Abstract
Background
Cardiovascular complications of pregnancy present an opportunity to assess risk for subsequent cardiovascular disease. We sought to determine whether peripartum cardiomyopathy and hypertensive disorder of pregnancy subtypes predict future myocardial infarction, heart failure or stroke independent of one another and of other risks such as gestational diabetes, preterm birth and intrauterine growth restriction.
Methods and results
The California Healthcare Cost and Utilization Project database was used to identify all hospitalised pregnancies from 2005 to 2009, with follow-up through 2011, for a retrospective cohort study. Pregnancies, exposures, covariates and outcomes were defined by International Classification of Diseases, Ninth Revision codes. Among 1.6 million pregnancies (mean age 28 years; median follow-up time to event excluding censoring 2.7 years), 558 cases of peripartum cardiomyopathy, 123 603 hypertensive disorders of pregnancy, 107 636 cases of gestational diabetes, 116 768 preterm births and 23 504 cases of intrauterine growth restriction were observed. Using multivariable Cox proportional hazards models, peripartum cardiomyopathy was independently associated with a 39.2-fold increase in heart failure (95% CI 30.0 to 51.9), resulting in ~1 additional hospitalisation per 1000 person-years. There was a 13.0-fold increase in myocardial infarction (95% CI 4.1 to 40.9) and a 7.7-fold increase in stroke (95% CI 2.4 to 24.0). Hypertensive disorders of pregnancy were associated with 1.4-fold (95% CI 1.0 to 2.0) to 7.6-fold (95% CI 5.4 to 10.7) higher risk of myocardial infarction, heart failure and stroke, resulting in a maximum of ~1 additional event per 1000 person-years. Gestational diabetes, preterm birth and intrauterine growth restriction had more modest associations.
Conclusion
These findings support close monitoring of women with cardiovascular pregnancy complications for prevention of early cardiovascular events and study of mechanisms underlying their development.
Keywords: peripartum cardiomyopathy, hypertensive disorders of pregnancy, cardiovascular disease, women
Key questions.
What is already known about this subject?
Peripartum cardiomyopathy and hypertensive disorders of pregnancy increase the risk for future heart failure.
Other conditions are associated with future risk of cardiovascular disease (CVD) but are also interrelated and coexistent, making it difficult to understand the independent effects of each condition.
What does this study add?
Peripartum cardiomyopathy is associated with risks for myocardial infarction (MI) and stroke in addition to recurrent heart failure.
Hypertensive disorders of pregnancy subtypes are associated with risks for MI, stroke and heart failure, and subtypes representing a longer duration of hypertension and a higher severity had the greatest magnitude of risk for MI, stroke and heart failure.
Gestational diabetes, preterm birth and intrauterine growth restriction were independently associated with risks for future CVD when accounting for peripartum cardiomyopathy and hypertensive disorders of pregnancy.
How might this impact on clinical practice?
Our findings support close monitoring of women with cardiovascular pregnancy complications for prevention of subsequent, near-term CVD events.
Introduction
Cardiovascular disease (CVD) is a significant and often underappreciated cause of morbidity and mortality in women.1 Pregnancy can often ‘unmask’ CVD in women.2 3 In the estimated 85% of women who experience pregnancy during their lifetime, cardiovascular complications during pregnancy and childbirth present an opportunity to assess risk for later CVD.4 Indeed, leveraging early phenotypes to identify those at risk for CVD as soon as possible is an aspirational goal for both precision medicine5 6 and for primary CVD disease prevention.7 8
Peripartum cardiomyopathy (PPCM) and hypertensive disorders of pregnancy (HDP) are two major cardiovascular complications of pregnancy that share common risk factors and that have been reported to share a common underlying pathophysiology in some animal studies.9–11 PPCM is characterised by the sudden onset of maternal heart failure (HF) presenting either in the last month of pregnancy or in the first 5 months post partum. Hypertension (HTN) in pregnancy is defined as a blood pressure equal to or greater than 140/90 mm Hg; gestational onset is defined at or after 20 weeks’ gestation (online supplementary table 2).
openhrt-2018-000927supp001.docx (238.6KB, docx)
We hypothesised that specific subtypes of PPCM and HDP may carry different CVD risks.12 13 Recent data from a Danish nationwide register-based cohort demonstrated that specific subtypes of HDP (ie, severe pre-eclampsia, moderate pre-eclampsia and gestational HTN) conferred differing risks of incident cardiomyopathy.14 Still, prior studies have not fully elucidated the independent risks of PPCM and HDP subtypes on specific CVD outcomes (ie, myocardial infarction [MI], HF and stroke). The availability of a large cohort of women directly representative of the general population of California in the California Healthcare Cost and Utilization Project (HCUP) allowed us to determine the risks of PPCM and HDP subtypes along a spectrum of chronicity and severity for subsequent MI, HF and stroke, while independently adjusting for other demographic and pregnancy-related factors that are known to be associated with later maternal CVD (ie, gestational diabetes mellitus [GDM], preterm delivery and intrauterine growth restriction [IUGR]).15–17
Methods
Study sample
We used the HCUP database (https://www.hcup-us.ahrq.gov/), which provides state-specific data for all inpatient, emergency and ambulatory visits. HCUP contains information collected as part of medical billing, including patient demographics, International Classification of Diseases, Ninth Revision (ICD-9) diagnoses, expected payer, dates of admission and discharge, and follow-up. We compared the number of deliveries in HCUP from 2005 to 2009 with the number of deliveries recorded by the California Office of Statewide Health Planning and Development, which records all vital statistics for the state, and found that HCUP represents approximately 92% of all deliveries state-wide. We identified the first delivery from all patients aged 18 and older in HCUP from 2005 to 2009 by ICD-9 code (n=1 686 601). During the 2005–2009 study period, after excluding those with a non-California residence (n=2734), missing information on age (n=2742) or race/ethnicity (n=11 434), data were available in California HCUP for 1 669 691 pregnancies. Among those 1 669 691 remaining pregnancies, we excluded those with pre-existing MI, HF, stroke, and congenital18 or valvular heart disease using ICD-9 codes (n=7646), leaving 1 662 045 eligible pregnancies for this study. Congenital heart disease diagnoses were classified according to methods previously described.18 The number of pregnancies in our study cohort represents 7.8% of all pregnancies in the USA within the same time period.19
Exposures
After pregnancies were identified, we defined instances of PPCM by ICD-9 codes (online supplementary table 1) present by 150 days (5 months) postdelivery, because the clinical definition of PPCM includes the first 5 months post partum. For the purposes of subanalysis, we further divided overall PPCM into diagnoses made prepartum (PPCM diagnoses already present at delivery) and those made post partum (PPCM diagnosis appearing after delivery but within 5 months postdelivery, as above).
HDP subtypes, GDM, preterm birth and IUGR were ascertained by ICD-9 codes (online supplementary table 1) present at delivery. We considered both principal and secondary diagnoses to identify these pregnancy exposures.
We defined HDP as one of the following: chronic HTN, gestational HTN, pre-eclampsia, chronic HTN with gestational HTN, chronic HTN with pre-eclampsia, and no HTN or PPCM (referent) (online supplementary table 2).
Outcomes
Primary CVD outcomes were admissions for MI, HF and stroke defined by ICD-9 codes (online supplementary table 1). MI subtypes were defined as MI with coronary artery disease (MICAD) and MI with non-obstructive coronary arteries (MINOCA). MINOCA includes MI from stress cardiomyopathy, hypercoagulable state, coronary artery dissection, coronary artery anomaly and coronary vasospasm.20 MINOCA was defined using its ICD-9 code as well as (1) having had an MI using ICD-9 codes 410.00–410.92; (2) having had a cardiac catheterisation or CT coronary angiogram (inpatient or outpatient, defined by CPT, or current procedural terminology, codes) within 7 days of index event; and (3) having had an ICD-9 code for a MINOCA subtype. ICD-9 codes used for MINOCA subtypes were 413.1 for coronary vasospasm, 414.12 for coronary artery dissection, 429.83 for Takotsubo/stress cardiomyopathy, 289.81 for hypercoagulable state and 746.85 for coronary anomaly.
Stroke was further subdivided into ischaemic, embolic and haemorrhagic aetiologies. HF was defined as recurrent HF hospitalisation. Among our study cohort, systolic versus diastolic HF and left versus right HF were not well specified, and thus we were unable to distinguish among these HF subtypes. ICD-9 codes defining the outcomes are found in online supplementary table 1.
Covariates
We adjusted for covariates known to be associated with PPCM, HTN, CVD, and peripartum morbidity and mortality21 which could potentially confound the associations we were interested in studying. Covariates included age, race, insurance status, median household income, chronic kidney disease, pre-existing diabetes, obesity, drug abuse, smoking, multiple gestations, as well as each of the exposures. Patients with pre-existing (prior to pregnancy) HTN were considered to have baseline chronic HTN and were included within the HDP variable; therefore, we did not include HTN as a covariate.
Statistical analyses
Primary analyses
We considered our baseline period in which initial delivery occurred from 2005 to 2009. Follow-up began 1 day after exposure criteria were fulfilled (ie, on the 151st day postdelivery for overall PPCM and for postpartum PPCM, and on the first day postdelivery for all other exposures; see the Exposures section) and lasted until the end of 2011. Descriptive statistics were performed including n, mean and percentage with SD, and IQR, as appropriate, among all participants and within PPCM and HDP subtypes. We tested the assumption of proportionality of hazards for PPCM and HDP subtypes and MI, HF and stroke. We found that one of the five HDP subtypes, pre-eclampsia, violated the assumption (p=0.02). Therefore, in the case of pre-eclampsia, our HR estimates represent an average across the length of study follow-up. We employed multivariable Cox proportional hazards models to determine the association between PPCM and HDP and subsequent CVD, adjusting for the potential confounders listed in the Covariates section. We considered GDM, preterm delivery and IUGR as secondary exposures and considered these in multivariable models adjusting for PPCM and HDP.
Secondary analyses
It is known that certain pregnancy complications beget further comorbidities: for example, HDP can be a risk factor for PPCM.14
Overlap
We assessed pregnancy exposures for overlap. Similarly, we assessed for overlap among our CVD outcomes of interest. Finally, since specific subtypes of MI and stroke may be more common in younger individuals and among women, we analysed outcomes by subtype. For example, we assessed how many of the MIs in our population were due to MICAD versus MINOCA, and how many of the incident strokes were from haemorrhagic versus ischaemic versus embolic aetiologies. As mentioned above, ICD-9 codes for HF subtypes were not specified in the study cohort. We also considered PPCM as intrapartum or post partum in order to determine whether timing of PPCM affected CVD outcomes.
Stratification
In addition to our original multivariable Cox proportional hazards model (see the Primary analyses section), we reran the model (1) using age as a time-varying covariate, and (2) stratifying by preterm birth and PPCM, pre-eclampsia and PPCM, and IUGR and PPCM.
All analyses were performed using SAS V.9.4X. A p value of 0.05 or less was considered statistically significant.
Results
Characteristics of the study population
Of the 1 662 045 study participants who delivered from 2005 to 2009 in California HCUP, 579 656 (35%) were non-Hispanic white women, 105 489 (6%) were African–American, 648 602 (39%) were Hispanic, and 200 908 (12%) were Asian or Pacific Islanders. Of the pregnancies, 20 765 (1.2%) were complicated by drug abuse and 11 248 (0.7%) were complicated by active smoking. Of the deliveries, 330 946 were second pregnancies within the study period. In terms of additional risk factors for cardiovascular complications, 23 108 (1.4%) of pregnant women had pre-existing HTN, 16 806 (1.0%) had pre-existing diabetes and 538 (0.3%) had chronic kidney disease. Notably, the prevalence of African–Americans, chronic HTN and obesity was higher in the PPCM group than in the general population. The prevalence of Asian and Pacific Islanders in the GDM group was 20%. Table 1 summarises these and other characteristics of the study cohort.
Table 1.
Patient characteristics
| PPCM | Gestational HTN | Pre-eclampsia | GDM | Uncomplicated pregnancies* | |
| n | 558 | 47 980 | 62 162 | 107 636 | 1 455 554 |
| Mean age (SD) | 31 (7) | 28 (7) | 28 (7) | 32 (6) | 28 (6) |
| Race/Ethnicity | |||||
| Non-Hispanic white | 174 (31%) | 18 801 (39%) | 19 994 (32%) | 28 153 (26%) | 515 843 (35%) |
| Black | 116 (21%) | 4427 (9%) | 5693 (9%) | 4774 (4%) | 91 344 (6%) |
| Hispanic | 133 (24%) | 17 198 (36%) | 26 458 (43%) | 44 794 (42%) | 565 042 (39%) |
| Asian/Pacific Islander | 82 (15%) | 4236 (9%) | 5572 (9%) | 21 705 (20%) | 171 072 (12%) |
| Other | 47 (8%) | 2658 (6%) | 3610 (6%) | 6553 (6%) | 77 394 (5%) |
| Native American | <10 (<2%)† | 660 (1%) | 835 (1%) | 1657 (2%) | 34 859 (2%) |
| Income | |||||
| First quartile | 155 (28%) | 13 088 (27%) | 18 729 (30%) | 26 292 (24%) | 389 006 (27%) |
| Second quartile | 145 (26%) | 12 637 (26%) | 16 252 (26%) | 27 992 (26%) | 378 219 (26%) |
| Third quartile | 132 (24%) | 11 509 (24%) | 14 619 (24%) | 26 721 (25%) | 350 629 (24%) |
| Fourth quartile | 126 (23%) | 10 746 (22%) | 12 562 (20%) | 26 631 (25%) | 337 700 (23%) |
| Insurance status | |||||
| Medicare | <10 (<2%)† | 166 (0.35%) | 317 (1%) | 441 (0.4%) | 4380 (0.3%) |
| Medicaid | 222 (40%) | 17 903 (37%) | 24 814 (40%) | 33 902 (32%) | 536 653 (37%) |
| Private insurance | 311 (56%) | 28 530 (59%) | 35 165 (57%) | 71 157 (66%) | 869 142 (60%) |
| Self-pay | 11 (2%) | 588 (1%) | 874 (1%) | 824 (0.8%) | 20 183 (1%) |
| Other | <10 (<2%)† | 793 (2%) | 992 (2%) | 1312 (1%) | 25 196 (2%) |
| Hospitalisation | |||||
| Mean cost (SD) | $36 979 ($49 863) | $17 316 ($15 642) | $30 194 ($29 414) | $21 068 ($22 192) | $16 458 ($13 948) |
| Days to rehospitalisation | 74 (204) | 679 (381) | 642 (399) | 676 (378) | 665 (386) |
| Comorbidities | |||||
| Drug abuse | 21 (4%) | 792 (2%) | 1244 (2%) | 691 (0.6%) | 18 145 (1%) |
| Diabetes | 22 (4%) | 1146 (2%) | 2554 (4%) | 656 (0.6%) | 12 570 (0.8%) |
| Chronic HTN | 50 (9%) | 4907 (10%) | 5743 (9%) | 4261 (4%) | 10 016 (0.7%) |
| Obesity | 71 (13%) | 3842 (8%) | 5215 (8%) | 7858 (7%) | 32 986 (2%) |
| Chronic kidney disease | <10 (<2%)† | 30 (0.07%) | 236 (0.4%) | 50 (0.05%) | 246 (0.02%) |
| Caesarean section | 87 (16%) | 4794 (10%) | 6323 (10%) | 20 619 (19%) | 182 361 (13%) |
| Multiple births | <10 (<2%)† | 44 (0.1%) | 237 (0.4%) | 164 (0.15%) | 644 (0.04%) |
| Smoking (active) | 12 (2%) | 374 (0.8%) | 537 (0.9%) | 605 (0.6%) | 9807 (0.7%) |
| Stillbirth | <10 (<2%)† | 286 (0.6%) | 621 (1%) | 485 (0.5%) | 8661 (0.6%) |
| Preterm birth | 100 (18%) | 4565 (10%) | 16 167 (26%) | 10 654 (10%) | 87 895 (6%) |
| Intrauterine growth restriction | 14 (3%) | 1267 (3%) | 3217 (5%) | 1609 (1%) | 17 779 (1%) |
| Family history of CVD | <10 (<2%)† | 94 (0.2%) | 303 (0.5%) | 346 (0.3%) | 2578 (0.2%) |
| Baseline DM | 15 (3%) | 881 (2%) | 2009 (3%) | 1979 (2%) | 10 081 (0.7%) |
Baseline characteristics for patients included in the analysis; those with pre-existing MI, heart failure, stroke, and congenital or valvular heart disease were excluded.
*Uncomplicated pregnancies in this context are defined as pregnancies uncomplicated by PPCM, gestational HTN, pre-eclampsia or GDM.
†In accordance with HCUP policies, categories in which there were fewer than 10 patients are represented simply as <10.
CVD, cardiovascular disease;DM, diabetes mellitus;GDM, gestational diabetes mellitus; HCUP, Healthcare Cost and Utilization Project; HTN, hypertension;MI, myocardial infarction; PPCM, peripartum cardiomyopathy.
Prevalence of pregnancy exposures in study population
There were 558 cases of PPCM, 97 of which were diagnosed before delivery and 461 were diagnosed post partum. There were 49 114 cases of gestational HTN and 62 162 cases of pre-eclampsia; broken down by HDP subtypes of interest, there were 12 458 cases of isolated chronic HTN, 43 073 cases of gestational HTN, 56 419 cases of pre-eclampsia, 4907 pregnancies with both chronic HTN and superimposed gestational HTN, and 5743 pregnancies with chronic HTN and superimposed pre-eclampsia (table 2). There were 107 636 cases of GDM, 23 504 cases of IUGR and 116 768 cases of preterm birth. The median follow-up time to event was 2.68 years for any CVD outcome (4.87 years to either event or censoring), ranging from 1 day to 6.84 years. By CVD outcome, the median follow-up time to MI was 2.68 years, the median follow-up time to HF was 2.00 years, and the median follow-up time to stroke was 2.48 years. The study follow-up period captured 0.99, 1.23 and 1.02 cases of MI, HF and stroke, respectively, per 1000 person-years. Online supplementary figure 1 reports the average annual event rates for each exposure and outcome. Online supplementary table 3 demonstrates the overlap and correlations among pregnancy exposure categories. Preterm birth and pre-eclampsia were the most highly correlated (Kendall-Tau correlation coefficient 0.146) whereas IUGR and PPCM had the lowest correlation (Kendall-Tau correlation coefficient 0.0017) among pregnancy exposures studied.
Table 2.
Multivariable-adjusted associations between pregnancy complications and myocardial infarction, heart failure and stroke
| All eligible pregnancies N=1 662 045 |
Myocardial infarction MV-adjusted HR (95% CI) n=558 |
Heart failure MV-adjusted HR (95% CI) n=3284 |
Stroke MV-adjusted HR (95% CI) n=973 |
| PPCM n=558 1.66 per 1000 PY |
13.0 (4.1 to 40.9) n=<10* PY NA |
39.2 (30.0 to 51.9) n=56 2.19 per 1000 PY |
7.7 (2.4 to 24.0) n=<10* PY NA |
| No PPCM (referent) n=1 661 487 0.58 per 1000 PY |
1.0 n=549 0.98 per 1000 PY |
1.0 n=3228 1.22 per 1000 PY |
1.0 n=964 1.01 per 1000 PY |
| HDP subtypes | |||
| Chronic HTN n=12 458 0.54 per 1000 PY |
6.3 (4.6 to 8.7) n=47 0.90 per 1000 PY |
3.9 (3.3 to 4.7) n=152 1.12 per 1000 PY |
3.4 (2.4 to 4.8) n=37 0.88 per 1000 PY |
|
Gestational HTN n=43 073 5.98 per 1000 PY |
2.3 (1.6 to 3.4) n=28 0.98 per 1000 PY |
2.5 (2.1 to 2.9) n=188 1.39 per 1000 PY |
1.4 (1.0 to 2.0) n=33 1.15 per 1000 PY |
| Pre-eclampsia n=56 419 0.59 per 1000 PY |
2.5 (1.9 to 3.4) n=49 0.89 per 1000 PY |
3.0 (2.7 to 3.4) n=355 1.69 per 1000 PY |
2.3 (1.8 to 3.0) n=79 1.08 per 1000 PY |
| Chronic HTN and gestational HTN n=4907 0.54 per 1000 PY |
2.0 (0.8 to 4.8) n=<10* PY NA |
3.3 (2.5 to 4.5) n=45 1.16 per 1000 PY |
3.0 (1.7 to 5.4) n=12 1.67 per 1000 PY |
| Chronic HTN and pre-eclampsia n=5743 0.61 per 1000 PY |
5.0 (3.1 to 8.0) n=22 1.01 per 1000 PY |
5.4 (4.5 to 6.6) n=135 1.36 per 1000 PY |
7.6 (5.4 to 10.7) n=43 1.03 per 1000 PY |
| No HTN/pre-eclampsia/gestational HTN (referent) n=1 539 445 0.57 per 1000 PY |
1.0 n=407 1.00 per 1000 PY |
1.0 n=2409 1.17 per 1000 PY |
1.0 n=769 1.01 per 1000 PY |
| Other adverse pregnancy outcomes | |||
| Gestational DM n=107 636 0.59 per 1000 PY |
1.0 (0.8 to 1.4) n=57 0.88 per 1000 PY |
1.3 (1.1 to 1.4) n=320 1.36 per 1000 PY |
1.42 (1.2 to 1.8) n=103 1.09 per 1000 PY |
| No gestational DM (referent) n=1 554 409 0.58 per 1000 PY |
1.0 n=501 1.00 per 1000 PY |
1.0 n=2964 1.22 per 1000 PY |
1.0 n=870 1.01 per 1000 PY |
| IUGR n=23 504 0.59 per 1000 PY |
1.6 (1.1 to 2.5) n=23 1.11 per 1000 PY |
1.1 (0.9 to 1.4) n=86 1.22 per 1000 PY |
1.5 (1.0 to 2.1) n=30 1.09 per 1000 PY |
| No IUGR (referent) n=1 638 541 0.58 per 1000 PY |
1.0 n=535 0.98 per 1000 PY |
1.0 n=3198 1.23 per 1000 PY |
1.0 n=943 1.02 per 1000 PY |
| Preterm birth n=116 768 0.58 per 1000 PY |
1.8 (1.4 to 2.3) n=105 1.05 per 1000 PY |
1.6 (1.4 to 1.7) n=532 1.25 per 1000 PY |
1.4 (1.1 to 1.6) n=134 1.05 per 1000 PY |
| No preterm birth(referent) n=1 545 277 0.58 per 1000 PY |
1.0 n=453 0.97 per 1000 PY |
1.0 n=2752 1.23 per 1000 PY |
1.0 n=839 1.01 per 1000 PY |
*In accordance with HCUP policies, categories in which there were fewer than 10 patients are represented simply as <10.
DM, diabetes mellitus;HCUP, Healthcare Cost and Utilization Project; HDP, hypertensive disorders of pregnancy;HTN, hypertension;IUGR, intrauterine growth restriction; MV, multivariate;NA, not available; PPCM, peripartum cardiomyopathy;PY, person-years.
Multivariable models demonstrated associations between exposures and future cardiovascular risk
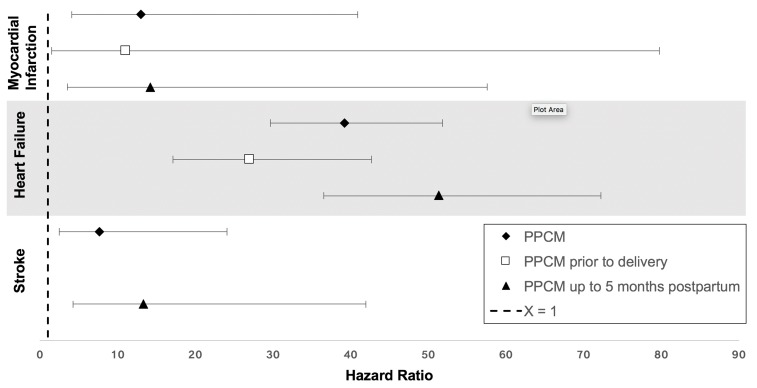
Among all women hospitalised during pregnancy from 2005 to 2009, there were 558 subsequent MIs, 3284 subsequent cases of HF and 973 subsequent strokes. Table 2 shows stroke, MI and HF event numbers by pregnancy exposure category. Of the 330 946 pregnancies (of the total ~1.6 million pregnancies) which were second pregnancies within the study period, 24 had MI (24 of 558), 133 had HF (133 of 3284) and 37 had stroke (37 of 973). Using multivariable Cox proportional hazards models adjusting for all exposures and covariates, we found that PPCM was independently associated with a 13.0-fold increased risk of MI (95% CI 4.1 to 40.9), a 39.2-fold increased risk of HF (95% CI 30.0 to 51.9) and a 7.7-fold increased risk of stroke (95% CI 2.4 to 24.0; figure 1, table 2). The HRs were similar for PPCM diagnosed at or before delivery when compared with PPCM diagnosed in the first 5 months post partum (figure 1). For PPCM, the above HRs corresponded to approximately one additional HF event per 1000 person-years (results could not be calculated for PPCM and MI or stroke due to a paucity of cases). For chronic HTN with superimposed gestational HTN, an HR of 3.0 (95% CI 1.7 to 5.4) corresponded to 0.66 additional stroke events per 1000 person-years, and for pre-eclampsia an HR of 3.0 (95% CI 2.7 to 3.4) corresponded to 0.52 additional HF events per 1000 person-years.
Figure 1.
Adjusted associations between peripartum cardiomyopathy (PPCM) and myocardial infarction, heart failure and stroke. HRs for all PPCM (black diamond), as well as PPCM diagnosed prior to delivery (open square) and post partum (black triangle). Covariates included age, race, insurance status, median household income, chronic kidney disease, pre-existing diabetes, obesity, drug abuse, smoking, multiple gestations, as well as for each of the exposures (PPCM, hypertensive disorders of pregnancy subtypes, gestational diabetes mellitus, intrauterine growth restriction and preterm birth).
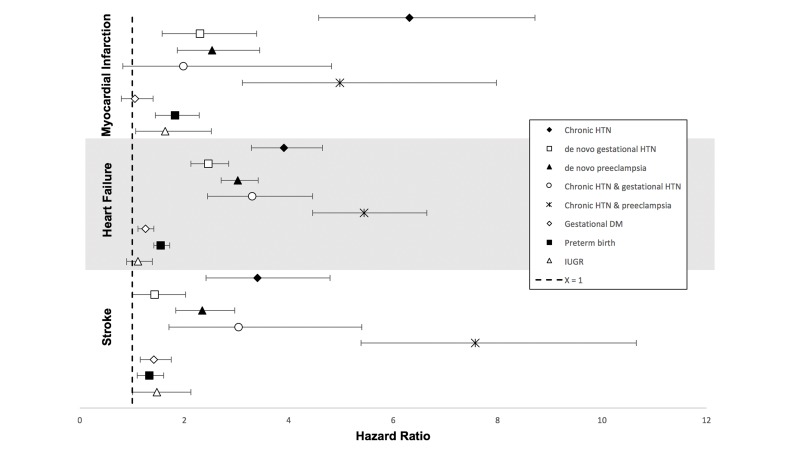
We investigated cardiovascular risks according to HDP subtypes of differing chronicity and severity. We examined five subtypes: chronic HTN alone, gestational HTN, pre-eclampsia, chronic HTN with superimposed gestational HTN and chronic HTN with superimposed pre-eclampsia (online supplementary table 2). These subtypes carried a 2.3–6.3-fold increased risk for MI, a 2.5–5.4-fold increased risk for HF and a 1.4–7.6-fold increased risk for stroke (figure 2, table 2). Although there was some overlap in 95% CIs, chronic HTN alone, and with superimposed pre-eclampsia, carried the highest risks of MI, HF and stroke compared with other HDP subtypes. Chronic HTN with superimposed gestational HTN did not demonstrate a statistically significant increased risk for MI; the n in this group was less than 10.
Figure 2.
Adjusted associations between hypertensive disorder of pregnancy subtypes and MI, heart failure and stroke. HRs for HDP subtypes, GDM, IUGR and preterm birth and MI, HF and stroke. Covariates included age, race, insurance status, median household income, chronic kidney disease, pre-existing diabetes, obesity, drug abuse, smoking, multiple gestations, as well as for each of the exposures (PPCM, HDP subtypes, GDM, IUGR and preterm birth). DM, diabetes mellitus; GDM, gestational diabetes mellitus; HDP, hypertensive disorders of pregnancy; HF, heart failure; HTN, hypertension; IUGR, intrauterine growth restriction; MI, myocardial infarction; PPCM, peripartum cardiomyopathy.
All of our models additionally included GDM, preterm birth and IUGR. IUGR was related to risk of MI but not HF or stroke (HR for MI=1.6, 95% CI 1.0 to 2.5). Preterm delivery was significantly associated with MI, stroke and HF (figure 2, table 2). GDM was associated with risks of stroke and HF but not with MI (figure 2, table 2). Overall, these three pregnancy exposures conferred comparatively lower risks for CVDs as compared with PPCM and HDP.
Tests for residual confounding by age in the multivariable model show robust associations between exposures and outcomes
Age is a well-known and strong predictor both for cardiovascular complications of pregnancy and for our outcomes of interest,21 22 and increased prevalence of exposures and outcomes by age was again demonstrated in our studyonline supplementary table 4 We therefore sought to test for residual confounding by age in our multivariable model. We compared HRs derived from our original model with those obtained using an alternate approach using age (available in HCUP as age rounded to the nearest year) as a time-varying covariate. We found that this alternate approach did not change HR point estimates or 95% CIs by more than a point (data not shown).
We also ran additional models stratifying by preterm birth and PPCM, pre-eclampsia and PPCM, and IUGR and PPCM, and did not find substantial differences in HR point estimates or 95% CIs (data not shown).
Finally, we ran additional models using the number of recurrent pregnancies as a time-varying covariate, and also did not find substantial differences in HR point estimates or 95% CIs (data not shown).
Secondary analyses
With respect to exposures, except the HDP subtypes of gestational HTN and chronic HTN and pre-eclampsia and chronic HTN, where clinical overlap was present by design (10.3% and 9.24% overlap, respectively), there was notable overlap between PPCM and pre-eclampsia (26.1%), pre-eclampsia and preterm birth (26.0%), and between chronic HTN and preterm birth (18.8%). Overlap among all other categories ranged from 1.49% to 10.6% (online supplementary table 3).
With respect to outcomes, PPCM is known to be a risk factor for HF but has not previously been described as a risk factor for MI or stroke (figure 1, table 2). To determine whether there was overlap in MI, HF and stroke outcome diagnoses in patients with PPCM that might explain our observations, we queried ICD-9 diagnoses for each individual patient in the PPCM exposure group. Most categories of overlap had fewer than 10 patients; for example, fewer than 10 out of 58 patients with PPCM (6.9%) had overlapping outcome diagnoses (for HF/stroke and HF/MI). No single PPCM exposure had all three outcome diagnoses. Among patients with HDP subtypes, GDM, preterm birth and IUGR exposures, the percentage of patients with more than one outcome diagnosis ranged from 5.74% to 11.37%. Therefore, the majority of observed outcomes were separate cardiovascular events.
We then considered whether HF could be a mediator of MI and stroke among patients with PPCM and overlapping outcome diagnoses. We refitted our MI and stroke models with HF as a time-dependent covariate. With HF as a mediator, the risk of MI decreased from 13.0 (95% CI 4.1 to 40.9) to 4.6 (95% CI 1.4 to 14.8), and the risk of stroke decreased from 7.7 (95% CI 2.4 to 24.0) to not significant (2.7, 95% CI 0.8 to 8.5). Therefore, although there were novel significant associations between PPCM and MI and PPCM and stroke, HF mediated much of those associations.
Finally, we analysed outcomes by MI and stroke subtypes (table 3). Based on ICD-9 coding, we found that the predominant type of MI experienced within the follow-up period was MICAD, and the most common type of stroke was ischaemic stroke.
Table 3.
Number of patients in the study cohort with CVD outcomes of interest by outcome subtype
| MI | Stroke | Heart failure | ||||
| MICAD | MINOCA | Embolic | Ischaemic | Haemorrhagic | ||
| PPCM (n=558) | <10* | <10* | <10* | <10* | 0 | 56 |
| Chronic HTN (n=12 458) | 14 | <10* | <10* | 28 | <10* | 152 |
| Gestational HTN (n=43 073) | <10* | <10* | <10* | 22 | <10* | 188 |
| Pre-eclampsia (n=56 419) | 16 | <10 | <10 | 60 | 14 | 355 |
| Chronic HTN and gestational HTN (n=4907) | <10* | 0 | <10* | <10* | <10* | 45 |
| Chronic HTN and pre-eclampsia (n=5743) | <10* | 0 | <10* | 35 | <10* | 135 |
| Gestational DM (n=107 636) | 24 | <10* | 12 | 62 | 29 | 320 |
| Preterm birth (n=116 768) | 42 | 15 | 13 | 96 | 25 | 532 |
| IUGR (n=23 504) | <10* | <10* | <10* | 17 | <10* | 86 |
| Total | 126 | 40 | 53 | 333 | 91 | 1251 |
*In accordance with HCUP policies, categories in which there were fewer than 10 patients are represented simply as <10.
CVD, cardiovascular disease; DM, diabetes mellitus;HCUP, Healthcare Cost and Utilization Project; HTN, hypertension;IUGR, intrauterine growth restriction; MI, myocardial infarction;MICAD, MI with obstructive coronary artery disease;MINOCA, MI without obstructive coronary artery disease;PPCM, peripartum cardiomyopathy.
Discussion
Summary of findings
In this retrospective cohort study of over 1.6 million deliveries representing nearly every completed pregnancy in California over a 5-year period, we report the following: (1) PPCM is associated with risks for MI and stroke, in addition to (and partly mediated by) HF; (2) HDP subtypes were associated with risks for MI, stroke and HF; (3) subtypes of HDP representing a longer duration of HTN and a higher severity (ie, chronic HTN and chronic HTN with superimposed pre-eclampsia) had the greatest magnitude of risks for MI, stroke and HF; (4) GDM, preterm birth and IUGR were independently associated with risks for future CVD when accounting for PPCM and HDP; and (5) PPCM and HDP were independently associated with CVD when accounting for these other CVD-related pregnancy complications. Stratification by age and by selected exposures did not significantly change observed HRs, suggesting these findings are robust to potential residual confounding. We recognise that associations described corresponded to only modest increases in absolute event rates, with a maximum additional event rate of ~1 per 1000 person-years (for PPCM and HF; for chronic HTN with superimposed gestational HTN and stroke; and about half that rate for pre-eclampsia and HF). However, given that some of the described pregnancy complications are common (eg, pre-eclampsia affects approximately 1 in 20 pregnancies worldwide) and that there are about 200 million pregnancies per year, even these small additional event rates may represent a public health opportunity to improve primary CVD prevention among affected women.
We found that the above cardiovascular pregnancy complications were associated with substantial risks for MI, HF and stroke even within the first 6 years postdelivery. In contrast to long-held belief that heart disease largely affects women who are long past their childbearing years, we demonstrate that the presence of cardiovascular complications of pregnancy can identify a subpopulation of younger women who are at high risk for premature CVD. Our findings are relevant given the recent call to attention and study concerning the growing epidemic of MI in younger women by scientific experts,7 and support initiatives designed to follow younger at-risk women more closely for CVD prevention.22
PPCM is hypothesised to have several different causes, including genetic mutations, fetal autoimmunity and vascular dysfunction, in addition to HDP itself.23–25 Furthermore, PPCM presenting during pregnancy may represent a different clinical entity as compared with PPCM presenting post partum12 and may have different underlying genetic underpinnings.13 Despite this, our findings did not suggest that intrapartum and postpartum PPCM as coded in HCUP conferred differing risks for CVD. Despite potentially overlapping pathophysiology between PPCM and HDP,26 PPCM has a relatively greater magnitude of risk for incident CVD than any of the HDP subtypes studied.
Given that preterm birth, IUGR and GDM have demonstrated associations with CVD27–29 and are commonly seen with HDP and PPCM, we studied these as secondary exposures and did find that each was significantly associated with one or more subtypes of CVD, confirming findings from prior investigations. HDP is a common reason for medically indicated preterm delivery. Several lines of evidence suggest that a common pathology in many cases of preterm birth and IUGR, as well as subtypes of HDP and PPCM and HDP, is vascular dysfunction of varying chronicity and severity.30–34 While pregnancy may cause vascular dysfunction, it may also just unmask dysfunction that predates the pregnancy.35 GDM is a risk factor for future diabetes and its attendant microvascular and macrovascular effects,36 which may explain the associations seen between GDM and stroke, as well as HF.
Strengths and limitations
As a state-wide record, California HCUP provided generalisable data from a large and ethnically diverse population and has been used to uncover several insights into CVD.18 37 38 Age, race and ethnicity in the study cohort were similar to those of national Centers for Disease Control birth records for the same time period.19 HCUP captures 98.5% of hospital discharges for California’s population, which comprises over 38 million people. Large data sets like HCUP can facilitate defining phenotypes at greater resolution, such as the pregnancy complication subtypes analysed in this paper and in other work from our group.18 In addition to several exposures, we were also able to adjust for several potential confounders, which clarified the effects of each exposure on future cardiovascular risk. Increasing phenotypic resolution at scale can help uncover new associations and inspire new hypotheses on the mechanisms of disease. Here we demonstrate for the first time that PPCM, a relatively rare event, carried risks for future HF and for MI and stroke as well, and that these associations, while mediated in part by HF, are not simply explained by overlap of outcome diagnoses.
In terms of magnitude, PPCM conferred the highest magnitude of HRs for future CVD, followed by chronic HTN with superimposed pre-eclampsia, and then chronic HTN alone. Whereas PPCM and HDP have been reported to exist on a common pathophysiological spectrum in two prior studies,11 26 we demonstrate that their respective association with later CVD differs in magnitude of risk. Further basic and translational studies are needed to determine the specific pathophysiological mechanisms that connect PPCM and HDP with later CVD.
Relatively short follow-up time (maximum of 6.84 years) likely resulted in an underestimation of risk along the lifetimes of the individuals studied. Furthermore, this short follow-up time allows us to highlight the risk of cardiovascular pregnancy complications not just on CVD at some later point in life, but on premature CVD events. This can be difficult to quantify in large cohort studies focused on the occurrence of CVD in later life, since a long follow-up period can allow an explanation of subsequent CVD events by later emergence of risk. Our study provides data on short-term events.
With small numbers in some groups after adjustment for several covariates, 95% CIs for several exposure subtypes were wide. Given the significant associations found despite these caveats, the HRs measured in this study may in fact be underestimates of true cardiovascular risk following a pregnancy complicated by PPCM and/or HDP.
Despite using a large data set like HCUP, our analysis of exposure and outcome subtypes was potentially limited due to misclassification bias and to a lack of availability of certain diagnostic codes. We attempted to assess the extent of misclassification bias in our study. HCUP data are anonymised, precluding the ability to perform manual chart review of the ICD-9 coding used to define exposures, covariates and outcomes. We found that prevalence of preterm birth was similar to national estimates; prevalence of PPCM and GDM was similar to previous reports in smaller California cohorts in which diagnoses were confirmed by manual chart review.19 21 Reports of substance abuse were lower in our cohort than in other reports.39 Literature review of ICD-9 coding for pre-eclampsia, gestational HTN and HDP shows low sensitivity and high specificity,40 while ICD-9 coding for outcomes such as MI, HF and stroke shows high sensitivity and specificity.41–43 If these trends are also true within HCUP, it would suggest that the HRs reported in this study are likely accurate, but could be underestimates of the true risk. Despite these shortcomings, our analysis shows several robust signals indicating increased CVD risk among exposures of interest.
Finally, the lack of certain diagnostic codes limited our ability to perform detailed analysis of outcome subtypes (eg, HF subtypes) or distinguish between HF class. When defining MI and stroke by outcome subtype, there were not enough numbers to perform statistical analysis; the national HCUP database may provide adequate numbers for such an analysis.
In this study, we demonstrated that PPCM is associated with near-term HF, MI and stroke in women in California, independent of HDP, GDM, preterm delivery and IUGR. Among HDP subtypes, chronicity and severity of HTN increased the risk of subsequent HF, MI and stroke. While these results are independent of age, the increased prevalence of exposures and outcomes with age in the face of national birth patterns with women giving birth at older ages means that we can expect even more PPCM, HDP and CVD in pregnant women. The importance of pregnancy as a cardiovascular ‘stress test’ will, therefore, only further expand in the future. Furthermore, it is worth noting that the presence of CVD events among those without cardiovascular pregnancy complications requires continued education and primary prevention for all women.
Conclusion
Our findings support close monitoring of women with cardiovascular pregnancy complications for prevention of subsequent, near-term CVD events, and beg further study of potential mechanisms underlying the development of these early CVD events.
Footnotes
Contributors: NP and RA conceived of the research questions and analysis with input from all authors. GN performed the analyses, with guidance and help from NP and RA. RA and NP wrote the manuscript with input from all authors.
Funding: RA was supported by NIH (K08HL125945) and the American Heart Association (15GPSPG238300004). ZT was supported by NIH (R01 Hl102090), NIH (R01 HL126555), CDC (DP14-1403) and NIH (R24 A1067039). NP was supported by NIH (R21 7R21HL115398).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: Analyses were performed in accordance with the HCUP Data Use Agreement and are considered exempt research per UCSF CHR. To preserve patient anonymity, any groups in which there were fewer than 10 patients are listed in the tables as <10. Certification to use de-identified HCUP data was obtained from the University of California San Francisco Committee on Human Research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1. Mosca L, Hammond G, Mochari-Greenberger H, et al. Fifteen-year trends in awareness of heart disease in women: results of a 2012 American Heart association national survey. Circulation 2013;127:1254–63. 10.1161/CIR.0b013e318287cf2f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khan NA, Daskalopoulou SS, Karp I, et al. Sex differences in prodromal symptoms in acute coronary syndrome in patients aged 55 years or younger. Heart 2017;103 10.1136/heartjnl-2016-309945 [DOI] [PubMed] [Google Scholar]
- 3. Hemal K, Pagidipati NJ, Coles A, et al. Sex differences in demographics, risk factors, presentation, and noninvasive testing in stable outpatients with suspected coronary artery disease: insights from the promise trial. JACC Cardiovasc Imaging 2016;9:337–46. 10.1016/j.jcmg.2016.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parikh NI, Jeppson RP, Berger JS, et al. Reproductive Risk Factors and Coronary Heart Disease in the Women’s Health Initiative Observational Study. Circulation 2016;133:2149–58. 10.1161/CIRCULATIONAHA.115.017854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Robinson PN. Deep phenotyping for precision medicine. Hum Mutat 2012;33:777–80. 10.1002/humu.22080 [DOI] [PubMed] [Google Scholar]
- 6. Delude CM. Deep phenotyping: the details of disease. Nature 2015;527:S14–S15. 10.1038/527S14a [DOI] [PubMed] [Google Scholar]
- 7. Nabel EG. Heart disease prevention in young women: sounding an alarm. Circulation 2015;132:989–91. 10.1161/CIRCULATIONAHA.115.018352 [DOI] [PubMed] [Google Scholar]
- 8. Goff DC, Lloyd-Jones DM, Bennett G, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation 2014;129(25 Suppl 2):S49–73. 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 9. Aoyama K, Suzuki Y, Sato T, et al. Cardiac failure caused by severe pre-eclampsia with placental abruption, and its treatment with anti-hypertensive drugs. J Obstet Gynaecol Res 2003;29:339–42. 10.1046/j.1341-8076.2003.00125.x [DOI] [PubMed] [Google Scholar]
- 10. Mann DL, Zipes DP, Libby P, et al. Braunwald's heart disease : a textbook of cardiovascular medicine. 10th edn Philadelphia, PA: Elsevier/Saunders, 2015. [Google Scholar]
- 11. Patten IS, Rana S, Shahul S, et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature 2012;485:333–8. 10.1038/nature11040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krishnamoorthy P, Garg J, Palaniswamy C, et al. Epidemiology and outcomes of peripartum cardiomyopathy in the United States: findings from the nationwide inpatient sample. J Cardiovasc Med 2016;17:756–61. [DOI] [PubMed] [Google Scholar]
- 13. Ware JS, Li J, Mazaika E, Seidman JG, Arany Z, et al. Shared genetic predisposition in Peripartum and dilated cardiomyopathies. N Engl J Med 2016;374:233–41. 10.1056/NEJMoa1505517 [DOI] [PubMed] [Google Scholar]
- 14. Behrens I, Basit S, Lykke JA, et al. Association between hypertensive disorders of pregnancy and later risk of cardiomyopathy. JAMA 2016;315:1026–33. 10.1001/jama.2016.1869 [DOI] [PubMed] [Google Scholar]
- 15. Savitz DA, Danilack VA, Elston B, et al. Pregnancy-induced hypertension and diabetes and the risk of cardiovascular disease, stroke, and diabetes hospitalization in the year following delivery. American Journal of Epidemiology 2014;180:41–4. 10.1093/aje/kwu118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wenger NK. Recognizing pregnancy-associated cardiovascular risk factors. The American Journal of Cardiology 2014;113:406–9. 10.1016/j.amjcard.2013.08.054 [DOI] [PubMed] [Google Scholar]
- 17. Manten GTR, Sikkema MJ, Voorbij HAM, et al. Risk factors for cardiovascular disease in women with a history of pregnancy complicated by preeclampsia or intrauterine growth restriction. Hypertension in Pregnancy 2007;26:39–50. 10.1080/10641950601146574 [DOI] [PubMed] [Google Scholar]
- 18. Hayward RM, Foster E, Tseng ZH. Maternal and fetal outcomes of admission for delivery in women with congenital heart disease. JAMA Cardiol 2017;2:664–71. 10.1001/jamacardio.2017.0283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martin JA, Hamilton BE, Ventura SJ, et al. Births: final data for 2010. Natl Vital Stat Rep 2012;61:1–72. [PubMed] [Google Scholar]
- 20. Pasupathy S, Tavella R, Beltrame JF. Myocardial infarction with nonobstructive coronary arteries (MINOCA): the past, present, and future management. Circulation 2017;135:1490–3. 10.1161/CIRCULATIONAHA.117.027666 [DOI] [PubMed] [Google Scholar]
- 21. Gunderson EP, Croen LA, Chiang V, et al. Epidemiology of peripartum cardiomyopathy: incidence, predictors, and outcomes. Obstet Gynecol 2011;118:583–91. 10.1097/AOG.0b013e318229e6de [DOI] [PubMed] [Google Scholar]
- 22. Cusimano MC, Pudwell J, Roddy M, et al. The maternal health clinic: an initiative for cardiovascular risk identification in women with pregnancy-related complications. American Journal of Obstetrics and Gynecology 2014;210:438.e1–438.e9. 10.1016/j.ajog.2013.12.001 [DOI] [PubMed] [Google Scholar]
- 23. Fett JD. Peripartum cardiomyopathy: a puzzle closer to solution. WJC 2014;6:87–99. 10.4330/wjc.v6.i3.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ntusi NBA, Badri M, Gumedze F, et al. Pregnancy-associated heart failure: a comparison of clinical presentation and outcome between hypertensive heart failure of pregnancy and idiopathic peripartum cardiomyopathy. Plos One 2015;10:e0133466 10.1371/journal.pone.0133466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ware JS, Li J, Mazaika E, et al. Shared genetic predisposition in Peripartum and dilated cardiomyopathies. N Engl J Med 2016;374:233–41. 10.1056/NEJMoa1505517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bello N, Rendon ISH, Arany Z. The relationship between pre-eclampsia and peripartum cardiomyopathy: a systematic review and meta-analysis. J Am Coll Cardiol 2013;62:1715–23. 10.1016/j.jacc.2013.08.717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tanz LJ, Stuart JJ, Williams PL, et al. Preterm delivery and maternal cardiovascular disease in young and middle-aged adult women. Circulation 2017;135:578–89. 10.1161/CIRCULATIONAHA.116.025954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Srinivas SK, Edlow AG, Neff PM, et al. Rethinking IUGR in preeclampsia: dependent or independent of maternal hypertension? J Perinatol 2009;29:680–4. 10.1038/jp.2009.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cirillo PM, Cohn BA. Pregnancy complications and cardiovascular disease death: 50-year follow-up of the child health and development studies pregnancy cohort. Circulation 2015;132:1234–42. 10.1161/CIRCULATIONAHA.113.003901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tomimatsu T, Mimura K, Endo M, et al. Pathophysiology of preeclampsia: an angiogenic imbalance and long-lasting systemic vascular dysfunction. Hypertens Res 2017;40:305–10. 10.1038/hr.2016.152 [DOI] [PubMed] [Google Scholar]
- 31. Grand'Maison S, Pilote L, Okano M, et al. Markers of vascular dysfunction after hypertensive disorders of pregnancy: a systematic review and meta-analysis. Hypertension 2016;68:1447–58. 10.1161/HYPERTENSIONAHA.116.07907 [DOI] [PubMed] [Google Scholar]
- 32. Di Martino D, Zullino S, Casati D, et al. C1. Maternal hemodynamic profile in hypertensive disorders of pregnancy (HDP) and intrauterine growth restriction. The Journal of Maternal-Fetal & Neonatal Medicine 2016;29 10.1080/14767058.2016.1234771 [DOI] [Google Scholar]
- 33. Boeldt DS, Bird IM. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J Endocrinol 2017;232:R27–R44. 10.1530/JOE-16-0340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Banerjee M, Cruickshank JK. Pregnancy as the prodrome to vascular dysfunction and cardiovascular risk. Nat Rev Cardiol 2006;3:596–603. 10.1038/ncpcardio0683 [DOI] [PubMed] [Google Scholar]
- 35. Lazdam M, Davis EF, Lewandowski AJ, et al. Prevention of vascular dysfunction after preeclampsia: a potential long-term outcome measure and an emerging goal for treatment. J Pregnancy 2012;2012:1–8. 10.1155/2012/704146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gunderson EP, Chiang V, Pletcher MJ, et al. History of gestational diabetes mellitus and future risk of atherosclerosis in Mid‐life: the coronary artery risk development in young adults study. J Am Heart Assoc 2014;3:e000490 10.1161/JAHA.113.000490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Walkey AJ, Wiener RS, Ghobrial JM, et al. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA 2011;306:2248–54. 10.1001/jama.2011.1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dewland TA, Olgin JE, Vittinghoff E, et al. Incident atrial fibrillation among Asians, Hispanics, blacks, and whites. Circulation 2013;128:2470–7. 10.1161/CIRCULATIONAHA.113.002449 [DOI] [PubMed] [Google Scholar]
- 39. Cook JL, Green CR, de la Ronde S, et al. Epidemiology and effects of substance use in pregnancy. Journal of Obstetrics and Gynaecology Canada 2017;39:906–15. 10.1016/j.jogc.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 40. Klemmensen Åse K., Olsen SF, Østerdal ML, et al. Validity of Preeclampsia-related diagnoses recorded in a National Hospital registry and in a postpartum interview of the women. Am J Epidemiol 2007;166:117–24. 10.1093/aje/kwm139 [DOI] [PubMed] [Google Scholar]
- 41. McCormick N, Bhole V, Lacaille D, et al. Validity of diagnostic codes for acute stroke in administrative databases: a systematic review. Plos One 2015;10:e0135834 10.1371/journal.pone.0135834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McCormick N, Lacaille D, Bhole V, et al. Validity of heart failure diagnoses in administrative databases: a systematic review and meta-analysis. PLoS ONE 2014;9:e104519 10.1371/journal.pone.0104519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McCormick N, Lacaille D, Bhole V, et al. Validity of myocardial infarction diagnoses in administrative databases: a systematic review. PLoS ONE 2014;9:e92286 10.1371/journal.pone.0092286 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2018-000927supp001.docx (238.6KB, docx)