Abstract
Background
Ethnic disparities have been shown in respiratory syncytial virus (RSV) bronchiolitis. However, it is unclear whether such differences are related to access to care. We compared demographic and clinical characteristics of Arab and Jewish children hospitalized for RSV bronchiolitis in Israel, a country with universal health insurance.
Methods
We reviewed the medical records of all children (n = 309) aged less than 24 months who were hospitalized with RSV between 2008 and 2011 in one medical center in Israel. Demographic, clinical, laboratory and radiological data were collected. The RSV antigen was identified using immunochromatography.
Results
The annual incidence of RSV hospitalization was 5.4/1000 and 6.8/1000 among Arab and Jewish children, respectively. Arab patients were significantly younger and had significantly younger parents; most lived in low socioeconomic status towns (93.7% vs. 13.3%; p<0.001) and had more siblings (median 2 vs. 1; p = 0.01) compared to Jewish patients. Disease severity did not differ between the two ethnic groups (p = 0.3). The main predictors of severe illness were having pneumonia (adjusted odds ratio [OR] 3.86; 95% confidence intervals [CI] 1.87–7.97) and history of respiratory diseases (adjusted OR 3.89; 95% CI 1.22–12.38).
Conclusions
The incidence of hospitalizations for RSV bronchiolitis tended to be higher among Jewish than Arab children, possibly due to differences in health care utilization patterns. Differences between the Jewish and Arab patients in demographic factors likely mirror differences between the groups in the general population. Pneumonia, and not ethnicity, affected the severity of RSV bronchiolitis.
Introduction
Respiratory syncytial virus (RSV), a single-stranded RNA virus of the Paramyxoviridae family [1, 2], is the most common pathogen identified in young children hospitalized with acute lower respiratory infection; winter outbreaks of RSV are typical [3, 4]. Worldwide, RSV is estimated to cause 34 million episodes of acute lower respiratory infection annually in children younger than age five years[3].
Treatment of RSV bronchiolitis is mainly supportive [5]. Immuno-prophylaxis with RSV specific neutralizing monoclonal antibody, palivizumab, is recommended for high-risk groups such as premature infants and individuals with bronchopulmonary dysplasia [6–9]. RSV immuno-prophylaxis is used in many high-income countries, including the United States, European countries and Israel. However, the requirement of monthly administration and high cost are major limitations in developing countries [10]. Currently, there is no licensed active vaccine against RSV, but several are under development [10, 11]. Therefore, understanding the epidemiology and clinical course of RSV bronchiolitis is important to inform clinical and public health decision-making.
Ethnic disparities in RSV bronchiolitis have been documented [12–15]. A study from the United States showed higher rates of RSV hospitalizations in black children than white children aged 12–23 months; yet differences were not found in incidence rates in the first year of life, nor in the severity of disease [14]. It is not clear whether ethnic disparities in RSV bronchiolitis are related to access to care or to health care utilization patterns; or whether true ethnic differences exist in disease incidence. Studies from Israel have demonstrated a high burden from RSV bronchiolitis [16–18], with a higher incidence rate of RSV hospitalization in Arab Bedouin children, a small Muslim minority, compared to Jewish children in southern Israel [18]. The aim of the current study was to compare demographic and clinical characteristics of Arab and Jewish children hospitalized for RSV bronchiolitis in a region of Israel, a country with universal health insurance.
Materials and methods
Study design and population
The Israeli population comprises two main ethnic groups; Arabs (20%) and Jews (75%), while ~5% belong to other ethnicities [19]. We reviewed the data of all children aged less than 24 months, hospitalized with laboratory-confirmed RSV bronchiolitis between January 1, 2008 and December 31, 2011 at Hillel Yaffe Medical Center. This center is a 506-bed university-affiliated hospital that mainly serves the population of Hadera sub-district. About 450,000 people were living in this region during the study period, 54.3% of whom were Arabs. The numbers of live births among Arabs and Jews in Hadera sub-district were 3,944 and 3,598, respectively, in 2011 [19].
Demographic and clinical information was collected from medical records for the following variables: age in months (categorized as 0–5, 6–11, 12–23), sex, population group (Arabs or Jews), number of siblings and parental age. Socioeconomic status (SES) of the town of residence defined by the Israel Central Bureau of Statistics [20] was used as a proxy of SES. Children who lived in towns with SES ranks of 1–4 and 5–10 were classified as living in low and high SES communities, respectively.
Clinical information included birth weight (in grams), gestational age at birth (in weeks), hospitalization in a neonatal intensive care unit after birth, background morbidity, feeding (breastfeeding, formula, other), prior hospitalizations and emergency room visits. Information was collected on symptoms of current illness (fever [≥38°C], cough, runny nose, dyspnea, tachypnea, saturation and eating difficulties), diagnosis of pneumonia (by chest x-ray), results of laboratory tests and duration of hospital stay. A summative disease severity score was constructed based on the following variables: dyspnea, tachypnea, hypoxia (oxygen saturation<92), cough, fever and length of hospitalization. For each symptom, the child was “accredited” one point. Children with the median score or above were classified as having a severe illness. Chest x-ray findings were grouped as pneumonia vs. no pneumonia. Since chest X-ray was performed based on a physician's' referral and findings in physical examination, children with normal chest X-ray were grouped together with children who were not referred to chest X-ray as not having pneumonia.
Laboratory methods
Patients’ respiratory samples (nasopharyngeal swabs or aspirates) were tested for the presence of RSV antigen at the laboratory of Hillel Yaffe Medical Center by immunochromatographic assay; BinaxNOW (Alere, Maine, USA). In children, the sensitivity and specificity of this kit were estimated at 90%-94% and 100%, respectively, compared to viral culture and/or reverse transcription polymerase chain reaction assay (RT-PCR) [21, 22].
Statistical analysis
The incidence (per 1000 children) and 95% confidence intervals (CIs) of hospitalization for RSV bronchiolitis were calculated, using the number of cases of laboratory-confirmed RSV bronchiolitis in the numerator and the estimated population served by Hillel Yaffe Medical Center in the denominator. The size of the population aged <24 months was estimated as 40% of the population aged 0–4 years living in Hadera sub-district. We estimated that 80%-90% of the population in Hadera sub-district receives hospitalization services from Hillel Yaffe Medical Center. The estimated population of children aged <24 months was 26,310 and 24,588 of Arab and Jewish children, respectively, during the 48-month study period [19] (S1 Table).
Differences between Arab and Jewish patients in demographic and clinical characteristics were examined using the chi-square test or Fisher's exact test for categorical variables, Student's t test for continuous variables and Mann-Whitney U-test for variables with skewed distribution. We examined differences in disease severity according to population group and other factors, using the chi-square test and multivariable logistic regression models. The variables age and population group were selected a-priori to be included in the multivariable model. Clinically relevant independent variables that were associated with disease severity with p<0.2 in bivariate analysis were also included in the multivariable analysis in a stepwise manner. Collinearity between the independent variables was assessed using the variance inflation factor (VIF). Since the variables population group and residential SES were highly correlated (Phi coefficient 0.80, p<0.001), they were analyzed in separate models, we present both models with parameters of model fit such as Hosmer-Lemeshow goodness-of-fit test and Nagelkerke R2. Odds ratios (ORs) and 95% CIs were obtained from logistic regression models. Statistical significance was set at p<0.05. Data were analyzed using SPSS version 25 (IBM, Armonk, New York, USA).
Ethical consideration
The study was approved by the Institutional Review Board (Helsinki) Committee of Hillel Yaffe Medical Center. Since this was a retrospective study, using archived medical records, an exempt from informed consent was granted by the Helsinki Committee.
Results
Overall, 369 children aged less than 24 months hospitalized with bronchiolitis were tested for RSV, of whom 309 children (55.5% boys) were positive to RSV and met study inclusion criteria. The study sample included 166 (53.7%) Jewish patients and 143 (46.3%) Arab patients (S1 Table). Children were hospitalized for a median of 3 days (range: 1–34). Most patients (50.3%) were younger than age three months. Overall, the annual hospitalization rate for RSV bronchiolitis was 5.7 per 1000 children aged <24 months: 6.8 per 1000 and 5.4 per 1000 among Jewish and Arab children, respectively (relative risk 1.24 [95% CI 0.99–1.55]; p = 0.05).
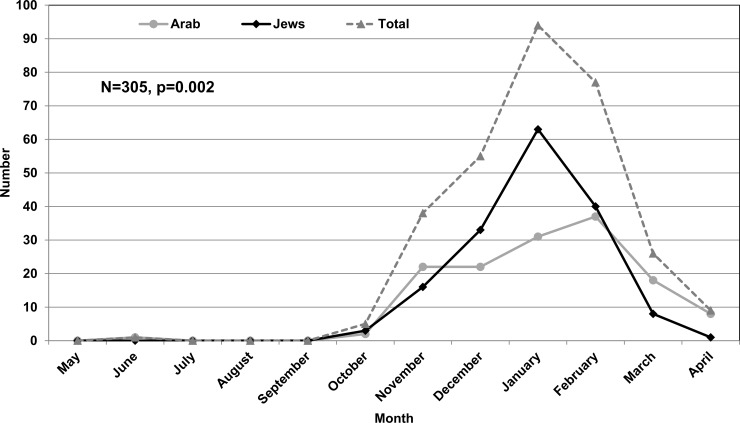
RSV bronchiolitis hospitalizations demonstrated typical winter seasonality. The rise in the number of cases began in November, peaked in January and decreased thereafter. Most (74.1%) cases occurred during December-February. The peak number of admissions was in January among Jewish children and February among Arabs (Fig 1).
Fig 1. Number of monthly hospitalizations for RSV-bronchiolitis in children aged <24 months by population group (2008–2011).
p = 0.002 for the difference between Arabs and Jews.
Sociodemographic and clinical characteristics by population group
The percentage of children younger than age 6 months was higher among Arab than Jewish patients. The majority (93.7%) of Arab children lived in low SES towns compared to 13.3% of Jewish patients (p<0.001). Parents of Arab children tended to be younger than parents of Jewish children (p<0.001 for maternal age, p = 0.03 for paternal age). The median number of siblings was higher among Arab than Jewish patients: 2 and 1, respectively (p = 0.01) (Table 1).
Table 1. Demographic characteristics of children hospitalized with RSV-bronchiolitis by population group*.
| Variable | All, N = 309 N (%) |
Arabs, N = 143 N (%) |
Jews, N = 166 N (%) |
P value |
|---|---|---|---|---|
| Sex, male | 172 (55.5) | 75 (52.4) | 68 (47.6) | 0.303 |
| Age, months | ||||
| 0–5 | 183 (60.2) | 93 (66.0) | 90 (55.2) | 0.115 |
| 6–11 | 81 (26.6) | 31 (22.0) | 50 (30.7) | |
| 12–23 | 40 (13.2) | 17 (12.0) | 23 (14.1) | |
| Missing | 5 (1.6) | 2 (1.4) | 3 (1.8) | |
| SES of place of residence | ||||
| Low (1–4) | 156 (50.6) | 134 (93.7) | 22 (13.3) | <0.001 |
| High (5–10) | 152 (49.4) | 9 (6.3) | 143 (86.7) | |
| Missing | 1 (0.3) | 0 (0.0) | 1 (0.6) | |
| Number of siblings § | ||||
| Median (min-max) | 1 (0–7) | 2 (0–6) | 1 (0–7) | 0.013 |
| Maternal age (years) § | ||||
| Mean (SD) | 29.6 (5.4) | 28.3 (5.5) | 30.7 (5.1) | <0.001 |
| Paternal age (years) § | ||||
| Mean (SD) | 33.3 (5.9) | 32.4 (6.2) | 34.1 (5.6) | 0.029 |
* Max: maximum; min: minimum; RSV: respiratory syncytial virus; SD: standard deviation; SES: socioeconomic status.
§ Information was missing on number of siblings, maternal age and paternal age for 51 (16.5%), 44 (14.2%) and 48 (15.5%) children, respectively (S2 Table).
Information on the child's feeding practices was available for 107 (34.7%) children (S2 Table), showing that breastfeeding was more common among Arab than Jewish children: 37/54 (68.5%) vs. 18/53 (34.0%); p<0.001. Arab and Jewish children did not differ significantly in birth weight, gestational age at birth (in weeks), background morbidity and family history of asthma. Disease symptoms did not differ significantly between the groups, expect for cough and fever, which were less common in Arab than Jewish patients (Table 2).
Table 2. Clinical characteristics of children hospitalized with RSV-bronchiolitis by population group.
| Variable | All (N = 309) | Arabs (N = 143) | Jews (N = 166) | P value |
|---|---|---|---|---|
| N/total (%) | N/total (%) | N/total (%) | ||
| Health and medical history | ||||
| Breastfeeding | 55/107 (51.4) | 37/54 (68.5) | 18/53 (34.0) | <0.001 |
| Family history of asthma | 53/259 (20.5) | 20/119 (16.8) | 33/140 (23.6) | 0.217 |
| Median weight at birth (grams) (min-max)* | 3080 (740–5505) | 3095 (893–5505) | 3100 (740–4925) | 0.715 |
| Gestational age at birth (weeks) | ||||
| <34 | 13/301 (4.3) | 8/139 (5.8) | 5/162 (3.1) | 0.503 |
| 34–36 | 37/301 (12.3) | 16/139 (11.5) | 21/162 (13.0) | |
| ≥37 | 251/301 (83.4) | 115/139 (82.7) | 136/162 (84.0) | |
| Background respiratory disorders | 27/309 (8.7) | 9/143 (6.3) | 18/166 (10.8) | 0.145 |
| Congenital heart disease | 14/309 (4.4) | 9/143 (6.3) | 5/166 (3.0) | 0.164 |
| Neurological defect | 12/309 (3.9) | 8/143 (5.6) | 4/166 (2.4) | 0.135 |
| Illness in the last month before hospitalization | 36/309 (11.6) | 15/143 (10.5) | 21/166 (12.6) | 0.597 |
| Past admission | 46/309 (15.4) | 22/143 (15.4) | 24/166 (14.5) | 0.873 |
| Symptoms, signs of current illness | ||||
| Dyspnea | 221/304 (72.5) | 105/141 (74.5) | 116/163 (71.2) | 0.519 |
| Strangulation | 26/298 (8.7) | 13/136 (9.6) | 13/162 (8.0) | 0.640 |
| Cough | 272/304 (89.2) | 119/140 (85.0) | 153/164 (92.7) | 0.041 |
| Fever (≥38°C) | 189/303 (62.4) | 77/139 (55.4) | 112/164 (68.3) | 0.024 |
| Runny nose | 155/298 (51.8) | 65/135 (48.1) | 89/163 (54.6) | 0.295 |
| Apathy | 22/300 (7.3) | 7/136 (5.1) | 15/164 (9.1) | 0.186 |
| Tachypnea | 262/303 (86.5) | 123/139 (88.5) | 139/164 (84.8) | 0.344 |
| Hypoxia (Saturation<92%) | 29/297 (9.7) | 14/136 (10.3) | 15/161 (9.3) | 0.777 |
| Eating difficulty | 117/302 (38.6) | 57/139 (41.0) | 60/163 (36.8) | 0.479 |
| Pneumonia by chest X-ray | 66/309 (21.4) | 28/143 (19.6) | 38/166 (22.9) | 0.479 |
| Co-infection | 84/309 (27.2) | 41/143 (28.7) | 43/166 (25.9) | 0.610 |
| Duration of hospital stay >4 days | 138/304 (45.4) | 69/140 (49.3) | 69/164 (42.1) | 0.248 |
Max: maximum; min: minimum; SD: standard deviation; RSV: respiratory syncytial virus.
* Data on birth weight were missing for 17 children.
Factors associated with the severity of RSV among hospitalized children
The median score of disease severity was 4 (range: 1–6), both among Arab and Jewish children. Significant differences were not found in disease severity between Arabs and Jews, and between males and females. A positive association was shown between child's age and disease severity (p for trend 0.024). The percentage of children who lived in high SES towns was higher among those with severe disease vs. children with less severe disease, but the difference was not statistically significant (53.8% vs. 43.7%; p = 0.08). Children with severe disease more often had background respiratory disorders (13.3% vs. 2.9%; p = 0.001), co-infection (32.4% vs. 20.8%; p = 0.02), and a diagnosis of pneumonia than children with less severe disease (Table 3).
Table 3. Factors associated with the severity of RSV-bronchiolitis in hospitalized children aged less than 24 months.
| Variable | Severe disease (N = 173) |
Less severe disease (N = 136) |
OR (95% CI) | P value |
|---|---|---|---|---|
| N (%) | N (%) | |||
| Population group | ||||
| Arabs | 75 (43.4) | 68 (50.0) | 0.77 (0.49–1.20) | 0.245 |
| Jews | 98 (56.6) | 68 (50.0) | Reference | |
| Sex | ||||
| Male | 99 (57.2) | 73 (53.7) | 1.08 (0.84–1.39) | 0.533 |
| Female | 74 (42.8) | 63 (46.3) | Reference | |
| Age, months | df = 2 | 0.026 | ||
| 0–5 | 93 (54.1) | 90 (68.2) | 0.39 (0.18–0.83) | 0.015 |
| 6–11 | 50 (29.1) | 31 (23.5) | 0.61 (0.26–1.39) | 0.244 |
| 12–23 | 29 (16.9) | 11 (8.3) | Reference | P trend 0.008 |
| Missing | 1 (0.6) | 4 (2.9) | ||
| Residential SES | ||||
| Low SES (1–4) | 80 (46.2) | 76 (56.3) | 0.67 (0.43–1.05) | 0.080 |
| High SES (5–10) | 93 (53.8) | 59 (43.7) | Reference | |
| Missing | 0 (0.0) | 1 (1.6) | ||
| Illness in the last month § | 24 (13.9) | 12 (8.8) | 1.66 (0.80–3.46) | 0.170 |
| Past admission § | 29 (16.8) | 17 (12.5) | 1.41 (0.74–2.69) | 0.296 |
| Co-infection § | 56 (32.4) | 28 (20.6) | 1.85 (1.09–3.12) | 0.021 |
| Chest X-ray | ||||
| Pneumonia | 53 (30.6) | 13 (9.6) | 4.18 (2.17–8.06) | <0.001 |
| No Pneumonia* | 120 (69.4%) | 123 (90.4%) | Reference | |
| Background respiratory disorders § | 23 (13.3) | 4 (2.9) | 5.06 (1.70–15.00) | 0.001 |
| Congenital heart disease § | 8 (4.6) | 6 (4.4) | 1.05 (0.36–3.10) | 0.929 |
CI: confidence interval; df: degrees of freedom; OR: odds ratio; RSV: respiratory syncytial virus; SES: socioeconomic status.
§ Reference category = not having the condition.
* No pneumonia by chest X-ray or by clinical judgment (chest X-ray was not ordered by a physician).
The variable child's age was positively correlated with having background respiratory disease (Phi correlation coefficient 0.26, p<0.001): 5/183 (2.7%) infants aged 0–5 months had a background respiratory disease, compared to 15/81 (18.5%) and 7/40 (17.5%) in those aged 6–11 and 12–23 months.
In a multivariable analysis that included 303 children (Model 1 Table 4), the associations of child's age and SES with disease severity were not statistically significant (p = 0.19, and 0.14, respectively). Background respiratory diseases and pneumonia (confirmed by chest X-ray), were strongly associated with a 4-fold increased risk for severe disease (adjusted OR 3.89 [95% CI 1.22–12.38]; p = 0.02 and adjusted OR 3.86 [95% CI 1.87–7.97] p<0.001, respectively). A second model that included the variable population group instead of residential SES showed similar results, as well as no significant difference in disease severity according to population group (Model 2 Table 4). VIF values ranged between 1.01–1.065, suggesting no collinearity between the independent variables.
Table 4. Multiple logistic regression models for factors associated with RSV severity.
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Variable | Adjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value |
| Age, months | df = 2 | 0.187 | df = 2 | 0.343 |
| 0–5 | 0.46 (0.20–1.06) | 0.068 | 0.55 (0.25–1.23) | 0.147 |
| 6–11 | 0.55 (0.22–1.36) | 0.196 | 0.65 (0.27–1.56) | 0.335 |
| 12–23 | Reference | Reference | ||
|
Residential SES Low (1–4) vs. high (5–10) |
0.69 (0.42–1.12) | 0.136 | Not included | |
| Population group (Jews vs. Arabs) | Not included | 1.20 (0.74–1.95) | 0.449 | |
|
Illness in the last month before hospitalization (yes vs. no) |
1.57 (0.72–3.44) | 0.256 | 1.62 (0.75–3.53) | 0.223 |
| Background respiratory disorders (yes vs. no) | 3.89 (1.22–12.38) | 0.022 | 4.05 (1.28–12.79) | 0.017 |
| Co-infection (yes vs. no) | 1.13 (0.61–2.08) | 0.698 | 1.20 (0.66–2.20) | 0.547 |
| Chest X-ray-pneumonia (yes vs. no)* | 3.86 (1.87–7.97) | <0.001 | 3.54 (1.76–7.15) | <0.001 |
Nagelkerke R2 model 1 = 0.161, Nagelkerke R2model 2 = 0.146
CI: confidence interval; df: degrees of freedom; OR: odds ratio; RSV: respiratory syncytial virus; SES: socioeconomic status.
* No pneumonia by chest X-ray or by clinical judgment (chest X-ray was not ordered by a physician)
Discussion
In a setting of universal health care insurance, we assessed ethnic differences in hospitalizations for RSV bronchiolitis in children aged less than 24 months. The incidence of hospitalizations for RSV bronchiolitis was higher among Jewish than Arab children. Arab and Jewish patients differed in sociodemographic factors but not in the clinical characteristics of their illness. Arab patients tended to be younger than Jewish patients. Parents of Arab patients also tended to be younger than parents of Jewish patients. Arab patients more often lived in communities of lower SES than Jewish patients and had more siblings. These differences likely reflect the existing general sociodemographic variation between the two population groups. Indeed, in Israel in general, and in the examined region in particular, the Arab population is younger than the Jewish population (median age; 21.8 vs. 33.2, respectively), has larger families and is of lower SES, as reflected by average income and education [19]. Although information on the child's feeding practices was available for only 35% of the sample, we found that the Arab patients were more often breastfed than the Jewish patients (68.5% vs. 34.0%; p<0.001). This observation is in agreement with a previous study that showed that the intention for exclusive breastfeeding was higher among Muslim than Jewish women [23], and with a national survey of children from birth until age 2 years, which showed higher breastfeeding rates among Arab than Jewish mothers [24].
Despite the above-mentioned sociodemographic disparities between Arab and Jewish RSV patients, overall, we found no significant differences in clinical characteristics or in the severity of RSV bronchiolitis between hospitalized Arab and Jewish children. Studies from the United States have shown that children belonging to minorities and those of lower SES were more likely to present with bronchiolitis to the emergency department and to subsequently be admitted compared to the general population [25, 26]. In contrast, other US studies [13, 14] did not find significant differences between black and white children in regard to RSV hospitalizations. Previous studies on SES disparities in RSV bronchiolitis showed higher rates in children from low vs. high SES, and in children from rural regions with limited access to care [13, 27–29]. In Australia, the risk of hospitalization due to RSV bronchiolitis was shown to be higher in Indigenous compared to non-Indigenous infants, and this was largely attributed to factors related to lower SES [27]. In both Indigenous and non-Indigenous children, better SES was related to lower risk for hospitalization due RSV bronchiolitis [30].
In southern Israel, Bedouin children are at higher risks of hospitalizations for infectious diseases, as well as for RSV bronchiolitis in early childhood, compared to Jewish children [18, 31]. The Bedouin population in southern Israel differs from the Arab population in the rest of Israel. About 50% of the Bedouin population lives in unrecognized villages and tribes, with limited access to health care facilities. This differs from other regions of Israel. Although in the current study, the SES was lower in the towns and villages where the Arabs compared to the Jews resided, all the residential areas have a basic and similar health care infrastructure. Considering the studies from various populations described above, the higher incidence of hospitalizations for RSV bronchiolitis in the Jewish population in our study likely reflects differences in referral or health care utilization patterns, or a combination of these factors.
In both population groups in the current study, most patients were under age 6 months (66% and 55% of the Arab and Jewish patients, respectively). Similarly, a review of studies of RSV hospitalizations reported that 49% to 70% of hospitalized infants with RSV are below age 6 months, and 66% to 100% younger than 1 year [32].
No significant differences were found between patients with more and less severe disease according to population group, sex, SES, birth week, birth weight, number of siblings and breastfeeding. The lack of correlation between these factors and severe bronchiolitis is consistent with findings reported by Papoff et al. [33]. We observed that children with more severe RSV were more likely to be admitted with evidence of lower respiratory involvement (e.g., higher frequency of pneumonia or an abnormal X-ray) and with background respiratory disorders, compared to those with less severe RSV. This finding is similar to that reported by Halasa et al. [34]. We found that co-infection is more likely to cause RSV severity in a bivariate analysis. Several studies have shown that co-infection results in higher hospitalization rates and longer hospital length of stay. However, up to one quarter of patients hospitalized with bronchiolitis were found to be co-infected with multiple agents [35, 36]. Unlike previous reports [33, 34, 37] we found that the percentage of children younger than age 6 months was significantly higher among those with less severe than more severe disease in bivariate analysis. A positive association between age and having background respiratory disease (Phi correlation coefficient 0.26, p<0.001). The association between age and disease severity became non-statistically significant in multivariable models after adding the variable background respiratory diseases, thus suggesting that age might be a marker of background diseases. Prevention of RSV disease in young children may ultimately be possible with active immunization or maternal immunization [38, 39]. The only currently available preventive measure for RSV disease is palivizumab, which reduces the risk of hospitalization caused by RSV in high-risk children [40]. Most of the children hospitalized with RSV in our study were full-term and otherwise healthy children, who did not meet the criteria for receiving the palivizumab vaccine. The latter is recommended only for a small proportion of infants who are born prematurely or who have exacerbating comorbidities, and thus this vaccination has little impact on overall RSV hospitalization rates. Therefore, the development of effective general preventive strategies such as infant or maternal vaccination or antiviral therapies specific for RSV is needed for all young infants, to reduce the burden of RSV hospitalizations.
Our study has limitations. First, not all children hospitalized due to respiratory illnesses underwent RSV testing; hence, the incidence of RSV bronchiolitis may have been underestimated. However, the decision to test for RSV is driven by clinical characteristics and not by ethnic group. Moreover, it is estimated that the study center serves 80%-90% of children residing in the region, therefore the representativeness of the study sample is high. Second, we used data from medical records over a 4-year study period. Differences could exist between physicians in obtaining and reporting medical information; however, such differences would likely not be related to children's ethnic background. Information on breastfeeding was available for 35% of the children, but this rate was similar among Arab (38%) and Jewish children (32%), (p = 0.2). Missing information on other variables was overall low, and the percentage of children with missing information was similar between Jewish and Arab children, the impact on the validity of our findings is limited.
The strengths of our study include the use of multi-year data, which yielded robust incidence estimates, in addition to the availability of comprehensive clinical and demographic information. This enabled broad comparisons between Arab and Jewish RSV patients. Lastly, since our study was conducted in a setting with universal health insurance, we were able to control for this factor as a potential contributor to ethnic disparities in RSV bronchiolitis.
In conclusion, the incidence of hospitalizations for RSV bronchiolitis tended to be higher among Jewish than Arab children in a setting of universal health insurance, likely reflecting health care utilization patterns. Infants less than 6 months of age comprised the majority of children hospitalized for RSV bronchiolitis, in both population groups. Differences in demographic characteristics between the Arab and Jewish children with RSV likely mirror differences between Arabs and Jews that exist in the general population, but they do not seem to affect disease severity. Most patients were ineligible for receiving the palivizumab vaccine; hence, the development of effective general preventive measure targeting all infants such as infant or maternal vaccination is warranted to reduce the burden of RSV bronchiolitis.
Supporting information
(XLSX)
(DOCX)
Acknowledgments
We would like to thank the medical archives department and Dr. Sarit Freman from the microbiology laboratory at Hillel Yaffe Medical Center for the assistance in retrieving the records.
Data Availability
The relevant, anonymized datapoints needed to replicate our analyses are within the paper and its Supporting Information. Given IRB and legal restrictions, we cannot provide any access to individual-level data, which we obtained from archived medical records from a single medical center and were collected as part of patients' clinical care, and not for research purposes. Legal and ethical restrictions apply for secondary usage of these data in research. Readers may contact Prof. Khitam Muhsen (the corresponding author) for further information.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Papadopoulos NG, Gourgiotis D, Javadyan A, Bossios A, Kallergi K, Psarras S, et al. Does respiratory syncytial virus subtype influences the severity of acute bronchiolitis in hospitalized infants? Respir Med. 2004;98:879–82. 10.1016/j.rmed.2004.01.009 [DOI] [PubMed] [Google Scholar]
- 2.Gilca R, De Serres G, Tremblay M, Vachon ML, Leblanc E, Bergeron MG, et al. Distribution and clinical impact of human respiratory syncytial virus genotypes in hospitalized children over 2 winter seasons. J Infect Dis. 2006;193:54–8. 10.1086/498526 [DOI] [PubMed] [Google Scholar]
- 3.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375(9725):1545–55. 10.1016/S0140-6736(10)60206-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6):588–98. 10.1056/NEJMoa0804877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Academy of Pediatrics subcommittee on diagnosis and management of bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118:1774–93. 10.1542/peds.2006-2223 [DOI] [PubMed] [Google Scholar]
- 6.Feltes TF, Cabalka AK, Meissner C, Piazza FM, Carlin DA, Top FH, et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. Journal of Pediatrics. 2003;143:532–40. 10.1067/S0022-3476(03)00454-2 [DOI] [PubMed] [Google Scholar]
- 7.Goddard NL, Cooke MC, Gupta RK, Nguyen-Van-Tam JS. Timing of monoclonal antibody for seasonal RSV prophylaxis in the United Kingdom. Epidemiol Infect. 2007;135:159–62. 10.1017/S0950268806006601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geskey JM, Thomas NJ, Brummel GL. Palivizumab: a review of its use in the protection of high risk infants against respiratory syncytial virus (RSV). Biologics. 2007;1:33–43. [PMC free article] [PubMed] [Google Scholar]
- 9.Fitzgerald DA. Preventing RSV bronchiolitis in vulnerable infants: the role of palivizumab. Paediatr Respir Rev. 2009;10:143–7. 10.1016/j.prrv.2009.06.002 [DOI] [PubMed] [Google Scholar]
- 10.Higgins D, Trujillo C, Keech C. Advances in RSV vaccine research and development—A global agenda. Vaccine. 2016;34(26):2870–5. 10.1016/j.vaccine.2016.03.109 [DOI] [PubMed] [Google Scholar]
- 11.Modjarrad K, Giersing B, Kaslow DC, Smith PG, Moorthy VS, WHO RSV Vaccine Consultation Expert Group. WHO consultation on respiratory syncytial virus vaccine development report from a World Health Organization meeting held on 23–24 March 2015. Vaccine. 2016;34:190–7. 10.1016/j.vaccine.2015.05.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grimwood K, Cohet C, Rich FJ, Cheng S, Wood C, Redshaw N, et al. Risk factors for respiratory syncytial virus bronchiolitis hospital admission in New Zealand. Epidemiol Infect. 2008;136:1333–41. 10.1017/S0950268807000180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sangare L, Curtis MP, Ahmad S. Hospitalization for respiratory syncytial virus among California infants: disparities related to race, insurance, and geography. J Pediatr. 2006;149(3):373–7. 10.1016/j.jpeds.2006.04.063 [DOI] [PubMed] [Google Scholar]
- 14.Iwane MK, Chaves SS, Szilagyi PG, Edwards KM, Hall CB, Staat MA, et al. Bisparities between black and white children in hospitalizations associated with acute respiratory illness and laboratory-confirmed influenza and respiratory syncytial virus in 3 US counties-2002-2009. Am J Epidemiol. 2013;177:656–65. 10.1093/aje/kws299 [DOI] [PubMed] [Google Scholar]
- 15.Valet RS, Gebretsadik T, Carroll KN, Minton PA, Woodward KB, Liu ZW, et al. Increased healthcare resource utilization for acute respiratory illness among Latino infants. J Pediatr. 2013;163:1186–91. 10.1016/j.jpeds.2013.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eidelman AI, Megged O, Feldman R, Toker O. The burden of respiratory syncytial virus bronchiolitis on a pediatric inpatient service. Isr Med Assoc J. 2009;11:533–6. [PubMed] [Google Scholar]
- 17.Gross I, Siedner-Weintraub Y, Abu Ahmad W, Bar-Oz B, Eventov-Friedman S. National evidence in Israel supporting reevaluation of respiratory syncytial virus prophylactic guidelines. Neonatology. 2017;111:240–6. 10.1159/000452196 [DOI] [PubMed] [Google Scholar]
- 18.Dagan R, Landau D, Haikin H, Tal A. Hospitalization of Jewish and Bedouin infants in southern Israel for bronchiolitis caused by respiratory syncytial virus. Pediatr Infect Dis J. 1993;12(5):381–6. [DOI] [PubMed] [Google Scholar]
- 19.Israel Central Bureau of Statistics. Statistical Abstracts of Israel. Jerusalem: State of Israel; 2012. [Google Scholar]
- 20.Israel Central Bureau of Statistics. Characterization and classification of geagraphic units by the socio-economic level of the population 2008. 2013. 1530. [Google Scholar]
- 21.Ohm-Smith MJ, Nassos PS, Haller BL. Evaluation of the Binax NOW, BD Directigen, and BD Directigen EZ assays for detection of respiratory syncytial virus. J Clin Microbiol. 2004;42:2996–9. 10.1128/JCM.42.7.2996-2999.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selvarangan R, Abel D, Hamilton M. Comparison of BD Directigen EZ RSV and Binax NOW RSV tests for rapid detection of respiratory syncytial virus from nasopharyngeal aspirates in a pediatric population. Diagn Microbiol Infect Dis. 2008;62:157–61. 10.1016/j.diagmicrobio.2008.05.005 [DOI] [PubMed] [Google Scholar]
- 23.Ben Natan M, Wiener A, Ben Haim Y. Womens intention to exclusively breast feed: The Israeli perspective. Midwifery. 2016;34:173–7. 10.1016/j.midw.2015.11.013 [DOI] [PubMed] [Google Scholar]
- 24.Health Status and nutrition among infants from birth until age 2 years in Israel, 2009–2012 Survey Israel Center for Disease Control, Ministry of Health, 2014. [Google Scholar]
- 25.Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr. 2003;143(5 Suppl):S127–32. [DOI] [PubMed] [Google Scholar]
- 26.Jansson L, Nilsson P, Olsson M. Socioeconomic environmental factors and hospitalization for acute bronchiolitis during infancy. Acta Paediatr. 2002;91:335–8. [DOI] [PubMed] [Google Scholar]
- 27.Reeve CA, Whitehall JS, Buettner PG, Norton R, Reeve DM, Francis F. Predicting respiratory syncytial virus hospitalisation in Australian children. J Paediatr Child Health. 2006;42:248–52. 10.1111/j.1440-1754.2006.00849.x [DOI] [PubMed] [Google Scholar]
- 28.Holman RC, Curns AT, Cheek JE, Bresee JS, Singleton RJ, Carver K, et al. Respiratory syncytial virus hospitalizations among American Indian and Alaska native infants and the general United States infant population. Pediatrics. 2004;114(4):E437–E44. 10.1542/peds.2004-0049 [DOI] [PubMed] [Google Scholar]
- 29.Bulkow LR, Singleton RJ, Karron RA, Harrison LH, Grp ARS. Risk factors for severe respiratory syncytial virus infection among Alaska Native children. Pediatrics. 2002;109(2):210–6. 10.1542/peds.109.2.210 [DOI] [PubMed] [Google Scholar]
- 30.Homaira N, Mallitt KA, Oei JL, Hilder L, Bajuk B, Lui K, et al. Risk factors associated with RSV hospitalisation in the first 2 years of life, among different subgroups of children in NSW: a whole-of-population-based cohort study. BMJ Open. 2016;6(6):e011398 10.1136/bmjopen-2016-011398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levy A, Fraser D, Vardi H, Dagan R. Hospitalizations for infectious diseases in Jewish and Bedouin children in southern Israel. Eur J Epidemiol. 1998;14:179–86. [DOI] [PubMed] [Google Scholar]
- 32.Simoes EAF. Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. J Pediatr. 2003;143:S118–S26. 10.1067/S0022-3476(03)00511-0 [DOI] [PubMed] [Google Scholar]
- 33.Papoff P, Moretti C, Cangiano G, Bonci E, Roggini M, Pierangeli A, et al. Incidence and predisposing factors for severe disease in previously healthy term infants experiencing their first episode of bronchiolitis. Acta Paediatr. 2011;100(7):e17–e23. 10.1111/j.1651-2227.2011.02181.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halasa N, Williams J, Faouri S, Shehabi A, Vermund SH, Wang L, et al. Natural history and epidemiology of respiratory syncytial virus infection in the Middle East: Hospital surveillance for children under age two in Jordan. Vaccine. 2015;33(47):6479–87. 10.1016/j.vaccine.2015.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aberle JH, Aberle SW, Pracher E, Hutter HP, Kundi M, Popow-Kraupp T. Single versus dual respiratory virus infections in hospitalized infants: impact on clinical course of disease and interferon-gamma response. Pediatr Infect Dis J. 2005;24:605–10. [DOI] [PubMed] [Google Scholar]
- 36.Jennings LC, Anderson TP, Werno AM, Beynon KA, Murdoch DR. Viral etiology of acute respiratory tract infections in children presenting to hospital—Role of polymerase chain reaction and demonstration of multiple infections. Pediatr Infect Dis J. 2004;23:1003–7. 10.1097/01.inf.0000143648.04673.6c [DOI] [PubMed] [Google Scholar]
- 37.Law B, Macdonald N, Langley J, Mitchell I, Stephens D, Wang E, et al. Severe respiratory syncytial virus infection among otherwise healthy prematurely born infants: What are we trying to prevent? Paediatr Child Health. 1998;3:402–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Englund JA. Prevention strategies for respiratory syncytial virus: passive and active immunization. J Pediatr. 1999;135(2 Pt 2):38–44. [PubMed] [Google Scholar]
- 39.Kaaijk P, Luytjes W, Rots NY. Vaccination against RSV Is maternal vaccination a good alternative to other approaches? Hum Vaccin Immunother. 2013;9:1263–7. 10.4161/hv.24096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brady MT, Byington CL, Davies HD, Edwards KM, Jackson MA, Maldonado YA, et al. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134:415–20. 10.1542/peds.2014-1665 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(DOCX)
Data Availability Statement
The relevant, anonymized datapoints needed to replicate our analyses are within the paper and its Supporting Information. Given IRB and legal restrictions, we cannot provide any access to individual-level data, which we obtained from archived medical records from a single medical center and were collected as part of patients' clinical care, and not for research purposes. Legal and ethical restrictions apply for secondary usage of these data in research. Readers may contact Prof. Khitam Muhsen (the corresponding author) for further information.