Abstract
The transforming growth factor β (TGF-β) superfamily participates in tumour proliferation, apoptosis, differentiation, migration, invasion, immune evasion and extracellular matrix remodelling. Genetic deficiency in distinct components of TGF-β and BMP-induced signalling pathways or their excessive activation has been reported to regulate the development and progression of some cancers. As more in-depth studies about this superfamily have been conducted, more evidence suggests that the TGF-β and BMP pathways play an opposing role. The cross-talk of these 2 pathways has been widely studied in kidney disease and bone formation, and the opposing effects have also been observed in some cancers. However, the antagonistic mechanisms are still insufficiently investigated in cancer. In this review, we aim to display more evidences and possible mechanisms accounting for the antagonism between these 2 pathways, which might provide some clues for further study in cancer.
Keywords: TGF-β BMP cancer antagonistic potential mechanism
Highlights
-
•
Describe the basics of TGF-β and BMP signalling
-
•
Summarize the potential mechanisms accounting for the antagonism between TGF-β and BMP pathways
-
•
Provide some evidence about the antagonistic effects between pathways observed in some cancers
1. Introduction
The transforming growth factor β (TGF-β) superfamily is a large group of molecules that includes transforming growth factor (TGF-β), bone morphogenetic protein (BMP), growth differentiation factor (GDF), activin, inhibin and Nodal. TGF-β and BMP are important members of this superfamily; they have similar downstream transduction pathways, and their regulation is closely related, which is the biological basis of their antagonistic interaction [1]. The antagonism between the TGF-β pathway and the BMP pathway was initially reported in kidney disease [2] and bone formation [3]. Recently, an increasing number of reports have focused on the opposing effect between these two pathways in the progression of cancers. Unfortunately, their antagonistic mechanisms in cancer are not very extensively discussed. Therefore, the rest of this review is organized as follows: in Section 1, we describe the basics of TGF-β and BMP signalling; in Section 2, due to a lack of studies on the antagonistic mechanisms of these two pathways in cancer, we provide some potential mechanism in other cell types for reference; in Section 3, we summarize the data that support the opposing effect of the two pathways in the progression of some cancers; and finally, we give our conclusion and highlight important questions for future research.
2. TGF-β and BMP signalling pathways
In general, the TGF-β and BMP ligands elicit their effects via binding to the dual serine/threonine kinase receptors on the surface of target cells, which are assembled into a complex of type I and type II receptors. Upon ligand binding, type I receptors specifically phosphorylate intracellular R-Smads (Receptor-regulated Smad proteins). Activated R-Smads form heteromeric Smad complexes with Smad4 and translocate into the nucleus, and the complexes then bind to transcription factors and transcriptional co-activators or co-repressors to regulate the transcription of target genes [4]. In addition, non-Smad signalling pathways are also initiated by the activated TGF-β and BMP receptors, including the PI3K-AKT-mTOR pathway, the Ras-ERK-MAPK pathway, the p38-MAPK pathway, Rho, Rac and Cdc42 GTPase pathways [5]. We will further elaborate on the specific TGF-β and BMP signalling pathways in the sections that follow.
2.1. Ligands and receptors along the TGF-β and BMP pathways
The TGF-β ligands include TGF-β1, TGF-β2, and TGF-β3. They exhibit high affinities for the TGF-β type II receptor (TβRII) but do not interact with TGF-β type I receptor (TβRI, also called ALK-5). In general, TGF-β ligands tightly bind to the extracellular domain of TβRII, and TβRI then participates in the formation of receptor complexes [6]. The BMP ligands can be further classified into 4 subgroups based on structural homology: the BMP-2/-4 subgroup, BMP-5/-6/-7/-8 subgroup, BMP-9/-10 subgroup, and BMP-12/-13/-14 subgroup [7]. There are three type II receptors for BMPs— the BMP type II receptor (BMPRII), the activin type II receptor (ActRII), and activin type IIB receptor (ActRIIB); BMPRII is specific for BMPs, while ActRII and ActRIIB are shared by BMPs, myostatin and activins [8]. The four activin receptor-like kinases (ALKs) ALK-1, ALK-2(ACVR1), ALK-3 (BMPRIA) and ALK-6 (BMPRIB) are termed type I receptors for BMPs [9]. In contrast to TGF-β, BMPs bind to type I and type II receptors with different affinities. For instance, BMP-2 and BMP-4 exhibit high affinity for the type I receptors and a comparably low affinity for BMPRII [10,11]. BMP-7, however, preferentially binds to ActRII and ActRIIB, while its affinity for the type I receptors is less pronounced [12]. In addition, different BMP ligands bind to different type I receptors; for example, BMP-7 effectively binds to ALK-2 and ALK-6 and has a lower affinity for ALK-3, while BMP-4 effectively binds to ALK-3 and ALK-6. Therefore, different BMP ligands bind to their corresponding receptors in a specific manner, which results in the activation of distinct signalling cascades [13]. Moreover, there are co-receptors, endoglin [14] and betaglycan [15], which do contribute to ligand binding and have multiple effects on TGF-β and BMP pathways and are also implicated in cancer. TGF-β can also bind ALK1 and the ALK-1 pathway is known to cross-talk with the ALK-5 pathway [16]. Differential potentiation of signal by co-receptors, endoglin in association with TβRII binds to TGF-β1 and TGF-β-β3 but not TGF-β2 [17] whereas betaglycan binds to all 3 isoforms [18]. In addition, the shedding of the membrane-bound endoglin and betaglycan can produce soluble forms, which bind and sequester ligands of TGF-β and BMPs to inhibit their downstream signalling [19]. Soluble endoglin binds with high affinity to BMP-9 and BMP-10 and forms a complex with these ligands to inhibit the effects of angiogenesis induced by BMP-9 and BMP-10 [20]. The balance of cell surface and soluble endoglin and betaglycan regulates TGF-β and BMP signalling and plays an important role in mediating their effects [21]. ALK1-Fc, a soluble chimeric protein targeting ALK-1, prevents binding of selective BMPs to ALK-1 and inhibits ALK-1 signalling; it is considered to be a powerful antiangiogenic agent capable of blocking vascularization [22].
2.2. The Smad proteins within the TGF-β and BMP pathways
Smad 2 and 3 are specifically activated by TGF-β receptors, and they can negatively regulate self-signalling by increasing the transcription of Smad7 [23], an inhibitory Smad (I-Smad). Smad7 not only interacts with activated TβRI to block the phosphorylation of Smad2 [24], but it also interferes with TGF-β-induced functional Smad-DNA complex formation in the nucleus to further inhibit TGF-β signalling [25]. In contrast, Smad1, Smad5 and Smad8 are activated by BMP receptors, and they can negatively regulate self-signalling by increasing the transcription of another I-Smad, Smad6 [26]. Smad6 impedes Smad1/5/8 phosphorylation by binding to BMP type I receptors [27] and competes with Smad4 to bind phosphorylated Smad1 to form an inactive Smad1-Smad6 complex [28]. Furthermore, at the transcriptional level, Smad6 also binds certain transcription factors to inhibit BMP-induced transcription in the nucleus [29]. In addition, Smad7 has also been reported to bind to BMP type I receptors and impede the phosphorylation of Smad1/5/8 [24].
2.3. Regulation of the TGF-β and BMP pathways
I-Smads can negatively regulate the TGF-β and BMP pathways in concert with Smurfs, the Smad ubiquitination regulators that display more obvious synergistic inhibitory effects than the direct inhibition of I-Smads [30]. Smurfs belong to the HECT family of E3 ubiquitin ligases, including Smurf1 and Smurf2, which specifically identify different substrates and initiate degradation through the ubiquitin-proteasome pathway [31]. Smurf1 negatively regulates both the TGF-β and BMP pathways; Smurf1 mediates ubiquitination of BMP type I receptors through either Smad6 or Smad7 [30], while it mediates degradation of TβRI exclusively through Smad7 [32] Moreover, Smurf1 can also directly degrade BMP type I receptors, Smad1 and Smad5 [33]. Unlike Smurf1, Smurf2 merely regulates the TGF-β pathway; Smad7 recruits and binds to Smurf2 in the nucleus and induces the translocation of Smurf2 to the cytoplasm, acting as an adaptor protein to mediate the degradation of TβRI through the proteasome pathway [34].
With regards to the negative regulators of Smad transcription, c-Ski and SnoN are two highly conserved members of the Ski family of oncoproteins. They directly interact with Smad4 to disrupt the formation of functional transcription complexes between Smad4 and R-Smads, thereby inhibiting the transcription of downstream genes along the TGF-β and BMP pathways [[35], [36], [37]]. In addition, Ski can further inhibit transcription by recruiting nuclear transcription co-repressors (N-CoR) and histone deacetylases (HDACs) or by interfering with the binding of Smad to the transcriptional co-activators p300/CBP [35].
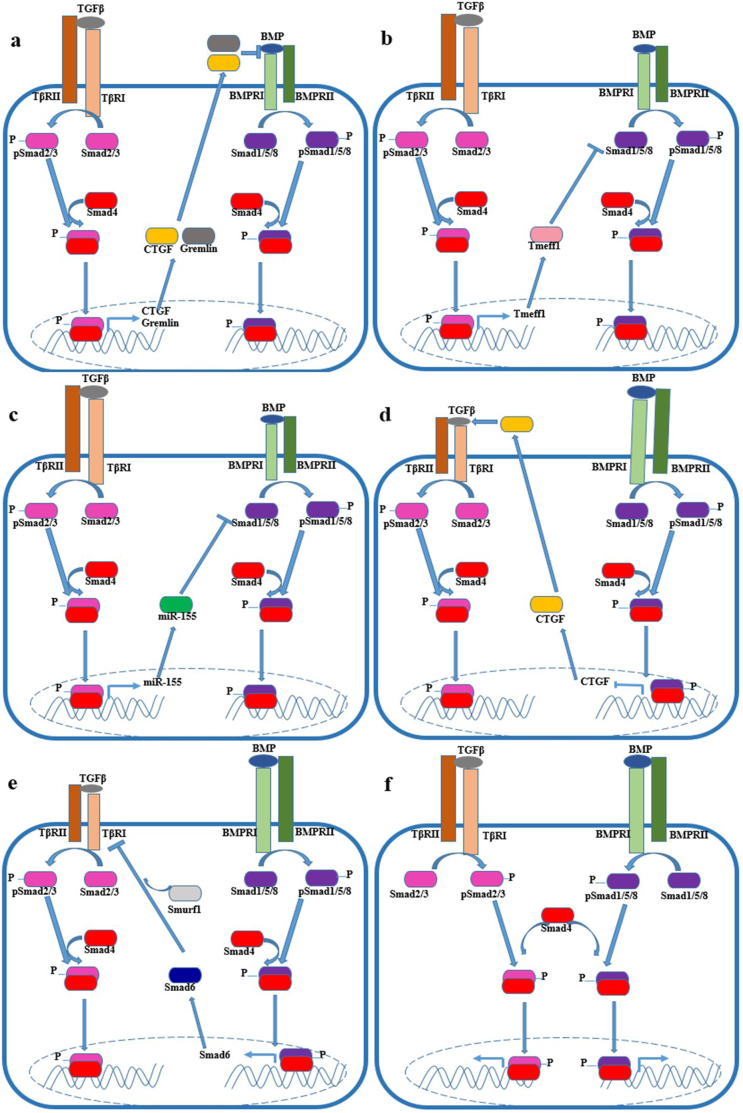
3. The potential mechanisms regulating the antagonism between TGF-β and BMP pathways (Fig. 1)
Fig. 1.
Antagonistic mechanism of TGF-β and BMP pathways: TGF-β signalling suppresses BMP signalling through upregulating CTGF, Gremlin, Tmeff1 and miR-155, as shown in a-c; BMP signalling suppresses TGF-β signalling through upregulating CTGF and Smad6, as shown in d-e; TGF-β and BMP signalling restrict each other by competing for limited Smad4, as shown in f.
3.1. Regulation of ligand-receptor interactions
3.1.1. Connective tissue growth factor (CTGF)
CTGF is a secretory protein, and it contains cysteine-rich (CR) domains, which have been reported to function as BMP and TGF-β binding domains in some cases [[38], [39], [40]]. CTGF can antagonize BMP signalling by preventing the binding of BMP ligands to BMP receptors in the extracellular space, but it facilitates TGF-β1 binding to its respective receptors [41]. To date, CTGF has been reported to antagonize BMP-2 [42], BMP-4 [41], BMP-6 [43], and BMP-7 [44] in different cell types. CTGF inhibits BMP and activates TGF-β signals by direct binding in the extracellular space and may explain why TGF-β signalling is enhanced and BMP signalling is weakened in certain CTGF-expressing tumours. In addition, CTGF can be induced by TGF-β1, and it promotes TGF-β-mediated responses by stimulating positive feedback mechanisms [45] It has been reported that TGF-β can antagonize the role of BMP-7 by upregulating CTGF in myogenic differentiation [46]. Contrarily, BMP-7 [46] and BMP-9 [47] have been described to downregulate CTGF expression.
3.1.2. Kielin/chordin-like protein
Kielin/chordin-like protein (KCP) contains 18 cysteine-rich domains. Unlike other cysteine-rich domain proteins that sequester ligands from the BMP receptor, KCP binds to BMP-7 and facilitate its binding to the type I receptor to enhance BMP signalling in a paracrine manner [48]. Subsequently, KCP is reported to inhibit TGF-β-mediated signalling through binding and blocking the interactions between TGF-β and its receptors [49]. The ability to enhance BMP activation while suppressing TGF-β signalling signifies a key role of KCP in preventing nonalcoholic fatty liver disease [50] and renal injury [51] in animal models.
3.1.3. Gremlin
Gremlin is a member of the cysteine knot superfamily and acts as a BMP antagonist [52]. In optic nerve cells, TGF-β2 can promote the synthesis of extracellular matrix (ECM) proteins, while BMP-4 can inhibit TGF-β2-induced ECM accumulation. It has been found that TGF-β2 can attenuate the effects of the BMP pathway by upregulating the BMP antagonist gremlin [53]. A similar mechanism is also observed in idiopathic pulmonary fibrosis (IPF); TGF-β1 induces gremlin expression to antagonize the effects of BMP-4 in promoting epithelial regeneration and reducing differentiation, and it sensitizes cells to TGF-β1-induced EMT and accelerates fibrosis in the lungs [54].
3.1.4. Tomoregulin-1 (Tmeff1)
Tmeff1 (originally named X7365) is a transmembrane protein containing two follistatin domains and an epidermal growth factor (EGF) domain in its extracellular region. Unlike follistatin and follistatin-related gene, Tmeff1 selectively inhibits BMP and nodal but not activin. Tmeff1 is considered to be a membrane-bound BMP antagonist that selectively inhibits BMP-2-mediated mesoderm induction by its cytoplasmic region in the Xenopus embryo [55]. Tmeff1 is also proven to be a direct target of TGF-β2/Smad2/3 signalling in hair follicle stem cell; as a result, TGF-β signalling counterbalances BMP-mediated effects by upregulating the expression of Tmeff1 [56]. Moreover, a study in prostate cancer shows that Tmeff1, which is induced by TGF-β signalling, also causes Smad1 and Smad5 to degrade through its interaction with Smurf1, thus inhibiting the BMP signalling pathway [57].
3.2. Regulation on the transcription of target genes
The activation of both the TGF-β and BMP signalling pathways sometimes exerts opposite effects on the expression of target genes. For instance, TGF-β stimulates the expression of connective tissue growth factor (CTGF), endothelin-1 (ET-1), plasminogen activator inhibitor-1 (PAI-1) in some types of cells, while BMP-7 downregulates the transcription and expression of these same target genes correspondingly [46,58,59].
SnoN, as a transcriptional repressor, is reported to participate in the antagonism of the TGF-β and BMP pathways at the transcriptional level in different cell types. For instance, TGF-β1 inhibits the target gene transcription of BMPs by upregulating SnoN in primary human osteoblasts and chondrocytes [60,61]. BMP-7 also specifically limits Smad3-DNA binding via increased SnoN expression [62] in proximal tubular epithelial cell.
The Smad transcription complex plays important roles in the regulation of target gene transcription. It has been extensively shown that TGF-β and BMP mutually regulate the transcription of each other through the Smad pathways. TGF-β inhibits BMP-mediated Smad signalling in pulmonary artery smooth muscle cells via Smad3 [63].The deficiency of Smad3 increases the expression of Smad1, Smad5, BMP-2 and BMP-6 in chondrocytes, which makes the effects of BMP-2 more obvious [64]. In addition, TGF-β can induce the formation of a non-functional transcription complex of phosphorylated Smad1/5 and Smad3, which leads to transcriptional repression of downstream target genes along the BMP signalling pathway [65]. Furthermore, some investigators proposed that the TGF-β and BMP signalling pathways compete for Smad4, which results in different functions of both pathways [[66], [67], [68]].
3.3. microRNA-related post-transcriptional regulation
It is estimated that more than 30% of the translation of encoded genes are regulated by miRNAs [69]. miRNAs are endogenous non-coding RNAs of ~23 nucleotides in length that inhibit protein translation by interacting with the 3′-untranslated regions (3′-UTRs) of the messenger RNAs (mRNAs) of target genes [70].
3.3.1. MiR-17~92 cluster
The MiR-17~92 cluster (including miR-17, miR-18a, miR-19a, miR-19b, miR-20a and miR-92a) is widely demonstrated to downregulate multiple components of the TGF-β pathway [71,72]. Inactivation of the TGF-β pathway, including downregulation of the TGF-β receptors Smad4 and Smad2, is common in gastrointestinal tumours [[73], [74], [75]], which is thought to be related to upregulation of the miR-17~92 cluster. In colorectal cancer [75,76] and glioblastoma, c-Myc mediates TGF-β pathway inactivation by upregulating the transcription of the miR-17~92 cluster, in which miR-17 and miR-20a reduce the expression of TβRII, while miR-18a inhibits the expression of Smad and Smad4. Moreover, the miR-17~92 cluster is also involved in the inhibition of downstream target genes of the TGF-β pathway, in which miR-17 and miR-20a inhibit the cell cycle inhibitor p21WAF1/CIP1 while miR-92 inhibits the anti-apoptosis gene BIM.
The MiR-17~92 cluster can enhance BMP signalling by targeting its negative regulators, such as Bambi and Crim1. Bambi is a pseudo-receptor that retains the ability to interact with functional BMP receptors and ligands and thus inactivates ligand-receptor complexes [77] Crim1 reduces the production and secretion of mature BMP proteins [78]. Both are targets of miR-20a [79] In addition, miR-17 can enhance the activity of the BMP signalling pathway by targeting Smurf1 and preventing the degradation of Smad1, Smad5 and BMP type I receptors.
3.3.2. MiR-302 cluster
The MiR-302∼367 gene cluster encodes four members of the miR-302 family (miR-302a/b/c/d) and a single transcript of miR-367. Both TβRII and BMPRII have been reported to be the target genes of miR-302 in different cases [80,81] In addition, miR-302 also targets Lefty-1 and Lefty-2, which have been shown to inhibit the TGF-β signalling pathway [82] BMP-4 reduces the transcription of the miRNA-302∼367 gene cluster [80], which then significantly inhibits the activation of the TGF-β signalling pathway.
3.3.3. MiR-21
MiR-21 has been proven to inhibit the BMP pathway since it targets Smad7 and suppresses its expression, although BMP-9 induces the expression of miR-21 [83] Similarly, miR-21 can also be induced by TGF-β and consequentially inhibits the translation of Smad7, which competitively binds to TβRI and prevents the activation of Smad2 and Smad3 upon TGF-β1 stimulation in carcinoma-associated fibroblasts (CAFs) [84]. Contrarily, BMP-6 inhibits miR-21 expression at the transcriptional level by reducing δEF1 and c-Fos/c-Jun binding to the miR-21 promoter in breast cancer [85] Moreover, a targeted relationship between miR-21 and BMPR2 was confirmed in bladder cancer and pulmonary hypertension [86,87].
3.3.4. MiR-155
In cancer, miR-155 can be upregulated by TGF-β1 and induce the epithelial-mesenchymal transition and acquisition of a cancer stem cell phenotype, as well as leading tumour cells to evade T cell surveillance [[88], [89], [90]] However, miR-155 targets the 3′ untranslated region (3′-UTR) of multiple components of the BMP signalling pathway, including BMPR2 [91], Smad1 and Smad5 [92], which induces the inhibition of the BMP signalling pathway.
It is worth noting that as a result of tissue-specific expression of miRNAs and variation in the 3′-UTR length of target mRNAs, miRNAs could target different mRNAs and play different roles in different cellular environments [69].
3.4. Regulation on ubiquitination-dependent degradation
TGF-β induces increased expression of Smurf1 protein, which can lead to the degradation of Smad1 and BMP downstream target genes [93] BMP-2 also effectively attenuates the TGF-β1 signalling pathway by promoting Smad6/Smurf1 complex-dependent TGF-β type I receptor degradation [94]. Moreover, 5-azacytidine upregulates the BMP signalling pathway and increases the expression of Smurf2, which induces the ubiquitination of Smad2/3 to make a shift from the TGF-β signalling pathway to the BMP signalling pathway [95].
4. The antagonistic effects between the TGF-β and BMP pathways in the progression of cancer
TGF-β inhibits cell proliferation and promotes differentiation in normal cells and some early stage tumours, thus serving as a tumour-suppressor factor. In advanced cancer, however, it aggravates tumour progression and metastasis, thus acting as an oncogenic factor. In some cases, tumour cells acquire somatic mutations along the TGF-β signalling pathway so that they escape from the growth inhibition of TGF-β. Mutational inactivation of TβRII and Smad4 are frequently observed in colorectal tumours [96]. Additionally, mutations in Smad4 have been observed in approximately 50% of pancreatic carcinoma (the highest rate of all tumour types) [97]. And 30% of breast tumours also harbour Smad4 mutations [98]. Furthermore, certain tumours without genetic mutations, such as glioblastoma, prostate cancers, melanomas and haematopoietic neoplasias [96] can overcome TGF-β-mediated growth inhibition through impairing the expression of anti-proliferative genes induced by TGF-β. TGF-β normally downregulates c-Myc expression, thus allowing upregulation of p15INK4A or p21WAF1; high levels of c-Myc can block the tumour-suppressive effect of TGF-β [99]. Hyperactive Akt activity and Ras/MAPK pathway signalling can also silence the growth inhibitory response to TGF-β [100,101]. Released from tumour-suppression constraints, cancer cells use the remaining TGF-β responses with impunity to support tumourigenic features, including cancer progression, which encompasses evasion of immune surveillance, tumour growth, migration, invasion, and metastasis. Aberrant expression of BMPs has been detected in various cancers, including lung cancer [102], hepatocellular carcinoma [103] and gastric cancer [104] and mutations in BMP signal components have been observed in colorectal cancers [105]. BMPs have a highly context-dependent effect on tumour progression and display bi-directional roles in different cancers. For example, BMP-9 promotes the cell proliferation of ovarian cancer [106] but induces apoptosis in prostate cancer cells [107]; BMPs promote the motility and invasiveness of various types of cancer cells, such as prostate cancer [108], colon cancer [109], and malignant melanoma [110], but they suppress tumour migration, invasion and bone metastasis of breast cancer cells [47,111]. Moreover, TGF-β and BMP pathways both have effects on angiogenesis. ALK-1 is specifically expressed in vascular endothelial cells. BMP-9 and BMP-10 bind to ALK-1 with high affinities and were demonstrated to activate the proliferation of endothelial cells [112]. TGF-β binds to ALK-5, inducing pSmad2/3 signalling, and leads to the inhibition of endothelial cell proliferation. Interestingly, TGF-β can also bind to ALK-1 and induce pSmad1/5 signalling in endothelial cells to promote proliferation like BMP-9 and BMP-10; the cellular response to TGF-β1 in endothelial cells has been described to depend on the balance of ALK-1 and ALK-5 [113]. In conclusion, it is worth noting that context-dependent factors determine the role of TGF-β and BMP pathways. Interestingly, one pathway enhancement is accompanied by another pathway weakening in the progression of some cancers and the 2 pathways have opposite effects in tumourigenesis and dissemination; thus, their cross-talk affects the carcinogenesis process. In the following paragraphs, we summarize the data that support an antagonism between the TGF-β and BMP pathways in glioblastoma, breast cancer, prostate cancer and hepatocellular carcinoma.
4.1. Glioblastoma
The interaction between TGF-β and BMP pathways is very important during brain cancer development, mostly in the case of glioblastomas (GBMs). GBMs have a high content of autocrine TGF-β levels, the hyperactive PI3K-AKT pathway leads to the loss of the TGF-β antiproliferative response in glioma. In addition, TGF-β promotes cell proliferation through inducing the expression of the platelet-derived growth factor (PDGF)-B [114]. Moreover, TGF-β also enhances the self-renewal capacity of glioma-initiating cells (GICs) in GBMs by upregulating the expression of the leukaemia inhibitory factor (LIF) [115], and the transcription factor Sox4 [116] and ID1 [117]. Unlike TGF-β, the expression of BMP is independently associated with a good prognosis in GBMs because it pushes the glioblastoma stem cell pool to differentiate towards different lineages. BMP-4 significantly reduces the numbers of stem-like tumour-initiating precursors of GBMs, and the delivery of BMP-4 in vivo effectively decreases the tumour growth that occurs in mice that have received intracerebral grafting of human GBM cells. This study suggests that BMP-4 could be a novel therapeutic effector to prevent the growth and recurrence of GBMs in humans [118]. In addition, BMP-2 and BMP-7 were also reported to decrease cancer stem cells by inducing differentiation [119].
4.2. Breast cancer
BMP signalling can antagonize TGF-β signalling both in normal mammary development and in breast cancer. TGF-β and Wnt signalling collaborate to induce EMT and thereafter function in an autocrine fashion to induce entrance of human mammary epithelial (HMLE) cells into the mesenchymal state and maintain residence in this state. The HMLE cells that have autocrine production of TGF-β express high levels of BMP-antagonists, Gremlin and Chordin-like proteins, to repress BMP signalling [120]. In breast cancer, BMP-7 is highly expressed in normal breast ducts, but it is significantly reduced in breast cancers with lymphatic and bone metastases, suggesting that reduced BMP-7 expression may confer the potential for breast cancer cell metastasis [121]. The exogenous addition of BMP-7 to human MDA-231 breast cancer cells counteracted TGF-β-mediated EMT and significantly inhibited the formation of osteolytic bone metastases in nude mice [122]. Another study demonstrated that BMP-7 may exert anti-invasive effects by inhibiting TGF-β-induced expression of integrin αvβ3 [123], which is strongly expressed by breast cancer cells residing in bone metastasis [124] and favours migration and invasion by mediating the adhesion of cells to the extracellular matrix [125]. In addition, it is also reported that BMP-9 can inhibit the bone metastasis of breast cancer cells by downregulating the expression of connective tissue growth factor (CTGF), which is a pivotal gene downstream of the TGF-β pathway that participates in tumour invasion and metastasis [47].
4.3. Prostate cancer
TGF-β signalling via Smad2/3 inhibits the proliferation of prostate cancer cells and exerts tumour suppressive effects [126]. BMP-7 works in contrast to attenuate TGF-β-mediated tumour suppression in proliferation and plays an anti-apoptotic role in some prostate cancer cells [127]. Owing to the biological complexity of the TGF-β and BMP signalling pathways in prostate cancer, investigators explored the function of these two pathways in prostate cancer development through genetic deletion of TβRII and BMPRII in the context of the PTEN-null prostate cancer mouse model. The opposing roles were determined in prostate cancer development, in which the TGF-β pathway suppressed cancer development by inducing either Smad-dependent or Smad-independent apoptosis, whereas the BMP pathway promoted progression by increasing the number of Ki67+ proliferating cells and promoting local inflammation [128]. A recent study revealed the mechanism of the interactions between TGF-β and BMP in prostate cancer, TβRII ablation disrupted the TGF-β pathway but increased the BMP pathway through downregulating the negative regulator Tmeff1, which interacts with the Smad1/Smad5 complex and induces the degradation of Smad1 and Smad5 by recruiting Smurf1 [57]. Moreover, endoglin, the TGF-β/BMP coreceptor, inhibits the pro-invasive ALK5-Smad3 pathway and facilitates anti-invasive ALK2-Smad1 activation in prostate cancer cells; the expression of endoglin regulates the balance between TGF-β signalling and BMP signalling, thereby suppressing prostate cancer metastasis [129,130].
4.4. Hepatocellular carcinoma (HCC)
The processes regulated by TGF-β in the liver are complex because TGF-β signalling operates in different hepatic cell types and their activation status varies during multiple stages of hepatic carcinogenesis. In recent years, more and more reports have proved the role of TGF pathways in inhibiting the progression of HCC. TGF-β signalling inhibits H19 expression via Sox2 in tumour-initiating hepatocytes to inhibit HCC development [131]. βII-Spectrin (SPTBN1) is an adapter protein for Smad3/Smad4 complex formation during TGF-β signal transduction and 40% SPTBN1(+/−) mice spontaneously develop HCC. SPTBN1 can decrease the expression of cyclin D1 [132] and upregulate the Wnt inhibitor kallistatin [133] to suppress proliferation and acquisition of stem cell-like of HCC. In clinical cases, reduced TβRII expression in HCC correlates with large tumour size, poor differentiation, aggressive intrahepatic metastasis, and a short time-to-recurrence, which suggests the tumour suppressive effects of the TGF-β signalling pathway in HCC [134]. Contrarily, the BMP signalling pathway seems to promote the development and progression of HCC, in which BMP-9 significantly promotes cell proliferation, enhances cell migration, and inhibits cell apoptosis in HCC cell lines [135,136]. Furthermore, BMP-4 is associated with a higher tumour stage and HCC cells with higher migratory and invasive potentials [137]. The expression of ACVR1 is associated with shorter recurrence-free survival in HCC, and ACVR1 was reported to activate Wnt signalling to promote the stem cell-like feature of HCC cells [138].
We address the TGF-β and BMP pathways with an emphasis on their opposing effects in some cancers above. However, it is worth noting that TGF-β and BMP pathways also have agonistic effects in cancer progression in some cases. In gastric carcinoma, BMP-2 and BMP-4 inhibit the proliferation of gastric carcinoma cells via p21WAF1 induction through the Smad pathway, which is similar to that of the TGF-β pathway [139]. BMP-7 was reported to improve lung cancer cell motility and invasiveness like the TGF-β pathway does [102]. Moreover, BMP-2 and TGF-β both increase the number of cancer stem cells in ovarian carcinoma, and inhibition of the 2 pathways showed an effective therapeutic effect on ovarian cancer [140,141].
As one can conclude from the different examples mentioned so far, TGF-β and BMP signalling promote different responses in different cell types. In glioblastoma and breast cancer, the BMP pathway inhibits EMT and the stem cell-like features, which are mediated by the TGF-β pathway. However, in prostate cancer and hepatocellular carcinoma, TGF-β inhibits proliferation and stemness and BMP promotes it. All these are related to the context-dependence of TGF-β and BMP pathways. The balance between these two pathways is probably involved in the progression of tumours, and correcting the imbalance between these two pathways may be beneficial for tumour treatment.
5. Conclusion
The roles of both the TGF-β and BMP pathways have been extensively studied in the development and progression of cancers. Here, we focused on the crosstalk between the 2 pathways and summarized the findings of the antagonistic effects in glioblastoma, prostate cancer, breast cancer and hepatocellular carcinoma. Moreover, in view of the lack of systemic mechanistic study between the 2 pathways in cancer, we summarized the potential mechanisms in other cell types for reference, including ligand-receptor interactions, the transcription of target genes, microRNA-related post-transcriptional regulation and ubiquitination-dependent degradation. In our opinion, more studies need to be conducted in order to fully demonstrate the panorama of the interactions between the two pathways in different tumour types in order to screen more effective therapeutic regimens in specific cancers.
Outstanding questions
In this review, we address the TGF-β and BMP pathways and their crosstalk, with an emphasis on opposing effects. In the light of the protumourigenic functions of TGF-β in cancer, focussing on the antagonistic mechanisms of BMP signalling is feasible with regard to a possible therapeutic use. However, the TGF-β and BMP pathways also have agonistic effects in cancer progression in some cases. It is worth noting that the roles of the 2 pathways in different cancers are in a context-dependent manner. Moreover, we also provide some potential mechanisms regulating the antagonism between TGF-β and BMP pathways that were discovered in non-cancerous cells; these mechanisms may help explain the antagonistic effects between the TGF-β and BMP pathways in some cancers and provide new clues for developing novel strategies for targeted therapy.
Search strategy and selection criteria
Data for this Review were identifed by searches of MEDLINE and PubMed, and references from relevant articles using the search terms “TGFbeta”, “TGF-β”, “BMP”, “pathway”, “cancer”, “tumour”, “Hepatocellular carcinoma”, “HCC”, “Glioblastoma”, “breast cancer”, “prostate cancer” and “opposite”, “antagonistic” and so on. Only articles published in English between 1991 and 2018 were included.
Conflict of interest
All authors state that there is no conflict of interest.
Acknowledgements
This work was supported by National Natural Science Foundation of China (grant numbers: 81872143,81472473, 81702280, 81272360) and Key Project of Tianjin Health and Family Planning Commission (16KG126).
References
- 1.Heldin C.H., Moustakas A. Signaling receptors for TGF-beta family members. CSH Perspect Biol. 2016;8(8) doi: 10.1101/cshperspect.a022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meng X.M., Chung A.C., Lan H.Y. Role of the TGF-beta/BMP-7/Smad pathways in renal diseases. Clin Sci (London, England: 1979) 2013;124(4):243–254. doi: 10.1042/CS20120252. [DOI] [PubMed] [Google Scholar]
- 3.Wu M., Chen G., Li Y. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016;4:16009. doi: 10.1038/boneres.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyazawa K., Shinozaki M., Hara T., Furuya T., Miyazono K. Two major Smad pathways in TGF-beta superfamily signalling. Genes Cells. 2002;7(12):1191–1204. doi: 10.1046/j.1365-2443.2002.00599.x. [DOI] [PubMed] [Google Scholar]
- 5.Moustakas A., Heldin C.H. Non-Smad TGF-beta signals. J Cell Sci. 2005;118(Pt 16):3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- 6.Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 7.Katagiri T., Watabe T. Bone Morphogenetic Proteins. Cold Spring Harb Perspect Biol. 2016;8(6) doi: 10.1101/cshperspect.a021899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenzweig B.L., Imamura T., Okadome T., Cox G.N., Yamashita H., ten Dijke P. Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc Natl Acad Sci U S A. 1995;92(17):7632–7636. doi: 10.1073/pnas.92.17.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyazono K., Kamiya Y., Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147(1):35–51. doi: 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- 10.Koenig B.B., Cook J.S., Wolsing D.H., Ting J., Tiesman J.P., Correa P.E. Characterization and cloning of a receptor for BMP-2 and BMP-4 from NIH 3T3 cells. Mol Cell Biol. 1994;14(9):5961–5974. doi: 10.1128/mcb.14.9.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knaus P., Sebald W. Cooperativity of binding epitopes and receptor chains in the BMP/TGFbeta superfamily. Biol Chem. 2001;382(8):1189–1195. doi: 10.1515/BC.2001.149. [DOI] [PubMed] [Google Scholar]
- 12.Greenwald J., Groppe J., Gray P., Wiater E., Kwiatkowski W., Vale W. The BMP7/ActRII extracellular domain complex provides new insights into the cooperative nature of receptor assembly. Mol Cell. 2003;11(3):605–617. doi: 10.1016/s1097-2765(03)00094-7. [DOI] [PubMed] [Google Scholar]
- 13.Nohe A., Hassel S., Ehrlich M., Neubauer F., Sebald W., Henis Y.I. The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J Biol Chem. 2002;277(7):5330–5338. doi: 10.1074/jbc.M102750200. [DOI] [PubMed] [Google Scholar]
- 14.Cheifetz S., Bellon T., Cales C., Vera S., Bernabeu C., Massague J. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem. 1992;267(27):19027–19030. [PubMed] [Google Scholar]
- 15.Lopez-Casillas F., Cheifetz S., Doody J., Andres J.L., Lane W.S., Massague J. Structure and expression of the membrane proteoglycan betaglycan, a component of the TGF-beta receptor system. Cell. 1991;67(4):785–795. doi: 10.1016/0092-8674(91)90073-8. [DOI] [PubMed] [Google Scholar]
- 16.Goumans M.-J., Valdimarsdottir G., Itoh S., Lebrin F., Larsson J., Mummery C. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFβ/ALK5 Signaling. Mol Cell. 2003;12(4):817–828. doi: 10.1016/s1097-2765(03)00386-1. [DOI] [PubMed] [Google Scholar]
- 17.Yamashita H., Ichijo H., Grimsby S., Moren A., ten Dijke P., Miyazono K. Endoglin forms a heteromeric complex with the signaling receptors for transforming growth factor-beta. J Biol Chem. 1994;269(3):1995–2001. [PubMed] [Google Scholar]
- 18.Lopez-Casillas F., Wrana J.L., Massague J. Betaglycan presents ligand to the TGF beta signaling receptor. Cell. 1993;73(7):1435–1444. doi: 10.1016/0092-8674(93)90368-z. [DOI] [PubMed] [Google Scholar]
- 19.Elderbroom J.L., Huang J.J., Gatza C.E., Chen J., How T., Starr M. Ectodomain shedding of TbetaRIII is required for TbetaRIII-mediated suppression of TGF-beta signaling and breast cancer migration and invasion. Mol Biol Cell. 2014;25(16):2320–2332. doi: 10.1091/mbc.E13-09-0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castonguay R., Werner E.D., Matthews R.G., Presman E., Mulivor A.W., Solban N. Soluble endoglin specifically binds bone morphogenetic proteins 9 and 10 via its orphan domain, inhibits blood vessel formation, and suppresses tumor growth. J Biol Chem. 2011;286(34):30034–30046. doi: 10.1074/jbc.M111.260133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gatza C.E., Elderbroom J.L., Oh S.Y., Starr M.D., Nixon A.B., Blobe G.C. The balance of cell surface and soluble type III TGF-β receptor regulates BMP signaling in normal and cancerous mammary epithelial cells. Neoplasia (New York, NY) 2014;16(6):489–500. doi: 10.1016/j.neo.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell D., Pobre E.G., Mulivor A.W., Grinberg A.V., Castonguay R., Monnell T.E. ALK1-fc inhibits multiple mediators of angiogenesis and suppresses tumor growth. Mol Cancer Ther. 2010;9(2):379–388. doi: 10.1158/1535-7163.MCT-09-0650. [DOI] [PubMed] [Google Scholar]
- 23.Denissova N.G., Pouponnot C., Long J., He D., Liu F. Transforming growth factor beta -inducible independent binding of SMAD to the Smad7 promoter. Proc Natl Acad Sci U S A. 2000;97(12):6397–6402. doi: 10.1073/pnas.090099297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Souchelnytskyi S., Nakayama T., Nakao A., Moren A., Heldin C.H., Christian J.L. Physical and functional interaction of murine and Xenopus Smad7 with bone morphogenetic protein receptors and transforming growth factor-beta receptors. J Biol Chem. 1998;273(39):25364–25370. doi: 10.1074/jbc.273.39.25364. [DOI] [PubMed] [Google Scholar]
- 25.Zhang S., Fei T., Zhang L., Zhang R., Chen F., Ning Y. Smad7 antagonizes transforming growth factor beta signaling in the nucleus by interfering with functional Smad-DNA complex formation. Mol Cell Biol. 2007;27(12):4488–4499. doi: 10.1128/MCB.01636-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishida W., Hamamoto T., Kusanagi K., Yagi K., Kawabata M., Takehara K. Smad6 is a Smad1/5-induced smad inhibitor. Characterization of bone morphogenetic protein-responsive element in the mouse Smad6 promoter. J Biol Chem. 2000;275(9):6075–6079. doi: 10.1074/jbc.275.9.6075. [DOI] [PubMed] [Google Scholar]
- 27.Imamura T., Takase M., Nishihara A., Oeda E., Hanai J., Kawabata M. Smad6 inhibits signalling by the TGF-beta superfamily. Nature. 1997;389(6651):622–626. doi: 10.1038/39355. [DOI] [PubMed] [Google Scholar]
- 28.Hata A., Lagna G., Massague J., Hemmati-Brivanlou A. Smad6 inhibits BMP/Smad1 signaling by specifically competing with the Smad4 tumor suppressor. Genes Dev. 1998;12(2):186–197. doi: 10.1101/gad.12.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bai S., Shi X., Yang X., Cao X. Smad6 as a transcriptional corepressor. J Biol Chem. 2000;275(12):8267–8270. doi: 10.1074/jbc.275.12.8267. [DOI] [PubMed] [Google Scholar]
- 30.Murakami G., Watabe T., Takaoka K., Miyazono K., Imamura T. Cooperative inhibition of bone morphogenetic protein signaling by Smurf1 and inhibitory Smads. Mol Biol Cell. 2003;14(7):2809–2817. doi: 10.1091/mbc.E02-07-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qureshi M.Z., Jabeen S., Butt G., Aslam A., Naqvi S.K., Attar R. Tudor tells about new twists in the story tale of SMURFs. Cell Mol Biol (Noisy-le-Grand, France) 2016;62(5):38–43. [PubMed] [Google Scholar]
- 32.Ebisawa T., Fukuchi M., Murakami G., Chiba T., Tanaka K., Imamura T. Smurf1 interacts with transforming growth factor-beta type I receptor through Smad7 and induces receptor degradation. J Biol Chem. 2001;276(16):12477–12480. doi: 10.1074/jbc.C100008200. [DOI] [PubMed] [Google Scholar]
- 33.Zhu H., Kavsak P., Abdollah S., Wrana J.L., Thomsen G.H. A SMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation. Nature. 1999;400(6745):687–693. doi: 10.1038/23293. [DOI] [PubMed] [Google Scholar]
- 34.Kavsak P., Rasmussen R.K., Causing C.G., Bonni S., Zhu H., Thomsen G.H. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF beta receptor for degradation. Mol Cell. 2000;6(6):1365–1375. doi: 10.1016/s1097-2765(00)00134-9. [DOI] [PubMed] [Google Scholar]
- 35.Liu X., Sun Y., Weinberg R.A., Lodish H.F. Ski/Sno and TGF-beta signaling. Cytokine Growth Factor Rev. 2001;12(1):1–8. doi: 10.1016/s1359-6101(00)00031-9. [DOI] [PubMed] [Google Scholar]
- 36.Wang W., Mariani F.V., Harland R.M., Luo K. Ski represses bone morphogenic protein signaling in Xenopus and mammalian cells. Proc Natl Acad Sci U S A. 2000;97(26):14394–14399. doi: 10.1073/pnas.97.26.14394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu J.W., Krawitz A.R., Chai J., Li W., Zhang F., Luo K. Structural mechanism of Smad4 recognition by the nuclear oncoprotein ski: insights on ski-mediated repression of TGF-beta signaling. Cell. 2002;111(3):357–367. doi: 10.1016/s0092-8674(02)01006-1. [DOI] [PubMed] [Google Scholar]
- 38.Zhu Y., Oganesian A., Keene D.R., Sandell L.J. Type IIA procollagen containing the cysteine-rich amino propeptide is deposited in the extracellular matrix of prechondrogenic tissue and binds to TGF-beta1 and BMP-2. J Cell Biol. 1999;144(5):1069–1080. doi: 10.1083/jcb.144.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larrain J., Bachiller D., Lu B., Agius E., Piccolo S., De Robertis E.M. BMP-binding modules in chordin: a model for signalling regulation in the extracellular space. Development. 2000;127(4):821–830. doi: 10.1242/dev.127.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakuta H., Suzuki R., Takahashi H., Kato A., Shintani T., Iemura S. Ventroptin: a BMP-4 antagonist expressed in a double-gradient pattern in the retina. Science (New York, NY) 2001;293(5527):111–115. doi: 10.1126/science.1058379. [DOI] [PubMed] [Google Scholar]
- 41.Abreu J.G., Ketpura N.I., Reversade B., De Robertis E.M. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol. 2002;4(8):599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mundy C., Gannon M., Popoff S.N. Connective tissue growth factor (CTGF/CCN2) negatively regulates BMP-2 induced osteoblast differentiation and signaling. J Cell Physiol. 2014;229(5):672–681. doi: 10.1002/jcp.24491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Falke L.L., Kinashi H., Dendooven A., Broekhuizen R., Stoop R., Joles J.A. Age-dependent shifts in renal response to injury relate to altered BMP6/CTGF expression and signaling. Am J Physiol Renal Physiol. 2016;311(5):F926–f34. doi: 10.1152/ajprenal.00324.2016. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen T.Q., Roestenberg P., van Nieuwenhoven F.A., Bovenschen N., Li Z., Xu L. CTGF inhibits BMP-7 signaling in diabetic nephropathy. J Am Soc Nephrol. 2008;19(11):2098–2107. doi: 10.1681/ASN.2007111261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Q., Usinger W., Nichols B., Gray J., Xu L., Seeley T.W. Cooperative interaction of CTGF and TGF-beta in animal models of fibrotic disease. Fibrogenesis Tissue Repair. 2011;4(1):4. doi: 10.1186/1755-1536-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meurer S.K., Esser M., Tihaa L., Weiskirchen R. BMP-7/TGF-beta1 signalling in myoblasts: components involved in signalling and BMP-7-dependent blockage of TGF-beta-mediated CTGF expression. Eur J Cell Biol. 2012;91(6–7):450–463. doi: 10.1016/j.ejcb.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Ren W., Sun X., Wang K., Feng H., Liu Y., Fei C. BMP9 inhibits the bone metastasis of breast cancer cells by downregulating CCN2 (connective tissue growth factor, CTGF) expression. Mol Biol Rep. 2014;41(3):1373–1383. doi: 10.1007/s11033-013-2982-8. [DOI] [PubMed] [Google Scholar]
- 48.Lin J., Patel S.R., Cheng X., Cho E.A., Levitan I., Ullenbruch M. Kielin/chordin-like protein, a novel enhancer of BMP signaling, attenuates renal fibrotic disease. Nat Med. 2005;11(4):387–393. doi: 10.1038/nm1217. [DOI] [PubMed] [Google Scholar]
- 49.Lin J., Patel S.R., Wang M., Dressler G.R. The cysteine-rich domain protein KCP is a suppressor of transforming growth factor beta/activin signaling in renal epithelia. Mol Cell Biol. 2006;26(12):4577–4585. doi: 10.1128/MCB.02127-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soofi A., Wolf K.I., Emont M.P., Qi N., Martinez-Santibanez G., Grimley E. The kielin/chordin-like protein (KCP) attenuates high-fat diet-induced obesity and metabolic syndrome in mice. J Biol Chem. 2017;292(22):9051–9062. doi: 10.1074/jbc.M116.771428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soofi A., Zhang P., Dressler G.R. Kielin/chordin-like protein attenuates both acute and chronic renal injury. J Am Soc Nephrol. 2013;24(6):897–905. doi: 10.1681/ASN.2012070759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Michos O., Panman L., Vintersten K., Beier K., Zeller R. Zuniga A. Gremlin-mediated BMP antagonism induces the epithelial-mesenchymal feedback signaling controlling metanephric kidney and limb organogenesis. Development. 2004;131(14):3401–3410. doi: 10.1242/dev.01251. [DOI] [PubMed] [Google Scholar]
- 53.Zode G.S., Clark A.F., Wordinger R.J. Bone morphogenetic protein 4 inhibits TGF-beta2 stimulation of extracellular matrix proteins in optic nerve head cells: role of gremlin in ECM modulation. Glia. 2009;57(7):755–766. doi: 10.1002/glia.20803. [DOI] [PubMed] [Google Scholar]
- 54.Koli K., Myllarniemi M., Vuorinen K., Salmenkivi K., Ryynanen M.J., Kinnula V.L. Bone morphogenetic protein-4 inhibitor gremlin is overexpressed in idiopathic pulmonary fibrosis. Am J Pathol. 2006;169(1):61–71. doi: 10.2353/ajpath.2006.051263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang C., Eggen B.J., Weinstein D.C., Brivanlou A.H. Regulation of nodal and BMP signaling by tomoregulin-1 (X7365) through novel mechanisms. Dev Biol. 2003;255(1):1–11. doi: 10.1016/s0012-1606(02)00075-1. [DOI] [PubMed] [Google Scholar]
- 56.Oshimori N., Fuchs E. Paracrine TGF-beta signaling counterbalances BMP-mediated repression in hair follicle stem cell activation. Cell Stem Cell. 2012;10(1):63–75. doi: 10.1016/j.stem.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao W., Tan P., Zhu Q., Ajibade A., Long T., Long W. Tgfbr2 inactivation facilitates cellular plasticity and development of Pten-null prostate cancer. J Mol Cell Biol. 2017;10(4):316–330. doi: 10.1093/jmcb/mjx052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fuchshofer R., Stephan D.A., Russell P., Tamm E.R. Gene expression profiling of TGFbeta2- and/or BMP7-treated trabecular meshwork cells: identification of Smad7 as a critical inhibitor of TGF-beta2 signaling. Exp Eye Res. 2009;88(6):1020–1032. doi: 10.1016/j.exer.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Star G.P., Giovinazzo M., Langleben D. ALK2 and BMPR2 knockdown and endothelin-1 production by pulmonary microvascular endothelial cells. Microvascular research. 2013;85:46–53. doi: 10.1016/j.mvr.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 60.Ehnert S., Zhao J., Pscherer S., Freude T., Dooley S., Kolk A. Transforming growth factor beta1 inhibits bone morphogenic protein (BMP)-2 and BMP-7 signaling via upregulation of ski-related novel protein N (SnoN): possible mechanism for the failure of BMP therapy? BMC Med. 2012;10:101. doi: 10.1186/1741-7015-10-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kawamura I., Maeda S., Imamura K., Setoguchi T., Yokouchi M., Ishidou Y. SnoN suppresses maturation of chondrocytes by mediating signal cross-talk between transforming growth factor-beta and bone morphogenetic protein pathways. J Biol Chem. 2012;287(34):29101–29113. doi: 10.1074/jbc.M112.349415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luo D.D., Phillips A., Fraser D. Bone morphogenetic protein-7 inhibits proximal tubular epithelial cell Smad3 signaling via increased SnoN expression. Am J Pathol. 2010;176(3):1139–1147. doi: 10.2353/ajpath.2010.090459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Upton P.D., Davies R.J., Tajsic T., Morrell N.W. Transforming growth factor-beta(1) represses bone morphogenetic protein-mediated Smad signaling in pulmonary artery smooth muscle cells via Smad3. Am J Respir Cell Mol Biol. 2013;49(6):1135–1145. doi: 10.1165/rcmb.2012-0470OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li T.F., Darowish M., Zuscik M.J., Chen D., Schwarz E.M., Rosier R.N. Smad3-deficient chondrocytes have enhanced BMP signaling and accelerated differentiation. J Bone Miner Res. 2006;21(1):4–16. doi: 10.1359/JBMR.050911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gronroos E., Kingston I.J., Ramachandran A., Randall R.A., Vizan P., Hill C.S. Transforming growth factor beta inhibits bone morphogenetic protein-induced transcription through novel phosphorylated Smad1/5-Smad3 complexes. Mol Cell Biol. 2012;32(14):2904–2916. doi: 10.1128/MCB.00231-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sartori R., Schirwis E., Blaauw B., Bortolanza S., Zhao J., Enzo E. BMP signaling controls muscle mass. Nat Genet. 2013;45(11):1309–1318. doi: 10.1038/ng.2772. [DOI] [PubMed] [Google Scholar]
- 67.Candia A.F., Watabe T., Hawley S.H., Onichtchouk D., Zhang Y., Derynck R. Cellular interpretation of multiple TGF-beta signals: intracellular antagonism between activin/BVg1 and BMP-2/4 signaling mediated by Smads. Development. 1997;124(22):4467–4480. doi: 10.1242/dev.124.22.4467. [DOI] [PubMed] [Google Scholar]
- 68.Yuan G., Zhan Y., Gou X., Chen Y., Yang G. TGF-beta signaling inhibits canonical BMP signaling pathway during palate development. Cell Tissue Res. 2018;371(2):283–291. doi: 10.1007/s00441-017-2757-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blahna M.T., Hata A. Smad-mediated regulation of microRNA biosynthesis. FEBS Lett. 2012;586(14):1906–1912. doi: 10.1016/j.febslet.2012.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Makeyev E.V., Maniatis T. Multilevel regulation of gene expression by microRNAs. Science (New York, NY) 2008;319(5871):1789–1790. doi: 10.1126/science.1152326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mestdagh P., Bostrom A.K., Impens F., Fredlund E., Van Peer G., De Antonellis P. The miR-17-92 microRNA cluster regulates multiple components of the TGF-beta pathway in neuroblastoma. Mol Cell. 2010;40(5):762–773. doi: 10.1016/j.molcel.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Petrocca F., Vecchione A., Croce C.M. Emerging role of miR-106b-25/miR-17-92 clusters in the control of transforming growth factor beta signaling. Cancer Res. 2008;68(20):8191–8194. doi: 10.1158/0008-5472.CAN-08-1768. [DOI] [PubMed] [Google Scholar]
- 73.Tang Y., Katuri V., Srinivasan R., Fogt F., Redman R., Anand G. Transforming growth factor-beta suppresses nonmetastatic colon cancer through Smad4 and adaptor protein ELF at an early stage of tumorigenesis. Cancer Res. 2005;65(10):4228–4237. doi: 10.1158/0008-5472.CAN-04-4585. [DOI] [PubMed] [Google Scholar]
- 74.Mishra L., Shetty K., Tang Y., Stuart A., Byers S.W. The role of TGF-beta and Wnt signaling in gastrointestinal stem cells and cancer. Oncogene. 2005;24(37):5775–5789. doi: 10.1038/sj.onc.1208924. [DOI] [PubMed] [Google Scholar]
- 75.Dews M., Fox J.L., Hultine S., Sundaram P., Wang W., Liu Y.Y. The myc-miR-17~92 axis blunts TGF{beta} signaling and production of multiple TGF{beta}-dependent antiangiogenic factors. Cancer Res. 2010;70(20):8233–8246. doi: 10.1158/0008-5472.CAN-10-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dews M., Tan G.S., Hultine S., Raman P., Choi J., Duperret E.K. Masking epistasis between MYC and TGF-beta pathways in antiangiogenesis-mediated colon cancer suppression. J Natl Cancer Inst. 2014;106(4) doi: 10.1093/jnci/dju043. dju043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen J., Bush J.O., Ovitt C.E., Lan Y., Jiang R. The TGF-beta pseudoreceptor gene Bambi is dispensable for mouse embryonic development and postnatal survival. Genesis (New York, NY: 2000) 2007;45(8):482–486. doi: 10.1002/dvg.20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wilkinson L., Kolle G., Wen D., Piper M., Scott J., Little M. CRIM1 regulates the rate of processing and delivery of bone morphogenetic proteins to the cell surface. J Biol Chem. 2003;278(36):34181–34188. doi: 10.1074/jbc.M301247200. [DOI] [PubMed] [Google Scholar]
- 79.Fabregat I. Dysregulation of apoptosis in hepatocellular carcinoma cells. World J Gastroenterol. 2009;15(5):513–520. doi: 10.3748/wjg.15.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kang H., Louie J., Weisman A., Sheu-Gruttadauria J., Davis-Dusenbery B.N., Lagna G. Inhibition of microRNA-302 (miR-302) by bone morphogenetic protein 4 (BMP4) facilitates the BMP signaling pathway. J Biol Chem. 2012;287(46):38656–38664. doi: 10.1074/jbc.M112.390898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Faherty N., Curran S.P., O'Donovan H., Martin F., Godson C., Brazil D.P. CCN2/CTGF increases expression of miR-302 microRNAs, which target the TGFbeta type II receptor with implications for nephropathic cell phenotypes. J Cell Sci. 2012;125(Pt 23):5621–5629. doi: 10.1242/jcs.105528. [DOI] [PubMed] [Google Scholar]
- 82.Barroso-delJesus A., Lucena-Aguilar G., Sanchez L., Ligero G., Gutierrez-Aranda I., Menendez P. The nodal inhibitor lefty is negatively modulated by the microRNA miR-302 in human embryonic stem cells. FASEB J. 2011;25(5):1497–1508. doi: 10.1096/fj.10-172221. [DOI] [PubMed] [Google Scholar]
- 83.Song Q., Zhong L., Chen C., Tang Z., Liu H., Zhou Y. miR-21 synergizes with BMP9 in osteogenic differentiation by activating the BMP9/Smad signaling pathway in murine multilineage cells. Int J Mol Med. 2015;36(6):1497–1506. doi: 10.3892/ijmm.2015.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Q., Zhang D., Wang Y., Sun P., Hou X., Larner J. MiR-21/Smad 7 signaling determines TGF-beta1-induced CAF formation. Sci Rep. 2013;3:2038. doi: 10.1038/srep02038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Du J., Yang S., An D., Hu F., Yuan W., Zhai C. BMP-6 inhibits microRNA-21 expression in breast cancer through repressing deltaEF1 and AP-1. Cell Res. 2009;19(4):487–496. doi: 10.1038/cr.2009.34. [DOI] [PubMed] [Google Scholar]
- 86.Martinez V.G., Rubio C., Martinez-Fernandez M., Segovia C., Lopez-Calderon F., Garin M.I. BMP4 induces M2 macrophage polarization and favors tumor progression in bladder cancer. Clin Cancer Res. 2017;23(23):7388–7399. doi: 10.1158/1078-0432.CCR-17-1004. [DOI] [PubMed] [Google Scholar]
- 87.Parikh V.N., Jin R.C., Rabello S., Gulbahce N., White K., Hale A. MicroRNA-21 integrates pathogenic signaling to control pulmonary hypertension: results of a network bioinformatics approach. Circulation. 2012;125(12):1520–1532. doi: 10.1161/CIRCULATIONAHA.111.060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu F., Kong X., Lv L., Gao J. TGF-beta1 acts through miR-155 to down-regulate TP53INP1 in promoting epithelial-mesenchymal transition and cancer stem cell phenotypes. Cancer Lett. 2015;359(2):288–298. doi: 10.1016/j.canlet.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 89.Zhou X., Mao Y., Zhu J., Meng F., Chen Q., Tao L. TGF-beta1 promotes colorectal cancer immune escape by elevating B7-H3 and B7-H4 via the miR-155/miR-143 axis. Oncotarget. 2016;7(41):67196–67211. doi: 10.18632/oncotarget.11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Johansson J., Berg T., Kurzejamska E., Pang M.F., Tabor V., Jansson M. MiR-155-mediated loss of C/EBPbeta shifts the TGF-beta response from growth inhibition to epithelial-mesenchymal transition, invasion and metastasis in breast cancer. Oncogene. 2013;32(50):5614–5624. doi: 10.1038/onc.2013.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu H., Zhong L., Yuan T., Chen S., Zhou Y., An L. MicroRNA-155 inhibits the osteogenic differentiation of mesenchymal stem cells induced by BMP9 via downregulation of BMP signaling pathway. Int J Mol Med. 2018;41(6):3379–3393. doi: 10.3892/ijmm.2018.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yin Q., Wang X., Fewell C., Cameron J., Zhu H., Baddoo M. MicroRNA miR-155 inhibits bone morphogenetic protein (BMP) signaling and BMP-mediated Epstein-Barr virus reactivation. J Virol. 2010;84(13):6318–6327. doi: 10.1128/JVI.00635-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun X., Xie Z., Ma Y., Pan X., Wang J., Chen Z. TGF-beta inhibits osteogenesis by upregulating the expression of ubiquitin ligase SMURF1 via MAPK-ERK signaling. J Cell Physiol. 2018;233(1):596–606. doi: 10.1002/jcp.25920. [DOI] [PubMed] [Google Scholar]
- 94.Wang S., Sun A., Li L., Zhao G., Jia J., Wang K. Up-regulation of BMP-2 antagonizes TGF-beta1/ROCK-enhanced cardiac fibrotic signalling through activation of Smurf1/Smad6 complex. J Cell Mol Med. 2012;16(10):2301–2310. doi: 10.1111/j.1582-4934.2012.01538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zuscik M.J., Baden J.F., Wu Q., Sheu T.J., Schwarz E.M., Drissi H. 5-azacytidine alters TGF-beta and BMP signaling and induces maturation in articular chondrocytes. J Cell Biochem. 2004;92(2):316–331. doi: 10.1002/jcb.20050. [DOI] [PubMed] [Google Scholar]
- 96.Seoane J., Gomis R.R. TGF-beta Family Signaling in Tumor Suppression and Cancer Progression. Cold Spring Harb Perspect Biol. 2017;9(12) doi: 10.1101/cshperspect.a022277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Goggins M., Shekher M., Turnacioglu K., Yeo C.J., Hruban R.H., Kern S.E. Genetic alterations of the transforming growth factor beta receptor genes in pancreatic and biliary adenocarcinomas. Cancer Res. 1998;58(23):5329–5332. [PubMed] [Google Scholar]
- 98.Kretzschmar M. Transforming growth factor-beta and breast cancer: transforming growth factor-beta/SMAD signaling defects and cancer. Breast Cancer Res. 2000;2(2):107–115. doi: 10.1186/bcr42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Warner B.J., Blain S.W., Seoane J., Massague J. Myc downregulation by transforming growth factor beta required for activation of the p15(Ink4b) G(1) arrest pathway. Mol Cell Biol. 1999;19(9):5913–5922. doi: 10.1128/mcb.19.9.5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Conery A.R., Cao Y., Thompson E.A., Townsend C.M., Jr., Ko T.C., Luo K. Akt interacts directly with Smad3 to regulate the sensitivity to TGF-beta induced apoptosis. Nat Cell Biol. 2004;6(4):366–372. doi: 10.1038/ncb1117. [DOI] [PubMed] [Google Scholar]
- 101.Kretzschmar M., Doody J., Timokhina I., Massague J. A mechanism of repression of TGFbeta/Smad signaling by oncogenic Ras. Genes Dev. 1999;13(7):804–816. doi: 10.1101/gad.13.7.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu Y., Chen J., Yang Y., Zhang L., Jiang W.G. Muolecular impact of bone morphogenetic protein 7, on lung cancer cells and its clinical significance. Int J Mol Med. 2012;29(6):1016–1024. doi: 10.3892/ijmm.2012.948. [DOI] [PubMed] [Google Scholar]
- 103.Maegdefrau U., Bosserhoff A.K. BMP activated Smad signaling strongly promotes migration and invasion of hepatocellular carcinoma cells. Exp Mol Pathol. 2012;92(1):74–81. doi: 10.1016/j.yexmp.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 104.Lei H., Wang J., Lu P., Si X., Han K., Ruan T. BMP10 inhibited the growth and migration of gastric cancer cells. Tumour Biol. 2016;37(3):3025–3031. doi: 10.1007/s13277-015-4116-5. [DOI] [PubMed] [Google Scholar]
- 105.Kodach L.L., Wiercinska E., de Miranda N.F., Bleuming S.A., Musler A.R., Peppelenbosch M.P. The bone morphogenetic protein pathway is inactivated in the majority of sporadic colorectal cancers. Gastroenterology. 2008;134(5):1332–1341. doi: 10.1053/j.gastro.2008.02.059. [DOI] [PubMed] [Google Scholar]
- 106.Herrera B., van Dinther M., Ten Dijke P., Inman G.J. Autocrine bone morphogenetic protein-9 signals through activin receptor-like kinase-2/Smad1/Smad4 to promote ovarian cancer cell proliferation. Cancer Res. 2009;69(24):9254–9262. doi: 10.1158/0008-5472.CAN-09-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ye L., Kynaston H., Jiang W.G. Bone morphogenetic protein-9 induces apoptosis in prostate cancer cells, the role of prostate apoptosis response-4. Mol Cancer Res. 2008;6(10):1594–1606. doi: 10.1158/1541-7786.MCR-08-0171. [DOI] [PubMed] [Google Scholar]
- 108.Lim M., Chuong C.M., Roy-Burman P. PI3K, Erk signaling in BMP7-induced epithelial-mesenchymal transition (EMT) of PC-3 prostate cancer cells in 2- and 3-dimensional cultures. Horm Cancer. 2011;2(5):298–309. doi: 10.1007/s12672-011-0084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim B.R., Oh S.C., Lee D.H., Kim J.L., Lee S.Y., Kang M.H. BMP-2 induces motility and invasiveness by promoting colon cancer stemness through STAT3 activation. Tumour Biol. 2015;36(12):9475–9486. doi: 10.1007/s13277-015-3681-y. [DOI] [PubMed] [Google Scholar]
- 110.Rothhammer T., Poser I., Soncin F., Bataille F., Moser M., Bosserhoff A.K. Bone morphogenic proteins are overexpressed in malignant melanoma and promote cell invasion and migration. Cancer Res. 2005;65(2):448–456. [PubMed] [Google Scholar]
- 111.Cao Y., Slaney C.Y., Bidwell B.N., Parker B.S., Johnstone C.N., Rautela J. BMP4 inhibits breast cancer metastasis by blocking myeloid-derived suppressor cell activity. Cancer Res. 2014;74(18):5091–5102. doi: 10.1158/0008-5472.CAN-13-3171. [DOI] [PubMed] [Google Scholar]
- 112.Brown M.A., Zhao Q., Baker K.A., Naik C., Chen C., Pukac L. Crystal structure of BMP-9 and functional interactions with pro-region and receptors. J Biol Chem. 2005;280(26):25111–25118. doi: 10.1074/jbc.M503328200. [DOI] [PubMed] [Google Scholar]
- 113.Goumans M.J., Valdimarsdottir G., Itoh S., Rosendahl A., Sideras P., ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 2002;21(7):1743–1753. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bruna A., Darken R.S., Rojo F., Ocana A., Penuelas S., Arias A. High TGFbeta-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell. 2007;11(2):147–160. doi: 10.1016/j.ccr.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 115.Penuelas S., Anido J., Prieto-Sanchez R.M., Folch G., Barba I., Cuartas I. TGF-beta increases glioma-initiating cell self-renewal through the induction of LIF in human glioblastoma. Cancer Cell. 2009;15(4):315–327. doi: 10.1016/j.ccr.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 116.Ikushima H., Todo T., Ino Y., Takahashi M., Miyazawa K., Miyazono K. Autocrine TGF-beta signaling maintains tumorigenicity of glioma-initiating cells through Sry-related HMG-box factors. Cell Stem Cell. 2009;5(5):504–514. doi: 10.1016/j.stem.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 117.Anido J., Saez-Borderias A., Gonzalez-Junca A., Rodon L., Folch G., Carmona M.A. TGF-beta receptor inhibitors target the CD44(high)/Id1(high) glioma-initiating cell population in human glioblastoma. Cancer Cell. 2010;18(6):655–668. doi: 10.1016/j.ccr.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 118.Piccirillo S.G., Reynolds B.A., Zanetti N., Lamorte G., Binda E., Broggi G. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444(7120):761–765. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- 119.Chirasani S.R., Sternjak A., Wend P., Momma S., Campos B., Herrmann I.M. Bone morphogenetic protein-7 release from endogenous neural precursor cells suppresses the tumourigenicity of stem-like glioblastoma cells. Brain J Neurol. 2010;133(Pt 7):1961–1972. doi: 10.1093/brain/awq128. [DOI] [PubMed] [Google Scholar]
- 120.Scheel C., Eaton E.N., Li S.H.-J., Chaffer C.L., Reinhardt F., Kah K.-J. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145(6):926–940. doi: 10.1016/j.cell.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schwalbe M., Sanger J., Eggers R., Naumann A., Schmidt A., Hoffken K. Differential expression and regulation of bone morphogenetic protein 7 in breast cancer. Int J Oncol. 2003;23(1):89–95. [PubMed] [Google Scholar]
- 122.Buijs J.T., Henriquez N.V., van Overveld P.G., van der Horst G., Que I., Schwaninger R. Bone morphogenetic protein 7 in the development and treatment of bone metastases from breast cancer. Cancer Res. 2007;67(18):8742–8751. doi: 10.1158/0008-5472.CAN-06-2490. [DOI] [PubMed] [Google Scholar]
- 123.Naber H.P., Wiercinska E., Pardali E., van Laar T., Nirmala E., Sundqvist A. BMP-7 inhibits TGF-beta-induced invasion of breast cancer cells through inhibition of integrin beta(3) expression. Cell Oncol (Dordr) 2012;35(1):19–28. doi: 10.1007/s13402-011-0058-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liapis H., Flath A., Kitazawa S. Integrin alpha V beta 3 expression by bone-residing breast cancer metastases. Diagn Mol Pathol. 1996;5(2):127–135. doi: 10.1097/00019606-199606000-00008. [DOI] [PubMed] [Google Scholar]
- 125.Hood J.D., Cheresh D.A. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2(2):91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 126.Bjerke G.A., Yang C.S., Frierson H.F., Paschal B.M., Wotton D. Activation of Akt signaling in prostate induces a TGFbeta-mediated restraint on cancer progression and metastasis. Oncogene. 2014;33(28):3660–3667. doi: 10.1038/onc.2013.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yang S., Zhong C., Frenkel B., Reddi A.H., Roy-Burman P. Diverse biological effect and Smad signaling of bone morphogenetic protein 7 in prostate tumor cells. Cancer Res. 2005;65(13):5769–5777. doi: 10.1158/0008-5472.CAN-05-0289. [DOI] [PubMed] [Google Scholar]
- 128.Lu X., Jin E., Cheng X., Feng S., Shang X., Deng P. Opposing roles of TGFβ and BMP signaling in prostate cancer development. Genes Dev. 2017;31(23–24):2337–2342. doi: 10.1101/gad.307116.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Romero D., Terzic A., Conley B.A., Craft C.S., Jovanovic B., Bergan R.C. Endoglin phosphorylation by ALK2 contributes to the regulation of prostate cancer cell migration. Carcinogenesis. 2010;31(3):359–366. doi: 10.1093/carcin/bgp217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lakshman M., Huang X., Ananthanarayanan V., Jovanovic B., Liu Y., Craft C.S. Endoglin suppresses human prostate cancer metastasis. Clin Exp Metastasis. 2011;28(1):39–53. doi: 10.1007/s10585-010-9356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang J., Han C., Ungerleider N., Chen W., Song K., Wang Y. A novel TGF-beta and H19 signaling axis in tumor-initiating hepatocytes that regulates hepatic carcinogenesis. Hepatology (Baltimore, Md) 2018 doi: 10.1002/hep.30153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kitisin K., Ganesan N., Tang Y., Jogunoori W., Volpe E.A., Kim S.S. Disruption of transforming growth factor-beta signaling through beta-spectrin ELF leads to hepatocellular cancer through cyclin D1 activation. Oncogene. 2007;26(50):7103–7110. doi: 10.1038/sj.onc.1210513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhi X., Lin L., Yang S., Bhuvaneshwar K., Wang H., Gusev Y. βII-Spectrin (SPTBN1) suppresses progression of hepatocellular carcinoma and Wnt signaling by regulation of Wnt inhibitor kallistatin. Hepatology (Baltimore, Md) 2015;61(2):598–612. doi: 10.1002/hep.27558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mamiya T., Yamazaki K., Masugi Y., Mori T., Effendi K., Du W. Reduced transforming growth factor-beta receptor II expression in hepatocellular carcinoma correlates with intrahepatic metastasis. Lab Invest. 2010;90(9):1339–1345. doi: 10.1038/labinvest.2010.105. [DOI] [PubMed] [Google Scholar]
- 135.Herrera B., Garcia-Alvaro M., Cruz S., Walsh P., Fernandez M., Roncero C. BMP9 is a proliferative and survival factor for human hepatocellular carcinoma cells. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0069535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li Q., Gu X., Weng H., Ghafoory S., Liu Y., Feng T. Bone morphogenetic protein-9 induces epithelial to mesenchymal transition in hepatocellular carcinoma cells. Cancer Sci. 2013;104(3):398–408. doi: 10.1111/cas.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Maegdefrau U., Amann T., Winklmeier A., Braig S., Schubert T., Weiss T.S. Bone morphogenetic protein 4 is induced in hepatocellular carcinoma by hypoxia and promotes tumour progression. J Pathol. 2009;218(4):520–529. doi: 10.1002/path.2563. [DOI] [PubMed] [Google Scholar]
- 138.Li L., Liu Y., Guo Y., Liu B., Zhao Y., Li P. Regulatory MiR-148a-ACVR1/BMP circuit defines a cancer stem cell-like aggressive subtype of hepatocellular carcinoma. Hepatology (Baltimore, Md) 2015;61(2):574–584. doi: 10.1002/hep.27543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shirai Y.T., Ehata S., Yashiro M., Yanagihara K., Hirakawa K., Miyazono K. Bone morphogenetic protein-2 and -4 play tumor suppressive roles in human diffuse-type gastric carcinoma. Am J Pathol. 2011;179(6):2920–2930. doi: 10.1016/j.ajpath.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.McLean K., Gong Y., Choi Y., Deng N., Yang K., Bai S. Human ovarian carcinoma–associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. J Clin Invest. 2011;121(8):3206–3219. doi: 10.1172/JCI45273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhu H., Gu X., Xia L., Zhou Y., Bouamar H., Yang J. A novel TGFbeta trap blocks chemotherapeutics-induced TGFbeta1 signaling and enhances their anticancer activity in gynecologic cancers. Clin Cancer Res. 2018;24(12):2780–2793. doi: 10.1158/1078-0432.CCR-17-3112. [DOI] [PMC free article] [PubMed] [Google Scholar]