Abstract
Inorganic arsenic exposure may be associated with diabetes, but the evidence at low-moderate levels is not sufficient. Polymorphisms in diabetes-related genes have been involved in diabetes risk. We evaluated the association of inorganic arsenic exposure on diabetes in the Hortega Study, a representative sample of a general population from Valladolid, Spain. Total urine arsenic was measured in 1,451 adults. Urine arsenic speciation was available in 295 randomly selected participants. To account for the confounding introduced by non-toxic seafood arsenicals, we designed a multiple imputation model to predict the missing arsenobetaine levels. The prevalence of diabetes was 8.3%. The geometric mean of total arsenic was 66.0 μg/g. The adjusted odds ratios (95% confidence interval) for diabetes comparing the highest with the lowest tertile of total arsenic were 1.76 (1.01, 3.09) and 2.14 (1.47, 3.11) before and after arsenobetaine adjustment, respectively. Polymorphisms in several genes including IL8RA, TXN, NR3C2, COX5A and GCLC showed suggestive differential associations of urine total arsenic with diabetes. The findings support the role of arsenic on diabetes and the importance of controlling for seafood arsenicals in populations with high seafood intake. Suggestive arsenic-gene interactions require confirmation in larger studies.
Keywords: Arsenic, diabetes, arsenic species, gene-environment interaction, multiple imputation
Brief summary of the main result of the work:
In a general population from Spain with low-moderate inorganic arsenic exposure, increased arsenic exposure, assessed as urine arsenic concentrations, was associated with higher diabetes prevalence. The reported association was stronger after adjustment for urine arsenobetaine reflecting the importance of accounting for seafood consumption in populations with high seafood intake. Carriers of specific genotypes may have increased susceptibility to arsenic-related diabetes, although larger studies are needed to confirm these suggestive findings.
Epidemiological studies support that people with higher inorganic arsenic exposure levels are more likely to have diabetes (Navas-Acien et al. 2006) but the evidence at low to moderate exposure levels is not sufficient. Moreover, most studies have been conducted in Asia and the Americas, with very few studies on arsenic and diabetes being conducted in Europe. Epidemiological studies in diverse populations are needed given the elevated burden of diabetes, a world-wide rapidly growing disease (World Health Organization 2016).
The sum of urine concentrations of inorganic arsenic (arsenite (AsIII) plus arsenate (AsV)) and its methylated species (monomethylarsonate (MMA) and dimethylarsinate (DMA)) is an established biomarker of inorganic arsenic exposure in populations with low seafood intake (Navas-Acien et al. 2011). It is well known that inorganic arsenic (iAs) is highly toxic to humans (World Health Organization 2001) and is associated with a wide range of adverse health effects, including cancer (Bates, Smith, and Hopenhayn-Rich 1992), cardiovascular disease (Moon, Guallar, and Navas-Acien 2012) and others (D’Ippoliti et al. 2015). People are exposed to inorganic arsenic mainly through drinking contaminated water (National Research Council 1999) and through food intake (Wei, Zhu, and Nguyen 2014). Organic arsenic species, such as arsenobetaine (Asb), arsenolipids and arsenosugars, are mostly found in saltwater finfish and shellfish and are considered not harmful to human health (Mozaffarian and Rimm 2006). Among the seafood arsenicals, arsenobetaine is the most common compound, which is rapidly cleared from the bloodstream and excreted unchanged in the urine (Molin et al. 2014), contributing to the total urinary arsenic concentrations. Arsenosugars and arsenolipids are metabolized in the body resulting in multiple metabolites in the urine, including DMA, and contributing to the total amount of inorganic arsenic species. As a result, in the presence of seafood intake, it is necessary to account for the potential residual confounding of organic arsenic in the interpretation of urinary arsenic concentrations as a biomarker of inorganic arsenic exposure (Navas-Acien et al. 2011).
The objective of this study was to evaluate the association of inorganic arsenic exposure, assessed as total urinary arsenic concentrations, with the prevalence of type 2 diabetes in a population from Spain with relatively high seafood consumption. This research was complicated by the presence of missing completely at random urine arsenobetaine concentrations for the ~80% of the study sample. We thus developed and implemented a multiple imputation model based in Markov Chain Monte Carlo (MCMC) methods using Gibbs sampling to account for the potential residual confounding by organic arsenicals in the whole study population. Given the well-established role of genetic variation on diabetes traits (Mahajan et al. 2014) and the paucity of gene-environmental interaction studies, we also evaluated the interaction of arsenic and diabetes-related candidate polymorphisms as a potential determinant of diabetes.
Methods
Study Population
The present study was conducted among adult participants of the Hortega Study, who were beneficiaries of the public health system assigned to the Rio Hortega University Hospital’s catchment area (Valladolid, northwestern Spain). The Hortega Study uses a complex sampling design to obtain a representative sample of the general population. The sampling design and methodology have been previously described (Mena-Martin et al. 2003). In 2001–2003, baseline information on socio-demographic, behavioral, dietary and other health-related factors were collected from in-person interviews, examinations, and review of health records (see e-Appendix 1). After signing an informed consent form, urine and blood samples were collected and stored at −80 ºC. A total of 1,502 individuals had sufficient urine and plasma samples available for metal determinations. After excluding 21 participants with missing total arsenic concentrations, 2 missing urine creatinine, 10 missing seafood intake, and 18 missing other relevant covariates, 1,451 participants were included in the present study.
Arsenic Measurements and Arsenobetaine Imputation
Urine arsenic determinations were conducted by the Laboratory of Analytical and Bioanalytical Chemistry of Huelva University (Spain) in 2013. Total urine arsenic and arsenic species concentrations were measured by inductively coupled-plasma mass spectrometry (ICPMS) on an Agilent 7500CEx ICPMS (Agilent Technologies, Santa Clara, California) and by anion exchange liquid chromatography coupled to ICPMS with octapole reaction cell, respectively, following a standardized protocol (see e-Appendix 2). Urine arsenic speciation, including AsIII, AsV, MMA, DMA, and Asb concentrations, occurred only in a random subsample of 295 individuals, leaving 1,156 (79.7%) of the 1,451 participants with missing completely at random urine arsenic species concentrations. Asb and DMA levels below the limit of detection (12 and 1 undetectable values, respectively) were imputed by the limit of detection divided by the square root of 2 (Hornung RW and Reed LD 1990). Total plasma arsenic levels were measured in the whole study population in 2010 by atomic absorption spectrometry with graphite furnace at Cerba International Laboratories Ltd. 42.6% of total plasma arsenic concentrations were under the limit of detection. See limits of detection in e-Appendix 2.
We used the arsenic speciation information available in the random subsample to generate 5 complete datasets (for the 1,451 participants) in which the Asb values missing completely at random where imputed as the 30th, 40th, 50th, 60th and 70th percentiles of each subject-specific posterior distribution obtained from a MCMC by Gibbs sampling nested linear model (Tellez-Plaza et al. 2010), implemented with WinBUGS software (Lunn et al. 2000) (see e-Appendix 3). Total plasma arsenic and DMA where strongly correlated with Asb (spearman correlation coefficients = 0.65 and 0.54, respectively). Thus, we incorporated the correlation of total plasma arsenic, DMA and Asb conditionally in our imputation model. We did not consider AsIII, AsV and MMA as potential Asb predictors. As a result, the MCMC arsenobetaine imputation model consisted of a tri-variate model with nested equations for Asb, DMA and total plasma arsenic, each based on strong predictors selected by a backward stepwise process starting from total urine concentrations and a list of socio-demographic and established arsenic exposure determinants (age, gender, smoking status, cotinine, body mass index, seafood, rice and chicken consumption) (e-Appendix 3, e-Table 1). For Asb and DMA, the predictive equations were standard linear models, whereas for total plasma arsenic the predictive equation was a tobit linear model for truncated data. The WinBUGS code of the imputation model is provided in e-Appendix 3. We conducted a post-hoc validation process to measure the predictive error of the MCMC imputation model using data from the Aragon Workers Health Study, an independent dataset with data on total urine arsenic, urine arsenic species and total plasma arsenic (e-Appendix 4 and e-Table 2).
Diabetes Determination
All participants provided blood samples after an average time of 3 hours from fasting (range 0–17). Plasma glucose was measured by an automated analyzer based on the glucose oxidase method. Participants with non-fasting glucose ≥ 140 mg/dL in the first determination, underwent an additional measure of glucose in fasting conditions. Glycosylated hemoglobin (HbA1c) was measured from capillary blood samples using a DCA 2000 HbA1c analyzer (Mena Martin et al. 2003). Prevalent type 2 diabetes was defined as fasting glucose levels ≥ 126 mg/dL, or HbA1c > 6.5%, or by physician diagnosis or glucose-lowering medication use.
Single Nucleotide Polymorphisms Selection and Genotyping
We selected 155 candidate genes, including 597 single nucleotide polymorphisms (SNPs), which were related with diabetes metabolic pathways. Detailed methods on DNA isolation and SNP genotyping have been previously published (Galan-Chilet et al. 2017). The mean genotyping coverage across all genotyped SNPs was 95%. Among the 597 selected SNPs, we excluded 42 because the coverage in the study sample was less than 90%, 59 with less than 3 genotypes, 25 with minor allele frequency <5%, 41 that did not meet the Hardy–Weinberg equilibrium (P<0.01), and 76 with minor genotype frequency <20 individuals. 354 SNPs were finally included in gene-environment interaction analyses. The complete list of the selected genes and SNPs is provided in e-Table 3.
Statistical Methods
Due to the different selection probabilities of the Hortega Study participants, all analyses were weighted to the underlying adult population aged 20 or older. Total urine arsenic and Asb concentrations were divided by urine creatinine and log-transformed for statistical analyses. We estimated the odds ratios and 95% confidence intervals (95% CI) for the association between total urine arsenic and diabetes prevalence using progressively adjusted logistic regression models. Model 1 was adjusted for age, gender and education. Model 2 was further adjusted for urine cotinine levels, smoking status, alcohol consumption, fish consumption and residence place. Model 3 was additionally adjusted for urine Asb levels. Total urine arsenic was modeled as tertiles and as restricted quadratic splines (see e-Appendix 5). We also explored whether the association between diabetes and arsenic was modified by gender, smoking status, abnormal albuminuria and reduced glomerular filtration rate status by including the interaction term of continuous total urine arsenic and the corresponding group variable in fully-adjusted models. Gene-environment interaction analyses were conducted by including each SNP and its interaction term with total urine arsenic levels. For each SNP, we estimated three separate models assuming dominant, recessive and additive inheritance. If only one model showed statistically significant SNP-arsenic interactions, we reported the associations in strata defined by the corresponding genotypes for this specific model. In cases where more than one model met statistically significant SNP-arsenic interactions, we reported the best fitting model selected by comparing to a general model that included separate dummy variables for the heterozygote and minor allele homozygote (reference major allele homozygote). The effective SNP number obtained with Plink software (Purcell et al. 2007) was 242, therefore we used a Bonferroni-corrected significance level of 0.0002 (0.05 divided by 242).
After the imputation of Asb and DMA missing completely at random and plasma arsenic levels below the detection limit, we obtained 5 databases with complete data for total urine arsenic and Asb concentrations. All statistical analyses above described were performed in each of the 5 data sets, and the results were combined using the method proposed by Rubin for multiple imputation procedures (Rubin 1978). In sensitivity analysis, we also evaluated the association of urine total arsenic and the sum of inorganic and methylated arsenic species in urine with diabetes within the subset of 295 individuals with arsenic speciation data (e-Table 4). See e-Appendix 5 for additional sensitivity analyses. All statistical tests were two-sided.
Results
Participant Characteristics
Among the 1,451 participants, 120 (8.3%) had prevalent type 2 diabetes (Table 1). Participants with type 2 diabetes were more likely to be men, older, former smoker, with lower education and to have lower fish intake. The overall geometric mean of total plasma arsenic and total urine arsenic in the complete study population were 2.6 μg/L and 66.0 μg/g, respectively. The overall geometric mean of the sum of inorganic arsenic species, i.e, AsIII+AsV+MMA+DMA (ΣiAs), and arsenobetaine in the randomly selected subsample of 295 participants was 11.1 μg/g and 47.3 μg/g, respectively. Participants with diabetes were older and showed higher values of all arsenic biomarkers (3.8 μg/L of total plasma arsenic, 106 μg/g of total urine arsenic, 14.9 μg/g of ΣiAs and 66.5 μg/g of Asb).
Table 1.
Participant characteristics by overall and type 2 diabetes status.
| Characteristics | No T2D | T2D | Overall |
|---|---|---|---|
| (N = 1331) | (N = 120) | (N = 1451) | |
| Age (years); mean (SE) | 48.5 (0.2) | 67.1 (1.4) | 49.7 (0.2) |
| Gender (male); % (SE) | 48.2 (0.3) | 60.6 (4.6) | 49.0 (0.0) |
| Education (< secondary education); % (SE) | 20.9 (1.0) | 43.8 (4.8) | 22.4 (1.0) |
| Body mass index (kg/m2); mean (SE)a | 26.0 (0.1) | 28.7 (0.5) | 26.2 (0.1) |
| Urine cotinine ng/mL; % (SE) | |||
| < 34 | 72.7 (1.3) | 85.1 (3.8) | 73.5 (1.2) |
| 34–500 | 5.6 (0.7) | 4.9 (2.2) | 5.6 (0.6) |
| > 500 | 21.7 (1.2) | 10.1 (3.4) | 20.9 (1.1) |
| Smoking status; % (SE) | |||
| Never | 44.5 (1.3) | 46.1 (4.8) | 44.6 (1.3) |
| Former | 27.9 (1.2) | 39.8 (4.8) | 28.6 (1.2) |
| Current | 27.7 (1.3) | 14.1 (3.8) | 26.8 (1.2) |
| Alcohol intake (mg/day); mean (SE) | 11.2 (0.6) | 11.2 (2.9) | 11.2 (0.6) |
| Fish intake (g/month); % (SE) | |||
| 0 | 12.4 (0.9) | 24.0 (4.2) | 13.1 (0.9) |
| 0 – 2000 | 46.0 (1.4) | 32.4 (4.6) | 45.2 (1.3) |
| > 2000 | 41.6 (1.4) | 43.7 (4.9) | 41.7 (1.3) |
| Residence place (urban); % (SE) | 76.6 (1.2) | 75.8 (4.3) | 76.6 (1.1) |
| Total plasma arsenic (μg/L); GM (95% CI) | 2.5 (2.4, 2.7) | 3.8 (3.0, 4.7) | 2.6 (2.4, 2.7) |
| Total urine arsenic (μg/L); GM (95% CI) | 59.5 (55.3, 64.0) | 106.4 (82.6, 137) | 61.7 (57.5, 66.2) |
| Urine creatinine (g/L); GM (95% CI) | 0.93 (0.90, 0.96) | 1.01 (0.91, 1.12) | 0.94 (0.91, 0.96) |
| Total urine arsenic (μg/g); GM (95% CI) | 64.0 (59.0, 69.3) | 106 (82.3, 136) | 66.0 (61.1, 71.2) |
| ΣiAs (μg/g); GM (95% CI) b,c | 10.9 (9.2, 12.9) | 14.9 (11.0, 20.2) | 11.1 (9.5, 13.0) |
| Arsenobetaine (μg/g); GM (95% CI) c | 46.1 (37.5, 56.7) | 66.5 (37.9, 116.6) | 47.3 (38.8, 57.6) |
| % iAs; median (IQR) c | 2.7 (1.1, 5.8) | 1.9 (1.4, 5.0) | 2.6 (1.2, 5.6) |
| % MMA; median (IQR) c | 11.0 (2.7, 29.7) | 6.3 (2.5, 18.5) | 10.4 (2.7, 29) |
| % DMA; median (IQR) c | 83.7 (61.1, 94.3) | 90.8 (79.5, 94.7) | 84.6 (62.5, 94.3) |
6 and 50 participants with and without T2D missed body mass index information.
ΣiAs=AsIII+AsV+MMA+DMA; iAs=AsIII+AsV.
Calculated in the 295 participants subsample with available arsenic speciation measurements (27 and 268 participants with and without T2D).
AsIII, AsV, MMA, DMA and Asb not detectable values were imputed as a LOD divided by the square root of 2.
Abbreviations: T2D, type 2 diabetes; SE, standard error; GM, geometric mean; CI, confidence interval; IQR, interquartile range; AsIII, arsenite; AsV, arsenate; iAs, inorganic arsenic, MMA monomethylarsonate; DMA, dimethylarsinate; Asb, arsenobetaine.
Total Urine Arsenic and Diabetes Prevalence
We found a positive significant association of total urine arsenic with diabetes prevalence before and after adjustment for arsenobetaine concentrations (Table 2). The fully adjusted odds ratio (95% CI) for diabetes comparing the second and the third tertiles with the first one of total urine arsenic were 2.03 (1.63–2.53) and 2.14 (1.47, 3.11), respectively (Table 2, model 3). In model 2, which was not adjusted for Asb, this relation was weaker and not strictly increasing (odds ratio (95% CI) comparing the second and third tertiles to the lowest were 1.85 (1.04, 3.30) and 1.76 (1.01, 3.09), respectively). The analyses repeated in the random subsample with originally measured arsenic species showed consistent findings (e-Table 4). Flexible models based on splines confirmed that multivariable adjusted odds ratio were higher after adjusting for Asb (model 2 versus model 3) (Figure 1). No significant differences for the odds ratio of diabetes were found for gender, ever-smoking status and abnormal albuminuria subgroups (Table 3). For reduced glomerular filtration rate status, however, the association between arsenic and diabetes was stronger among participants without kidney disease (P of interaction = 0.02).
Table 2.
Odds ratio (95% confidence interval) for type 2 diabetes by total urine arsenic concentrations (N=1,451)
| Model 1a | Model 2b | Model 3c | |||||
|---|---|---|---|---|---|---|---|
| Total Urine Arsenic, μg/g |
Cases / Non cases |
OR | 95% CI | OR | 95% CI | OR | 95% CI |
| Tertile 1 (0 – 33.77) | 25 / 437 | 1 Ref. | 1 Ref. | 1 Ref. | |||
| Tertile 2 (33.77 – 123.90) | 41 / 432 | 1.85 | 1.04, 3.28 | 1.85 | 1.04, 3.30 | 2.03 | 1.63, 2.53 |
| Tertile 3 (> 123.90) | 54 / 462 | 1.75 | 1.01, 3.03 | 1.76 | 1.01, 3.09 | 2.14 | 1.47, 3.11 |
| p80th vs. p20th | 120 / 1331 | 1.30 | 0.94, 1.80 | 1.31 | 0.94, 1.81 | 1.76 | 1.54, 2.02 |
Abbreviations: OR, odds ratio; CI, confidence interval.
p80th and p20th are 229.93 and 19.25 μg/g of creatinine, respectively.
Model 1: Adjusted for age (years), gender (male, female) and education (<secondary education, >= secondary education).
Model 2: Model 1 further adjusted for urine cotinine levels (<34, 34–500 and >500 ng/mL), smoking status (non-smoker, former and current), alcohol consumption (mg/day), fish consumption (g/month), and residence place (rural, urban).
Model 3: Model 2 further adjusted for log-transformed urine arsenobetaine levels (μg/g).
Figure 1. Odds ratio of type 2 diabetes by total urine arsenic concentrations, before and after adjustment for arsenobetaine (Asb).
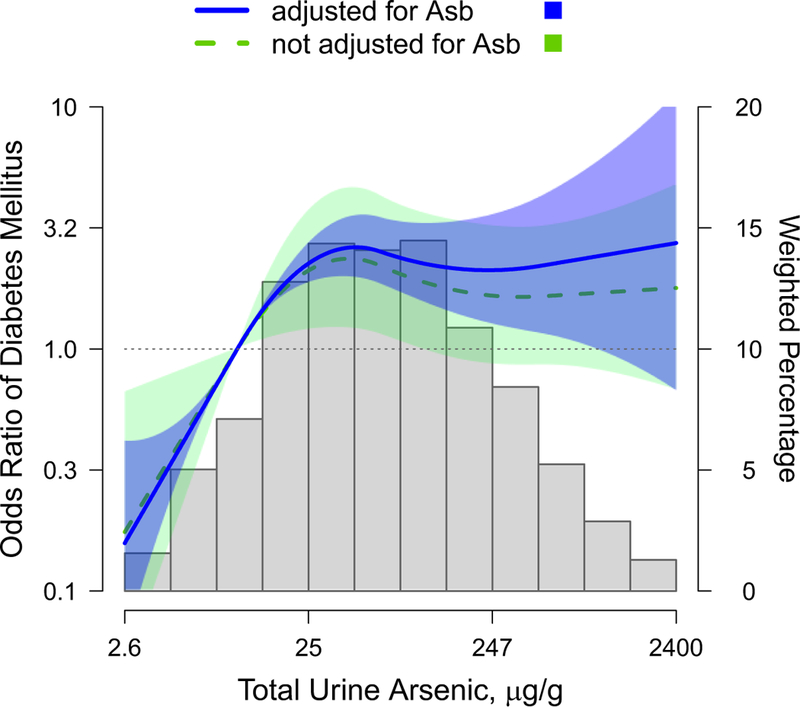
Curves represent the odds ratios for type 2 diabetes based on restricted quadratic splines with knots at the 10th, 50th, and 90th percentiles (10.4, 61.1 and 450.1 μg/g, respectively) of the total urine arsenic distribution. The reference was set at the 10th percentile of the total urine arsenic distribution. Dotted line and light gray area represent the odds ratio and the 95% confidence interval before adjustment for arsenobetaine levels, i.e., adjusted for age, gender, education (<secondary education, ≥secondary education), urine cotinine levels (<34, 34–500 and >500 ng/mL), smoking status (non-smoker, former, current), alcohol intake, fish intake, and residence place (rural, urban). Solid line and dark gray area represent the odds ratio and the 95% confidence interval after further adjustment for log-transformed urine arsenobetaine (μg/g). Bars represent the weighted distribution of creatinine corrected total urine arsenic concentrations (μg/g). Tails were truncated by excluding 1% of the study sample with total urine arsenic concentrations below 2.6 μg/g and 1% of the study sample with total urine arsenic concentratiions above 2400 μg/g.
Table 3.
Odds ratio (95% confidence interval) for type 2 diabetes comparing the 80th and the 20th percentiles of total urine arsenic by participant subgroups (N=1,451).
| N | Cases / Non cases |
OR | 95% CI | Interaction P |
|
|---|---|---|---|---|---|
| Overall | 1451 | 120 / 1331 | 1.76 | 1.54, 2.02 | |
| Gender | |||||
| Female | 722 | 46 / 676 | 1.68 | 1.45, 1.94 | |
| Male | 729 | 74 / 655 | 1.85 | 1.57, 2.19 | 0.77 |
| Smoking | |||||
| Never | 681 | 59 / 622 | 2.08 | 1.78, 2.42 | |
| Ever | 770 | 61 / 709 | 1.52 | 1.30, 1.78 | 0.35 |
| Abnormal Albuminuria | |||||
| No | 1421 | 108 / 1313 | 1.92 | 1.67, 2.21 | |
| Yes | 26 | 14 / 12 | 2.79 | 1.67, 4.66 | 0.61 |
| Reduced GFR | |||||
| No | 1331 | 94 / 1237 | 2.19 | 1.89, 2.54 | |
| Yes | 120 | 26 / 94 | 0.99 | 0.85, 1.15 | 0.02 |
Abbreviations: OR, odds ratio; CI, confidence interval; GFR, glomerular filtration rate. p80th and p20th are 229.93 and 19.25 μg/g of creatinine, respectively. Models were adjusted for further adjusted for age (years), gender (male, female), educational level (<secondary education, >= secondary education), urine cotinine (<34, 34–500 and >500 ng/mL), smoking status (non-smoker, former and current), alcohol consumption (mg/day), fish consumption (g/month), residence place (rural, urban), and log-transformed urine arsenobetaine levels (μg/g).
Gene-environment interaction
In gene-environment interaction analyses, while no polymorphism showed significant interactions with total urine arsenic after correcting for multiple comparisons, several genotypes showed marginally significant differential associations with diabetes (Figure 2 and Table 4). The top 5 genes showing the highest statistical interactions were IL8RA (rs1008562 [P=0.004]), TXN (rs4135168 [P=0.004]), NR3C2 (rs13117325 [P=0.007]), COX5A (rs1133322 [P =0.01]) and GCLC (rs11415624 [P =0.01]).
Figure 2. Candidate genes-arsenic interaction –log10 P-values.
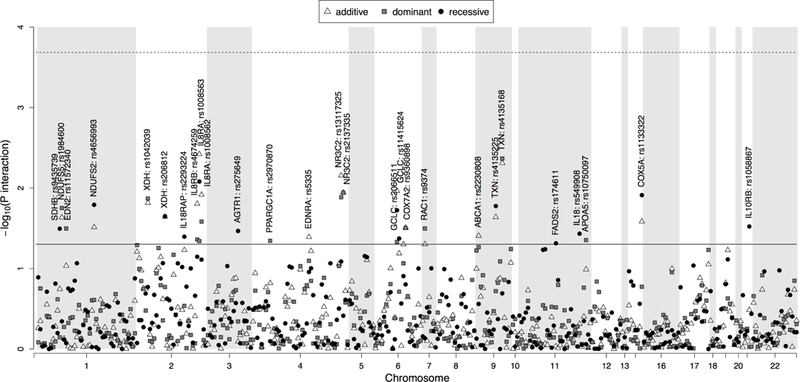
P-values for 354 SNPs derived from linear regression models (dominant, recessive and additive model) adjusted for age, sex, education, urine cotinine levels, smoking status, alcohol consumption, fish consumption, residence place and log-transformed arsenobetaine, are presented on the left Y axis on the logarithmic scale according to the position of the SNPs on chromosome (X axis). The horizontal solid line corresponds to a nominal p-value of significance equal to 0.05. Horizontal dashed line corresponds the effective SNP number corrected p-value equal to 0.0002.
Table 4.
Odds ratio (95% CI) of type 2 diabetes comparing the 80th vs the 20th percentiles of total urine arsenic distribution by genotypes among the 5 genes with top interaction p-values.
| Chr. | Gene | SNP | Model | Cases / Non cases |
Genotype | OR | 95% CI |
P Interaction |
|---|---|---|---|---|---|---|---|---|
| 2 | IL8RA | rs1008563 | ADD | 40/329 | T/T | 2.26 | 2.18, 2.34 | 0.004 |
| 46/608 | T/C | 3.38 | 3.29, 3.46 | |||||
| 27/313 | C/C | 5.04 | 4.69, 5.42 | |||||
| rs1008562 | ADD | 49/451 | C/C | 2.48 | 2.40, 2.56 | 0.01 | ||
| 44/602 | C/G | 3.48 | 3.39, 3.58 | |||||
| 20/207 | G/G | 4.89 | 4.48, 5.33 | |||||
| 9 | TXN | rs4135168 | DOM | 71/732 | A/A | 4.19 | 4.01, 4.38 | 0.004 |
| 47/566 | A/G+G/G | 2.48 | 2.38, 2.58 | |||||
| 4 | NR3C2 | rs13117325 | ADD | 48/572 | G/G | 3.87 | 3.73, 4.02 | 0.007 |
| 46/554 | G/A | 2.82 | 2.76, 2.88 | |||||
| 18/138 | A/A | 2.06 | 1.93, 2.19 | |||||
| rs2137335 | DOM | 82/779 | T/T | 2.70 | 2.63, 2.76 | 0.01 | ||
| 33/492 | T/C+C/C | 4.65 | 4.30, 5.03 | |||||
| 6 | GCLC | rs11415624 | ADD | 58/585 | D/D | 3.80 | 3.67, 3.93 | 0.01 |
| 54/574 | D/A | 2.73 | 2.67, 2.79 | |||||
| 8/164 | A/A | 1.96 | 1.84, 2.09 | |||||
| 15 | COX5A | rs1133322 | REC | 96/978 | T/C+T/T | 3.44 | 3.36, 3.53 | 0.01 |
| 21/323 | C/C | 2.03 | 1.90, 2.17 | |||||
Abbreviations: OR, odds ratio; CI, confidence interval; ADD, DOM, and REC, additive, dominant and recessive model, respectively.
The Bonferroni-corrected significance level was 0.0002.
Odds ratio for diabetes comparing the 80th and 20th percentiles of urine arsenic distributions (229.93 and 19.24 μg/g respectively) and associated test for interaction were obtained from linear regression models with log-transformed arsenic as a continuous variable. Models were adjusted for age (years), sex (male, female), education (<secondary education, >=secondary education), urine cotinine levels (<34, 34–500 and >500 ng/mL), smoking status (non-smoker, former and current), alcohol consumption (mg/day), fish consumption (g/month), residence place (rural, urban) and log-transformed arsenobetaine (μg/g).
Discussion
We found a positive association between increasing levels of total urine arsenic and the prevalence of type 2 diabetes in a representative sample of Spanish adults characterized by exposure to low arsenic levels. Our results support the relevance of adjusting for arsenobetaine levels in populations with high seafood consumption in order to estimate the association with arsenic exposure not derived from seafood. We also observed suggestive statistical interactions of urine arsenic levels with genetic variation in several genes involved in diabetes metabolic pathways, especially genes involved in regulation of oxidative stress response or inflammation.
The arsenobetaine levels in our study population (median: 160.3 μg/L, interquartile range: 15.8–124.5 μg/L) were much higher than those observed in a study of the 2003–2004 National Health and Nutrition Examination Survey (NHANES) (median: 0.9 μg/L, interquartile range: 0.3–3.5 μg/L) (Navas-Acien et al. 2008), reflecting a higher seafood intake. In populations with low seafood consumption, subtracting arsenobetaine concentration from total urine arsenic levels may provide a valid estimate of inorganic arsenic, since it removes the most common part of organic arsenic in seafood (Steinmaus et al. 2009). In populations with high seafood consumption, however, this approach may not be sufficient to remove the contribution of other seafood organic arsenic compounds, such as arsenosugars and arsenolipids and their metabolites, including DMA (Navas-Acien et al. 2011). Arsenobetaine is specific to seafood, it is excreted in the urine untransformed, and it is correlated with other seafood arsenicals. Using arsenobetaine as a covariate in the statistical regression models can thus indirectly control for other seafood arsenic species and their metabolites (Navas-Acien et al. 2009).
In 2003, the maximum admissible arsenic levels in drinking water in Spain decreased from 50 μg/L to 10 μg/L (Ministerio de la Presidencia 2003) as recommended by the World Health Organization. Our study participants were recruited between 1997 and 2003 in Valladolid, in central Spain. Most participants were most likely exposed to low levels of inorganic arsenic in drinking water. It is possible, however, that some participants coming from rural areas could have been exposed to moderate-high arsenic levels from the groundwater. A study evaluating groundwater quality in rural areas of Valladolid found concentrations of arsenic reaching levels as high as 260 μg/L in 2001 (mean=93 μg/L), 187 μg/L in 2003 (mean=48 μg/L) and 51 μg/L in 2007 (mean=15 μg/L) (Mayorga P, Moyano A, and García-Sánchez 2014). In our study population, the sum of inorganic and methylated arsenic concentrations was similar in participants from rural (geometric mean=8.8 μg/g) and urban areas (geometric mean=11.9 μg/g), even after adjustment for Asb and seafood intake (data not shown).
Several epidemiologic studies have found positive associations between arsenic exposure levels and diabetes in adults, both cross-sectionally (Navas-Acien et al. 2008) and prospectively (Brauner et al. 2014; James et al. 2013; Kim et al. 2013). In the US, increasing levels of urine arsenic were associated with increasing odds of diabetes after adjustment for urine arsenobetaine (Navas-Acien et al. 2008). Long-term exposure to low arsenic levels, assessed as arsenic concentrations in drinking water, has been prospectively associated with diabetes in central Italy (D’Ippoliti et al. 2015) and Denmark (Brauner et al. 2014). Studies using biomarkers of arsenic exposure also found positive relationship between arsenic and incident diabetes in Colorado (James et al. 2013) and American Indians from South-West of the United States (Kim et al. 2013), but not in American Indians 45–74 years of age participating in the Strong Heart Study (Kuo et al. 2015).
Experimental evidence supports the role of arsenic as a diabetes risk factor. In rodents, exposure to arsenic through contaminated water has shown to induce insulin resistance (Palacios, Roman, and Cifuentes 2012) and beta-cell dysfunction (Liu et al. 2014). The liver is the major target organ of the regulation of arsenic exposure and metabolism levels, and several experimental studies have demonstrated that chronic arsenic exposure induces severe toxic effects in the liver (Santra et al. 1999; Mazumder 2005). The liver also plays a major role in the regulation of glucose metabolism (Cotrozzi et al. 1997). In vivo evidence suggests that arsenic-induced diabetes may be mediated by increased oxidative stress in hepatic and pancreatic tissue (Patel and Kalia 2013). Interestingly, in our gene-environment interaction analysis, we observed differential associations of arsenic with polymorphisms in genes involved in redox-related pathways, including SNPs in TXN and GCLC, genes that are highly expressed in the pancreas and liver (Diaz, Krejsa, and Kavanagh 2002; Tran et al. 2004; Yamamoto et al. 2008; Okuyama et al. 2008), although the evidence was only suggestive. Some studies, however, support a potential biological link between proteins encoded by these genes and arsenic.
A positive correlation was observed between serum thioredoxin1 (TXN1) and total water arsenic intake or urinary arsenic species in humans (Li et al. 2012). Arsenic inhibits thioredoxin reductase, and this may contribute to TXN oxidation (Lin et al. 2001). Another mechanistic study showed that arsenic exposure can disrupt the GCLC expression, since low arsenic exposure increased the expression of GCLC in liver tissues of rats while high arsenic exposure levels reduced its expression (Ren et al. 2015). In addition, the interaction of arsenic with the polymorphism rs1008563 in IL8RA (also known as CXCR2), a chemokine receptor for interleukin-8 (IL8), showed the highest p-value. Several lines of evidence support that IL8RA inhibitors play a key role in the etiopathogenesis of type 1 diabetes (Diana et al. 2013; Valle et al. 2013). Interestingly, an experimental study found that urothelial cells chronically exposed to trivalent monomethylarsonate showed an over-expression of IL8 (Escudero-Lourdes et al. 2012).
Despite the limited power to detect interactions at the Bonferroni-corrected level, we found marginally significant associations for some polymorphisms, which, given the biological relevance on diabetes-related pathways, should be evaluated in larger studies. There are limited studies evaluating the interaction of genetic variation and arsenic on diabetes. A prospective study in Bangladesh (N=957) reported a significant interaction of SNPs in NOTCH2 with arsenic exposure (assessed in drinking water) on the association with type 2 diabetes (Pan et al. 2013). Unfortunately, we did not genotype candidate SNPs in NOTCH2. Additional and larger prospective studies, especially with an extensive panel of candidate genes, are needed.
One of the limitations of our study is the cross-sectional design, which cannot determine the direction of the associations. Elevated arsenic exposure levels could increase diabetes risk by inducing liver damage, as well as liver dysfunction related to diabetes might influence arsenic metabolism and result in higher arsenic excretion in the urine (Ahmadieh and Azar 2014). Another limitation is the use of one single urine sample given the within-subject variability in urinary arsenic excretion. Our study has also some strengths, including the complex sampling design; the adjustment for relevant diabetes risk factors, potential confounders and markers of seafood intake; the use of a multiple imputation model to adjust total urine arsenic for arsenobetaine, which enabled the use of urinary arsenic as a proxy for inorganic arsenic exposure; and the availability of SNPs in 155 candidate genes for gene-environmental evaluation.
In conclusion, arsenic exposure was associated with increased prevalence of type 2 diabetes in a general population from Spain. Our findings support the hypothesis that arsenic plays a biological role as a diabetogenic risk factor. Our analytical strategy also shows the importance of controlling for seafood arsenicals in populations with high seafood consumption. While the evaluated gene-environment interactions were only close to statistical significance after multiple comparisons correction, and should be only considered exploratory, our results provide the basis for gene-environment interaction studies in larger epidemiologic studies.
Supplementary Material
Acknowledgments
Funding:This work was supported by the Strategic Action for Research in Health Sciences from the Institute of Health Carlos III [CP12/03080, PI10/0082, PI13/ 01848, PI15/00071 and PI11/00726], GRUPOS 03/101; PROMETEO/ 2009/029 and ACOMP/2013/039 from the Valencia Government; GRS/279/A/08 from Castilla-Leon Government; the European Network of Excellence Ingenious Hypercare (EPSS- 037093) from the European Commission; CIBER Fisiopatologia Obesidad y Nutricion (CIBERobn) [CIBER-02–08-2009, CB06/03 and CB12/03/30016], and CIBER de Diabetes y Enfermedades Metabolicas Relacionadas (CIBERDEM). The Strategic Action for Research in Health Sciences, CIBEROBN and CIBERDEM are initiatives from Carlos III Health Institute Madrid and the Spanish Ministry of Economy and Competitiveness and are co-funded with European Funds for Regional Development (FEDER). Maria Grau-Perez and Ana Navas-Acien were supported by National Institute of Environmental Health Sciences [R01ES025216, R01ES021367, P42ES10349].
Abbreviations:
- HbA1C
glycosylated hemoglobin
- ICPMS
inductively coupled-plasma mass spectrometry
- MCMC
Markov Chain Monte Carlo
- SNPs
single nucleotide polymorphisms
Footnotes
Conflict of interests: All authors declared that they do not have any conflict of interest.
Data and computing code: Code for MCMC imputation is provided in the Supplemental Material. The computing code for other statistical models is available upon request to the corresponding author. The individual-level data are not available as the steering committee and the participants did not approve unrestricted data sharing at the time of ethical approval of the study and data sharing was not included in the consent form.
References
- Ahmadieh H, and Azar ST. 2014. ‘Liver disease and diabetes: association, pathophysiology, and management’, Diabetes Res Clin Pract, 104: 53–62. [DOI] [PubMed] [Google Scholar]
- Bates MN, Smith AH, and Hopenhayn-Rich C. 1992. ‘Arsenic ingestion and internal cancers: a review’, Am J Epidemiol, 135: 462–76. [DOI] [PubMed] [Google Scholar]
- Brauner EV, Nordsborg RB, Andersen ZJ, Tjonneland A, Loft S, and Raaschou-Nielsen O. 2014. ‘Long-term exposure to low-level arsenic in drinking water and diabetes incidence: a prospective study of the diet, cancer and health cohort’, Environ Health Perspect, 122: 1059–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrozzi G, Casini Raggi V, Relli P, and Buzzelli G. 1997. ‘[Role of the liver in the regulation of glucose metabolism in diabetes and chronic liver disease]’, Ann Ital Med Int, 12: 84–91. [PubMed] [Google Scholar]
- D’Ippoliti D, Santelli E, De Sario M, Scortichini M, Davoli M, and Michelozzi P. 2015. ‘Arsenic in Drinking Water and Mortality for Cancer and Chronic Diseases in Central Italy, 1990–2010’, PLoS One, 10: e0138182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana J, Simoni Y, Furio L, Beaudoin L, Agerberth B, Barrat F, and Lehuen A. 2013. ‘Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes’, Nat Med, 19: 65–73. [DOI] [PubMed] [Google Scholar]
- Diaz D, Krejsa CM, and Kavanagh TJ. 2002. ‘Expression of glutamate-cysteine ligase during mouse development’, Mol Reprod Dev, 62: 83–91. [DOI] [PubMed] [Google Scholar]
- Escudero-Lourdes C, Wu T, Camarillo JM, and Gandolfi AJ. 2012. ‘Interleukin-8 (IL-8) over-production and autocrine cell activation are key factors in monomethylarsonous acid [MMA(III)]-induced malignant transformation of urothelial cells’, Toxicology and Applied Pharmacology, 258: 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan-Chilet I, Grau-Perez M, De Marco G, Guallar E, Martin-Escudero JC, Dominguez-Lucas A, Gonzalez-Manzano I, Lopez-Izquierdo R, Briongos-Figuero LS, Redon J, Chaves FJ, and Tellez-Plaza M. 2017. ‘A gene-environment interaction analysis of plasma selenium with prevalent and incident diabetes: The Hortega study’, Redox Biol, 12: 798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung RW, and Reed LD. 1990. ‘Estimation of Average Concentration in the Presence of Nondetectable Values’, Applied occupational and environmental hygiene, 5: 46–51. [Google Scholar]
- James KA, Marshall JA, Hokanson JE, Meliker JR, Zerbe GO, and Byers TE. 2013. ‘A case-cohort study examining lifetime exposure to inorganic arsenic in drinking water and diabetes mellitus’, Environ Res, 123: 33–8. [DOI] [PubMed] [Google Scholar]
- Kim NH, Mason CC, Nelson RG, Afton SE, Essader AS, Medlin JE, Levine KE, Hoppin JA, Lin C, Knowler WC, and Sandler DP. 2013. ‘Arsenic exposure and incidence of type 2 diabetes in Southwestern American Indians’, Am J Epidemiol, 177: 962–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CC, Howard BV, Umans JG, Gribble MO, Best LG, Francesconi KA, Goessler W, Lee E, Guallar E, and Navas-Acien A. 2015. ‘Arsenic exposure, arsenic metabolism, and incident diabetes in the strong heart study’, Diabetes Care, 38: 620–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gao Y, Zhao L, Wei Y, Feng H, Wang C, Wei W, Ding Y, and Sun D. 2012. ‘Changes in serum thioredoxin among individuals chronically exposed to arsenic in drinking water’, Toxicol Appl Pharmacol, 259: 124–32. [DOI] [PubMed] [Google Scholar]
- Lin S, Del Razo LM, Styblo M, Wang C, Cullen WR, and Thomas DJ. 2001. ‘Arsenicals inhibit thioredoxin reductase in cultured rat hepatocytes’, Chem Res Toxicol, 14: 305–11. [DOI] [PubMed] [Google Scholar]
- Liu S, Guo X, Wu B, Yu H, Zhang X, and Li M. 2014. ‘Arsenic induces diabetic effects through beta-cell dysfunction and increased gluconeogenesis in mice’, Sci Rep, 4: 6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn D, Thomas A, Best N, and Spiegelhalter D. 2000. ‘WinBUGS - a Bayesian modelling framework: Concepts, structure, and extensibility.’, Statistics and Computing, 10: 325–37. [Google Scholar]
- Mahajan A, Go MJ, Zhang W, Below JE, Gaulton KJ, Ferreira T, Horikoshi M, et al. 2014. ‘Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility’, Nat Genet, 46: 234–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayorga P, Moyano A, and García-Sánchez A. 2014. ‘Arsenic in groundwater of Castilla and León and its impact on soils and carrots crops’, Av. cien. ing, 5: 19–36. [Google Scholar]
- Mazumder DN. 2005. ‘Effect of chronic intake of arsenic-contaminated water on liver’, Toxicol Appl Pharmacol, 206: 169–75. [DOI] [PubMed] [Google Scholar]
- Mena Martin FJ, Martin Escudero JC, Simal Blanco F, Carretero Ares JL, and Herreros Fernandez V. 2003. ‘[Cardiovascular risk factors in diabetic patients. Cross-sectional study in general population: Hortega study]’, An Med Interna, 20: 292–6. [PubMed] [Google Scholar]
- Mena-Martin FJ, Martin-Escudero JC, Simal-Blanco F, Carretero-Ares JL, Arzua-Mouronte D, and Herreros-Fernandez V. 2003. ‘Health-related quality of life of subjects with known and unknown hypertension: results from the population-based Hortega study’, J Hypertens, 21: 1283–9. [DOI] [PubMed] [Google Scholar]
- Ministerio de la Presidencia. 2003. ‘REAL DECRETO 140/2003, de 7 de febrero, por el que se establecen los criterios sanitarios de lacalidad del agua de consumo humano.’, BOE, 45: 7228–45. [Google Scholar]
- Molin M, Ulven SM, Dahl L, Goessler W, Fliegel D, Holck M, Sloth JJ, Oshaug A, Alexander J, Meltzer HM, and Ydersbond TA. 2014. ‘Urinary excretion of arsenicals following daily intake of various seafoods during a two weeks intervention’, Food Chem Toxicol, 66: 76–88. [DOI] [PubMed] [Google Scholar]
- Moon K, Guallar E, and Navas-Acien A. 2012. ‘Arsenic exposure and cardiovascular disease: an updated systematic review’, Curr Atheroscler Rep, 14: 542–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, and Rimm EB. 2006. ‘Fish intake, contaminants, and human health: evaluating the risks and the benefits’, JAMA, 296: 1885–99. [DOI] [PubMed] [Google Scholar]
- National Research Council. 1999. ‘Arsenic in Drinkin Water’, Washington, DC: National Academy Press. [Google Scholar]
- Navas-Acien A, Francesconi KA, Silbergeld EK, and Guallar E. 2011. ‘Seafood intake and urine concentrations of total arsenic, dimethylarsinate and arsenobetaine in the US population’, Environ Res, 111: 110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, and Guallar E. 2008. ‘Arsenic exposure and prevalence of type 2 diabetes in US adults’, JAMA, 300: 814–22. [DOI] [PubMed] [Google Scholar]
- Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, and Guallar E. 2009. ‘Arsenic exposure and Prevalence of Type 2 Diabetes: Updated Findings from the National Health Nutrition and Examination Survey, 2003–2006’, Epidemiology, 20: 816–e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, Silbergeld EK, Streeter RA, Clark JM, Burke TA, and Guallar E. 2006. ‘Arsenic exposure and type 2 diabetes: a systematic review of the experimental and epidemiological evidence’, Environ Health Perspect, 114: 641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyama H, Son A, Ahsan MK, Masutani H, Nakamura H, and Yodoi J. 2008. ‘Thioredoxin and thioredoxin binding protein 2 in the liver’, IUBMB Life, 60: 656–60. [DOI] [PubMed] [Google Scholar]
- Palacios J, Roman D, and Cifuentes F. 2012. ‘Exposure to low level of arsenic and lead in drinking water from Antofagasta city induces gender differences in glucose homeostasis in rats’, Biol Trace Elem Res, 148: 224–31. [DOI] [PubMed] [Google Scholar]
- Pan WC, Kile ML, Seow WJ, Lin X, Quamruzzaman Q, Rahman M, Mahiuddin G, Mostofa G, Lu Q, and Christiani DC. 2013. ‘Genetic susceptible locus in NOTCH2 interacts with arsenic in drinking water on risk of type 2 diabetes’, PLoS One, 8: e70792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel HV, and Kalia K. 2013. ‘Role of hepatic and pancreatic oxidative stress in arsenic induced diabetic condition in Wistar rats’, J Environ Biol, 34: 231–6. [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, and Sham PC. 2007. ‘PLINK: a tool set for whole-genome association and population-based linkage analyses’, Am J Hum Genet, 81: 559–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Gaile DP, Gong Z, Qiu W, Ge Y, Zhang C, Huang C, Yan H, Olson JR, Kavanagh TJ, and Wu H. 2015. ‘Arsenic responsive microRNAs in vivo and their potential involvement in arsenic-induced oxidative stress’, Toxicol Appl Pharmacol, 283: 198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DB. 1978. ‘Multiple imputations in sample surveys - a phenomenological Bayesian approach to nonresponse.’, Proceeding of the survey research methods section, ASA: 20–34. [Google Scholar]
- Santra A, Das Gupta J, De BK, Roy B, and Guha Mazumder DN. 1999. ‘Hepatic manifestations in chronic arsenic toxicity’, Indian J Gastroenterol, 18: 152–5. [PubMed] [Google Scholar]
- Steinmaus C, Yuan Y, Liaw J, and Smith AH. 2009. ‘Low-level population exposure to inorganic arsenic in the United States and diabetes mellitus: a reanalysis’, Epidemiology, 20: 807–15. [DOI] [PubMed] [Google Scholar]
- Tellez-Plaza M, Navas-Acien A, Crainiceanu CM, Sharrett AR, and Guallar E. 2010. ‘Cadmium and peripheral arterial disease: gender differences in the 1999–2004 US National Health and Nutrition Examination Survey’, Am J Epidemiol, 172: 671–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PO, Parker SM, LeRoy E, Franklin CC, Kavanagh TJ, Zhang T, Zhou H, Vliet P, Oseid E, Harmon JS, and Robertson RP. 2004. ‘Adenoviral overexpression of the glutamylcysteine ligase catalytic subunit protects pancreatic islets against oxidative stress’, J Biol Chem, 279: 53988–93. [DOI] [PubMed] [Google Scholar]
- Valle A, Giamporcaro GM, Scavini M, Stabilini A, Grogan P, Bianconi E, Sebastiani G, et al. 2013. ‘Reduction of circulating neutrophils precedes and accompanies type 1 diabetes’, Diabetes, 62: 2072–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Zhu J, and Nguyen A. 2014. ‘Rice consumption and urinary concentrations of arsenic in US adults’, Int J Environ Health Res, 24: 459–70. [DOI] [PubMed] [Google Scholar]
- World Health Organization. 2001. ‘Arsenic and arsenic compounds’. [Google Scholar]
- World Health Organization. 2016. ‘Global Report on Diabetes’. [Google Scholar]
- Yamamoto M, Yamato E, Toyoda S, Tashiro F, Ikegami H, Yodoi J, and Miyazaki J. 2008. ‘Transgenic expression of antioxidant protein thioredoxin in pancreatic beta cells prevents progression of type 2 diabetes mellitus’, Antioxid Redox Signal, 10: 43–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


