Abstract
Background
Subclinical thyroid dysfunction, defined as thyroid-stimulating hormone (TSH) levels outside the reference range with normal free thyroxine levels in asymptomatic patients, is associated with alterations in cardiac hemodynamics. We used Mendelian randomization to assess the role of thyroid dysfunction for cardiovascular disease.
Methods
Single-nucleotide polymorphisms associated with thyroid function were identified from a genome-wide association meta-analysis in up to 72 167 individuals. Data for genetic associations with cardiovascular disease were obtained from meta-analyses of genome-wide association studies of atrial fibrillation (n=537 409 individuals), coronary artery disease (n=184 305 individuals), and ischemic stroke (n=438 847) as well as from the UK Biobank (n=367 703 individuals).
Results
Genetically predicted TSH levels and hyperthyroidism were statistically significantly associated with atrial fibrillation but no other cardiovascular diseases at the Bonferroni-corrected level of significance (P <7.8×10-4). The odds ratios of atrial fibrillation were 1.15 (95% confidence interval 1.11-1.19, P=2.4×10-14) per genetically predicted one standard deviation decrease in TSH levels and 1.05 (95% confidence interval 1.03-1.08, P=5.4×10-5) for genetic predisposition to hyperthyroidism. Genetically predicted free thyroxin levels were not statistically significantly associated with any cardiovascular disease.
Conclusions
This Mendelian randomization study supports evidence for a causal association of decreased TSH levels in the direction of a mild form of hyperthyroidism with increased risk of atrial fibrillation but no other cardiovascular diseases.
Keywords: atrial fibrillation, cardiovascular disease, hyperthyroidism, Mendelian randomization, thyrotropin
Journal Subject Terms: Atrial Fibrillation; Coronary Artery Disease; Ischemic Stroke; Genetic, Association Studies
Subclinical thyroid dysfunction, defined as serum thyroid-stimulating hormone (TSH) levels below (hyperthyroidism) or above (hypothyroidism) the reference interval with normal free thyroxine (FT4) levels in asymptomatic patients,1 is a common condition and is particularly prevalent in older women.2–6 Subclinical thyroid dysfunction is associated with alterations in cardiac hemodynamics, such as impaired cardiac contractility, increased heart rate, systolic hypertension, increased left ventricular mass, and diastolic dysfunction.1,7–12
Data from observational prospective studies on subclinical thyroid dysfunction in relation to risk of cardiovascular disease (CVD) are inconclusive. Subclinical hyperthyroidism was reported to be positively associated with risk of atrial fibrillation (AF),4,13–18 coronary heart disease,18,19 overall stroke,20 and heart failure5 in several studies. Subclinical hypothyroidism has been found to be associated with increased risk of coronary heart disease,3,21,22 heart failure5 and cardiac mortality3,21,22 but not with risk of AF or stroke.21 However, residual confounding or reverse causality may have affected those associations and may also explain the inconsistent results.
Genetic variants with an explicit association with a potential risk factor (e.g., TSH levels) can be used as unbiased proxies for the risk factor to determine causality.23,24 This method, known as Mendelian randomization (MR), builds on Mendel’s second law and the fact that genetic variants are randomly distributed at conception and thus unlikely related to possible confounders. In addition, reverse causality is avoided because disease cannot affect genotype.
Given the controversy regarding the role of thyroid dysfunction for CVD, we used the MR design to determine the associations of TSH levels and hyper- and hypothyroidism with CVD. Our primary aim was to assess the associations of TSH levels and hyper- and hypothyroidism with AF, coronary artery disease (CAD), and ischemic stroke using data from meta-analyses of genome-wide association studies (GWAS) of these outcomes25–27 and data from the UK Biobank.28 In secondary analyses, we investigated the associations of TSH levels and hyper- and hypothyroidism with other CVD outcomes in UK Biobank. Finally, we examined whether there is an association between FT4 levels and any CVD outcome.
Methods
The methods are available as supplemental material. This study is based on publicly available summary-level data, which are available in the supplemental material. All studies included in the analyses received ethics approval from a relevant institutional review board, and all participants had provided informed consent.
Results
Genetic Consortia
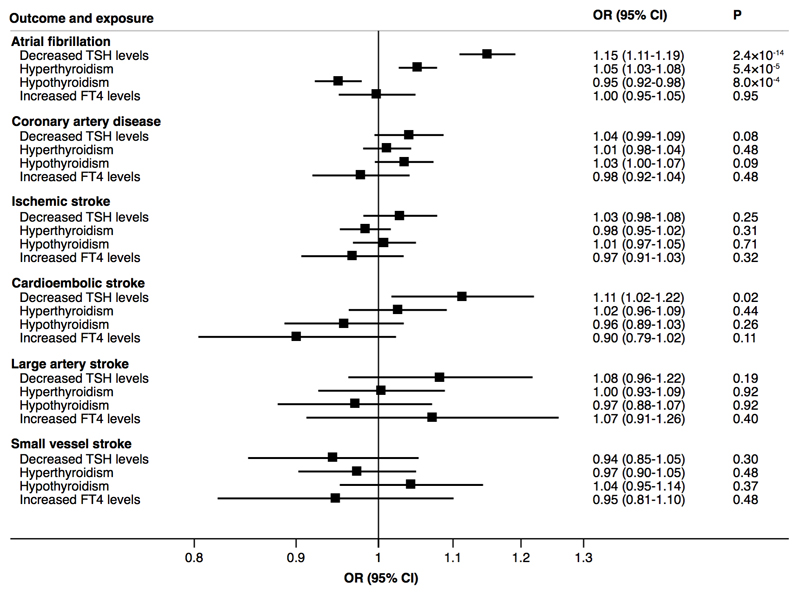
Analyses using data from three large-scale genetic consortia (Atrial Fibrillation Consortium [AFGen] 2018 GWAS dataset, Coronary ARtery DIsease Genome-wide Replication and Meta-analysis plus The Coronary Artery Disease consortium’s 1000 Genomes-based GWAS [CARDIoGRAMplusC4D], and MEGASTROKE) revealed a statistical significant association between genetically decreased TSH levels and higher odds of AF (odds ratio [OR] 1.15, 95% confidence interval [CI] 1.11-1.19, P=2.4×10-14) but not CAD or ischemic stroke as a whole (Figure 1). Among ischemic stroke subtypes, genetically decreased TSH levels were associated with higher odds of cardioembolic stroke (OR 1.11, 95% CI 1.02-1.22, P=0.02) (Figure 1) but the association did not achieve statistical significance at the Bonferroni-corrected threshold. Hyperthyroidism was associated with higher odds of AF (OR 1.05, 95% CI 1.03-1.08, P=5.4×10-5), whereas hypothyroidism was associated with lower odds of AF (OR 0.95, 95% CI 0.92-0.98, P=8.0×10-4) (Figure 1). Genetically higher FT4 levels were not associated with any outcome (Figure 1).
Figure 1.
Associations of genetically decreased TSH levels, hyperthyroidism, hypothyroidism and increased FT4 levels with odds ratio of AF, CAD, and ischemic stroke and its subtypes based on data from the AFGen, CARDIoGRAMplusC4D and MEGASTROKE consortia. Odds ratios are expressed per one SD decrease of TSH levels, per one unit higher log-odds of hyperthyroidism and hypothyroidism and per one SD increase of FT4 levels. Hyperthyroidism = TSH levels below the reference range in the population; hypothyroidism = TSH levels above the reference range in the population.
Results for AF were similar when using data from the AFGen 2017 GWAS dataset. In these analyses, the ORs were 1.16 (95% CI 1.10-1.23, P=7.1×10-7) per one SD decrease of TSH levels, 1.05 (95% CI 1.01-1.09, P=0.02) for hyperthyroidism, 0.94 (95% CI 0.89-0.99, P=0.01) for hypothyroidism, and 1.00 (95% CI 0.92-1.09, P=0.93) per one SD increase of FT4 levels.
UK Biobank
In the UK Biobank, genetically decreased TSH levels were statistically significantly associated with higher odds of AF (OR 1.20, 95% CI 1.13-1.27, P=1.4×10-9) but not the other outcomes (Table 1). There was suggestive evidence of associations between hyperthyroidism and higher odds of AF and lower odds of thoracic aortic aneurysm; hypothyroidism and higher odds CAD and hypertension; and increased FT4 levels and higher odds of peripheral arterial disease (Table 1).
Table 1. Associations of genetically predicted decreased TSH levels, hyperthyroidism, hypothyroidism, and increased FT4 levels with odds ratio of 13 cardiovascular diseases in UK Biobank.
| TSH levels | Hyperthyroidism | Hypothyroidism | FT4 levels | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | Cases* | OR (95% CI)† | P | OR (95% CI)† | P | OR (95% CI)† | P | OR (95% CI)† | P |
| Atrial fibrillation | 13 538 | 1.20 (1.13-1.27) | 1.4×10-9 | 1.06 (1.02-1.10) | 0.005 | 0.95 (0.90-1.01) | 0.11 | 0.97 (0.90-1.05) | 0.47 |
| Coronary artery disease | 24 531 | 1.00 (0.96-1.05) | 0.91 | 0.99 (0.96-1.02) | 0.40 | 1.06 (1.02-1.11) | 0.008 | 0.99 (0.93-1.05) | 0.63 |
| Ischemic stroke | 3554 | 1.02 (0.91-1.14) | 0.75 | 1.03 (0.95-1.11) | 0.51 | 1.10 (0.99-1.22) | 0.09 | 1.01 (0.87-1.17) | 0.91 |
| Intracerebral hemorrhage | 1655 | 0.99 (0.84-1.17) | 0.91 | 1.04 (0.93-1.17) | 0.47 | 0.99 (0.84-1.16) | 0.89 | 1.03 (0.83-1.28) | 0.81 |
| Subarachnoid hemorrhage | 1834 | 1.06 (0.91-1.24) | 0.44 | 1.08 (0.97-1.20) | 0.17 | 0.92 (0.79-1.07) | 0.28 | 1.10 (0.89-1.36) | 0.36 |
| Heart failure | 4803 | 1.02 (0.92-1.12) | 0.71 | 0.98 (0.91-1.04) | 0.46 | 1.04 (0.95-1.14) | 0.41 | 1.01 (0.89-1.15) | 0.91 |
| Aortic valve stenosis | 1252 | 1.01 (0.99-1.04) | 0.35 | 1.01 (0.99-1.03) | 0.18 | 1.00 (0.97-1.02) | 0.84 | 1.01 (0.98-1.05) | 0.51 |
| Abdominal aortic aneurysm | 758 | 0.90 (0.71-1.15) | 0.41 | 1.02 (0.86-1.20) | 0.84 | 0.86 (0.68-1.08) | 0.20 | 1.05 (0.76-1.46) | 0.75 |
| Thoracic aortic aneurysm | 231 | 0.65 (0.42-1.01) | 0.06 | 0.60 (0.44-0.81) | 0.001 | 1.47 (0.97-2.23) | 0.07 | 0.64 (0.36-1.13) | 0.13 |
| Deep vein thrombosis | 3514 | 1.07 (1.00-1.15) | 0.05 | 1.00 (0.95-1.05) | 0.93 | 1.04 (0.97-1.11) | 0.28 | 0.98 (0.89-1.08) | 0.65 |
| Pulmonary embolism | 8891 | 1.09 (0.99-1.20) | 0.07 | 1.03 (0.96-1.10) | 0.39 | 1.06 (0.96-1.16) | 0.23 | 0.94 (0.83-1.06) | 0.31 |
| Peripheral arterial disease | 5097 | 1.06 (0.94-1.18) | 0.34 | 0.99 (0.92-1.07) | 0.86 | 1.06 (0.95-1.18) | 0.33 | 0.83 (0.71-0.96) | 0.01 |
| Hypertension | 119 500 | 1.01 (0.98-1.03) | 0.66 | 1.01 (1.00-1.03) | 0.14 | 1.04 (1.01-1.06) | 0.003 | 0.99 (0.96-1.02) | 0.65 |
Total number of participants is 367 703.
Odds ratios are expressed per one SD decrease of TSH levels, per one unit higher log-odds of hyperthyroidism and hypothyroidism, and per one SD increase of FT4 levels. Hyperthyroidism = TSH levels below the reference range in the population; hypothyroidism = TSH levels above the reference range in the population.
Sensitivity Analyses
The associations of TSH and FT4 levels with the outcomes were robust when limiting the analysis to the lead SNP of each locus (Table S5). When excluding the three loci associated with both hyper- and hypothyroidism, the ORs of AF (using data from 2018 AFGen GWAS) were 1.07 (95% CI 1.04-1.11, P=3.6×10-5) for hyperthyroidism and 0.99 (95% CI 0.95-1.03, P=0.59) for hypothyroidism. The results for TSH levels using the weighted median and MR-Egger approaches were similar to those of the primary analysis (inverse-variance weighted method), but the precision of the estimates was as expected lower (Table S6). The MR-Egger analysis provided no evidence of directional pleiotropy (Table S6). The MR-PRESSO analysis identified no outlying single-nucleotide polymorphisms (SNPs) in the analysis of TSH levels and AF. However, there was one outlying SNP in the analyses of TSH levels in relation to CAD and aortic valve stenosis and three outlying SNPs in the analyses of TSH and hypertension (Table S7). Exclusion of those SNPs did not change the results appreciably (Table S7). Likewise, inclusion of the pleiotropic SNP in the ABO gene did not change the interpretation of the results for TSH levels and any CVD outcome (Table S8).
Discussion
For the first time, this study uses a MR approach to systematically investigate the relationship of thyroid function and dysfunction with a wide range of CVD outcomes. Our results showed that genetically decreased TSH levels and hyperthyroidism were robustly associated with higher odds of AF. We found no statistically significant and consistent associations of TSH levels, hyper- or hypothyroidism, or FT4 levels with the other CVD outcomes, though there was suggestive evidence of possible associations between decreased TSH levels and higher odds of cardioembolic stroke, hyperthyroidism and lower odds of thoracic aortic aneurysm, hypothyroidism and higher odds AF, CAD and hypertension, and increased FT4 levels and higher odds of peripheral arterial disease. However, those suggestive associations were only observed in one dataset (one of the genetic consortia or UK Biobank) or were not consistent in sensitivity analyses.
Our findings support prior observational studies associating decreased TSH levels and hyperthyroidism to increased risk of AF4,13–17 as well as results from two recent MR studies associating genetically higher TSH levels to decreased risk of AF.29,30 However, an individual patient data analysis of 30 085 participants (including 2574 AF cases) from 11 cohorts showed only a suggestive association of lower TSH levels within the reference range with increased risk of AF (P for linear trend = 0.054).31 That analysis further showed that FT4 levels were positively associated with AF risk in euthyroid individuals.31 A positive association between genetically higher FT4 levels and AF was not detected in the present MR analysis, but we were unable to simultaneously control for TSH levels. In addition, these MR results reflect the association between lifelong higher FT4 levels on AF risk and it is unknown whether high FT4 levels during different periods in the life course differently affect the risk of developing AF.
An association between decreased TSH levels and increased risk of AF may in part be mediated by increased left ventricular mass8,10–12 and diastolic dysfunction,7 both of which are associated with an increased risk of AF,32,33 though the causal relationships remain unclear. Findings from an experimental study in mice showed that hyperthyroidism, with suppression of circulating TSH levels, leads to impaired Pitx2>Wnt>microRNA signaling,34 thus providing a molecular link between hyperthyroidism and AF since PITX2 is one of the strongest genetic locus related to AF.25
A chief strength of this study is the MR approach, which reduces systematic biases (e.g., confounding and reverse causality) that can distort the results of conventional observational studies. Another major strength is that we examined the associations of thyroid function and dysfunction with AF, CAD and ischemic stroke using data from large-scale genetic consortia, which included a large number of cases. Hence, we had high statistical power to detect weak associations of the examined exposures and those outcomes. However, the power was low in the analyses of several CVD outcomes measured in UK Biobank, in particular intracerebral and subarachnoid hemorrhage, abdominal and thoracic aortic aneurysm, and aortic valve stenosis. We thus cannot rule out that the lack of association with those outcomes are due to insufficient power. We could restrict the study populations (except in the CARDIoGRAMplusC4D consortium) to individuals of European ancestry, a constraint that reduced bias from population stratification. A limitation of any MR analysis is that pleiotropy cannot be ruled out as an explanation for an observed association. Nevertheless, we found no evidence that directional pleiotropy may have influenced the results. A further shortcoming is that we could not examine U-shaped associations or effects of very low or high TSH and FT4 levels or different combinations of TSH and FT4 levels on CVD risk.
Conclusions
This MR study supports evidence for a causal association between decreased TSH levels in the direction of a mild form of hyperthyroidism and increased risk of AF. Suggestive evidence of possible associations was found between thyroid dysfunction and some other CVD outcomes, including cardioembolic stroke. These findings may have clinical implications because they suggest that treatment of subclinical hyperthyroidism may be a complement to other possible prevention strategies for AF, such as reducing excessive alcohol consumption, tobacco control, reducing blood pressure, blood glucose, and body mass, and therapy for myocardial infarction and heart failure.35
Supplementary Material
Acknowledgments
The analyses of UK Biobank data were conducted under UK Biobank application number 29202. The authors thank the Atrial Fibrillation Consortium, the CARDIoGRAMplusC4D consortium, and the MEGASTROKE consortium for providing summary-level data for AF, CAD, and ischemic stroke, respectively. The MEGASTROKE project received funding from sources specified at megastroke.org/acknowledgements.html. MEGASTROKE authors are listed in the Supplementary material.
Sources of Funding: This work was supported by the Swedish Research Council for Health, Working Life and Welfare (FORTE) [Grant Number 2018-00123] and the Swedish Research Council [Grant Number 2016-01042]. Stephen Burgess is supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society [Grant Number 204623/Z/16/Z].
Footnotes
Disclosures: None.
References
- 1.Floriani C, et al. Subclinical thyroid dysfunction and cardiovascular diseases: 2016 update. Eur Heart J. 2018;39:503–507. doi: 10.1093/eurheartj/ehx050. [DOI] [PubMed] [Google Scholar]
- 2.Hollowell JG, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 3.Rodondi N, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304:1365–1374. doi: 10.1001/jama.2010.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cappola AR, et al. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. 2006;295:1033–1041. doi: 10.1001/jama.295.9.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gencer B, et al. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation. 2012;126:1040–1049. doi: 10.1161/circulationaha.112.096024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor PN, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14:301–316. doi: 10.1038/nrendo.2018.18. [DOI] [PubMed] [Google Scholar]
- 7.Smit JW, et al. Reversible diastolic dysfunction after long-term exogenous subclinical hyperthyroidism: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2005;90:6041–6047. doi: 10.1210/jc.2005-0620. [DOI] [PubMed] [Google Scholar]
- 8.Klein I, et al. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501–509. doi: 10.1056/nejm200102153440707. [DOI] [PubMed] [Google Scholar]
- 9.Roef GL, et al. Thyroid hormone levels within reference range are associated with heart rate, cardiac structure, and function in middle-aged men and women. Thyroid. 2013;23:947–954. doi: 10.1089/thy.2012.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biondi B, et al. Endogenous subclinical hyperthyroidism affects quality of life and cardiac morphology and function in young and middle-aged patients. J Clin Endocrinol Metab. 2000;85:4701–4705. doi: 10.1210/jcem.85.12.7085. [DOI] [PubMed] [Google Scholar]
- 11.Sgarbi JA, et al. The effects of early antithyroid therapy for endogenous subclinical hyperthyroidism in clinical and heart abnormalities. J Clin Endocrinol Metab. 2003;88:1672–1677. doi: 10.1210/jc.2002-021046. [DOI] [PubMed] [Google Scholar]
- 12.Iida M, et al. Thyroid hormone within the normal range is associated with left ventricular mass in patients with hypertension. J Am Soc Hypertens. 2012;6:261–269. doi: 10.1016/j.jash.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Selmer C, et al. The spectrum of thyroid disease and risk of new onset atrial fibrillation: a large population cohort study. BMJ. 2012;345:e7895. doi: 10.1136/bmj.e7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heeringa J, et al. High-normal thyroid function and risk of atrial fibrillation: the Rotterdam study. Arch Intern Med. 2008;168:2219–2224. doi: 10.1001/archinte.168.20.2219. [DOI] [PubMed] [Google Scholar]
- 15.Gammage MD, et al. Association between serum free thyroxine concentration and atrial fibrillation. Arch Intern Med. 2007;167:928–934. doi: 10.1001/archinte.167.9.928. [DOI] [PubMed] [Google Scholar]
- 16.Auer J, et al. Subclinical hyperthyroidism as a risk factor for atrial fibrillation. Am Heart J. 2001;142:838–842. doi: 10.1067/mhj.2001.119370. [DOI] [PubMed] [Google Scholar]
- 17.Sawin CT, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med. 1994;331:1249–1252. doi: 10.1056/nejm199411103311901. [DOI] [PubMed] [Google Scholar]
- 18.Collet TH, et al. Subclinical hyperthyroidism and the risk of coronary heart disease and mortality. Arch Intern Med. 2012;172:799–809. doi: 10.1001/archinternmed.2012.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun J, et al. Relationship between subclinical thyroid dysfunction and the risk of cardiovascular outcomes: a systematic review and meta-analysis of prospective cohort studies. Int J Endocrinol. 2017;2017 doi: 10.1155/2017/8130796. 8130796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaker L, et al. Thyroid function within the reference range and the risk of stroke: an individual participant data analysis. J Clin Endocrinol Metab. 2016;101:4270–4282. doi: 10.1210/jc.2016-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ning Y, et al. What is the association of hypothyroidism with risks of cardiovascular events and mortality? A meta-analysis of 55 cohort studies involving 1,898,314 participants. BMC Med. 2017;15:21. doi: 10.1186/s12916-017-0777-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moon S, et al. Subclinical Hypothyroidism and the Risk of Cardiovascular Disease and All-Cause Mortality: A Meta-Analysis of Prospective Cohort Studies. Thyroid. 2018;28:1101–1110. doi: 10.1089/thy.2017.0414. [DOI] [PubMed] [Google Scholar]
- 23.Smith GD, et al. 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32:1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- 24.Burgess S, et al. Mendelian randomization: methods for using genetic variants in causal estimation. Chapman and Hall/CRC Press; 2015. [Google Scholar]
- 25.Roselli C, et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat Genet. 2018;50:1225–1233. doi: 10.1038/s41588-018-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nikpay M, et al. A comprehensive 1,000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 2015;47:1121–1130. doi: 10.1038/ng.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malik R, et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. 2018;50:524–537. doi: 10.1038/s41588-018-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sudlow C, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellervik C, et al. Assessment of the relationship between genetic determinants of thyroid function and atrial fibrillation: a Mendelian randomization study. JAMA Cardiol. 2019 doi: 10.1001/jamacardio.2018.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salem JE, et al. Association of thyroid function genetic predictors with atrial fibrillation: a phenome-wide association study and inverse-variance weighted average meta-analysis. JAMA Cardiol. 2019 doi: 10.1001/jamacardio.2018.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baumgartner C, et al. Thyroid function within the normal range, subclinical hypothyroidism, and the risk of atrial fibrillation. Circulation. 2017;136:2100–2116. doi: 10.1161/circulationaha.117.028753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenberg MA, et al. Diastolic dysfunction and risk of atrial fibrillation: a mechanistic appraisal. Circulation. 2012;126:2353–2362. doi: 10.1161/circulationaha.112.113233. [DOI] [PubMed] [Google Scholar]
- 33.Chatterjee S, et al. Meta-analysis of left ventricular hypertrophy and sustained arrhythmias. Am J Cardiol. 2014;114:1049–1052. doi: 10.1016/j.amjcard.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 34.Lozano-Velasco E, et al. Hyperthyroidism, but not hypertension, impairs PITX2 expression leading to Wnt-microRNA-ion channel remodeling. PLoS One. 2017;12:e0188473. doi: 10.1371/journal.pone.0188473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Du X, et al. Is atrial fibrillation a preventable disease? J Am Coll Cardiol. 2017;69:1968–1982. doi: 10.1016/j.jacc.2017.02.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.