Abstract
T lymphocyte proliferation and differentiation are controlled by signaling pathways initiated by the T cell antigen receptor. Here we explore how key serine-threonine kinases and their substrates mediate T cell signaling and coordinate T cell metabolism to meet the metabolic demands of participating in an immune response.
T lymphocyte proliferation and differentiation are controlled by signaling pathways initiated by the T cell antigen receptor (TCR). The initial events in TCR signaling are mediated by cytosolic tyrosine kinases and adaptors that function to couple the TCR to a network of serine-threonine kinases that propagate the signal from the cell membrane to the nucleus and determine the transcriptional changes that support effector T cell differentiation. In this context, the immune activation of T lymphocytes is a metabolically demanding process, and emerging evidence suggests that serine-threonine kinases that control the metabolic programs that determine T cell function are themselves regulated by extracellular nutrient availability and the cytokine milieu. Here we describe key serine-threonine kinases that mediate TCR signaling and explore how serine kinase networks can coordinate T cell metabolism to meet the metabolic demands of participation in an immune response.
Diacylglycerol-regulated serine-threonine kinases in T cells
A key event in the TCR signaling cascade is phospholipase C-γ (PLC-γ)-mediated hydrolysis of phosphatidylinositol-(4,5)-bisphosphate (PtdIns(4,5)P2) at the cell membrane, which generates the second messengers inositol-(1,4,5)-trisphosphate (Ins(1,4,5)P3) and polyunsaturated diacyglycerols (DAGs) (Fig. 1). T lymphocytes express multiple DAG-binding serine-threonine kinases in the protein kinase C (PKC) family1,2. The importance of these enzymes for T cell activation was first recognized after experiments showing that phorbol esters, pharmacological mimics of DAG that activate PKCs, could regulate cytokine production and TCR expression in T cells3,4. Another observation that drew the attention of immunologists to PKCs was that the PKC-θ isozyme selectively polarized to the contact area between a T cell and an antigen-presenting cell (APC) loaded with antigen. This formed the basis for the concept of the now well-studied immunological synapse. In this context5,6, it is now recognized the recruitment of PKC-θ to the immune synapse is regulated by other PKC isozymes, notably PKC-ε and PKC-η7. It is also now well known that PKCs are not the sole targets for DAG signaling in T cells. Other TCR-regulated DAG binding proteins in T cells are guanine nucleotide–exchange factors (RasGRPs)8 and the protein kinase D (PKD) serine-threonine kinases9.
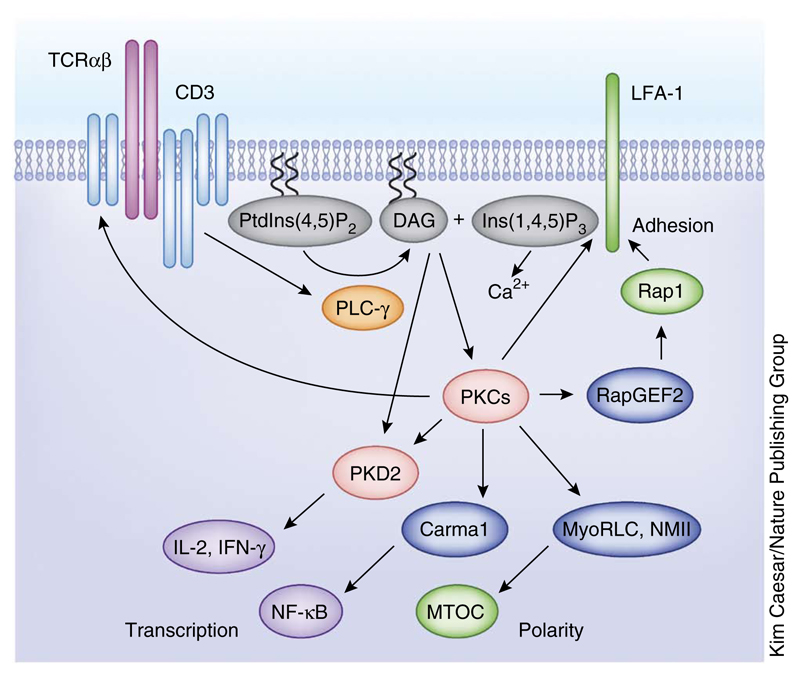
Figure 1.
DAG-regulated serine-threonine kinases in T cells. TCR triggering activates PLC-γ, which cleaves PtdIns(4,5)P2 to generate two key second messenger molecules, DAG and Ins(1,4,5)P3. The accumulation of DAG in the plasma membrane mediates the activation kinases PKC and PKD. PKC substrates regulate different aspects of the T cell activation process, such as cell adhesion, establishment of polarity and transcription. MTOC, microtubule organizing center; MyoRLC, myosin regulatory light chain; NMII, nonmuscle myosin.
DAG probes have been used to define the spatial dynamics of DAG production in T lymphocytes responding to antigen presented by major histocompatibility complex (MHC) molecules on the surface of APCs10–13. When T cells make contacts with antigen-loaded APCs, a sharp gradient of DAG rapidly develops, resulting in an accumulation of this lipid in a focused area of T cell membrane, at the point of contact with the APC. This polarized concentration of DAG permits the localized recruitment and activation of PKCs and PKDs7,11,13. The rate-limiting step for PKC activation by TCR triggering is thus production of DAG and the localization of PKCs to the plasma membrane. PKC activity is also dependent on the phosphorylation of a conserved T-loop motif within the catalytic domain of the enzyme by phosphoinositide-dependent protein kinase 1 (PDK1)2. However, there is high basal activity of PDK1 in T cells, and the T-loop phosphorylation of PKCs is a priming phosphorylation that occurs before TCR engagement14. In addition, the PKC α, βI and βIIm isoforms have a calcium-binding site, but the concentrations of calcium found in quiescent T cells (70–100 nM) are sufficient to support enzyme catalytic activity. Mature T loop–phosphorylated PKCs and/or calcium-bound PKCs are thus dependent on DAG and phospholipid binding at membranes to achieve full catalytic function.
Several substrates identified for PKCs explain why these kinases are important for TCR signal transduction (Fig. 1). For example, PKC-θ phosphorylates caspase recruitment domain–containing MAGUK protein 1 (Carma1), allowing this scaffolding protein to bind to signaling protein Bcl-10 and the IκB kinase IKKγ, promoting the assembly of the IKK signaling complex and coupling the TCR to the activation of the transcription factor NF-κB15,16. PKCs also have an essential role in effector cytotoxic T lymphocytes (CTLs) for antigen receptor–induced reorientation of the centrosome13,17 and the polarized exocytosis of the cytolytic granules that kill target cells18. The relevant PKC substrate for centrosome polarization is the myosin regulatory light chain, which controls the localization of the nonmuscle myosin motor complex17. PKCs also allow the TCR to control T cell adhesion and cell motility. The phosphorylation of the guanine nucleotide exchange protein RapGEF2 by PKC-θ thus triggers activation of the guanine nucleotide–binding protein Rap1 that in turn activates the integrin LFA-1 (ref. 19) (Fig. 1). PKCs also phosphorylate the β-chain of LFA-1 on sites that regulate the interaction of LFA-1 with cytoskeletal proteins and controls LFA-1–mediated cell adhesion20. DAG-PKC signaling cascades thus regulate cell adhesion and cell migration at multiple levels and control T cell positioning within lymphoid tissues, T cell transmigration across endothelial membranes and T cell contacts with APCs.
Another DAG-binding serine-threonine kinase expressed abundantly in T cells is PKD2. This kinase is rapidly activated by TCR engagement in a response that is initiated directly by antigen receptor–regulated increases in DAG binding to an N-terminal regulatory domain in PKD2 (ref. 21). The activation of PKD2 is then completed and stabilized by PKC-mediated phosphorylation of Ser707 and Ser711 within the activation loop of the PKD2 catalytic domain22,23. The allosteric regulation of PKD2 by PKC phosphorylation affords a mechanism for this molecule to act as a signal amplifier for PKC. PKDs are substrates for most classical and novel PKCs and are thus regulated by antigen receptors irrespective of the repertoire of PKC isoform expression in a particular cell9. Importantly, PKC-phosphorylated PKD2 is catalytically active in the absence of DAG binding and hence does not need to be localized to the plasma membrane to remain active. Indeed, during the sustained response to antigen receptor engagement, phosphorylated and active PKD2 molecules are found localized in the cytosol11. The allosteric activation of PKD2 by PKC-mediated phosphorylation thus allows this kinase to transduce TCR-mediated increases in DAG from the cell membrane to the cytosol. The importance of PKD2 as a PKC substrate and an effector of DAG-PKC signaling in T cells has been demonstrated in vivo through T cell–mediated immune responses in mice expressing PKD2 variants that cannot be phosphorylated by PKC (S707A and S711A in PKD). These PKD-mutant mice showed a striking reduction in the ability of the TCR to induce the production of proinflammatory cytokines such as interleukin 2 (IL-2) and interferon-γ (IFN-γ)24,25 (Fig. 1).
Ras-regulated serine-threonine kinase pathways
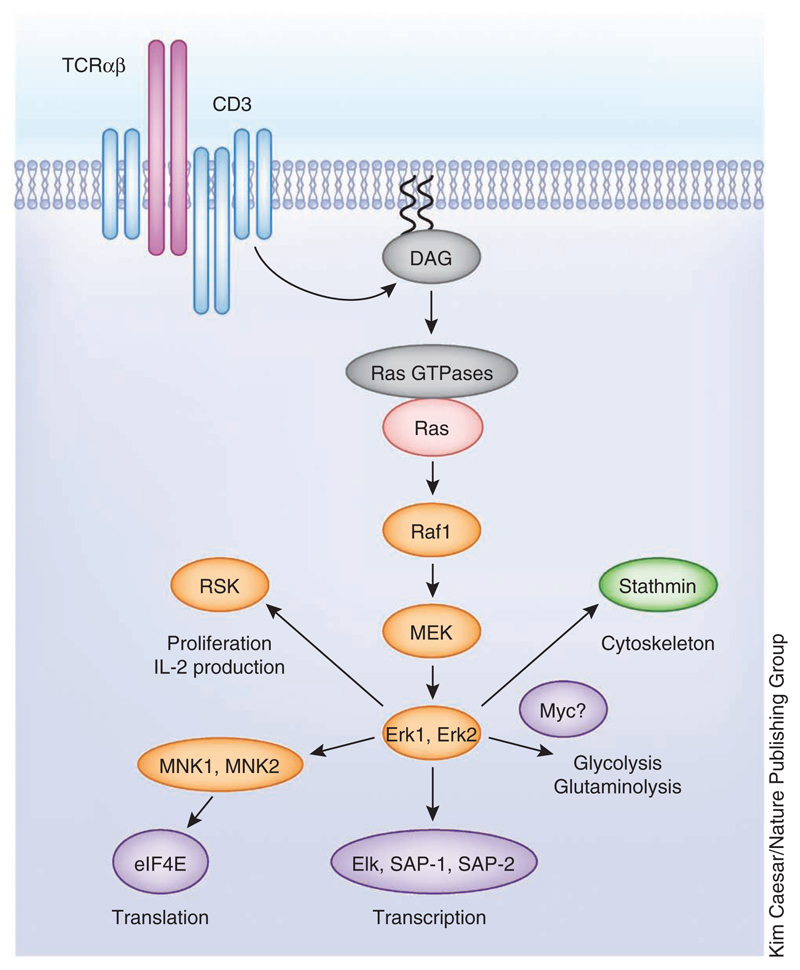
Triggering of the TCR is known to activate serine-threonine kinase signaling cascades regulated by Ras guanine nucleotide–binding proteins. TCR-mediated DAG production controls Ras activity by regulating the intracellular localization and PKC-mediated phosphorylation of RasGRPs8. Ras rapidly cycles between GDP-bound (inactive) and GTP-bound (active) states. When active, Ras proteins bind to the serine-threonine kinase Raf-1, initiating the mitogen-activated protein kinase (MAPK) cascade that phosphorylates and activates the serine-threonine kinases Erk1 and Erk2 (Fig. 2). The Ras-Raf-Erk1-Erk2 pathway is important for TCR function during positive selection in the thymus and in peripheral T cells26,27. The importance of Erk1 and Erk2 in T cells is partially explained by their ability to phosphorylate transcription factors and thus regulate their activity. For example, Erk1 and Erk2 activate the ternary complex factor subfamily of ETS-domain transcription factors Elk-1, SAP-1 and SAP-2, which control expression of immediate early genes (such as those encoding c-Fos, Egr1 and Egr3) in T cells28. The Erk-mediated regulation of c-Fos expression contributes to the activator protein-1 (AP-1) transcription complex formed by the transcription factors c-Fos and Jun29. The ternary complex factors also control the transcription of dual-specificity (threonine/tyrosine) MAPK phosphatases that initiate a negative regulatory feedback pathway that acts to limit the duration and magnitude of Erk activity30. Erk1 and Erk2 also have a role in regulating microtubule remodeling through the phosphorylation and regulation of stathmin31. However, it should be emphasized that Erk1 and Erk2 kinases are not the endpoint of the kinase signaling cascade. In this context, the 90-kilodalton ribosomal protein S6 kinase (RSK) is an Erk1 and Erk2 substrate that has been implicated in cell cycle progression and cytokine production in T cells32. The initiating step for RSK activation is thus Erk1- and Erk2-mediated phosphorylation of Ser369, Thr365 and Thr577 in its C-terminal catalytic domain. The activated C-terminal catalytic domain of RSK then autophosphorylates its Ser386 to create a docking site for PDK1, which then phosphorylates Ser227 in the N-terminal RSK kinase domain, thereby activating the enzyme33. In addition, Erk1 and Erk2 phosphorylate and activate the MAPK signal–integrating kinases 1 and 2 (MNK1 and MNK2). Erk1 and Erk2 phosphorylate regulatory sites outside the T activation loop in MNK1 and MNK2 that are required for efficient activation34,35. When active, MNK1 and MNK2 regulate protein translation through the phosphorylation of the eukaryotic translation initiation factor eIF4E35,36. The ability of DAG to couple the TCR to diverse serine-threonine kinases is thus a mechanism that allows the TCR to coordinate the regulation of key processes for T cell activation such as transcription, translation and proliferation (Figs. 1 and 2).
Figure 2.
Ras-regulated serine-threonine kinase pathways. TCR-mediated DAG generation activates Ras GTPases, which promote Ras activation and start the MAPK signaling cascades required for Erk1 and Erk2 activation. Erk1 and Erk2 kinase activity controls T cell activation through different substrates that control cytoskeleton remodeling, cell metabolism, transcription, translation and proliferation.
The PtdIns-(3,4,5)P3-regulated serine-threonine kinase Akt
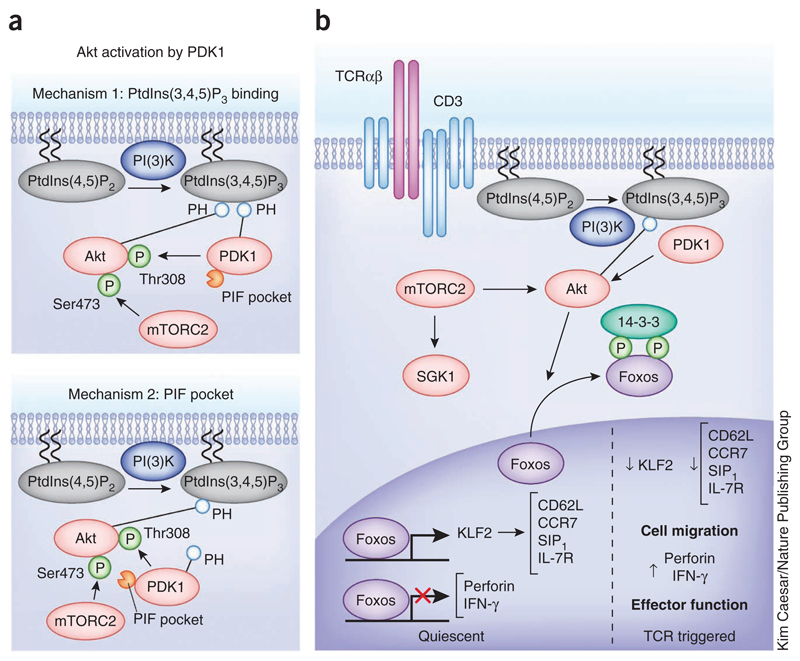
One other lipid second messenger that couples the TCR to pathways of serine-threonine phosphorylation is phosphatidylinositol-(3,4,5)-trisphosphate (PtdIns(3,4,5)P3)37. In naive T cells the intracellular abundance of PtdIns(3,4,5)P3 is low, but it increases rapidly after triggering of the TCR38,39. T cells can sustain production of large amounts of PtdIns(3,4,5)P3 for many hours in a response that requires continual engagement of the TCR, costimulatory molecules such as CD28 and continual activation of class 1 phosphatidylinositol-3-OH kinase (PI(3)K) p110δ38,40,41. One crucial function for PtdIns(3,4,5)P3 is that of regulating the activity of the serine-threonine protein kinase B, also known as Akt. A rate-limiting step for Akt activation is phosphorylation of Thr308 within the Akt catalytic domain by PDK1. PtdIns(3,4,5)P3 binding to the Akt pleckstrin homology (PH) domain causes a conformational change that allows PDK1 to phosphorylate Thr308 within the Akt catalytic domain and activate the enzyme42. A key step in Akt activation is the colocalization of PDK1 and Akt, and two mechanisms ensure that this happens efficiently. One is mediated by high-affinity PtdIns(3,4,5)P3 binding to the PDK1 PH domain, which is not essential for PDK1 catalytic activity but promotes translocation of PDK1 to the plasma membrane where it can colocalize with Akt43 (Fig. 3a). The second mechanism is controlled by Akt phosphorylation on its C-terminal Ser473 by the kinase complex mTORC2, which allows phosphorylated Akt to dock to a substrate-docking motif on PDK1 termed the PIF pocket44 (Fig. 3a).
Figure 3.
The PtdIns(3,4,5)P3-regulated serine-threonine kinase Akt. (a) Mechanisms of Akt activation by PDK1. Two described mechanisms for colocalization of Akt and PDK1; one is dependent on PDK1 binding to PtdIns(3,4,5)P3 and the other is dependent on PDK1 recruitment to Akt via mTORC2-mediated Ser473 phosphorylation. (b) Role of Akt in TCR signal transduction. Akt activation by PDK1 has a key role in T cell activation, regulating the subcellular localization and function of the Foxo family of transcription factors. Foxos regulate the expression of key molecules that control T cell trafficking and effector functions.
mTORC2 comprises the mTOR serine-threonine kinase in complex with RICTOR, MLST8 and mSIN1 (ref. 45). The signals that control the activity of mTORC2 in T cells are not understood, but the PI(3)K sensitivity of TCR-induced phosphorylation of Akt at Ser473, its mTORC2 site, suggests that mTORC2 function is regulated downstream of PI(3)K. Thus mTORC2 has a vital role in controlling the hydrophobic motif phosphorylation of other members of the AGC family of protein kinases (protein kinase A, protein kinase G and PKC), which need to colocalized and phosphorylated by PDK1 (ref. 14). For example, mTORC2 can couple the TCR to serum- and glucocorticoid-induced protein kinase 1 (SGK1)46, and this is important for the differentiation of type 2 helper T (TH2)47 and TH17 cells48,49 (Fig. 3b).
How important is mTORC2 for Akt activation in T cells? In T cells lacking mTORC2 complexes, there is no TCR-induced phosphorylation of Ser473 in Akt; however, phosphorylation of Thr308 in Akt is lower but not ablated, which suggests that phosphorylation of this residue is not obligatory for Akt activation50. Akt activity is also low but not ablated in T cells that express a PDK1 mutant that cannot bind PtdIns(3,4,5)P3 (ref. 51). Collectively, these results support the argument that there are two mechanisms by which T cells can colocalize PDK1 and Akt: one is dependent on mTORC2 and the PDK1 PIF pocket, and the other is dependent on PtdIns(3,4,5)P3-mediated colocalization of PDK1 and Akt (Fig. 3a).
The role of Akt in TCR signal transduction
The paradigmatic role of Akt in many cells is that it functions to control cell metabolism. Numerous experiments using PI(3)K inhibitors in different T cell populations support the idea that PI(3)K, and hence Akt, are important for glucose uptake, protein synthesis or both52–54. However, many of the PI(3)K inhibitors used in these studies, such as LY294002 and wortmanin, have off-target effects on other kinases, such as mTOR and PIMs55–57. Moreover, there is no direct evidence in mature T cells that Akt functions to couple the TCR to key metabolic programs. Indeed, PI(3)K and Akt direct the transcriptional program of T cells, but that they are not required for TCR-mediated initiation of glucose uptake or glycolysis in naive T cells58. There is also no evidence that Akt is essential for peripheral T cell survival or proliferation, which is inconsistent with the idea that this kinase links the TCR to metabolic programs58.
A major role for Akt in the context of TCR signaling is to control the phosphorylation and localization of the Foxo transcription factors, including Foxo1, Foxo3a and Foxo4. The Foxo transcription factors are nuclear and active in quiescent T cells, but when phosphorylated by Akt they exit the nucleus and form a complex with 14-3-3 proteins in the cytosol, thereby terminating their transcriptional activity59 (Fig. 3b). Foxos target genes in naive T cells, including genes encoding cytokines, cytokine receptors and transcription factors60–62. For example, Foxos drive expression of the α-chain of the receptor for IL-7, an essential homeostatic cytokine for T cells61,63. Foxo1 also induces expression of the transcription factor KLF2, which directly regulates the transcription of genes encoding chemokine receptors and adhesion molecules that control T cell trafficking51,61,64–66. TCR triggering causes T cells to activate Akt, which results in phosphorylation and inactivation of Foxos. The loss of Foxo transcriptional activity immediately causes loss of expression of KLF2 and of its target genes encoding L-selectin (CD62L), the chemokine receptor CCR7 and sphingosine 1-phosphate receptor S1P1 (refs. 61,67). CD62L is an adhesion molecule that is crucial for the transmigration of T cells across high endothelial venules, CCR7 controls lymphocyte entry and retention into secondary lymphoid tissues, and S1P1 controls T cell egress from lymph nodes68. Consequently, the loss of Foxos activity in TCR-triggered T cells changes the normal trafficking program of T cells61,69.
The role of Akt in T cells is not limited to controlling T cell trafficking. For example, a key role for Akt in CD8+ T cells is to promote effector cell differentiation by driving expression of cytolytic effector molecules and controlling the cytokine receptor profile of effector T cells58. Akt-regulated genes in CD8+ T cells include those encoding multiple granzymes, Fas ligand, the cytolytic effector perforin and IFN-γ (Ifng). It is also notable that Akt positively regulates expression of genes encoding the IL-12 receptor β-chain while negatively regulating expression of genes encoding IL-6 receptors and the CD70 coreceptor CD27 (refs. 58,70). Akt can thus act to couple the TCR to the transcriptional programs that control effector T cell differentiation and, indeed, is necessary for the TCR to initiate expression of key effector molecules in CTLs. For example, inhibition of Akt blocks TCR-mediated induction of IFN-γ expression in both naive CD8+ T cells and effector CTLs58. The molecular mechanism for the diverse effects of Akt on T cell transcriptional programs resides in the ability of nonphosphorylated Foxos to both transactivate and repress gene expression. For example, Foxos act as positive regulators in the context of Klf2 (encoding KLF2) and Il7r (encoding IL-7Rα), whereas in the context of Ifng, Foxos are repressive. Accordingly, in TCR-activated T cells, Akt-regulated nuclear export of Foxo transcription factors is required for IFN-γ production. Thus, in the context of TCR signaling, Akt regulates expression of cytolytic effector molecules and determines the repertoire of cytokine receptors, adhesion molecules and chemokine receptors expressed by T cell populations58,61,69.
Serine-threonine kinases control T cell metabolism
T cells use serine-threonine kinases to modulate the key metabolic programs that meet the demands of participating in an immune response. The metabolic reprogramming of immune-activated T cells is triggered by antigen receptors but sustained by costimulatory signals and inflammatory cytokines. TCR engagement initiates increases in glucose uptake and a glycolytic switch whereby activated T cells swap from metabolizing glucose primarily through oxidative phosphorylation to using the glycolytic pathway71. The activation of the Erk1 and Erk2 initiates this switch in glucose metabolism by regulating the expression of c-Myc, a key transcription factor for this process72–74 (Fig. 2). T cell activation also increases cellular amino acid uptake to support high levels of protein synthesis75. These TCR-regulated increases in amino acid uptake are not only required for protein synthesis; glutamine can be diverted into glutaminolysis. It is also important that T cells control the intracellular supply of leucine, because this controls the activity of the serine-threonine kinase complex mTORC1 (ref. 76). In this context, glutamine metabolism in T cells is mediated through Erk1 and Erk2, Akt and mTORC1 control of c-Myc expression72–74, whereas the uptake of leucine is mediated through system L amino acid transporters whose expression is controlled by calcium- and calcineurin-mediated signals75 (Figs. 2 and 4).
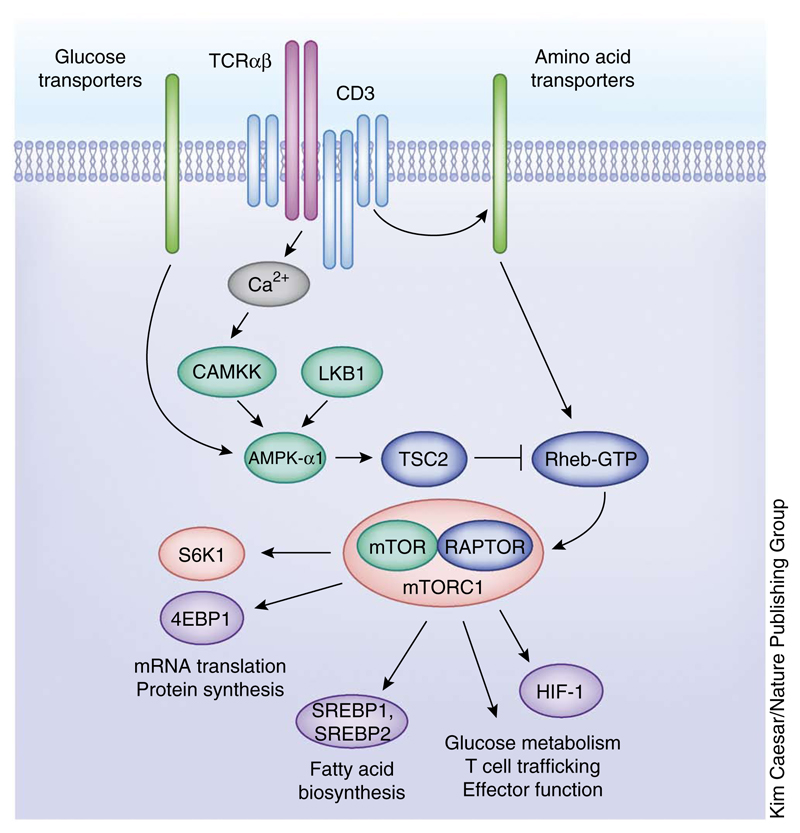
Figure 4.
Nutrient-sensing serine-threonine kinases in T cells. Cartoon depiction of the complex regulation of T cell metabolic pathways by different nutrient sensing serine-threonine kinases such as AMPK-α1 and the mTORC1 complex. Through the regulation of the expression or function of different transcription factors, mTORC1 controls key aspects of T cell function such as protein and fatty acid biosynthesis, glucose metabolism, T cell trafficking and effector function.
One kinase that is crucial for T cell metabolism is mTORC1. This protein complex is composed of mTOR and RAPTOR, MLST8, PRA40 and DEPTOR45,77. The mTORC1 complex is activated in response to TCR engagement and has two known direct substrates: p70 S6-kinase 1 and eIF4E-binding protein 1, which have selective roles in regulating mRNA translation, protein synthesis and ribosome biogenesis78,79 (Fig. 4). mTORC1 signaling also activates the transcription factors SREBP-1 and SREBP-2, which induce the expression of enzymes involved in de novo fatty acid and sterol biosynthesis79,80. Moreover, mTORC1 has a key role in regulating the translation of the hypoxia-inducible factor 1 (HIF-1) transcription factor complex. The HIF-1 pathway functions to couple mTORC1 to a diverse transcriptional program that sustains expression of glucose transporters and multiple rate-limiting glycolytic enzymes81. The mTORC1–HIF-1 pathway is thus not required to initiate glucose metabolism in TCR-activated cells, but it is required to sustain glucose metabolism and glycolysis in activated T cells74,81. HIF-1 also controls effector T cell differentiation by coupling mTORC1 to multiple transcriptional programs that control expression of inflammatory cytokines, cytolytic effector molecules and essential chemokine and adhesion receptors that regulate T cell trafficking81–83.
Nutrient-sensing serine-threonine kinases in T cells
The discussion above focuses on how pathways of serine-threonine phosphorylation control T cell metabolism. However, there are key serine-threonine kinases that respond to cellular energy demands to modulate T cell responses. In simple terms, these kinases sense rates of nutrient transport into T cells, and the activity of these nutrient sensors affects T cell activation and function. For example, evolutionarily conserved pathways whereby mTORC1 activity is regulated by glucose and amino acid availability45,84 exist in T cells75,85,86. T cells also express the AMP-activated kinase AMPK-α1, which stimulates the conservation and production of ATP in response to metabolic stress87,88. AMPK-α1 is activated in response to TCR triggering via a pathway mediated by calcium and calcium-calmodulin-dependent protein kinase kinase 2 (CAMKK)87. Moreover, AMPK-α1 can also be phosphorylated and activated in T cells by liver kinase B1 (LKB1) in response to changes in glucose metabolism that increase cellular AMP/ATP ratios89,90. The LKB1-AMPK-α1 pathway thus functions as a sensitive sensor of glucose transport in T cells and one of its functions is to regulate mTORC1 activity90.
Triggering of the TCR activates mTORC1, and the molecular basis for this process is not fully understood but is known to be dependent on TCR regulation of the expression of system L amino acid transporters, which ensure the effective transport of leucine into activated T cells75. How does leucine control mTORC1 activity in mammalian cells? The current model holds that amino acid sensing regulates the recruitment of mTORC1 to lysosomal membranes76. A crucial step is amino acid–dependent activation of the guanine nucleotide exchange activity of the Ragulator complex. This process results in the accumulation of active GTP-bound Rag GTPases, which then recruit mTORC1 to the lysosomal surface, where the complex interacts with Rheb-GTP, a potent direct stimulator of mTORC1 kinase activity76. In T cells, the rate of intracellular leucine uptake in T cells is determined by TCR control of system L amino acid transporter expression; that is, the TCR has ‘assumed’ control of the evolutionarily conserved pathway whereby amino acids control mTORC1 activity by controlling the transport of leucine into the T cell75. In a similar context, the activity of mTORC1 is controlled by glutamine availability, and the TCR also controls mTORC1 activity by regulating the expression of the glutamine transporter ASCT2 and hence controlling the cellular uptake of glutamine86.
Most models of lymphocyte signal transduction invariably link mTORC1 activation to PI(3)K and Akt. The reason for this link is that Akt can phosphorylate tuberous sclerosis complex 2 (TSC2), a Rheb GTPase-activating protein (GAP) that negatively regulates the accumulation of the Rheb-GTP complexes required to activate mTORC1 (refs. 45,77). It was originally proposed that phosphorylation of TSC2 by Akt inhibited its GAP activity and allowed the accumulation of Rheb-GTP. It was also proposed that Akt phosphorylation promoted TSC2 binding to 14-3-3 proteins to cause the sequestration of TSC2 in the cytosolic fraction away from lysosomes and, hence, away from Rheb91,92. It is clear that deletion of TSC complex in T lymphocytes makes these cells hyper-responsive for TCR-mediated activation of mTORC1 (ref. 93). However, although TSC2 phosphorylation frequently correlates with an increase in mTORC1 activation, it is not yet proven that Akt-mediated phosphorylation of TSC2 leads to its repression94. Hence there is no direct biochemical evidence that the regulation of phosphorylation of TSC2 by Akt suppresses its GAP activity toward Rheb. Similarly, the regulation of TSC2 localization to the lysosome by Akt has yet to be proven to be a key regulatory event in T cells. Indeed, selective inhibition of PtdIns(3,4,5)P3 production and activation of Akt does not compromise mTORC1 activation in T cells81. The GTP and GDP loading of Rheb, which is controlled by the TSC complex, is thus essential for mTORC1 activity in T cells50, and the TSC complex acts as a negative regulator of mTORC1 activation93. However, TCR control of amino acid signaling to mTORC1 seems to dominate mTORC1 regulation rather than TCR control of TSC via Akt75,81.
Another key regulatory input that controls mTORC1 is mediated by AMPK-α145,77. AMPKα1 acts as a crucial glucose sensor in activated effector T cells and monitors T cell energy status to restrict mTORC1 activity to metabolically sufficient T cells90. AMPK-α1 can regulate mTORC1 activity because it can phosphorylate and activate TSC2, the Rheb GAP95. Accordingly, the TCR will be unable to trigger mTORC1 activity unless it can induce expression of glucose transporters and allow sufficient glucose supply for cells to maintain cellular AMP/ATP ratios to restrict AMPKα1 activity and allow the accumulation of active Rheb-GTP. The restraint of mTORC1 activity by AMPK-α1 seems to be important in the effector-to-memory transition of T cells. Effector T cells need to revert from a metabolically active state to a quiescent catabolic state to produce memory T cells. The regulation of mTORC1 activity by AMPK-α1 seems to be a pivotal control switch for this transition90,96.
Complexities and conclusions
Here we present a relatively linear, simplistic view of how a few conserved serine kinases and their substrates are activated by the TCR and regulate T cell function. However, high-resolution quantitative mass-spectrometry experiments are beginning to reveal the real scale and complexity of the TCR-regulated serine-threonine phosphoproteome. Phosphoproteomic experiments have been performed using clonal cytotoxic T cell populations triggered with cognate peptide-MHC complexes and primary and transformed human T cells polyclonally activated with antibodies to the TCR complex97–99. These experiments have identified thousands of serine-threonine–phosphorylated proteins in T cells and shown that triggering of TCR complexes induces increases or decreases in the phosphorylation of ~20% of the serine-threonine phosphoproteome of a T cell population97,99. One key insight from these studies is that there is a complicated web of basal protein phosphorylation in T cells before TCR engagement, including links between serine-threonine kinases and chromatin regulators, that maintains the transcriptional programs permissive for TCR function. These data sets also afford insights about the scope of TCR-regulated phosphorylation. The TCR controls phosphorylation of proteins that control all the significant events associated with T cell activation, such as transcriptional regulation, cytoskeleton reorganization, the cell cycle, survival and cell growth97. It is noteworthy that transcription factors and chromatin regulators are overrepresented.
The power of unbiased global approaches to study protein phosphorylation is that they unequivocally identify which protein isoforms or splice variants are expressed in a T cell population and which isoforms are regulated by TCRs. Unbiased global phosphoproteomic approaches can yield new insights about known TCR signaling pathways, but the real power resides in their potential to identify TCR-regulated phosphorylations that can direct new targeted, hypothesis-driven experiments to dissect molecular mechanisms controlling T cell function. TCR-regulated phosphoproteomic data sets available in the literature97–99 contain highly valuable information about previously unknown phosphorylation sites on key transcription factors for T cell function and links between TCR-regulated serine-threonine kinases and transcriptional regulators97–99. They show that current knowledge of how serine-threonine kinases mediate TCR signal transduction is at a nascent stage, but they highlight that understanding the function of TCR-regulated serine-threonine phosphorylations is essential for an understanding of how the TCR regulates the essential epigenetic, transcriptional and metabolic programs that control T cell participation in an immune response.
Acknowledgments
We thank members of the D.A.C. laboratory for critical discussion during the preparation of the manuscript.
Footnotes
Competing Financial Interests
The authors declare no competing financial interests.
Reprints and permissions information is available online at http://www.nature.com/reprints/index.html.
References
- 1.Matthews SA, Cantrell DA. New insights into the regulation and function of serine/threonine kinases in T lymphocytes. Immunol Rev. 2009;228:241–252. doi: 10.1111/j.1600-065X.2008.00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosse C, et al. PKC and the control of localized signal dynamics. Nat Rev Mol Cell Biol. 2010;11:103–112. doi: 10.1038/nrm2847. [DOI] [PubMed] [Google Scholar]
- 3.Weiss A, Wiskocil RL, Stobo JD. The role of T3 surface molecules in the activation of human T cells: a two-stimulus requirement for IL 2 production reflects events occurring at a pre-translational level. J Immunol. 1984;133:123–128. [PubMed] [Google Scholar]
- 4.Cantrell DA, Davies AA, Crumpton MJ. Activators of protein kinase C down-regulate and phosphorylate the T3/T-cell antigen receptor complex of human T lymphocytes. Proc Natl Acad Sci USA. 1985;82:8158–8162. doi: 10.1073/pnas.82.23.8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monks CR, Kupfer H, Tamir I, Barlow A, Kupfer A. Selective modulation of protein kinase C-θ during T-cell activation. Nature. 1997;385:83–86. doi: 10.1038/385083a0. [DOI] [PubMed] [Google Scholar]
- 6.Dustin ML, Depoil D. New insights into the T cell synapse from single molecule techniques. Nat Rev Immunol. 2011;11:672–684. doi: 10.1038/nri3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quann EJ, Liu X, Altan-Bonnet G, Huse M. A cascade of protein kinase C isozymes promotes cytoskeletal polarization in T cells. Nat Immunol. 2011;12:647–654. doi: 10.1038/ni.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jun JE, Rubio I, Roose JP. Regulation of Ras exchange factors and cellular localization of Ras activation by lipid messengers in T cells. Front Immunol. 2013;4:239. doi: 10.3389/fimmu.2013.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rozengurt E. Protein kinase D signaling: multiple biological functions in health and disease. Physiology (Bethesda) 2011;26:23–33. doi: 10.1152/physiol.00037.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrasco S, Merida I. Diacylglycerol-dependent binding recruits PKCθ and RasGRP1 C1 domains to specific subcellular localizations in living T lymphocytes. Mol Biol Cell. 2004;15:2932–2942. doi: 10.1091/mbc.E03-11-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spitaler M, Emslie E, Wood CD, Cantrell D. Diacylglycerol and protein kinase D localization during T lymphocyte activation. Immunity. 2006;24:535–546. doi: 10.1016/j.immuni.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Huse M, et al. Spatial and temporal dynamics of T cell receptor signaling with a photoactivatable agonist. Immunity. 2007;27:76–88. doi: 10.1016/j.immuni.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 13.Quann EJ, Merino E, Furuta T, Huse M. Localized diacylglycerol drives the polarization of the microtubule-organizing center in T cells. Nat Immunol. 2009;10:627–635. doi: 10.1038/ni.1734. [DOI] [PubMed] [Google Scholar]
- 14.Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol. 2010;11:9–22. doi: 10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto R, et al. Phosphorylation of CARMA1 plays a critical role in T cell receptor-mediated NF-κB activation. Immunity. 2005;23:575–585. doi: 10.1016/j.immuni.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Dustin ML. PKC-θ: hitting the bull’s eye. Nat Immunol. 2011;12:1031–1032. doi: 10.1038/ni.2141. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Kapoor TM, Chen JK, Huse M. Diacylglycerol promotes centrosome polarization in T cells via reciprocal localization of dynein and myosin II. Proc Natl Acad Sci USA. 2013;110:11976–11981. doi: 10.1073/pnas.1306180110. [This article, together with refs. 7 and 13, provides evidence that DAG gradients generated at the immunological synapse promote the recruitment of different PKC isozymes and the microtubule-organizing center and links these phenomena, identifying the myosin regulatory light chain as the PKC substrate relevant in the regulation of the localization of the nonmuscle myosin motor complex.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma JSY, Haydar TF, Radoja S. Protein kinase C-δ localizes to secretory lysosomes in CD8+ CTL and directly mediates TCR signals leading to granule exocytosis-mediated cytotoxicity. J Immunol. 2008;181:4716–4722. doi: 10.4049/jimmunol.181.7.4716. [DOI] [PubMed] [Google Scholar]
- 19.Letschka T, et al. PKC-θ selectively controls the adhesion-stimulating molecule Rap1. Blood. 2008;112:4617–4627. doi: 10.1182/blood-2007-11-121111. [DOI] [PubMed] [Google Scholar]
- 20.Fagerholm S, Morrice N, Gahmberg CG, Cohen P. Phosphorylation of the cytoplasmic domain of the integrin CD18 chain by protein kinase C isoforms in leukocytes. J Biol Chem. 2002;277:1728–1738. doi: 10.1074/jbc.M106856200. [DOI] [PubMed] [Google Scholar]
- 21.Matthews SA, Rozengurt E, Cantrell D. Protein kinase D. A selective target for antigen receptors and a downstream target for protein kinase C in lymphocytes. J Exp Med. 2000;191:2075–2082. doi: 10.1084/jem.191.12.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waldron RT, et al. Activation loop Ser744 and Ser748 in protein kinase D are transphosphorylated in vivo. J Biol Chem. 2001;276:32606–32615. doi: 10.1074/jbc.M101648200. [DOI] [PubMed] [Google Scholar]
- 23.Rey O, Reeve JR, Zhukova E, Sinnett-Smith J, Rozengurt E. G protein-coupled receptor-mediated phosphorylation of the activation loop of protein kinase D dependence on plasma membrane translocation and protein kinase Cε. J Biol Chem. 2004;279:34361–34372. doi: 10.1074/jbc.M403265200. [DOI] [PubMed] [Google Scholar]
- 24.Matthews SA, et al. Unique functions for protein kinase D1 and protein kinase D2 in mammalian cells. Biochem J. 2010;432:153–163. doi: 10.1042/BJ20101188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navarro MN, et al. Protein kinase D2 has a restricted but critical role in T-cell antigen receptor signalling in mature T-cells. Biochem J. 2012;442:649–659. doi: 10.1042/BJ20111700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischer AM, Katayama CD, Pagès G, Pouysségur J, Hedrick SM. The role of erk1 and erk2 in multiple stages of T cell development. Immunity. 2005;23:431–443. doi: 10.1016/j.immuni.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 27.D’Souza WN, Chang C-F, Fischer AM, Li M, Hedrick SM. The Erk2 MAPK regulates CD8 T cell proliferation and survival. J Immunol. 2008;181:7617–7629. doi: 10.4049/jimmunol.181.11.7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costello PS, Nicolas RH, Watanabe Y, Rosewell I, Treisman R. Ternary complex factor SAP-1 is required for Erk-mediated thymocyte positive selection. Nat Immunol. 2004;5:289–298. doi: 10.1038/ni1038. [DOI] [PubMed] [Google Scholar]
- 29.Wagner EF, Eferl R. Fos/AP-1 proteins in bone and the immune system. Immunol Rev. 2005;208:126–140. doi: 10.1111/j.0105-2896.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- 30.Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26:3203–3213. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- 31.Lovrić J, Dammeier S, Kieser A, Mischak H, Kolch W. Activated raf induces the hyperphosphorylation of stathmin and the reorganization of the microtubule network. J Biol Chem. 1998;273:22848–22855. [PubMed] [Google Scholar]
- 32.Lin J-X, Spolski R, Leonard WJ. Critical role for Rsk2 in T-lymphocyte activation. Blood. 2008;111:525–533. doi: 10.1182/blood-2007-02-072207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romeo Y, Zhang X, Roux PP. Regulation and function of the RSK family of protein kinases. Biochem J. 2012;441:553–569. doi: 10.1042/BJ20110289. [DOI] [PubMed] [Google Scholar]
- 34.Fukunaga R, Hunter T. MNK1, a new MAP kinase-activated protein kinase, isolated by a novel expression screening method for identifying protein kinase substrates. EMBO J. 1997;16:1921–1933. doi: 10.1093/emboj/16.8.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waskiewicz AJ, Flynn A, Proud CG, Cooper JA. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 1997;16:1909–1920. doi: 10.1093/emboj/16.8.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueda T, et al. Combined deficiency for MAP kinase-interacting kinase 1 and 2 (Mnk1 and Mnk2) delays tumor development. Proc Natl Acad Sci USA. 2010;107:13984–13990. doi: 10.1073/pnas.1008136107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okkenhaug K, Bilancio A, Emery JL, Vanhaesebroeck B. Phosphoinositide 3-kinase in T cell activation and survival. Biochem Soc Trans. 2004;32:332–335. doi: 10.1042/bst0320332. [DOI] [PubMed] [Google Scholar]
- 38.Costello PS, Gallagher M, Cantrell DA. Sustained and dynamic inositol lipid metabolism inside and outside the immunological synapse. Nat Immunol. 2002;3:1082–1089. doi: 10.1038/ni848. [DOI] [PubMed] [Google Scholar]
- 39.Harriague J, Bismuth G. Imaging antigen-induced PI3K activation in T cells. Nat Immunol. 2002;3:1090–1096. doi: 10.1038/ni847. [DOI] [PubMed] [Google Scholar]
- 40.Huppa JB, Gleimer M, Sumen C, Davis MM. Continuous T cell receptor signaling required for synapse maintenance and full effector potential. Nat Immunol. 2003;4:749–755. doi: 10.1038/ni951. [DOI] [PubMed] [Google Scholar]
- 41.Garçon F, et al. CD28 provides T-cell costimulation and enhances PI3K activity at the immune synapse independently of its capacity to interact with the p85/p110 heterodimer. Blood. 2008;111:1464–1471. doi: 10.1182/blood-2007-08-108050. [DOI] [PubMed] [Google Scholar]
- 42.Calleja V, et al. Intramolecular and intermolecular interactions of protein kinase B define its activation in vivo. PLoS Biol. 2007;5:e95. doi: 10.1371/journal.pbio.0050095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bayascas JR, et al. Mutation of the PDK1 PH domain inhibits protein kinase B/Akt, leading to small size and insulin resistance. Mol Cell Biol. 2008;28:3258–3272. doi: 10.1128/MCB.02032-07. [This article describes the generation of knock-in mice expressing a PDK1 mutant incapable of binding PtdIns(3,4,5)P3, and demonstrates that PDK1 binding to PtdIns(3,4,5)P3 is required for efficient Akt activation, but some Akt activation is detected in absence of PDK1-PtdIns(3,4,5)P3 binding.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Najafov A, Shpiro N, Alessi DR. Akt is efficiently activated by PIF-pocket- and PtdIns(3,4,5)P3-dependent mechanisms leading to resistance to PDK1 inhibitors. Biochem J. 2012;448:285–295. doi: 10.1042/BJ20121287. [Together with ref. 42, this article provides evidence of an alternative mechanism for Akt activation by PDK1 that is independent of PDK1-PtdIns(3,4,5)P3 binding. This work shows how mTORC2-mediated phosphorylation of Ser473 in Akt recruits PDK1 via a substrate-docking motif termed the PIF-pocket, allowing phosphorylation of Akt Thr308 by PDK1 and efficient Akt activation.] [DOI] [PubMed] [Google Scholar]
- 45.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.García-Martínez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1) Biochem J. 2008;416:375–385. doi: 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- 47.Heikamp EB, et al. The AGC kinase SGK1 regulates TH1 and TH2 differentiation downstream of the mTORC2 complex. Nat Immunol. 2014;15:457–464. doi: 10.1038/ni.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu C, et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496:513–517. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kleinewietfeld M, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496:518–522. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delgoffe GM, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [This article provides key evidence about the specific role of mTORC1 and mTORC2 complexes in T cell function. In Rheb-deficient mice with disabled mTORC1 function, TH1 and TH17 responses are impaired both in vitro and in vivo; in RICTOR-deficient mice in which mTORC2 function is ablated, T cells fail to generate TH2 responses.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waugh C, Sinclair L, Finlay D, Bayascas JR, Cantrell D. Phosphoinositide (3,4,5)-triphosphate binding to phosphoinositide-dependent kinase 1 regulates a protein kinase B/Akt signaling threshold that dictates T-cell migration, not proliferation. Mol Cell Biol. 2009;29:5952–5962. doi: 10.1128/MCB.00585-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 53.Barata JT, et al. Activation of PI3K is indispensable for interleukin 7–mediated viability, proliferation, glucose use, and growth of T cell acute lymphoblastic leukemia cells. J Exp Med. 2004;200:659–669. doi: 10.1084/jem.20040789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cornish GH, Sinclair LV, Cantrell DA. Differential regulation of T-cell growth by IL-2 and IL-15. Blood. 2006;108:600–608. doi: 10.1182/blood-2005-12-4827. [DOI] [PubMed] [Google Scholar]
- 55.Brunn GJ, et al. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J. 1996;15:5256–5267. [PMC free article] [PubMed] [Google Scholar]
- 56.Jacobs MD, et al. Pim-1 ligand-bound structures reveal the mechanism of serine/threonine kinase inhibition by LY294002. J Biol Chem. 2005;280:13728–13734. doi: 10.1074/jbc.M413155200. [DOI] [PubMed] [Google Scholar]
- 57.Bain J, et al. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Macintyre AN, et al. Protein kinase B controls transcriptional programs that direct cytotoxic T cell fate but is dispensable for T cell metabolism. Immunity. 2011;34:224–236. doi: 10.1016/j.immuni.2011.01.012. [This work provides key evidence that Akt does not mediate T cell metabolic responses. Using mice deficient in PI3K and PDK1 function and Akt-specific pharmacologic inhibitors, the authors show that Akt catalytic activity controls expression of key effector molecules but is not required for glucose uptake or IL-2–mediated proliferation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eijkelenboom A, Burgering BMT. FOXOs: signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol. 2013;14:83–97. doi: 10.1038/nrm3507. [DOI] [PubMed] [Google Scholar]
- 60.Fabre S, et al. FOXO1 regulates L-Selectin and a network of human T cell homing molecules downstream of phosphatidylinositol 3-kinase. J Immunol. 2008;181:2980–2989. doi: 10.4049/jimmunol.181.5.2980. [DOI] [PubMed] [Google Scholar]
- 61.Kerdiles YM, et al. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat Immunol. 2009;10:176–184. doi: 10.1038/ni.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ouyang W, et al. Novel Foxo1-dependent transcriptional programs control T(reg) cell function. Nature. 2012;491:554–559. doi: 10.1038/nature11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ouyang W, Beckett O, Flavell RA, Li MO. An essential role of the Forkhead-box transcription factor Foxo1 in control of T cell homeostasis and tolerance. Immunity. 2009;30:358–371. doi: 10.1016/j.immuni.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carlson CM, et al. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006;442:299–302. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- 65.Finlay DK, et al. Phosphoinositide-dependent kinase 1 controls migration and malignant transformation but not cell growth and proliferation in PTEN-null lymphocytes. J Exp Med. 2009;206:2441–2454. doi: 10.1084/jem.20090219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Preston GC, Feijoo-Carnero C, Schurch N, Cowling VH, Cantrell DA. The impact of KLF2 modulation on the transcriptional program and function of CD8 T cells. PLoS ONE. 2013;8:e77537. doi: 10.1371/journal.pone.0077537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takada K, et al. Kruppel-like factor 2 is required for trafficking but not quiescence in postactivated T cells. J Immunol. 2011;186:775–783. doi: 10.4049/jimmunol.1000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cyster JG, Schwab SR. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu Rev Immunol. 2012;30:69–94. doi: 10.1146/annurev-immunol-020711-075011. [DOI] [PubMed] [Google Scholar]
- 69.Sinclair LV, et al. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008;9:513–521. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hand TW, et al. Differential effects of STAT5 and PI3K/AKT signaling on effector and memory CD8 T-cell survival. Proc Natl Acad Sci USA. 2010;107:16601–16606. doi: 10.1073/pnas.1003457107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang R, Green DR. Metabolic checkpoints in activated T cells. Nat Immunol. 2012;13:907–915. doi: 10.1038/ni.2386. [DOI] [PubMed] [Google Scholar]
- 72.Marko AJ, Miller RA, Kelman A, Frauwirth KA. Induction of glucose metabolism in stimulated T lymphocytes is regulated by mitogen-activated protein kinase signaling. PLoS ONE. 2010;5:e15425. doi: 10.1371/journal.pone.0015425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carr EL, et al. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J Immunol. 2010;185:1037–1044. doi: 10.4049/jimmunol.0903586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang R, et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [This article shows how TCR signaling induces metabolic reprogramming in naive T cells. In this work, the authors show that acute deletion of the transcription factor c-Myc inhibits TCR-mediated glycolysis and glutaminolysis, impairing T cell proliferation. Together with refs. 72 and 73, this study shows that activation of the Erk1 and Erk2 kinases regulates the expression of c-Myc.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sinclair LV, et al. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat Immunol. 2013 doi: 10.1038/ni.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bar-Peled L, Sabatini DM. Regulation of mTORC1 by amino acids. Trends Cell Biol. 2014 doi: 10.1016/j.tcb.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dibble CC, Manning BD. Signal integration by mTORC1 coordinates nutrient input with biosynthetic output. Nat Cell Biol. 2013;15:555–564. doi: 10.1038/ncb2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Powell JD, Delgoffe GM. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity. 2010;33:301–311. doi: 10.1016/j.immuni.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ricoult SJH, Manning BD. The multifaceted role of mTORC1 in the control of lipid metabolism. EMBO Rep. 2013;14:242–251. doi: 10.1038/embor.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kidani Y, et al. Sterol regulatory element-binding proteins are essential for the metabolic programming of effector T cells and adaptive immunity. Nat Immunol. 2013;14:489–499. doi: 10.1038/ni.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Finlay DK, et al. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J Exp Med. 2012;209:2441–2453. doi: 10.1084/jem.20112607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shi LZ, et al. HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [This work shows that TH17 differentiation requires the upregulation of glycolytic activity and that the mTORC1-regulated expression of the transcription factor HIF1α is a crucial mediator of glycolytic pathways. Together with ref. 81 this article defines key role for mTORC1-HIF1α axis in the maintenance of glucose metabolism and glycolysis in effector T cells] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang H, et al. Negative regulation of Hif1a expression and TH17 differentiation by the hypoxia-regulated microRNA miR-210. Nat Immunol. 2014;15:393–401. doi: 10.1038/ni.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shimobayashi M, Hall MN. Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat Rev Mol Cell Biol. 2014;15:155–162. doi: 10.1038/nrm3757. [DOI] [PubMed] [Google Scholar]
- 85.Cham CM, Gajewski TF. Glucose availability regulates IFN-γ-production and p70S6 kinase activation in CD8+ effector T cells. J Immunol. 2005;174:4670–4677. doi: 10.4049/jimmunol.174.8.4670. [This article shows how glucose availability controls key effector functions, such as IFN-δ production, in CD8 T cells. This work also shows that glucose deprivation decreases the phosphorylation of the mTORC1 substrate p70S6 kinase] [DOI] [PubMed] [Google Scholar]
- 86.Nakaya M, et al. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity. 2014 doi: 10.1016/j.immuni.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tamás P, et al. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J Exp Med. 2006;203:1665–1670. doi: 10.1084/jem.20052469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hardie DG, Ross FA, Hawley SA. AMP-activated protein kinase: a target for drugs both ancient and modern. Chem Biol. 2012;19:1222–1236. doi: 10.1016/j.chembiol.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tamás P, et al. LKB1 is essential for the proliferation of T-cell progenitors and mature peripheral T cells. Eur J Immunol. 2010;40:242–253. doi: 10.1002/eji.200939677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rolf J, et al. AMPKα 1 A glucose sensor that controls CD8 T-cell memory. Eur J Immunol. 2013;43:889–896. doi: 10.1002/eji.201243008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cai S-L, et al. Activity of TSC2 is inhibited by AKT-mediated phosphorylation and membrane partitioning. J Cell Biol. 2006;173:279–289. doi: 10.1083/jcb.200507119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Menon S, et al. Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell. 2014;156:771–785. doi: 10.1016/j.cell.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang K, Neale G, Green DR, He W, Chi H. The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function. Nat Immunol. 2011;12:888–897. doi: 10.1038/ni.2068. [This article describes the role of TSC1-dependent control of mTOR using Tsc1-deficient mice. Tsc1-deficient T cells had more mTORC1 activity, causing the disruption of immune homeostasis] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Efeyan A, Sabatini DM. Nutrients and growth factors in mTORC1 activation. Biochem Soc Trans. 2013;41:902–905. doi: 10.1042/BST20130063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gwinn DM, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Araki K, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [This work reports how unexpectedly, the mTORC1 pharmacological inhibitor rapamycin enhances the generation of CD8 memory T cells. The regulation of mTORC1 activity appears to be a control switch for effector-to-memory transition in CD8 T cells] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Navarro MN, Goebel J, Feijoo-Carnero C, Morrice N, Cantrell DA. Phosphoproteomic analysis reveals an intrinsic pathway for the regulation of histone deacetylase 7 that controls the function of cytotoxic T lymphocytes. Nat Immunol. 2011;12:352–361. doi: 10.1038/ni.2008. [This article describes the application of large-scale quantitative phosphoproteomic approaches to analyze the TCR-regulated phosphoproteome of primary cytotoxic T cells. This work reports the existence of a basal web protein phosphorylation and also affords new insights about the scope of TCR-regulated phosphorylations] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ruperez P, Gago-Martinez A, Burlingame AL, Oses-Prieto JA. Quantitative phosphoproteomic analysis reveals a role for serine and threonine kinases in the cytoskeletal reorganization in early T cell receptor activation in human primary T cells. Mol Cell Proteomics. 2012;11:171–186. doi: 10.1074/mcp.M112.017863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mayya V, et al. Quantitative phosphoproteomic analysis of T cell receptor signaling reveals system-wide modulation of protein-protein interactions. Sci Signal. 2009;2:ra46. doi: 10.1126/scisignal.2000007. [DOI] [PubMed] [Google Scholar]