Abstract
Biodiversity and ecosystem service losses driven by land use change are expected to intensify as a growing and more affluent global population requires more agricultural and forestry products, and teleconnections in the global economy lead to increasing remote environmental responsibility. By combining global biophysical and economic models, we show that between the years 2000-2011 overall population and economic growth resulted in increasing total impacts on bird diversity and carbon sequestration globally, despite a reduction of land–use impacts per unit of GDP. The exceptions were North America and Western Europe, where there was a reduction of forestry and agriculture impacts on nature, accentuated by the 2007-2008 financial crisis. Biodiversity losses occurred predominantly in Central and Southern America, Africa and Asia with international trade an important and growing driver. In 2011, 33% of Central and Southern America and 26% of Africa’s biodiversity impacts were driven by consumption in other world regions. Overall, cattle farming is the major driver of biodiversity loss, but oil seeds production showed the largest increases in biodiversity impacts. Forestry activities exerted the highest impact on carbon sequestration, and also showed the largest increase in the 2000-2011 period. Our results suggest that to address the biodiversity crisis, governments should take an equitable approach recognizing remote responsibility, and promote a shift of economic development towards activities with low biodiversity impacts.
Agriculture and forestry activities are major drivers of biodiversity loss and ecosystem degradation1–3. Population growth and economic development will continue to increase the demand for agricultural and forestry products, and shift consumption patterns towards products with higher overall environmental burdens1,4. If unchecked, such strong demand-side drivers will cause higher pressures on biodiversity and ecosystems and put future well-being at risk5. Ensuring sustainable production and consumption patterns, by decoupling economic growth from natural resource use and environmental impacts, is fundamental to sustainable development6. However, teleconnections between world regions through international trade lead to an increasing disconnect between production and consumption, resulting in complex causal interrelationships, hampering straightforward analyses and resulting in governance challenges2,7–12. In this study we systematically analyse the global impacts of agricultural and forestry activities on biodiversity and a key ecosystem service, the sequestration of atmospheric carbon in ecosystems, taking these complex production-consumption interlinkages into account. We quantify the magnitude and dynamics of these pressures from agriculture, forestry and the consumption of biomass products between 2000 and 2011 and analyse the role of underlying drivers such as population growth, economic development and technological progress.
Assessing the impacts of socioeconomic activities on biodiversity and ecosystem services is complex due to their multidimensional nature13,14; this work covers one dimension of biodiversity and one ecosystem service. To assess the biodiversity impacts we focus on bird species richness, the species group best characterized in terms of responses to land-use activities2. We estimated, for each year, impending bird extinctions (i.e., number of species that would become extinct if land-use activities would be maintained in the long run) based on the number of endemic bird species in each biogeographical region (Methods and Supplementary Tables 1-3) and the amount and type of land being used for agriculture and forestry activities in each country or region (Methods and Supplementary Figures 1-2). We computed two estimates for the biodiversity impacts due to the uncertainties associated with the spatial information of the forestry activities. The non-conservative estimates are quantified for an upper bound estimation of forestry areas whereas the conservative estimates assume a smaller area of forestry activities by considering biomass harvest volumes and typical rotation times for managed forests (see Methods). In the manuscript text we refer to the conservative estimates unless explicitly stated otherwise. To assess the impacts on ecosystem services, we focused on net carbon sequestration, a key ecosystem service for climate change mitigation15. We estimated the biomass carbon sequestration lost each year, by calculating the potential additional carbon that would be sequestered if current land use ceased and natural vegetation was allowed to regrow (Supplementary Tables 4-5). We used the IPAT identity16 to study the role of the indirect socioeconomic drivers (population growth, economic development and technological progress) on biodiversity and ecosystem services losses. In order to quantify the consumption drivers we linked the two impact indicators to a multi-regional input-output (MRIO) model based on EXIOBASE 3, a new time series of MRIO tables (see Methods)17.
Results and discussion
Globally, between 2000 and 2011 we found increasing impacts of agriculture and forestry on biodiversity and ecosystem services; the number of bird species with impending extinction due to land-use activities increased 3% in our non-conservative estimates (from 118 to 121, Supplementary Tables 6-7) and 7% in our conservative estimates (from 69 to 74, Supplementary Tables 2-3), and the amount of carbon sequestration lost increased 6% (from 3.2GtC/year to 3.4GtC/year, Supplementary Tables 4-5). As a comparison, 140 bird species are estimated to have been lost since the beginning of the 16th century from all drivers combined18, and in the period 2002 – 2010, global carbon emissions were estimated at 8 ± 2 GtC/year (30 ± 8 GtCO2/year)19.
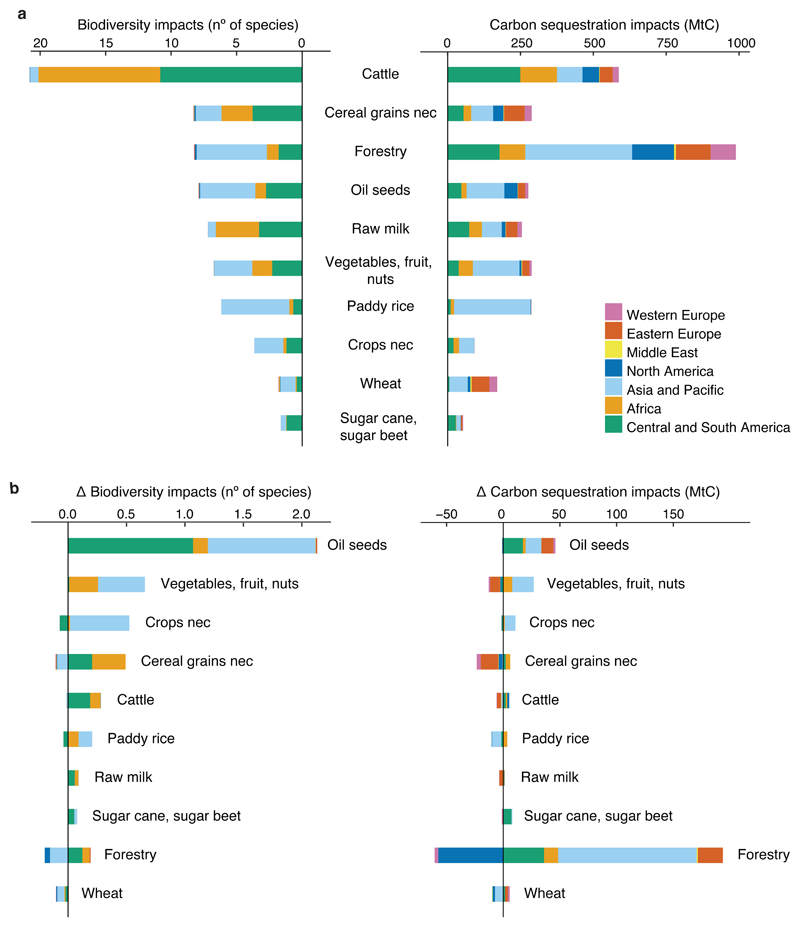
Our conservative estimates show that cattle farming had the highest impact on biodiversity, contributing to approximately 28% of total impending extinctions in 2011, mostly in Central and South America and in Africa (Fig. 1a and Supplementary Table 3). The production of oil seeds (including soy beans) was the activity with the highest contribution to the increase in impacts on biodiversity from 2000 to 2011 (Fig. 1b). The expansion of oil seeds production typically occurs at the expense of tropical forests20 rich in biodiversity. The activities with highest biodiversity impacts per unit area were non-specificed crops (crops nec), sugar crops and paddy rice (see Supplementary Table 8). Forestry activities, i.e. the use of forests for timber and woodfuel extraction, had the highest impact on carbon sequestration, contributing approximately 30% of the total carbon sequestration lost (Fig. 1a and Supplementary Table 5), and contributed most to the increasing losses from 2000 to 2011, albeit a strong reduction of forestry impacts occurred in North America (Fig. 1b). The activity with the highest carbon sequestration impacts per unit area was paddy rice, followed by non-specified crops (crops nec) and sugar crops (see Supplementary Table 9).
Figure 1. Production impacts on biodiversity and carbon sequestration per economic sectors.
a, Impacts in absolute terms for the year 2011; b, the difference between the impacts in 2011 and 2000. Negative values imply a decrease of their impacts by 2011. The left side are represents impending global bird extinctions (number of species) and on the right side carbon sequestration lost (MtC per year). Results are sorted by decreasing biodiversity impacts from production activities. The impacts of sectors accounting for less than 1% of the total are not shown. Nec stands for not elsewhere classified.
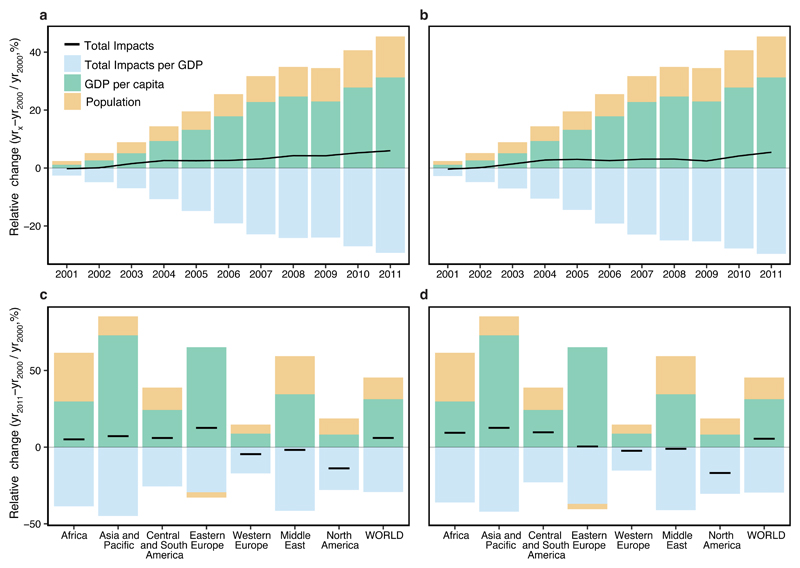
Economic and population growth have been driving the upward trend of impacts on biodiversity and ecosystem services, despite a reduction of the impacts per unit of GDP (Fig. 2a-b). We found in all world regions consistent reductions of the biodiversity and ecosystem services impacts per unit of GDP (Fig. 2c-d and Supplementary Figures 3-4); in Africa, Asia and Pacific, Central and South America and Eastern Europe, these were not sufficient to enable a reduction of the impacts caused by agricultural and forestry production activities. The reduction of biodiversity and ecosystem services impacts per unit of GDP reported in this work is a result of the higher increase of GDP in relation to the increase of biodiversity and ecosystem services impacts (due to increases in land use, Supplementary Table 10).
Figure 2. Decomposition of changes in impacts of agriculture and forestry on biodiversity and carbon sequestration into the contribution of the changes in population, GDP per capita and impact per GDP.
Biodiversity impacts are measured in terms of impending global bird extinctions, and ecosystem services impacts in terms of carbon sequestration lost. Impacts can be decomposed as (Methods): Δ Impacts = Δ Population × Δ GDP per capita (i.e., affluence) × Δ Impacts per GDP (i.e., land-use efficiency). Annual changes in production impacts relative to 2000 (Δ) at the global level for biodiversity (a) and ecosystem services (b), overall changes between 2000-2011 for different world regions for biodiversity (c) and ecosystem services (d).
The overall decrease of the production impacts in Western Europe, Middle East and North America could indicates a decoupling of biodiversity and carbon sequestration impacts from economic growth. However, analysing decoupling trends only by assessing impacts from production activities taking place within a region might be misleading; a region may effectively import the environmental impacts from another region (“displacement effects”)21. Therefore, we used a MRIO model to assess the impacts from consumption activities.
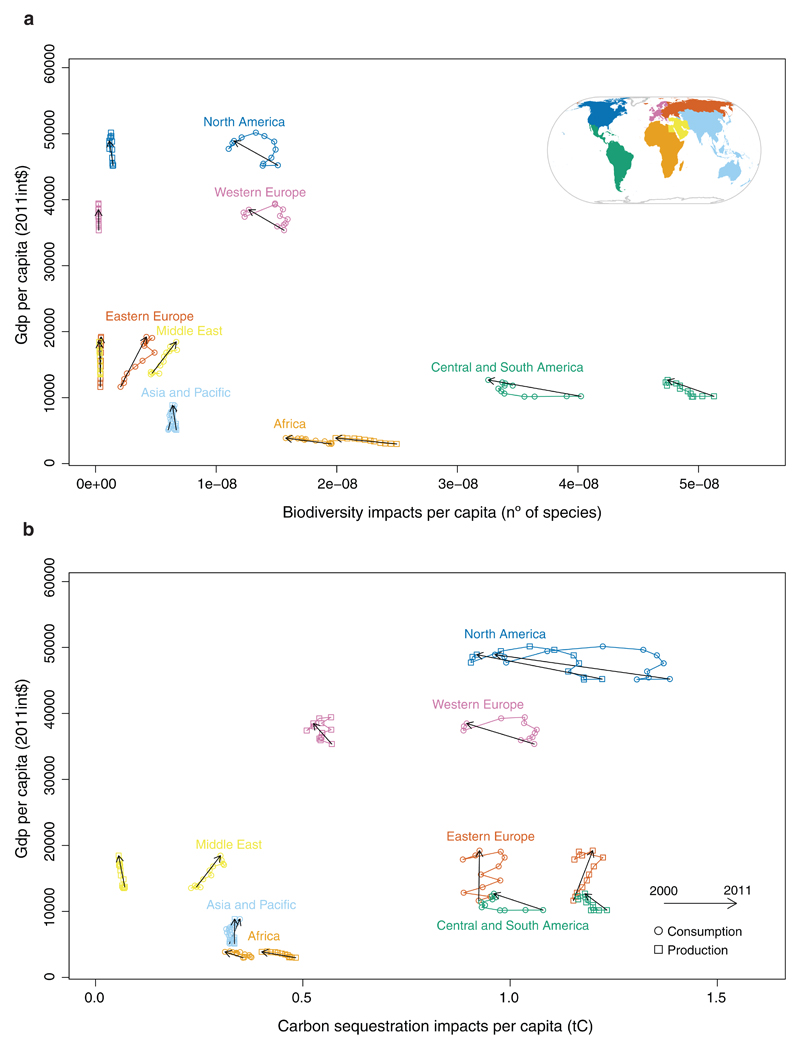
The comparison between per capita impacts from a production and consumption perspective for the different world regions shows that the consumption patterns of an average citizen in North America, Western Europe, Eastern Europe and Middle East is driving biodiversity impacts elsewhere, i.e. consumption impacts are up to an order of magnitude greater than the production impacts for those regions, (Fig. 3a), and the same happens for carbon sequestration except for Eastern Europe (Fig. 3b). Interestingly, between 2000 and 2011, per capita consumption impacts decreased in North America, Western Europe, Africa and Central and South America (Fig. 3a-b). In contrast, in Eastern Europe, Asia and Pacific and Middle East consumption impacts per capita increased (Fig. 3a-b), reflecting the recent rapid economic expansion of these regions.
Figure 3. GDP per capita (in constant 2011 international$) and per capita impacts on biodiversity and carbon sequestration, per world region.
Consumption and production impacts on biodiversity (a) as global impending bird extinctions (number of species per capita and year) and ecosystem services (b) as carbon sequestration lost (tC per capita and year). Consumption impacts are represented by a circle, production impacts by a square. The arrows show the trend on the impacts between 2000 (starting point) and 2011 (tip of the arrow). Inset map was created based on Natural Earth countries boundaries and the United Nations regional groups using ArcGIS software version 10.2.1.
The decrease on the biodiversity and ecosystem services impacts per unit of GDP from both a production and consumption perspective shows that decoupling between economic growth and impacts occurs in Western Europe and North America, but not in the Middle East (Supplementary Figures 3-4). While the decoupling in production impacts is expected, due to decreases in land use in both regions during the period analysed (Supplementary Table 10), the decoupling in per capita consumption impacts is surprising and requires a reduction of consumption and/or an increase of the efficiency in the regions exporting to Western Europe and North America. In Western Europe, the consumption impacts on biodiversity and carbon sequestration decreased between 2007 and 2009 and in North America between 2006 and 2009. After 2009 there is again an increase in impacts for biodiversity, although by 2011 they were still below their 2001 levels. These results reflect the financial crisis and consequent decrease in consumption that occurred in these regions. The decreases of the biodiversity impacts associated with agricultural activities are mainly due to decreases of impacts associated with food consumption in hotels and restaurants and clothing purchases by consumers, both in Western Europe and North America (Supplementary Figures 5-6). These sectors are amongst those whose consumption was most affected during the financial crisis22,23. The decreases of the biodiversity and carbon sequestration impacts associated with forestry activities are mainly due to decreases of impacts associated with manufacturing, construction and products of forestry sectors (Supplementary Figures 5-6). Such findings reflect the reduction of the activity of the construction sector in both regions as a direct consequence of the financial crisis23–25.
In any case, consumption based on internationally traded goods was driving 25% and 21% of the global impacts on biodiversity and carbon sequestration in 2011, representing a 3% and 1%, increase in relation to 2000, respectively (Fig. 4 and Supplementary Table 11-12). In 2000, Western Europe and North America were responsible for 69% and 58%, of the biodiversity and carbon sequestration impacts transferred through international trade; in 2011 these shares were reduced to 48% in the case of biodiversity impacts and 41% in the case of the carbon sequestration impacts (Fig. 4). In contrast the shares of other regions were increasing fast: for example, Asia and Pacific drove 13% in 2000 and 23% in 2011 of the biodiversity impacts embodied in international trade; and 20% in 2000 and 29% in 2011 of the carbon sequestration impacts embodied in international trade (Fig. 4 and Supplementary Table 11-12).
Figure 4. Biodiversity (a,2000; b,2011) and carbon sequestration (c,2000; d,2011) impacts embodied in international trade.
On the left is the region where the impacts occur and on the right is the region whose consumption is driving the impacts. The width of the flows represents the magnitude of the impacts. Exact values can be found in Supplementary Tables 11-12. Impacts arising from domestic production and consumption are not included in this figure. The visualized impacts represent 22%, 25%, 19% and 21% of the yearly global totals, respectively for biodiversity and carbon sequestration lost.
A complex analysis as the one presented here has several associated uncertainties and limitations, some of which we discuss in the Methods section, particularly those related with identifying forest areas under active management, the affinity parameter values of the countryside species-area relationship, and the necessity to follow two different approaches for the impacts on carbon sequestration from agriculture and forestry activities, which make these values comparable only on the short-term. In addition, it is particularly important to highlight that our analysis does not account for the effects of agriculture intensification (e.g., the response of biodiversity to different intensification levels of farmland was not discriminated in our calculations). Therefore, our estimates of impending extinctions due to land-use activities can be considered a lower bound for the likely range of values. As some of the recent trends in land-use change have been on intensifying levels of production (i.e., yields per area of farmland use) we may also overestimate the reductions of biodiversity and ecosystem services impacts per unit of GDP of the last decade26,27. In addition, the decomposition of the impacts into the product of population growth component, economic growth, and efficiency change has been criticized for not considering other driving forces and for ignoring more complex interactions between these three components28.
Decoupling economic development and population growth from environmental impacts and natural resource use, e.g. via technological progress, is often seen as the solution to the current sustainability challenges6,29. Our analysis highlights several intricacies related to such a perspective. In developed regions, a relative decoupling is observed, however it occurred associated with the financial crisis. Following the financial crisis there was again an increase in impacts, suggesting that this effect might be transient. In developed regions more than 90% of the biodiversity impacts from consumption as well as 40% of the carbon sequestration impacts from consumption, on average between 2000 and 2011, were outsourced. This is of particular concern in terms of global equity. The upcoming discussion of the parties to the Convention on Biological Diversity on the post-2020 biodiversity strategy should consider remote responsibility in an equitable way. Policies need to be tailored for each region and biodiversity and ecosystem services need to be mainstreamed into specific sectors. For developing regions, continuous population growth and rapid economic development outweigh the decrease in impacts per unit of GDP. In these regions biodiversity issues might co-benefit from the progress towards other SDG goals which might attenuate population growth30. For developed regions and emerging economies, policies need to address the increasing teleconnection through designing policies based on consumption-based accounting to avoid any biodiversity and ecosystem services impact leakage. Our work supports recent calls for changes in production and consumption patterns31,32, and it shows the importance of taking into account time trends as well as all economic sectors’ processes to properly identify the drivers of increasing impacts on biodiversity and ecosystem services.
Methods
The starting point for the quantification of the drivers of biodiversity and ecosystem services loss was a spatially-explicit land-use dataset, with information on 14 categories of land-use activities which cover all the agricultural and forestry production reported in authoritative international databases (FAOSTAT). This enabled determining the impacts to biodiversity and ecosystem services per km2 of land-use activity (the so-called characterization factors). The characterization factors together with a time series of land-use data for 49 countries/world regions were used to determine the total impacts on biodiversity and ecosystem services, for the period 2000-2011. These are the impacts driven by the production activities (agriculture and forestry). To determine the consumption patterns driving biodiversity and ecosystem services loss we coupled the impacts from production activities to a multi-regional input-output model. We used the IPAT identity to distinguish the influence of population growth (P), economic development (A) and technological progress (T) on the evolution of the drivers of biodiversity loss and ecosystem degradation. The results were aggregated into 7 world regions, using EXIOBASE’s world regions and the United Nations regional groups33. In the following sections the methods are presented in detail.
Land-use spatially explicit dataset
A spatially explicit land-use dataset for the year 2000, matching the sectoral resolution (for land-use activities) of the EXIOBASE dataset (see below Multi-regional input-output analysis and Supplementary Table 13), was developed to assess the biodiversity impacts as well as carbon sequestration lost due to agriculture and forestry activities17. The starting point of the assessment was the construction of a consistent and comprehensive set of layers at the spatial resolution of 5 arc minutes. We followed a previously published approach34 and used a series of recent datasets for the year 2000 (restricted to this year by the availability of comprehensive cropland maps which currently are only available for the year 2000) to create the individual layers. A cropland layer35 was adjusted to reproduce newly published national statistics for cropland area for the year 2000 (based on the regular updates by FAO36 and data on cropland distribution35) by increasing or decreasing cell values equally distributed to match the updated national sums. The cropland layer was split into nine sub-layers (corresponding to crop-categories in EXIOBASE) using the distribution of major crop groups37: (a) paddy rice, (b) wheat, (c) cereals, grains nec (not elsewhere classified) (d) vegetables, fruit and nuts, (e) oil seeds, (f) sugar cane, sugar beet (g) plant-based fibres, (h) crops nec such as herbs and spices and (i) fodder crops (Supplementary Figures 1-2 and Supplementary Table 13). Next, a recent global forest map was integrated into the dataset38. This dataset is based on the integration of recent high-resolution tree cover maps and a validation procedure through citizen science approaches, and applies a single definition of “forest” globally. Compared to FAO data this leads to a lower global forest cover estimate (32 Mkm2 vs 42 Mkm2). Individual input data and maps for the construction of the land-use dataset origin from different sources. The resulting inconsistencies have been solved the following way: in grid cells where the sum of all allocated layers (cropland, built-up and infrastructure, and the forest layer) exceeded 100%, the forest layer was capped so that all land-use types fill 100% of the grid cell, assuming that information on cropland and built-up areas was more reliable than on forests. Information on intact forests39 was used to identify unused forests. The layer of permanent pastures was derived from35 and added to the grid, also here capping the pasture layer at 100% total land use coverage in each grid cell. The permanent pasture dataset is largely consistent with FAO statistics for permanent pastures, but uses national and subnational statistics and corrects the FAO data based on top-down considerations (e.g., on the maximum extent of grazing activities, corrections based on alternative statistics, and plausibility checks, e.g., with remote sensing data35). In consequence, the total sum for permanent pastures is 27Mkm2 (in contrast to 35Mkm2 in FAO). By taking non-productive areas (aboveground NPP below 20gC m-2 yr-1) into account34, permanent pasture land was further reduced to 23km2. This reduction occurs mainly in dryland areas of Australia and central Asia and assumes that permanent pastures at a very low productivity do not contribute to grazing. Fodder crops were split into five separate layers (raw milk, cattle meat, pig meat, poultry and other meat), and permanent pastures into three layers (raw milk, cattle meat, other meat)40, matching the available livestock sectors in EXIOBASE (Supplementary Figures 1-2). The remaining areas can be considered under extensive, sporadic use, mainly for temporary livestock grazing and wood fuel collection. However, no biodiversity or ecosystem service impacts were allocated to them due to large uncertainties about the dimension and nature of the impacts of land use on these lands.
Correction of forest areas for quantification of biodiversity impacts
The approach described above gives an estimate of all forest areas that are not considered areas of wilderness. These are in many cases an overestimation of the areas that are actively managed for forestry activities. Which are the activities considered in our economic model (see Multi-regional input-output analysis). To account for this, we used an alternative approach to estimate the area of managed forests: we first estimated the forest area that would have to be cleared to produce the harvest volumes (see Characterization factors for carbon sequestration impacts for details on how biomass harvest data were assessed), assuming clear-cut regimes. To convert the estimates of harvest volumes into areas we assumed that biomass stocks at the time of harvest equal the average national potential biomass stocks (i.e., the stock that would prevail without land use but under current climatic conditions; from refs.41,42). In order to determine an estimate of forest area actively managed, we multiplied the amount of clear cut area by the estimates of typical rotation times43,44 (Supplementary Table 14). Following this procedure yearly correction coefficients for each country were determined (Supplementary Table 15).
In general, this estimate should give areas smaller or similar to the area calculated via the spatially explicit land-use datasets. In a few cases the numbers were higher, owing to uncertainties in all the data involved. To arrive at a conservative estimate, we used the smaller number of the two approaches as the area of managed forests considered in the biodiversity impact assessment, with the affinity parameter of the countryside species area relationship set for intensive forestry use (see Characterization factors for biodiversity impacts). We have also computed the biodiversity impacts associated with the higher non-conservative estimates of forest area under active management, for these estimates the affinity parameter of the countryside species-area relationship was set as the average value between the affinities for intensive and extensive forest use. (Supplementary Table 16). The results are reported in Supplementary Tables 6-7.
The correction of the forest area for the quantification of the impending bird extinctions resulted in a smaller biodiversity impact. With the conservative estimates the number of bird species with impending extinction due to land-use activities was 69 and 74 in the year 2000 and 2011, respectively. With the non-conservative estimates these values were 118 and 121, for 2000 and 2011 respectively. The results presented and discussed in the paper are the conservative estimates.
Characterization factors for biodiversity impacts
In order to quantify potential global bird species extinctions due to different land-use activities, we started by computing characterization factors (CFs) for each land-use activity (number of birds potentially extinct per km2 of area used by land-use activity), based on the land-use dataset described in the previous section. To compute the extinctions associated to each individual land-use activity we used the countryside species-area relationship (cSAR)45–47. Species-area relationship models have been classically used to assess species extinctions after habitat loss, however this approach has a number of limitations. One issue is assuming that the number of species is mainly determined by habitat area, and that the habitat is uniform and continuous48,49. Another issue, that we believe to be even more prevalent, is that the classic SAR only captures the species richness response to changes in native habitat area, overlooking the diversity of species responses to changes in habitat composition. The countryside species-area relationship45 describes the use of both human-modified and natural habitats by different functional species groups. Consider a completely natural landscape where habitat conversion takes place and only a single functional group of species is present. Then, according to the cSAR, the proportion of species remaining after habitat conversion is46,47
| (1) |
where n is the number of habitat types, hj is the affinity of species to non-natural habitat j (hereafter called land-use activity j), h1 is the affinity of species to the natural habitat, Aj is the area occupied by the different land-use activities j, A1 the area of natural habitat before conversion takes place and z is a constant indicating the rate at which species richness increases with area. The superscript 0 indicates the natural state, and the superscript 1 indicates the modified state (i.e., after land-use change occurred). We used a value of z = 0.20, as it is an appropriate value for the spatial scales used in this work (biogeographical region)50,51. We assumed that species have maximum affinity for the natural habitat (h1=1) For human-modified habitats we calculated affinities as46:
| (2) |
where σj is the mean sensitivity of the species to each land-use activity j. Sensitivity values (σ) were retrieved from previously published global databases52–54 of studies of biodiversity responses to human-modified landscapes (Supplementary Table 17). From these databases, we selected studies that provided data on bird species richness on both natural habitat and at least one human-modified habitat (i.e., land-use activity), as σj is the difference between the plot scale species richness found in the modified habitat of type j and the species richness in the native habitat (i.e., the proportion of species disappearing at the plot-scale in modified habitats), which led to a total of 319 pairwise comparisons. The data was subset into four land use classes based on the description of the habitat given in the source dataset: managed forest (extensive and intensive use), cropland, permanent crops and pastures; and two major biomes, tropical and temperate (Supplementary Table 17). From these σj values and hj were computed (see Supplementary Tables 16-17). The correspondence between the habitats types used for the computation of the hj values and the categories in our land-use dataset can be found in Supplementary Table 13.
Using ArcGIS version 10.255, we overlaid the land-use layers (see previous section for details on the spatially explicit land-use dataset), with a biogeographic region layer56 to derive the current share of each of the fourteen land-use activities (13 agricultural types and forestry), Aj, per biogeographic region g, Ag,j. We used equation (1) to calculate the proportion of endemic species remaining after land-use change in each of the 19 biogeographical regions, with as the area of the biogeographic region g. Bird species’ distribution maps57 were used to derive the number of endemic species present in each of the biogeographic regions (Sg), 1295 endemic bird species were identify across all biogeographic regions (Supplementary Table 1), which represents approximately 12% of the total number of bird species reported in ref.57. The total number of endemic species lost in each biogeographic region, ΔSg was calculated as:
| (3) |
where Sg is the number of endemic species in a biogeographic region as determined through bird species distribution maps57. Then, the total number of species lost per land-use activity j in each biogeographic region g was computed as follows,
| (4) |
where wj = (1 - hj) is a weight that reflects the impacts of the different land-use activities and n the number of land-use activities considered. For each biogeographic region g, the number of species lost due to each land-use activity j in each country i was then determined by taking into account the area of each land-use activity in each country that crosses the biogeographic region, Ag,i,j:
| (5) |
If a country was covered by more than one biogeographic region, the total impacts consisted on the sum of the impacts per biogeographic region:
| (6) |
where Gi is the number of different biogeographic regions in country i. The biodiversity characterization factors, CFs, were then determined by dividing the ΔSi,j by the area of each land-use activity j in each country i:
| (7) |
The biodiversity CFs (bird species potentially lost per km2 of land use) were multiplied by the land-use data time series (see Multi-regional input-output analysis) to obtain the impending birds extinctions in every year. All calculations were performed using Python58.
Previous studies52,59, applying the countryside species area relationship at the global level, determined that the parameter associated with the responses of species to habitat changes was the one contributing the most to the uncertainty of the characterization factors. This is mostly a result of the broad range of values reported for species response to habitat changes spanning from positive to negative (i.e., from a detrimental effect to a beneficial one) and a heterogeneous distribution of the data in terms of taxa and biogeographical regions covered. In this study we focused on the birds group, the one which is best covered in terms of number of studies assessing their response to land-use change2. Despite limiting the uncertainty of our results by covering just one species group, it is still important to mention that the range of the values and the unbalanced geographical distribution (Supplementary Figure 7) (e.g., for temperate biogeographical regions there are 82 data points whereas for tropical there are 237 data points) are still important sources of uncertainty in the determination of the characterization factors. By using birds as a single functional group, we assume that all bird species respond equally to land use and habitat loss, also by considering broad geographic areas we ignore the effects of the particular characteristics of habitats48.
Characterization factors for carbon sequestration impacts
Ecosystems store large amounts of carbon in living biomass providing a crucial climate regulation service. Globally, the largest amounts of biomass carbon are stored in forest systems42. Agricultural activities replace these natural ecosystems with agro-ecosystems (cropland and pasture) that provide higher amounts of biomass flows useful for society, but massively reduce vegetation carbon stocks. Forestry lowers biomass carbon stocks through wood harvests, even if practiced sustainably, as forestry operations optimize the annual wood increment, which leads to lower biomass carbon stocks compared to forests not under harvest regimes42,60. When agricultural and forestry practices cease, systems can regenerate towards a more natural state. We estimated the carbon sequestration potential on land currently under use that would prevail in the absence of land use: the carbon sequestration potential lost. It is important to note that this potential is expressed as annual flow, but these flows cannot be expected to continue infinitely as biomass carbon stocks in ecosystem without land use will saturate at some point. Thus, the indicator reflects short-to-medium term conditions only. This assumption, however, allows to unambiguously link carbon stock impacts and current land-use activities, irrespective of the long legacy effects of past land uses on biomass carbon stocks42,61,62, and thus avoids incorrect attributions.
For agricultural land use, we assign the effect of land conversion (i.e., clearing of forests to agricultural fields) to the agricultural sectors in EXIOBASE (Supplementary Table 13). We based our calculations on the land-use maps described in the land-use dataset section (see Land-use spatially explicit dataset) and combine them with a map of the biomass carbon stocks in the potential natural vegetation41 (i.e., the vegetation that would prevail without human land use). Due to large uncertainties relating to biomass carbon stocks of non-forest ecosystems we perform the assessment only for agricultural land on potentially forested areas. These sites were identified by combining three biome maps63–65, and assuming potential forest cover where two of the three maps report a forest biome. Because of the omission of lands without potential forest cover, our estimate on the impact of agriculture on biomass carbon stocks should be considered conservative.
We assume that in absence of agricultural land use, vegetation would grow back to 75% of the potential natural carbon stock value within 50 years61. The calculations are performed on a global grid with a resolution of five arc minutes. The annual carbon sequestration lost (ΔC) in agricultural land-uses activities j, per grid cell m is calculated as:
| (8) |
where is the potential biomass carbon stock per unit area in the grid cell m and Am,j is the area of agricultural land-use activity j in the grid cell m. In equation (8) we implicitly assume that the biomass stock of agricultural land is negligible compared with the potential carbon stock42. To link the indicator to the multi-regional input-output model an indicator per country i and land-use activity j was computed:
| (9) |
where ΔCi,j represents the amount of carbon sequestration lost due to each land-use activity j in each country i, and Mi is the number of grid cells per country i.
For forestry a different approach was required to account for the effect of forest management on biomass carbon stocks. The difference between potential biomass carbon stocks and current biomass carbon stocks is not a good proxy for this effect, as this difference is largely influenced by land-use histories and not solely by present use42. To unambiguously account for the effect of forestry on biomass carbon socks, we focus on wood harvest, the main purpose of forestry activities. We assume that, at the national level, annual carbon sequestration lost due to forestry equals the biomass removed by wood harvest (industrial roundwood and fuelwood) activities in a given year62. For this we convert annual wood harvest quantities from ref.36 into carbon, taking into account bark and other biomass destroyed in the harvest process, but not removed from the forests, correcting for the fact that part of this biomass was foliage and would not have contributed to long term carbon sequestration (factors from ref.66). Part of the harvested wood is stored in long lived products, representing a form of carbon sequestration. We account for this, by deducting amount of industrial roundwood that ends up in such products (about 20% of harvested industrial roundwood globally, based on ref.67). The national level data for annual carbon sequestration lost due to forestry, ΔCi,forestry, were aggregated where necessary to match EXIOBASE’s regional resolution (Supplementary Table 18). This approach disregards ecosystem effects such as compensatory growth and thus only holds for a short term perspective, but gives an indication on how forestry practices currently lower the potential sink function of biomass in ecosystems60,68,69.
The ecosystem services characterization factors, CFs, were then determined by dividing the ΔCi,j by the area of each land-use activity j in each country i:
| (10) |
Similarly to the biodiversity CFs, the ecosystem services CFs (carbon sequestration lost per km2 of land use) were multiplied by the land-use data time series (see Multi-regional input-output analysis) to obtain carbon sequestration lost in every year. The calculations were performed using MATLAB.
Multi-regional input-output analysis
Multi-regional input-output (MRIO) analysis has been increasingly used to identify the consumption drivers of environmental impacts. Environmental impacts analysed within a MRIO framework include emissions of pollutants, appropriation of natural resources and loss of biodiversity7,70–72. Environmentally-extended MRIO (EEMRIO) models are particularly suited to track the spatial disconnection between environmental pressures from production processes and the consumption drivers behind them as they cover the world economy and the international trade relations between different countries and sectors. In this work we followed the standard Leontief model to compute the biodiversity and ecosystem services impacts from consumption activities. The standard environmentally extended Leontief pull model is formulated as follows73:
| (11) |
Where (for i countries and m economic sectors):
E is the (1 x i) matrix of environmental impacts associated with final demand of each country.
f is a (1 x i.m) direct intensity vector, which gives the environmental pressures (biodiversity and ecosystem services losses) associated with 1€ of production of the economic sectors. Since in this work we quantified the biodiversity and ecosystem services losses associated with land-use activities this vector will be a sparse vector only populated in the entries for land-use activities. The biodiversity and ecosystem services losses are calculated by multiplying the previously determined characterization factors (CFs) by the amount of land used in each year by a given land-use activity. The amount of annual land used was extracted from the MRIO database used (see below for more details).
A is the (i.m x i.m) matrix of technical coefficients, which gives the amount of inputs that are required to produce 1€ of production.
Y is the (i.m x i) matrix of final demand in monetary terms.
I is the (i.m x i.m) identity matrix.
The matrix inversion is represented by the exponent -1.
More details on the calculations underlying environmental input-output analysis can be found elsewhere 8,74,75.
The MRIO database used in this work was EXIOBASE 3; this database provides a harmonized time series of MRIO tables and environmental extensions ranging from 1995 to 201117, sectoral disaggregation of 200 products and 49 regions/countries (Supplementary Table 18-19). Particular important to this work and for the time-series calculation of the biodiversity and ecosystem services are the land-use accounts, developed consistently to the spatial explicitly land-use data set17.
MRIO models are top-down models that assume a linear relationship between a unit of demand, and the production (and, in this case) land use required to produce goods and services along the supply chain. Accuracy of MRIO analysis is estimated to be in the order of 10-20% at the national level76,77, given a consistent coverage of the account for the environmental pressure (in this case, land use). High sector detail helps to reduce this uncertainty78,79, and the EXIOBASE MRIO model provides the highest harmonized sector detail available80. Regional aggregation affects results in a similar way to product aggregation81. Whilst many comparative MRIO studies find quantitative differences between databases, they also point to robust trends for consumption based accounts observed in all EEMRIO studies such that qualitative conclusions from the quantitative data are reliable76–83.
IPAT Identity
We used the IPAT identity81 to distinguish the influence of population growth (P), economic development (A) and technological progress (T) on the evolution of the drivers of biodiversity loss and ecosystem degradation through time:
| (13) |
I refers to impacts (on biodiversity and ecosystem services), in this work the absolute amount of impacts was determined from a supply side perspective, by multiplying the CFs with land-use data, and from a demand side perspective through multi-regional input-output analysis. P refers to population. A refers to affluence measured as Gross Domestic Product (GDP). is the metric of affluence in per capita terms. is a metric of technological progress and it measures the impacts per unit of GDP. The higher the value, the lower the economic efficiency as more impacts are generated per unit GDP. Population data was retrieved from ref.84 and GDP data was collected in 2011 international dollars (corrected for purchasing power parity) from ref.85.
Supplementary Material
Acknowledgements
Authors would like to thank the financial support provided by EU-FP7 project DESIRE (project no. FP7-ENV-2012–308552). K.H.E. and T.K. have been funded by the Austrian Science Fund project GELUC (project no. P29130), the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) project no. KA 4815/1-1 and ERC grant (ERC-2010–263522 LUISE). K.H.E., T.K. and C.P. have been funded by the Vienna Science and Technology Fund (WWTF) through project no. ESR17-014. T.K. acknowledges support from the Swedish Research Council Formas (project no. 231–2014–1181). M.A.J.H. was supported by the ERC grant (ERC—CoG SIZE 647224).
Footnotes
Data availability
The authors declare that all the data, except the land use spatially explicit dataset, supporting the findings of this study are available within the paper and its supplementary information files. The land-use spatially explicit dataset and the computer code used in this work are available upon request.
Author Contributions: All authors provided input into the final manuscript. A.M., I.S.M., M.B., M.A.J.H., T.K., K.E. and H.M.P. designed the study. A.M., I.S.M., T.K., C.P., M.T., N.E., K.H.E., R.W. and K.S. contributed data. A.M., I.S.M. and T.K. performed the analysis. A.M. and H.M.P. wrote the paper with help from all the authors and coordinated the study.
Competing interests
The authors declare no competing interests.
References
- 1.Venter O, et al. Sixteen years of change in the global terrestrial human footprint and implications for biodiversity conservation. Nat Commun. 2016;7 doi: 10.1038/ncomms12558. 12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newbold T, et al. Global effects of land use on local terrestrial biodiversity. Nature. 2015;520:45–50. doi: 10.1038/nature14324. [DOI] [PubMed] [Google Scholar]
- 3.MA. Millenium Ecosystem Assesment - Ecosystems and human well-being. Island Press; 2005. [Google Scholar]
- 4.West PC, et al. Leverage points for improving global food security and the environment. Science. 2014;345:325–328. doi: 10.1126/science.1246067. [DOI] [PubMed] [Google Scholar]
- 5.Cardinale BJ, et al. Biodiversity loss and its impact on humanity. Nature. 2012;486:59–67. doi: 10.1038/nature11148. [DOI] [PubMed] [Google Scholar]
- 6.UN. Transforming our World: the 2030 Agenda for Sustainable Development. United Nations; 2015. [Google Scholar]
- 7.Lenzen M, et al. International trade drives biodiversity threats in developing nations. Nature. 2012;486:109–112. doi: 10.1038/nature11145. [DOI] [PubMed] [Google Scholar]
- 8.Wilting HC, Schipper AM, Bakkenes M, Meijer JR, Huijbregts MAJ. Quantifying Biodiversity Losses Due to Human Consumption: A Global-Scale Footprint Analysis. Environ Sci Technol. 2017;51:3298–3306. doi: 10.1021/acs.est.6b05296. [DOI] [PubMed] [Google Scholar]
- 9.Verones F, Moran D, Stadler K, Kanemoto K, Wood R. Resource footprints and their ecosystem consequences. Sci Rep. 2017;7 doi: 10.1038/srep40743. 40743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laurance WF, Sayer J, Cassman KG. Agricultural expansion and its impacts on tropical nature. Trends Ecol Evol. 2014;29:107–116. doi: 10.1016/j.tree.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Phalan B, Onial M, Balmford A, Green RE. Reconciling Food Production and Biodiversity Conservation: Land Sharing and Land Sparing Compared. Science. 2011;333:1289–1291. doi: 10.1126/science.1208742. [DOI] [PubMed] [Google Scholar]
- 12.Erb K-H, Krausmann F, Lucht W, Haberl H. Embodied HANPP: Mapping the spatial disconnect between global biomass production and consumption. Ecol Econ. 2009;69:328–334. [Google Scholar]
- 13.Pereira HM, et al. Essential Biodiversity Variables. Science. 2013;339:277–278. doi: 10.1126/science.1229931. [DOI] [PubMed] [Google Scholar]
- 14.Reyers B, Stafford-Smith M, Erb K-H, Scholes RJ, Selomane O. Essential Variables help to focus Sustainable Development Goals monitoring. Curr Opin Environ Sustain. 2017;26–27:97–105. [Google Scholar]
- 15.Pan Y, et al. A Large and Persistent Carbon Sink in the World’s Forests. Science. 2011;333:988–993. doi: 10.1126/science.1201609. [DOI] [PubMed] [Google Scholar]
- 16.Ehrlich PR, Holdren JP. Impact of Population Growth. Science. 1971;171:1212–1217. doi: 10.1126/science.171.3977.1212. [DOI] [PubMed] [Google Scholar]
- 17.Stadler K, et al. EXIOBASE 3: Developing a Time Series of Detailed Environmentally Extended Multi-Regional Input-Output Tables. J Ind Ecol. 2018 doi: 10.1111/jiec.12715. n/a-n/a. [DOI] [Google Scholar]
- 18.Ceballos G, et al. Accelerated modern human–induced species losses: Entering the sixth mass extinction. Sci Adv. 2015;1:e1400253. doi: 10.1126/sciadv.1400253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanco G, et al. Drivers, Trends and Mitigation. In: Edenhofer O, et al., editors. Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press; 2014. [Google Scholar]
- 20.Vijay V, Pimm SL, Jenkins CN, Smith SJ. The Impacts of Oil Palm on Recent Deforestation and Biodiversity Loss. PLOS ONE. 2016;11:e0159668. doi: 10.1371/journal.pone.0159668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ward JD, et al. Is Decoupling GDP Growth from Environmental Impact Possible? PLOS ONE. 2016;11:e0164733. doi: 10.1371/journal.pone.0164733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eurostat. Archive:Household consumption expenditure - national accounts - Statistics Explained. [Accessed: 22nd March 2018];2013 Available at: http://ec.europa.eu/eurostat/statistics-explained/index.php/Archive:Household_consumption_expenditure_-_national_accounts.
- 23.Shao Q, Schaffartzik A, Mayer A, Krausmann F. The high ‘price’ of dematerialization: A dynamic panel data analysis of material use and economic recession. J Clean Prod. 2017;167:120–132. [Google Scholar]
- 24.Eurostat. EU-27 construction activity falls by 16 % from its precrisis high by the second quarter of 2011. 2011.
- 25.USDA. U.S. Forest Resource Facts and Historical Trends. United States Department of Agriculture; 2014. [Google Scholar]
- 26.Seppelt R, Manceur A, Liu J, Fenichel E, Klotz S. Synchronized peak-rate years of global resources use. Ecol Soc. 2014;19 [Google Scholar]
- 27.Gerstner K, Dormann CF, Stein A, Manceur AM, Seppelt R. EDITOR’S CHOICE: REVIEW: Effects of land use on plant diversity – A global meta-analysis. J Appl Ecol. 2014;51:1690–1700. [Google Scholar]
- 28.Alcott B. Impact caps: why population, affluence and technology strategies should be abandoned. J Clean Prod. 2010;18:552–560. [Google Scholar]
- 29.UNEP. Decoupling natural resource use and environmental impacts from economic growth, A Report of the Working Group on Decoupling to the International Resource Panel. UNEP; 2011. [Google Scholar]
- 30.Abel GJ, Barakat B, Kc S, Lutz W. Meeting the Sustainable Development Goals leads to lower world population growth. Proc Natl Acad Sci. 2016;113:14294–14299. doi: 10.1073/pnas.1611386113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Godfray HCJ, et al. Meat consumption, health, and the environment. Science. 2018;361 doi: 10.1126/science.aam5324. eaam5324. [DOI] [PubMed] [Google Scholar]
- 32.Poore J, Nemecek T. Reducing food’s environmental impacts through producers and consumers. Science. 2018;360:987–992. doi: 10.1126/science.aaq0216. [DOI] [PubMed] [Google Scholar]
- 33.UN. United Nations regional groups of member states. United Nations; 2014. [Google Scholar]
- 34.Erb K-H, et al. A comprehensive global 5 min resolution land-use data set for the year 2000 consistent with national census data. J Land Use Sci. 2007;2:191–224. [Google Scholar]
- 35.Ramankutty N, Evan AT, Monfreda C, Foley JA. Farming the planet: 1. Geographic distribution of global agricultural lands in the year 2000. Glob Biogeochem Cycles. 2008;22 GB1003. [Google Scholar]
- 36.FAOSTAT. Statistical Databases. FAO; 2014. http://faostat.fao.org/ [Google Scholar]
- 37.Monfreda C, Ramankutty N, Foley JA. Farming the planet: 2. Geographic distribution of crop areas, yields, physiological types, and net primary production in the year 2000. Glob Biogeochem Cycles. 2008;22:GB1022. [Google Scholar]
- 38.Schepaschenko D, et al. Development of a global hybrid forest mask through the synergy of remote sensing, crowdsourcing and FAO statistics. Remote Sens Environ. 2015;162:208–220. [Google Scholar]
- 39.Potapov P, et al. The last frontiers of wilderness: Tracking loss of intact forest landscapes from 2000 to 2013. Sci Adv. 2017;3:e1600821. doi: 10.1126/sciadv.1600821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Müller M, Pérez Dominguez I, Gay SH. Construction of Social Accounting Matrices for EU27 with disaggregated Agricultural Sectors (agroSAM) 2009.
- 41.Erb KH, et al. Biomass turnover time in terrestrial ecosystems halved by land use. Nat Geosci. 2016;9:674–678. [Google Scholar]
- 42.Erb K-H, et al. Unexpectedly large impact of forest management and grazing on global vegetation biomass. Nature. 2018;553:73–76. doi: 10.1038/nature25138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Planted forests: uses, impacts and sustainability. CABI Publishing; 2009. [Google Scholar]
- 44.Penna I. Understanding the FAO’s ‘Wood Supply from Planted Forests’ Projections. Centre for Environmental Management, University of Ballarat; 2010. [Google Scholar]
- 45.Pereira HM, Daily GC. Modeling biodiversity dynamics in countryside landscapes. Ecology. 2006;87:1877–1885. doi: 10.1890/0012-9658(2006)87[1877:mbdicl]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 46.Pereira HM, Ziv G, Miranda M. Countryside Species–Area Relationship as a Valid Alternative to the Matrix-Calibrated Species–Area Model. Conserv Biol. 2014;28:874–876. doi: 10.1111/cobi.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martins IS, Pereira HM. Improving extinction projections across scales and habitats using the countryside species-area relationship. Sci Rep. 2017;7 doi: 10.1038/s41598-017-13059-y. 12899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanski I, Zurita GA, Bellocq MI, Rybicki J. Species–fragmented area relationship. Proc Natl Acad Sci. 2013;110:12715–12720. doi: 10.1073/pnas.1311491110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rybicki J, Hanski I. Species–area relationships and extinctions caused by habitat loss and fragmentation. Ecol Lett. 2013;16:27–38. doi: 10.1111/ele.12065. [DOI] [PubMed] [Google Scholar]
- 50.Rosenzweig ML. Species diversity in space and time. Cambridge Univ Pr; 1995. [Google Scholar]
- 51.Storch D, Keil P, Jetz W. Universal species–area and endemics–area relationships at continental scales. Nature. 2012;488:78–81. doi: 10.1038/nature11226. [DOI] [PubMed] [Google Scholar]
- 52.Chaudhary A, Verones F, de Baan L, Hellweg S. Quantifying Land Use Impacts on Biodiversity: Combining Species–Area Models and Vulnerability Indicators. Environ Sci Technol. 2015;49:9987–9995. doi: 10.1021/acs.est.5b02507. [DOI] [PubMed] [Google Scholar]
- 53.Sodhi NS, Lee TM, Koh LP, Brook BW. A Meta-Analysis of the Impact of Anthropogenic Forest Disturbance on Southeast Asia’s Biotas. Biotropica. 2009;41:103–109. [Google Scholar]
- 54.Hudson LN, Newbold T, Contu S, Hill SLL. The 2016 release of the PREDICTS database. Natural History Museum Data Portal; 2016. [Google Scholar]
- 55.ESRI. ArcGIS. Environmental Systems Resource Institute; 2009. [Google Scholar]
- 56.Holt BG, et al. An Update of Wallace’s Zoogeographic Regions of the World. Science. 2013;339:74–78. doi: 10.1126/science.1228282. [DOI] [PubMed] [Google Scholar]
- 57.BirdLife International & NatureServe. Bird species distribution maps of the world. BirdLife International and NatureServe; 2014. [Google Scholar]
- 58.Python Software Foundation. Python Language Reference, version 2.7
- 59.de Baan L, Mutel CL, Curran M, Hellweg S, Koellner T. Land Use in Life Cycle Assessment: Global Characterization Factors Based on Regional and Global Potential Species Extinction. Environ Sci Technol. 2013;47:9281–9290. doi: 10.1021/es400592q. [DOI] [PubMed] [Google Scholar]
- 60.Holtsmark B. Harvesting in boreal forests and the biofuel carbon debt. Clim Change. 2011;112:415–428. [Google Scholar]
- 61.Houghton RA. Revised estimates of the annual net flux of carbon to the atmosphere from changes in land use and land management 1850-2000. Tellus B. 2003;55:378–390. [Google Scholar]
- 62.Kastner T, Erb K-H, Nonhebel S. International wood trade and forest change: A global analysis. Glob Environ Change. 2011;21:947–956. [Google Scholar]
- 63.FAO. Global Ecological Zoning for the Global Forest Resources Assessment, 2000. Food and Agriculture Organization of the United Nations; 2001. [Google Scholar]
- 64.Ramankutty N, Foley J. Estimating historical changes in global land cover: croplands from 1700 to 1992. Glob Biogeochem Cycles. 1999;13:997–1027. [Google Scholar]
- 65.Olson DM, et al. Terrestrial Ecoregions of the World: A New Map of Life on Earth A new global map of terrestrial ecoregions provides an innovative tool for conserving biodiversity. BioScience. 2001;51:933–938. [Google Scholar]
- 66.Krausmann F, Erb K-H, Gingrich S, Lauk C, Haberl H. Global patterns of socioeconomic biomass flows in the year 2000: A comprehensive assessment of supply, consumption and constraints. Ecol Econ. 2008;65:471–487. [Google Scholar]
- 67.Lauk C, Haberl H, Erb K-H, Gingrich S, Krausmann F. Global socioeconomic carbon stocks in long-lived products 1900–2008. Environ Res Lett. 2012;7 034023. [Google Scholar]
- 68.Schlesinger WH. Are wood pellets a green fuel? Science. 2018;359:1328–1329. doi: 10.1126/science.aat2305. [DOI] [PubMed] [Google Scholar]
- 69.Pingoud K, Ekholm T, Sievänen R, Huuskonen S, Hynynen J. Trade-offs between forest carbon stocks and harvests in a steady state – A multi-criteria analysis. J Environ Manage. 2018;210:96–103. doi: 10.1016/j.jenvman.2017.12.076. [DOI] [PubMed] [Google Scholar]
- 70.Davis SJ, Caldeira K. Consumption-based accounting of CO2 emissions. Proc Natl Acad Sci. 2010;107:5687–5692. doi: 10.1073/pnas.0906974107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wiedmann TO, et al. The material footprint of nations. Proc Natl Acad Sci. 2015;112:6271–6276. doi: 10.1073/pnas.1220362110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marques A, Verones F, Kok MT, Huijbregts MA, Pereira HM. How to quantify biodiversity footprints of consumption? A review of multi-regional input–output analysis and life cycle assessment. Curr Opin Environ Sustain. 2017;29:75–81. [Google Scholar]
- 73.Kitzes J. An Introduction to Environmentally-Extended Input-Output Analysis. Resources. 2013;2:489–503. [Google Scholar]
- 74.Kanemoto K, Lenzen M, Peters GP, Moran DD, Geschke A. Frameworks for Comparing Emissions Associated with Production, Consumption, And International Trade. Environ Sci Technol. 2012;46:172–179. doi: 10.1021/es202239t. [DOI] [PubMed] [Google Scholar]
- 75.Miller R, Bair P. Input-Ouput Analysis. Foundations and Extensions. Cambridge University Press; 2009. [Google Scholar]
- 76.Lenzen M, Wood R, Wiedmann T. Uncertainty Analysis for Multi-Region Input–Output Models – a Case Study of the Uk’s Carbon Footprint. Econ Syst Res. 2010;22:43–63. [Google Scholar]
- 77.Moran D, Wood R. Convergence Between the Eora, Wiod, Exiobase, and Openeu’s Consumption-Based Carbon Accounts. Econ Syst Res. 2014;26:245–261. [Google Scholar]
- 78.de Koning A, et al. Effect of aggregation and disaggregation on embodied material use of products in input–output analysis. Ecol Econ. 2015;116:289–299. [Google Scholar]
- 79.Lenzen M. Aggregation Versus Disaggregation in Input–Output Analysis of the Environment. Econ Syst Res. 2011;23:73–89. [Google Scholar]
- 80.Wood R, Hawkins TR, Hertwich EG, Tukker A. Harmonising National Input—Output Tables for Consumption-Based Accounting — Experiences from Exiopol. Econ Syst Res. 2014;26:387–409. [Google Scholar]
- 81.Stadler K, Steen-Olsen K, Wood R. The ‘Rest of the World’ – Estimating the Economic Structure of Missing Regions in Global Multi-Regional Input–Output Tables. Econ Syst Res. 2014;26:303–326. [Google Scholar]
- 82.Owen A, Wood R, Barrett J, Evans A. Explaining value chain differences in MRIO databases through structural path decomposition. Econ Syst Res. 2016;28:243–272. [Google Scholar]
- 83.Steen-Olsen K, Owen A, Hertwich EG, Lenzen M. Effects of Sector Aggregation on Co2 Multipliers in Multiregional Input–Output Analyses. Econ Syst Res. 2014;26:284–302. [Google Scholar]
- 84.World Bank. World Development Indicators, SP.POP.TOTL Population, total. World Bank; 2015. [Google Scholar]
- 85.World Bank. World Bank. 2015b. World Development Indicators, NY.GDP.MKTP.PP.KD, GDP, PPP (constant 2011 international $) World Bank; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.