6 years (E and F). Events are depicted as cumulative incidence curves. The P values indicated were calculated with the Fisher exact test. CI: confidence interval; OR: odds ratio; TE: thromboembolism; UFH: unfractionated heparin.
Exploratory as-treated analyses stratified by age (Figure 2D,F) demonstrated a significantly reduced risk of thromboembolism in patients 6 years of age or older when treated in one of the experimental arms compared to the risk in the control group [UFH: 18/158, 11.4%; enoxaparin: 5/120, 4.2%, P(versus UFH)=0.001; antithrombin 4/150, 2.7%, P(versus UFH)<0.001]. No significant differences were found among patients below 6 years of age (UFH 7/214, 3.3%; enoxaparin 2/96, 2.1%; antithrombin 5/191, 2.6%).
No formal test for interaction was done for the subgroup analysis by age. Applying Fine-Gray models with interaction terms for age older than 6 years and enoxaparin/antithrombin, the interactions were not significant. This, however, does not entirely exclude interactions since the power of such tests is low.
Hemorrhage
Eight bleeding episodes were documented among the 949 randomized patients (0.9%). Four of them occurred during induction chemotherapy under antithrombotic prophylaxis and four during consolidation after termination of the anticoagulants. All hemorrhages were classified as major (7 gastrointestinal, 1 cerebral). Four patients with hemorrhage were treated in the UFH group (1.1%), three in the antithrombin group [0.9%, P(versus UFH)=1.0] and one patient in the enoxaparin group [0.5%, P(versus UFH)=0.66].
Leukemia outcome and survival
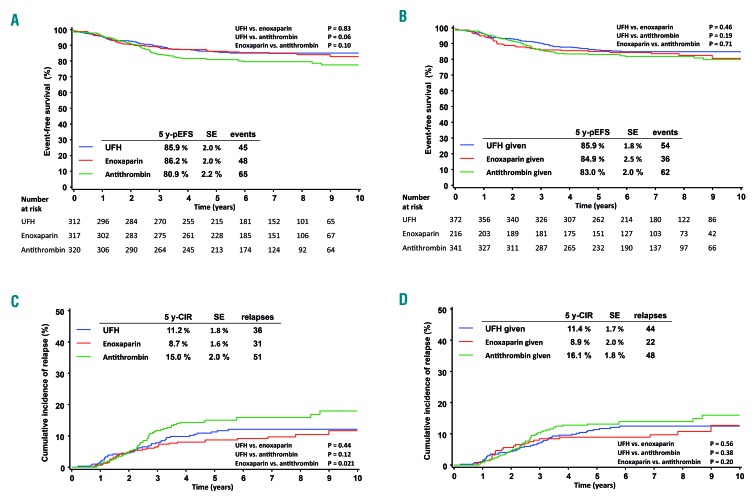
The 5-year probability of event-free survival and cumulative incidence of relapse of the THROMBOTECT cohort were comparable to those of the 577 non-randomized patients (THROMBOTECT cohort: 5-year probability of event-free survival 84.3±1.2%, 5-year cumulative incidence of relapse 11.7±1.1%; non-randomized patients: 5-year probability of event-free survival 84.0±1.6%, 5-year cumulative incidence of relapse 11.8±1.4). Patients randomized to the antithrombin arm had a 5-year probability of event-free survival of 80.9±2.2% compared with those assigned to enoxaparin (86.2±2.0%, P=0.10) or UFH (85.9±2.0%, P=0.06) (Figure 3A) with a hazard ratio of 1.40 (1.02-1.92; P=0.040) for the antithrombin arm versus the remaining patients. The probability of overall survival at 5 years was similar in all three arms (antithrombin 89.8±1.7%, enoxaparin 90.9±1.6%, UFH 92.4±1.5%). The differences observed in the event-free survival were due to a higher incidence of late relapses in the antithrombin group that in the other groups (Figure 3C); the as-treated analyses showed no statistically significant differences between the three groups [hazard ratio for the antithombin group versus the other groups: 1.16 (0.84-1.59); P=0.37) (Figure 3B,D). Retrospective exploratory subgroup analyses revealed a higher incidence of relapse among the antithrombin-treated patients, but only within the medium-risk group (Online Supplementary Figure S3). Multivariate Cox regression analyses on event-free survival were performed including risk group according to respective trial criteria, TEL-AML1 status, initial white blood cell count, age and the THROMBOTECT arm as covariates. Hazard ratios for the antithrombin arm were 1.38 (0.99-1.91; P=0.054) for the intention-to-treat analysis and 1.19 (0.86-1.66; P=0.269) for the as-treated analysis and thus comparable with those of the univariate analyses (Online Supplementary Table S6).
Figure 3.
Outcome of acute lymphoblastic leukemia according to the THROMBOTECT randomization arms. (A,B) Event-free survival and (C,D) cumulative incidence of relapse are shown by intention to treat (A,C) and by treatment as given (B,D). Numbers of patients at risk in the event-free survival graphs also apply to the respective relapse incidence graphs. 5 y-pEFS: 5-year probability of event-free survival; 5 y-CIR: 5-year cumulative incidence of relapse; SE: standard error; UFH: unfractionated heparin.
To test for a potential dose effect of antithrombin, doses given were analyzed in patients treated in the antithrombin arm. Data available for 248 of 341 patients (72.7%) did not disclose a dose-related effect on the relapse incidence (Online Supplementary Figure S4).
Discussion
Reliable data on thromboembolism during induction therapy of childhood ALL are scarce. The only randomized interventional trial was the PARKAA trial (Prophylactic antithrombin replacement in kids with ALL treated with L-asparaginase), designed to determine whether there was a trend to efficacy and safety of antithrombin treatment but not powered to prove it.16 To our knowledge, no other data from adequately designed and powered studies have been available so far to provide sufficient evidence that would allow valid recommendations.4,5,9,19,20,23,43,44
The THROMBOTECT trial shows, for the first time, that prophylactic antithrombotic interventions significantly reduce thromboembolism during ALL induction therapy as compared to a control. Both interventions, enoxaparin and activity-adapted Antithrombin substitution, were equally effective. Asparaginase-induced antithrombin deficiency is assumed to be the most important mechanism for the development of thromboembolism during ALL induction therapy.45 As a consequence of asparagine depletion, asparaginase therapy leads to intracellular retention of a misfolded antithrombin, resulting in acquired antithrombin deficiency.45,46 The THROMBOTECT trial demonstrated that maintaining antithrombin activity at 80% or higher throughout the induction phase could significantly protect patients from thromboembolism. Thus, correction of low antithrombin activity seems to be one effective way to prevent thromboembolism, this being consistent with clinical and laboratory data on antithrombin supplementation.10,16,18,19,47
A considerable number of patients eligible for the study were not randomized. In this group the rate of prednisone poor-responders was significantly higher than in the THROMBOTECT cohort. This may be attributed to a tendency of the doctors or parents to avoid additional burden from interventions of an add-on trial in particular in those patients with very poor response during the first days of treatment. However, patients’ characteristics were comparable between the three randomization groups except for a slight underrepresentation of younger patients assigned to enoxaparin. The main reason for not participating was refusal to accept the daily subcutaneous enoxaparin injections. Not surprisingly, the proportion of patients and parents refusing the assigned enoxaparin was highest in young children. This demonstrates not only their reluctance to receive injections but also underlines a considerable drawback in practical use, irrespective of the antithrombotic efficacy of enoxaparin.
Older age proved to be an important risk factor for thromboembolism, as has been reported earlier by others.1,13,48 The best cut-off in our data was the age of 6 years. Exploratory analyses suggested that the benefit from either experimental arm was more pronounced in older patients than in young children. The significant benefit in risk reduction of thromboembolism with either intervention, enoxaparin or antithrombin, as compared to UFH, provides a convincing rationale for thromboprophylaxis in this age group. For younger children, the incidence of thromboembolism was low and comparable in all three randomization arms. The need for thromboprophylaxis in ALL patients below 6 years of age could, therefore, be questioned. However, the study was not powered for subgroup analyses and the lack of statistical difference in the incidences of thromboembolism between the treatment groups in younger children may be due to insufficient power caused by the number of patients as well as the lower incidence of thromboembolism. Furthermore, in younger children thromboembolism may be missed as symptoms are often subtle. This is in line with the findings of the PARKAA study, which showed that children with symptomatic thromboembolism tend to be older than those with clinically asymptomatic thromboembolism.16 Even if clinically not diagnosed, asymptomatic thromboembolism may be associated with significant vessel occlusion.16 This, in turn, can lead to destruction of the vessel wall, causing long-term morbidity in terms of post-thrombotic syndrome, likely becoming apparent years after the end of ALL therapy. Whether this applies to young patients with ALL remains unknown.17 Future studies with sufficient statistical power are needed to ascertain whether such interventions in small children are justified. Nevertheless, although the high proportion of patients who refused allocation to the enoxaparin arm may complicate the interpretation of the results in this treatment arm, the reduction of thromboembolism in the global analysis appears to be sufficiently convincing to recommend thromboprophylaxis not only for older patients but for all age groups, all the more as hemorrhage is of no concern.
Most thrombotic events occurred between induction treatment day 9 and 36, the latter marking the start of induction consolidation. This confirms our experience that thromboembolism only rarely occurs at the time of ALL diagnosis but rather in the course of induction therapy. Furthermore, not all centers were able to get a CVC inserted at the time of ALL diagnosis. For these reasons, thromboprophylaxis was started after the prednisone prephase on day 8 of induction therapy. The primary objective of the THROMBOTECT trial was to evaluate efficacy and safety of different prophylactic antithrombotic interventions during ALL induction therapy. The duration of thromboprophylaxis was, therefore, limited to induction therapy until day 33. Some of the thromboembolic events occurred after the end of the induction phase. However, only a few of these patients had already started the consolidation phase when the thrombosis was diagnosed. Factors that may have contributed to these late thromboses could be concurrent medical issues such as infections. Given the gradual development of a clot, a still asymptomatic thrombosis might have started to develop towards the end of induction therapy and only become symptomatic in early induction consolidation. Since pegylated asparaginase is presently used more frequently - in the AIEOP-BFM ALL 2009 trial, the second dose of this drug was given on day 26 of induction - late thromboses in induction consolidation might become more relevant as the use of pegylated asparaginase may lead to longer asparagine depletion with disturbed coagulation patterns, including extended dysfunction of antithrombin. Irrespective of possible concomitant prothrombotic risk situations, the hypercoagulable state seems to remain beyond the end of induction therapy. Given the very low rate of hemorrhage it might, therefore, be advisable to extend thromboprophylaxis accordingly.
The open label assignment as well as the diagnosis of thromboembolism made on clinical suspicion only are drawbacks of the THROMBOTECT study design. However, masking the antithrombotic intervention would have meant that all patients in all randomization groups would have had to have been given subcutaneous injections, including those in the UFH and antithrombin groups containing placebo. To conduct the study as a double-blinded trial with double dummy subcutaneous injections was not considered feasible in a large pediatric population.
Similar concerns apply to the primary outcome defined as thromboembolism based on clinical suspicion. The PARKAA study showed that a high incidence of clinically not recognized thromboses can be found by routine imaging screening.16 To overcome observer bias, various and repeated routine imaging screening for vessel occlusion at all possible anatomical sites would have been mandatory at predefined time points. This comprises ultrasound but also magnetic resonance imaging which, in young children, often requires general anesthesia. In addition, for the time being the appropriate time points to look for vessel occlusion are not known and hence the possibility of missing a thrombosis at arbitrarily chosen time points would be high. Exposing children to repeated extra anesthesia with a questionable benefit was considered too high an additional burden. The study design chosen was, therefore, in favor of an open-label treatment. Imaging was performed on clinical suspicion despite the acknowledged inherent drawbacks.
Evaluation of event-free survival and relapse rate within the THROMBOTECT randomization groups revealed the unexpected finding that patients randomized to the antithrombin group had a higher incidence of relapse compared to those in the enoxaparin and UFH groups. The differences were no longer obvious in the as-treated analysis and were apparent in the medium-risk group only. Although a causal relationship between the cumulative antithrombin dose and the relapse rate could not be established, the possibility that antithrombin substitution might affect leukemia outcome cannot be entirely excluded.
In conclusion, the THROMBOTECT study has, for the first time, demonstrated that activity-targeted antithrombin replacement as well as the use of enoxaparin lead to a significant risk reduction for thromboembolism during ALL induction therapy when compared with low-dose UFH. Bleeding was not a major concern. Thromboprophylaxis during induction therapy can, therefore, be recommended for children and adolescents with ALL. The higher incidence of late relapses in children with medium-risk ALL assigned to the antithrombin group remains to be resolved and leads us to recommend, at present, primarily enoxaparin. Whether thromboprophylaxis contributes to minimize not only clinical but also silent thromboses and by that long-term morbidity in terms of post-thrombotic syndrome remains to be determined. The THROMBOTECT results provide the rationale for new studies, both to elucidate a possible impact of antithrombin on leukemia outcome and to further determine the best practice to prevent thromboembolism during ALL induction chemotherapy.
Supplementary Material
Acknowledgments
We thank the investigators, the medical staff, the data managers and the patients of all participating centers for their sustained support of the THROMBOTECT study.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/104/4/756
References
- 1.Athale UH, Chan AK. Thrombosis in children with acute lymphoblastic leukemia. Part I. Epidemiology of thrombosis in children with acute lymphoblastic leukemia. Thromb Res. 2003;111(3):125–131. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell L, Hoogendoorn H, Giles AR, Vegh P, Andrew M. Increased endogenous thrombin generation in children with acute lymphoblastic leukemia: risk of thrombotic complications in L-asparaginase-induced antithrombin III deficiency. Blood. 1994;83(2):386–391. [PubMed] [Google Scholar]
- 3.Mitchell LG, Sutor AH, Andrew M. Hemostasis in childhood acute lymphoblastic leukemia: coagulopathy induced by disease and treatment. Semin Thromb Hemost. 1995;21(4):390–401. [DOI] [PubMed] [Google Scholar]
- 4.Nowak-Göttl U, Ahlke E, Fleischhack G, et al. Thromboembolic events in children with acute lymphoblastic leukemia (BFM protocols): prednisone versus dexamethasone administration. Blood. 2003;101(7):2529–2533. [DOI] [PubMed] [Google Scholar]
- 5.Caruso V, Iacoviello L, di Castelnuovo A, et al. Thrombotic complications in childhood acute lymphoblastic leukemia: a meta-analysis of 17 prospective studies comprising 1752 pediatric patients. Blood. 2006;108(7):2216–2222. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell LG, Andrew M, Hanna K, et al. A prospective cohort study determining the prevalence of thrombotic events in children with acute lymphoblastic leukemia and a central venous line who are treated with L-asparaginase: results of the Prophylactic Antithrombin Replacement in Kids with Acute Lymphoblastic Leukemia Treated with Asparaginase (PARKAA) study. Cancer. 2003;97(2):508–516. [DOI] [PubMed] [Google Scholar]
- 7.Journeycake JM, Buchanan GR. Catheter-related deep venous thrombosis and other catheter complications in children with cancer. J Clin Oncol. 2006;24(28):4575–4580. [DOI] [PubMed] [Google Scholar]
- 8.Male C, Chait P, Andrew M, Hanna K, Julian J, Mitchell L. Central venous line-related thrombosis in children: association with central venous line location and insertion technique. Blood. 2003;101(11):4273–4278. [DOI] [PubMed] [Google Scholar]
- 9.Athale UH, Chan AK. Thrombosis in children with acute lymphoblastic leukemia. Part II. Pathogenesis of thrombosis in children with acute lymphoblastic leukemia: effects of the disease and therapy. Thromb Res. 2003;111(4–5):199–212. [DOI] [PubMed] [Google Scholar]
- 10.Astwood E, Vora A. Personal practice: how we manage the risk of bleeding and thrombosis in children and young adults with acute lymphoblastic leukaemia. Br J Haematol. 2011;152(5):505–511. [DOI] [PubMed] [Google Scholar]
- 11.Vidal E, Sharathkumar A, Glover J, Faustino EV. Central venous catheter-related thrombosis and thromboprophylaxis in children: a systematic review and meta-analysis: reply. J Thromb Haemost. 2015;13(1):161–162. [DOI] [PubMed] [Google Scholar]
- 12.Wiernikowski JT, Athale UH. Thromboembolic complications in children with cancer. Thromb Res. 2006;118(1):137–152. [DOI] [PubMed] [Google Scholar]
- 13.Athale UH, Siciliano SA, Crowther M, Barr RD, Chan AK. Thromboembolism in children with acute lymphoblastic leukaemia treated on Dana-Farber Cancer Institute protocols: effect of age and risk stratification of disease. Br J Haematol. 2005;129(6):803–810. [DOI] [PubMed] [Google Scholar]
- 14.Tuckuviene R, Ranta S, Albertsen BK, et al. Prospective study of thromboembolism in 1038 children with acute lymphoblastic leukemia: a Nordic Society of Pediatric Hematology and Oncology (NOPHO) study. J Thromb Haemost. 2016;14(3):485–494. [DOI] [PubMed] [Google Scholar]
- 15.Nowak-Göttl U, Wermes C, Junker R, et al. Prospective evaluation of the thrombotic risk in children with acute lymphoblastic leukemia carrying the MTHFR TT 677 genotype, the prothrombin G20210A variant, and further prothrombotic risk factors. Blood. 1999;93(5):1595–1599. [PubMed] [Google Scholar]
- 16.Mitchell L, Andrew M, Hanna K, et al. Trend to efficacy and safety using antithrombin concentrate in prevention of thrombosis in children receiving l-asparaginase for acute lymphoblastic leukemia. Results of the PAARKA study. Thromb Haemost. 2003;90(2):235–244. [DOI] [PubMed] [Google Scholar]
- 17.Avila ML, Duan L, Cipolla A, et al. Postthrombotic syndrome following upper extremity deep vein thrombosis in children. Blood. 2014;124(7):1166–1173. [DOI] [PubMed] [Google Scholar]
- 18.Gugliotta L, D’Angelo A, Mattioli BM, et al. Hypercoagulability during L-asparaginase treatment: the effect of antithrombin III supplementation in vivo. Br J Haematol. 1990;74(4):465–470. [DOI] [PubMed] [Google Scholar]
- 19.Nowak-Göttl U, Kuhn N, Wolff JE, et al. Inhibition of hypercoagulation by antithrombin substitution in E. coli L-asparaginase-treated children. Eur J Haematol. 1996;56(1–2):35–38. [DOI] [PubMed] [Google Scholar]
- 20.Elhasid R, Lanir N, Sharon R, et al. Prophylactic therapy with enoxaparin during L-asparaginase treatment in children with acute lymphoblastic leukemia. Blood Coagul Fibrinolysis. 2001;12(5):367–370. [DOI] [PubMed] [Google Scholar]
- 21.Harlev D, Zaidman I, Sarig G, Ben Arush MW, Brenner B, Elhasid R. Prophylactic therapy with enoxaparin in children with acute lymphoblastic leukemia and inherited thrombophilia during L-asparaginase treatment. Thromb Res. 2010;126(2):93–97. [DOI] [PubMed] [Google Scholar]
- 22.Ruud E, Holmstrom H, de Lange C, Natvig S, Albertsen BK, Wesenberg F. Thrombotic effects of asparaginase in two acute lymphoblastic leukemia protocols (NOPHO ALL-1992 versus NOPHO ALL-2000): a single-institution study. Pediatr Hematol Oncol. 2006;23(3):207–216. [DOI] [PubMed] [Google Scholar]
- 23.Meister B, Kropshofer G, Klein-Franke A, Strasak AM, Hager J, Streif W. Comparison of low-molecular-weight heparin and antithrombin versus antithrombin alone for the prevention of symptomatic venous thromboembolism in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2008;50(2):298–303. [DOI] [PubMed] [Google Scholar]
- 24.Goossens GA. Flushing and locking of venous catheters: available evidence and evidence deficit. Nurs Res Pract. 2015;2015: 985686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hentschel R, Sutor AH. Catheter-related thrombosis and its prevention in infancy. Hamostaseologie. 2002;22(4):167–173. [PubMed] [Google Scholar]
- 26.Lersch C. Prevention of catheter-induced thromboses by low molecular weight heparins. Hamostaseologie. 2002;22(4):161–166. [PubMed] [Google Scholar]
- 27.Randolph AG, Cook DJ, Gonzales CA, Andrew M. Benefit of heparin in central venous and pulmonary artery catheters: a meta-analysis of randomized controlled trials. Chest. 1998;113(1):165–171. [DOI] [PubMed] [Google Scholar]
- 28.Conter V, Bartram CR, Valsecchi MG, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010;115(16):3206–3214. [DOI] [PubMed] [Google Scholar]
- 29.Möricke A, Zimmermann M, Valsecchi MG, et al. Dexamethasone vs prednisone in induction treatment of pediatric ALL: results of the randomized trial AIEOP-BFM ALL 2000. Blood. 2016;127(17):2101–2112. [DOI] [PubMed] [Google Scholar]
- 30.Schrappe M, Valsecchi MG, Bartram CR, et al. Late MRD response determines relapse risk overall and in subsets of childhood T-cell ALL: results of the AIEOP-BFM-ALL 2000 study. Blood. 2011;118(8):2077–2084. [DOI] [PubMed] [Google Scholar]
- 31.Massicotte P, Adams M, Marzinotto V, Brooker LA, Andrew M. Low-molecular-weight heparin in pediatric patients with thrombotic disease: a dose finding study. J Pediatr. 1996;128(3):313–318. [DOI] [PubMed] [Google Scholar]
- 32.Punzalan RC, Hillery CA, Montgomery RR, Scott CA, Gill JC. Low-molecular-weight heparin in thrombotic disease in children and adolescents. J Pediatr Hematol Oncol. 2000;22(2):137–142. [DOI] [PubMed] [Google Scholar]
- 33.Dix D, Andrew M, Marzinotto V, et al. The use of low molecular weight heparin in pediatric patients: a prospective cohort study. J Pediatr. 2000;136(4):439–445. [DOI] [PubMed] [Google Scholar]
- 34.Schneppenheim R, Greiner J. Thrombosis in infants and children. Hematology Am Soc Hematol Educ Program. 2006;86–96. [DOI] [PubMed] [Google Scholar]
- 35.Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692–694. [DOI] [PubMed] [Google Scholar]
- 36.Levine MN, Raskob G, Landefeld S, Kearon C. Hemorrhagic complications of anticoagulant treatment. Chest. 2001;119(1 Suppl):108S–121S. [DOI] [PubMed] [Google Scholar]
- 37.Committee for proprietary medicinal products (CPMP) Notes for guidance on clinical investigation of medicinal products for treatment of venous thromboembolic disease. http://www.emaeuropeeu2016;CPMP/EWP/563/98
- 38.Kaplan ES, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 39.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50(3):163–170. [PubMed] [Google Scholar]
- 40.Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methodol. 1972;42(2):187–220. [Google Scholar]
- 41.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. 1980;1st 163–188. [Google Scholar]
- 42.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141–1154. [Google Scholar]
- 43.Santoro N, Colombini A, Silvestri D, et al. Screening for coagulopathy and identification of children with acute lymphoblastic leukemia at a higher risk of symptomatic venous thrombosis: an AIEOP experience. J Pediatr Hematol Oncol. 2013;35(5):348–355. [DOI] [PubMed] [Google Scholar]
- 44.Giordano P, Santoro N, Del Vecchio GC, Rizzari C, Masera G, de Mattia D. T-immunophenotype is associated with an increased prevalence of thrombosis in children with acute lymphoblastic leukemia. A retrospective study. Haematologica. 2003;88(9):1079–1080. [PubMed] [Google Scholar]
- 45.Hernandez-Espinosa D, Minano A, Ordonez A, et al. Dexamethasone induces a heat-stress response that ameliorates the conformational consequences on antithrombin of L-asparaginase treatment. J Thromb Haemost. 2009;7(7):1128–1133. [DOI] [PubMed] [Google Scholar]
- 46.Bushman JE, Palmieri D, Whinna HC, Church FC. Insight into the mechanism of asparaginase-induced depletion of antithrombin III in treatment of childhood acute lymphoblastic leukemia. Leuk Res. 2000;24(7):559–565. [DOI] [PubMed] [Google Scholar]
- 47.Payne JH, Vora AJ. Thrombosis and acute lymphoblastic leukaemia. Br J Haematol. 2007;138(4):430–445. [DOI] [PubMed] [Google Scholar]
- 48.Appel IM, Hop WC, van Kessel-Bakvis C, Stigter R, Pieters R. L-asparaginase and the effect of age on coagulation and fibrinolysis in childhood acute lymphoblastic leukemia. Thromb Haemost. 2008;100(2):330–337. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.