Abstract
Objective:
To compare the effects of cognitive-behavioral therapy (CBT) and lifestyle modification (LS) versus LS alone on weight, depressive and anxiety symptoms, and stress response in women with polycystic ovary syndrome (PCOS), overweight/obesity, and depressive symptoms.
Design:
A 16-week pilot randomized clinical trial.
Setting:
Tertiary-care PCOS center.
Patient(s):
Overweight/obese women with PCOS and depressive symptoms.
Intervention(s):
Weekly CBT (n = 7) or contact only/no therapy (n = 8) for 8 weeks. Both groups received weekly LS for 16 weeks.
Main Outcome Measure(s):
Changes in weight, depression (Center for Epidemiologic Studies Depression Scale [CES-D]), anxiety (State-Trait Anxiety Inventory [STAI]), quality of life (Polycystic Ovary Syndrome Health-Related Quality of Life Questionnaire [PCOSQ]), laboratory tests, and response to a Trier Social Stress Test (TSST).
Result(s):
The CBT+LS group lost more weekly weight (−0.35 kg/wk vs. −0.16 kg/wk) compared with the LS group. Overall, the CBT+LS group lost 3.2 kg versus 1.8 kg for the LS group. The CBT+LS group had greater improvement in PCOSQ at 8 weeks (+3.7 vs. +1.2 points). In the overall cohort, STAI and CES-D decreased by −0.27 points per week and −0.31 points/wk, respectfully, and total and free T decreased at week 8. Heart rate response to TSST was lower at 15 minutes after stressor in the CBT+LS group.
Conclusion(s):
Weekly CBT+LS for 8 weeks compared with LS alone resulted in significant weight loss and improved quality of life in overweight/obese women with PCOS and depressive symptoms. These interventions were associated with a decreased autonomic response to a laboratory stressor, suggesting a potential link between CBT, weight loss, and modulation of the stress response.
Keywords: PCOS, weight loss, depression, CBT, nutrition
Abstract
Objetivo:
Comparar los efectos de la terapia cognitivo-conductual (CBT) y las modificaciones del estilo de vida (LS) frente a la modificación únicamente del estilo de vida (LS) en el peso, síntomas de ansiedad y depresión, y respuesta al estrés en mujeres con síndrome de ovario poliquístico (PCOS), sobrepeso/obesidad, y síntomas de depresion.
Diseño:
Ensayo clínico piloto aleatorizado de 16 semanas.
Entorno:
Centro de cuidado terciario para pacientes con PCOS.
Paciente (s):
Mujeres obesas/con sobrepeso con PCOS y síntomas depresivos.
Intervencion (s):
CBT semanal (n:7) o solo control/sin terapia (n:8) durante 8 semanas. Ambos grupos recibieron modificación de su estilo de vida semanal durante 16 semanas.
Principales medidas de resultado:
Cambios en el peso, grado de depresión (Escala de depresión del centro de estudios epidemiológicos [CES-D]), grado de ansiedad (Cuestionario de ansiedad estado-rasgo [STAI]), calidad de vida (cuestionario de calidad de vida relacionado con la salud en síndrome de ovario poliquístico [PCOSQ]), pruebas de laboratorio y respuesta al test de clasificación social del estrés (TSST).
Resultado (s):
El grupo CBT+LS perdió más peso semanalmente ( −0.35 kg/semana vs. − 0.16 Kg/semana) comparado con el grupo LS. En conjunto, el grupo CBT+LS perdió 3.2 Kg vs. 1.8 Kg en el grupo LS. El grupo CBT+LS tuvo una mayor mejoría en el PCOSQ a las 8 semanas (+ 3.7 puntos vs +1.2 puntos). En la cohorte general STAI y CES-D disminuyeron en −0.27 puntos por semana y −0.31 puntos por semana respectivamente, además de una disminución en la testosterona total y libre a las 8 semanas. La respuesta de la frecuencia cardíaca al TSST fue menor a los 15 minutos del estímulo estresante en el grupo CBT+LS.
Conclusion (s):
La CBT+LS semanal durante 8 semanas comparado con solo LS, produjo una significativa pérdida de peso y una mejoría en la calidad de vida de mujeres con sobrepeso/obesidad, con PCOS y síntomas depresivos. Estas intervenciones estuvieron asociadas a una disminución de la respuesta autónoma al estímulo estresante de laboratorio, sugiriendo una asociación potencial entre la CBT, la pérdida de peso, y la modulación de respuesta al estrés.
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders affecting reproductive-age women worldwide (1). Women with PCOS have higher rates of obesity and an increased prevalence of cardiovascular disease (CVD) risk factors, such as insulin resistance, dyslipidemia, and diabetes (2, 3). Given that women with PCOS often present to health care providers early in their reproductive years, there is an opportunity to modify these risk factors. Lifestyle modifications (LS) are recommended as first-line treatment in women with PCOS (4, 5), with a recent study showing that lifestyle interventions associated with weight loss can also improve pregnancy rates (6).
Women with PCOS also have a high prevalence of depression and anxiety. A recent meta-analysis showed median prevalences of depressive symptoms of 36.6% (interquartile range [IQR] 22.3%–50.0%) and anxiety symptoms of 41.9% (IQR 13.6%–52.0%) in women with PCOS. Compared with women without PCOS, these numbers represented a more than threefold increase in odds of depressive symptoms (odds ratio [OR] 3.78–95% CI 3.03–4.72; 18 studies) and a more than fivefold increase in odds of anxiety symptoms (OR 5.62–95% CI 3.22–9.80; 9 studies) (7). Other studies have also shown that women with PCOS have a decreased quality of life (QoL) compared with control women (8). Depression and anxiety are independently associated with CVD (9), as well as CVD risk factors, such as obesity (10), insulin resistance (11), and diabetes (12). In addition, obesity and psychiatric disorders are both associated with hypothalamic-pituitary-adrenal (HPA) axis disturbances and dysregulated inflammatory pathways (13). Studies on the use of LS in women with PCOS have not addressed comorbid depression or anxiety, so we do not know the success of these interventions in women with PCOS who also have depression or anxiety.
A small study in adolescents with PCOS showed improvement in both weight and depression scores after cognitive-behavioral therapy (CBT); however, there was no control group (14). CBT is a psychotherapy that focuses on changing dysfunctional thoughts that lead to negative mood states and maladaptive behaviors (15). CBT is recommended by the American Psychological Association and the American College of Physicians as a first-line treatment for depression (16, 17), and a recent meta-analysis showed moderate to large treatment effects for both major depressive disorder (MDD) and generalized anxiety disorder (18). CBT techniques have also been successful in achieving weight loss in various populations (19–22), although very few studies have included women with concurrent obesity and depression (14, 23, 24). Also, CBT has not been evaluated in adult women with PCOS to improve weight loss or depressive and anxiety symptoms. Therefore, our overall aim was to compare the feasibility and preliminary efficacy of an intervention which combined CBT and LS versus LS alone in the concurrent treatment of overweight/obesity and depressive symptoms in women with PCOS. Our primary outcome was change in weight. Secondary outcomes were changes in depression, anxiety, and quality of life scores. Tertiary outcomes included changes in metabolic risk, inflammation, perceived stress, and autonomic and endocrine response to a laboratory stressor.
MATERIALS AND METHODS
Study Design
We conducted an open-label 16-week randomized clinical pilot study at a single tertiary care center from August 2013 to October 2015 to investigate the use of CBT to treat symptoms of depression and improve cardiovascular risk factors in overweight/obese women with PCOS. The study protocol was approved by the Institutional Review Board at the University of Pennsylvania. The trial was registered at clinicaltrials.gov (NCT01899001).
Participants
Women were eligible if they met National Institutes of Health criteria for PCOS (25), had a body mass index (BMI) of 27–50 kg/m2 and had a positive screen for depression symptoms, defined as a Center for Epidemiologic Studies Depression Scale (CES-D) score ≥14 (26). Subjects with depression and anxiety disorders that were currently being treated were included if their medication had not been changed for ≥2 months and their CES-D score was still ≥14. Patients with an active eating disorder, currently participating in a weight loss program, or receiving pharmacotherapy for dyslipidemia, hypertension, or diabetes/impaired glucose tolerance, or on hormonal therapy were excluded. The washout period for oral contraceptive pills or metformin was 4 weeks. Given the association between nicotine and cortisol levels (27, 28), which we measured during the Trier Social Stress Test (TSST), women who smoked on average five or more cigarettes per day were excluded. Pregnant women and women who were planning pregnancy within the 16-week period were also excluded.
Lifestyle Modification
All women received in-person individual 30-minute weekly nutrition/exercise counseling by a trained counselor for 16 visits at Penn’s Center for Weight and Eating Disorders. Subjects were recommended a self-selected diet of 1,500–1,800 kcal/d of conventional foods based on the Food Guide Pyramid and an exercise goal starting at 50 minutes per week and increasing to 175 minutes per week. Counseling included standard weight loss skills including self-monitoring, problem-solving, enlisting social support, and overcoming negative thoughts. Subjects kept daily food intake and exercise logs which were reviewed at each counseling session. Previous studies have shown significant weight loss after 16 weeks of LS in women with PCOS (6).
Cognitive-Behavioral Therapy
Participants randomized to the CBT group received weekly 30-minute sessions with a CBT-trained clinical psychologist (L.H. or S.K.) from the Penn Center for Women’s Behavioral Wellness for the first eight visits. Sessions included behavioral components, such as activity scheduling and homework, and cognitive skills, such as identifying automatic thoughts and cognitive distortions (15, 17). Sessions were highly standardized and followed The Brief Cognitive Therapy Manual (Supplemental Table 1 available online at www.fertstert.org) (29). This 8-week time frame of brief CBT has been successful in other randomized controlled trials (22). To control for contact time during CBT, the subjects randomized to the LS-only arm met with a team member at the same time intervals to correspond with the CBT sessions (weekly 30-minute sessions for the first eight visits). During these sessions, subjects were queried about symptoms but no active intervention was administered. Randomization was blinded and performed with the use of the Research Electronic Data Capture program. We had a higher dropout rate in the CBT group before 8 weeks. Given that this was a pilot study, we wanted to be able to analyze similar numbers of subjects who had completed at least 8 weeks of intervention (i.e., CBT therapy). After discussion with our statistician (M.D.S.), randomization was overridden for three subjects.
Measurements
Anthropometric and laboratory measurements.
Subjects were weighed weekly in light clothing with the use of a Tanita scale. Blood pressure and waist (WC) and hip (HC) circumferences were measured at baseline, visit 8 to and visit 16 (Supplemental Fig. 1 available online at www.fertstert.org). Blood was drawn at baseline, visit 8 to and visit 16 (Supplemental Fig. 1) for fasting lipid profile (total cholesterol, low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), and triglycerides), fasting glucose, insulin, total testosterone, free testosterone, high-sensitivity C-reactive protein (hs-CRP), interleukin-6 (IL-6), apolipoprotein A1 (Apo A1) and apolipoprotein B (Apo B). All blood samples were drawn the morning after an overnight fast in the follicular phase of the menstrual cycle. The samples were analyzed in the Translational Core Laboratory at the Children’s Hospital of Philadelphia (CHOP) or the University of Pennsylvania Diabetes Research Center. See Supplemental Table 2 (available online at www.fertstert.org) for assay platforms and range.
Psychiatric scales.
All enrolled subjects completed multiple validated screening tools. The CES-D detects, assesses, and monitors changes in depressive symptoms (range 0–60; positive screen defined as ≥14). The State-Trait Anxiety Inventory (STAI) measures trait (longstanding/general) and state (current/situation based) anxiety (range 20–80). The Polycystic Ovary Syndrome Health-Related Quality of Life Questionnaire (PCOSQ) is a QoL scale validated for PCOS women. QoL is a multidimensional concept that encompasses the physical, emotional, and social aspects associated with a disease. For PCOS, the five domains that were identified to be important during scale validation were emotions, hair growth, body weight, infertility, and menstruation (overall score range 0–35; domain score range 0–7). Higher scores indicate better function, and a change of 0.5 units is clinically significant (30). The Mini International Neuropsychiatric Interview (MINI) is a structured interview to assess current and past mood disorders and psychiatric conditions. The Perceived Stress Scale (PSS) measures the degree to which situations in one’s life are appraised as stressful (range 0–40). The Adverse Childhood Experiences questionnaire determines a person’s number of experienced adverse events before the age of 18 years, on a scale of 0–9 based on the number of traumas experienced. All scales were administered at baseline. In addition, the PCOSQ and PSS were administered at visits 8 and 16 to and the CES-D and STAI scales were administered weekly for 16 visits (Supplemental Fig. 1).
Trier Social Stress Test.
At baseline and visit 8 to the TSST (31) was performed by trained study personnel to induce stress in the participants in a reliable, validated, and safe manner. The TSST is a standardized and commonly used test to induce acute stress in experimental settings and has been shown to reliably increase activation of the HPA axis. The test was administered during the follicular phase of the subject’s menstrual cycle. The test period lasts 20 minutes and is divided into three components: 1) anticipatory stress phase/preparation (10 min); 2) presentation component, where the subject gives a presentation to a panel of three “judges” (5 min); and 3) mental arithmetic component (5 min); followed by a recovery period. Salivary cortisol was collected and heart rate (HR) was recorded before and after the stressor at a total of six time points: two before stressor start (−30 min and −15 min), one immediately on completion of the stressor (0 min), and three after stressor completion (+15 min, +30 min, and +60 min). Cortisol was measured by the Translational Core Laboratory at CHOP (Supplemental Table 2).
Statistical Analysis
Data analysis was performed with the use of Stata version 14.2. Continuous variables are reported as median (interquartile range [IQR]). Group differences were analyzed with the use of chi-square or Fisher exact tests for categoric variables and Wilcoxon rank sum or Kruskall-Wallis tests for continuous variables. For comparisons between baseline and visit 8/visit 16 only subjects with measurements at both time points were analyzed. Effect size was calculated using the eta-squared statistic, which measures the contribution of a variable (CBT group) on the observed dependent variable (change in weight). Using Cohen’s 1988 definition, an eta-squared statistic of 0.01 is considered to be small, 0.6 medium, and 0.14 large (32, 33).
Analysis of longitudinal data.
Mixed-effects linear regression with a random slope and Markov correlation between the residuals was used to compare weekly changes in weight, CES-D scores, and STAI-State (STAI-S) scores. The weight and STAI-S analyses were adjusted for baseline values. Baseline CES-D scores were not associated with weekly changes and thus baseline scores were not included in the final model. According to intention-to-treat analysis, all subjects that contributed data were included in the analysis, even if they did not complete the full intervention. Various assumptions for the random-effects structure were explored and compared with the use of likelihood ratio test and the Akaike information criterion (AIC). To verify the assumption of normality, histograms of the post-model residuals were plotted (data not shown) and were consistent with normality. A priori, we had chosen to use the random-effects model because our focus was on within-woman change over time. For all three outcomes (weight, CES-D, STAI-S), sensitivity analyses comparing the primary model with other mixed-effects models showed that the primary model had the best fit based on likelihood ratio test, AIC, and bayesian information criterion (BIC). Results were unchanged when a generalized estimating equations (GEE) model was used (data not shown).
Trier Social Stress Test.
GEE was used to compare the HR and cortisol values (log-transformed) at each time point between baseline and visit 8. The model included a three-way interaction term between group (CBT+LS vs. LS), week (baseline vs. visit 8), and time of measurement (six total time points) as well as each two-way interaction term and each main effect.
RESULTS
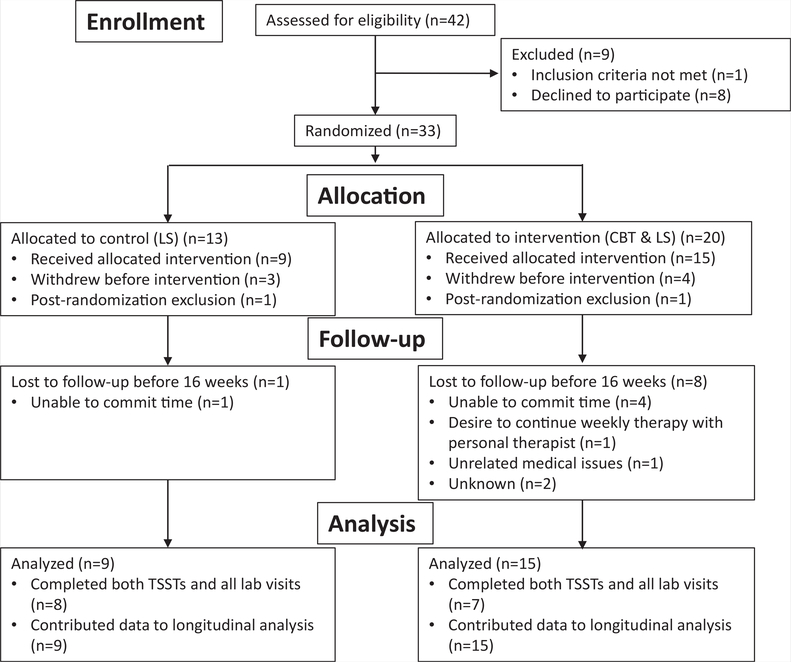
Figure 1 shows the flow diagram for the number of subjects screened. Twenty women were randomized to CBT+LS and 13 to LS alone; however, only seven women in the CBT+LS group and eight in the LS group completed the entire study.
FIGURE 1.
CONSORT flow diagram. CBT = cognitive-behavioral therapy; LS = lifestyle modification; TSST = Trier Social Stress Test.
Cooney. Mood and nutrition intervention in PCOS. Fertil Steril 2018.
There were no statistically significant differences in PCOS diagnostic criteria, scores on psychiatric scales, or anthropometric and laboratory measurements between groups (Table 1). The majority of subjects (≥75%) in each group met criteria for MDD based on the MINI. There were no differences in baseline characteristics between those who did and did not complete the study (data not presented).
Table 1.
Baseline demographics.
| Characteristic | LS (n = 12) | CBT + LS (n = 19) |
|---|---|---|
| Age (y) | 32 (27–34) | 29 (25–33) |
| Race white | 7(58.3) | 9 (47.4) |
| Ethnicity Hispanic | 1 (8.3) | 4(21.1) |
| Nulliparous | 9(75.0) | 13 (68.4) |
| PCOS phenotypea | ||
| A (HA, OA, and PCOM) | 10 (83.3) | 15 (83.3) |
| B (HA with OA only) | 1 (8.3) | 1 (5.6) |
| C (HA with PCOM only) | 1 (8.3) | 2 (11.1) |
| D (OA and PCOM) | 0 | 0 |
| MINI diagnoses | ||
| Major depressive disorder | 10 (83.3) | 15 (79.0) |
| Generalized anxiety disorder | 4(33.3) | 4(21.1) |
| Biometrics | ||
| Screening weight (kg) | 94 (77–111) | 100 (88–114) |
| Screening BMI (kg/m2) | 35 (31–40) | 38 (35–42) |
| Waist circumference (cm) | 108(102–125) | 111 (106–119) |
| Hip circumference (cm) | 123 (112–131) | 121 (112–130) |
| Waist-hip ratio | 0.93 (0.88–0.97) | 0.93 (0.90–0.97) |
| SBP (mm Hg) | 127 (119–131) | 122 (115–126) |
| DBP (mm Hg) | 79 (70–83) | 74 (69–87) |
| Screening scores | ||
| CES-D score (0–60) | 24(19–27) | 23 (22–29) |
| STAI-State (20–80) | 41 (39–50) | 42 (37–53) |
| STAI-Trait (20–80) | 52 (44–54) | 49 (47–48) |
| PSS (0–56) | 32 (24–36) | 33 (31–37) |
| ACE (0–40) | 1.5 (0–3) | 2(1–5) |
| PCOSQ overall (0–35) | 15 (13–19) | 15 (13–16) |
| PCOSQ domains | ||
| Emotion | 3.3 (2.6–4.1) | 3.3 (2.6–3.8) |
| Hair | 2.4 (1.5–3.4) | 3.2 (2.4–4.2) |
| Weight | 1.5 (1.2–2.3) | 1.4 (1.0–2.4) |
| Infertility | 4.1 (2.1 –4.6) | 3.0 (1.5–4.0) |
| Menstrual | 4.0 (3.6–4.6) | 3.5 (3.0–4.8) |
| Androgensb | ||
| Total T (ng/dL) | 61 (53–64) | 39 (34–80) |
| Free T (pg/mL) | 2.5 (2.3–3.1) | 2.4 (1.7–3.0) |
| SHBG (nmol/L) | 535 (260–773) | 320(210–836) |
| Metabolic laboratory valuesb | ||
| Total cholesterol (mg/dL) | 173 (170–200) | 186(135–197) |
| Triglycerides (mg/dL) | 79(52–103) | 71 (36–113) |
| HDL (mg/dL) | 52 (41 –59) | 45 (37–59) |
| LDL (mg/dL) | 106 (101–13) | 1 17 (96–127) |
| HOMA-IR | 4.3 (3.7–5.8) | 4.0 (3.5–4.2) |
| Inflammatory markersb | ||
| IL-6 (pg/mL) | 1.3 (0.9–2.0) | 1.3 (0.9–1.5) |
| ApoA1 (g/L) | 2.0 (1.5–2.2) | 2.1 (1.2–2.5) |
| ApoB (μg/mL) | 770 (691 –929) | 716 (647–928) |
| hs-CRP (mg/L) | 5.7 (3.2–14.3) | 4.9 (1.4–7.6) |
Note: Categorie data presented as n (%), continuous data as median (interquartile range). P>.05 for all comparisons. ACE = Adverse Childhood Experiences questionnaire; Apo = apolipoprotein; BMI = body mass index; CBT = cognitive-behavioral therapy; CES-D = Cen- terfor Epidemiologic Studies Depression Scale; DBP = diastolic blood pressure; HDL = high- density lipoprotein; HOMA-IR = homeostasis-model assessment of insulin resistance; hs-CRP = high-sensitivotyC-reactive protein; IL-6 = interleukin-6; LDL = low-densitylipopro- tein; LS = lifestyle modification; MINI = Mini International Neuropsychiatric Interview; PCOSQ = Polycystic Ovary Syndrome Health-Related Quality of Life Questionnaire; PSS = Perceived Stress Scale; SBP = systolic blood pressure; STAI = State-Trait Anxiety Inventory.
PCOS phenotypes based on which diagnostic features are met: clinical or biochemical hyperandrogenism (HA), oligoanovulation (OA), and polycystic ovarian morphologyon ultrasound (PCOM).
Enrollment laboratory tests were only run on those who completed at least eight visits (LS: n = 8; CBT+LS: n = 7).
Cooney. Mood and nutrition Intervention in PCOS. Fertil Steril 2018.
Effect on Weight, Androgens, and Metabolic and Inflammatory Markers
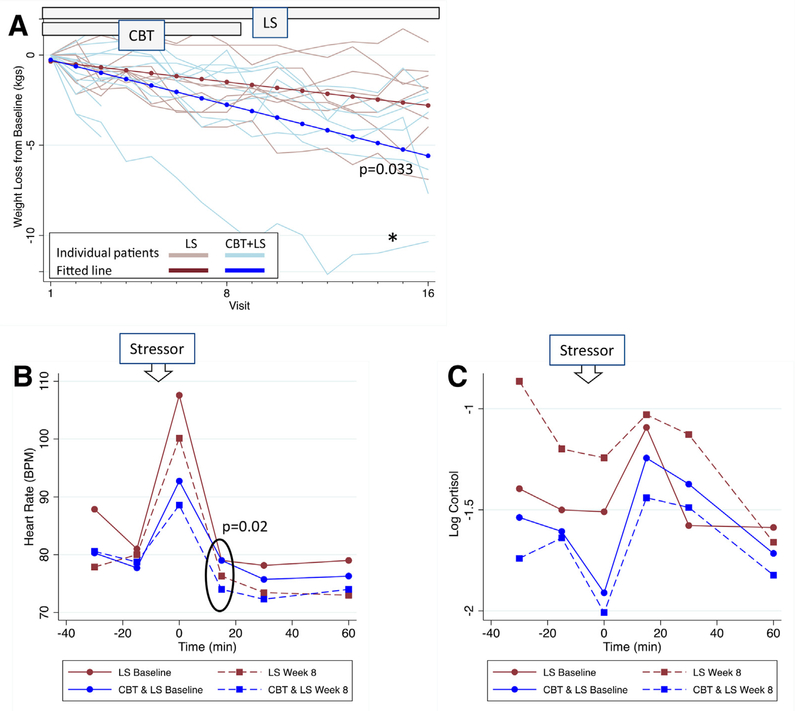
In the overall group, the median weight loss (from visit 1 to visit 16) was 2.4 kg (IQR 6.4 to 1.8; P<.001). The CBT+LS group showed more than twice as much weight loss per week over the study period compared with the LS-only group (0.35 kg/wk [95% CI 0.47 to 0.23; n ¼ 15] vs. 0.16 kg/ wk [95% CI 0.28 to 0.04; n ¼ 9]; P¼.033; Fig. 2A). To confirm that the significant results were not driven by a subject in the CBT+LS group who lost a greater than average amount of weight (10 kg; Fig. 2A), the longitudinal analysis was repeated without this potential outlier and the results were similar. Overall, the CBT+LS group lost almost twice as much weight as the LS group (3.2 kg [IQR 7.7 to 2.1 mean percentage loss 4.3%] vs. 1.8 kg [IQR 3.8 to 1.0 mean percentage loss 1.5%]; P¼.08; Table 2), although this difference may not be clinically significant. An effect size (eta-squared statistic) for CBT+LS of 0.18 was observed, which is considered to be large (32).
FIGURE 2.
(A) Weekly weight loss compared with baseline for each subject overlaid with the fitted line after random-effects linear regression: CBT+LS: −0.35 kg/wk (95% confidence interval [CI] −0.47 to −0.23), blue; LS: −0.16 kg/wk (95% CI −0.28 to −0.04), red. P value for three-way interaction among group, time, and week. Results unchanged when repeated after excluding the outlier (*). (B, C) Results of Trier Social Stress Test (TSST): values for (B) heart rate and (C) log cortisol in the LS versus CBT+LS groups at baseline and week 8. Time 0 is immediately following the stressor. P value is for three-way interaction among group, time, and week. Abbreviations as in Figure 1.
Cooney. Mood and nutrition intervention in PCOS. Fertil Steril 2018.
Table 2.
Comparison of biometrics, scores, and laboratory values.
| Difference (week 8, baseline) | Difference (week 16, baseline) | |||||
|---|---|---|---|---|---|---|
| Parameter | LS (n = 8) | CBT + LS (n = 7) | P valuea | LS (n = 8) | CBT + LS (n = 7) | P valuea |
| Anthropometrics | ||||||
| Weight (kg) | −2.3 (−3.2 to −1.6) | −1.4 (−3.0 to −0.6) | .60 | −1.8 (−3.8 to −1.0) | −3.2 (−7.7 to −2.1) | .08 |
| BMI (kg/m2) | −0.8 (−1.1 to −0.6) | −0.5 (−1.2 to −0.2) | .56 | −0.6 (−1.5 to −0.4) | −0.9 (−3.1 to −0.8) | .08 |
| Waist circumference (cm) | 0.3 (−0.8 to 4.0) | −3 (−13 to 0) | .09 | 0(−2to 1.5) | −1 (−3 to 1) | .45 |
| Hip circumference (cm) | −0.8 (−2to 2) | 1 (−6 to 2) | .72 | −4(−6to 2) | −1 (−3 to 1) | .42 |
| Waist-hip ratio | 0.03 (−0.01 to 0.4) | −0.02 (−0.05 to 0) | .04a | 0.05 (0 to 0.05) | 0(−0.04 to 0.01) | .04a |
| SBP (mm Hg) | −11 (−18 to −7) | 1 (−2 to 7) | < .01a | −5 (−11 to 2) | 1 (−14 to 7) | .49 |
| DBP (mm Hg) | −11 (−16 to −9) | −5(−15 to −2) | .18 | −5 (−10 to −1) | 1 (−6 to 2) | .27 |
| Scores | ||||||
| CES-Db | −5 (−13 to −3) | −5 (−11 to −8) | .95 | −6(−12 to 3) | −1 (−9to 8) | .45 |
| STAI-Stateb | −5(−14 to −1) | 0 (−3 to 3) | .35 | −6 (−11 to 1) | 1 (−14 to 11) | .56 |
| PSS | −1 (−8to 6) | −4 (−8 to 1) | .49 | −4.5 (−6.5 to 2) | −3(−6to −1) | .91 |
| PCOSQ | 1.2 (−0.9 to 2.7) | 3.7 (2.9 to 5.0) | .02a | 2.3 (0.3 to 3.7) | 3.0 (1.9 to 3.5) | .56 |
| PCOSQ domains | ||||||
| Emotion | 0.1 (−0.1 to 0.9) | 0.8 (1.0 to 1.1) | .09 | 0.3 (−0.3 to 0.9) | 0.8 (0.3 to 1) | .32 |
| Hair | 0.1 (−0.5 to 0.5) | 0.6 (0.4 to 1.0) | .10 | 0.1 (−0.3 to 0.3) | 0.6 (0.2 to 1) | .049a |
| Weight | 0.1 (−0.1 to 1.4) | 0.6 (0.2 to 0.8) | .49 | 0.7 (0 to 1.6) | 0.6 (0.4 to 1) | .95 |
| Infertility | 0.6 (0.3 to 0.8) | 0.5 (0.5 to 0.8) | .86 | 0.5 (−0.1 to 1.1) | 0.3 (−0.5 to 1) | .77 |
| Menstrual | −0.8 (−1.4 to 0.1) | 0.5 (−0.3 to 1.3) | .02a | 0.4(0 to 0.8) | 0 (−0.5 to 0.5) | .44 |
| Androgens | ||||||
| Total T (ng/dL) | −0.1 (−0.3 to 0) | −0.1 (−0.4 to −0.1) | .73 | 10 (− 18to20) | 0 (−40 to 1.6) | .13 |
| Free T (pg/mL) | −0.2 (−0.9 to −0.1) | −0.5 (−1.1 to 0) | .73 | 0.3 (−0.1 to 0.4) | −0.1 (−1.2 to 0.5) | .25 |
| SHBG (nmol/L) | 14 (−256 to 54) | 41 (−59 to 85) | .56 | −21 (−184 to 63) | 43 (23 to 283) | .11 |
| Metabolic laboratory valuesc | ||||||
| Total cholesterol (mg/dL) | −6(−13 to 2) | −6(−18 to 8) | .32 | −19(−19 to −11) | 3 (−5 to 7) | .03 |
| Triglycerides (mg/dL) | 34 (−8 to 85) | 1 (−15 to 17) | .06 | −9 (−32 to 42) | 2 (−20 to 15) | .70 |
| HDL (mg/dL) | 1 (−3 to 3) | 0(−2 to 1) | .75 | 0 (0 to 5) | 2 (−4 to 6) | .61 |
| LDL (mg/dL) | − 10 (−15 to −5) | 6 (−17 to 8) | .11 | − 15 (−27 to −12) | −2 (−15 to 9) | .08 |
| HOMA-IR | −0.1 (−1.5 to 5.3) | 0.4 (−0.9 to 1.2) | .85 | −0.2 (−1.6 to 0.4) | −0.1 (−1.2 to 1.9) | .56 |
| Inflammatory markers | ||||||
| IL-6 (pg/mL) | 0 (−0.5 to 0.3) | −0.3 (−0.8 to 0.1) | .42 | −0.1 (−0.3 to 0.1) | 0(−0.1 to 0.5) | .42 |
| ApoA1 (g/L) | 0.4 (−0.3 to 0.6) | −0.2 (−0.3 to −0.1) | .42 | 0.4 (−0.01 to 0.8) | 0 (−0.3 to 0.4) | .20 |
| ApoB (μg/mL) | −253 (−306 to −216) | −215 (−247 to −164) | .13 | −42 (−88 to 24) | − 16 (−157 to 12) | 1.0 |
| hs-CRP (mg/L) | 1.5 (−1.8 to 3.8) | 0.2 (−2.7 to 0.4) | .30 | − 1.3 (−2.7 to −0.5) | −0.2 (−2.9 to 3.5) | .13 |
Note: Continuous data presented as median (interquartile range [IQR]). A change in the POCS-QOL domain score by ≥0.5 is considered clinically significant. Abbreviations as in Table 1.
P< .05 (Wilcoxon rank sum P value for comparison of differences between LS and CBT+LS groups).
One subject in the CBT+LS group did not haveCES-D or STAI-State testing atweek 16, soweek 15 valueswere used to calculate difference from baseline. Two subjects in the CBT+LS group did not have CES-D or STAI-State testing at week 8, so week 7 values were used to calculate difference from baseline.
Only seven subjects in the LS group had metabolic laboratory values measured at both week 0 and week 16.
Cooney. Mood and nutrition Intervention in PCOS. Fertil Steril 2018.
Changes in androgens, metabolic and inflammatory markers are shown in Table 2. In the overall group, total T decreased by −0.14 ng/dL (IQR −0.36 to −0.06; P=.004), free T decreased by −0.26 pg/mL (IQR −0.86 to −0.12; P=.003), and apolipoprotein B decreased by −244 μg/mL (IQR −257 to −187; P<.001) from baseline to visit 8 to but these decreases were not maintained by visit 16. Total cholesterol decreased more in the LS group (−19 mg/dL [IQR −19 to −11] vs. 3 mg/dL [IQR −5 to 7]; P=.03) from baseline to visit 16. There were no other significant changes in laboratory values during the study period.
Effect on Depression, Anxiety, and Quality of Life
In the overall group, CES-D scores decreased by 0.31 points per week (95% CI −0.55 to −0.07; P=.01) with no significant differences between CBT+LS (−0.24 points/wk, 95% CI −0.58 to 0.11; n = 15) and LS groups (−0.34 points/wk, 95% CI −0.68 to 0.01; n = 9; P=.68). In the overall group, STAI-S scores decreased by 0.27 points per week (95% CI −0.50 to −0.03; P=.03) with no differences between CST+LS (−0.16 points/wk, 95% CI −0.50 to 0.18; n = 15) and LS groups (−0.32 points/wk, 95% CI −0.65 to 0.00; n = 9; P=.49). Although statistically significant, these improvements are overall small and may not be clinically significant. Clinically significant improvement in depression can be assessed by improvement of scores from a positive screening for depressive symptoms (CES-D ≥14) to a normal screen (CESD <14). All subjects were depressed at baseline as part of the inclusion criteria, and by the end of 16 weeks approximately one-half of the subjects in the overall group (7/15) had a normal depression screening.
At visit 8 to the CBT+LS group had greater improvement in PCOSQ scores than the LS group (3.7 points [IQR 2.9–5.0; n = 7] vs. 1.2 points [IQR −0.9 to −2.7; n = 8]; P=.021) and a clinically significant improvement (≥0.5 points) in all PCOSQ domains except menstrual domain (Table 2). By study end, PCOSQ scores increased by 3 points (IQR 1.3–3.5; P=.005) and PSS scores decreased by 3 points (IQR −6 to −1; P=.06) in the overall cohort, but there were no differences between groups.
Trier Social Stress Test
The results of the GEE model comparing HR at baseline and week 8 during each time point of the stress test are plotted in Figure 2B. Both groups show the typical pattern of response with a peak in HR immediately following the stressor (31). For both groups, HR was lower at each time point at visit 8 than baseline (P=.029 for interaction between week and time). When the two groups were compared, the HR difference between visit 8 and baseline was significantly lower in the CBT+LS group at 15 minutes after the stressor than in the LS group (−5 beats/min vs. −3 beats/min; P=.020), indicating a quicker return to baseline HR after the stressor in the CBT+LS group.
Figure 2C shows results of the GEE model for log cortisol. Both groups had a similar cortisol response to the stressor at baseline (P>.3 for the interaction term between group and time at each time point) with peak log cortisol at 15 minutes after stressor completion, the typical pattern of response (31). In the CBT+LS group there were no differences between visit 8 and baseline cortisol at each time point (−0.2–95% CI −0.9 to 0.5; P=.58). In contrast, in the LS group, the average log cortisol levels were higher at visit 8 compared with baseline (+0.5–95% CI −0.01 to 1.1; P=.05), suggesting that this group was not sensitized to the stressor. When the difference in log cortisol (visit 8 − baseline) was averaged across time, there was a significant difference between groups (P=.026).
Adherence to Exercise and Diet Goals
The seven women in the CBT+LS group and eight women in the LS group attended an average of 14 total sessions each. Although women in both groups were equally likely to report exercising in the past week (84.5% vs. 74.6%; P=.072), women in the CBT+LS group were more likely to meet their weekly exercise goal (59% vs. 38% of sessions; P=.002) and to exercise a higher median number of minutes per week (102 min vs. 90 min; P=.003). Women in the CBT+LS group were more likely to keep a weekly food diary (83% vs. 66% of sessions; P=.007); however, of the sessions when a food diary was kept, the groups reported a similar number of days per week where they met their calorie goals (CBT+LS 3 d/wk vs. LS 4 d/wk; P=.08).
DISCUSSION
This pilot randomized clinical study is the first to evaluate the use of CBT as an adjunct to standard of care lifestyle counseling in overweight/obese women with PCOS and depression. Given the high prevalence of obesity, depression, and their coexistence in women with PCOS, standard treatments can pose unique challenges. We demonstrated that women who received CBT+LS lost twice as much weight on a weekly basis than those who received LS alone, had significant improvement in QoL scores at 8 weeks, and had improvement in stress responsiveness.
Although CBT is primarily recommended for the treatment of MDD and anxiety, some studies have demonstrated improvement in weight with the use of CBT+LS versus LS alone in obese subjects (without concurrent diagnosis of depression) (20–22). In fact, obese individuals who suffer from depressive disorders are typically screened out of weight loss trials. We found two studies, both noncontrolled prospective cohorts, that evaluated CBT+LS in subjects with both obesity and depression (14, 23). Despite small sample sizes, their results supported our findings of increased weight loss with CBT+LS. Faulconbridge et al. studied 12 women with both obesity and MDD who attended longer CBT sessions for depression as well as behavioral weight management skills (16 weekly 90-min group sessions). Mean weight loss was 11.4 ± 5.9% of initial weight (P<.001), and mean reduction of Hamilton Depression Rating Scale score was 11.1 ± 4.8 points (P<.001). That study also showed a reduction of Framingham 10-year risk scores from 4.2% to 1.7% (P<.01) (23). Another study assessed CBT in 12 adolescents with PCOS, obesity, and depression who underwent eight weekly sessions of individual CBT with lifestyle counseling and three family sessions (45–60 min each). That study showed a reduction in mean weight (10.6% loss of initial weight; P<.05) as well as improvement in depressive symptoms as measured by the Children’s Depression Inventory (from 17 ± 3 to 9.6 ± 2; P<.01) (14). Collectively, these studies show the feasibility of achieving successful weight loss with 8–16 weeks of CBT intervention in subjects with both depression and obesity. Our duration of CBT (30 min/wk for 8 weeks) was shorter than other studies that evaluated effects of CBT for obesity management and included up to an hour of CBT per week for 16 weeks and sometimes included subsequent maintenance periods of less frequent therapy (19, 24, 34). Despite a short duration of CBT intervention, our CBT+LS group showed a quicker and more significant weight loss than LS alone.
Given that this was a pilot study, one of our goals was to assess feasibility and acceptability of the CBT+LS intervention in our population. Despite including women with high depressive scores (75% had MDD, based on the MINI) our dropout rate was similar to that seen in other LS studies in overweight or obese women (35, 36). The most common reason for drop out after randomization was time commitment to weekly visits for 16 weeks. We found that a significantly higher proportion of women in the CBT-LS arm met their weekly exercise goal and kept a food diary. Identification of strategies to improve adherence to LS modifications will be critical to long term weight maintenance.
Body image distress is a recognized feature of PCOS (37) and persists even after controlling for BMI in some (38–40) but not all (41) studies. In addition, in women with PCOS, poor body image is associated with an increased risk of depression (38). As seen in previous studies, we noted the lowest baseline scores on the PCOSQ in the weight and hair domains, both of which are involved in the concept of “body image.” Poor body image makes weight loss more difficult and increases the risk of relapse (42). A meta-analysis of weight loss interventions in the general population showed greater improvement in body image in the intervention groups (43). We did not measure body image in our study, but we did show improvement in QoL. Further studies are needed to investigate the effect of the interaction between CBT and body image on weight loss in women with PCOS.
At 8 weeks, after the CBT intervention phase, the CBT+LS groups showed an improvement in PCOSQ scores in all domains, with a significant improvement in the overall score, compared with the LS group. It is well recognized that women with PCOS have low QoL, and previous studies have shown an improvement in PCOSQ scores with LS alone (44, 45). Our pilot data indicates a positive impact on PCOSQ scores when CBT is added to an intensive lifestyle intervention in women with multiple comorbidities such as obesity and depression. By 16 weeks, the differential improvement in scores in the CBT+LS group was no longer significant, because by the end of the study both groups had a >2-point improvement in PCOSQ scores. Our results suggest that the addition of CBT results in a quicker improvement in QoL during CBT administration, but additional studies are needed to evaluate the duration of CBT that is most effective in creating lasting improvement in QoL. Importantly, there were also small improvements in depression and anxiety scores during our study and almost one-half of the women were no longer depressed at the completion. Previous studies have shown an improvement in these scores with weight loss interventions (45), suggesting the feasibility of these interventions in obese women with PCOS with depression.
Women in both groups in our study had high baseline perceived stress scores (31.8 ± 8.2) compared with other populations of young women (23.6 ± 7.6 to 25.7 ± 6.2) (46). In the general population, stress and the associated alterations in the HPA axis, notably increased corticotropin-releasing hormone and increased cortisol, have been implicated as a possible mechanism contributing to depression (47). A few older studies have shown a greater increase in cortisol after a stressor in women with PCOS compared with control women (48, 49). In the present study, both groups showed a decrease in HR at 8 weeks compared with baseline, a result that can be interpreted as either an improvement in the sympathetic response to stress or habituation due to repeated measures. Studies have shown that both the sympathetic (HR) and HPA (cortisol) response to stress habituate to the TSST when the tests are repeated, even when separated by a time span of 4 or 10 weeks (50, 51). To control for this, our model evaluated not only the difference in response between week 8 and baseline, which could be due to habituation, but also whether this difference varied by group, which is more likely to be related to our intervention. We showed that after eight sessions of CBT+LS, women had a decreased HR response and a constant, rather than an increased, cortisol response to a laboratory stressor compared with the LS group. The increased cortisol response in the control women was unexpected, and more studies need to be done to evaluate whether this is a response unique to women with PCOS. Overall, our results suggest a positive effect of CBT on stress responses and a role for CBT in the treatment of depression and anxiety in women with PCOS.
Strengths of our study are that it is the first randomized controlled trial focusing on treatment interventions for women with PCOS who are overweight/obese and have concurrent depression. Psychiatric symptoms were thoroughly evaluated via a structured interview and CBT was standardized based on a commonly used treatment manual and thus could be generalizable to clinical practice. To control for CBT in the intervention group, nondirective supportive therapy was provided to all subjects in the LS-only group by a master- or doctorate-level counselor. Nondirective therapy has been shown to be moderately effective in the treatment of depression in adults (18). Although we view this as a strength of our study, this intervention could potentially make it harder to differentiate between the general benefit of having someone listen to one’s concerns and the more specific benefit of CBT. To assess the impact of CBT, we completed validated surveys for anxiety, depression, and QoL and in addition assessed responses to stressors, such as the TSST. To our knowledge, this is the first study to assess the impact of lifestyle interventions on TSST in women with PCOS.
Weaknesses of this study include the time-intensive nature of the three-component intervention (nutrition, exercise, and CBT). In addition, unlike lifestyle modifications, including nutrition and exercise that subjects can complete during their own time, in-person CBT involves regular meetings with a clinical psychologist, which can be expensive and time consuming. There was higher early dropout in the CBT+LS group, although the reasons for dropout (Fig. 1) were not specific to the CBT aspect of treatment. Dropout in future studies could be decreased with the use of computerized CBT, which can be completed online and has been shown to be effective, reducing time commitment by the patient (52, 53). Despite the CBT+LS group having a significant improvement in weight loss, this did not translate into differential improvement in other metabolic parameters, androgens, or inflammatory markers. Other studies have demonstrated that antiinflammatory effects of weight loss may not be observed for up to 6 months (13), so our 16 weeks of intervention may not have been sufficient to note potential improvements. In addition, weight loss can be difficult to maintain even when CBT is used for treatment of obesity (54). Because this was a pilot study, we did not follow subjects after 16 weeks to determine if the improved weight loss in the CBT+LS group was maintained.
CONCLUSION
Weight loss is difficult in all populations, but it can be harder in those with comorbid medical conditions, particularly psychiatric disorders. Our study showed that the addition of CBT to treat depressed mood improves weight loss, short-term QoL scores, and response to stress in women with PCOS undergoing LS counseling. Future studies should include a larger sample size, focus on tracking CBT adherence, use a higher dose of CBT and use less frequent in-person visits interspersed with telephone counseling and internet-based weighing. In addition, longer follow-up could be used to evaluate the sustainability of weight loss and potential improvement in long-term metabolic and inflammatory markers.
Supplementary Material
Acknowledgments:
The authors thank the University of Pennsylvania Diabetes Research Center (P30-DK19525) and the Translational Core Laboratory at CHOP for running all laboratories and the National Institutes of Health Office of Research on Women’s Health for their funding support.
Supported by a National Institutes of Health (NIH) Reproductive Epidemiology Training Grant (T32-HD007440; L.G.C.), University of Pennsylvania Penn Presbyterian Harrison award (A.D.), and NIH P50 MH099910 and K12 HD085848 (C.N.E.).
Footnotes
L.G.C. has nothing to disclose. L.W.M. has nothing to disclose. L.H. has nothing to disclose. S.K. has nothing to disclose. M.D.S. has nothing to disclose. K.C.A. has nothing to disclose. C.N.E. has nothing to disclose. A.D. has nothing to disclose.
Clinical Trial Registration Number: NCT01899001. (Fertil Steril® 2018;110:161–71.
Número de registro del ensayo clínico: NCT01899001.
REFERENCES
- 1.Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod 2016;31:2841–55. [DOI] [PubMed] [Google Scholar]
- 2.Behboudi-Gandevani S, Ramezani Tehrani F, Rostami Dovom M, Farahmand M, Bahri Khomami M, Noroozzadeh M, et al. Insulin resistance in obesity and polycystic ovary syndrome: systematic review and meta-analysis of observational studies. Gynecol Endocrinol 2016;32:343–53. [DOI] [PubMed] [Google Scholar]
- 3.Zhao L, Zhu Z, Lou H, Zhu G, Huang W, Zhang S, et al. Polycystic ovary syndrome (PCOS) and the risk of coronary heart disease (CHD): a meta-analysis. Oncotarget 2016;7:33715–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgess Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 2. Endocr Pract 2015;21:1415–26. [DOI] [PubMed] [Google Scholar]
- 5.Moran LJ, Hutchison SK, Norman RJ, Teede HJ. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev 2011: CD007506. [DOI] [PubMed] [Google Scholar]
- 6.Legro RS, Dodson WC, Kris-Etherton PM, Kunselman AR, Stetter CM, Williams NI, et al. Randomized controlled trial of preconception interventions in infertile women with polycystic ovary syndrome. J Clin Endocrinol Metab 2015;100:4048–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooney LG, Lee I, Sammel MD, Dokras A. High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod 2017;32:1075–91. [DOI] [PubMed] [Google Scholar]
- 8.Veltman-Verhulst SM, Boivin J, Eijkemans MJ, Fauser BJ. Emotional distress is a common risk in women with polycystic ovary syndrome: a systematic review and meta-analysis of 28 studies. Hum Reprod Update 2012;18:638–51. [DOI] [PubMed] [Google Scholar]
- 9.van der Kooy K, van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry 2007;22:613–26. [DOI] [PubMed] [Google Scholar]
- 10.Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry 2010;67:220–9. [DOI] [PubMed] [Google Scholar]
- 11.Kan C, Silva N, Golden SH, Rajala U, Timonen M, Stahl D, et al. A systematic review and meta-analysis of the association between depression and insulin resistance. Diabetes Care 2013;36:480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mezuk B, Eaton WW, Golden SH, Ding Y. The influence of educational attainment on depression and risk of type 2 diabetes. Am J Public Health 2008;98:1480–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopresti AL, Drummond PD. Obesity and psychiatric disorders: commonalities in dysregulated biological pathways and their implications for treatment. Prog Neuropsychopharmacol Biol Psychiatry 2013;45:92–9. [DOI] [PubMed] [Google Scholar]
- 14.Rofey DL, Szigethy EM, Noll RB, Dahl RE, Lobst E, Arslanian SA. Cognitive-behavioral therapy for physical and emotional disturbances in adolescents with polycystic ovary syndrome: a pilot study. J Pediatr Psychol 2009;34:156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Work Group on Major Depressive Disorder. Practice guideline for the treatment of patients with major depressive disorder, 2010. Available at: http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf.
- 16.American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry 2000;157:1–45. [PubMed] [Google Scholar]
- 17.Qaseem A, Barry MJ, Kansagara D, Clinical Guidelines Committee of the American College of Physicians. Nonpharmacologic versus pharmacologic treatment of adult patients with major depressive disorder: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2016;164:350–9. [DOI] [PubMed] [Google Scholar]
- 18.Cuijpers P, Cristea IA, Karyotaki E, Reijnders M, Huibers MJ. How effective are cognitive behavior therapies for major depression and anxiety disorders? A meta-analytic update of the evidence. World Psychiatry 2016;15:245–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abiles V, Rodriguez-Ruiz S, Abiles J, Obispo A, Gandara N, Luna V, et al. Effectiveness of cognitive-behavioral therapy in morbidity obese candidates for bariatric surgery with and without binge eating disorder. Nutr Hosp 2013;28:1523–9. [DOI] [PubMed] [Google Scholar]
- 20.Gade H, Hjelmesaeth J, Rosenvinge JH, Friborg O. Effectiveness of a cognitive behavioral therapy for dysfunctional eating among patients admitted for bariatric surgery: a randomized controlled trial. J Obes 2014;2014:127936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mefferd K, Nichols JF, Pakiz B, Rock CL. A cognitive behavioral therapy intervention to promote weight loss improves body composition and blood lipid profiles among overweight breast cancer survivors. Breast Cancer Res Treat 2007;104:145–52. [DOI] [PubMed] [Google Scholar]
- 22.Pimenta F, Leal I, Maroco J, Ramos C. Brief cognitive-behavioral therapy for weight loss in midlife women: a controlled study with follow-up. Int J Womens Health 2012;4:559–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faulconbridge LF, Wadden TA, Berkowitz RI, Pulcini ME, Treadwell T. Treatment of comorbid obesity and major depressive disorder: a prospective pilot study for their combined treatment. J Obes 2011;2011:870385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jelalian E, Jandasek B, Wolff JC, Seaboyer LM, Jones RN, Spirito A. Cognitive-behavioral therapy plus healthy lifestyle enhancement for depressed, overweight/obese adolescents: results of a pilot trial. J Clin Child Adolesc Psychol 2016:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evidence-based methodology workshop on polycystic ovary syndrome, 2012. Executive summary. Available at: https://prevention.nih.gov/docs/programs/pcos/FinalReport.pdf Accessed September 20, 2017.
- 26.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab 2006;91:4237–45. [DOI] [PubMed] [Google Scholar]
- 27.Cohen LM, al’Absi M, Collins FL Jr. Salivary cortisol concentrations are associated with acute nicotine withdrawal. Addict Behav 2004;29:1673–8. [DOI] [PubMed] [Google Scholar]
- 28.Kirschbaum C, Wust S, Strasburger CJ. “Normal” cigarette smoking increases free cortisol in habitual smokers. Life Sci 1992;50:435–42. [DOI] [PubMed] [Google Scholar]
- 29.Cully JA, Teten AL. A therapist’s guide to brief cognitive behavioral therapy. Houston: Department of Veterans Affairs South Central MIRECC; 2008. [Google Scholar]
- 30.Cronin L, Guyatt G, Griffith L, Wong E, Azziz R, Futterweit W, et al. Development of a health-related quality-of-life questionnaire (PCOSQ) for women with polycystic ovary syndrome (PCOS). J Clin Endocrinol Metab 1998;83:1976–87. [DOI] [PubMed] [Google Scholar]
- 31.Allen AP, Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Biological and psychological markers of stress in humans: focus on the Trier Social Stress Test. Neurosci Biobehav Rev 2014;38:94–124. [DOI] [PubMed] [Google Scholar]
- 32.Cohen J Statistical power for the behavioral sciences. 2nd ed. Hillsdale NY: Erbaum; 1988. [Google Scholar]
- 33.Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen 2012;141:2–18. [DOI] [PubMed] [Google Scholar]
- 34.Rapoport L, Clark M, Wardle J. Evaluation of a modified cognitive-behavioural programme for weight management. Int J Obes Relat Metab Disord 2000;24:1726–37. [DOI] [PubMed] [Google Scholar]
- 35.Mutsaerts MA, Kuchenbecker WK, Mol BW, Land JA, Hoek A. Dropout is a problem in lifestyle intervention programs for overweight and obese infertile women: a systematic review. Hum Reprod 2013;28:979–86. [DOI] [PubMed] [Google Scholar]
- 36.Naderpoor N, Shorakae S, de Courten B, Misso ML, Moran LJ, Teede HJ. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. Hum Reprod Update 2015;21:560–74. [DOI] [PubMed] [Google Scholar]
- 37.Bazarganipour F, Ziaei S, Montazeri A, Foroozanfard F, Kazemnejad A, Faghihzadeh S. Body image satisfaction and self-esteem status among the patients with polycystic ovary syndrome. Iran J Reprod Med 2013;11:829–36. [PMC free article] [PubMed] [Google Scholar]
- 38.Himelein MJ, Thatcher SS. Depression and body image among women with polycystic ovary syndrome. J Health Psychol 2006;11:613–25. [DOI] [PubMed] [Google Scholar]
- 39.Pastore LM, Patrie JT, Morris WL, Dalal P, Bray MJ. Depression symptoms and body dissatisfaction association among polycystic ovary syndrome women. J Psychosom Res 2011;71:270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiner CL, Primeau M, Ehrmann DA. Androgens and mood dysfunction in women: comparison of women with polycystic ovarian syndrome to healthy controls. Psychosom Med 2004;66:356–62. [DOI] [PubMed] [Google Scholar]
- 41.Karacan E, Caglar GS, Gursoy AY, Yilmaz MB. Body satisfaction and eating attitudes among girls and young women with and without polycystic ovary syndrome. J Pediatr Adolesc Gynecol 2014;27:72–7. [DOI] [PubMed] [Google Scholar]
- 42.Ohsiek S, Williams M. Psychological factors influencing weight loss maintenance: an integrative literature review. J Am Acad Nurse Pract 2011;23:592–601. [DOI] [PubMed] [Google Scholar]
- 43.Chao HL. Body image change in obese and overweight persons enrolled in weight loss intervention programs: a systematic review and meta-analysis. PLoS One 2015;10:e0124036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomson RL, Buckley JD, Brinkworth GD. Perceived exercise barriers are reduced and benefits are improved with lifestyle modification in overweight and obese women with polycystic ovary syndrome: a randomised controlled trial. BMC Womens Health 2016;16:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dokras A, Sarwer DB, Allison KC, Milman L, Kris-Etherton PM, Kunselman AR, et al. Weight loss and lowering androgens predict improvements in health-related quality of life in women with PCOS. J Clin Endocrinol Metab 2016;101:2966–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385–96. [PubMed] [Google Scholar]
- 47.Belmaker RH, Agam G. Major depressive disorder. N Engl J Med 2008;358:55–68. [DOI] [PubMed] [Google Scholar]
- 48.Benson S, Arck PC, Tan S, Hahn S, Mann K, Rifaie N, et al. Disturbed stress responses in women with polycystic ovary syndrome. Psychoneuroendocrinology 2009;34:727–35. [DOI] [PubMed] [Google Scholar]
- 49.Gallinelli A, Matteo ML, Volpe A, Facchinetti F. Autonomic and neuroendocrine responses to stress in patients with functional hypothalamic secondary amenorrhea. Fertil Steril 2000;73:812–6. [DOI] [PubMed] [Google Scholar]
- 50.Petrowski K, Wintermann GB, Siepmann M. Cortisol response to repeated psychosocial stress. Appl Psychophysiol Biofeedback 2012;37:103–7. [DOI] [PubMed] [Google Scholar]
- 51.Schommer NC, Hellhammer DH, Kirschbaum C. Dissociation between reactivity of the hypothalamus-pituitary-adrenal axis and the sympatheticadrenal-medullary system to repeated psychosocial stress. Psychosom Med 2003;65:450–60. [DOI] [PubMed] [Google Scholar]
- 52.National Institute for Health and Care Excellence. Computerised cognitive behaviour therapy for depression and anxiety. Technology appraisal guidance [TA97], 2013. Available at: https://www.nice.org.uk/guidance/ta97. Accessed October 23, 2017.
- 53.Olthuis JV, Watt MC, Bailey K, Hayden JA, Stewart SH. Therapist-supported Internet cognitive behavioural therapy for anxiety disorders in adults. Cochrane Database Syst Rev 2015:CD011565. [DOI] [PubMed] [Google Scholar]
- 54.Cooper Z, Doll HA, Hawker DM, Byrne S, Bonner G, Eeley E, et al. Testing a new cognitive behavioural treatment for obesity: a randomized controlled trial with three-year follow-up. Behav Res Ther 2010;48:706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.