Abstract
STUDY QUESTION
Is marijuana smoking associated with semen quality, sperm DNA integrity or serum concentrations of reproductive hormones among subfertile men?
SUMMARY ANSWER
Men who had ever smoked marijuana had higher sperm concentration and count and lower serum FSH concentrations than men who had never smoked marijuana; no differences were observed between current and past marijuana smokers.
WHAT IS KNOWN ALREADY
Studies of marijuana abuse in humans and animal models of exposure to marijuana suggest that marijuana smoking adversely impacts spermatogenesis. Data is less clear for moderate consumption levels and multiple studies have found higher serum testosterone concentrations among marijuana consumers.
STUDY DESIGN, SIZE, DURATION
This longitudinal study included 662 subfertile men enroled at the Massachusetts General Hospital Fertility Center between 2000 and 2017. The men provided a total of 1143 semen samples; 317 men also provided blood samples in which we measured reproductive hormones.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Use of marijuana and other drugs was self-reported at baseline. Standard protocols were followed for measuring semen quality, sex hormones and DNA integrity. We used linear mixed effect models with a random intercept to evaluate the associations of self-reported marijuana smoking at enrolment with semen parameters from subsequently collected samples, and linear regression models for sperm DNA integrity and serum reproductive hormones, while adjusting for confounders including smoking and cocaine use.
MAIN RESULTS AND THE ROLE OF CHANCE
Men who had ever smoked marijuana (N = 365) had significantly higher sperm concentration (62.7 (95% confidence interval: 56.0, 70.3) million/mL) than men who had never smoked marijuana (N = 297) (45.4 (38.6, 53.3) million/mL) after adjusting for potential confounders (P = 0.0003). There were no significant differences in sperm concentration between current (N = 74) (59.5 (47.3, 74.8) million/mL) and past marijuana smokers (N = 291) (63.5 (56.1, 72.0) million/mL; P = 0.60). A similar pattern was observed for total sperm count. Furthermore, the adjusted prevalence of sperm concentration and total sperm motility below WHO reference values among marijuana smokers was less than half that of never marijuana smokers. Marijuana smokers had significantly lower follicle stimulating hormone (FSH) concentrations than never marijuana smokers (−16% (−27%, −4%)) and there were no significant differences between current and past marijuana smokers (P = 0.53). Marijuana smoking was not associated with other semen parameters, with markers of sperm DNA integrity or with reproductive hormones other than FSH. Chance findings cannot be excluded due to the multiple comparisons.
LIMITATIONS, REASONS FOR CAUTION
Our results may not be generalisable to men from the general population. Marijuana smoking was self-reported and there may be misclassification of the exposure.
WIDER IMPLICATIONS OF THE FINDINGS
These findings are not consistent with a deleterious effect of marijuana on testicular function. Whether these findings are reflective of the previously described role of the endocannabinoid system in spermatogenesis or a spurious association requires confirmation in further studies.
STUDY FUNDING/COMPETING INTEREST(S)
The project was funded by grants R01ES009718 and P30ES000002 from the National Institute of Environmental Health Sciences (NIEHS). None of the authors has any conflicts of interest to declare.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: marijuana, semen parameters, male infertility, hormone, drugs, testicular function
Introduction
Approximately 183 million people reported using of marijuana (Cannabis sativa) in 2015 (UNODC, 2017) making marijuana the most commonly used drug worldwide. In the USA, its estimated prevalence of use among adults was 16.5% (19.4% in men and 13.6% in women) (UNODC, 2017). Furthermore, support for legal recreational use of marijuana in the USA increased 5-fold (12% to 61%) between 1969 and 2017 and nearly doubled (31% to 61%) between 2000 and 2017 (Geiger, 2018), coinciding with a growing perception that marijuana poses few health hazards and with increased legalisation and decriminalisation of recreational marijuana use worldwide.
Most of the literature on the health effects of marijuana and Delta-9-tetrahydrocannabinol (THC), its active component, has focused on its neurological effects (Scott et al., 2018). Yet, animal models suggest a critical role of the endocannabinoid system on spermatogenesis (Grimaldi et al., 2009, 2013). A few human studies have assessed the reproductive effects of marijuana smoking, including its potential effects on the male reproductive system. However, most have focused on men with drug abuse history, thus limiting the generalisability of the results (Hembree et al., 1978; Close et al., 1990; el-Gothamy and el-Samahy, 1992). A 2015 study assessed this question among healthy young Danish men finding that men who regularly smoked marijuana more than once per week had significantly lower sperm count but significantly higher serum testosterone concentrations (Gundersen et al., 2015). To further evaluate the role of marijuana on male reproductive function, we studied the association between self-reported marijuana smoking and markers of testicular function as measured by semen quality parameters, sperm DNA fragmentation and serum reproductive hormones. Based on the preponderance of previous findings, we hypothesised that marijuana smoking would be associated with worse semen quality and lower serum testosterone.
Materials and Methods
Study population
Men from couples presenting for evaluation at the Massachusetts General Hospital (MGH) Fertility Center between 2000 and 2017 were invited to participate in an ongoing study aimed at identifying environmental determinants of fertility (Meeker et al., 2011; Messerlian et al., 2018). Of the approached men, ~55% agreed to participate. All men signed an informed consent. The studies were approved by institutional review boards at the Harvard T. H. Chan School of Public Health and MGH.
Of the 1011 men recruited, 280 men did not answer questions regarding drug use. Furthermore, 18 men were azospermic and excluded. We also excluded 51 men who did not have complete semen analysis data. The remaining 662 men contributed 1143 semen samples between 2000 and 2017 (Supplementary Fig. 1). This included 296 semen samples, from men enroled between 2000 and 2004, that were previously analysed for sperm DNA damage. Of the 662 men, 317 also provided serum samples that were analysed for reproductive hormones. Due to limited resources, not all 1143 semen samples were analysed but selection was unrelated to semen analysis results, type or outcome of any infertility treatments, marijuana smoking status or any other participant’s characteristic. Differences in participant characteristics between men included and men excluded from the analysis were minor (Supplementary Table I).
Marijuana smoking and covariate assessment
At baseline, men reported marijuana smoking in a self-administered questionnaire. Specifically, they first reported if they had ever smoked marijuana (more than two joints/cigarettes or the equivalent amount of marijuana in your lifetime) and if they were current marijuana smokers. Among ever smokers, we also assessed the average number of joints/cigarettes (or equivalent amount of marijuana) they smoked per week, whether they ever quit and for how many years, age of starting to smoke marijuana, last time they smoked marijuana, and the total duration of marijuana smoking. The questionnaire had similar questions about cocaine use. Men also self-reported demographic information, data on other lifestyle factors and medical history. A research nurse abstracted clinical information from medical records and measured their height and weight to calculate body mass index (BMI) (kg/m2) at the time of enrolment.
Semen analysis
Men provided a semen sample onsite at the MGH andrology laboratory by masturbation into a sterile plastic specimen cup. Men were asked to abstain from ejaculation for 2–5 days before providing the semen sample. Men reported the duration of abstinence before providing the sample. All semen samples were analysed using standardised protocols and quality control was as described previously (Nassan et al., 2016). Before analysis, the sample was liquefied at 37°C for 20 min after collection. Ejaculate semen volume (mL) was measured using a graduated serological pipet. Sperm concentration (million/mL) and % motility were assessed using a computer-aided semen analyser (CASA; 10HTM-IVOS, Hamilton-Thorne Research, Beverly, MA, USA). We calculated the total sperm count (million/ejaculate) as semen volume × sperm concentration. Sperm morphology (% normal) was assessed on two slides per specimen (with a minimum of 200 cells assessed per slide) via a microscope with an oil-immersion ×100 objective (Nikon, Tokyo, Japan). Strict Kruger scoring criteria were used to classify men as having normal or below normal morphology (Kruger et al., 1988). The andrologists participate regularly in internal and external quality control checks.
Sperm DNA integrity
The neutral comet assay was used following the previously described protocol (Meeker, Yang, Ye, Calafat and Hauser, 2011; McAuliffe et al., 2014; Nassan et al., 2018). Briefly, 50 μL of a semen/agarose mixture was embedded between two additional layers of agarose on microgel electrophoresis glass slides. Slides were immersed in a cold lysing solution to dissolve the sperm cell membranes and make sperm chromatin available. After 1 h of cold lysis, slides were transferred to a solution for enzyme treatment with RNAse (Amresco, Solon, OH) and incubated at 37°C for 4 h. Slides were transferred to a second enzyme treatment with proteinase K (Amresco) and incubated at 37°C for 18 h then placed on a horizontal slab in an electrophoretic unit toundergo electrophoresis for 1 h. DNA in the gel was subsequently precipitated, fixed in ethanol and dried. Slides were stained and observed using a fluorescence microscope.
Comet extent (CE), DNA percent in the tail (%tail) and tail distributed moment (TDM) were assessed in 100 sperm cells in each semen sample using the VisComet software (Impulus Computergestutze Bildanalyse GmbH, Gilching, Germany). CE represents the average total comet length in μm from the beginning of the head to the last visible pixel in the tail. %Tail represents the average proportion of DNA that is in the tail of the comet. TDM represents an integrated measure that takes into account the distance and intensity of comet fragments (Nassan et al., 2018). TDM is calculated as Σ(I × X)ΣI, where ΣI is the sum of all intensity measures for the head, body or tail, and X is the x-position of the intensity measure. An additional measure of sperm DNA damage used was the counted number of sperm cells with CE > 300μm, i.e. too long to measure with VisComet.
Reproductive hormones
A non–fasting blood sample was drawn between 9 a.m. and 4 p.m. on the same day of the first semen sample in a subset of the men. Blood was centrifuged, and serum was stored at −80°C until analysis. Serum was thawed and analysed in one batch for follicle-stimulating hormone (FSH), luteinising hormone (LH), estradiol, inhibin-B, total testosterone and sex hormone-binding globulin (SHBG). FSH, LH, and estradiol concentrations were determined by microparticle enzyme immunoassay using an automated Abbot AxSYM system (Abbott Laboratories, Chicago, IL). The assay sensitivities were 1.1 IU/L for FSH and 1.2 IU/L for LH. The intra-assay coefficient of variation (CV) for FSH and LH was <5% and <3%, respectively with inter-assay CVs for both hormones of <9%. The assay sensitivity for estradiol was 20 pg/mL with a within-run CV between 3% and 11%, and the total CV was between 5% and 15%. Total testosterone was directly measured using the Coat-A-Count RIA kit (Diagnostic Products, Los Angeles, CA), which had a sensitivity of 4 ng/dL, inter-assay CV of 12% and intra-assay CV of 10%. Inhibin-B was measured using a double-antibody enzyme-linked immunosorbent assay (Oxford Bioinnovation, Oxford, UK) with inter-assay CV of 20% and intra-assay CV of 8%. SHBG was measured using an automated system (Immulite; DPC Inc, Los Angeles, CA), which used a solid phase two site chemiluminescent enzyme immunometric assay and had an inter-assay CV of <8%.
Statistical analysis
We calculated descriptive statistics for baseline characteristics across categories of marijuana smoking and tested for differences across categories. We natural-log transformed ejaculate volume, sperm concentration, total sperm count, CE, %tail, TDM and serum hormone concentrations. We used linear mixed effect models to evaluate the associations of marijuana smoking with semen parameters and included a random intercept for each man to account for the longitudinal collection of multiple semen samples per man. For sperm DNA fragmentation measures and serum hormones, we used linear regression models. We used Poisson regression to model the number of cells with high DNA damage while accounting for over-dispersion. All results are presented as adjusted marginal means (Searle et al., 1980). The primary analyses consisted of evaluating men’s marijuana smoking at enrolment (never/ever and never/past/current) in relation to study outcomes. Among the marijuana smokers, we also analysed the association of joint-years of marijuana smoking (joints/day for the total duration of marijuana smoking in years) with the same outcomes. In addition, we evaluated the association of time since last use of marijuana and sample collection, and age at the start of marijuana smoking. Potential confounders were selected based on prior knowledge and descriptive statistics in the study population. The final model adjusted for age, race, sexual abstinence time, BMI, tobacco smoking, coffee and alcohol intake, cocaine use and calendar year. In the sperm motility models, we further adjusted for duration elapsed between semen sample collection and analysis. We also conducted an additional analysis in which semen parameters were dichotomised as above or below WHO-2010 lower reference limits (WHO, 2010) using the first semen sample per man (closest to marijuana assessment). In this analysis, we used generalised linear models with a binary distribution and logit link adjusting for the same covariates as above.
To assess the robustness of our results, we conducted a series of sensitivity analyses including (1) re-categorising the marijuana smoking status based on last time reported of smoking marijuana (recent if ≤2 years, and past of >2 years), (2) restricting analyses to men who did not receive a male factor infertility diagnosis, (3) restricting analyses to the first semen sample per man which was closest to reporting the marijuana smoking, (4) further adjustment for history of sexually transmitted diseases (STDs), and stress levels as assessed by the standardised perceived stress scale 4 (Cohen et al., 1983; Cohen and Janicki-Deverts, 2012) and (5) further adjusting the testosterone models for time of serum sample collection. In addition, to address the possibility of selection bias, we compared the characteristics at enrolment and the semen parameters between men included in the main analysis versus those who were excluded. Finally, we calculated the E-value (VanderWeele and Ding, 2017) to quantitatively assess the potential impact of unmeasured confounding on the observed associations, conditional on the measured covariates. We conducted all statistical analyses using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Men had a mean (standard deviation, SD) age of 36.3 (5.11) years and BMI of 27.5 (4.70) kg/m2. Most were Caucasian (88%), had a university degree (84%), and did not currently smoke tobacco (94%). Of the 662 men in our study, 455 (69%) provided one semen sample, 90 (14%) provided two samples, and 117 (18%) provided ≥3 samples. Most (88%) semen samples were analysed within 30 min after specimen collection and 72% of the men had a sexual abstinence of 2–4 days (Table I). Fifty five percent of the men reported having ever smoked marijuana; 44% of men were past and 11% were current marijuana smokers. Marijuana smokers were more likely to be white, overweight or obese and tobacco smokers. They also had higher intakes of alcohol and coffee and were more likely to have ever used cocaine (Table I). All but one of the men who reported ever use of cocaine also reported marijuana smoking. The distributions of the semen parameters, sperm DNA damage measures and hormone concentrations are shown in Supplementary Table II
Table I.
Demographic and semen sample characteristics by marijuana smoking categories among 662 men (1143 semen samples) participating in the study (2000–2017).
| Baseline characteristics | Marijuana smoking | ||||
|---|---|---|---|---|---|
| Total (N = 662) | Never N = 297 (45%) | Past N = 291 (44%) | Current N = 74 (11%) | P valueb | |
| Age, years | 36.3 (5.11) | 36.0 (5.30) | 36.7 (5.02) | 35.6 (4.57) | 0.09 |
| BMI, kg/m2 | 27.5 (4.70) | 27.0 (4.30) | 28.0 (5.35) | 27.5 (3.12) | 0.14 |
| Race | 0.001 | ||||
| Caucasian | 581 (88) | 245 (82) | 272 (93) | 64 (86) | – |
| Black/African American | 15 (2) | 10 (3) | 2 (1) | 3 (4) | – |
| Asian | 33 (5) | 22 (7) | 10 (3) | 1 (1) | – |
| Native American/Alaska Native | 33 (5) | 20 (7) | 7 (2) | 6 (8) | – |
| Education | 0.09 | ||||
| Below college | 106 (16) | 41 (14) | 47 (16) | 18 (24) | – |
| College or Graduate Degree | 554 (84) | 256 (86) | 242 (84) | 56 (76) | – |
| Tobacco smoking Status | – | – | <0.0001 | ||
| Never | 472 (71) | 251 (85) | 185 (64) | 36 (49) | – |
| Former | 153 (23) | 36 (12) | 92 (32) | 25 (34) | – |
| Current | 37 (6) | 10 (3) | 14 (5) | 13 (18) | – |
| Coffee ≥5 cup/week | 407 (61) | 154 (52) | 200 (69) | 53 (72) | <0.0001 |
| Alcohol ≥1 days/week | 453 (68) | 169 (57) | 218 (75) | 66 (89) | <0.0001 |
| Cocaine ever use | 148 (22) | 1 (0.3) | 107 (73) | 40 (54) | <0.0001 |
| History of reproductive tract diseasesc | 199 (30) | 83 (28) | 88 (30) | 28 (38) | 0.25 |
| History of sexually transmitted diseasesd | 67 (10) | 30 (10) | 28 (10) | 9 (12) | 0.81 |
| History of reproductive tract surgeriese | 78 (12) | 41 (14) | 28 (10) | 9 (12) | 0.29 |
| Infertility Diagnosis | 0.37 | ||||
| Male Factor | 79 (25) | 37 (28) | 31 (20) | 11 (33) | |
| Female Factor | 110 (35) | 41 (31) | 59 (39) | 10 (30) | |
| Unexplained | 128 (40) | 55 (41) | 62 (41) | 12 (36) | |
| Average Marijuana joints smoked/ weekf | 2.07 (4.32) | 0 | 1.76 (2.58) | 2.98 (7.31) | – |
| Marijuana joint-yearf | 1.81 (8.11) | 0 | 2.67 (5.31) | 9.76 (25.0) | – |
| Age of Marijuana smoking initiation, yearsf | 17.5 (3.20) | NA | 17.5 (2.93) | 17.4 (4.08) | – |
| Duration of marijuana smoking, yearsf | 10.4 (7.54) | 0 | 8.52 (6.72) | 18.1 (5.54) | – |
| Duration of marijuana quit history, yearsf | 4.06 (5.47) | NA | 4.62 (6.01) | 2.36 (2.72) | – |
| Duration since last time marijuana smoking, yearsf | 8.78 (8.05) | NA | 11.5 (7.61) | 0.85 (1.16) | – |
| Time-varying characteristics (semen samples) | 1143 | 490 (43) | 526 (46) | 127 (11) | |
| Calendar Year of the semen sample | 2008 (5) | 2007 (5) | 2009 (5) | 2007 (5) | <0.0001 |
| Warm Season (April through September) | 555 (49) | 241 (49) | 261 (50) | 53 (42) | 0.26 |
| Sexual Abstinence | – | – | – | 0.95 | |
| <2 Days | 244 (21) | 106 (22) | 114 (22) | 24 (19) | – |
| 2 ≤ Days < 4 | 381 (33) | 162 (33) | 174 (33) | 45 (35) | – |
| ≥4 Days | 442 (39) | 189 (39) | 201 (38) | 52 (41) | – |
| Unknown | 76 (7) | 33 (7) | 37 (7) | 6 (5) | |
| Time elapsed between sample collection and analysis | 0.15 | ||||
| ≤30 min | 1002 (88) | 417 (85) | 473 (90) | 112 (88) | |
| >30 min | 65 (6) | 37 (8) | 22 (4) | 6 (5) | |
| Unknown | 76 (7) | 36 (7) | 31 (6) | 9 (7) | |
Abbreviations: BMI; body mass index, mins; minutes.
aN (%) is presented for categorical/binary variables and mean (standard deviation) is presented for continuous variables.
bFrom Chi-square (or Fisher’s exact test when appropriate) for discrete variables and Kruskal–Wallis for continuous variables.
cGroin injury, testes not always in scrotum, varicocele, testicular torsion, testicular injury, hernia, epididymitis, prostatitis and seminal vesicle infection.
dSyphilis, gonorrhoea, mycoplasma/ureaplasma, chlamydia, trichomonas, herpes, human papilloma virus, lymphogranuloma, group-B strep or other sexually transmitted diseases.
eVaricocelectomy, orchidopexy, hydrocelectomy, repair of hernia, urethra, or hypospadias, sympathectomy, or bladder neck surgery.
fThe numbers presented for the entire cohort are restricted to ever marijuana smokers.
Infertility diagnosis was missing before 2004.
Education was missing for two men.
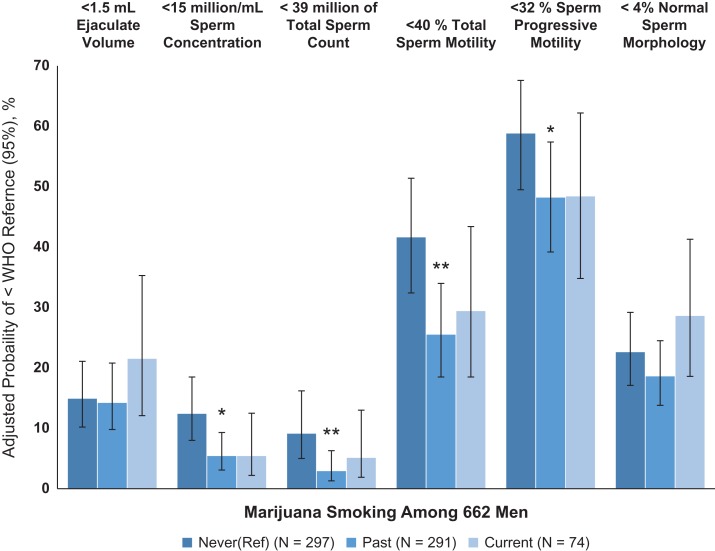
Men who had ever smoked marijuana had significantly higher sperm concentration than men who had never smoked marijuana in unadjusted (Supplementary Table III) and multivariable-adjusted analyses (62.7 (95% confidence interval (CI): 56.0, 70.3) million/mL vs. 45.4 (38.6, 53.3) million/mL; P = 0.0003) (Table II). There were no statistically significant differences in sperm concentration between current and past marijuana smokers (P = 0.60). Similar patterns were observed for total sperm count. Men who had ever smoked marijuana also had 16% (−27%, −4%) lower serum FSH concentrations than men who had never smoked it, with no significant differences between past and current marijuana smokers (P = 0.53) (Table II). There were no associations of marijuana smoking status with other semen parameters, markers of sperm DNA integrity or other reproductive hormone concentrations. Of note, cocaine use was associated with a higher adjusted proportion of sperm concentration and count below the WHO reference values. In these analyses, marijuana smokers had an estimated 5% (95% CI: 3%, 9%) of semen samples with concentrations below 15 million/mL while never marijuana smokers had 12% (95% CI: 8%, 19%) (Fig. I and Supplementary Table SIV).
Table II.
Adjusted semen quality parameters and serum reproductive hormone concentrations according to marijuana smoking status.
| Reproductive parameters | Marijuana smoking Adjusted means (95% CI)a | |||
|---|---|---|---|---|
| Never (Reference) | Ever | Past | Current | |
| Semen Quality Parameters | N = 297 men, 490 samples | N = 365 men, 653 samples | N = 291 men, 526 samples | N = 74 men, 127 samples |
| Ejaculate volume, mL | 2.52 (2.31, 2.74) | 2.39 (2.25, 2.54) | 2.41 (2.26, 2.57) | 2.30 (2.04, 2.59) |
| Sperm concentration, million/mL | 45.4 (38.6, 53.3) | 62.7 (56.0, 70.3)* | 63.5 (56.1, 72.0)* | 59.5 (47.3, 74.8)* |
| Total sperm count, million | 114 (97.0, 134) | 150 (133, 168)* | 152 (134, 173)* | 139 (110, 175) |
| % Total Sperm Motilityb | 45.6 (41.6, 49.5) | 49.3 (46.2, 52.4) | 49.3 (46.0, 52.6) | 49.3 (43.9, 54.7) |
| % Progressive Sperm Motilityb | 27.2 (24.5, 29.9) | 29.6 (27.5, 31.7) | 29.6 (27.4, 31.9) | 29.4 (25.7, 33.1) |
| % Normal Sperm Morphology | 6.51 (5.89, 7.13) | 6.79 (6.35, 7.23) | 6.91 (6.43, 7.39) | 6.32 (5.43, 7.20) |
| Sperm DNA Damage | N = 146 | N = 150 | N = 113 | N = 37 |
| Comet Extent, μm | 124 (109, 142) | 125 (111, 142) | 127 (112, 144) | 119 (102, 139) |
| Comet Tail DNA, % | 29.6 (25.4, 34.5) | 27.5 (23.9, 31.7) | 27.2 (23.5, 31.4) | 28.9 (24.2, 34.5) |
| Comet Tail Distributed Moment (TDM), μm | 54.6 (48.8, 61.1) | 55.7 (50.1, 61.9) | 56.8 (51.0, 63.2) | 51.8 (45.4, 59.0) |
| Comet Cells with High DNA damage, N | 3.90 (0.69, 22.0) | 3.21 (0.58, 17.9) | 3.31 (0.59, 18.5) | 2.87 (0.49, 16.8) |
| Hormone Concentrations | N = 149 | N = 168 | N = 131 | N = 37 |
| FSH, IU/L | 7.77 (6.23, 9.68) | 6.49 (5.28, 7.98)* | 6.56 (5.32, 8.09)* | 6.18 (4.77, 8.00)* |
| LH, IU/L | 10.6 (8.60, 13.0) | 10.2 (8.38, 12.3) | 10.3 (8.45, 12.5) | 9.68 (7.60, 12.3) |
| Inhibin-B, pg/mL | 138 (112, 170) | 150 (123, 183) | 147 (121, 180) | 163 (127, 208) |
| Estradiol, pg/mL | 23.8 (19.3, 29.3) | 25.7 (21.1, 31.2) | 26.1 (21.5, 31.9) | 23.6 (18.5, 30.1) |
| Testosterone, ng/dL | 368 (321, 421) | 376 (331, 426) | 375 (330, 427) | 378 (323, 443) |
| SHBG, nmol/L | 23.6 (20.1, 27.7) | 24.9 (21.4, 29.0) | 24.2 (20.7, 28.2) | 28.6 (23.7, 34.6)* |
Abbreviations: CI; confidence interval, N; number, FSH; follicle stimulating hormone, LH; luteinizing hormone, and SHBG; sex hormone-binding globulin; DNA, deoxyribonucleic acid.
aAdjusted marginal means were estimated using linear mixed models and a random intercept for each man for the semen quality parameters and linear regression models for the reproductive hormone concentrations and DNA integrity. The adjusted marginal means in each exposure category were adjusted for the covariates at their average levels for continuous variables and weighted average level of categorical variable in the model including age (years, continuous), race (white/ not), sexual abstinence time (days, categorical), body mass index (kg/m2, continuous), tobacco smoking (yes/no), coffee (binary) and alcohol intake (binary), cocaine use (yes/no), and calendar year (continuous).
Motility models were further adjusted for time elapsed between semen collection and analysis.
* P < 0.05 compared to never.
Figure 1.
Adjusted means of the proportions (95% CI) of semen parameters below the WHO lower reference values associated with marijuana smoking status among 662 men (using the first semen samples given per man closest to assessment of marijuana smoking). Abbreviations: WHO; World Health Organization; 95% CI, 95% confidence interval. 1Adjusted mean proportions were estimated using generalised linear models with binary distribution and logit link. Models were adjusted for age (years, continuous), race (white/ not), sexual abstinence time (days, categorical), body mass index (kg/m2, continuous), tobacco smoking (yes/no), coffee (binary) and alcohol intake (binary), cocaine use (yes/no), and calendar year (continuous). Motility models were further adjusted for time elapsed between semen collection and analysis. 2WHO lower reference limits (2010): ejaculate volume <1.5 mL; sperm concentration <15 million/mL; total sperm count <39 million; total motile sperm <40%; progressive motile sperm <32%, and normal sperm morphology <4% using ‘strict’ Tygerberg method. *P value < 0.05, **P value < 0.01 compared to never.
In analyses restricted to ever marijuana smokers, increasing marijuana smoking by 20 joint-years was associated with significantly higher serum concentrations of testosterone of 8% (2%, 15%), inhibin B of 11% (0.30%, 23%) and SHBG of 9% (2%, 17%) and a non-statistically significant higher sperm concentration of 12.6 (−6.55, 35.6) million/mL and total sperm count of 10.7 (−8.12, 33.4) million (Table III). In addition, a later age of initiation of marijuana smoking was associated with a non-statistically significant lower sperm count (−2.56 (−5.46, 0.42) million) and concentration (−2.94 (−5.84, 0.06) million/mL). Furthermore, each additional year since last smoking marijuana was associated with a 2.21 (0.13, 4.34) million higher sperm count, a 1.03% (0.16, 1.91) higher TDM and lower (−1.75% (−3.21, −0.27)) estradiol concentrations. The association between marijuana smoking and sperm concentration persisted after adjustment for serum testosterone (data not shown).
Table III.
Adjusted difference (95% confidence interval) in semen quality parameters and serum reproductive hormone concentrations associated with intensity of marijuana smoking among ever marijuana smoking men.
| Semen Quality Parameters | Per 20 additional Marijuana joint-year N = 262 men, 439 samples | Per 1 additional year elapsed since last time smoked Marijuana N = 296 men, 507 samples | Per 1 additional year delay in start of Marijuana smoking N = 356 men, 636 samples |
|---|---|---|---|
| Ejaculate volume, mL | −1.52 (−10.8, 8.73) | 1.07 (0, 2.19)* | 0.46 (−1.20, 2.15) |
| Sperm concentration, million/mL | 12.6 (−6.55, 35.6) | 1.26 (−0.80, 3.40) | −2.94 (−5.84, 0.06) |
| Total sperm count, million | 10.7 (−8.12, 33.4) | 2.21 (0.13, 4.34)* | −2.56 (−5.46, 0.42) |
| % Total Sperm Motilityb | −0.78 (−5.06,3.50) | 0.22 (−0.24, 0.69) | −0.63 (−1.34, 0.09) |
| % Progressive Sperm Motilityb | −0.94 (−3.89,2.00) | 0.16 (−0.17, 0.48) | −0.30 (−0.78, 0.19) |
| % Normal Sperm Morphologyb | −0.48 (−1.27,0.31) | 0.03 (−0.06, 0.11) | −0.03 (−0.16, 0.09) |
| Sperm DNA Damage | N = 133 | N = 146 | N = 145 |
| Comet Extent, μm | −1.06 (−7.21, 5.50) | 0.99 (−0.03, 2.03) | 0.58 (−1.12, 2.31) |
| Comet Tail DNA, % | −4.86 (−11.1, 1.86) | 0.37 (−0.75, 1.51) | −0.25 (−2.11, 1.64) |
| Comet Tail Distributed Moment (TDM), μm | −0.67 (−5.98, 4.95) | 1.03 (0.16, 1.91)* | 0.87 (−0.62, 2.39) |
| Comet Cells with High DNA damage, N | −16.5 (−44.4, 25.5) | 3.33 (−1.20, 8.04) | 0.16 (−7.09, 8.00) |
| Hormone Concentrations | N = 152 | N = 165 | N = 163 |
| FSH, IU/L | 2.74 (−7.84, 14.5) | 0.16 (−1.52, 1.86) | 2.06 (−0.83, 5.04) |
| LH, IU/L | 4.41 (−5.96, 15.9) | 0.42 (−1.19, 2.07) | −0.95 (−3.72, 1.90) |
| Inhibin-B, pg/mL | 10.9 (0.30, 22.6)* | 0.14 (−1.41, 1.72) | −1.81 (−4.40, 0.84) |
| Estradiol, pg/mL | 3.47 (−6.36, 14.3) | −1.75 (−3.21, −0.27) | −0.01 (−2.65, 2.70) |
| Testosterone, ng/dL | 8.22 (2.02, 14.8)* | 0.23 (−0.71, 1.17) | −0.64 (−2.23, 0.98) |
| SHBG, nmol/L | 9.00 (1.65, 16.9)* | −0.16 (−1.24, 0.94) | −0.85 (−2.74, 1.07) |
Abbreviations: CI; confidence interval, n; number, FSH; follicle stimulating hormone, LH; luteinizing hormone, and SHBG; sex hormone-binding globulin; DNA, deoxyribonucleic acid.
aThe adjusted effect estimates are adjusted for the covariates at their average levels for continuous variables and weighted average level of categorical variable in the model including age (years, continuous), race (white/not), sexual abstinence time (days, categorical), body mass index (kg/m2, continuous), tobacco smoking (yes/no), coffee (binary) and alcohol intake (binary), cocaine use (yes/no), and calendar year (continuous). Motility models were further adjusted for time elapsed between semen collection and analysis.
bEffect estimates are presented as percent changes for all reproductive parameters except for motility and morphology sperm parameters.
The associations between marijuana smoking status and markers of testicular function persisted after re-categorising exposure status based on last time of reported use, after restricting analyses to men without a diagnosis of male factor infertility, in analyses restricted to the first semen sample per man, after further adjustment for stress levels (Supplementary Tables V–VIII) or history of STDs, and after further adjusting for time of serum sample collection for testosterone (data not shown). A sensitivity analysis to quantify the impact of unmeasured confounding showed that in order for an unmeasured confounder to explain the observed relation between marijuana smoking status and sperm concentration, it would have to be associated with both sperm concentration and marijuana smoking status by a risk ratio ≥2.08 (or ≥1.59 to exclude the lower bound of the confidence interval) above and beyond the measured confounders.
Discussion
Contrary to our hypothesis, we observed that men who had ever smoked marijuana had higher sperm concentration and total sperm count, lower prevalence of sperm parameters below the WHO reference values, and lower FSH concentrations than men who had never smoked marijuana. These findings were robust after conducting several sensitivity analyses and considering different metrics of marijuana smoking. Specifically, more intense use was associated with significantly higher concentrations of testosterone, SHBG and inhibin-B, and later initiation of marijuana had an association with lower sperm count of marginal statistical significance. These results are consistent with a direct pro-spermatogenic testicular effect and secondary compensation in FSH secretion. On the other hand, the associations of marijuana smoking with sperm count and FSH concentrations were stronger for past smokers than for current smokers even though these two groups did not differ significantly from each other. Furthermore, longer duration since last use of marijuana was related to higher sperm count. These other results raise the possibility that our findings are not explained by a true underlying biologic mechanism but are instead spurious associations.
Let us first consider the possibility that the observed relations are spurious. While we considered a large number of potential confounders, residual confounding must still be considered. Our analysis suggests that, in order to account for the observed relations, an unmeasured confounder would have to be positively related to marijuana smoking and simultaneously positively related to semen quality by ≥2.08 risk ratio. In other words, for an unmeasured confounder to explain the observed associations, it would have to have a relation with marijuana smoking of greater magnitude than the association between marijuana and tobacco smoking (RR = 1.6) (one of the potential confounders most strongly related to marijuana smoking in our data) and a similarly strong positive association with semen quality, independently of all measured confounders. This seems unlikely. Selection bias does not seem likely either. Although we observed some differences in BMI, race and primary infertility diagnosis between men included and excluded from analysis, we did not observe systematic differences in terms of frequency of cocaine use, sperm concentration or sperm count between included and excluded men from analysis. The close match in the frequency of marijuana smoking in this population and the general USA population (Azofeifa et al., 2016), as well and the lack of difference in semen quality between men who joined this study and men from the same clinic who did not join the study (Hauser et al., 2005) also argues against selection bias possibility.
Another possibility is that the assumed causal structure is incorrect and the association reflects reverse causation. Specifically, we had assumed that marijuana use would have a negative effect on the testis impairing spermatogenesis and, secondarily, affecting concentrations of reproductive hormones (Supplementary Fig. S2A). In an equally plausible alternate causal structure (Supplementary Fig. 2B), men with higher circulating testosterone concentrations are more likely to engage in risk-seeking behaviours (Campbell et al., 2010), including marijuana and cocaine use, and testosterone is positively related to sperm count to the extent that testosterone reflects the normal gonadotropic activity to maintain intra-testicular testosterone concentrations and sustain spermatogenesis (Walker, 2011). These two causal structures are difficult to differentiate with only the available data. If anything, the lack of substantial change in estimates of the relation between marijuana and semen quality after adjusting for testosterone concentrations in addition to the opposite relations of cocaine and marijuana in our data argue more strongly for the first causal structure (Supplementary Fig. S2A). In the absence of randomised trials of marijuana use, new studies with detailed information on within-person changes in marijuana use over time will be necessary to identify the correct causal structure.
Our results are also consistent with a true biological association whereby the effect of marijuana smoking on testicular function, both in terms of spermatogenesis and hormone production, is dose dependent and non-linear. Specifically, and similar to the relation between alcohol intake and cardiovascular disease risk (Chiuve et al., 2006), we hypothesise that moderate use of marijuana may be related to improved testicular function but this relation reverses at higher doses, resulting in adverse effects (Supplementary Fig. 2C). This possible scenario is consistent not only with our results but also with past data in humans and experimental models. If this hypothesis is correct, the apparent discrepancy in the association between marijuana use and sperm counts between this study and the report among young Danish men (Gundersen et al., 2015) could be explained by the differences in intensity of marijuana use between populations. Gundersen et al. (2015) reported that among 1215 healthy young men, men in the highest frequency of marijuana use had a 28% (95% CI: −48, −1) lower sperm concentration than non-users. Similar deleterious effects at high levels of exposure have been documented by others studying men with a history of drug abuse (Hembree et al., 1978; Issidorides, 1978; Singer et al., 1986; el-Gothamy and el-Samahy, 1992; Vescovi et al., 1992), although a positive correlation between marijuana use and percentage of motile sperm has also been reported (Close et al., 1990). Similarly, animal models have shown disruption of spermatogenesis associated with marijuana exposure (du Plessis et al., 2015; Alagbonsi et al., 2016; Di Giacomo et al., 2016). However, cannabinoid receptor 1 (CB1) knockout mice have a reproductive phenotype that strongly suggests an important effect of endocannabinoids, and potentially exogenous cannabinoids, on testicular function including decreased testicular production of testosterone, low numbers of Leydig cells in adulthood and abnormal spermatogenesis (Cacciola et al., 2008, 2013). CB1 receptors are found in the testis, vas deferens, and human sperm cells, and anterior pituitary, and activation of CB1 in spermatozoa by THC is different at low doses (hyper-activation) and high doses (inactivation) (Rossato et al., 2005). Clearly, additional research is needed to evaluate whether the effects of marijuana smoking on testicular function are dose dependent as suggested.
The most important limitation of the study is the possibility of underreporting of marijuana use given its status as an illegal drug during most of the study, its social stigma and potential effects on insurance coverage for infertility services of disclosing this information. In addition, we did not have information about other forms of marijuana use other than marijuana smoking. However, it has been shown that the self-report of marijuana was highly correlated with the blood and urinary cannabinoids levels (Fried, 1980; Greenland et al., 1982). Also, our results may not generalisable to men in the general population because men in the current study were enroled from a fertility centre. Strengths of our study include its prospective design with multiple semen samples in a large proportion of men and our ability to adjust for a wide range of potential confounders. We had data for many reproductive outcomes including semen parameters, sperm DNA integrity and serum reproductive hormones, which allow for more comprehensive assessments of testicular function.
In conclusion, marijuana smokers had higher sperm concentration and sperm count, lower prevalence of sperm parameters below the WHO reference values, and lower FSH concentrations than never marijuana smokers. These findings are not consistent with a deleterious role of marijuana smoking on testicular function as initially hypothesised. The findings are equally also consistent, however, with a non-causal interpretation. Whether these findings are reflective of the previously described role of cannabinoids in spermatogenesis and dose-dependent effects of the activation of endocannabinoid receptors on testicular function or are, instead, reflective of a spurious association, requires further work.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the contributions of all members of the EARTH study team, specifically research nurse Jennifer B. Ford, Myra G. Keller, senior research staff Ramace Dadd and Patricia Morey, and the physicians and staff at the Massachusetts General Hospital Fertility Center. A special thank you is due to all of the study participants.
Contributor Information
EARTH Study Team:
Authors’ roles
All the authors of this manuscript have made substantial contributions to the conception or design of the work or the acquisition, analysis or interpretation of data for the work and have contributed to drafting the work or revising it critically for important intellectual content. All authors have approved the final version to be published and have agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
The project was financed by grants R01ES009718 and P30ES000002 from the National Institute of Environmental Health Sciences (NIEHS).
Conflict of interest
None of the authors has any conflicts of interest to declare.
References
- Alagbonsi IA, Olayaki LA, Salman TM. Melatonin and vitamin C exacerbate Cannabis sativa-induced testicular damage when administered separately but ameliorate it when combined in rats. J Basic Clin Physiol Pharmacol 2016;27:277–287. [DOI] [PubMed] [Google Scholar]
- Azofeifa A, Mattson ME, Schauer G, McAfee T, Grant A, Lyerla R National Estimates of Marijuana Use and Related Indicators—National Survey on Drug Use and Health, United States, 2002–2014. MMWR Surveill Summ 2016. 2016. Morbidity and Mortality Weekly Report (MMWR), pp. 65(No. SS-11)61–25. [DOI] [PubMed]
- Cacciola G, Chioccarelli T, Fasano S, Pierantoni R, Cobellis G. Estrogens and spermiogenesis: new insights from Type 1 cannabinoid receptor knockout mice. Int J Endocrinol 2013;2013:501350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciola G, Chioccarelli T, Mackie K, Meccariello R, Ledent C, Fasano S, Pierantoni R, Cobellis G. Expression of type-1 cannabinoid receptor during rat postnatal testicular development: possible involvement in adult leydig cell differentiation. Biol Reprod 2008;79:758–765. [DOI] [PubMed] [Google Scholar]
- Campbell BC, Dreber A, Apicella CL, Eisenberg DT, Gray PB, Little AC, Garcia JR, Zamore RS, Lum JK. Testosterone exposure, dopaminergic reward, and sensation-seeking in young men. Physiol Behav 2010;99:451–456. [DOI] [PubMed] [Google Scholar]
- Chiuve SE, McCullough ML, Sacks FM, Rimm EB. Healthy lifestyle factors in the primary prevention of coronary heart disease among men: benefits among users and nonusers of lipid-lowering and antihypertensive medications. Circulation 2006;114:160–167. [DOI] [PubMed] [Google Scholar]
- Close CE, Roberts PL, Berger RE. Cigarettes, alcohol and marijuana are related to pyospermia in infertile men. J Urol 1990;144:900–903. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D. Who’s stressed? Distributions of psychological stress in the United States in probability samples from 1983, 2006, and 2009. J Appl Soc Psychol 2012;42:1320–1334. [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385–396. [PubMed] [Google Scholar]
- Di Giacomo D, De Domenico E, Sette C, Geremia R, Grimaldi P. Type 2 cannabinoid receptor contributes to the physiological regulation of spermatogenesis. FASEB J 2016;30:1453–1463. [DOI] [PubMed] [Google Scholar]
- du Plessis SS, Agarwal A, Syriac A. Marijuana, phytocannabinoids, the endocannabinoid system, and male fertility. J Assist Reprod Genet 2015;32:1575–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Gothamy Z, el-Samahy M. Ultrastructure sperm defects in addicts. Fertil Steril 1992;57:699–702. [DOI] [PubMed] [Google Scholar]
- Fried PA. Marihuana use by pregnant women: neurobehavioral effects in neonates. Drug Alcohol Depend 1980;6:415–424. [DOI] [PubMed] [Google Scholar]
- Geiger A. About six-in-ten Americans support marijuana legalization. 2018. Pew Research Center, http://www.pewresearch.org/fact-tank/2018/01/05/americans-support-marijuana-legalization/.
- Greenland S, Staisch KJ, Brown N, Gross SJ. The effects of marijuana use during pregnancy. I. A preliminary epidemiologic study. Am J Obstet Gynecol 1982;143:408–413. [DOI] [PubMed] [Google Scholar]
- Grimaldi P, Di Giacomo D, Geremia R. The endocannabinoid system and spermatogenesis. Front Endocrinol 2013;4:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi P, Orlando P, Di Siena S, Lolicato F, Petrosino S, Bisogno T, Geremia R, De Petrocellis L, Di Marzo V. The endocannabinoid system and pivotal role of the CB2 receptor in mouse spermatogenesis. Proc Natl Acad Sci U S A 2009;106:11131–11136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen TD, Jorgensen N, Andersson AM, Bang AK, Nordkap L, Skakkebaek NE, Priskorn L, Juul A, Jensen TK. Association between use of marijuana and male reproductive hormones and semen quality: a study among 1,215 healthy young men. Am J Epidemiol 2015;182:473–481. [DOI] [PubMed] [Google Scholar]
- Hauser R, Godfrey-Bailey L, Chen Z. Does the potential for selection bias in semen quality studies depend on study design? Experience from a study conducted within an infertility clinic. Hum Reprod 2005;20:2579–2583. [DOI] [PubMed] [Google Scholar]
- Hembree WC 3rd, Nahas GG, Zeidenberg P, Huang HF. Changes in human spermatozoa associated with high dose marihuana smoking. Adv Biosci 1978;22–23:429–439. [DOI] [PubMed] [Google Scholar]
- Issidorides MR. Observations in chronic hashish users: nuclear aberrations in blood and sperm and abnormal acrosomes in spermatozoa. Adv Biosci 1978;23:377–388. [DOI] [PubMed] [Google Scholar]
- Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Oehninger S. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril 1988;49:112–117. [DOI] [PubMed] [Google Scholar]
- McAuliffe ME, Williams PL, Korrick SA, Dadd R, Marchetti F, Martenies SE, Perry MJ. Human sperm sex chromosome disomy and sperm DNA damage assessed by the neutral comet assay. Hum Reprod (Oxford, England) 2014;29:2148–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Yang T, Ye X, Calafat AM, Hauser R. Urinary concentrations of parabens and serum hormone levels, semen quality parameters, and sperm DNA damage. Environ Health Perspect 2011;119:252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerlian C, Williams PL, Ford JB, Chavarro JE, Minguez-Alarcon L, Dadd R, Braun JM, Gaskins AJ, Meeker JD, James-Todd T et al. The Environment and Reproductive Health (EARTH) Study: a prospective preconception cohort. Hum Reprod Open 2018;2018:hoy001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassan FL, Chavarro JE, Minguez-Alarcon L, Williams PL, Tanrikut C, Ford JB, Dadd R, Perry MJ, Hauser R, Gaskins AJ. Residential distance to major roadways and semen quality, sperm DNA integrity, chromosomal disomy, and serum reproductive hormones among men attending a fertility clinic. Int J Hyg Environ Health 2018;221:830–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassan FL, Coull BA, Skakkebaek NE, Williams MA, Dadd R, Minguez-Alarcon L, Krawetz SA, Hait EJ, Korzenik JR, Moss AC et al. A crossover-crossback prospective study of dibutyl-phthalate exposure from mesalamine medications and semen quality in men with inflammatory bowel disease. Environ Int 2016;95:120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossato M, Ion Popa F, Ferigo M, Clari G, Foresta C. Human sperm express cannabinoid receptor Cb1, the activation of which inhibits motility, acrosome reaction, and mitochondrial function. J Clin Endocrinol Metab 2005;90:984–991. [DOI] [PubMed] [Google Scholar]
- Scott JC, Slomiak ST, Jones JD, Rosen AFG, Moore TM, Gur RC. Association of cannabis with cognitive functioning in adolescents and young adults: a systematic review and meta-analysis. JAMA Psychiatry 2018;75:585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle SR, Speed FM, Milliken GA. Population marginal means in the linear model: an alternative to least squares means. Am Stat 1980;34:216–221. [Google Scholar]
- Singer R, Ben-Bassat M, Malik Z, Sagiv M, Ravid A, Shohat B, Livni E, Mamon T, Segenreich E. Servadio C. Oligozoospermia, asthenozoospermia, and sperm abnormalities in ex-addict to heroin, morphine, and hashish. Arch Androl 1986;16:167–174. [DOI] [PubMed] [Google Scholar]
- UNODC World Drug Report 2017 – United Nations Office on Drugs and Crime. 2017.
- VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the e-value. Ann Int Med 2017;167:268–274. [DOI] [PubMed] [Google Scholar]
- Vescovi PP, Pedrazzoni M, Michelini M, Maninetti L, Bernardelli F, Passeri M. Chronic effects of marihuana smoking on luteinizing hormone, follicle-stimulating hormone and prolactin levels in human males. Drug Alcohol Depend 1992;30:59–63. [DOI] [PubMed] [Google Scholar]
- Walker WH. Testosterone signaling and the regulation of spermatogenesis. Spermatogenesis 2011;1:116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO WHO laboratory manual for the examination and processing of human semen In: World Health Organization Department of Reproductive Health and Research, 5th edn Geneva: Switzerland, 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.