Abstract
Tumor cell interactions with the bone microenvironment are vital for the establishment and progression of bone metastases. Recently in Cancer Cell, Wang et al. (2018) showed that cells of the osteoblast lineage are critical for the delivery of calcium to tumor cells through gap junctions, pointing toward potential therapies for bone metastases.
Bone is a common site for metastases from breast, prostate, lung, and other cancers. The associated morbidity of bone pain, fractures, hypercalcemia, nerve compression, and muscle weakness is devastating, can last for years, and renders the disease incurable. A current therapy directed against the tumor and the bone-resorbing osteoclast improves this morbidity but does not cure the disease. The lack of effective therapies for bone metastases is partially due to incomplete understanding of the underlying mechanisms that drive the metastatic process. Tumor cells arrive in the bone microenvironment and interact with resident cells, including osteoclasts, osteoblasts, osteocytes, hematopoietic cells, and other stromal cells. Cancer cells stimulate osteoclastic bone resorption, which causes release of growth factors, such as transforming growth factor beta (TGFβ) and calcium from the mineralized bone matrix. These factors drive tumor growth and a feedforward vicious cycle of continuous bone destruction (Weilbaecher et al., 2011).
The role of osteogenic cells (defined as cells of the osteoblast lineage and mesenchymal stem cells) in initiation and progression of bone metastases has become increasingly evident. Osteoblasts secrete factors such as TGFβ, which are deposited into the mineralized bone matrix and can induce the metastatic potential of prostate cancer cells (Karlsson et al., 2018). Osteoblasts secrete factors (Kinder et al., 2008) and also differentiate into osteocytes, both of which can initiate osteoclastogenesis. Wang et al. previously showed that tumor cells utilize the osteogenic niche (Wang et al., 2015), which consists of osteoprogenitors, preosteoblasts, mature osteoblasts, osteocytes, and osteoclasts, in early colonization of the bone. Cancer cells make contact with the mesenchymal stem cells through N-cadherin/E-cadherin junctions, which activates mTOR signaling in cancer cells to support growth and metastasis (Wang et al., 2015). mTOR signaling blockade prevented metastasis to bone. Thus, the osteogenic niche is an intermediate space that allows disseminated tumor cells to develop into bone metastases.
In a recent issue of Cancer Cell, the same group established a detailed molecular mechanism to explain how cancer cells hijack the osteogenic niche to survive. Specifically, tumor cells utilize these osteogenic cells (cells of the osteoblast lineage and mesenchymal stem cells) to absorb calcium and increase intracellular calcium concentration in a process achieved via Connexin 43 (Cx43) gap junctions (Wang et al., 2018). Intracellular calcium is an important second messenger that regulates gene transcription, cell proliferation, migration, and apoptosis. Intracellular calcium signaling is altered in cancer cells, and this change plays a role in all of the hallmarks of cancer (Hanahan and Weinberg, 2011). Using an intra-iliac model of bone metastases, Wang et al. showed upregulation of calcium-dependent transcription factor activities, including NFAT and MEF2 in bone metastases compared to metastases to other organs from the same tumor cells. In addition, they discovered that activation of calcium signaling led to epigenetic changes. MeCP2, which binds methylated DNA and acts as a transcriptional repressor, was found to be expressed at a lower level in bone metastases. In ex vivo cultures they found that breast cancer cells have a limited ability to absorb calcium directly but exhibit a dramatic increase in calcium absorption when co-cultured with osteoblasts. Cx43 expression was also higher in breast cancer cells colonizing the bone compared to other metastatic sites. Overall, Wang et al. found that in addition to mTOR signaling, calcium signaling can promote osteolytic bone metastases from breast and prostate cancer. Importantly, inhibition of these two pathways using a combined treatment strategy of the mTOR inhibitor everolimus and a gap junction inhibitor, arsenic trioxide (As2O3), was able to eliminate the bone micrometastases in a mouse model of estrogen receptor-positive (ER+) breast cancer. Importantly, the authors supported these findings using human samples. Bone metastases exhibited the highest level of Cx43 expression among all sites of metastases of both breast and prostate cancer. High Cx43 expression in human primary tumors was associated with a lower probability of bone metastasis-free survival. The combination of in vivo data from multiple bone metastatic models as well as human sample analysis elegantly tied together the work.
Bone is a rich source of calcium, which is responsible for its mineral properties. Wang et al. have shown how cancer cells use this stored calcium to promote their own growth. These findings provide significant insight into our understanding of how the osteogenic niche promotes the early stages of cancer cell growth and indicate that the inhibition of calcium signaling via Cx43 blockade may restrain the development of bone metastases. Previous studies show that extracellular calcium, acting via calcium-sensing receptors, prominently affects tumor growth (Boudot et al., 2017; Ahearn et al., 2016). This is the first finding to describe how cancer cells access calcium through interactions with osteogenic and mesenchymal stem cells, highlighting the unique aspects of the bone microenvironment that may be targeted to treat and prevent bone metastases (Figure 1).
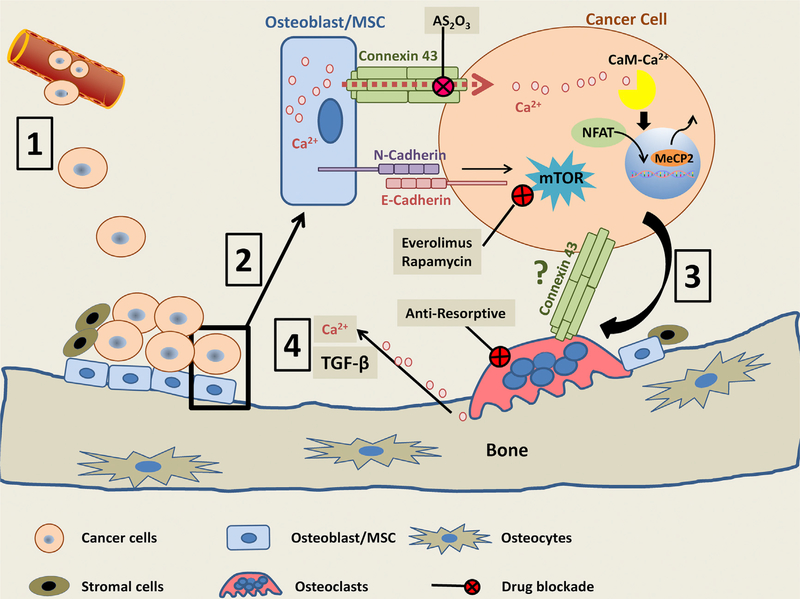
Figure 1. Bone Metastasis and the Metastatic Niche.
Circulating tumor cells extravasate and seed into the bone (1). These cells interact with the osteogenic niche in the early stages of metastasis, through connexin 43 and E/N-cadherin, activating calcium and mTOR signaling to promote tumor growth (2). Later, during disease progression, tumor cells interact with the bone-resorbing osteoclasts through various mechanisms, thereby stimulating bone destruction (3). TGFβ and calcium are released as a consequence of osteoclastic bone destruction (4). This can enhance connexin 43 expression and provide more calcium to perpetuate the cycle of growth.
By digging deep into the mechanisms by which the osteogenic niche promotes the early stages of bone metastases development, this study effectively raises new questions. Does this mechanism play a role in dormancy? Does inhibition of bone destruction (by bisphosphonates or denosumab) impact tumor utilization of the osteogenic niche by reducing the extracellular calcium concentrations? What is the role of osteocytes in this process, since these cells display some of the highest expression of Cx43 in bone? Recent data show that TGFβ increases expression of Cx43 (Liu et al., 2018). Could bone-derived TGFβ further increase tumor utilization of the osteogenic niche in states of increased bone destruction and further fuel the vicious cycle? What other cells in the microenvironment may interact with cancer cells to participate in calcium uptake through gap junctions, such as hematopoietic cells or osteocytes present in bone metastatic niche? Finally, considering that the timing of treatment strategies is crucial, when is the best time to use a gap junction inhibitor to block calcium signaling? These and many other questions remain, the answers of which will bring us closer to a cure for this devastating complication of cancer.
ACKNOWLEDGMENTS
T.A.G. and K.S.M. acknowledge support from NCI R01CA206025 and DOD BC150678. D.L.W. acknowledges support from NCI R21CA205437 and from the Phi Beta Psi National Project.
REFERENCES
- Ahearn TU, Tchrakian N, Wilson KM, Lis R, Nuttall E, Sesso HD, Loda M, Giovannucci E, Mucci LA, Finn S, and Shui IM (2016). Calcium-sensing receptor tumor expression and lethal prostate cancer progression. J. Clin. Endocrinol. Metab 101, 2520–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudot C, Hénaut L, Thiem U, Geraci S, Galante M, Saldanha P, Saidak Z, Six I, Clézardin P, Kamel S, and Mentaverri R (2017). Overexpression of a functional calcium-sensing receptor dramatically increases osteolytic potential of MDA-MB-231 cells in a mouse model of bone metastasis through epiregulin-mediated osteoprotegerin downregulation. Oncotarget 8, 56460–56472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, and Weinberg RA (2011). Hallmarks of cancer: the next generation. Cell 144, 646–674. [DOI] [PubMed] [Google Scholar]
- Karlsson T, Sundar R, Widmark A, Landström M, and Persson E (2018). Osteoblast-derived factors promote metastatic potential in human prostate cancer cells, in part via non-canonical transforming growth factor β (TGFβ) signaling. Prostate 78, 446–456. [DOI] [PubMed] [Google Scholar]
- Kinder M, Chislock E, Bussard KM, Shuman L, and Mastro AM (2008). Metastatic breast cancer induces an osteoblast inflammatory response. Exp. Cell Res 314, 173–183. [DOI] [PubMed] [Google Scholar]
- Liu W, Cui Y, Sun J, Cai L, Xie J, and Zhou X (2018). Transforming growth factor-β1 up-regulates connexin43 expression in osteocytes via canonical Smad-dependent signaling pathway. Biosci. Rep 38, BSR20181678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yu C, Gao X, Welte T, Muscarella AM, Tian L, Zhao H, Zhao Z, Du S, Tao J, et al. (2015). The osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells. Cancer Cell 27, 193–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Tian L, Liu J, Goldstein A, Bado I, Zhang W, Arenkiel BR, Li Z, Yang M, Du S, et al. (2018). The osteogenic niche is a calcium reservoir of bone micrometastases and confers unexpected therapeutic vulnerability. Cancer Cell 34, 823–839.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weilbaecher KN, Guise TA, and McCauley LK (2011). Cancer to bone: a fatal attraction. Nat. Rev. Cancer 11, 411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]