Abstract
11ß-Hydroxysteroid dehydrogenase type 2 is a glucocorticoid metabolizing enzyme that catalyzes rapid inactivation of corticosterone and cortisol to inert 11-keto derivatives. As 11ß-hydroxysteroid dehydrogenase type 2 is highly expressed in the developing brain, but not in the adult CNS, we hypothesized that it may represent a protective barrier to the deleterious actions of corticosteroids on proliferating cells. To test this hypothesis we have investigated the development and growth of the cerebellum in neonatal C57BL/6 mice and mice lacking 11ß -hydroxysteroid dehydrogenase type 2. 11ß-Hydroxysteroid dehydrogenase type 2-/- mice had consistently ower body weight throughout the neonatal period, coupled with a smaller brain size although this was normalized when corrected for body weight. The cerebellar size was smaller in 11ß-hydroxysteroid dehydrogenase type 2-/- mice, due to decreases in size of both the molecular and internal granule layers. When exogenous corticosterone was administered to the pups between postnatal days 4 and 13, 11ß-hydroxysteroid dehydrogenase type 2-/ mice were more sensitive, showing further inhibition of cerebellar growth while the wildtype mice were not affected. Upon withdrawal of exogenous steroid, there was a rebound growth spurt so that at day 21 postnatally, the cerebellar size in 11ß-hydroxysteroid dehydrogenase type 2-/- mice was similar to untreated mice of the same genotype. Furthermore, 11ß-hydroxysteroid dehydrogenase type 2-/- had a delay in the attainment of neurodevelopmental landmarks such as negative geotaxis and eye opening. We therefore suggest that 11ß-hydroxysteroid dehydrogenase type 2 acts as to protect the developing nervous system from the deleterious consequences of glucocorticoid overexposure.
Keywords: corticosterone, 11(-HSD2 knockout mice, growth, developmental landmarks, BrdU
Glucocorticoids have profound effects on brain development both pre-and postnatally. Elevated glucocorticoid levels inhibit neuronal proliferation, differentiation and migration as well as dendritic arborization (de Kloet et al., 1988; Baud, 2004). Maintenance of low glucocorticoid exposure during critical periods of brain development is essential. Postnatally, this is ensured, in part, by the stress hyporesponsive period, during which the hypothalamic– pituitary–adrenal (HPA) axis is relatively unresponsive (Walker, 1986; Schmidt et al., 2003). However, the stress hyporesponsive period is not a complete protection as corticosteroid binding globulin levels are also very low, so ‘free’ steroids are not minimized. Moreover, the HPA axis is still responsive to some stressors (Viau et al., 1996; Dent et al., 2000). Additional mechanisms restraining glucocorticoid action are uncertain.
11ß-Hydroxysteroid dehydrogenase type 2 (11ß-HSD2) catalyzes the rapid inactivation of corticosterone (cortisol in humans) to inert 11-dehydrocorticosterone (cortisone). In the distal nephron, 11ß-HSD2 protects mineralocorticoid receptors (MR) from activation by corticosterone (Edwards et al., 1988). Its deficiency leads to apparent mineralocorticoid excess in which glucocorticoids illicitly activate MR causing sodium retention, hypertension and hypokalemia. 11ß-HSD2 is also expressed in the placenta (Benediktsson et al., 1997) where it protects the developing fetus from excess maternal glucocorticoid exposure (Benediktsson et al., 1997).
Although 11ß-HSD2 is barely expressed in the adult brain, the enzyme is highly expressed in the developing CNS, until the end of mid-gestation in rats, mice and humans (Brown et al., 1996; Diaz et al., 1998). Thereafter expression becomes more restricted as each brain area ceases to proliferate and differentiates. After birth, high 11ß-HSD2 expression occurs only in the proliferating, external granule layer (EGL) of the cerebellum and in several differentiating and migrating nuclei of the thalamus (Roland et al., 1995; Robson et al., 1998). Cerebellar development is particularly sensitive to high levels of glucocorticoids produced by either exogenous administration or in response to the stress induced by maternal separation (Bohn and Lauder, 1978; Mirescu et al., 2004). Furthermore, elevating maternal corticosterone levels in the lactating rat is anticipated to saturate the 11[-HSD2 barrier and indeed results in profound lifelong effects on brain biochemistry and behavior (Meerlo et al., 2001).
In this paper we test the hypothesis that 11ß-HSD2 expression in the early postnatal brain acts as a further level of protection for the still developing cerebellum. To achieve this we investigated neonatal cerebellar development in mice omozygous for targeted disruption of the 11ß-HSD2 gene, 11ß-HSD2-/- mice (Kotelevtsev et al., 1999), back-crossed onto the C57BL/6J strain for 10 generations (Paterson, Bailey, Hadoke, Brownstein, Bellamy, Fleming, Seckl, Mullins, unpublished). Furthermore, we investigated whether exogenous corticosterone administered to the neonate mimicked effects observed in mice lacking the 11ß-HSD2 protective barrier and whether the 11ß-HSD2 null mice were more sensitive to this treatment.
Experimental Procedures
Animals
Male and female 11ß-HSD2-/- mice (congenic—10 generations— on the C57BL/6J background) were housed in pairs in breeding cages with bedding for nest building. The resulting offspring were compared with offspring from similarly housed C57Bl/6J control mice. The light/dark cycle was kept constant with lights on from 07:00 h to 19:00 h. Animals were given standard chow and water ad libitum, and all studies were carried out to the highest standards of humane care in strict accordance with the UK Animals (Scientific Procedures) Act, 1986, and international guidelines on the ethical use of animals. All efforts were made to minimize the number of animals used and their suffering. All pups were weighed on day of birth and at time of kill. One or two pups were taken from each litter and decapitated on postnatal days (P) 7, 14 and 21 (preweaning) and at P28 (postweaning).
Corticosterone treatment
To assess sensitivity to exogenous physiological glucocorticoids and determine if exogenous glucocorticoids mimic or exacerbate the phenotype observed in 11ß-HSD2-/- mice, some litters of 11ß-HSD2-/- and control pups were treated with corticosterone (50 ng/g i.p.) daily between P4 and 13. Pups were weighed daily at time of injection and two mice per litter were killed at P14 or P21.
Cell proliferation studies
BrdU (40 fg/g i.p.) was injected 24 h prior to kill at P7, 14 and 21.
In situ hybridization histochemistry
In situ hybridization histochemistry to determine 11ß-HSD2 mRNA expression patterns in the cerebellum of C57BL/6 mice was carried out according to the method described in Brown et al. (1996). Briefly, a 666 –base pair mouse 11[-HSD2 complementary DNA (cDNA) fragment corresponding to bases +393–1059, was amplified from a 2.74,61BS clone containing 1.6 kilobase mouse 11[-HSD2 cDNA clone, with unique primers incorporating T3/T7 polymerase binding sites at their 5= ends. The polymerase chain reaction fragment was purified on Chromaspin columns (Clontech, Palo Alto, CA, USA) and used as a template for mouse 35S-labeling of 11ß-HSD2 antisense and sense riboprobes. Sagittal 20 fm cryostat sections were thaw mounted onto 3-amino propyl silane coated slides. Tissue sections fixed, prehybridized, hybridized (3X106 cpm/section 35S-uridine triphosphate (UTP)-labeled probe at 50 °C for 12–14 h) and washed as described previously (Holmes et al., 1997).
Morphometric and immunocytochemical analysis of cerebella
Brains from decapitated neonates were quickly dissected and immersion fixed in 4 ml neutral-buffered formalin (Sigma, Poole, Dorset, UK) for 24 h. Brains were then washed in 0.1 M phosphate buffer and stored at 8 °C in 70% ethanol until wax-embedded. Samples were wax-embedded in a standard protocol, 6 fm sections from the midsagittal region of the cerebellum were cut using a microtome, collected on electrostatically charged microscope slides (Superfrost, VWR Int., Lutterworth, Leicestershire, UK) and stored at RT prior to staining or immunocytochemical analysis.
Morphometric analyses
Sections at the midline of the cerebellum were stained with hematoxylin and eosin. Total area of the section (total cerebellar area), area of the molecular layer (ML) and area of the granule layer were measured using computer-assisted image analysis (MCID, Research Imaging, St. Catherines, Ontario, Canada). Mean measurements were calculated from three mid-sagittal sections for each brain.
Immunocytochemistry for cell proliferation (bromodeoxyuridine, BrdU) and glial projections (glial fibrillary acidic protein, GFAP) and TUNEL (terminal-deoxynucleotidyl transferase dUTP nick-end labeling) staining for assessment of apoptosis measurement were carried out on adjacent mid-sagittal sections of the cerebellum. Primary antibodies for BrdU and GFAP were monoclonal antibodies raised in mice (Sigma). Immunohistochemistry was carried out using a mouse-on-mouse peroxidase kit (Vector Laboratories, Peterborough, UK) and processed in a Sequenza (Thermo Shandon, Runcorn, Cheshire, UK). In brief, slides were heated to 50 °C for 15 min in an oven, deparaffinized in xylene for 5 min, then rehydrated through decreasing ethanols to be washed in tap water. Slides were then placed in an antigen unmasking solution (Vector Laboratories), microwaved (850 W) for 15 min, cooled under running water for 20 min, washed in PBS, treated with 0.01% Triton for 5 min, endogenous peroxidase activity was inactivated by treatment with 3% hydrogen peroxide for 5 min, blocking serum added for 1 h prior to incubation with the primary antibody for 30 min. Sections were then incubated with biotinylated secondary antibody for 10 min and finally with an avidin-bound biotinylated peroxidase complex for 5 min. Visualization of the antibody complex was possible with diaminobenzidine (DAB) staining. Control slides were processed without primary antibody and, in the case of BrdU, sections from mice not having a BrdU injection. These slides revealed no non-specific staining (data not shown).
TUNEL analysis was carried out using a commercially available kit (DeadEnd Colorimetric System; Promega, Southampton, UK), according to the manufacturer’s protocol. In brief, sections were dewaxed in xylene and rehydrated in graded ethanols before antigen retrieval was performed with proteinase K and endogenous peroxidase quenched with hydrogen peroxide. Sections were then incubated with terminal-deoxynucleotidyl transferase (TdT) and biotinylated nucleotide. Nuclear DNA containing incorporated biotinylated nucleotides was then labeled by avidin bound horseradish-peroxidase using DAB as the chromogen. Sections were counterstained in hemotoxylin before mounting. Positive controls were created by pre-treating tissue with DNAse 1, while negative controls omitted the TdT enzyme step.
Quantification of the immunostained sections was carried out in three midsagittal sections from each cerebellum by counting labeled and non-labeled cells in each cerebellar layer in lobule 2 (indicated by a box in Fig. 3a). This was carried out using a color capture, computer driven image analysis package (KS Imaging, Imaging Associates, Bicester, UK). For hippocampal cell proliferation assessment, BrdU-labeled cells were counted in three coronal sections (DBregma -2.06 mm) in the hilus and dentate gyrus for each brain.
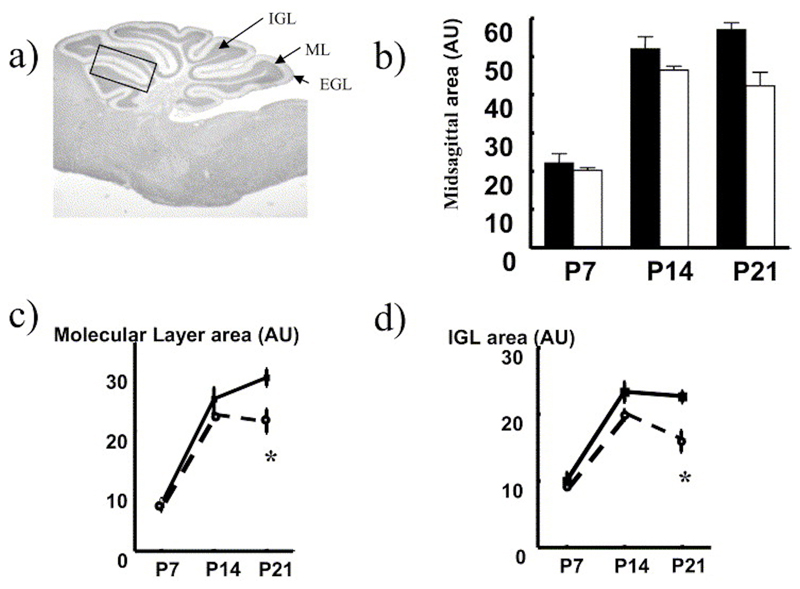
Fig. 3. Altered growth of the cerebellum in 11ß-HSD2-/- compared with controls.
(a) Midsagittal section of mouse cerebellum indicating zonations. Box indicates area used in cell counting (see Table 2). Comparative measurements of mid-sagittal area of (b) total cerebellum, (c) ML and (d) IGL removed from C57BL/6 control (black columns and solid lines with closed symbols) and 11ß-HSD2-/- (open columns and dotted line with open symbols) mice at P7, 14 and 21. Values are means±S.E.M., n=4– 6/group. * P<0.05 compared with respective C57BL/6 value.
Assessment of neurodevelopment
Negative geotaxis
At P7 pups were removed from their mothers and placed on an inclined plane of approx 30° with their head facing downwards. The number of mice which completed a reflex turn to face up the slope within a 1 min period was recorded.
Physical development, eyes opening
At P14 mice were observed and the number with both eyes open was recorded.
Analysis of plasma corticosterone
Trunk blood was collected at time of decapitation at P14, P21 and adult male mice, into EDTA-coated Microvette tubes (Sarstedt, Leicester, UK) and stored at 4 °C until centrifugation. Plasma was then stored at -20 °C until corticosterone determination by radioimmunoassay, as described previously (Holmes et al., 1997).
Statistical analysis
Data are expressed as means±S.E.M. Statistical significance was determined by multiple or one-way ANOVA as appropriate, followed by post hoc test for multiple comparisons. A P value <0.5 was considered significant.
Results
Ontogeny of 11ß-HSD2 expression in mouse neonatal cerebellum
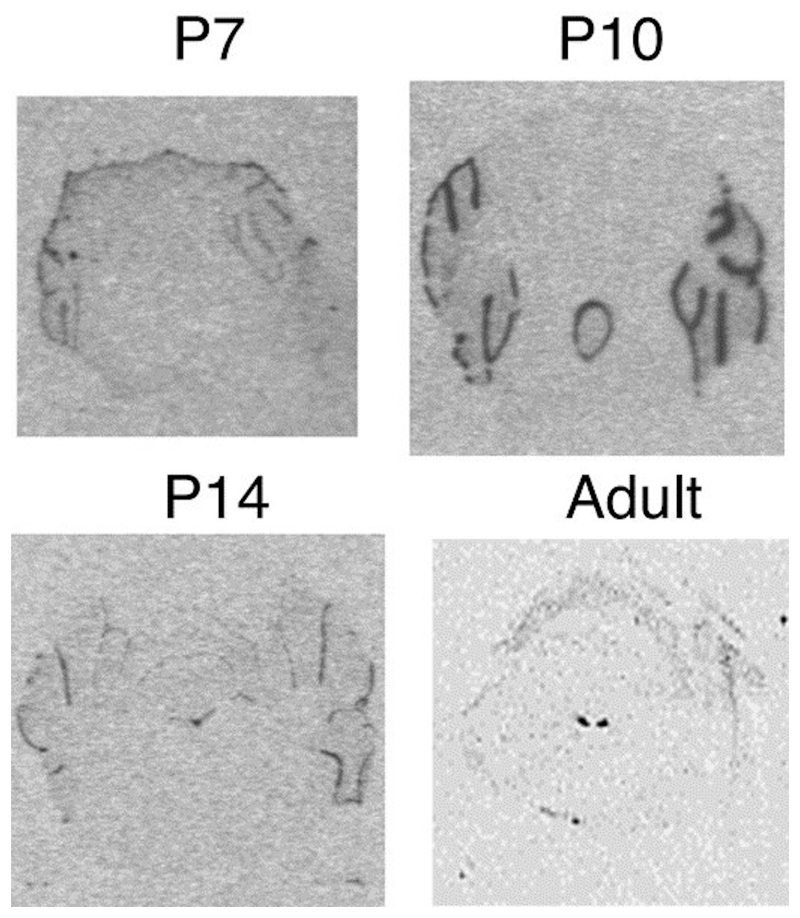
11ß-HSD2 mRNA is highly expressed in the proliferating, EGL of the postnatal cerebellum (Fig. 1). As the EGL decreases in size, the 11ß-HSD2 mRNA expression also decreases, until after P14 no detectable expression is observed in the cerebellum throughout the rest of life. In the adult mouse brain high expression of 11ß-HSD2 is only seen in the nucleus of the tractus solitarius, an area of the brain involved in blood pressure regulation.
Fig. 1. Pattern of 11ß-HSD2 mRNA expression in neonatal cerebellum.
11ß-HSD2 mRNA expression in EGL cerebellum at P7, 10 and 14. No expression is seen in adult cerebellum. The only significant expression of 11ß-HSD2 mRNA in adult mouse brain is in nucleus tractus solitarius.
Neonatal morphometry
There was no difference in the litter size or gestation length between 11ß-HSD2-/- and C57BL/6 controls (not shown). 11ß-HSD2-/- pups were smaller (lower body weight) throughout the postnatal period studie (effect of genotype F(1,64)=54.66, P=0.001; two-way ANOVA) (Fig. 2a). The significant interaction between genotype and age (P=0.004) shows that the effects of loss of 11ß-HSD2 on body weight are different depending on age (i.e. the effect becomes greater with age). The brain weight was consistently smaller in the 11ß-HSD2-/- pups (F(1,29)=4.578, P=0.04) at all postnatal time points (Fig. 2b), but when the brain weights were corrected for body weight differences, the effect of genotype on brain weight was abolished (Fig. 2c).
Fig. 2. Neonatal growth curve of 11ß-HSD2-/- and C57BL/6 controls.
(a) Body weights, (b) brain weights and (c) brain/body weight ratios of control C57BL/6 mice (solid columns) and 11ß-HSD2-/- mice (open columns) at 7, 14 and 21 postnatally. Values are means±S.E.M., n= 5–12/group. * P<0.05 compared with respective C57BL/6 value.
Neonatal cerebellar growth
Cerebellar growth was determined in wax-embedded brains at P7,14 and 21, by measuring the cross-sectional area of midsagittal sections of the cerebellum as depicted in Fig. 3a. The postnatal cerebellar growth curve was significantly reduced in 11ß-HSD2-/- mice compared with controls (P=0.009 for genotype; two-way ANOVA) (Fig. 3b). When each of the cerebellar layers were considered separately, 11ß-HSD2-/- mice had reduced growth of both the ML (P=0.009, Fig. 3c) and the internal granule layer (IGL, P=0.007, Fig. 3d), effects which showed an interaction with age (P=0.045) indicating that the greatest difference between genotypes was seen at P21.
Cell proliferation studies
In order to assess whether the alteration in cerebellar growth in 11ß-HSD2-/- mice reflected an alteration in proliferation of the cerebellar EGL, previously reported to be affected by glucocorticoids, BrdU was injected 24 h prior to kill at P7 and P14 to mark cells dividing within a 24 h period. At P7, the EGL is larger with a high number of cells proliferating in 24 h, whereas at P14 the EGL is reduced in width giving a lower number of cells proliferating. The number of BrdU-labeled cells in the EGL was unaffected by genotype (Table 1). Furthermore, there was no effect of genotype on the number of cells/unit area in the EGL or ML at P7 or 14. However, there was a reduction in the number of granule cells within the IGL at P7 (P<0.05; Table 1).
Table 1. Cell number and proliferating cell number in zones of the neonatal cerebellum of 11β-HSD2−/− mice and C57BL/6 controls.
| Age | Genotype | BrdU-labeled cells in EGL | No. cells in EGL | No. cells in ML | No cells in IGL |
|---|---|---|---|---|---|
| P7 | C57BL/6 | 77±5.9 | 193±11.0 | 22±2.5 | 50±1.6 |
| 11β-HSD2−/− | 80±6.3 | 167±8.6 | 20±3.1 | 34±1.3* | |
| P14 | C57BL/6 | 13±1.7 | 59±5.6 | 57±4.6 | 55±3.0 |
| 11β-HSD2−/− | 16±2.0 | 55±6.2 | 54±6.3 | 61±1.4 | |
| Corticosterone treated | |||||
| P14 | C57BL/6 | 15±2.6 | 53±6.9 | 56±4.2 | 52±2.8 |
| 11β-HSD2−/− | 18±1.4 | 63±3.7 | 48±3.2 | 55±2.0 | |
The number of proliferating cells was assessed by BrdU labeling. Cell counts were assessed in a standard area for all cerebellar zones and values for each cerebellum are means of counts from three sections across the midsagittal cerebellum at P7 and 14. Values are means±S.E.M., n=4–5/group. * P<0.05 compared to C57BL/6 control
Cell proliferation was also assessed in the hilus, the proliferating layer of the hippocampal dentate gyrus at P7, 14 and 21. The number of cells that have undergone division in 24 h over this neonatal period was not different in 11ß-HSD2-/- mice or C57BL/6 mice. However, at 21 days the number of proliferating cells, though greatly reduced in both genotypes is less (P<0.05) in the 11ß-HSD2-/- compared with controls (Table 2).
Table 2. Number of proliferating cells in hilus region of the dentate gyrus of neonates.
| Age | C57BL/6 | 11β-HSD2−/− |
|---|---|---|
| P7 | 105±9 (5) | 133±19 (3) |
| P14 | 51±7 (4) | 59±7 (5) |
| P21 | 19±3 (5) | 11±1 (4)* |
The number of proliferating cells was assessed by BrdU labeling. Cell counts were averaged for each horn of the dentate gyrus of three coronal sections (~Bregma −2.06mm) for each brain. Values are means±S.E.M. (n). *P<0.05 compared to C57BL/6 control.
Apoptosis
The neonatal period is a period of proliferation in the cerebellum, but we wished to determine if the increased local tissue glucocorticoid exposure due to lack of 11ß-HSD2 in the cerebellum altered apoptosis, visualized with TUNEL stain. Apoptotic cells were extremely rare. However, there were no apparent differences in cerebellar apoptosis between the genotypes (data not shown).
GFAP staining
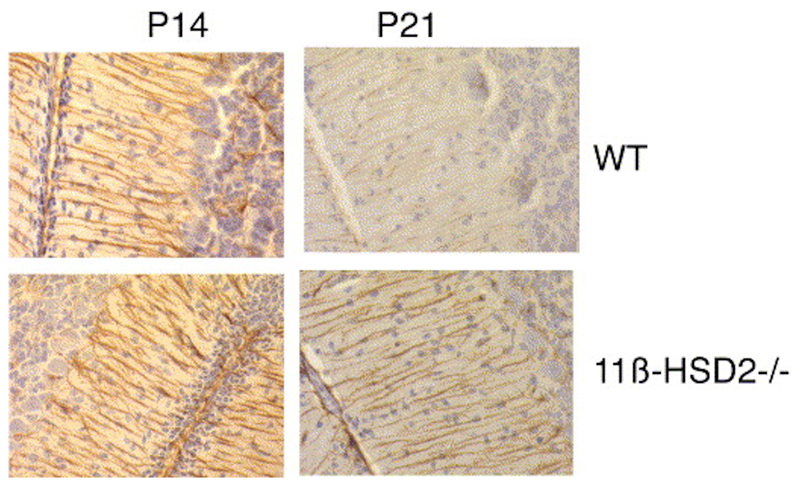
GFAP staining of the sagittal section of cerebellum shows striking Bergman glial projections emanating from cell bodies within the IGL and projecting down to endfeet in the EGL. At P14, there was no difference in the strong dense GFAP staining between 11ß-HSD2-/- and wildtype cerebella. However, at P21 the glial projections were less densely stained and were more fragmented in wildtype mice, whereas 11ß-HSD2-/- mice maintained a dense pattern of GFAP staining typical of the earlier stage of development (Fig. 4).
Fig. 4. Immunohistochemistry for GFAP staining of Bergman glia projections in cerebellum from C57BL/6 and 11ß-HSD2-/- neonates.
Representative midsagittal sections of cerebellum removed from C57BL/6 control or 11ß-HSD2-/- mice and stained for GFAP expression at P14 and 21. Glia projections appear more fragmented at P21 in C57BL/6 sections compared with 11ß-HSD2.
Exogenous corticosterone administration
A three-way ANOVA for genotype, age and corticosterone treatment revealed that there was a significant effect of genotype (F(1,69)=18.66, P=0.0001), and age (P=0.0001) but there was no significant effect of corticosterone treatment overall. However, exogenous corticosterone treatment abolished the difference in postnatal growth seen between untreated 11ß-HSD2-/- and control mice. Specifically, corticosterone decreased the weight gain in control mice, but had no additional effects on growth in 11ß-HSD2-/- mice (interaction of treatment and genotype F=16,67, P=0.0001; Table 3).
Table 3. Effect of corticosterone treatment on neonatal body weight (g) in 11β-HSD2−/−mice and C57BL/6 controls.
| C57BL/6 | 11β-HSD2−/− | |||
|---|---|---|---|---|
| −cort | +cort | −cort | +cort | |
| P7 | 3.91±0.14 (16) | 3.76±0.08 (35) | 3.07±0.18 (9) | 3.52±0.9 (13) |
| P14 | 7.87±0.17 (14) | 7.1±0.23 (10) | 6.4±0.20 (25) | 7.46±0.51 (5) |
| P21 | 10.01±0.21 (15) | 8.8±0.25 (10) | 8.32±0.65 (17) | 8.3±0.49 (6) |
Pups were weighed at P7, 14 and 21. Corticosterone-treated pups were given 50ng/g corticosterone i.p.. from P4–13. In the absence of corticosterone treatment, the 11β-HSD2−/− mice were significantly smaller (P=0.001, two-way ANOVA); however, when corticosterone was administered there was no significant difference between genotypes. Values are means±S.E.M. (n/group).
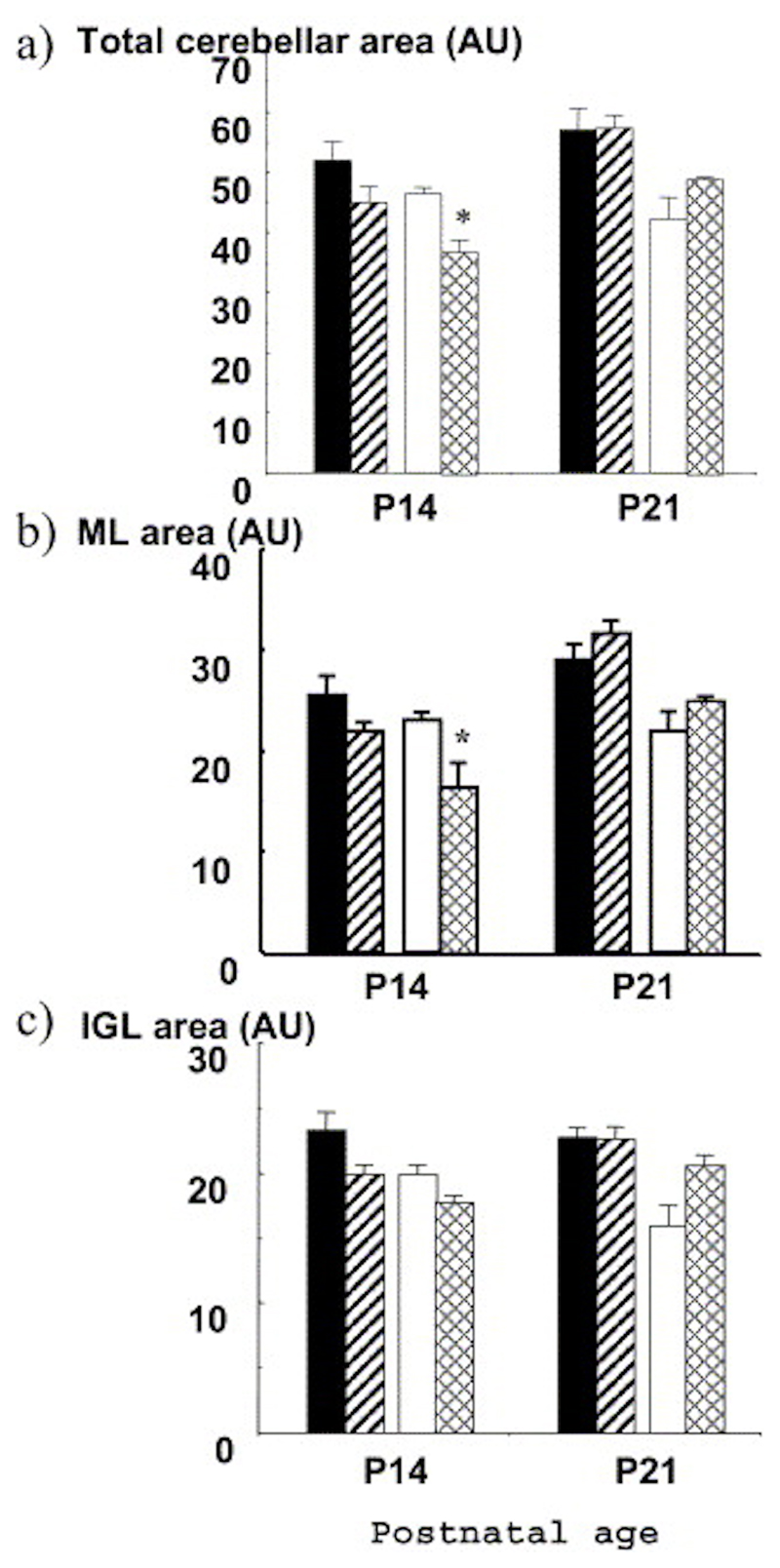
The size of the cerebellum was measured at P14 (24 h after the last administration of corticosterone) and at P21. At P14, there was a significant effect of genotype and corticosterone treatment on the size of the cerebellum, as well as on the ML and IGL individually. Post hoc examination revealed that the effect of exogenous corticosterone was only significant in 11ß-HSD2-/- mice, affecting both total cerebellar (P=0.005; Fig. 5a) and ML size (P=0.02; Fig. 5b), but not IGL size (Fig. 5c). By P21, the effects of exogenous corticosterone treatment were abolished as the size of the cerebellum in the 11ß-HSD2 ko mice showed a late catch-up growth spurt after the termination of the steroid treatment (Fig. 5).
Fig. 5. Altered cerebellar size following treatment with exogenous corticosterone. Corticosterone was administered to mice from P4-13.
The size of (a) cerebellum, (b) the ML and (c) IGL measured in midsagittal sections was compared in untreated C57BL/6 (black columns) and 11ß-HSD2-/- (open columns) mice with corticosterone treated C57BL/6 (striped columns) and 11ß-HSD2-/- (hatched columns) mice. Values are means±S.E.M., n=4– 6/group. * P<0.05 compared with untreated controls of the same genotype.
Numbers of proliferating cells in the EGL were also measured at the end of the corticosterone treatment (P14) as these are reported to be specifically sensitive to glucocorticoid manipulations, at least in the rat. Corticosterone did not affect the number of BrdU-labeled cells/area in the EGL of either genotype. Furthermore, there was no apparent difference in numbers of cells in ML and IGL per area with corticosterone treatment in either genotype (Table 1). Hence, although corticosterone decreased cerebellar size in 11ß--HSD2-/- mice, cell density remained unaltered.
Developmental landmarks
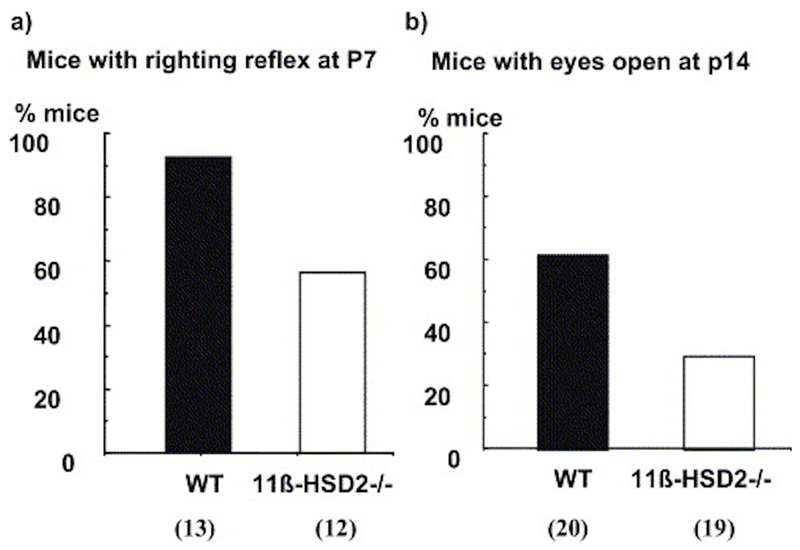
To determine the rate of development of postnatal functional neural maturation, two behavioral tests were used. Negative geotaxis, associated with cerebellar function, was examined at 7 days of age. Nearly all control C57BL/6 pups were competent at turning to face up a slope at P7 (92%), whereas only 58% of 11ß-HSD2-/- pups achieved this criterion (chi square=3.94, df=1, P<0.05; Fig. 6a). At 14 days of age, significantly less 11ß-HSD2-/- pups had full eyes opening compared with controls (chi square=4.36, df=1, P<0.05; Fig. 6b).
Fig. 6. Developmental landmarks.
Percentage of C57BL/6 mice (black columns) and 11[-HSD2-/- mice (open columns) that complete negative geotaxis within one minute at P7 and have both eyes open at P14. Number of animals/group is shown in parentheses.
Discussion
11ß-HSD2-/- pups are smaller during early postnatal growth, but overall brain weight is spared throughout. However the cerebellum, a region of the brain which expresses 11ß-HSD2 into the postnatal period and is still proliferating at this time, has reduced postnatal growth in 11ß-HSD2-/ mice. The reduction in cerebellar size was observed in both the granule and MLs and was coupled with delayed maturation of developmental reflexes.
We have demonstrated that the neonatal growth of the mouse cerebellum is sensitive to glucocorticoids, paralleling findings in the rat (Bohn and Lauder, 1978; Bohn, 1980). 11ß-HSD2-null mice show reduced growth in the cerebellum over the neonatal period. The loss of the 11ß-HSD2 barrier will allow glucocorticoids to access the cerebellum unhindered throughout this crucial period reducing the size of both the ML and IGL. Bohn and Lauder (Bohn and Lauder, 1978; Bohn, 1980) have shown that differential effects on the two layers can occur with discreet timing of the glucocorticoid exposure to coincide with the formation of the two zones. Exposing the mice to a further insult of exogenous corticosterone produces an additional effect on the cerebellar growth, that is more apparent in the 11ß-HSD2 knockout mice than the wildtypes, presumably because the lack of the enzyme allows greater access of active glucocorticoids to cerebellar cells. Also, following the removal of the exogenous steroid there is a rebound increase in growth, at a time where proliferation and growth are usually tailing off (between P14 and 21). This rebound growth has also been seen in rats subjected to exogenous glucocorticoids (Bohn and Lauder, 1978), and has been postulated to be due to prolongation of the proliferation period and EGL function by glucocorticoids.
In this study, although we saw dramatic effects on cerebellar size in the 11ß-HSD2-/- mice, we did not observe an alteration in neuronal proliferation. This is unlikely to be due to a species difference, as exogenous glucocorticoids have been found to alter proliferation in the mouse (Scheepens et al., 2003), albeit given prenatally. The failure to see differences in cell proliferation in this study may be due to the small observational windows (24 h around P7 and P14) chosen for analysis from the literature on the rat, which may not be at optimal times for cerebellar turnover in the mouse. In future studies, we will increase the time of BrDU exposure effectively enlarging the time windows and increasing the sample size of dividing cells. However, it has been reported recently, that glucocorticoids alter neonatal blood–brain barrier permeability and therefore [3H]thymidine incorporation may give a more realistic assessment of cell proliferation in the presence of glucocorticoids (Scheepens et al., 2003). Alternatively, the reduction in cerebellar size might reflect a predominant reduction in cell processes, an effect noted in hippocampal and prefrontal cortex neurons which show ‘pruning’ of dendrites when exposed to elevated glucocorticoid (Woolley et al., 1990; Magarinos et al., 1998; Cook and Wellman, 2004) coupled with a reduction in glia (O’Callaghan et al., 1991; Krugers et al., 1994). The additional explanation for reduced cerebellar size, an increase in apoptosis in response to glucocorticoids, does not seem likely as apoptotic cells were undetectable in either genotype or with exogenous glucocorticoid treatment.
The hippocampus is known to be an area of the brain that is particularly sensitive to the effects of glucocorticoids on cell proliferation and death (Sapolsky et al., 1985; Gould et al., 1990). Rats exposed to the stress of maternal separation or exogenous glucocorticoid administration during the neonatal period, show decreased hippocampal cell births (Gould et al., 1991) and decreased hippocampal neurogenesis as adults (Mirescu et al., 2004). This effect should be independent of 11[-HSD2 activity as this enzyme is not present in the hippocampus. In 11[-HSD2-/ mice, there was a small decrease in hippocampal neurogenesis at P21 confirming in this model that early-life (prenatal and/or postnatal) exposure to elevated glucocorticoids has adverse effects on hippocampal cell births.
11ß-HSD2-/- mice showed normal gross development, but exhibited a delay in attainment of developmental landmarks such as negative geotaxis or eye opening. It has previously been shown that cortisol administration in the neonatal rat delays the attainment of normal swimming posture (Anderson and Schanberg, 1975) and that maternal stress delays neonatal behavioral landmarks in rats (Patin et al., 2004) and humans (Rieger et al., 2004). The growth reduction in neonatal rats exposed to exogenous glucocorticoids, is proposed to be a consequence of elevated leptin levels and an increase in glucose: insulin ratio reflective of a catabolic state (He et al., 2004). As adults these rats have elevated NPY in hypothalamus, eat more and become obese, have a risk of diabetes (increased glucose:insulin ratios) and neurological impairment (loss of cerebral volume) (He et al., 2004). Hence, exposure of a neonatal rodent to elevated glucocorticoids by giving exogenous steroid, stressing the mother or removing 11ß-HSD2 activity (by gene targeting or enzyme inhibition), will alter growth of brain regions still proliferating during this period. The consequences of this are evident in the delay of developmental landmarks and other long-lasting behavioral and metabolic abnormalities. Intriguingly, postnatal dexamethasone therapy in premature human infants has a substantial deleterious effect on both neuromotor and cognitive function later in life (Yeh et al., 2004), suggesting that the developmental consequences of neonatal glucocorticoid excess, demonstrated here in the mouse cerebellum, are conserved in humans and persist.
Conclusion
In conclusion, loss of 11ß-HSD2 results in elevated exposure of the developing brain to active glucocorticoids. The consequence of this exposure is a smaller cerebellar size, a greater sensitivity to exogenous corticosterone and delayed developmental landmarks. We, therefore, suggest that 11ß-HSD2 acts to protect the developing nervous system against the deleterious consequences of glucocorticoid overexposure which may otherwise result in long-lasting behavioral and functional defects.
Acknowledgments
This work was supported by a project grant from the Wellcome Trust.
Abbreviations
- BrdU
bromodeoxyuridine
- cDNA
complementary DNA
- DAB
diaminobenzidine
- EGL
external granule layer of the cerebellum
- GFAP
glial fibrillary acidic protein
- HPA
hypothalamic–pituitary–adrenal
- IGL
internal granule layer of the cerebellum
- ML
molecular layer of the cerebellum
- MR
mineralocorticoid receptor
- P
postnatal day
- TdT
terminal-deoxynucleotidyl transferase
- TUNEL
terminal-deoxynucleotidyl transferase dUTP nick-end labeling
- UTP
uridine triphosphate
- 11[HSD2
11[-hydroxysteroid dehydrogenase type 2
References
- Anderson TR, Schanberg SM. Effect of thyroxine and cortisol on brain ornithine decarboxylase activity and swimming behavior in developing rat. Biochem Pharmacol. 1975;24:495–501. doi: 10.1016/0006-2952(75)90136-7. [DOI] [PubMed] [Google Scholar]
- Baud O. Postnatal steroid treatment and brain development. Arch Dis Child Fetal Neonatal Ed. 2004;89:F96–F100. doi: 10.1136/adc.2003.028696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benediktsson R, Calder AA, Edwards CRW, Seckl JR. Placental 11[-hydroxysteroid dehydrogenase type 2 is the placental barrier to maternal glucocorticoids: ex vivo studies. Clin Endocrinol. 1997;46:161–166. doi: 10.1046/j.1365-2265.1997.1230939.x. [DOI] [PubMed] [Google Scholar]
- Bohn MC. Granule cell genesis in the hippocampus of rats treated neonatally with hydrocortisone. Neuroscience. 1980;5:2003–2012. doi: 10.1016/0306-4522(80)90045-7. [DOI] [PubMed] [Google Scholar]
- Bohn MC, Lauder JM. The effects of neonatal hydrocortisone on rat cerebellar development: an autoradiographic and light microscopic study. Dev Neurosci. 1978;1:250–266. [Google Scholar]
- Brown RW, Diaz R, Robson AC, Kotolevtsev Y, Mullins JJ, Kaufman MH, Seckl JR. The ontogeny of 11[-hydroxysteroid dehydrogenase type 2 and mineralocorticoid receptor gene expression reveal intricate control of glucocorticoid action in development. Endocrinology. 1996;137:794–797. doi: 10.1210/endo.137.2.8593833. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Rosenfeld P, van Eekelen JAM, Sutanto W, Levine S. Stress, glucocorticoids and development. Prog Brain Res. 1988;73:101–116. doi: 10.1016/S0079-6123(08)60500-2. [DOI] [PubMed] [Google Scholar]
- Dent GW, Okimoto DK, Smith MA, Levine S. Stress-induced alterations in corticotropin-releasing hormone and vasopressin gene expression in the paraventricular nucleus during ontogeny. Neuroendocrinology. 2000;71:333–342. doi: 10.1159/000054554. [DOI] [PubMed] [Google Scholar]
- Diaz R, Brown RW, Seckl JR. Distinct ontogeny of glucocorticoid and mineralocorticoid receptor and 11[-hydroxysteroid dehydrogenase types I and II mRNAs in the fetal rat brain suggest a complex control of glucocorticoid actions. J Neurosci. 1998;18:2570–2580. doi: 10.1523/JNEUROSCI.18-07-02570.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards CRW, Stewart PM, Burt D, Brett L, McIntyre MA, Sutanto WS, de Kloet ER, Monder C. Localisation of 11[-hydroxysteroid dehydrogenase-tissue specific protector of the mineralocorticoid receptor. Lancet. 1988;ii:986–989. doi: 10.1016/s0140-6736(88)90742-8. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Cameron HA, Daniels DC, McEwen BS. Adrenal steroids regulate postnatal development of the rat dentate gyrus: II. Effects of glucocorticoids and mineralocorticoids on cell birth. J Comp Neurol. 1991;313:486–493. doi: 10.1002/cne.903130309. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, McEwen BS. Short-term glucocorticoid manipulations affect neuronal morphology and survival in the adult dentate gyrus. Neuroscience. 1990;37:367–375. doi: 10.1016/0306-4522(90)90407-u. [DOI] [PubMed] [Google Scholar]
- He J, Varma A, Weissfeld LA, Devaskar SU. Postnatal glucocorticoid exposure alters the adult phenotype. Am J Physiol Regul Integr Comp Physiol. 2004;287:R198–R208. doi: 10.1152/ajpregu.00349.2003. [DOI] [PubMed] [Google Scholar]
- Holmes MC, French KL, Seckl JR. Dysregulation of diurnal rhythms of serotonin 5-HT2C and corticosteroid receptor gene expression in the hippocampus with food restriction and glucocorticoids. J Neurosci. 1997;17:4056–4065. doi: 10.1523/JNEUROSCI.17-11-04056.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotelevtsev Y, Brown RW, Fleming S, Kenyon C, Edwards CRW, Seckl JR, Mullins JJ. Hypertension in mice lacking 11[hydroxysteroid dehydrogenase type 2. J Clin Invest. 1999;103:683–689. doi: 10.1172/JCI4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugers HJ, Medema RM, Postema F, Korf J. Induction of glial fibrillary acidic protein immunoreactivity in the rat dentate gyrus after adrenalectomy: comparison with neurodegenerative changes using silver impregnation. Hippocampus. 1994;4:307–314. doi: 10.1002/hipo.450040314. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, Orchinik M, McEwen BS. Morphological changes in the hippocampal CA3 region induced by non-invasive glucocorticoid administration: a paradox. Brain Res. 1998;809:314–318. doi: 10.1016/s0006-8993(98)00882-8. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Horvath KM, Luiten PG, Angelucci L, Catalani A, Koolhaas JM. Increased maternal corticosterone levels in rats: effects on brain 5-HT1A receptors and behavioral coping with stress in adult offspring. Behav Neurosci. 2001;115:1111–1117. [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nat Neurosci. 2004;7:841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP, Brinton RE, McEwen BS. Glucocorticoids regulate the synthesis of glial fibrillary acidic protein in intact and adrenalectomized rats but do not affect its expression following brain injury. J Neurochem. 1991;57:860–869. doi: 10.1111/j.1471-4159.1991.tb08230.x. [DOI] [PubMed] [Google Scholar]
- Patin V, Vincent A, Lordi B, Caston J. Does prenatal stress affect the motoric development of rat pups? Brain Res Dev Brain Res. 2004;149:85–92. doi: 10.1016/j.devbrainres.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Rieger M, Pirke KM, Buske-Kirschbaum A, Wurmser H, Papousek M, Hellhammer DH. Influence of stress during pregnancy on HPA activity and neonatal behavior. Ann N Y Acad Sci. 2004;1032:228–230. doi: 10.1196/annals.1314.026. [DOI] [PubMed] [Google Scholar]
- Robson AC, Leckie CM, Seckl JR, Holmes MC. 11beta-Hydroxysteroid dehydrogenase type 2 in the postnatal and adult rat brain. Mol Brain Res. 1998;61:1–10. doi: 10.1016/s0169-328x(98)00161-2. [DOI] [PubMed] [Google Scholar]
- Roland BL, Li KXZ, Funder JW. Hybridization histochemical localization of 11[-hydroxysteroid dehydrogenase type 2 in rat brain. Endocrinology. 1995;136:4697–4700. doi: 10.1210/endo.136.10.7664691. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. J Neurosci. 1985;5:1221–1226. doi: 10.1523/JNEUROSCI.05-05-01222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheepens A, van de Waarenburg M, van den Hove D, Blanco CE. A single course of prenatal betamethasone in the rat alters postnatal brain cell proliferation but not apoptosis. J Physiol. 2003;552:163–175. doi: 10.1113/jphysiol.2003.043414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Enthoven L, van der Mark M, Levine S, de Kloet ER, Oitzl MS. The postnatal development of the hypothalamic-pituitary-adrenal axis in the mouse. Int J Dev Neurosci. 2003;21:125–132. doi: 10.1016/s0736-5748(03)00030-3. [DOI] [PubMed] [Google Scholar]
- Viau V, Sharma S, Meaney MJ. Changes in plasma adrenocorticotropin, corticosterone, corticosteroid-binding globulin, and hippocampal glucocorticoid receptor occupancy/translocation in rat pups in response to stress. J Neuroendocrinol. 1996;8:1–8. doi: 10.1111/j.1365-2826.1996.tb00680.x. [DOI] [PubMed] [Google Scholar]
- Walker C-D. Ontogeny of the stress response in the rat: Role of the pituitary and hypothalamus. Endocrinology. 1986;118:1445–1451. doi: 10.1210/endo-118-4-1445. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- Yeh TF, Lin YJ, Lin HC, Huang CC, Hsieh WS, Lin CH, Tsai CH. Outcomes at school age after postnatal dexamethasone therapy for lung disease and prematurity. N Engl J Med. 2004;350:1304–1313. doi: 10.1056/NEJMoa032089. [DOI] [PubMed] [Google Scholar]