Abstract
Background
Native aortic and mitral valve infective endocarditis (AVE and MVE, respectively) are usually grouped together as left-sided native valve infective endocarditis (LNVE), while the differences between AVE and MVE have not yet been properly investigated. We aimed to compare AVE and MVE in regard to patient characteristics, microbiology and determinants of survival.
Methods
We conducted a retrospective study using the Swedish national registry on infective endocarditis, which contains nationwide patient data. The study period was 2007‒2017, and included cases were patients who had either AVE or MVE.
Results
We included 649 AVE and 744 MVE episodes. Staphylococcus aureus was more often the causative pathogen in MVE (41% vs 31%, p<0.001), whereas enterococci were more often the causative pathogen in AVE (14% vs 7.4%, p<0.001). Perivalvular involvement occurred more frequently in AVE (8.5% vs 3.5%, p<0.001) and brain emboli more frequently in MVE (21% vs 13%, p<0.001). Surgery for IE was performed more often (35% vs 27%, p<0.001) and sooner after diagnosis (6.5 days vs 9 days, p=0.012) in AVE than in MVE. Several risk predictors differed between the two groups.
Conclusions
The microbiology seems to differ between AVE and MVE. The causative pathogen was not associated with mortality in AVE. The between-group differences regarding clinical presentation and predictors of survival indicate that it may be important to differentiate AVE from MVE in the treatment of LNVE.
Keywords: endocarditis, left-sided native valve infective endocarditis, surgery, outcome, microbiology
Key messages.
What is already known about this subject?
Native valve infective endocarditis of the aortic valve (AVE) and mitral valve (MVE) are usually studied as one entity, although the valves differ in anatomy and pressure conditions.
AVE is known to be associated with higher rates of invasiveness, while MVE is associated with higher rates of brain emboli.
What does this study add?
In this nationwide registry study, important differences between AVE and MVE were reported. Staphylococcus aureus was more common in MVE, whereas enterococci more common in AVE. S. aureus and enterococci were independent predictors of short-term mortality in MVE, whereas in AVE the causative pathogen did not predict outcome. S. aureus AVE was associated with a decrease in surgical intervention, and the short-term outcome of operated and non-operated S. aureus AVE patients was similar.
How might this impact on clinical practice?
The between-group differences regarding clinical presentation and predictors of survival suggest that a more individualised treatment approach may be needed depending on which valve is affected.
In AVE, the causative pathogen itself should not influence the decision whether or not to operate a patient.
Introduction
Infective endocarditis (IE) is a severe infection of the heart, with a reported incidence of 3‒10 cases per 100 000 person-years.1 2 Despite advances in diagnostics and therapeutic management, in-hospital mortality (10%‒20%)3–5 and 1-year mortality (>30%)3 6 remain high.
IE is traditionally categorised either according to the site of infection into right-sided or left-sided IE; to the underlying structure into native or prosthetic valve IE or to the causative pathogen.7 Thus, native valve IE of the aortic valve (AVE) and mitral valve (MVE) are usually grouped into one entity: left-sided native valve infective endocarditis (LNVE). However, there are important differences in anatomy, physiology and ageing of the two valves: (1) the mitral valve is composed of two leaflets anchored to the left ventricular wall through chordae tendineae, while the aortic valve is composed of three cusps that are anchored to the walls of the aortic root; (2) the aortic valve is constantly subject to high pressure, while the atrial side of the mitral valve is never exposed to high pressure and (3) the most frequent disease affecting the aortic valve is aortic stenosis due to calcification, while mitral insufficiency due to leaflet prolapse is the most common pathology of the mitral valve.8 9
To better understand determinants of outcome, and to give a more precise understanding of native valve IE, patients with AVE and MVE should be studied separately. Previous studies on AVE and MVE have dealt with heterogeneous patient groups,10 11 including those with prosthetic valve IE and involvement of multiple valves. The aim of this study was to compare patient characteristics, microbiology, outcomes and predictors of mortality and surgery in patients with native valve IE involving either the aortic valve or the mitral valve.
Methods
Study design and population
We conducted a retrospective cohort study using the Swedish national registry on IE (SRIE), which is estimated to cover approximately 75% of all in-hospital treated IE episodes in Sweden.12 The registry contains a wide set of variables on patient characteristics, clinical presentation and treatment strategies. We looked at the time period from 2007 to 2017, which encompassed 4151 episodes of possible or definite IE, corresponding to an estimate of 5.5 cases/100,000 residents/year. Episodes from 2007 had been reported using a standardised questionnaire by regular post, while episodes from the period 2008‒2017 had been submitted using an internet-based form with a more detailed description of the episodes. Our study protocol was approved by the Regional Ethical Review Board of Lund.
Patient selection
Patient selection was done by consecutively using the following exclusion criteria: possible IE or missing information on the modified Duke criteria (n=1123); prosthetic valve endocarditis (n=354); right-sided native valve infective endocarditis (n=292); IE affecting both sides of the heart (n=158); implantable cardioverter defibrillator, permanent pacemaker or pacemaker endocarditis (n=309); unknown location or no valves affected (n=106); history of cardiac surgery with synthetic or non-autologous biological material implanted (n=235); duplicate of episode entry, not a resident of Sweden, or patient identification errors (n=56) and double valve IE (n=125). Thus, a total of 1393 IE episodes comprised the study material.
Follow-up and study variables
Follow-up was defined as the period between the start of antimicrobial treatment for IE and the latest status of the patient and was carried out in July 2017 using the Swedish National Population Registry. Follow-up was complete in all cases and comprised 4563 patient-years, with a median follow-up of 2.8 years (IQR 1‒5.2 years). Patient groups were compared regarding clinical characteristics, microbiology, short-term outcome and predictors of mortality and surgery.
Statistical analysis
Descriptive statistics are presented as median with IQR in parentheses for continuous variables and as number of cases with percentage in parentheses for dichotomous variables. Continuous variables were regarded as non-normally distributed and were compared between the AVE and MVE group using the Mann Whitney U test. Categorical variables were analysed using the χ² test. The cumulative probability of survival was calculated and plotted using the Kaplan-Meier estimator. The log-rank test was used to compare the between-group difference in survival rate. Diabetes and end-stage renal disease (ESRD) from 2007 to 2012 were not negated and the variables were only filled if the outcome was positive. Cells with missing values were regarded as negative. From 2012 onwards, remaining missing values for diabetes and ESRD were eligible for multiple imputation. Variables were examined for the patterns of absence and 10% of all cases had at least one missing value. Variables with the highest proportions of missing values were diabetes, ESRD, and the length of time from onset of symptoms to diagnosis. All the variables that were assessed for missing values were subsequently entered into the multiple imputation model. SPSS found no monotonicity and opted by default for the “Fully Conditional Specification” imputation method. Missing values were imputed into five variables through five imputations with 10 iterations for each imputation. Linear regression was used to impute continuous variables and logistic regression was used to impute categorical variables. The risk-adjusted survival was assessed with Cox proportional hazards regression model, where we included known risk factors and also covariates that the authors deemed important. Univariable and multivariable logistic regression analyses of in-hospital mortality and surgery for IE were conducted in the same manner as Cox regression with respect to inclusion of the variables. Log-minus-log survival plots were used to assess the proportional hazards assumption graphically for each categorical variable, and no important violation was found. Pearson correlation was performed to identify possible interactions between variables. All statistical analyses were conducted using the statistical software package SPSS (V.24.0; IBM, Amonk, New York, USA).
Results
Patient characteristics
Baseline characteristics are listed in table 1. One-third of the patients (n=458) were female; they comprised 26% (n=171) of all AVE cases and 39% (n=287) of all MVE cases (p<0.001). Brain emboli were more common in MVE (p<0.001), whereas perivalvular involvement occurred more often in AVE (p<0.001). We did not find any significant between-group differences regarding patient age, prevalence of diabetes, previous episode of IE, injection drug use (IDU), mode of acquisition or heart failure.
Table 1.
Patient characteristics
| Variable | N | AVE n=649 |
MVE n=744 |
P value |
| Female sex | 1393 | 171 (26%) | 287 (39%) | <0.001 |
| Age | 1393 | 66 (56‒77) | 68 (57‒79) | 0.090 |
| Diabetes* | 1338 | 117 (19%) | 118 (17%) | 0.26 |
| ESRD† | 1335 | 20 (3.2%) | 35 (4.9%) | 0.13 |
| Pre-existing valve disease | 1393 | |||
| Bicuspid aortic valve | 75 (12%) | 5 (0.7%) | <0.001 | |
| Mitral valve prolapse | 0 (0%) | 105 (14%) | <0.001 | |
| Rheumatic heart disease | 4 (0.6%) | 6 (0.8%) | 0.68 | |
| Previous IE | 1393 | 26 (4.0%) | 29 (3.9%) | 0.92 |
| IDU | 1393 | 40 (6.2%) | 51 (6.9%) | 0.60 |
| Time from symptoms to diagnosis | 1393 | 11 (4‒28) | 9 (4‒22) | 0.048 |
| TEE | 1393 | 554 (85%) | 621 (84%) | 0.33 |
| Healthcare-associated | 1393 | 80 (12%) | 98 (13%) | 0.64 |
| Community-acquired | 1393 | 557 (86%) | 636 (86%) | 0.86 |
| Heart failure‡ | 1393 | 190 (29%) | 199 (27%) | 0.29 |
| Emboli | 1393 | |||
| Brain | 84 (13%) | 154 (21%) | <0.001 | |
| Meninges | 6 (0.9%) | 13 (1.7%) | 0.19 | |
| Vertebral column | 66 (10%) | 57 (7.7%) | 0.10 | |
| Other skeletal parts | 48 (7.4%) | 54 (7.3%) | 0.92 | |
| Skin | 39 (6.0%) | 57 (7.7%) | 0.23 | |
| Coronary arteries | 3 (0.5%) | 6 (0.8%) | 0.42 | |
| Spleen | 1393 | 22 (3.4%) | 22 (3.0%) | 0.65 |
| Liver | 1393 | 7 (1.1%) | 5 (0.7%) | 0.41 |
| Vegetation§ | 1393 | 559 (86%) | 671 (90%) | 0.019 |
| Perivalvular involvement¶ | 1393 | 55 (8.5%) | 26 (3.5%) | <0.001 |
N represents the total number of responses per variable.
Dichotomous variables are expressed as number of cases with percentage in parentheses. Continuous variables are expressed as median with IQR in parentheses.
*Includes diabetes mellitus types 1 and 2. There were 27/649 and 28/744 missing responses for diabetes in the AVE and MVE group, respectively.
†There were 31/649 and 27/744 missing responses for ESRD in the AVE and MVE group, respectively.
‡Before or during treatment of IE.
§Any size visualised.
¶This includes any periannular spread beyond the confinements of the valve.
AVE, aortic valve infective endocarditis; ESRD, end-stage renal disease;IDU, injection drug use;IE, infective endocarditis; MVE, mitral valve infective endocarditis; TEE, transoesophageal echocardiography.
Microbiological aetiology of IE
A causative pathogen was identified in 99% of the cases. The microbiological findings are summarised in table 2 and figure 1. Staphylococcus aureus was the single most common causative pathogen, with a higher prevalence in MVE than in AVE (p<0.001). In contrast, coagulase-negative staphylococci and enterococci were more common in AVE than in MVE (p=0.010 and p<0.001, respectively).
Table 2.
Microbiological aetiology of IE
| Pathogen | AVE n=649 |
MVE n=744 |
P value |
| Staphylococcus aureus* | 202 (31%) | 302 (41%) | <0.001 |
| Coagulase-negative staphylococci | 30 (4.6%) | 16 (2.2%) | 0.010 |
| Alpha-haemolytic streptococci† | 212 (33%) | 239 (32%) | 0.83 |
| Beta-haemolytic streptococci | 30 (4.6%) | 54 (7.3%) | 0.039 |
| Streptococcus pneumoniae | 11 (1.7%) | 12 (1.6%) | 0.91 |
| Streptococcus bovis | 17 (2.6%) | 14 (1.9%) | 0.35 |
| Enterococci | 89 (14%) | 55 (7.4%) | <0.001 |
| HACEK | 11 (1.7%) | 12 (1.6%) | 0.91 |
| Other gram-positive bacteria | 28 (4.3%) | 30 (4.0%) | 0.79 |
| Other gram-negative bacteria | 5 (0.80%) | 2 (0.3%) | 0.19 |
| Fungi | 3 (0.50%) | 3 (0.4%) | 0.87 |
| Pathogen unknown | 11 (1.7%) | 5 (0.70%) | 0.074 |
*Methicillin-sensitive (n=495), methicillin-resistant (AVE, n=4; MVE, n=5).
†Includes digestive streptococci (AVE, n=7; MVE, n=13), oral streptococci (AVE, n=121; MVE, n=117) and unclassified alpha-haemolytic streptococci (AVE, n=84; MVE, n=109).
AVE, aortic valve infective endocarditis; HACEK, Haemophilus spp., Aggregatibacter actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens and Kingella kingae; IE, infective endocarditis; MVE, mitral valve infective endocarditis.
Figure 1.
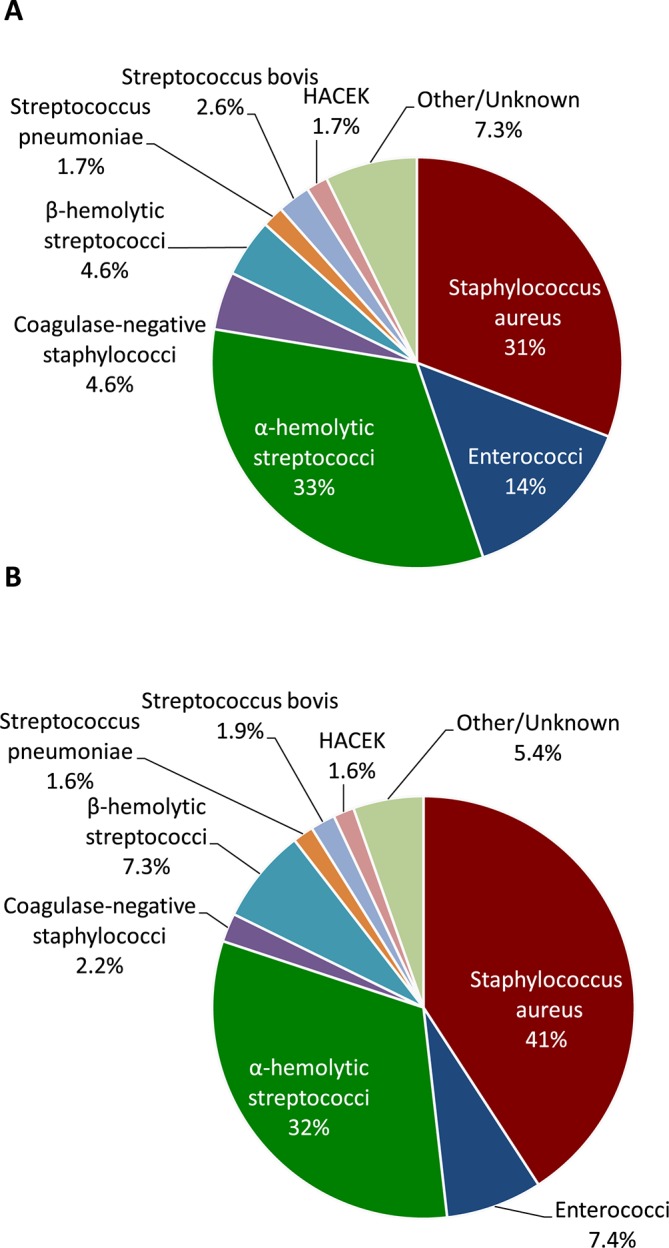
Microbiology: native aortic valve IE (A); native mitral valve IE (B). HACEK, Haemophilus spp., Aggregatibacter actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens and Kingella kingae; IE, infective endocarditis.
Antimicrobial treatment of IE
The median time of antimicrobial treatment was 30 days in both groups, in patients who completed intravenous treatment (p=0.44). An aminoglycoside was administered in 61% (n=395) of the AVE cases and in 58% (n=431) of the MVE cases (p=0.27).
Short-term outcomes and predictors
Short-term outcomes are presented in table 3. The overall in-hospital mortality was 11%. Surgery was performed in 35% (n=224) of the AVE cases and in 27% (n=201) of the MVE cases (p=0.002). There were no significant between-group differences in the length of hospitalisation and in mortality rates. A posthoc analysis of S. aureus AVE showed no significant difference in in-hospital mortality between the operated and the conservatively treated AVE subgroup (8.7% vs 12.8%, respectively, p=0.45). Predictors of in-hospital mortality are presented in table 4. Increasing age per 1 year increment, brain emboli and heart failure were associated with an increase in in-hospital mortality in both AVE and MVE. In addition, female sex, S. aureus infection and enterococcal infection were also associated with an increase in in-hospital mortality, but only in MVE. Among the studied variables, surgery for AVE was the only factor significantly associated with a decrease in in-hospital mortality (p<0.001).
Table 3.
Short-term outcome
| AVE n=649 |
MVE n=744 |
P value | |
| Surgery for IE | 224 (35%) | 201 (27%) | 0.002 |
| Length of hospitalisation | 32 (25‒42) | 32 (23‒42) | 0.77 |
| 30-day mortality | 57 (8.8%) | 66 (8.9%) | 0.95 |
| 90-day mortality | 86 (13%) | 105 (14%) | 0.64 |
| In-hospital mortality | 64 (9.9%) | 90 (12%) | 0.18 |
Dichotomous variables are expressed as number of cases with percentage in parentheses. Continuous variables are expressed as median with IQR in parentheses. Time is expressed in days.
AVE, aortic valve infective endocarditis; IE, infective endocarditis; MVE, mitral valve infective endocarditis.
Table 4.
Multivariable logistic regression of in-hospital mortality
| Predictor | AVE | MVE | ||||
| OR | 95% CI | P value | OR | 95% CI | P value | |
| ESRD | 3.6 | 0.95 to 13 | 0.060 | 2.2 | 0.81 to 5.9 | 0.12 |
| Female sex | 1.2 | 0.65 to 2.2 | 0.55 | 2.2 | 1.3 to 3.6 | 0.002 |
| Age* | 1.04 | 1.01 to 1.06 | 0.003 | 1.04 | 1.02 to 1.07 | <0.001 |
| Diabetes† | 0.91 | 0.44 to 1.9 | 0.81 | 0.74 | 0.38 to 1.5 | 0.38 |
| IDU | 1.2 | 0.25 to 5.4 | 0.84 | 1.3 | 0.33 to 5.0 | 0.72 |
| Healthcare-associated | 1.2 | 0.54 to 2.6 | 0.66 | 1.4 | 0.69 to 2.6 | 0.38 |
| Pathogen | ||||||
| Alpha-haemolytic streptococci | (ref) | (ref) | ||||
| Staphylococcus aureus | 0.67 | 0.37 to 1.6 | 0.47 | 3.2 | 1.5 to 6.6 | 0.002 |
| Enterococci | 1.1 | 0.44 to 2.8 | 0.84 | 3.5 | 1.3 to 9.7 | 0.015 |
| Other | 0.86 | 0.36 to 2.0 | 0.73 | 1.6 | 0.64 to 3.8 | 0.33 |
| Surgery for IE | 0.16 | 0.061 to 0.40 | <0.001 | 0.75 | 0.39 to 1.4 | 0.38 |
| Brain emboli | 3.4 | 1.6 to 7.2 | 0.001 | 2.3 | 1.3 to 4.0 | 0.004 |
| Heart failure‡ | 4.8 | 2.7 to 8.8 | <0.001 | 1.9 | 1.2 to 3.2 | 0.012 |
| Vegetation§ | 1.2 | 0.47 to 3.2 | 0.69 | 0.75 | 0.32 to 1.7 | 0.50 |
| Perivalvular involvement¶ | 2.9 | 0.93 to 9.0 | 0.067 | 0.74 | 0.22 to 2.5 | 0.63 |
*Increasing age per 1 year increment.
†Includes diabetes mellitus types 1 and 2.
‡Before or during treatment of IE.
§Any size visualised.
¶This includes any periannular spread beyond the confinements of the valve.
AVE, aortic valve infective endocarditis; ESRD, end-stage renal disease;IDU, injection drug use; IE, infective endocarditis; MVE, mitral valve infective endocarditis.
Long-term survival and predictors of long-term mortality
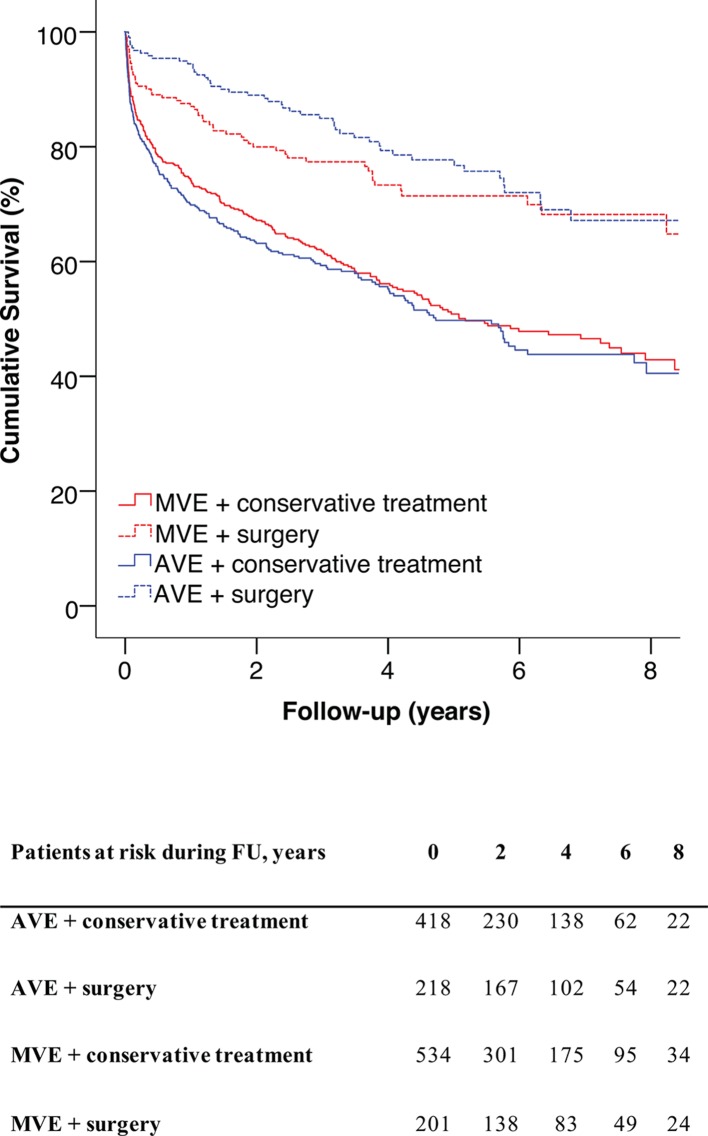
The 1-year, 5-year and 10-year survival rates were 78% (95% CI 75 to 81), 59% (95% CI 55 to 63) and 50% (95% CI 44 to 55), respectively, in AVE and 78% (95% CI 75 to 81), 56% (95% CI 52 to 60) and 46% (95% CI 40 to 52), respectively, in MVE. There were no significant differences in survival rates between AVE and MVE (log-rank, p=0.56). Long-term survival was better in operated patients than in patients who only received conservative treatment (figure 2, p<0.001). Predictors of long-term mortality are given in table 5. Several factors were associated with increased long-term mortality, and these varied somewhat between AVE and MVE. Surgery for IE was associated with reduced long-term mortality in both groups.
Figure 2.
Survival of operated versus non-operated patients. AVE, aortic valve infective endocarditis; MVE, mitral valve infective endocarditis.
Table 5.
Multivariable Cox regression for predictors of long-term mortality
| Predictor | AVE | MVE | ||||
| HR | 95% CI | P value | HR | 95% CI | P value | |
| ESRD | 4.2 | 2.4 to 7.5 | <0.001 | 1.9 | 1.1 to 3.3 | 0.025 |
| Female sex | 0.98 | 0.74 to 1.3 | 0.90 | 1.3 | 1.0 to 1.6 | 0.057 |
| Age* | 1.05 | 1.04 to 1.07 | <0.001 | 1.05 | 1.04 to 1.07 | <0.001 |
| Diabetes† | 0.95 | 0.69 to 1.3 | 0.76 | 1.4 | 1.0 to 1.8 | 0.050 |
| IDU | 3.6 | 2.0 to 6.4 | <0.001 | 2.6 | 1.4 to 4.8 | 0.004 |
| Healthcare-associated | 1.6 | 1.2‒2.3 | 0.004 | 1.4 | 1.0 to 2.0 | 0.033 |
| Pathogen | ||||||
| Alpha-haemolytic streptococci | (ref) | (ref) | ||||
| Staphylococcus aureus | 1.1 | 0.78 to 1.6 | 0.59 | 2.2 | 1.6 to 3.1 | <0.001 |
| Enterococci | 1.3 | 0.86 to 2.0 | 0.22 | 1.9 | 1.2 to 3.0 | 0.01 |
| Other | 1.1 | 0.73 to 1.6 | 0.70 | 1.7 | 1.1 to 2.4 | 0.007 |
| Surgery for IE | 0.37 | 0.25 to 0.54 | <0.001 | 0.61 | 0.43 to 0.86 | 0.004 |
| Brain emboli | 1.5 | 1.0 to 2.1 | 0.041 | 1.3 | 0.97 to 1.7 | 0.083 |
| Heart failure‡ | 2.1 | 1.6 to 2.9 | <0.001 | 1.8 | 1.4 to 2.3 | <0.001 |
| Vegetation§ | 1.1 | 0.75 to 1.7 | 0.54 | 1.2 | 0.79 to 1.9 | 0.36 |
| Perivalvular involvement¶ | 1.4 | 0.84 to 2.5 | 0.19 | 1.0 | 0.58 to 1.8 | 0.92 |
*Increasing age per 1-year increment.
†Includes diabetes mellitus types 1 and 2.
‡Before or during treatment of IE.
§Any size visualised.
¶This includes any periannular spread beyond the confinements of the valve.
AVE, aortic valve infective endocarditis; ESRD, end-stage renal disease;IDU, injection drug use; IE, infective endocarditis; MVE, mitral valve infective endocarditis.
Surgery for IE
The predictors of surgery for IE (table 6) were similar between the two groups, with exception of valve vegetation and S. aureus. Valve vegetation was associated with an increased likelihood of surgery for IE in MVE, but not in AVE. S. aureus was associated with a decrease in surgical intervention in AVE but not in MVE. Details on surgical cases are presented in table 7. The time delay between symptoms onset and diagnosis was longer in the surgically treated AVE subgroup than in the surgically treated MVE subgroup (p<0.001), whereas the time delay between diagnosis and surgery was shorter in the AVE group than in the MVE group (p=0.012). In-hospital mortality was 3.6% (n=8) and 9.0% (n=18) in patients who underwent surgery for AVE and MVE, respectively (p=0.021). There was no significant between-group difference in long-term survival in the subgroup of patients who were operated (figure 2, p=0.2).
Table 6.
Multivariable logistic regression for operation during treatment
| Predictor | AVE | MVE | ||||
| OR | 95% CI | P value | OR | 95% CI | P value | |
| ESRD | 2.0 | 0.57 to 7.2 | 0.27 | 0.77 | 0.27 to 2.2 | 0.63 |
| Female sex | 0.73 | 0.46 to 1.2 | 0.19 | 1.3 | 0.87 to 1.9 | 0.21 |
| Age* | 0.95 | 0.94 to 0.97 | <0.001 | 0.95 | 0.93 to 0.96 | <0.001 |
| Diabetes† | 0.47 | 0.26 to 0.87 | 0.015 | 0.65 | 0.38 to 1.1 | 0.12 |
| IDU | 0.79 | 0.35 to 1.8 | 0.57 | 0.33 | 0.15 to 0.71 | 0.005 |
| Healthcare-associated | 0.40 | 0.20 to 0.82 | 0.012 | 0.46 | 0.24 to 0.87 | 0.017 |
| Pathogen | ||||||
| Alpha-haemolytic streptococci | ref | ref | ||||
| Staphylococcus aureus | 0.56 | 0.32 to 0.96 | 0.036 | 1.1 | 0.69 to 1.8 | 0.66 |
| Enterococci | 1.7 | 0.88 to 3.1 | 0.12 | 1.9 | 0.93 to 4.0 | 0.076 |
| Other | 2.2 | 1.3 to 3.7 | 0.002 | 1.7 | 0.99 to 2.8 | 0.056 |
| Brain emboli | 2.6 | 1.5 to 4.5 | 0.001 | 1.6 | 1.0 to 2.4 | 0.037 |
| Heart failure‡ | 4.3 | 2.8 to 6.5 | <0.001 | 3.3 | 2.2 to 4.9 | <0.001 |
| Vegetation§ | 1.2 | 0.64 to 2.1 | 0.61 | 4.6 | 2.0 to 10 | <0.001 |
| Perivalvular involvement¶ | 8.8 | 4.0 to 19 | <0.001 | 4.7 | 1.8 to 12 | 0.001 |
*Increasing age per 1 year increment.
†Includes diabetes mellitus types 1 and 2.
‡Before or during treatment of IE.
§Any size visualised.
¶This includes any periannular spread beyond the confinements of the valve.
AVE, aortic valve infective endocarditis; ESRD, end-stage renal disease;IDU, injection drug use; IE, infective endocarditis; MVE, mitral valve infective endocarditis.
Table 7.
Subgroup analysis of patients who underwent surgery
| Variable | AVE n=224 |
MVE n=201 |
P value |
| Time from symptoms to diagnosis | 18 (6.3‒37) | 9 (4‒26) | <0.001 |
| Time from diagnosis to operation | 6.5 (3‒14) | 9 (4‒18) | 0.012 |
| 30-day mortality | 5 (2.2%) | 11 (5.5%) | 0.08 |
| 90-day mortality | 8 (3.6%) | 19 (9.5%) | 0.013 |
| In-hospital mortality | 8 (3.6%) | 18 (9%) | 0.021 |
Dichotomous variables are expressed as number of patients with percentage in parentheses. Continuous variables are expressed as median with IQR in parentheses. Time is expressed in days.
AVE, aortic valve infective endocarditis; MVE, mitral valve infective endocarditis.
Discussion
We identified several between-group differences in the microbiological aetiology of AVE and MVE. Enterococci were found to be the causative pathogen twice as often in AVE than in MVE, which is supported by a previous observation that was partially based on the same registry.13 S. aureus was the single most common causative pathogen and had a higher prevalence in MVE than in AVE. The aortic valve and mitral valve differ in flow, pressure and calcification conditions. Therefore, it does not surprise that pathogens such as S. aureus and enterococci, that display different adherence strategies to host tissues, have apparent different propensities to attach and colonise different valves.14–16
Our findings indicate that S. aureus is an independent predictor of in-hospital and long-term mortality in MVE, whereas S. aureus was not associated with mortality in AVE. This finding was unexpected, since S. aureus has generally been regarded as one of the most aggressive pathogens in IE and a cause of high mortality.17 18 However, S. aureus is also a common cause of right-sided IE, which is rarely invasive. One explanation may be that the high-pressure environment of the aortic valve plays a more important role in determining whether an infection becomes invasive, rather than the causative pathogen itself. This notion is supported by a very recent report by Hussain et al, which hypothesised that chamber pressure may be the driving force behind invasiveness and tissue destruction in IE.19 Despite the finding that S. aureus was associated with mortality only in MVE, the overall survival rate was not significantly different between the two patient groups, showing that there is a complex interplay between different risk factors.
The male predominance in IE has been well-documented.11 20 Furthermore, we found that the proportion of females was higher in the MVE than in the AVE group, which was also previously reported.11 21 The reasons for these differences in sex distribution in IE are unclear, but deserve further investigation in future studies.
We sought to investigate the between-group differences regarding surgery. AVE was operated on more often, which may have been due in part to the higher frequency of perivalvular involvement in AVE. The time interval between onset of symptoms and diagnosis was twice as long in operated AVE cases compared with operated MVE cases. However, the time delay between diagnosis and surgery was significantly longer in MVE than in AVE. Intuitively, one might expect the patient group with a short interval between onset of symptoms and diagnosis to have a more aggressive course of infection and to require early surgery. In-hospital mortality was higher in surgical MVE cases than in surgical AVE cases, which appears logical since invasive MVE is more difficult to treat surgically than invasive AVE.10 Thus, one reason for the delayed timing of surgery in MVE might be due to logistical reasons, because centres with cardiac surgery typically have only a few surgeons who perform mitral valve surgery on a regular basis. Alternatively, the risk of brain emboli is the highest during the early stage of IE,22 and European guidelines advice to delay cardiac surgery if there is extensive brain damage or intracranial haemorrhage.23 Thus, the higher prevalence of brain emboli in MVE may be another contributing factor to the delayed timing of surgery in MVE. Surgery was associated with a decrease in in-hospital mortality in AVE, whereas it was not associated with in-hospital mortality in MVE. However, interpreting the effect of surgery on outcome is not straightforward. Surgical results are subject to multiple factors, such as timing of surgery,24 25 the intrinsic complexity of the procedure and the surgeons’ experience.10 Surgical cases were not stratified according to their eligibility for surgery, and we were not able to determine in which cases surgery was not performed despite clear indications for surgery. This further complicates the interpretation of our results. This being said, it is possible that earlier surgery in a centre with a high annual volume of surgical MVE cases might be associated with a better in-hospital outcome in MVE.
S. aureus was the only causative pathogen independently associated with surgery and, surprisingly, S. aureus was associated with a decrease in surgical intervention in AVE. This is counterintuitive, since the aggressive nature of S. aureus should prompt surgery in a higher proportion of cases with AVE; however, this finding has been previously reported as well.26 Moreover, radical debridement and reconstruction are more straightforward in AVE than in MVE,10 and our results showed that surgery for AVE was associated with better survival rates. The apparent practice of not invoking surgery in S. aureus AVE is clearly different from recent American guidelines,7 27 which recommend early surgery in patients with left-sided IE caused by S. aureus. European and Swedish guidelines, however, have not specifically pointed out S. aureus infection as an indication for surgery in native valve endocarditis.23 28 Our results have showed no significant difference in in-hospital mortality regarding how S. aureus AVE was treated and S. aureus infection itself did not predict mortality in AVE, thus indicating that conservative treatment of S. aureus AVE may be successful and that S. aureus infection should not necessarily be regarded as an indication for surgery.
Most studies on prognostic factors in IE are susceptible to referral centre bias,29–31 whereas it would be more valid to generalise from our results because of our use of data on IE that was collected on a nationwide basis. We identified several prognostic factors, some of which showed clear differences between groups, while others showed significant overlap in effect size. IDU was the strongest predictor of long-term mortality in both groups, which can be explained by continued IDU after initially successful treatment results, resulting in a higher risk of reinfection.32 The younger age of these patients, and therefore less frailty and comorbidities, may be the reason why IDU was not associated with in-hospital mortality.
Strengths and limitations
This study was conducted using homogenous contemporary patient data from a nationwide registry on IE. Data in the registry originate from both local and tertiary care hospitals, and all of the cases included in our study had definite IE. Although population-based, our study only included cases that had been reported to the SRIE, and we did not perform an active search for IE cases. The SRIE has not undergone formal validation. Our results are mainly generalisable to industrialised countries with similar low incidences of rheumatic heart disease and IDU-related IE. The retrospective design of this study, and the possibility of unknown confounders, may have affected our results.
Conclusion
We found interesting differences regarding microbiology: MVE had a higher proportion of S. aureus, whereas AVE a higher proportion of enterococci. This difference in location predilection between enterococci and S. aureus might be explained by the unique flow, pressure and calcification conditions of the two valves. Our results also suggest that the causative pathogen itself plays a lesser role than pressure conditions around the valves in tissue invasiveness. In AVE, the causative pathogen did not predict outcome and should therefore not influence the decision whether or not to operate a patient. AVE and MVE also differed regarding their clinical presentation. The differences in the prevalence of perivalvular involvement, brain emboli and time delay between onset of symptoms and diagnosis indicate that it is important to consider which valve is affected in patients with LNVE. The authors believe that these findings can serve as a stepping-stone for further research into the different disease processes of AVE and MVE and thus may be an important step towards a more individualised approach to patients with IE.
Acknowledgments
We thank the quality assurance study group of the Swedish Infectious Diseases Society.
Footnotes
Contributors: AVV: planning of study design, conducting statistical analyses, data interpretation, has contributed to all the sections of the manuscript. SR: planning of study design, conducting statistical analyses, data interpretation, has contributed to all the sections of the manuscript, guarantor of the submitted work. MR: advice on statistical analyses, data interpretation, has contributed to multiple sections of the manuscript. JN: advice on statistical analyses, data interpretation and writing of the methods chapter. LO: data interpretation, advice on the use of the registry.
Funding: This work was supported by the Marianne and Marcus Wallenberg Foundation and by the Swedish Heart-Lung Foundation.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data available.
References
- 1. Thuny F, Grisoli D, Collart F, et al. . Management of infective endocarditis: challenges and perspectives. The Lancet 2012;379:965–75. 10.1016/S0140-6736(11)60755-1 [DOI] [PubMed] [Google Scholar]
- 2. Muñoz P, Kestler M, De Alarcon A, et al. . Current epidemiology and outcome of infective endocarditis: a multicenter, prospective, cohort study. Medicine 2015;94:e1816 10.1097/MD.0000000000001816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murdoch DR, Corey GR, Hoen B, et al. . Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International collaboration on Endocarditis-Prospective cohort study. Arch Intern Med 2009;169:463–73. 10.1001/archinternmed.2008.603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fedeli U, Schievano E, Buonfrate D, et al. . Increasing incidence and mortality of infective endocarditis: a population-based study through a record-linkage system. BMC Infect Dis 2011;11 10.1186/1471-2334-11-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoen B, Alla F, Selton-Suty C, et al. . Changing profile of infective endocarditis: results of a 1-year survey in France. JAMA 2002;288:75–81. [DOI] [PubMed] [Google Scholar]
- 6. Cresti A, Chiavarelli M, Scalese M, et al. . Epidemiological and mortality trends in infective endocarditis, a 17-year population-based prospective study. Cardiovasc Diagn Ther 2017;7:27–35. 10.21037/cdt.2016.08.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pettersson GB, Coselli JS, Pettersson GB, et al. . 2016 the American Association for thoracic surgery (AATS) consensus guidelines: surgical treatment of infective endocarditis: Executive summary. J Thorac Cardiovasc Surg 2017;153:e29:1241–58. 10.1016/j.jtcvs.2016.09.093 [DOI] [PubMed] [Google Scholar]
- 8. Freed LA, Levy D, Levine RA, et al. . Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med 1999;341:1–7. 10.1056/NEJM199907013410101 [DOI] [PubMed] [Google Scholar]
- 9. David TE, Armstrong S, McCrindle BW, et al. . Late outcomes of mitral valve repair for mitral regurgitation due to degenerative disease. Circulation 2013;127:1485–92. 10.1161/CIRCULATIONAHA.112.000699 [DOI] [PubMed] [Google Scholar]
- 10. Hussain ST, Shrestha NK, Gordon SM, et al. . Residual patient, anatomic, and surgical obstacles in treating active left-sided infective endocarditis. J Thorac Cardiovasc Surg 2014;148:981–8. 10.1016/j.jtcvs.2014.06.019 [DOI] [PubMed] [Google Scholar]
- 11. Curlier E, Hoen B, Alla F, et al. . Relationships between sex, early valve surgery and mortality in patients with left-sided infective endocarditis analysed in a population-based cohort study. Heart 2014;100:1173–8. 10.1136/heartjnl-2013-304916 [DOI] [PubMed] [Google Scholar]
- 12. Lindell F, Söderquist B, Sundman K, et al. . Prosthetic valve endocarditis caused by Propionibacterium species: a national registry-based study of 51 Swedish cases. Eur J Clin Microbiol Infect Dis 2018;37:765–71. 10.1007/s10096-017-3172-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McDonald JR, Olaison L, Anderson DJ, et al. . Enterococcal endocarditis: 107 cases from the International collaboration on endocarditis merged database. Am J Med 2005;118:759–66. 10.1016/j.amjmed.2005.02.020 [DOI] [PubMed] [Google Scholar]
- 14. Foster TJ, Geoghegan JA, Ganesh VK, et al. . Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol 2014;12:49–62. 10.1038/nrmicro3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. George NPE, Wei Q, Shin PK, et al. . Staphylococcus aureus adhesion via spa, ClfA, and SdrCDE to immobilized platelets demonstrates shear-dependent behavior. Arterioscler Thromb Vasc Biol 2006;26:2394–400. 10.1161/01.ATV.0000237606.90253.94 [DOI] [PubMed] [Google Scholar]
- 16. Nallapareddy SR, Singh KV, Sillanpää J, et al. . Endocarditis and biofilm-associated pili of Enterococcus faecalis. J Clin Invest 2006;116:2799–807. 10.1172/JCI29021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fernández-Hidalgo N, Almirante B, Tornos P, et al. . Immediate and long-term outcome of left-sided infective endocarditis. A 12-year prospective study from a contemporary cohort in a referral hospital. Clin Microbiol Infect 2012;18:E522–E530. 10.1111/1469-0691.12033 [DOI] [PubMed] [Google Scholar]
- 18. Lauridsen TK, Park L, Tong SYC, et al. . Echocardiographic findings predict in-hospital and 1-year mortality in left-sided native valve Staphylococcus aureus endocarditis: analysis from the International collaboration on Endocarditis-Prospective echo cohort study. Circ Cardiovasc Imaging 2015;8:e003397 10.1161/CIRCIMAGING.114.003397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hussain ST, Shrestha NK, Witten J, et al. . Rarity of invasiveness in right-sided infective endocarditis. J Thorac Cardiovasc Surg 2018;155:54–61. 10.1016/j.jtcvs.2017.07.068 [DOI] [PubMed] [Google Scholar]
- 20. Olmos C, Vilacosta I, Fernández-Pérez C, et al. . The Evolving Nature of Infective Endocarditis in Spain: A Population-Based Study (2003 to 2014). J Am Coll Cardiol 2017;70:2795–804. 10.1016/j.jacc.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 21. Sambola A, Fernández-Hidalgo N, Almirante B, et al. . Sex differences in native-valve infective endocarditis in a single tertiary-care hospital. Am J Cardiol 2010;106:92–8. 10.1016/j.amjcard.2010.02.019 [DOI] [PubMed] [Google Scholar]
- 22. Kang D-H. Timing of surgery in infective endocarditis. Heart 2015;101:1786–91. 10.1136/heartjnl-2015-307878 [DOI] [PubMed] [Google Scholar]
- 23. Habib G, Lancellotti P, Antunes MJ, et al. . 2015 ESC guidelines for the management of infective endocarditis: the task Force for the management of infective endocarditis of the European Society of cardiology (ESC). endorsed by: European association for Cardio-Thoracic surgery (EACTS), the European association of nuclear medicine (EANM). Eur Heart J 2015;36:3075–128. 10.1093/eurheartj/ehv319 [DOI] [PubMed] [Google Scholar]
- 24. Kang D-H, Kim Y-J, Kim S-H, et al. . Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012;366:2466–73. 10.1056/NEJMoa1112843 [DOI] [PubMed] [Google Scholar]
- 25. Bannay A, Hoen B, Duval X, et al. . The impact of valve surgery on short- and long-term mortality in left-sided infective endocarditis: do differences in methodological approaches explain previous conflicting results? Eur Heart J 2011;32:2003–15. 10.1093/eurheartj/ehp008 [DOI] [PubMed] [Google Scholar]
- 26. Fowler VG, Miro JM, Hoen B, et al. . Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 2005;293:3012–21. 10.1001/jama.293.24.3012 [DOI] [PubMed] [Google Scholar]
- 27. Nishimura RA, Otto CM, Bonow RO, et al. . 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014;63:e57–185. 10.1016/j.jacc.2014.02.536 [DOI] [PubMed] [Google Scholar]
- 28. Westling K, Aufwerber E, Ekdahl C, et al. . Swedish guidelines for diagnosis and treatment of infective endocarditis. Scand J Infect Dis 2007;39:929–46. 10.1080/00365540701534517 [DOI] [PubMed] [Google Scholar]
- 29. Thuny F, Giorgi R, Habachi R, et al. . Excess mortality and morbidity in patients surviving infective endocarditis. Am Heart J 2012;164:94–101. 10.1016/j.ahj.2012.04.003 [DOI] [PubMed] [Google Scholar]
- 30. Chu VH, Cabell CH, Benjamin DK, et al. . Early predictors of in-hospital death in infective endocarditis. Circulation 2004;109:1745–9. 10.1161/01.CIR.0000124719.61827.7F [DOI] [PubMed] [Google Scholar]
- 31. Hill EE, Herijgers P, Claus P, et al. . Infective endocarditis: changing epidemiology and predictors of 6-month mortality: a prospective cohort study. Eur Heart J 2007;28:196–203. 10.1093/eurheartj/ehl427 [DOI] [PubMed] [Google Scholar]
- 32. Shrestha NK, Jue J, Hussain ST, et al. . Injection drug use and outcomes after surgical intervention for infective endocarditis. Ann Thorac Surg 2015;100:875–82. 10.1016/j.athoracsur.2015.03.019 [DOI] [PubMed] [Google Scholar]