Abstract
Background
Socioeconomic experiences are recognized determinants of health, and recent work has shown that social disadvantages in early life may induce sustained biological changes at molecular level that are detectable later in life. However, the dynamics and persistence of biological embedding of socioeconomic position (SEP) remains vastly unexplored.
Methods
Using the data from the ALSPAC birth cohort, we performed epigenome-wide association studies of DNA methylation changes at three life stages (birth, n = 914; childhood at mean age 7.5 years, n = 973; and adolescence at mean age 15.5 years, n = 974), measured using the Illumina HumanMethylation450 Beadchip, in relation to pregnancy SEP indicators (maternal and paternal education and occupation).
Results
Across the four early life SEP metrics investigated, only maternal education was associated with methylation levels at birth, and four CpGs mapped to SULF1, GLB1L2 and RPUSD1 genes were identified [false discovery rate (FDR)-corrected P-value <0.05]. No epigenetic signature was found associated with maternal education in child samples, but methylation levels at 20 CpG loci were found significantly associated with maternal education in adolescence. Although no overlap was found between the differentially methylated CpG sites at different ages, we identified two CpG sites at birth and during adolescence which are 219 bp apart in the SULF1 gene that encodes an heparan sulphatase involved in modulation of signalling pathways. Using data from an independent birth cohort, the ENVIRONAGE cohort, we were not able to replicate these findings.
Conclusions
Taken together, our results suggest that parental SEP, and particularly maternal education, may influence the offspring’s methylome at birth and adolescence.
Keywords: Social class, DNA methylation, occupations, education
Key Messages
Recent evidence suggests that DNA methylation may play a key role in the embedding of SEP experiences during the life course.
In this study, we found that SEP has a modest influence on the methylome of the offspring at birth, with the strongest effects seen for maternal education.
We have observed more differentially methylated CpG loci related to maternal education in adolescents than in newborns.
We sought independent validation of the CpG sites found differentially methylated in relation to maternal education in cord blood, using neonatal biosamples from the ENVIRONAGE study. Although one CpG site was found to be nominally significant, we did not consistently replicate the direction of this association.
Although no overlap was found between the differentially methylated CpG sites at different ages, we identified two CpG sites at birth and during adolescence to be associated with SEP, which are 219 bp apart in the SULF1 gene that encodes an heparan sulphatase and is involved in modulation of signalling pathways.
Introduction
Individual chronic disease risk profiles in adulthood are not only driven by recent experiences (e.g. behaviours such as smoking and diet in adult life) but also, as formalized in the developmental origin of adult disease hypothesis, by combinations of in utero and early life exposures that influence health in a long-term fashion through processes known as biological embedding.1,2 Socioeconomic experiences are recognized determinants of health,3,4 and recent work has shown that social disadvantages in early life may induce sustainable biological changes such as increased burden of inflammation.5,6 Whereas evidence is accumulating to highlight the importance of the inflammatory response in the mediation of the SEP effect, a better understanding of the biological embedding may elucidate mechanisms that contribute to the early life influence of health inequalities.7 DNA methylation may play a key role in the embedding of SEP experiences during the life course.8–10 Several studies have investigated methylation changes associated with early life socioeconomic experiences in adults.11–20
With few exceptions,14,15,17,19 research found early life SEP to be associated with differential methylation in adulthood of gene promoters,11 repetitive elements,12 candidate genes involved in inflammatory and neuroendocrine responses13,16 and, more recently, with epigenetic age acceleration.18,20
In children, evidence of an effect of early life SEP is still sparse.21–28 Maternal education was found associated with: placental hypomethylation of HSD11B2, which is involved in converting cortisol into inactive cortisone21; cord blood hypomethylation of imprinted genes;25 and hypermethylation of INSIGF and LEP genes, involved in growth and metabolism,22,23 in children at the age of 17 months. However, no effect on global methylation was detected either at birth or at 3 years.28 Neighbourhood-level poverty during pregnancy but not individual maternal education was found to be associated with (higher) methylation of repetitive elements in cord blood,26 and another study found positive association with maternal education only in schoolboys.24 Also, maternal socioeconomic position (SEP) was associated in newborns with epigenetic acceleration.27
Apart from being limited to candidate genes, a major limitation of previous research lays in study design. In practice, adult biosamples were retrospectively related to reported early life SEP,11–20 and biosamples collected at birth, childhood or adolescence were related to cross-sectional information on early life SEP.21–28 By construction, these approaches did not allow an appraisal of the temporal sequence of the events and might represent reverse causation due to the dynamic nature of epigenetic patterns.29 The epigenome, in fact, varies over time as a function of environmental exposures, random processes and ageing.30,31 Longitudinal studies based on repeated measures from the same individuals across life from birth onwards overcome these issues, and may allow us to assess the temporal relationship between early life SEP and epigenetic changes.32
In this context, we propose to use data from the Avon Longitudinal Study of Parents and Children (ALSPAC) birth cohort, where methylation profiles are available at three time points in early life, to identify the early life SEP indicator most associated with epigenetic profiles at birth and to assess whether SEP-associated methylation changes at birth persist during childhood and adolescence.
Methods
Study population and methylation profiles
Our study population arises from the Accessible Resource for Integrated Epigenomics Studies (ARIES) project,33 a sub-study drawn from the ALSPAC mother-child cohort34,35 on a subset of 1018 mother-child pairs, which has DNA methylation available. Ethical approval was obtained from the ALSPAC Ethics and Law Committee and the local research ethics committees, and mothers gave written informed consent. Characteristics of the ALSPAC and ARIES mother-child cohorts are summarized in the Table 1. A searchable data dictionary provides the full information available on the ALSPAC study website [http://www.bris.ac.uk/alspac/researchers/data-access/data-dictionary/].
Table 1.
Descriptive characteristics of all the ALSPAC mother-child cohort, the ARIES subset at birth, the ARIES study population by maternal educational level and the ENVIRONAGE cohort at birth
| ALSPAC | ARIES | Study population (ARIES) by maternal education | ENVIRONAGE | |||
|---|---|---|---|---|---|---|
| n = 15 445 | n = 914 |
n = 860 |
n = 180 | |||
| Low (O level/ vocational/CSE) | Medium (A level) | High (degree) | ||||
| n = 431 | n = 249 | n = 180 | ||||
| Child characteristics | ||||||
| Sex, female | 7219 (48.5) | 469 (51.3) | 228 (52.9) | 119 (47.8) | 88 (48.9) | 85 (47.2) |
| Birthweight, gramsa | 3381 ± 580.9 | 3485 ± 486.8 | 3479 ± 494.5 | 3474 ± 470.9 | 3505 ± 470.1 | 3401 ± 471.9 |
| Gestational age, weeksa | 38.36 ± 5.5 | 39.56 ± 1.5 | 39.5 ± 1.6 | 39.42 ± 1.5 | 39.80 ± 1.4 | 39.11 ± 1.6 |
| Parent characteristics | ||||||
| Maternal age, yearsa,b | 28.35 ± 4.8 | 29.59 ± 4.49 | 28.37 ± 4.4 | 30.39 ± 4.1 | 31.67 ± 3.6 | 29.37 ± 4.2 |
| Maternal BMI, kg/m2b | 22.93 ± 3.9 | 22.82 ± 3.7 | 23.35 ± 4.2 | 22.52 ± 3.2 | 21.85 ± 2.6 | 23.97 ± 4.3 |
| Maternal smoking during pregnancy, yesa,b | 1854 (24.7) | 121 (13.2) | 79 (18.3) | 24 (9.6) | 11 (6.1) | 25 (13.9) |
| Maternal alcohol consumption during pregnancy, yes | 9382 (60.7) | 708 (77.5) | 337 (78.2) | 196 (78.7) | 148 (82.2) | 19 (10.5)c |
| Parity, multiparous | 7252 (55.2) | 465 (50.9) | 228 (52.9) | 139 (55.8) | 89 (49.4) | 81 (45) |
| Maternal education | ||||||
| Low (O level/vocational/CSE)a | 8084 (52.3) | 450 (49.2) | 431 (50.1) | – | – | 91 (50.6) |
| Medium (A level) | 2802 (18.1) | 260 (28.4) | – | 249 (28.9) | – | 62 (34.4) |
| High (degree) | 1610 (10.4) | 184 (20.1) | – | – | 180 (20.9) | 27 (15) |
| Paternal education, | ||||||
| Low (O level/vocational/CSE)b | 6709 (43.4) | 393 (43) | 264 (61.2) | 86 (34.5) | 24 (13.3) | 62 (34.4) |
| Medium (A level) | 3123 (20.2) | 262 (28.7) | 132 (30.6) | 98 (39.4) | 25 (13.9) | 72 (40) |
| High (degree) | 2182 (14.1) | 227 (24.8) | 130 (30.2) | 63 (25.3) | 27 (15) | 30 (16.7) |
| Maternal occupation, manuala,b | 2870 (18.6) | 143 (15.6) | 102 (23.7) | 31 (12.4) | 6 (3.3) | – |
| Paternal occupation, manualb | 4987 (32.3) | 305 (33.4) | 214 (49.7) | 67 (26.9) | 11 (6.1) | – |
Counts (percentages) and means ± standard deviations are reported for categorical and continuous variables, respectively.
Significant P-value for difference in proportion (chi square test) and mean (t test) of ALSPAC versus ARIES population.
Significant P-value between maternal education categories of the study population using chi-square (for categorical dependent variables) and ANOVA test (for continuous dependent variables).
In ENVIRONAGE occasional alcohol use was reported.
We analysed DNA methylation data of the offspring at the three time points (at birth, n = 914; at mean age 7.5 years, n = 973; and at mean age 15.5 years, n = 974). A description of the data and sample collection and analyses of DNA methylation can be found in Supplementary Methods S1, available as Supplementary data at IJE online.
Early life socioeconomic position indicators and covariates
Early life SEP was measured by parental education and occupation during pregnancy. Maternal and paternal educations were collected from a self-reported questionnaire at 32 weeks of gestation, and were coded in three categories according to educational achievement: (i) low: Certificate of Secondary Education (CSE), Vocational or Ordinary- (O-) level, educational qualifications generally obtained at 16 years of age; (ii) intermediate: Advanced- (A-) level, subject-specific qualification most commonly attained at 18 years of age and required for admission to higher education; (iii) high: university degree and above.
Maternal occupation was collected from mothers’ self-reported antenatal (18-week) questionnaire, and paternal occupation from fathers’ antenatal (32-week) questionnaire. Occupation was categorized according to the UK Registrar General’s classification36 and dichotomized into: (i) manual, including unskilled, semi-skilled manual and skilled manual occupations; (ii) non-manual, including skilled non-manual, managerial, technical and professional occupations. Information on covariates collection can be found in Supplementary Methods S1, available as Supplementary data at IJE online.
Replication study
As an independent dataset from which to seek validation, we used the ENVIRonmental influence ON AGEing (ENVIRONAGE) birth-cohort.37 Data and sample collection information and analyses of DNA methylation can be found in Supplementary Methods S1, available as Supplementary data at IJE online.
Statistical analysis
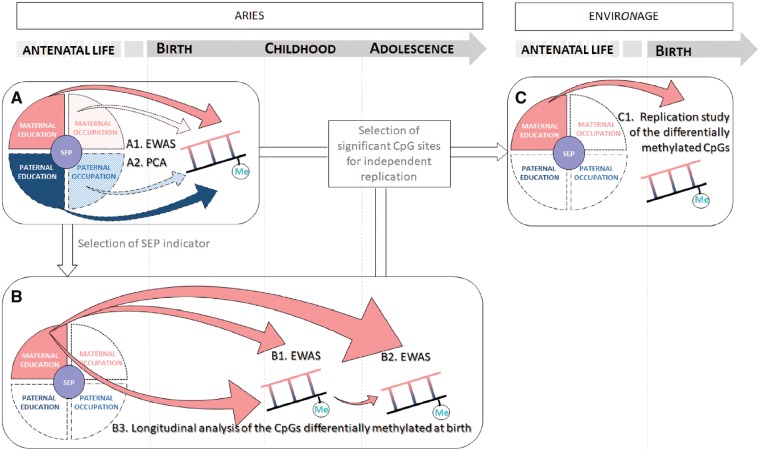
Figure 1 depicts the study workflow, which is structured in three phases.
Figure 1.
Study workflow. The figure depicts the study workflow which is structured in three phases. First, the association between DNA methylation levels at birth and the four indicators of early life SEP was investigated performing EWAS (1 A1) and then regressing DNA methylation PCs against each indicators of SEP (1 A2). Second, based on significance from the PC analyses, a SEP indicator was selected. EWASs were performed for this selected indicator and DNA methylation status at childhood (1 B1) and at adolescence (1 B2), and methylation levels of the probes significant in cord blood were integrated over the three time points in a longitudinal model (1 B3). Finally, we adopted a targeted approach to seek independent validation of the CpG sites differentially methylated in relation to the selected SEP indicator both at birth and later in life, using neonatal biosamples from the ENVIRONAGE study (1 C1). EWAS, epigenome-wide association study; PCA, principal component analysis; SEP, socioeconomic position.
Using the full resolution methylation data, we investigated the association between DNA methylation levels at birth and the four indicators of early life SEP: maternal and paternal education, and maternal and paternal occupation (Figure 1 A1). DNA methylation levels were modelled as dependent variable in a generalized linear model with beta-distributed response using the parameterization of Ferrari and Cribari-Neto,38 and we accounted for multiple testing by controlling the false discovery rate (FDR)39 at a level below 0.05. As a lower resolution alternative, we ran principal component (PC) analyses of the methylome using the prcomp function in R. We then regressed the PCs against each of the indicators of SEP (Figure 1 A2).
For the followings two steps, we selected one indicator of SEP based on its statistical significance in the PC analyses. We ran epigenome-wide association studies (EWASs) for the selected SEP indicator and DNA methylation status at childhood (Figure 1 B1) and adolescence (Figure 1 B2). Methylation levels of the probes significant in cord blood were integrated over the three time points (Figure 1 B3), according to the method described in Supplementary Methods S1, available as Supplementary data at IJE online.
Finally, we adopted a targeted approach to seek independent validation of the CpG sites found to be differentially methylated in relation to the selected SEP indicator, using neonatal biosamples from the ENVIRONAGE study (Figure 1C).
All the analyses were adjusted for birthweight,40 parity,41 gestational age40,42 and sex of the newborn,43 in addition to technical variables: bead array row and bisulphite conversion batch.
To assess the robustness of our findings, we ran sensitivity analyses stratified by sex and including additional adjustment: (i) on the possible explanatory variables of SEP: maternal age,44 body mass index (BMI),40,45 smoking status46 and alcohol consumption during pregnancy47; (ii) on blood cell composition which were estimated through an established deconvolution approach48; (iii) on delivery mode and self-reported maternal health during the pregnancy; and (iv) for analyses at 7 and 15 years on offspring life course characteristics: own BMI, own use of tobacco and alcohol (only for the analysis at 15 years).
To compare our results with previous targeted studies, we performed look-up analyses of methylation profiles at the three time points, based on a list of 281 probes derived by CpG sites and genes previously associated with early life SEP.13,16,21–23,25
Results
Compared with the ALSPAC mothers, those included in ARIES were slightly older and more likely to have a higher educational level and non-manual occupation and to be a non-smoker during pregnancy. In the ARIES subset, smoking during pregnancy, higher BMI and younger age of the mothers at birth were more prevalent in lowest SEP group, and alcohol consumption was higher in the highest SEP group although not significantly (Table 1).
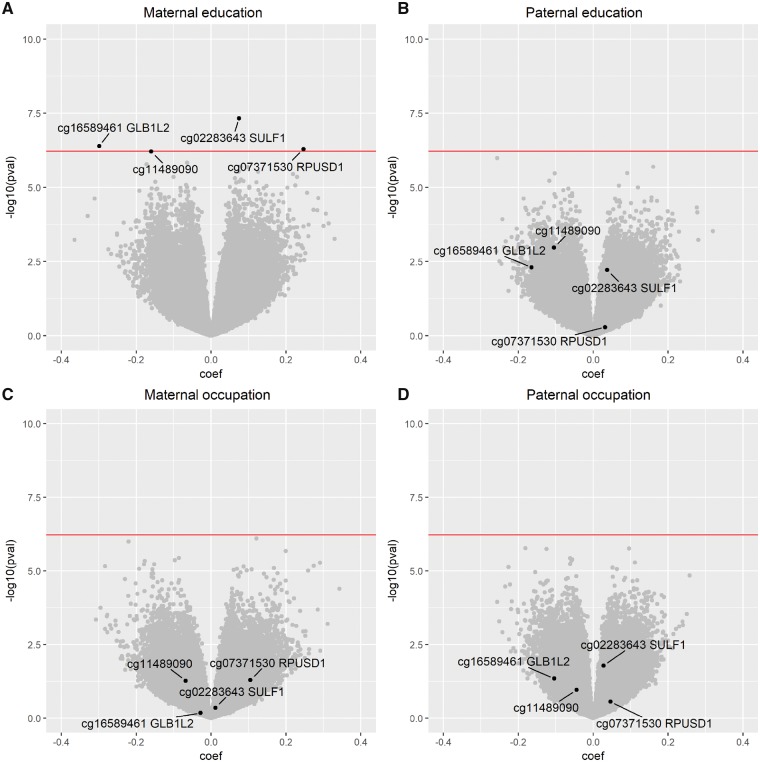
These variables may act as mediators in the relationship between SEP and DNA methylation and were therefore excluded from the main analyses although shown to affect cord blood DNA methylation (Supplementary Figure S2, available as Supplementary data at IJE online). The SEP indicators were all significantly positively correlated with each other (r range = 0.41–0.68) (Supplementary Figure S3, available as Supplementary data at IJE online). Results of EWAS of DNA methylation in cord blood in relation to parental SEP indicators (maternal and paternal education and occupation) are reported in Figure 2.
Figure 2.
Volcano plots for EWAS of parental early life SEP indicators and cord DNA methylation. The figure shows the volcano plots for EWAS of cord DNA methylation and parental early life SEP indicators (2A, maternal education; 2B, paternal education; 2C, maternal occupation; 2D, paternal occupation). β values (coefficients) are reported on the x-axis as a function of the −log10 P-values on the y-axis. The horizontal line represents the FDR level of 0.05. CpG sites whose methylation levels were found statistically differentially methylated in the analysis of maternal education are highlighted in black, and located also in the plots of maternal occupation and paternal education and occupation. Models were adjusted for birthweight, parity, gestational age and sex of the newborn in addition to technical variables: bead array row and bisulphite conversion batch.
Below the FDR level of 0.05, we identified (four) differentially methylated sites only in relation to maternal education (Table 2). The regression coefficients for these CpG sites for all the other SEP indicators are reported in Supplementary Table S4, available as Supplementary data at IJE online.
Table 2.
CpG sites associated with maternal education (FDR-adjusted P-values <0.05) in ARIES from EWAS at birth and at 15 years
| Probe | Closest gene | Genomic location | Relation to CpG island | β | Standard error | P-value | q-value |
|---|---|---|---|---|---|---|---|
| Birth | |||||||
| cg02283643 | SULF1 | TSS200 | – | 0.075 | 0.014 | 4.67e-08 | 0.011 |
| cg16589461 | GLB1L2 | Body | South shore | −0.299 | 0.059 | 4.08e-07 | 0.032 |
| cg07371530 | RPUSD1 | TSS1500 | North shore | 0.247 | 0.049 | 5.10e-07 | 0.034 |
| cg11489090 | – | – | – | −0.160 | 0.032 | 6.20e-07 | 0.036 |
| 15 years | |||||||
| cg21013866 | EFS | TSS200 | Island | 0.121 | 0.023 | 2.39e-07 | 0.034 |
| cg27187881 | NAGA | 1st Exon | North shore | 0.070 | 0.014 | 3.67e-07 | 0.034 |
| cg01122167 | CAMK2A | Body | – | 0.189 | 0.037 | 4.20e-07 | 0.034 |
| cg13483196 | – | – | – | −0.149 | 0.030 | 6.96e-07 | 0.039 |
| cg16582803 | – | – | South shore | −0.114 | 0.023 | 9.19e-07 | 0.040 |
| cg05806180 | SULF1 | 5'UTR | – | 0.106 | 0.022 | 1.29e-06 | 0.042 |
| ch.10.295680R | – | – | – | −0.088 | 0.018 | 1.41e-06 | 0.042 |
| cg13093989 | EFCAB2 | Body | – | 0.168 | 0.035 | 1.51e-06 | 0.043 |
| cg12050497 | FAM84A | 5'UTR | Island | −0.061 | 0.013 | 1.80e-06 | 0.043 |
| cg22091037 | STARD13 | TSS200 | – | −0.083 | 0.018 | 1.98e-06 | 0.044 |
| cg11066033 | THAP4 | 1st Exon | – | −0.083 | 0.018 | 2.07e-06 | 0.044 |
| cg06237983 | HOXA6 | 1st Exon | Island | 0.064 | 0.014 | 2.38e-06 | 0.044 |
| cg25316853 | SLC1A3 | TSS200 | – | −0.084 | 0.018 | 2.47e-06 | 0.044 |
| cg20483690 | LBR | TSS1500 | South shore | −0.085 | 0.018 | 2.69e-06 | 0.045 |
| cg06974483 | SPRY1 | TSS200 | North shore | −0.057 | 0.012 | 2.72e-06 | 0.045 |
| cg05585947 | – | – | North shelf | −0.142 | 0.030 | 3.38e-06 | 0.046 |
| cg05076221 | HOXA5 | Body | Island | 0.072 | 0.016 | 3.44e-06 | 0.046 |
| cg11367267 | – | – | North shelf | 0.187 | 0.040 | 3.45e-06 | 0.046 |
| cg22891600 | – | – | – | −0.097 | 0.021 | 3.57e-06 | 0.046 |
| cg25397818 | MAD1L1 | Body | North shore | −0.203 | 0.044 | 3.77e-06 | 0.046 |
No probe was significant in blood collected from 7-year-old children, hence no probe is presented for children. Models were adjusted for birthweight, parity, gestational age and sex of the newborn in addition to technical variables: bead array row and bisulphite conversion batch.
TSS, transcription start site; UTR, untranslated region; closest gene, UCSC annotated gene; genomic location, UCSC gene region feature category; relation to CpG island, UCSC relation to CpG islands; β, regression coefficient; standard error, standard error for regression coefficient.
EWAS using alternative early life SEP indicators yielded lower effect size estimates and weaker associations (Figure 2B–D, for maternal occupation and paternal education and occupations, Supplementary Figure S5A and B, available as Supplementary data at IJE online for household highest education and occupation, and Supplementary Figure S5C, available as Supplementary data at IJE online for alternative coding of the occupations) than the analysis of maternal education. Additional adjustment of the full resolution analyses of the four indicators of SEP for possible explanatory variables, including maternal age, maternal BMI before the pregnancy, maternal smoking and alcohol consumption during pregnancy, did not yield additional associations except for three probes in relation to paternal occupation (Supplementary Figure S6, available as Supplementary data at IJE online).
Among the four probes significantly associated with maternal education, only two sites (cg02283643, β = 0.075, P-value = 4.67e-8, q-value = 0.011; cg11489090, β=-0.160, P-value = 6.20e-7, q-value = 0.036) remained statistically significant upon adjustment for maternal age and BMI, smoking status and alcohol consumption during pregnancy (cg02283643, β = 0.082, P-value = 4.91e-08, q-value = 0.016; cg11489090, β=-0.179, P-value = 7.29e-7, q-value = 0.049) (Supplementary Figure S7, available as Supplementary data at IJE online). None of the four probes have been previously reported to be associated with maternal age,44 BMI,49 smoking50 or alcohol consumption51 during pregnancy by larger studies, including the Pregnancy and Childhood epigenetics consortium. Albeit mitigated, consistent results were observed in both males and females for three CpG sites (cg02283643, cg165894161 and cg11489090). Only cg07371530 had a much stronger association in females (β = 0.40, P-value = 1.33e-8) compared with males (β = 0.06, P-value = 0.43) and for this CpG site interaction between sex and maternal education (P-value for interaction = 0.01) was identified (Supplementary Table S8, available as Supplementary data at IJE online).
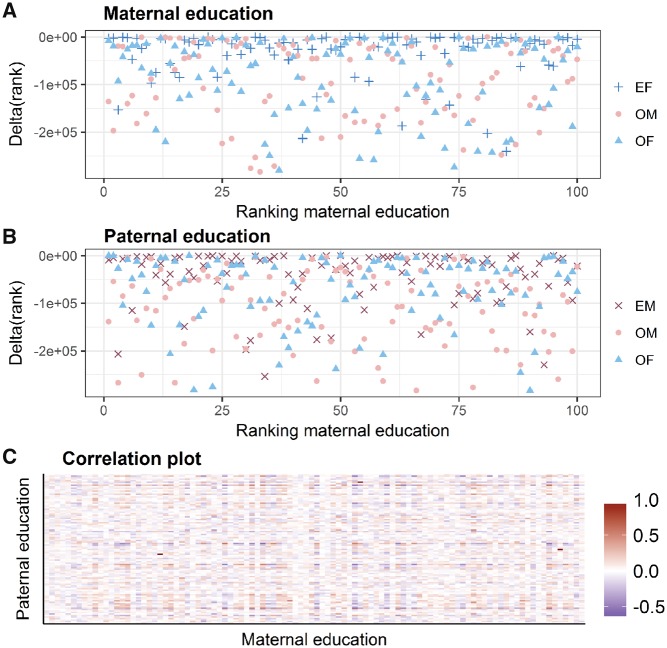
Figure 3A shows that a considerable number (n = 27) of the 100 strongest associations found with maternal education (x-axis) consistently ranked high (within the first percentile) in the analysis of paternal education. Paternal education showed a similar behaviour (Figure 3B), whereas maternal or paternal occupation did seem to yield inconsistent ranking. Correlation between the strongest association from the analyses of maternal and paternal education in cord blood are reported in Figure 3C.
Figure 3.
Delta rank of the top 100 CpG loci for the four SEP indicators. The upper part of the plot represents the difference in the rank of the first 100 CpG loci from the EWAS of (3A) maternal and (3B) paternal education and the rank of the same CpG loci in the EWAS of the other SEP indicators in cord blood identified by colours and shapes of the dots (maternal education, cross; paternal education, plus; maternal occupation, circle; paternal occupation, triangle). The lower part of the plot (3C) shows the correlation plot of the first 100 strongest associations from the EWAS of maternal and paternal education in cord blood. Ranks are derived from models adjusted for birthweight, parity, gestational age and sex of the newborn in addition to technical variables: bead array row and bisulphfite conversion batch. EF, education of the father; OM, occupation of the mother; OF, occupation of the father; EM, education of the mother.
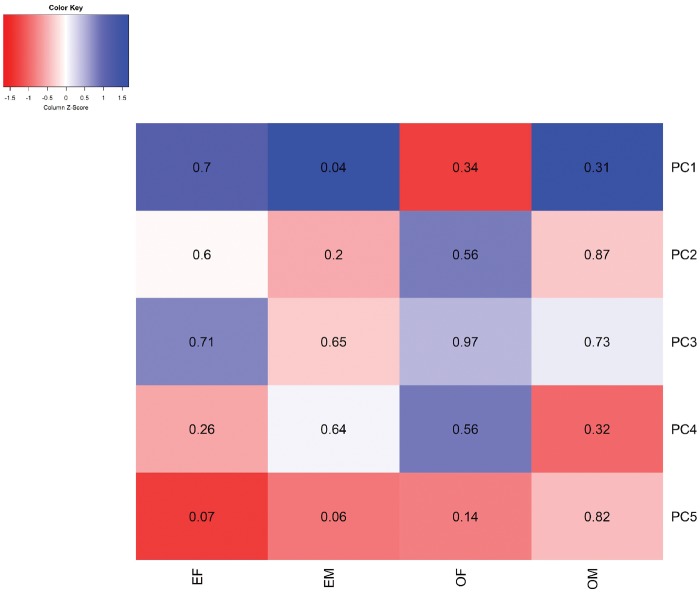
To capture the SEP influence on the overall methylome, we ran principal component (PC) analyses of the methylome as a lower resolution alternative to our full-resolution analyses. Regressing the PCs against the four early life SEPs under investigation, education of the mother was found significantly associated to the scores of the first PC, which explained 12.44% of the variability of cord blood DNA methylation, whereas none of the other components yielded significant associations (Figure 4 shows the first five components that explain 22% of the variance).
Figure 4.
Heatmap of associations between SEP indicators and principal components of cord blood DNA methylation. The heatmap depicts the estimates of associations, represented by shades, and corresponding P-values, displayed as numbers, between the four SEP indicators and the first five principal components of cord blood DNA methylation. Models were adjusted for birthweight, parity, gestational age and sex of the newborn in addition to technical variables: bead array row and bisulphite conversion batch. EF, education of the father; EM, education of the mother; OF, occupation of the father; OM, occupation of the mother.
We did not identify any differentially methylated sites in relation to the education of the mother in 7-year-olds, but found 20 significant associations in adolescents (Table 2). No CpG site of this set of 20 CpG sites was significantly differentially methylated in either cord blood or childhood biosamples (Table 3). As for cord blood analysis, results were consistent in both males and females, although significance was weaker especially for males (Supplementary Table S9, available as Supplementary data at IJE online). Adjustment on child life course characteristics (BMI, smoking and alcohol consumption) did not affect direction and strength of associations although in general it slightly increased the P-value (Supplementary Table S10, available as Supplementary data at IJE online).
Table 3.
Results from the ARIES analyses of maternal education and DNA methylation at 15 years, at 7 years and at birth, for the 20 probes identified as associated with maternal education by EWAS at 15 years
| DNA methylation |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 15 years |
7 years |
Birth |
||||||||
| Probe | Gene | Rank | β | P-value | Rank | β | P-value | Rank | β | P-value |
| cg21013866 | EFS | 1 | 0.121 | 2.39e-07 | 142 039 | −0.015 | 0.471 | 84 269 | −0.021 | 0.200 |
| cg27187881 | NAGA | 2 | 0.070 | 3.67e-07 | 161 256 | 0.008 | 0.540 | 126 301 | −0.015 | 0.343 |
| cg01122167 | CAMK2A | 3 | 0.189 | 4.20e-07 | 181 763 | −0.017 | 0.616 | 68 182 | −0.056 | 0.150 |
| cg13483196 | – | 4 | −0.149 | 6.96e-07 | 142 225 | 0.022 | 0.472 | 239 441 | −0.009 | 0.802 |
| cg16582803 | – | 5 | −0.114 | 9.19e-07 | 82 871 | −0.025 | 0.261 | 57 578 | −0.039 | 0.119 |
| cg05806180 | SULF1 | 6 | 0.106 | 1.29e-06 | 34 426 | 0.035 | 0.099 | 49 454 | 0.041 | 0.097 |
| ch.10.295680R | – | 7 | −0.088 | 1.41e-06 | 133 477 | 0.013 | 0.440 | 68 485 | 0.026 | 0.151 |
| cg13093989 | EFCAB2 | 8 | 0.168 | 1.51e-06 | 162 044 | −0.021 | 0.543 | 77 789 | −0.048 | 0.179 |
| cg12050497 | FAM84A | 9 | −0.061 | 1.80e-06 | 227 015 | −0.003 | 0.781 | 240 217 | −0.003 | 0.805 |
| cg22091037 | STARD13 | 10 | −0.083 | 1.98e-06 | 204 341 | −0.006 | 0.697 | 104 306 | 0.020 | 0.265 |
| cg11066033 | THAP4 | 11 | −0.083 | 2.07e-06 | 73 680 | −0.018 | 0.229 | 22 593 | 0.045 | 0.035 |
| cg06237983 | HOXA6 | 12 | 0.064 | 2.38e-06 | 705 | 0.044 | 0.001 | 17 659 | 0.037 | 0.026 |
| cg25316853 | SLC1A3 | 13 | −0.084 | 2.47e-06 | 208 276 | −0.006 | 0.712 | 53 709 | 0.032 | 0.109 |
| cg20483690 | LBR | 14 | −0.085 | 2.69e-06 | 78 050 | 0.021 | 0.244 | 58 245 | 0.033 | 0.121 |
| cg06974483 | SPRY1 | 15 | −0.057 | 2.72e-06 | 24 573 | 0.024 | 0.068 | 88 379 | 0.018 | 0.213 |
| cg05585947 | – | 16 | −0.142 | 3.38e-06 | 253 540 | 0.005 | 0.879 | 233 307 | −0.010 | 0.775 |
| cg05076221 | HOXA5 | 17 | 0.072 | 3.44e-06 | 4686 | 0.042 | 0.010 | 2514 | 0.054 | 0.002 |
| cg11367267 | – | 18 | 0.187 | 3.45e-06 | 105 426 | 0.036 | 0.340 | 236 211 | −0.012 | 0.788 |
| cg22891600 | – | 19 | −0.097 | 3.57e-06 | 181 340 | 0.008 | 0.614 | 95 169 | 0.022 | 0.235 |
| cg25397818 | MAD1L1 | 20 | −0.203 | 3.77e-06 | 210 800 | 0.015 | 0.721 | 182 977 | 0.026 | 0.564 |
Models were adjusted for birthweight, parity, gestational age and sex of the newborn in addition to technical variables: bead array row and bisulphite conversion batch.
Gene, UCSC annotated gene; rank, rank of methylation at birth, 7 and 15 years of age; β, regression coefficient.
Also, the CpGs identified in cord blood were not found to be significantly differentially methylated in either childhood or adolescent biosamples (Supplementary Table S11, available as Supplementary data at IJE online). Using a longitudinal model confirmed non-persistence of the neonatal epigenetic marks at later life time points (Supplementary Table S12, available as Supplementary data at IJE online).
Nevertheless, from our EWAS in cord and in adolescent blood, we identified differentially methylated CpG sites on the same gene: one site located in SULF1 gene (cg02283643, located in the TSS200 region, P-value = 4.67e-08) for cord blood samples, and another site for adolescents (cg05806180, located in the 5’UTR region, P-value = 1.29e-06). Correlation of these sites was significant both in the analyses of cord (r = 0.21, P-value = 4.65e-10) and adolescent blood (r = 0.17, P-value = 4.80e-08) (Supplementary Figure S13, available as Supplementary data at IJE online). These two CpG sites are only 219 bp distant and show a similar magnitude and direction of methylation (cg02283643, β = 0.07; cg05806180, β = 0.10). The probe (cg02283643), located on SULF and found significant in cord blood, is the only one to remain significant even after adjustment for delivery mode and maternal health during the pregnancy and white blood cells composition (Supplementary Table S14, available as Supplementary data at IJE online).
We interrogated the methylation levels at the four CpG loci found differentially methylated in cord blood in relation to maternal education in the ENVIRONAGE cohort, and were not able to replicate the findings. Compared with results from ARIES, the same direction of association was detected for only one CpG cg02283643 (ENVIRONAGE, β = 0.017; ARIES, β = 0.075) (Tables 2 and 4); however, the P-value was >0.05 (P-value = 0.76).
Table 4.
Results from replication analysis in ENVIRONAGE cohort of the four probes found associated with maternal education at birth in the ARIES study population
| Probe | Closest gene | Genomic location | Relation to CpG island | β | Standard error | P-value |
|---|---|---|---|---|---|---|
| cg02283643 | SULF1 | TSS200 | – | 0.017 | 0.055 | 0.756 |
| cg16589461 | GLB1L2 | Body | South shore | 0.033 | 0.040 | 0.399 |
| cg07371530 | RPUSD1 | TSS1500 | North shore | −0.047 | 0.024 | 0.048 |
| cg11489090 | – | – | – | 0.002 | 0.037 | 0.965 |
Models were adjusted for birthweight, parity, gestational age and sex of the newborn in addition to technical variables: bead array row and bisulphite conversion batch.
TSS, transcription start site; closest gene, UCSC annotated gene; genomic location, UCSC gene region feature category; relation to CpG island, UCSC relation to CpG islands; β, regression coefficient; standard error, standard error for regression coefficient.
At the opposite, another CpG site (cg07371530) was found nominally significant (p-value < 0.05) but the direction of association did not consistently replicate (ENVIRONAGE, β=-0.047; ARIES, β = 0.247) (Tables 2 and 4) Also, none of the 20 CpG sites found significant in ARIES adolescents was replicated in ENVIRONAGE (Supplementary Table S15, available as Supplementary data at IJE online).
In the look-up analyses we did not identify any significant probe; however, BDNF gene appeared to be the top hit in the analyses at all the three time points (Supplementary Figure S16, available as Supplementary data at IJE online).
Discussion
One of the main findings of our study was that the impact of maternal education may be embedded in the offspring’s methylome.
Education attainment, occupation and income are valid indicators to define SEP and social inequality.52 As expected, the measures of SEP we used in our study were all significantly correlated to each other; however, maternal education was less correlated with maternal occupation as compared with paternal education with occupation. This can be partly attributable to the fact that our classification of occupation into manual and non-manual, according the UK Registrar General’s classification, was developed for male worker and may poorly apply to females.
Each indicator measures different, often related aspects of socioeconomic stratification and may be more or less relevant to different health outcomes at different stages in the life course.53
Occupational levels reflect access to material resources, prestige and exposure to occupational toxicants or physical workload.52 Specifically for infants, maternal employment reflects prestige, access to material resources and has been associated with better pregnancy outcomes.54 However specific maternal occupations, such as those involving exposure to endocrine disruptors55 or heavy physical work,56 may directly affect pregnancy outcomes, although effect sizes are generally small.57 Intuitively, maternal occupation has a larger effect on birth outcomes than paternal occupation, especially when considering occupation with specific toxic risks,58 whereas the contrary seems to happen later in life59 because prestige and access to resources become more influential. Despite this, in our study we were not able to detect any epigenetic signal in relation to maternal or paternal occupation. A possible explanation could be that we used a broad classification of occupation into manual and non-manual classes, which may have led to misclassification of occupational exposures. Similarly, previous studies in the ALSPAC cohort failed to detect adverse pregnancy outcomes in relation to maternal60 or paternal occupation.61
The level of education has been postulated as the dimension of the SEP that most strongly and consistently predicts health, especially for women and their children.53,62,63 In support of these observations, we found an epigenetic link between education and the methylome. A lower level of education might affect birth outcomes directly by limiting the capacity to integrate within society and increasing the risk of poverty, or indirectly through maternal health behaviours.64 The knowledge and skills achieved through education may affect a person’s cognitive functioning, making one more amenable to health information messages or more able to access appropriate health services, which might be advantageous for the offspring. For example, before the pregnancy, adverse birth effects can be mediated by unhealthy lifestyle such as maternal smoking, alcohol consumption, malnutrition and stress. In this regard, a recent EWAS meta-analysis found overlaps between the epigenetic signals associated with education attainment and those previously described to be associated with own or prenatal smoking, suggesting that the associations with education attainment could be due to correlation with smoking.65 After the birth, maternal behaviour in child care may mediate negative effects on health outcomes in infants and children. For example, mothers with lower level of education are less likely to be aware of the benefits of maternal milk for very preterm infants,66 or to provide child immunization.67
We found that maternal education was the most important SEP variable significantly affecting the offspring’s methylome, considering both CpG loci (Figure 2) and principal components analyses of cord blood DNA methylation (Figure 4). These results suggest that the association of maternal SEP with offspring methylation at birth are likely to be driven via in utero mechanisms. The epigenome is thought to be particularly vulnerable to environmental factors during embryogenesis, and there is increasing evidence for a developmental plasticity in response to toxicological, hormonal, nutritional, social and broad ecological environmental exposures.68 A wealth of epidemiological data supports the associations between maternal BMI or malnutrition and smoking with intrauterine growth retardation and birthweight.69–71 Studies on the ARIES cohort, here also under study, have found that maternal obesity and underweight as well as smoking affect the neonatal epigenome.49,50,72
We found more robust effects in females than males. Similarly, a study of the literature found SES risk in childhood to be more robustly associated with methylation in young adult females than in males,73 although in placenta samples the opposite trend has been described.21
We have identified CpG sites differentially methylated in cord blood associated with maternal education, but we did not observe persistence of these methylation differences at later time points, suggesting that these associations fade during the first years of life. These specific epigenetic signals at birth might have downstream effects in early life rather than be persistent across the life course, yet this does not exclude the involvement of epigenetic mechanisms. Studies on the variation of methylation markers in the population and their stability over time are limited, especially in early life.31,74–78 Previous studies demonstrated that intra-individual variability of the methylome during the first 2 years of life is mainly located within genes with important biological functions, including immunity and inflammation.31 These results have been confirmed in a study within the first 5 years after birth.79 In a different study based on the ARIES cohort, there was also little evidence of an association between methylation during childhood or in adolescence and either birthweight or gestational age; the authors speculated correspondingly that there appears to be a phase of rapid ‘catch-up’ in methylation differences.80 Similarly, non-persistence of associations over time is acknowledged as one possible reason of the lack of association of early life SEP with the methylation acceleration in adulthood found in ALSPAC mothers.17 Besides, in the life course perspective it is possible that the time span considered in this study is too short to identify biological changes that become evident only in adulthood and older ages, according to duration and intensity of exposure to favourable or unfavourable SEP exposures throughout life.81
We have observed 20 significant differentially methylated CpG loci related to maternal education in adolescents, but only four CpGs in newborns. The maternal SEP might be associated with stronger effects on DNA methylation over time compared with only during the pregnancy, though additional research using early life SEP trajectories are warranted to explore these observations. In fact, we cannot exclude that these effects are associated with adolescent SEP, which in turn is related to childhood SEP. In this regard, adjustment for adolescent BMI, alcohol and tobacco consumption, which are associated with own SEP, lowered the significance of the epigenetic associations although did not affect direction and effect sizes.
Of particular interest were two loci in the SULF1 gene, which were significantly associated with maternal education in either cord blood or during adolescence, and which were only 219 bp distant from each other. SULF1 encodes an extracellular heparan sulphate endosulphatase that catalyzes the 6-O-desulphation of heparan sulphate proteoglycans co-receptors for heparin-binding growth factors and cytokine signalling pathways, and therefore has an important role in many biological processes, such as embryogenesis, cell signalling, angiogenesis and tumourigenesis.82–84 In experimental studies, the SULF1 gene has been found hypermethylated in cancers, and in humans it was differentially methylated in essential hypertension cases in young adults.85 We could also not replicate the CpG located on SULF1 and the other three CpG loci in the ENVIRONAGE birth cohort. In this regard, it might be spurious to generalize the maternal education of the two cohorts because: there are more than 20 years between their sampling; public health information might evolve over time; and the cohorts are in two different countries. Although both cohorts are representative for their respective areas, the participants are on average somewhat more highly educated than is general in the geographical area they represent. For example, the ALSPAC population has a shortfall in less affluent families compared with the Avon area, and those in ARIES were more highly educated compared with those not in ARIES.33,34 In this regard, the ARIES sub-sample has been reported to be reasonably representative of the main study population33; however, we cannot exclude a bias in the selection which in turn could be related to different parameters.86 In this study, which fits in a discovery framework, we are focusing on potential methylation targets, and the reliability of the targets we identified should be further assessed by other population studies. Further, since the epigenome is under both genetic and environmental influences, the epigenetics response to an exposure can be variable between individuals, populations, over time and so forth. Mechanistic pathways through which parental SEP (behavioural, occupational exposures, psychosocial stress) can affect the offspring CpG methylation may differ between the two cohorts. Nevertheless, heterogeneous methylation patterns can have similar phenotypic consequences over the life course.87
Findings from this study should be interpreted with caution due to certain limitations. DNA methylation has been measured in peripheral blood cells and not in specific tissues; although tissue specificity is a well-established attribute of DNA methylation, there is no clear consensus on which tissue might be most relevant to study when considering the impact of SEP.30 SEP embedding involves several processes,15,88 and hence DNA methylation of brain or immune cells could potentially provide more insight. Moreover, in a mixed cell population such as (cord) blood, cells may demonstrate similar phenotypes but with distinct methylation patterns,89 and SEP-linked differences in B to T cell ratios might account for some of our observations.90 We did additionally adjust the significant CpG sites for the estimated blood cell composition,48 and the magnitude of the associations remained. To our knowledge, this is the first study exploring the relationship between early life SEP and epigenome-wide DNA methylation at birth and subsequently during childhood.
Conclusion
Understanding the differences in methylation patterns across ages and the consistency across independent studies could be the key to interpret the biological pathways through which the socioeconomic environment relates to molecular changes in the body. Taken together, our study provides some evidence that parental SEP has a modest influence on the methylome of the offspring early in life, with the strongest effects seen for maternal education on the offspring’s methylome at birth and adolescence.
Funding
This work was supported by the UK Medical Research Council and the Wellcome Trust (102215/2/13/2), the University of Bristol, the UK BBSRC (BB/I025751/1 and BB/I025263/1), the UK ESRC (ES/N000498/1), the Erasmus Plus Programme (to R.A.), the COLT foundation (to F.G.), the ‘Lifepath’ grant (European Commission H2020 grant number 633666 to P.V.), and the People Program (Marie Curie Actions) of the European Union's Seventh Framework Program FP7/2007–2013/ (under REA grant agreement 628858 to M.P.).
Supplementary Material
Acknowledgements
We acknowledge Silvia Stringhini for her contribution in the classification of the socioeconomic position. Michelle Plusquin vouched for the integrity of the data and the accuracy of their reporting in the present paper. Rossella Alfano is responsible for accuracy and completeness of the reference list.
Conflict of interest: None declared.
References
- 1. Barker DJ. The developmental origins of adult disease. J Am Coll Nutr 2004;23(Suppl 6):588s–95s. [DOI] [PubMed] [Google Scholar]
- 2. Hertzman C. The biological embedding of early experience and its effects on health in adulthood. Ann N Y Acad Sci 1999;896:85–95. [DOI] [PubMed] [Google Scholar]
- 3. Galobardes B, Lynch JW, Davey Smith G.. Childhood socioeconomic circumstances and cause-specific mortality in adulthood: systematic review and interpretation. Epidemiol Rev 2004;26:7–21. [DOI] [PubMed] [Google Scholar]
- 4. Galobardes B, Davey Smith G, Lynch JW.. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Ann Epidemiol 2006;16:91–104. [DOI] [PubMed] [Google Scholar]
- 5. Pillas D, Marmot M, Naicker K, Goldblatt P, Morrison J, Pikhart H.. Social inequalities in early childhood health and development: a European-wide systematic review. Pediatr Res 2014;76:418–24. [DOI] [PubMed] [Google Scholar]
- 6. Marmot M, Allen J, Bell R, Bloomer E, Goldblatt P.. WHO European review of social determinants of health and the health divide. Lancet 2012;380:1011–29. [DOI] [PubMed] [Google Scholar]
- 7. Hertzman C, Boyce T.. How experience gets under the skin to create gradients in developmental health. Annu Rev Public Health 2010;31:329–47. [DOI] [PubMed] [Google Scholar]
- 8. Demetriou CA, van Veldhoven K, Relton C, Stringhini S, Kyriacou K, Vineis P.. Biological embedding of early-life exposures and disease risk in humans: a role for DNA methylation. Eur J Clin Invest 2015;45:303–32. [DOI] [PubMed] [Google Scholar]
- 9. Vineis P, Kelly-Irving M, Rappaport S, Stringhini S.. The biological embedding of social differences in ageing trajectories. J Epidemiol Community Health 2016;70:111–13. [DOI] [PubMed] [Google Scholar]
- 10. Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet 2012;13:484–92. [DOI] [PubMed] [Google Scholar]
- 11. Borghol N, Suderman M, McArdle W. et al. Associations with early-life socioeconomic position in adult DNA methylation. Int J Epidemiol 2012;41:62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tehranifar P, Wu HC, Fan X. et al. Early life socioeconomic factors and genomic DNA methylation in mid-life. Epigenetics 2013;8:23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Needham BL, Smith JA, Zhao W. et al. Life course socioeconomic status and DNA methylation in genes related to stress reactivity and inflammation: the multi-ethnic study of atherosclerosis. Epigenetics 2015;10:958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Subramanyam MA, Diez-Roux AV, Pilsner JR. et al. Social factors and leukocyte DNA methylation of repetitive sequences: the multi-ethnic study of atherosclerosis. PLoS One 2013;8:e54018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stringhini S, Polidoro S, Sacerdote C. et al. Life-course socioeconomic status and DNA methylation of genes regulating inflammation. Int J Epidemiol 2015;44:1320–30. [DOI] [PubMed] [Google Scholar]
- 16. McDade TW, Ryan C, Jones MJ. et al. Social and physical environments early in development predict DNA methylation of inflammatory genes in young adulthood. Proc Natl Acad Sci U S A 2017;114:7611–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lawn RB, Anderson EL, Suderman M. et al. Psychosocial adversity and socioeconomic position during childhood and epigenetic age: analysis of two prospective cohort studies. Hum Mol Genet 2018;27:1301–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fiorito G, Polidoro S, Dugue PA. et al. Social adversity and epigenetic aging: a multi-cohort study on socioeconomic differences in peripheral blood DNA methylation. Sci Rep 2017;7:16266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Terry MB, Ferris JS, Pilsner R. et al. Genomic DNA methylation among women in a multiethnic New York City birth cohort. Cancer Epidemiol Biomarkers Prev 2008;17:2306–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hughes A, Smart M, Gorrie-Stone T. et al. Socioeconomic position and DNA methylation age acceleration across the lifecourse. Am J Epidemiol 2018;187:2346–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Appleton AA, Armstrong DA, Lesseur C. et al. Patterning in placental 11-B hydroxysteroid dehydrogenase methylation according to prenatal socioeconomic adversity. PLoS One 2013;8:e74691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Obermann-Borst SA, Heijmans BT, Eilers PH. et al. Periconception maternal smoking and low education are associated with methylation of INSIGF in children at the age of 17 months. J Dev Orig Health Dis 2012;3:315–20. [DOI] [PubMed] [Google Scholar]
- 23. Obermann-Borst SA, Eilers PH, Tobi EW. et al. Duration of breastfeeding and gender are associated with methylation of the LEPTIN gene in very young children. Pediatr Res 2013;74:344–49. [DOI] [PubMed] [Google Scholar]
- 24. Perng W, Rozek LS, Mora-Plazas M. et al. Micronutrient status and global DNA methylation in school-age children. Epigenetics 2012;7:1133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. King K, Murphy S, Hoyo C.. Epigenetic regulation of newborns' imprinted genes related to gestational growth: patterning by parental race/ethnicity and maternal socioeconomic status. J Epidemiol Community Health 2015;69:639–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coker ES, Gunier R, Huen K, Holland N, Eskenazi B.. DNA methylation and socioeconomic status in a Mexican-American birth cohort. Clin Epigenetics 2018;10:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Javed R, Chen W, Lin F, Liang H.. Infant's DNA methylation age at birth and epigenetic aging accelerators. Biomed Res Int 2016;2016:4515928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Herbstman JB, Wang S, Perera FP. et al. Predictors and consequences of global DNA methylation in cord blood and at three years. PLoS One 2013;8:e72824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ng JW, Barrett LM, Wong A, Kuh D, Davey Smith G, Relton CL.. The role of longitudinal cohort studies in epigenetic epidemiology: challenges and opportunities. Genome Biol 2012;13:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Christensen BC, Houseman EA, Marsit CJ. et al. Aging and environmental exposures alter tissue-specific DNA methylation dependent upon CpG island context. PLoS Genet 2009;5:e1000602.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang D, Liu X, Zhou Y. et al. Individual variation and longitudinal pattern of genome-wide DNA methylation from birth to the first two years of life. Epigenetics 2012;7:594–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Birney E, Davey Smith G, Greally JM.. Epigenome-wide association studies and the interpretation of disease -Omics. PLoS Genet 2016;12:e1006105.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Relton CL, Gaunt T, McArdle W. et al. Data Resource Profile: Accessible Resource for Integrated Epigenomic Studies (ARIES). Int J Epidemiol 2015;44:1181–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fraser A, Macdonald-Wallis C, Tilling K. et al. Cohort Profile: The Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol 2013;42:97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boyd A, Golding J, Macleod J. et al. Cohort Profile: The ‘children of the 90s’—the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol 2013;42:111–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Office of population Censuses & Surveys. Standard Occupational Classification. London: HM Stationery Office, 1991. [Google Scholar]
- 37. Janssen BG, Madhloum N, Gyselaers W. et al. Cohort Profile: The ENVIRonmental influence ON early AGEing (ENVIRONAGE): a birth cohort study. Int J Epidemiol 2017;46:1386–87m. [DOI] [PubMed] [Google Scholar]
- 38. Ferrari S, Cribari-Neto F.. Beta regression for modelling rates and proportions. J Appl Stat 2004;31:799–815. [Google Scholar]
- 39. Strimmer K. fdrtool: a versatile R package for estimating local and tail area-based false discovery rates. Bioinformatics 2008;24:1461–62. [DOI] [PubMed] [Google Scholar]
- 40. Burris HH, Baccarelli AA, Byun HM. et al. Offspring DNA methylation of the aryl-hydrocarbon receptor repressor gene is associated with maternal BMI, gestational age, and birth weight. Epigenetics 2015;10:913–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nilormee O, Lockett GA, Iqbal S, Holloway JW, Arshad SH, Karmaus W.. Cord blood DNA methylation of Treg cytokine genes differs with parity. J Allergy Clin Immunol 2015;135:AB99. [Google Scholar]
- 42. Schroeder JW, Conneely KN, Cubells JC. et al. Neonatal DNA methylation patterns associate with gestational age. Epigenetics 2011;6:1498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yousefi P, Huen K, Dave V, Barcellos L, Eskenazi B, Holland N.. Sex differences in DNA methylation assessed by 450 K BeadChip in newborns. BMC Genomics 2015;16:911.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Markunas CA, Wilcox AJ, Xu Z. et al. Maternal age at delivery is associated with an epigenetic signature in both newborns and adults. PLoS One 2016;11:e0156361.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu X, Chen Q, Tsai HJ. et al. Maternal preconception body mass index and offspring cord blood DNA methylation: exploration of early life origins of disease. Environ Mol Mutagen 2014;55:223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Joubert BR, Håberg SE, Nilsen RM. et al. 450K epigenome-wide scan identifies differential DNA methylation in newborns related to maternal smoking during pregnancy. Environ Health Perspect 2012;120:1425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mandal C, Halder D, Jung KH, Chai YG.. Gestational alcohol exposure altered DNA methylation status in the developing fetus. Int J Mol Sci 2017, Jun 28. doi: 10.3390/ijms18071386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Houseman EA, Accomando WP, Koestler DC. et al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics 2012;13:86.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sharp GC, Lawlor DA, Richmond RC. et al. Maternal pre-pregnancy BMI and gestational weight gain, offspring DNA methylation and later offspring adiposity: findings from the Avon Longitudinal Study of Parents and Children. Int J Epidemiol 2015;44:1288–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Joubert BR, Felix JF, Yousefi P. et al. DNA methylation in newborns and maternal smoking in pregnancy: genome-wide consortium meta-analysis. Am J Hum Genet 2016;98:680–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sharp GC, Arathimos R, Reese SE. et al. Maternal alcohol consumption and offspring DNA methylation: findings from six general population-based birth cohorts. Epigenomics 2018;10:27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Galobardes B, Lynch J, Davey Smith G.. Measuring socioeconomic position in health research. Br Med Bull 2007;81–82:21–37. [DOI] [PubMed] [Google Scholar]
- 53. Kramer MS, Seguin L, Lydon J, Goulet L.. Socio-economic disparities in pregnancy outcome: why do the poor fare so poorly? Paediatr Perinat Epidemiol 2000;14:194–210. [DOI] [PubMed] [Google Scholar]
- 54. Casas M, Cordier S, Martinez D. et al. Maternal occupation during pregnancy, birth weight, and length of gestation: combined analysis of 13 European birth cohorts. Scand J Work Environ Health 2015;41:384–96. [DOI] [PubMed] [Google Scholar]
- 55. Birks L, Casas M, Garcia AM. et al. Occupational exposure to endocrine-disrupting chemicals and birth weight and length of gestation: a European meta-analysis. Environ Health Perspect 2016;124:1785–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. van Beukering MD, van Melick MJ, Mol BW, Frings-Dresen MH, Hulshof CT.. Physically demanding work and preterm delivery: a systematic review and meta-analysis. Int Arch Occup Environ Health 2014;87:809–34. [DOI] [PubMed] [Google Scholar]
- 57. Palmer KT, Bonzini M, Harris EC, Linaker C, Bonde JP.. Work activities and risk of prematurity, low birth weight and pre-eclampsia: an updated review with meta-analysis. Occup Environ Med 2013;70:213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Spycher BD, Lupatsch JE, Huss A. et al. Parental occupational exposure to benzene and the risk of childhood cancer: a census-based cohort study. Environ Int 2017;108:84–91. [DOI] [PubMed] [Google Scholar]
- 59. Pinilla J, Lopez-Valcarcel BG, Urbanos-Garrido RM.. Estimating direct effects of parental occupation on Spaniards’ health by birth cohort. BMC Public Health 2017;17:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Farrow A, Shea KM, Little RE.. Birthweight of term infants and maternal occupation in a prospective cohort of pregnant women. The ALSPAC Study Team. Occup Environ Med 1998;55:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shea KM, Farrow A, Little R.. An investigation of the effect of paternal occupation group at conception on birth weight and gestational age. ALSPAC Study Team of Pregnancy and Childhood. Am J Ind Med 1997;31:738–43. [DOI] [PubMed] [Google Scholar]
- 62. Bloomberg L, Meyers J, Braverman MT.. The importance of social interaction: a new perspective on social epidemiology, social risk factors, and health. Health Educ Q 1994;21:447–63; discussion 65–69. [DOI] [PubMed] [Google Scholar]
- 63. Parker JD, Schoendorf KC, Kiely JL.. Associations between measures of socioeconomic status and low birth weight, small for gestational age, and premature delivery in the United States. Ann Epidemiol 1994;4:271–78. [DOI] [PubMed] [Google Scholar]
- 64. Davey TM, Cameron CM, Ng SK, McClure RJ.. The relationship between maternal education and child health outcomes in urban Australian children in the first 12 months of life. Matern Child Health J 2015;19:2501–11. [DOI] [PubMed] [Google Scholar]
- 65. Karlsson Linner R, Marioni RE, Rietveld CA. et al. An epigenome-wide association study meta-analysis of educational attainment. Mol Psychiatry 2017;22:1680–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Herich LC, Cuttini M, Croci I. et al. Maternal education is associated with disparities in breastfeeding at time of discharge but not at initiation of enteral feeding in the neonatal intensive care unit. J Pediatr 2017;182:59–65.e7. [DOI] [PubMed] [Google Scholar]
- 67. Forshaw J, Gerver SM, Gill M, Cooper E, Manikam L, Ward H.. The global effect of maternal education on complete childhood vaccination: a systematic review and meta-analysis. BMC Infect Dis 2017;17:801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Champagne FA. Epigenetics and developmental plasticity across species. Dev Psychobiol 2013;55:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Abraham M, Alramadhan S, Iniguez C. et al. A systematic review of maternal smoking during pregnancy and fetal measurements with meta-analysis. PLoS One 2017;12:e0170946.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pereira PP, Da Mata FA, Figueiredo AC, de Andrade KR, Pereira MG.. Maternal active smoking during pregnancy and low birth weight in the Americas: a systematic review and meta-analysis. Nicotine Tob Res 2017;19:497–505. [DOI] [PubMed] [Google Scholar]
- 71. Stamnes Koepp UM, Frost Andersen L, Dahl-Joergensen K, Stigum H, Nass O, Nystad W.. Maternal pre-pregnant body mass index, maternal weight change and offspring birthweight. Acta Obstet Gynecol Scand 2012;91:243–49. [DOI] [PubMed] [Google Scholar]
- 72. Richmond RC, Simpkin AJ, Woodward G. et al. Prenatal exposure to maternal smoking and offspring DNA methylation across the lifecourse: findings from the Avon Longitudinal Study of Parents and Children (ALSPAC). Hum Mol Genet 2015;24:2201–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Beach SR, Dogan MV, Brody GH, Philibert RA.. Differential impact of cumulative SES risk on methylation of protein-protein interaction pathways as a function of SLC6A4 genetic variation in African American young adults. Biol Psychol 2014;96:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Flanagan JM, Brook MN, Orr N. et al. Temporal stability and determinants of white blood cell DNA methylation in the breakthrough generations study. Cancer Epidemiol Biomarkers Prev 2015;24:221–29. [DOI] [PubMed] [Google Scholar]
- 75. Acevedo N, Reinius LE, Vitezic M. et al. Age-associated DNA methylation changes in immune genes, histone modifiers and chromatin remodeling factors within 5 years after birth in human blood leukocytes. Clin Epigenetics 2015;7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Alisch RS, Barwick BG, Chopra P. et al. Age-associated DNA methylation in pediatric populations. Genome Res 2012;22:623–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Martino D, Loke YJ, Gordon L. et al. Longitudinal, genome-scale analysis of DNA methylation in twins from birth to 18 months of age reveals rapid epigenetic change in early life and pair-specific effects of discordance. Genome Biol 2013;14:R42.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Martino DJ, Tulic MK, Gordon L. et al. Evidence for age-related and individual-specific changes in DNA methylation profile of mononuclear cells during early immune development in humans. Epigenetics 2011;6:1085–94. [DOI] [PubMed] [Google Scholar]
- 79. Urdinguio RG, Torro MI, Bayon GF. et al. Longitudinal study of DNA methylation during the first 5 years of life. J Transl Med 2016;14:160.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Simpkin AJ, Suderman M, Gaunt TR. et al. Longitudinal analysis of DNA methylation associated with birth weight and gestational age. Hum Mol Genet 2015;24:3752–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Power C, Kuh D, Morton S.. From developmental origins of adult disease to life course research on adult disease and aging: insights from birth cohort studies. Annu Rev Public Health 2013;34:7–28. [DOI] [PubMed] [Google Scholar]
- 82. Lai JP, Sandhu DS, Shire AM, Roberts LR.. The tumor suppressor function of human sulfatase 1 (SULF1) in carcinogenesis. J Gastrointest Cancer 2008;39:149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zahraei M, Sheikhha MH, Kalantar SM. et al. The association of arylendosulfatase 1 (SULF1) gene polymorphism with recurrent miscarriage. J Assist Reprod Genet 2014;31:157–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Narita K, Staub J, Chien J. et al. HSulf-1 inhibits angiogenesis and tumorigenesis in vivo. Cancer Res 2006;66:6025–32. [DOI] [PubMed] [Google Scholar]
- 85. Wang X, Falkner B, Zhu H. et al. A genome-wide methylation study on essential hypertension in young African American males. PLoS One 2013;8:e53938.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Munafò MR, Tilling K, Taylor AE, Evans DM, Davey Smith G.. Collider scope: when selection bias can substantially influence observed associations. Int J Epidemiol 2018;47:226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Maleszka R, Mason PH, Barron AB.. Epigenomics and the concept of degeneracy in biological systems. Brief Funct Genomics 2014;13:191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Rutter M. Achievements and challenges in the biology of environmental effects. Proc Natl Acad Sci U S A 2012;109(Suppl 2):17149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Adalsteinsson BT, Gudnason H, Aspelund T. et al. Heterogeneity in white blood cells has potential to confound DNA methylation measurements. PLoS One 2012;7:e46705.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Castagne R, Delpierre C, Kelly-Irving M. et al. A life course approach to explore the biological embedding of socioeconomic position and social mobility through circulating inflammatory markers. Sci Rep 2016;6:25170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.