Elevated trimethylamine-N-oxide is an established cardiovascular and metabolic risk factor
To date, at least five prospective cohort studies have concluded that increased plasma levels of trimethylamine N-oxide (TMAO) predict increased risk for major adverse cardiovascular (CV) events in patients with pre-existing coronary heart disease.1–5 Moreover, though some epidemiology does not support a connection between plasma TMAO and CV risk,6 7 a recent meta-analysis of 11 prospective cohort studies concludes that higher plasma TMAO correlates with a 23% increase in risk for CV events (HR 1.23, 95% CI 1.07 to 1.42), as well as a 55% increase in all-cause mortality.8 The possibility that TMAO may be a mediating factor in this regard is raised by rodent studies in which plasma levels have been raised either by direct oral administration of TMAO, or by administration of very high doses (proportionately very much higher than would be employed in human supplementation) of its precursors phosphatidylcholine and carnitine; in these studies, in which the achieved plasma level of TMAO was at least an order of magnitude higher than commonly observed in humans, a proatherogenic effect was documented.9–14 In vitro studies, likewise employing supraphysiological concentrations of TMAO, have demonstrated effects suggesting proatherogenic potential.12 13 15–17
In case–control epidemiology, elevated TMAO has also been linked to substantially increased risk for type 2 diabetes and metabolic syndrome.18–20 Indeed, the correlations between TMAO and diabetes risk appear to be stronger than those for CV risk.
Nutritional intakes of TMAO and its precursors do not correlate with CV risk
Yet the notion that TMAO acts as a human vascular toxin at the plasma concentrations seen in people with reasonably normal renal function is difficult to square with other recent findings. Preformed TMAO is notably high in fish, in which it serves to maintain osmotic balance; levels tend to be higher in deep-sea fish, which must survive at higher pressures.21–24 This TMAO can be directly absorbed after fish consumption.25 However, at least in those who do not ingest a very large amount of fish, a high proportion of their plasma TMAO arises from bacterial metabolism of dietary choline (usually ingested as phosphatidylcholine) and carnitine; trimethyllysine also makes a minor contribution in this regard.9 10 26 Certain gut bacteria can metabolise these compounds to trimethylamine (TMA) via TMA lyase activity; inhibition of this lyase activity prevents induction of atherosclerosis in mice fed high-dose choline.11 27 28 This TMA can then be absorbed; its subsequent oxidation by hepatic flavin-containing monooxygenases (FMOs) converts it to TMAO.29 30 Unless choline has cardioprotective properties, we are currently unaware of, a diet relatively rich in choline would be expected to increase CV risk if physiological levels of plasma TMAO can indeed provoke CV disease or CV events. Yet a recent meta-analysis of prospective epidemiological studies concluded that dietary choline intake has no significant impact on risk for incident CV disease or CV mortality; with respect to CV mortality, only two pertinent studies were available, so the conclusion in this respect might not be definitive.31 Likewise, a recent meta-analysis failed to associate consumption of eggs–a rich source of phosphatidylcholine–with increased CV risk.32
With respect to carnitine and CV risk, a meta-analysis of prospective clinical trials in patients who had recently experienced a myocardial infarction (MI) concluded that carnitine supplementation is markedly protective with respect to total mortality, ventricular arrhythmias and new-onset angina; trends for lower incidence of reinfarction or heart failure did not achieve statistical significance, possibly owing to the modest size of the studies included.33 Clinical trials have also reported favourable effects of supplemental carnitine or carnitine esters on angina, intermittent claudication and heart failure.34–39 Moreover, rodent atherogenesis studies, in which carnitine has been administered in doses reasonably proportional to the supplementation doses used clinically, have found that carnitine is antiatherogenic, despite its propensity to raise TMAO.22 40–42 With respect to fish, the primary dietary source of preformed TMAO, a meta-analysis found that fish consumption correlates dose dependently with CV protection, likely because of the long-chain omega-3 content of fish.43 While it might be argued that the benefits of omega-3 ingestion are masking a genuine adverse impact of TMAO on CV risk, the impact of moderate supplemental intakes of fish omega-3 on this risk seems to be rather modest in the context of current drug therapy, primarily influencing risk for sudden death arrhythmias.44–46 Hence, these benefits would seem unlikely to overwhelm the adverse effects of TMAO if these were of important magnitude. In aggregate, these findings are difficult to square with the notion that TMAO is a mediating CV risk factor, at least in commonly occurring levels, since increased ingestion of choline, carnitine or fish would be expected to increase TMAO levels, but is not associated with increased CV risk.
It is, therefore, reasonable to suspect that moderately elevated TMAO, rather than being a mediator of the associated CV risk, is a marker for factors which both promote CV events and increase plasma TMAO.47 48 Indeed, after a plethora of multicentre supplementation trials, we have learnt something precisely comparable about moderately elevated homocysteine and coronary risk.49 50 Whereas the highly elevated homocysteine levels seen in genetic hyperhomocysteinaemia are evidently directly pathogenic to the vascular system, and homocysteine at comparably high levels exerts proinflammatory effects on vascular cells in vitro, we were never presented with evidence that the moderately elevated levels of homocysteine associated with increased CV risk–roughly an order of magnitude lower–exerted important effects in vitro. Currently, TMAO appears to be in an analogous position.22
Diminished renal function can markedly elevate TMAO
Evidently, an increase in plasma TMAO can reflect an increase in TMAO synthesis or a reduction in its renal clearance. A promising lead is offered by the observation that plasma TMAO levels are highly dependent on renal function. A study examining plasma TMAO levels in patients with varying degrees of renal compromise and in healthy controls found that TMAO averaged 5.8 µM/L in the controls (with average measured glomerular filtration rate [mGFR]–83 mL/min), 14.6 µM/L in patients with stages 3–4 kidney disease (mGFR 28 mL/min) and 75.5 µM/L (mGFR 7 mL/min) in stage 5 patients.51 Hence, as mGFR falls, plasma TMAO tends to rise almost proportionally.
Although it is well known that severe kidney disease is associated with a considerable increase in CV risk, a meta-analysis of general population cohort studies has found that even a mild reduction in estimated GFR (eGFR) is a risk factor for CV mortality.52 Thus, whereas risk for CV mortality was found to be relatively flat for eGFRs in the range of 75–120 mL/min, a significantly higher risk was seen at eGFR 60 mL/min, and this mortality rose progressively as eGFR fell. Hence, even relatively modest reductions of eGFR sometimes considered to be within the ‘normal’ range of kidney function (eGFR of 60 mL/min or greater) are associated with increased CV risk. This increased risk could presumably reflect an impact of suboptimal kidney function per se (leading to increased levels of phosphate or other uraemic toxins), as well as of vasculotoxic factors inducing reduction of kidney function. These may not have been adequately corrected for in epidemiological analyses focusing on TMAO.
Nonetheless, there is good reason to believe that, whereas uncorrected correlations of TMAO with CV risk are explained in part by the CV risk associated with diminished renal function, this is not the sole explanation for the utility of TMAO as a risk factor. That is because the five cohort studies cited above-included analyses which adjusted for eGFR as a covariate; while this correction markedly decreased the calculated CV risk associated increased TMAO, it by no means eliminated it.1–5 We can, therefore, conclude that factors, which boost TMAO synthesis, are mediators of some of the risk associated with elevated TMAO.
Are bad bacteria the culprit?
Two steps are involved in the synthesis of TMAO: generation of TMA from certain dietary precursors–most notably, choline and carnitine–by the TMA lyase activity of gastrointestinal (GI) bacteria; and oxidation of circulating TMA to TMAO by hepatic FMOs, by far the most active of which in this regard is FMO3.29 With respect to GI bacteria, rodent studies have led to increasing awareness of the fact that microbiota can notably modulate metabolic health.53–56 Is it possible that certain commonly occurring GI bacteria are quite proficient at generating TMA, while simultaneously increasing CV risk by certain mechanisms–for example, by suppressing incretin synthesis or maximising bile acid reabsorption (which might elevate LDL cholesterol)? Or could some dietary factor–soluble fibre, perhaps–suppress the capacity of GI bacteria to generate TMA, while simultaneously protecting CV health?
While this is an intriguing hypothesis that merits further follow-up, research studies to date provide little support for it. Controlled clinical studies of supplementation with probiotic micro-organisms linked to improved intestinal health–Lactobacillus casei Shirota and another preparation providing a Lactobacillus, Bifidobacterium and Streptococcus thermophilus–have so far failed to demonstrate reductions in plasma TMAO.57 58 Faecal microbiota transplantation from vegan donors to recipients with metabolic syndrome, while it did succeed in altering the latter’s GI flora, did not lower their plasma TMAO levels.59 Administration of the cholesterol-lowering prebiotic glucaro-1,4-lactone to rats fed a high-fat diet, which markedly boosted intestinal levels of Lactobacillus, Bifidobacteria and Enterococcus, while suppressing Escherichia coli, was associated with an increase of TMAO in urine.60 Supplementation of mouse diets with either galacto-oligosaccharides/inulin or polydextrose and insoluble bran fibre increased serum TMAO levels, whereas supplementation with both simultaneously failed to influence TMAO.61
In one mouse study, supplementation with soluble fibre from wheat bran did lower colonic TMA lyase activity as well as serum cholesterol.62 However, it seems unlikely that an increased intake of protective soluble fibre explains the association of TMAO with vascular risk, since very ample intakes of soluble fibre are required to achieve a modest reduction in Low-density lipoprotein (LDL) cholesterol–intakes which very few people ingest; and in any case the associated risk persists after adjustment for lipid risk factors such as LDL cholesterol.63
While it is feasible to produce mice whose intestines have been colonised with bacteria with limited capacity to generate TMA, there so far is no evidence that this confers any special vascular protection on these mice when they eat normal diets.27
Elevated hepatic FMO3 activity can reflect hepatic insulin resistance
Which brings us to the alternative thesis: that modulation of hepatic FMO3 activity by certain factors that can influence CV health, can rationalise the epidemiology of TMAO. The regulation of hepatic FMO3 requires much further research, but several intriguing findings have emerged. Insulin suppresses FMO3 expression at both the messenger RNA (mRNA) and protein level; conversely, glucagon elevates FMO3 expression.64 Also, the FXR receptor, for which many bile acids serve as activating ligands, stimulates transcription of the FMO3 gene.29 65 With respect to the impact of insulin, genetically modified mice in which hepatic expression of the insulin receptor has been selectively ablated (Liver-specific insulin receptor knockout mice) have greatly enhanced hepatic expression of FMO3.64 These mice develop marked hypercholesterolaemia and are exceptionally prone to atherosclerosis when fed a proatherogenic diet, and also understandably have an elevated hepatic glucose output.66 The pertinence of these findings to humans has been clarified by a study in which liver biopsies were obtained both from obese subjects and lean controls; mRNA expression of FMO3 was about twice as high in the obese subjects, likely reflecting hepatic insulin resistance in the context of hyperinsulinaemia.64
Recent studies suggest that the hepatic insulin resistance associated with obesity and metabolic syndrome is mediated by increased hepatic influx of free fatty acids (FFAs), giving rise to increased levels of diacylglycerol; the latter promotes activation of protein kinase C-epsilon, which in term hampers the tyrosine kinase activity of the insulin receptor by phosphorylating threonine-1160 of the beta-chain.67–69 Other kinase or phosphatase activities stimulated by lipid overload may also impair insulin signalling at points downstream from the insulin receptor.70 71 Excess FFA influx also drives increased triglyceride synthesis, giving rise to the hepatic steatosis often associated with hepatic insulin resistance. However, increased hepatic triglyceride levels per se may not promote hepatic insulin resistance; such resistance correlates with hepatocyte levels of diacylglycerol, rather than of triglycerides.69 72
Hepatic insulin resistance and its common concomitant hepatic steatosis are associated with increased CV risk, as well as elevated risk for type 2 diabetes—risks likewise associated with elevated TMAO.66 73–77 It is, therefore, straightforward to postulate that TMAO can serve as a marker for hepatic insulin resistance, and that this explains at least a portion of the risk for CV events and diabetes linked to TMAO. Although studies establishing TMAO as an independent CV risk factor have often corrected for certain correlates of obesity, such as body mass index or diabetes, it is unlikely that such corrections fully capture the impact of hepatic insulin resistance.
Correcting hepatic insulin resistance
This analysis suggests that healthful measures which tend to correct hepatic insulin resistance may favourably impact the vascular and metabolic health of subjects with high TMAO. Evidently, sustained remediation of the visceral obesity which often underlies hepatic insulin resistance should be helpful in this regard; nonetheless, it is easier to recommend this than to achieve it! By improving the insulin sensitivity of hypertrophied adipocytes, thiazolidinediones such as pioglitazone tend to improve hepatic insulin resistance in people with diabetes by quelling excessive fatty acid efflux from adipocytes, even though they tend to increase body fat mass somewhat.78–81
Hormones and medications which boost hepatic AMPK activity tend to improve impaired hepatic insulin sensitivity. AMPK achieves this, at least in part, by downregulating mTORC1 activity, which acts indirectly to promote phosphorylations of insulin receptor substrate-1 that impede transmission of the insulin signal.82 Also, by promoting oxidative disposal of FFAs while suppressing lipogenesis, AMPK could be expected to lessen hepatic diacylglycerol synthesis, thereby getting to the root of hepatic insulin resistance.83 84 The favourable impact of metformin on hepatic insulin resistance in diabetes is thought to be mediated by activation of AMPK.85–88 The phytochemical nutraceutical berberine, widely used in China for the management of type 2 diabetes, is likewise thought to improve glycaemic control via activation of AMPK, and has been shown to counter hepatic insulin resistance in diabetic hamsters.89–93
Both adiponectin and glucagon-like peptide-1 (GLP-1) act on the liver to stimulate AMPK activity; moreover, they have been shown to combat hepatic insulin resistance, and work in various ways to promote vascular and metabolic health.94–106 Hence, elevated TMAO may often be a marker for suboptimal adiponectin and/or GLP-1 activity. The antidiabetic drug pioglitazone tends to boost the diminished adiponectin secretion of hypertrophied adipocytes.107 108 It seems likely that plant-based diets of rather low-protein content can increase adiponectin production, as these boost the liver’s production of fibroblast growth factor-21, one of whose major functions is to promote adiponectin secretion by adipocytes.109 110 Such diets are also useful for preventing or correcting the obesity that often underlies hepatic insulin resistance.111–113
With respect to GLP-1, acarbose, dietary lente carbohydrate, bile acid sequestrants and certain prebiotics can boost GLP-1 production, drugs inhibiting plasma dipeptidyl peptidase-4 can prolong its half-life, and injectable GLP-1 receptor agonists can mimic its bioactivity.114–117
PPARalpha agonists, such as fenofibrate, also promote hepatic fatty acid oxidation, owing to induction of a range of mitochondrial enzymes (including carnitine palmitoyl transferases-1a and -2, fatty acyl-CoA dehydrogenase, UCP-2) which catalyse such oxidation.118 119 Moreover, PPARalpha agonism also acts indirectly to stimulate AMPK in the liver and other tissues by boosting adiponectin production in adipose tissue; PPARalpha enhances hepatic synthesis and release of fibroblast growth factor-21, which in turn stimulates adiponectin synthesis in adipocytes.120–123 Not surprisingly, fenofibrate has been shown to decrease hepatic levels of diacylglycerol and alleviate hepatic insulin resistance in rodents fed diets high in fat and/or fructose.124–128 Moreover, fenofibrate therapy has been shown to reduce risk for CV events in patients with metabolic syndrome.118
There is recent evidence that the carotenoid antioxidant astaxanthin can also serve as a PPARalpha agonist, and, both in rodents and humans, alleviate the dyslipidaemia associated with metabolic syndrome.129–135 In obese mice, astaxanthin has been reported to improve hepatic insulin resistance.136 Krill oil provides esterified forms of astaxanthin which have superior bioavailability, as well as health-protective omega-3 fatty acids, oxidised forms of which likewise serve as PPARalpha agonists.137–140 Moreover, krill oil supplementation has been found to beneficially modulate serum lipid profile–including, intriguingly, a reduction in LDL cholesterol–in controlled clinical trials.141 Krill oil, even when compared with fish oil, suppresses hepatic steatosis in rodents.142–144 This may be due to its astaxanthin content, which is not found in fish oil. Moreover, krill oil, but not fish oil, reduces diacylglycerol and ceramide content in the liver.145 The phospholipid fraction of krill oil has also been noted to reduce hepatic glucose production, unlike fish oil.146 Thus, krill oil, being a source of highly bioavailable form of astaxanthin, appears to have additional advantages for reducing hepatic steatosis and hepatic insulin resistance compared with fish oil.
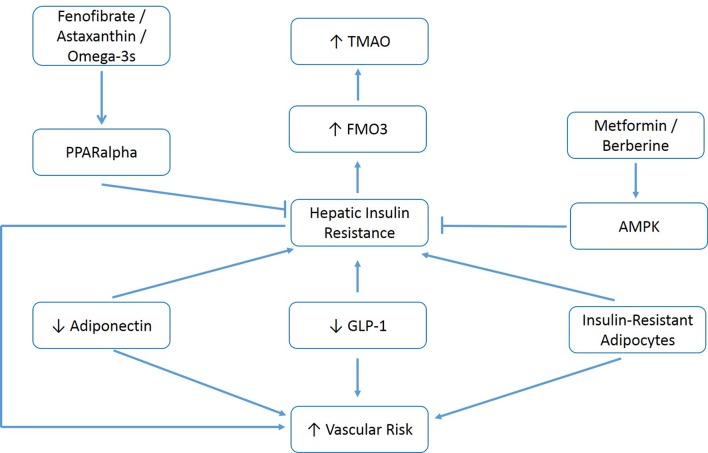
In brief, if this analysis is accurate, various measures which alleviate hepatic insulin resistance–correction of visceral obesity, activation of 5' adenosine monophosphate-activated protein kinase (AMPK) with metformin or berberine, activation of PPARalpha with fenofibrate or astaxanthin, amplification of adiponectin production with pioglitazone or plant-based diets, and clinical strategies which boost the production or bioactivity of GLP-1, could be expected to decrease elevated TMAO while also decreasing the risk for vascular events and diabetes associated with this risk factor. Figure 1 summarises these relationships.
Figure 1.
Measures which increase adiponectin, increase GLP-1 activity, control metabolic syndrome and activate hepatic AMPK or PPARalpha may decrease elevated TMAO and associated vascular/metabolic risk. GLP-1, glucagon-like peptide-1; TMAO, trimethylamine-N-oxid.
FMO3 might also mediate risk associated with elevated TMAO
One intriguing observation to emerge from TMAO research is that elevated hepatic expression of FMO3 boosts hepatic lipogenesis and gluconeogenesis, independent of its impact on TMAO levels; this might reflect FMO3’s ability to somehow support expression of FoxO1.30 64 This raises the interesting prospect that drugs selectively targeting FMO3 might have some utility in diabetes and hyperlipidaemia, particularly when elevated TMAO levels suggest that hepatic FMO3 expression is high. However, since FMO3 plays a systemic role in catecholamine metabolism, suppressing its function might not prove to be innocuous; genetic absence of FMO3 activity has been associated with hypertension.147 In any case, when hepatic insulin resistance is present, correcting this should lessen hepatic FMO3 expression.
Overview
Accumulating evidence points to elevated plasma TMAO as a risk factor for both atherosclerosis, CV events and type 2 diabetes, and rodent studies have found that extremely high dietary intakes of TMAO per se or its dietary precursors choline and carnitine are proatherogenic. Moreover, supraphysiological concentrations of TMAO exert proinflammatory effects in cell culture studies. These findings have led some observers to recommend that dietary or supplementary consumption of choline and carnitine should be minimised–although these analysts have rarely recommended abstinence from fish, the richest dietary source of preformed TMAO. In fact, a meta-analysis of pertinent nutritional epidemiology has failed to observe an impact of dietary choline on CV risk. Supplemental use of carnitine has been found to reduce mortality and diminish risk for arrhythmias and new-onset angina in patients who have suffered a previous MI, has shown clinical utility in angina, intermittent claudication and heart failure, and exerts antiatherogenic effects in rodents when fed at moderate levels comparable to human supplemental intake. And, fish consumption correlates dose dependently with favourable vascular outcomes. These findings point ineluctably to the conclusion that TMAO is not a mediating risk factor, at least in the concentrations seen in people whose renal function is not severely defective.
Hence, moderately elevated TMAO must be viewed as a marker for other factors that both raise TMAO and confer increased risk for vascular disease and diabetes. Plasma levels of TMAO are highly reflective of renal function, and hence a portion of the risk associated with elevated TMAO is mediated either by impaired renal function, or renotoxic factors that are also vasculotoxic or promote diabetes. Nonetheless, TMAO remains predictive of vascular risk after statistical correction for eGFR; factors influencing TMAO synthesis evidently mediate some of this risk. While it is theoretically possible that certain strains of GI bacteria possessing high TMA lyase activity exert adverse effects on vascular and metabolic health, this remains to be demonstrated, and efforts to lower plasma TMAO with probiotics thought to be health protective have so far failed.
Factors which upregulate hepatic expression and activity of FMO3, chiefly responsible for conversion of TMA to TMAO, therefore, fall under suspicion. In this regard, it is notable that subnormal hepatic insulin activity reflecting hepatic insulin resistance has been found to boost hepatic FMO3 expression. Hepatic insulin resistance is typically induced by the excessive FFA influx associated with metabolic syndrome and visceral obesity, well-known risk factors for vascular disease and diabetes. This excessive FFA influx also gives rise to hepatic steatosis; although excessive accumulation of triglycerides in the liver does not appear to mediate hepatic insulin resistance, it serves as a marker for the increased FFA influx that does. Subnormal activities of either adiponectin or GLP-1–both of which exert favourable vascular and metabolic effects–can also promote hepatic insulin resistance. It is, therefore, reasonable to speculate that lifestyle measures which reverse visceral obesity, or nutraceutical/drug/dietary measures which boost the production or bioactivity of adiponectin and/or GLP-1, will alleviate the risk associated with elevated TMAO by ameliorating hepatic insulin resistance. Activation of AMPK with metformin or berberine, or of PPARalpha with fenofibrate or astaxanthin, could also be expected to have a favourable impact in this regard, in part by accelerating the oxidative disposal of excessive hepatic FFAs. Finally, elevated FMO3 activity per se may mediate some of the risk associated with high TMAO via upregulation of hepatic lipogenesis and gluconeogenesis.
Importantly, this analysis does not exclude the possibility that TMAO might be directly pathogenic at the very elevated levels typically seen in severe kidney dysfunction. Indeed, cell culture studies suggest that TMAO can be proinflammatory in the plasma concentrations achieved during kidney failure. It generally is wise to minimise the consumption of nitrogenous compounds in this context.
In conclusion, there is a reason to suspect that the elevated risk for vascular events and type 2 diabetes associated with elevated TMAO, after correction for recognised risk factors, is mediated largely by hepatic insulin resistance and the metabolic factors which induce it. This implies that a range of measures which typically improve hepatic insulin sensitivity, as catalogued above, could be expected to decrease elevated TMAO–a proposition that is readily clinically testable–while ameliorating the vascular and metabolic risk associated with high TMAO.
Footnotes
Contributors: All authors contributed to the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: JJD is the author of The Salt Fix and Superfuel. MM: owner and science director of NutriGuard Research, a nutraceutical company which, among other things, sells berberine and astaxanthin supplements. JO: chief medical officer and founder of CardioTabs, a nutraceutical company which sells omega-3 supplements, and has a major ownership interest in the company.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Tang WHW, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med Overseas Ed 2013;368:1575–84. 10.1056/NEJMoa1109400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Senthong V, Wang Z, Li XS, et al. Intestinal Microbiota‐Generated Metabolite Trimethylamine‐ N‐ Oxide and 5‐Year Mortality Risk in Stable Coronary Artery Disease: The Contributory Role of Intestinal Microbiota in a COURAGE‐Like Patient Cohort. J Am Heart Assoc 2016;5 10.1161/JAHA.115.002816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lever M, George PM, Slow S, et al. Betaine and trimethylamine-N-oxide as predictors of cardiovascular outcomes show different patterns in diabetes mellitus: an observational study. PLoS One 2014;9:e114969 10.1371/journal.pone.0114969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Suzuki T, Heaney LM, Jones DJL, et al. Trimethylamine N-oxide and risk stratification after acute myocardial infarction. Clin Chem 2017;63:420–8. 10.1373/clinchem.2016.264853 [DOI] [PubMed] [Google Scholar]
- 5. Li XS, Obeid S, Klingenberg R, et al. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J 2017;38:814–24. 10.1093/eurheartj/ehw582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meyer KA, Benton TZ, Bennett BJ, et al. Microbiota‐Dependent metabolite trimethylamine N‐Oxide and coronary artery calcium in the coronary artery risk development in young adults study (cardia). J Am Heart Assoc 2016;5 10.1161/JAHA.116.003970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yin J, Liao Shuo‐Xi, He Y, et al. Dysbiosis of gut microbiota with reduced Trimethylamine‐N‐Oxide level in patients with Large‐Artery atherosclerotic stroke or transient ischemic attack. J Am Heart Assoc 2015;4 10.1161/JAHA.115.002699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qi J, You T, Li J, et al. Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: a systematic review and meta-analysis of 11 prospective cohort studies. J Cell Mol Med 2018;22:185–94. 10.1111/jcmm.13307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472:57–63. 10.1038/nature09922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013;19:576–85. 10.1038/nm.3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Z, Roberts AB, Buffa JA, et al. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 2015;163:1585–95. 10.1016/j.cell.2015.11.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boini KM, Hussain T, Li P-L, et al. Trimethylamine-N-oxide instigates NLRP3 inflammasome activation and endothelial dysfunction. Cell Physiol Biochem 2017;44:152–62. 10.1159/000484623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen Ming‐liang, Zhu Xiao‐hui, Ran L, et al. Trimethylamine‐N‐Oxide induces vascular inflammation by activating the NLRP3 inflammasome through the SIRT3‐SOD2‐mtROS signaling pathway. J Am Heart Assoc 2017;6 10.1161/JAHA.117.006347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen M-liang, Yi L, Zhang Y, et al. Resveratrol Attenuates Trimethylamine-N-Oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. MBio 2016;7:e02210–5. 10.1128/mBio.02210-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seldin MM, Meng Y, Qi H, et al. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κB. J Am Heart Assoc 2016;5 10.1161/JAHA.115.002767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ma G, Pan B, Chen Y, et al. Trimethylamine N-oxide in atherogenesis: impairing endothelial self-repair capacity and enhancing monocyte adhesion. Biosci Rep 2017;37 10.1042/BSR20160244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sun X, Jiao X, Ma Y, et al. Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem Biophys Res Commun 2016;481:63–70. 10.1016/j.bbrc.2016.11.017 [DOI] [PubMed] [Google Scholar]
- 18. Tang WHW, Wang Z, Li XS, et al. Increased trimethylamine N-oxide portends high mortality risk independent of glycemic control in patients with type 2 diabetes mellitus. Clin Chem 2017;63:297–306. 10.1373/clinchem.2016.263640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dambrova M, Latkovskis G, Kuka J, et al. Diabetes is associated with higher trimethylamine N-oxide plasma levels. Exp Clin Endocrinol Diabetes 2016;124:251–6. 10.1055/s-0035-1569330 [DOI] [PubMed] [Google Scholar]
- 20. Shan Z, Sun T, Huang H, et al. Association between microbiota-dependent metabolite trimethylamine-N-oxide and type 2 diabetes. Am J Clin Nutr 2017;106:ajcn157107–94. 10.3945/ajcn.117.157107 [DOI] [PubMed] [Google Scholar]
- 21. Samerotte AL, Drazen JC, Brand GL, et al. Correlation of trimethylamine oxide and habitat depth within and among species of teleost fish: an analysis of causation. Physiol Biochem Zool 2007;80:197–208. 10.1086/510566 [DOI] [PubMed] [Google Scholar]
- 22. McCarty MF. L-carnitine consumption, its metabolism by intestinal microbiota, and cardiovascular health. Mayo Clin Proc 2013;88:786–9. 10.1016/j.mayocp.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 23. Ussher JR, Lopaschuk GD, Arduini A. Gut microbiota metabolism of L-carnitine and cardiovascular risk. Atherosclerosis 2013;231:456–61. 10.1016/j.atherosclerosis.2013.10.013 [DOI] [PubMed] [Google Scholar]
- 24. Landfald B, Valeur J, Berstad A, et al. Microbial trimethylamine-N-oxide as a disease marker: something fishy? Microb Ecol Health Dis 2017;28 10.1080/16512235.2017.1327309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang AQ, Mitchell SC, Smith RL. Dietary precursors of trimethylamine in man: a pilot study. Food Chem Toxicol 1999;37:515–20. 10.1016/S0278-6915(99)00028-9 [DOI] [PubMed] [Google Scholar]
- 26. XS L, Wang Z, Cajka T, et al. Untargeted metabolomics identifies trimethyllysine, a TMAO-producing nutrient precursor, as a predictor of incident cardiovascular disease risk. JCI Insight 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Romano KA, Vivas EI, Amador-Noguez D, et al. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. MBio 2015;6:e02481 10.1128/mBio.02481-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gregory JC, Buffa JA, Org E, et al. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J Biol Chem 2015;290:5647–60. 10.1074/jbc.M114.618249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bennett BJ, de Aguiar Vallim TQ, Wang Z, et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab 2013;17:49–60. 10.1016/j.cmet.2012.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shih DM, Wang Z, Lee R, et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res 2015;56:22–37. 10.1194/jlr.M051680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meyer K, Shea J. Dietary choline and betaine and risk of CVD: a systematic review and meta-analysis of prospective studies. Nutrients 2017;9 10.3390/nu9070711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alexander DD, Miller PE, Vargas AJ, et al. Meta-analysis of egg consumption and risk of coronary heart disease and stroke. J Am Coll Nutr 2016;35:704–16. 10.1080/07315724.2016.1152928 [DOI] [PubMed] [Google Scholar]
- 33. DiNicolantonio JJ, Lavie CJ, Fares H, et al. L-carnitine in the secondary prevention of cardiovascular disease: systematic review and meta-analysis. Mayo Clin Proc 2013;88:544–51. 10.1016/j.mayocp.2013.02.007 [DOI] [PubMed] [Google Scholar]
- 34. Cherchi A, Lai C, Angelino F, et al. Effects of L-carnitine on exercise tolerance in chronic stable angina: a multicenter, double-blind, randomized, placebo controlled crossover study. Int J Clin Pharmacol Ther Toxicol 1985;23:569–72. [PubMed] [Google Scholar]
- 35. Cacciatore L, Cerio R, Ciarimboli M, et al. The therapeutic effect of L-carnitine in patients with exercise-induced stable angina: a controlled study. Drugs Exp Clin Res 1991;17:225–35. [PubMed] [Google Scholar]
- 36. Brevetti G, Chiariello M, Ferulano G, et al. Increases in walking distance in patients with peripheral vascular disease treated with L-carnitine: a double-blind, cross-over study. Circulation 1988;77:767–73. 10.1161/01.CIR.77.4.767 [DOI] [PubMed] [Google Scholar]
- 37. Andreozzi GM. Propionyl L-carnitine: Intermittent claudication and peripheral arterial disease. Expert Opin Pharmacother 2009;10:2697–707. 10.1517/14656560903215871 [DOI] [PubMed] [Google Scholar]
- 38. Anand I, Chandrashekhan Y, De Giuli F, et al. Acute and chronic effects of propionyl-L-carnitine on the hemodynamics, exercise capacity, and hormones in patients with congestive heart failure. Cardiovasc Drugs Ther 1998;12:291–9. 10.1023/A:1007721917561 [DOI] [PubMed] [Google Scholar]
- 39. Mancini M, Rengo F, Lingetti M, et al. Controlled study on the therapeutic efficacy of propionyl-L-carnitine in patients with congestive heart failure. Arzneimittelforschung 1992;42:1101–4. [PubMed] [Google Scholar]
- 40. Spagnoli LG, Orlandi A, Marino B, et al. Propionyl-L-carnitine prevents the progression of atherosclerotic lesions in aged hyperlipemic rabbits. Atherosclerosis 1995;114:29–44. 10.1016/0021-9150(94)05460-Z [DOI] [PubMed] [Google Scholar]
- 41. Sayed-Ahmed MM, Khattab MM, Gad MZ, et al. L-carnitine prevents the progression of atherosclerotic lesions in hypercholesterolaemic rabbits. Pharmacol Res 2001;44:235–42. 10.1006/phrs.2001.0852 [DOI] [PubMed] [Google Scholar]
- 42. Collins HL, Drazul-Schrader D, Sulpizio AC, et al. L-Carnitine intake and high trimethylamine N-oxide plasma levels correlate with low aortic lesions in ApoE(-/-) transgenic mice expressing CETP. Atherosclerosis 2016;244:29–37. 10.1016/j.atherosclerosis.2015.10.108 [DOI] [PubMed] [Google Scholar]
- 43. He K, Song Y, Daviglus ML, et al. Accumulated evidence on fish consumption and coronary heart disease mortality: a meta-analysis of cohort studies. Circulation 2004;109:2705–11. 10.1161/01.CIR.0000132503.19410.6B [DOI] [PubMed] [Google Scholar]
- 44. Rizos EC, Ntzani EE, Bika E, et al. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA 2012;308:1024–33. 10.1001/2012.jama.11374 [DOI] [PubMed] [Google Scholar]
- 45. Khoueiry G, Abi Rafeh N, Sullivan E, et al. Do omega-3 polyunsaturated fatty acids reduce risk of sudden cardiac death and ventricular arrhythmias? A meta-analysis of randomized trials. Heart Lung 2013;42:251–6. 10.1016/j.hrtlng.2013.03.006 [DOI] [PubMed] [Google Scholar]
- 46. Casula M, Soranna D, Catapano AL, et al. Long-term effect of high dose omega-3 fatty acid supplementation for secondary prevention of cardiovascular outcomes: A meta-analysis of randomized, placebo controlled trials [corrected]. Atheroscler Suppl 2013;14:243–51. 10.1016/S1567-5688(13)70005-9 [DOI] [PubMed] [Google Scholar]
- 47. Cho CE, Caudill MA. Trimethylamine-N-oxide: friend, foe, or simply caught in the cross-fire? Trends Endocrinol Metab 2017;28:121–30. 10.1016/j.tem.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 48. Velasquez MT, Ramezani A, Manal A, et al. Trimethylamine N-oxide: the good, the bad and the unknown. Toxins 2016;8 10.3390/toxins8110326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bazzano LA, Reynolds K, Holder KN, et al. Effect of folic acid supplementation on risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. JAMA 2006;296:2720–6. 10.1001/jama.296.22.2720 [DOI] [PubMed] [Google Scholar]
- 50. Martí-Carvajal AJ, Solà I, Lathyris D, et al. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev 2017;96 10.1002/14651858.CD006612.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Missailidis C, Hällqvist J, Qureshi AR, et al. Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS One 2016;11:e0141738 10.1371/journal.pone.0141738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010;375:2073–81. 10.1016/S0140-6736(10)60674-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lippi G, Danese E, Mattiuzzi C, et al. The intriguing link between the intestinal microbiota and cardiovascular disease. Semin Thromb Hemost 2017;43:609–13. 10.1055/s-0036-1597903 [DOI] [PubMed] [Google Scholar]
- 54. Kobyliak N, Virchenko O, Falalyeyeva T. Pathophysiological role of host microbiota in the development of obesity. Nutr J 2015;15 10.1186/s12937-016-0166-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Simon M-C, Strassburger K, Nowotny B, et al. Intake of Lactobacillus reuteri improves incretin and insulin secretion in glucose-tolerant humans: a proof of concept. Diabetes Care 2015;38:1827–34. 10.2337/dc14-2690 [DOI] [PubMed] [Google Scholar]
- 56. Costabile A, Buttarazzi I, Kolida S, et al. An in vivo assessment of the cholesterol-lowering efficacy of Lactobacillus plantarum ECGC 13110402 in normal to mildly hypercholesterolaemic adults. PLoS One 2017;12:e0187964 10.1371/journal.pone.0187964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tripolt NJ, Leber B, Triebl A, et al. Effect of Lactobacillus casei Shirota supplementation on trimethylamine-N-oxide levels in patients with metabolic syndrome: an open-label, randomized study. Atherosclerosis 2015;242:141–4. 10.1016/j.atherosclerosis.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 58. Boutagy NE, Neilson AP, Osterberg KL, et al. Probiotic supplementation and trimethylamine-N-oxide production following a high-fat diet. Obesity 2015;23:2357–63. 10.1002/oby.21212 [DOI] [PubMed] [Google Scholar]
- 59. Smits LP, Kootte RS, Levin E, et al. Effect of vegan fecal microbiota transplantation on Carnitine- and Choline-Derived trimethylamine-N-oxide production and vascular inflammation in patients with metabolic syndrome. J Am Heart Assoc 2018;7 10.1161/JAHA.117.008342. [Epub ahead of print: 26 Mar 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Xie B, Liu A, Zhan X, et al. Alteration of gut bacteria and metabolomes after Glucaro-1,4-lactone treatment contributes to the prevention of hypercholesterolemia. J Agric Food Chem 2014;62:7444–51. 10.1021/jf501744d [DOI] [PubMed] [Google Scholar]
- 61. Cheng W, Lu J, Li B, et al. Effect of functional oligosaccharides and ordinary dietary fiber on intestinal microbiota diversity. Front Microbiol 2017;8 10.3389/fmicb.2017.01750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li Q, Wu T, Liu R, et al. Soluble dietary fiber reduces trimethylamine metabolism via gut microbiota and co-regulates host AMPK pathways. Mol Nutr Food Res 2017;61 10.1002/mnfr.201700473 [DOI] [PubMed] [Google Scholar]
- 63. Doi K. Effect of konjac fibre (glucomannan) on glucose and lipids. Eur J Clin Nutr 1995;49 Suppl 3(Suppl 3):S190–S197. [PubMed] [Google Scholar]
- 64. Miao J, Ling AV, Manthena PV, et al. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat Commun 2015;6 10.1038/ncomms7498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jiang Y, Jin J, Iakova P, et al. Farnesoid X receptor directly regulates xenobiotic detoxification genes in the long-lived little mice. Mech Ageing Dev 2013;134:407–15. 10.1016/j.mad.2013.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Biddinger SB, Hernandez-Ono A, Rask-Madsen C, et al. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab 2008;7:125–34. 10.1016/j.cmet.2007.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jornayvaz FR, Shulman GI. Diacylglycerol activation of protein kinase Cε and hepatic insulin resistance. Cell Metab 2012;15:574–84. 10.1016/j.cmet.2012.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Petersen MC, Madiraju AK, Gassaway BM, et al. Insulin receptor Thr1160 phosphorylation mediates lipid-induced hepatic insulin resistance. J Clin Invest 2016;126:4361–71. 10.1172/JCI86013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ter Horst KW, Gilijamse PW, Versteeg RI, et al. Hepatic diacylglycerol-associated protein kinase Cε translocation links hepatic steatosis to hepatic insulin resistance in humans. Cell Rep 2017;19:1997–2004. 10.1016/j.celrep.2017.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Park E, Wong V, Guan X, et al. Salicylate prevents hepatic insulin resistance caused by short-term elevation of free fatty acids in vivo. J Endocrinol 2007;195:323–31. 10.1677/JOE-07-0005 [DOI] [PubMed] [Google Scholar]
- 71. Sajan MP, Ivey RA, Lee MC, et al. Hepatic insulin resistance in ob/ob mice involves increases in ceramide, aPKC activity, and selective impairment of Akt-dependent FoxO1 phosphorylation. J Lipid Res 2015;56:70–80. 10.1194/jlr.M052977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cantley JL, Yoshimura T, Camporez JPG, et al. CGI-58 knockdown sequesters diacylglycerols in lipid droplets/ER-preventing diacylglycerol-mediated hepatic insulin resistance. Proceedings of the National Academy of Sciences 2013;110:1869–74. 10.1073/pnas.1219456110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jin R, Le N-A, Cleeton R, et al. Amount of hepatic fat predicts cardiovascular risk independent of insulin resistance among Hispanic-American adolescents. Lipids Health Dis 2015;14 10.1186/s12944-015-0038-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ballestri S, Lonardo A, Bonapace S, et al. Risk of cardiovascular, cardiac and arrhythmic complications in patients with non-alcoholic fatty liver disease. World J Gastroenterol 2014;20:1724–45. 10.3748/wjg.v20.i7.1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Privitera G, Spadaro L, Alagona C, et al. Hepatic insulin resistance in NAFLD: relationship with markers of atherosclerosis and metabolic syndrome components. Acta Diabetol 2016;53:449–59. 10.1007/s00592-015-0816-y [DOI] [PubMed] [Google Scholar]
- 76. Agouni A, Tual-Chalot S, Chalopin M, et al. Hepatic protein tyrosine phosphatase 1B (PTP1B) deficiency protects against obesity-induced endothelial dysfunction. Biochem Pharmacol 2014;92:607–17. 10.1016/j.bcp.2014.10.008 [DOI] [PubMed] [Google Scholar]
- 77. Lallukka S, Yki-Järvinen H. Non-alcoholic fatty liver disease and risk of type 2 diabetes. Best Pract Res Clin Endocrinol Metab 2016;30:385–95. 10.1016/j.beem.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 78. Mayerson AB, Hundal RS, Dufour S, et al. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes 2002;51:797–802. 10.2337/diabetes.51.3.797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Defronzo RA. Dysfunctional fat cells, lipotoxicity and type 2 diabetes. Int J Clin Pract 2004;58(Suppl. 3):9–21. 10.1111/j.1368-504X.2004.00389.x [DOI] [PubMed] [Google Scholar]
- 80. Sugiyama Y, Shimura Y, Ikeda H. Effects of pioglitazone on hepatic and peripheral insulin resistance in Wistar fatty rats. Arzneimittelforschung 1990;40:436–40. [PubMed] [Google Scholar]
- 81. Miyazaki Y, Mahankali A, Matsuda M, et al. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab 2002;87:2784–91. 10.1210/jcem.87.6.8567 [DOI] [PubMed] [Google Scholar]
- 82. Mordier S, Iynedjian PB. Activation of mammalian target of rapamycin complex 1 and insulin resistance induced by palmitate in hepatocytes. Biochem Biophys Res Commun 2007;362:206–11. 10.1016/j.bbrc.2007.08.004 [DOI] [PubMed] [Google Scholar]
- 83. Wang Q, Liu S, Zhai A, et al. AMPK-mediated regulation of lipid metabolism by phosphorylation. Biol Pharm Bull 2018;41:985–93. 10.1248/bpb.b17-00724 [DOI] [PubMed] [Google Scholar]
- 84. Viollet B, Guigas B, Leclerc J, et al. AMP-activated protein kinase in the regulation of hepatic energy metabolism: from physiology to therapeutic perspectives. Acta Physiol 2009;196:81–98. 10.1111/j.1748-1716.2009.01970.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zabielski P, Hady HR, Chacinska M, et al. The effect of high fat diet and metformin treatment on liver lipids accumulation and their impact on insulin action. Sci Rep 2018;8 10.1038/s41598-018-25397-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gómez-Sámano Miguel Ángel, Gulias-Herrero A, Cuevas-Ramos D, et al. Metformin and improvement of the hepatic insulin resistance index independent of anthropometric changes. Endocr Pract 2012;18:8–16. 10.4158/EP11072.OR [DOI] [PubMed] [Google Scholar]
- 87. Tiikkainen M, Häkkinen A-M, Korsheninnikova E, et al. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes 2004;53:2169–76. 10.2337/diabetes.53.8.2169 [DOI] [PubMed] [Google Scholar]
- 88. Lin HZ, Yang SQ, Chuckaree C, et al. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med 2000;6:998–1003. 10.1038/79697 [DOI] [PubMed] [Google Scholar]
- 89. Lan J, Zhao Y, Dong F, et al. Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J Ethnopharmacol 2015;161:69–81. 10.1016/j.jep.2014.09.049 [DOI] [PubMed] [Google Scholar]
- 90. Dong H, Wang N, Zhao L, et al. Berberine in the treatment of type 2 diabetes mellitus: a systemic review and meta-analysis. Evidence-Based Complementary and Alternative Medicine 2012;2012:1–12. 10.1155/2012/591654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lee YS, Kim WS, Kim KH, et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes 2006;55:2256–64. 10.2337/db06-0006 [DOI] [PubMed] [Google Scholar]
- 92. Turner N, Li J-Y, Gosby A, et al. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes 2008;57:1414–8. 10.2337/db07-1552 [DOI] [PubMed] [Google Scholar]
- 93. Liu X, Li G, Zhu H, et al. Beneficial effect of berberine on hepatic insulin resistance in diabetic hamsters possibly involves in SREBPs, LXRα and PPARα transcriptional programs. Endocr J 2010;57:881–93. 10.1507/endocrj.K10E-043 [DOI] [PubMed] [Google Scholar]
- 94. Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 2002;8:1288–95. 10.1038/nm788 [DOI] [PubMed] [Google Scholar]
- 95. Nawrocki AR, Rajala MW, Tomas E, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem 2006;281:2654–60. 10.1074/jbc.M505311200 [DOI] [PubMed] [Google Scholar]
- 96. Viollet B, Foretz M, Guigas B, et al. Activation of AMP-activated protein kinase in the liver: a new strategy for the management of metabolic hepatic disorders. J Physiol 2006;574:41–53. 10.1113/jphysiol.2006.108506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Whitehead JP, Richards AA, Hickman IJ, et al. Adiponectin--a key adipokine in the metabolic syndrome. Diabetes Obes Metab 2006;8:264–80. 10.1111/j.1463-1326.2005.00510.x [DOI] [PubMed] [Google Scholar]
- 98. Ben-Shlomo S, Zvibel I, Shnell M, et al. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J Hepatol 2011;54:1214–23. 10.1016/j.jhep.2010.09.032 [DOI] [PubMed] [Google Scholar]
- 99. Yamazaki S, Satoh H, Watanabe T. Liraglutide enhances insulin sensitivity by activating AMP-activated protein kinase in male Wistar rats. Endocrinology 2014;155:3288–301. 10.1210/en.2013-2157 [DOI] [PubMed] [Google Scholar]
- 100. Svegliati-Baroni G, Saccomanno S, Rychlicki C, et al. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int 2011;31:1285–97. 10.1111/j.1478-3231.2011.02462.x [DOI] [PubMed] [Google Scholar]
- 101. He Q, Sha S, Sun L, et al. GLP-1 analogue improves hepatic lipid accumulation by inducing autophagy via AMPK/mTOR pathway. Biochem Biophys Res Commun 2016;476:196–203. 10.1016/j.bbrc.2016.05.086 [DOI] [PubMed] [Google Scholar]
- 102. Armstrong MJ, Hull D, Guo K, et al. Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. J Hepatol 2016;64:399–408. 10.1016/j.jhep.2015.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Cani PD, Knauf C, Iglesias MA, et al. Improvement of glucose tolerance and hepatic insulin sensitivity by oligofructose requires a functional glucagon-like peptide 1 receptor. Diabetes 2006;55:1484–90. 10.2337/db05-1360 [DOI] [PubMed] [Google Scholar]
- 104. Rizzo M, Nikolic D, Patti AM, et al. GLP-1 receptor agonists and reduction of cardiometabolic risk: Potential underlying mechanisms. Biochim Biophys Acta Mol Basis Dis 2018;1864:2814–21. 10.1016/j.bbadis.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 105. Ji Q. Treatment strategy for type 2 diabetes with obesity: focus on glucagon-like peptide-1 receptor agonists. Clin Ther 2017;39:1244–64. 10.1016/j.clinthera.2017.03.013 [DOI] [PubMed] [Google Scholar]
- 106. Achari AE, Jain SK, Adiponectin JSK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci 2017;18 10.3390/ijms18061321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bodles AM, Banga A, Rasouli N, et al. Pioglitazone increases secretion of high-molecular-weight adiponectin from adipocytes. Am J Physiol Endocrinol Metab 2006;291:E1100–E1105. 10.1152/ajpendo.00187.2006 [DOI] [PubMed] [Google Scholar]
- 108. Pereira RI, Leitner JW, Erickson C, et al. Pioglitazone acutely stimulates adiponectin secretion from mouse and human adipocytes via activation of the phosphatidylinositol 3'-kinase. Life Sci 2008;83:638–43. 10.1016/j.lfs.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 109. Fontana L, Cummings NE, Arriola Apelo SI, et al. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep 2016;16:520–30. 10.1016/j.celrep.2016.05.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. McCarty MF. The moderate essential amino acid restriction entailed by low-protein vegan diets may promote vascular health by stimulating FGF21 secretion. Horm Mol Biol Clin Investig 2016;30 10.1515/hmbci-2015-0056 [DOI] [PubMed] [Google Scholar]
- 111. McCarty MF. GCN2 and FGF21 are likely mediators of the protection from cancer, autoimmunity, obesity, and diabetes afforded by vegan diets. Med Hypotheses 2014;83:365–71. 10.1016/j.mehy.2014.06.014 [DOI] [PubMed] [Google Scholar]
- 112. Spencer EA, Appleby PN, Davey GK, et al. Diet and body mass index in 38 000 EPIC-Oxford meat-eaters, fish-eaters, vegetarians and vegans. Int J Obes 2003;27:728–34. 10.1038/sj.ijo.0802300 [DOI] [PubMed] [Google Scholar]
- 113. Toohey ML, Harris MA, DeWitt W, Williams D, et al. Cardiovascular disease risk factors are lower in African-American vegans compared to lacto-ovo-vegetarians. J Am Coll Nutr 1998;17:425–34. 10.1080/07315724.1998.10718789 [DOI] [PubMed] [Google Scholar]
- 114. McCarty MF, DiNicolantonio JJ. Acarbose, lente carbohydrate, and prebiotics promote metabolic health and longevity by stimulating intestinal production of GLP-1. Open Heart 2015;2:e000205 10.1136/openhrt-2014-000205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Holst JJ, McGill MA. Potential new approaches to modifying intestinal GLP-1 secretion in patients with type 2 diabetes mellitus. Clin Drug Investig 2012;32:1–14. 10.2165/11595370-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 116. Li N, Wang L-J, Jiang B, et al. Recent progress of the development of dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes mellitus. Eur J Med Chem 2018;151:145–57. 10.1016/j.ejmech.2018.03.041 [DOI] [PubMed] [Google Scholar]
- 117. Men P, Li X-T, Tang H-L, et al. Efficacy and safety of saxagliptin in patients with type 2 diabetes: a systematic review and meta-analysis. PLoS One 2018;13:e0197321 10.1371/journal.pone.0197321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Tenenbaum A, Fisman EZ. Fibrates are an essential part of modern anti-dyslipidemic arsenal: spotlight on atherogenic dyslipidemia and residual risk reduction. Cardiovasc Diabetol 2012;11 10.1186/1475-2840-11-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Botta M, Audano M, Sahebkar A, et al. PPAR agonists and metabolic syndrome: an established role? Int J Mol Sci 2018;19 10.3390/ijms19041197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Badman MK, Pissios P, Kennedy AR, et al. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 2007;5:426–37. 10.1016/j.cmet.2007.05.002 [DOI] [PubMed] [Google Scholar]
- 121. Lundåsen T, Hunt MC, Nilsson L-M, et al. PPARalpha is a key regulator of hepatic FGF21. Biochem Biophys Res Commun 2007;360:437–40. 10.1016/j.bbrc.2007.06.068 [DOI] [PubMed] [Google Scholar]
- 122. Lin Z, Tian H, Lam KSL, et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metabolism 2013;17:779–89. 10.1016/j.cmet.2013.04.005 [DOI] [PubMed] [Google Scholar]
- 123. Hui X, Feng T, Liu Q, et al. The FGF21-adiponectin axis in controlling energy and vascular homeostasis. J Mol Cell Biol 2016;8:110–9. 10.1093/jmcb/mjw013 [DOI] [PubMed] [Google Scholar]
- 124. Chan SMH, Zeng X-Y, Sun R-Q, et al. Fenofibrate insulates diacylglycerol in lipid droplet/ER and preserves insulin signaling transduction in the liver of high fat fed mice. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 2015;1852:1511–9. 10.1016/j.bbadis.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 125. Chan SMH, Sun R-Q, Zeng X-Y, et al. Activation of PPARα ameliorates hepatic insulin resistance and steatosis in high fructose-fed mice despite increased endoplasmic reticulum stress. Diabetes 2013;62:2095–105. 10.2337/db12-1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kostapanos MS, Kei A, Elisaf MS. Current role of fenofibrate in the prevention and management of non-alcoholic fatty liver disease. World J Hepatol 2013;5:470–8. 10.4254/wjh.v5.i9.470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. de la Monte SM, Pang M, Chaudhry R, et al. Peroxisome proliferator-activated receptor agonist treatment of alcohol-induced hepatic insulin resistance. Hepatol Res 2011;41:386–98. 10.1111/j.1872-034X.2011.00775.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Neschen S, Morino K, Dong J, et al. N-3 fatty acids preserve insulin sensitivity in vivo in a peroxisome proliferator-activated receptor-alpha-dependent manner. Diabetes 2007;56:1034–41. 10.2337/db06-1206 [DOI] [PubMed] [Google Scholar]
- 129. Jia Y, Kim J-Y, Jun H-J, et al. The natural carotenoid astaxanthin, a PPAR-α agonist and PPAR-γ antagonist, reduces hepatic lipid accumulation by rewiring the transcriptome in lipid-loaded hepatocytes. Mol Nutr Food Res 2012;56:878–88. 10.1002/mnfr.201100798 [DOI] [PubMed] [Google Scholar]
- 130. Jia Y, Wu C, Kim J, et al. Astaxanthin reduces hepatic lipid accumulations in high-fat-fed C57BL/6J mice via activation of peroxisome proliferator-activated receptor (PPAR) alpha and inhibition of PPAR gamma and Akt. J Nutr Biochem 2016;28:9–18. 10.1016/j.jnutbio.2015.09.015 [DOI] [PubMed] [Google Scholar]
- 131. Hussein G, Nakagawa T, Goto H, et al. Astaxanthin ameliorates features of metabolic syndrome in SHR/NDmcr-cp. Life Sci 2007;80:522–9. 10.1016/j.lfs.2006.09.041 [DOI] [PubMed] [Google Scholar]
- 132. Ikeuchi M, Koyama T, Takahashi J, et al. Effects of astaxanthin in obese mice fed a high-fat diet. Biosci Biotechnol Biochem 2007;71:893–9. 10.1271/bbb.60521 [DOI] [PubMed] [Google Scholar]
- 133. Yoshida H, Yanai H, Ito K, et al. Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis 2010;209:520–3. 10.1016/j.atherosclerosis.2009.10.012 [DOI] [PubMed] [Google Scholar]
- 134. Choi HD, Youn YK, Shin WG. Positive effects of astaxanthin on lipid profiles and oxidative stress in overweight subjects. Plant Foods Hum Nutr 2011;66:363–9. 10.1007/s11130-011-0258-9 [DOI] [PubMed] [Google Scholar]
- 135. Yang Y, Pham TX, Wegner CJ, et al. Astaxanthin lowers plasma tag concentrations and increases hepatic antioxidant gene expression in diet-induced obesity mice. Br J Nutr 2014;112:1797–804. 10.1017/S0007114514002554 [DOI] [PubMed] [Google Scholar]
- 136. Ni Y, Nagashimada M, Zhuge F, et al. Astaxanthin prevents and reverses diet-induced insulin resistance and steatohepatitis in mice: a comparison with vitamin E. Sci Rep 2015;5 10.1038/srep17192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Takaichi S, Matsui K, Nakamura M, et al. Fatty acids of astaxanthin esters in krill determined by mild mass spectrometry. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 2003;136:317–22. 10.1016/S1096-4959(03)00209-4 [DOI] [PubMed] [Google Scholar]
- 138. Aoi W, Maoka T, Abe R, et al. Comparison of the effect of non-esterified and esterified astaxanthins on endurance performance in mice. J Clin Biochem Nutr 2018;62:161–6. 10.3164/jcbn.17-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Sethi S, Ziouzenkova O, Ni H, et al. Oxidized omega-3 fatty acids in fish oil inhibit leukocyte-endothelial interactions through activation of PPAR alpha. Blood 2002;100:1340–6. 10.1182/blood-2002-01-0316 [DOI] [PubMed] [Google Scholar]
- 140. Mishra A, Chaudhary A, Sethi S. Oxidized omega-3 fatty acids inhibit NF-κB activation via a PPARα-Dependent pathway. Arterioscler Thromb Vasc Biol 2004;24:1621–7. 10.1161/01.ATV.0000137191.02577.86 [DOI] [PubMed] [Google Scholar]
- 141. Ursoniu S, Sahebkar A, Serban M-C, et al. Lipid-modifying effects of krill oil in humans: systematic review and meta-analysis of randomized controlled trials. Nutr Rev 2017;75:361–73. 10.1093/nutrit/nuw063 [DOI] [PubMed] [Google Scholar]
- 142. Vigerust NF, Bjørndal B, Bohov P, et al. Krill oil versus fish oil in modulation of inflammation and lipid metabolism in mice transgenic for TNF-α. Eur J Nutr 2013;52:1315–25. 10.1007/s00394-012-0441-2 [DOI] [PubMed] [Google Scholar]
- 143. Tandy S, Chung RWS, Wat E, et al. Dietary krill oil supplementation reduces hepatic steatosis, glycemia, and hypercholesterolemia in high-fat-fed mice. J Agric Food Chem 2009;57:9339–45. 10.1021/jf9016042 [DOI] [PubMed] [Google Scholar]
- 144. Ferramosca A, Conte L, Zara V. A krill oil supplemented diet reduces the activities of the mitochondrial tricarboxylate carrier and of the cytosolic lipogenic enzymes in rats. J Anim Physiol Anim Nutr 2012;96:295–306. 10.1111/j.1439-0396.2011.01135.x [DOI] [PubMed] [Google Scholar]
- 145. Skorve J, Hilvo M, Vihervaara T, et al. Fish oil and krill oil differentially modify the liver and brain lipidome when fed to mice. Lipids Health Dis 2015;14 10.1186/s12944-015-0086-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Burri L, Berge K, Wibrand K, et al. Differential effects of krill oil and fish oil on the hepatic transcriptome in mice. Front Genet 2011;2 10.3389/fgene.2011.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Dolan C, Shields DC, Stanton A, et al. Polymorphisms of the flavin containing monooxygenase 3 (FMO3) gene do not predispose to essential hypertension in Caucasians. BMC Med Genet 2005;6 10.1186/1471-2350-6-41 [DOI] [PMC free article] [PubMed] [Google Scholar]