Abstract
Cellular elongation requires the defined coordination of intra‐ and extracellular processes, but the underlying mechanisms are largely unknown. The vacuole is the biggest plant organelle, and its dimensions play a role in defining plant cell expansion rates. Here, we show that the increase in vacuolar occupancy enables cellular elongation with relatively little enlargement of the cytosol in Arabidopsis thaliana. We demonstrate that cell wall properties are sensed and impact on the intracellular expansion of the vacuole. Using vacuolar morphology as a quantitative read‐out for intracellular growth processes, we reveal that the underlying cell wall sensing mechanism requires interaction of extracellular leucine‐rich repeat extensins (LRXs) with the receptor‐like kinase FERONIA (FER). Our data suggest that LRXs link plasma membrane‐localised FER with the cell wall, allowing this module to jointly sense and convey extracellular signals to the cell. This mechanism coordinates the onset of cell wall acidification and loosening with the increase in vacuolar size.
Keywords: cell expansion, cell wall, growth, plant, vacuole
Subject Categories: Membrane & Intracellular Transport, Plant Biology
Introduction
Primary plant cell walls are composed of the polysaccharides cellulose, hemicelluloses and pectin along with structural proteins. The extracellular matrix features considerable tensile strength, withstanding the internal hydraulic turgor pressure of cells. This stiff construction provides stability to the plant body, yet obviously conflicts with cellular enlargements. Remarkably, acidic pH of the apoplast (extracellular space) allows cell walls to extend and accordingly coincides with cellular elongation (Barbez et al, 2017). Nuclear signalling of the phytohormone auxin is crucial for the control of extracellular pH and plant cell expansion in a concentration‐ and tissue‐dependent manner (Spartz et al, 2014; Fendrych et al, 2016; Barbez et al, 2017). In hypocotyls, the auxin‐induced SMALL AUXIN UP RNA 19 (SAUR19) inhibits PP2C‐D phosphatases, thereby stimulating the activity of plasma membrane H+‐ATPases, which promote cellular expansion in aerial tissues (Spartz et al, 2014). Expansins and other cell wall remodelling enzymes have been proposed as mediators of this acid growth mechanism. Developmentally defined cell wall loosening and concomitant water uptake are important pre‐requisites to enable turgor‐driven cell expansion (reviewed in Braidwood et al, 2013). Accordingly, cellular elongation requires a complex coordination of several internal and external processes; however, relatively little is known how cell wall changes are molecularly sensed and translated into intracellular signals. Receptor kinases are predestined to have a role in linking the intra‐ and extracellular compartments. THESEUS1 (THE1) attenuates defects in cellulose deficiency, proposing a function of Catharanthus roseus receptor‐like kinase 1‐likes (CrRLK1Ls) in cell wall sensing (Hématy et al, 2007). In agreement, the receptor kinase CrRLK1L FERONIA (FER) also controls the extensibility of the cell wall (Höfte, 2015) and functions as a mechano‐sensor (Shih et al, 2014). CrRLK1Ls are transmembrane proteins, typically with an extracellular domain, consisting of two adjacent malectin‐like domains, for signal perception and a cytoplasmic kinase domain for intracellular signal transduction (Escobar‐Restrepo et al, 2007). Besides its contribution to mechanical sensing of the extracellular space, FER is a versatile growth integrator in plants with important contributions to a wide range of responses (reviewed in Li et al, 2016). The malectin domains bind to the peptide ligand RAPID ALKALINISATION FACTOR1 (RALF1), which subsequently triggers the FER kinase‐dependent repression of cellular expansion (Haruta et al, 2014). However, the importance of RALF peptides for the FER‐dependent mechano‐sensing is unknown.
Besides cell wall loosening and turgor pressure, the dimension of the biggest plant organelle, the vacuole, is another determinant for cellular enlargement. The size of the vacuole correlates with cell size in plants and is controlled by the phytohormone auxin, thereby impacting cellular elongation rates (Löfke et al, 2015; Scheuring et al, 2016). Despite its eminent importance, it remains largely unknown how intracellular processes, such as the regulation of vacuolar size, are coordinated with cell wall loosening to ensure the coordination of cellular elongation in Arabidopsis thaliana. Here, we show that extracellular leucine‐rich repeat extensin (LRX) proteins contribute to cell wall sensing via interaction with FER. Our work proposes that LRX proteins bridge the plasma membrane‐localised FER with the cell wall, enabling it to sense wall rigidity. This LRX/FER‐dependent mechanism conveys extracellular signals to the underlying cell and thereby suppresses growth‐relevant processes, such as the intracellular expansion of the vacuole.
Results
Here, we use epidermal atrichoblast (non‐root hair forming) root cells to record vacuole expansion during cellular elongation. To assess the relative dilation of the vacuole, we combined the fluorescent dye BCECF (2′,7′‐Bis‐(2‐carboxyethyl)‐5‐(and‐6)‐carboxyfluorescein), which accumulates in the vacuolar lumen of plant cells, with propidium iodide, which stains the exterior of the cells (Scheuring et al, 2015). Subsequently, we performed defined z‐stack imaging and 3D rendering of the cell (Movies EV1, EV2, EV3 and EV4), allowing quantification of how much cellular space is filled by the vacuole (also defined as vacuolar occupancy of the cell; Scheuring et al, 2016). In young/early meristematic cells, the vacuole occupied about 30–40% of the cellular space (Fig 1A and B; Movies EV1 and EV2). By contrast, the size of the vacuole and its relative occupancy of the cell dramatically increased during cellular elongation, ultimately taking up 80–90% of the cellular volume (Fig 1A and B; Movies EV3 and EV4). While the epidermal cells enlarged their volume by a factor of approximately 14, the absolute space between the vacuole and the cell boundary increased only about twofold to threefold (Fig 1C and Appendix Fig S1A and B). The 3D imaging of a cytosolic fluorescent protein also confirmed that the cytosol shows relatively minor volume expansion during epidermal elongation (Appendix Fig S1A and B). We illustrate that the relative increase in vacuolar size has a dramatic impact on cytosol homeostasis, consequently requiring relatively little de novo production of cytosolic components during cellular enlargement. Moreover, we propose that the vacuolar size is a suitable intracellular marker for cellular expansion dynamics.
Figure 1. Vacuolar occupancy of the cell enables cytosol homeostasis during rapid growth.
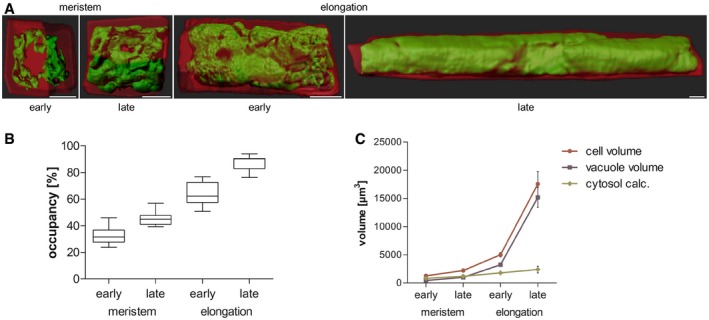
- 3‐D reconstructions of propidium iodide (PI)‐stained cell walls (red) and BCECF‐stained vacuoles (green) of epidermal atrichoblasts in the early and late meristem and in the early and late elongation zone. Scale bars: 5 μm.
- Boxplots showing vacuolar occupancy of cells in the defined zones (n = 7–11). Box limits represent 25th percentile and 75th percentile; horizontal line represents median. Whiskers display min. to max. values.
- Graph depicts absolute values of cell volume and vacuolar volume in the depicted root zones (data points display mean, error bars depict s.e.m., n = 7–11). “cytosol calc.” is approximated as the difference between cell volume and vacuolar volume.
Source data are available online for this figure.
Cell wall acidification is central in activating a cascade of events, ultimately leading to cell wall loosening and subsequent cellular elongation (Fendrych et al, 2016; Barbez et al, 2017). Accordingly, the cell wall acidification/loosening in elongating cells (Barbez et al, 2017) is coinciding with growth‐relevant intracellular processes, such as the increase in vacuolar size (Fig 1A–C). Here, we used the vacuolar size as a suitable intracellular read‐out to study the coordination of extracellular and intracellular processes during cellular elongation. We investigated the possibility that apoplast acidification/cell wall loosening is sensed and provides a feedback for vacuolar morphogenesis. We either directly acidified the rhizosphere (by lowering the pH of the media) or genetically (SAUR19 induction) as well as pharmacologically (fusicoccin treatment) induced the activity of the plasma membrane H+‐ATPase (Marre, 1979; Spartz et al, 2014, 2017). All these conditions have recently been shown to acidify the pH of epidermal cell walls, consequently inducing cellular elongation (Barbez et al, 2017). We quantified the vacuolar morphology, depicting the dimension of the biggest luminal vacuolar structure (Löfke et al, 2015; Scheuring et al, 2016), in root epidermal cells of the late meristematic zone. This allows us to assess the impact of cell wall acidification before the actual onset of elongation. Using the tonoplast stain MDY‐64 or the tonoplast marker line pUBQ10::VAMP711‐YFP (Geldner et al, 2009; Löfke et al, 2015; Scheuring et al, 2015), we revealed that cell wall acidification correlated with a dramatic alteration in vacuolar morphology (Fig 2A, C and E), leading to an increased vacuolar occupancy of the cell (Fig 2B, D and F). Fusicoccin‐induced cell wall acidification had an immediate (within 30 min) influence on the vacuolar lumen (Appendix Fig S2A and B), suggesting that cell wall acidification/loosening directly impacts on vacuolar morphogenesis and size.
Figure 2. Vacuolar size correlates with cell wall modifications.
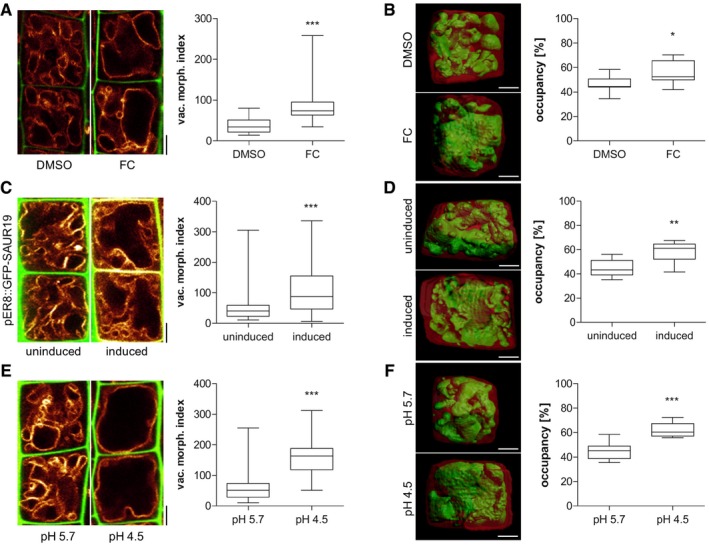
- Representative images and quantification of vacuolar morphology of late meristematic cells. PI (green) and pUBQ10::VAMP711 (yellow) depict cell wall and tonoplast, respectively. Seedlings were treated with DMSO (solvent control) or 5 μM FC (Fusicoccin) for 2.5 h in liquid medium (n = 24). Mann–Whitney U‐test (***P < 0.001).
- Left: 3‐D reconstructions of PI‐stained cell wall (red) and BCECF‐stained vacuole (green) of late meristematic cells. Right: Boxplot depicts vacuolar occupancy of the cell treated with the solvent DMSO or 5 μM FC for 2.5 h in liquid medium (n = 11). Student's t‐test (*P < 0.05).
- Cell wall and vacuolar membrane were visualised with PI (green) and MDY‐64 (yellow). pER8::GFP‐SAUR19 seedlings were treated with DMSO (n = 60) or 10 μM β‐estradiol (n = 56) for 6 h in liquid medium. Mann–Whitney U‐test (***P < 0.001).
- Left: 3‐D cell reconstructions of PI‐stained cell wall (red) and BCECF‐stained vacuole (green) of late meristematic cells in pER8::GFP‐SAUR19 lines. Right: Boxplot depicts vacuolar occupancy of the cell. Seedlings were treated with the solvent control DMSO (n = 11) or 10 μM β‐estradiol (n = 8) for 6 h in liquid medium. Student's t‐test (**P < 0.01).
- Cell wall and vacuolar membrane were visualised with PI (green) and pUBQ10::VAMP711 (E) (yellow). Col‐0 wild‐type seedlings were treated for 3 h in liquid medium adjusted to pH 5.7 (n = 44) or pH 4.5 (n = 40). Mann–Whitney U‐test (***P < 0.001).
- Left: 3‐D reconstructions of PI‐stained cell wall (red) and BCECF‐stained vacuole (green) of late meristematic cells. Right: Boxplot depicts vacuolar occupancy of cell. Seedlings were treated for 3 h in liquid medium adjusted to pH 5.7 or pH 4.5 (n = 11). Student's t‐test (***P < 0.001).
The inhibition of pectin methyl esterases (PMEs) leads to reduced cellular elongation, presumably due to stiffening of the cell walls (Wolf et al, 2012). Therefore, we used epigallocatechin gallate (EGCG), which is a natural inhibitor for PMEs (Lewis et al, 2008), to inducibly interfere with the properties of the cell wall. The application of EGCG‐induced smaller vacuolar structures and 3D imaging revealed an overall reduced vacuolar occupancy of the cell (Fig 3A and B). Similarly, roots that penetrate a relatively stiff medium also showed smaller vacuoles, when compared to surface‐grown roots (Fig 3C and D). These findings suggest that extracellular constraints restrict intracellular enlargements of vacuoles.
Figure 3. Extracellular constraints impact on vacuolar appearance.
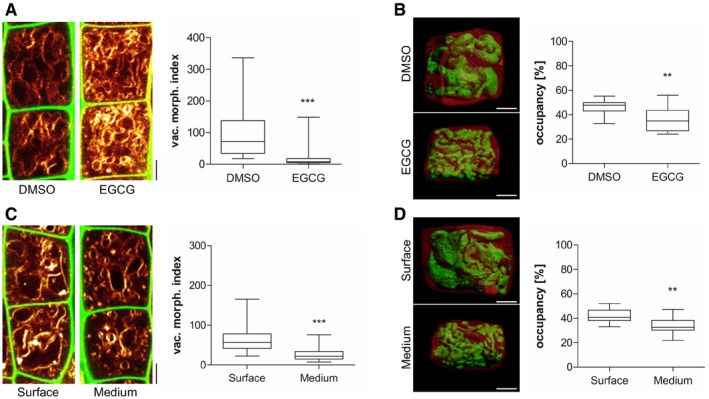
- Representative images and quantification of vacuolar morphology of late meristematic cells. PI (green) and MDY‐64 (yellow) staining depict cell wall and vacuolar membrane, respectively. Seedlings were treated with DMSO solvent control or 50 μM EGCG for 22 h on solid medium (n = 40). Mann–Whitney U‐test (***P < 0.001).
- 3‐D reconstructions of PI‐stained cell wall (red) and BCECF‐stained vacuole (green) of late meristematic cells. Boxplot depicts vacuolar occupancy of the cell. Seedlings were treated with DMSO (n = 16) or 50 μM EGCG (n = 15) for 22 h on solid medium. Student's t‐test (**P < 0.01).
- Representative images and quantification of vacuolar morphology of late meristematic cells. PI (green) and MDY‐64 (yellow) staining depict cell wall and vacuolar membrane, respectively. Seedling roots were grown on the surface (n = 28) or into the matrix (n = 28) of 2% agar‐containing solid medium. Mann–Whitney U‐test (***P < 0.001).
- 3‐D reconstructions of PI‐stained cell wall (red) and BCECF‐stained vacuole (green) of late meristematic cells. Boxplot depicts vacuolar occupancy of surface (n = 10) and into the medium (n = 11) grown seedling roots. Student's t‐test (**P < 0.01).
We assume that cell wall properties are sensed, which generates a signal to subsequently modulate vacuolar size. To test this hypothesis, we focused on FER receptor‐like kinase, which is required for mechanical cell wall sensing (Shih et al, 2014; Feng et al, 2018). Compared to wild‐type seedlings, the fer‐2 and fer‐4 loss‐of‐function mutants showed enlarged, roundish vacuoles (Fig 4A; Appendix Fig S3A) and increased vacuolar occupancy of the epidermal cells (Fig 4B; Appendix Fig S3B). Notably, epidermal cell length was tendentially slightly enlarged in the root meristem of fer mutant when compared to wild type (Appendix Fig S3C). Importantly, fer mutant vacuoles were markedly less affected by EGCG treatments or by extracellular constraints of the substrate (Fig 4C and D). In agreement, fer‐4 mutants were insensitive to the root growth inhibitory effect of EGCG when compared to wild type (Appendix Fig S3D and E). Accordingly, we conclude that an extracellular, FER‐dependent signal impacts intracellular expansion of the vacuole. Notably, an engineered fer mutant, carrying a point mutation in the intracellular kinase domain, was not able to fully complement the vacuolar phenotype of fer4 mutants (Appendix Fig S3F). These data support a role for the FER kinase activity and, hence, FER‐dependent signalling in restricting intracellular expansion of the vacuole.
Figure 4. Putative cell wall sensor FERONIA impacts on vacuolar size.
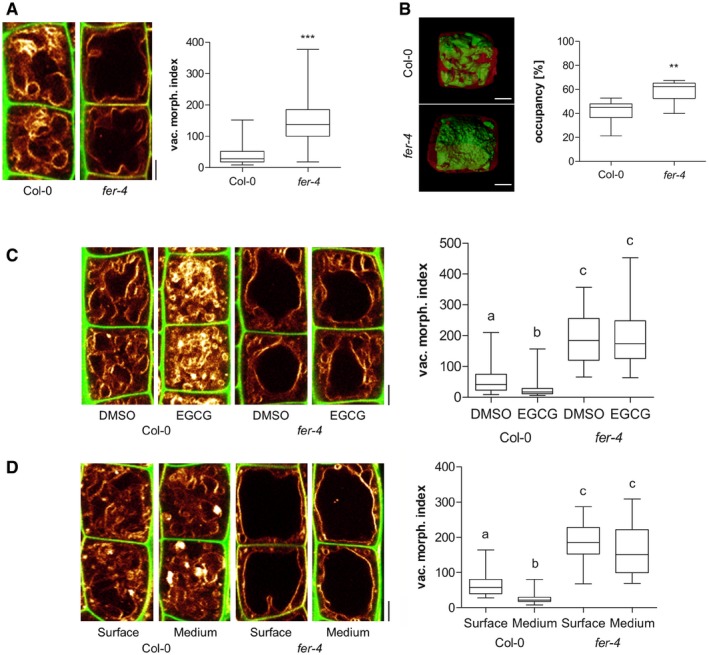
-
A–DRepresentative images and quantification of vacuolar morphology of late meristematic atrichoblast cells. In panels (A, C and D), PI (green) and MDY‐64 (yellow) staining depicts cell wall and vacuolar membrane, respectively. (A) Vacuolar morphology of Col‐0 (n = 64) and fer‐4 (n = 60). Mann–Whitney U‐test (***P < 0.001). (B) 3‐D reconstructions of PI‐stained cell wall (red) and BCECF‐stained vacuole (green). Boxplot depicts vacuolar occupancy of Col‐0 control (n = 12) and fer‐4 (n = 10) mutant cells. Mann–Whitney U‐test (**P < 0.01). (C) Col‐0 (n = 52) and fer‐4 (n = 44) seedlings were treated with solvent DMSO or 50 μM EGCG for 22 h on solid medium. Kruskal–Wallis test followed by Dunn's multiple comparison test (b: P < 0.05, c: P < 0.001). (D) Col‐0 (n = 28) and fer‐4 (n = 28) mutant seedling roots were grown on the surface or into the matrix of 2% agar‐containing solid medium. Kruskal–Wallis test followed by Dunn's multiple comparison test (b: P < 0.05, c: P < 0.001). In all panels scale bars: 5 μm. Boxplots: Box limits represent 25th percentile and 75th percentile; horizontal line represents median. Whiskers display min. to max. values. Representative experiments are shown.
Source data are available online for this figure.
We, subsequently, used the vacuolar morphology as a quantitative read‐out to further our knowledge on FER‐dependent cell wall sensing mechanisms and turned our attention to extracellular proteins with a possible role in cell wall sensing. Interestingly, leucine‐rich repeat extensins (LRXs) are extracellular proteins (Baumberger et al, 2003a) and showed co‐expression with FER and THE1 as well as several RALF peptides (Appendix Fig S4A and B). LRX proteins display an N‐terminal leucine‐rich repeat (LRR) and a C‐terminal extensin (EXT) domain (Draeger et al, 2015). While the LRR domain is presumably involved in protein–protein interactions, the EXT domain allows the LRX proteins to bind cell wall components (Ringli, 2010). This domain structure also envisioned the hypothetical role of LRX in cell wall sensing (Humphrey et al, 2007). LRX1 and LRX2 are mainly associated with root hair growth, while LRX8‐LRX11 are pollen specific (Baumberger et al, 2003a,b). Here, we concentrated on LRX3, LRX4 and LRX5, because they have been suggested to redundantly impact root and shoot growth (Draeger et al, 2015). In agreement, lrx3 lrx4 lrx5 triple mutants displayed a pronounced enlargement of the vacuolar lumina when compared to the wild type (Fig 5A) and the lrx single and double mutants (Appendix Fig S5A). Similar to fer mutants, these changes also resulted in vacuoles occupying more space in the late meristematic, epidermal cells (Fig 5B). Notably, epidermal cell length was mostly unaffected in lrx3 lrx4 lrx5 mutant background (Appendix Fig S5B). lrx3 lrx4 lrx5 triple mutant vacuoles were, moreover, resistant to EGCG treatments as well as to external constraints by the substrate (Fig 5C and D). In agreement, lrx3 lrx4 lrx5 mutants displayed increased resistance to the root growth inhibitory effect of EGCG when compared to wild type (Appendix Fig S5C and D) as well as lrx single and double mutants (Appendix Fig S5E). We accordingly conclude that extracellular LRX proteins are redundantly involved in setting the intracellular expansion of the vacuole.
Figure 5. Extracellular LRX proteins are required to constrain vacuolar expansion.
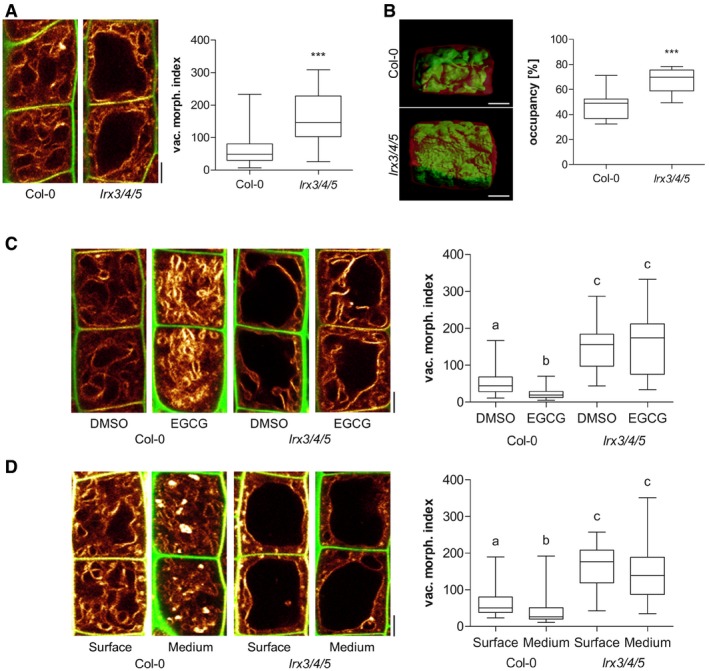
-
A–DRepresentative images and quantification of vacuolar morphology of late meristematic atrichoblast cells. In panels (A, C and D), PI (green) and MDY‐64 (yellow) staining depicts cell wall and vacuolar membrane, respectively. (A) Vacuolar morphology of Col‐0 control (n = 52) and lrx3/4/5 triple mutants (n = 48). Mann–Whitney U‐test (***P < 0.001). (B) 3‐D reconstructions of PI‐stained cell wall (red) and BCECF‐stained vacuole (green) of late meristematic cells. Boxplot depicts vacuolar occupancy of the cell in Col‐0 control (n = 11) and lrx3/4/5 (n = 10). Student's t‐test (***P < 0.001). (C) Col‐0 (n = 40–44) and lrx3/4/5 (n = 36) seedlings were treated with DMSO or 50 μM EGCG for 22 h on solid medium. Kruskal–Wallis test followed by Dunn's multiple comparison test (b: P < 0.01, c: P < 0.001). (D) Col‐0 (n = 40–48) and fer‐4 (n = 28–32) seedlings were grown on the surface or into the matrix of 2% agar‐containing solid medium. Kruskal–Wallis test followed by Dunn's multiple comparison test (b: P < 0.05, c: P < 0.001). In all panels scale bars: 5 μm. Boxplots: Box limits represent 25th percentile and 75th percentile; horizontal line represents median. Whiskers display min. to max. values. Representative experiments are shown.
Source data are available online for this figure.
We noted that not only the vacuoles, but also the overall plant phenotype, of lrx3 lrx4 lrx5 triple mutants closely resembled the appearance of fer mutants (Fig 6A). Notably, salt stress in the root has been recently shown to damage, among others, the cell wall. Even though it cannot be ruled out that salt stress also triggers additional defects in the plasma membrane or cytoplasm, it seems that the salt‐induced defects in the cell wall are sensed by FER (Feng et al, 2018). In agreement with our assumptions, salt sensitivity of lrx3 lrx4 lrx5 triple mutant largely resembled fer single mutants, suggesting that the FER and LRX proteins might function in the same signalling process. In agreement with these assumptions, the morphological and cellular phenotypes of fer lrx3 lrx4 lrx5 quadruple mutants were not distinguishable from the fer single mutants (Fig 6A). Likewise, the root growth response to salt stress was not enhanced in fer lrx3 lrx4 lrx5 quadruple mutants when compared to fer single mutants (Appendix Fig S6). Collectively, this set of data indicates that FER and LRX reside in the same pathway.
Figure 6. LRX and FERONIA jointly sense extracellular signals.

- Rosette phenotype of 3‐week‐old Col‐0, fer‐4, lrx3/4/5 and fer‐4/lrx3/4/5 (upper panel). Vacuolar morphology (MDY‐64‐stained) of late meristematic atrichoblast cells of Col‐0, fer‐4, lrx3/4/5 and fer‐4/lrx3/4/5 (lower panel). Scale bars: 1 cm (upper row) and 5 μm (lower row).
- LRR4‐HA and RALF1‐FLAG were transiently expressed (as indicated by + and −) in Nicotiana benthamiana. Immunoprecipitation and subsequent detection of the proteins by Western blotting were done as labelled.
- Relative root length [% of control] of Col‐0 (n = 11–13), lrx3/4/5 (n = 9–13) and fer‐4 (n = 11–12) after 3 days of RALF1 treatment. Statistical analyses were performed for each concentration using one‐way ANOVA followed by Bonferroni post test (1 and 1.25 μM b: P < 0.001; 1.5 μM b: P < 0.05, c: P < 0.001). Boxplots: Box limits represent 25th percentile and 75th percentile; horizontal line represents median. Whiskers display min. to max. values. Representative experiment is shown.
- Nicotiana benthamiana was transiently transformed with LRR4‐HA and NtermFER‐FLAG. Immunoprecipitation and subsequent detection of the proteins by Western blotting were done as labelled.
- Yeast was transformed with either empty plasmids (e/e), NtermFER‐FLAG or LRR4‐HA with the empty plasmid, or both NtermFER‐FLAG and LRR4‐HA. Growth of yeast on quadruple drop‐out medium was only observed in cells transformed with NtermFER‐FLAG and LRR4‐HA.
Source data are available online for this figure.
Subsequently, we assessed how LRX could function together with FER in extracellular sensing. Other pollen‐specific members of the LRX protein family (LRX8‐LRX11) have been recently shown to bind to RALF4 and RALF19 via its LRR domains (Mecchia et al, 2017). We confirmed such a potential interaction for seedling expressed LRX proteins by co‐immunoprecipitating the HA‐tagged N‐terminal part of LRX4 (LRR4‐HA) together with FLAG‐tagged RALF1 (Fig 6B).
As FER was also shown to bind RALF1 (Haruta et al, 2014), the LRX proteins could be in principle linked to FER‐dependent processes via RALF peptide binding. We, hence, tested the contribution of LRX3‐5 to the RALF1‐sensitive root growth. Root growth of fer mutants is strongly resistant to RALF1 application, whereas lrx3 lrx4 lrx5 triple mutant remained sensitive and showed only slight resistance to RALF1 at higher concentrations when compared to a wild‐type control (Fig 6C). In addition, the rapid impact of RALF1 on apoplast alkalinisation was completely abolished in fer mutants, but still detectable in lrx3 lrx4 lrx5 triple mutants (Appendix Fig S7). Accordingly, we conclude that root meristem‐expressed LRXs, such as LRX3, LRX4 and LRX5, modulate sensitivity to RALF1, but are not absolutely required for the FER‐dependent perception of RALF1.
The lrx3 lrx4 lrx5 largely resembles fer mutant phenotypes, but the comparably weak resistance to RALF1 suggests that LRX may have additional functions in FER‐dependent processes. Interestingly, truncated LRX proteins that lack the EXT cell wall binding domain associate with membranes (Fabrice et al, 2018), suggesting that the N‐terminus of LRX4 interacts with some membrane component. Therefore, we next assessed whether LRX and FER may reside within a complex. Accordingly, we expressed the LRR4‐HA together with a GFP‐tagged FER in Nicotiana benthamiana. Co‐immunoprecipitation of full‐length FER and LRR4 indicated an association of these two proteins in a complex (Appendix Fig S8). Strikingly, LRR4‐HA co‐immunoprecipitated also with a truncated N‐terminal part of FER (NtermFER; Fig 6D), suggesting that a part of the extracellular malectin‐like domains is sufficient for the association with LRX4. In addition, the LRR4 domain also interacted in a yeast two‐hybrid approach with the NtermFER (Fig 6E), suggesting that FER and LRX4 can directly interact with each other.
Next, we overexpressed a truncated, EXT‐lacking version of LRX4 in Arabidopsis to further assess the contribution of the EXT domain. Overexpression of the citrine‐tagged LRR4 (p35S::LRR4‐citrine) caused dominant negative phenotypes in Arabidopsis, which were morphologically reminiscent to the lrx3 lrx4 lrx5 triple or fer single mutants (Fig 7A). In agreement, the expression of the LRR4 domain was also sufficient to induce bigger luminal vacuoles in late meristematic, epidermal cells (Fig 7B). Moreover, LRR4 overexpressors were insensitive to EGCG‐induced reduction in vacuolar size (Fig 7B). This set of data suggests that the EXT cell wall binding domain is critically important for the LRX/FER‐dependent cell wall sensing mechanism.
Figure 7. EXT‐domain of LRXs is crucial for cell wall sensing.
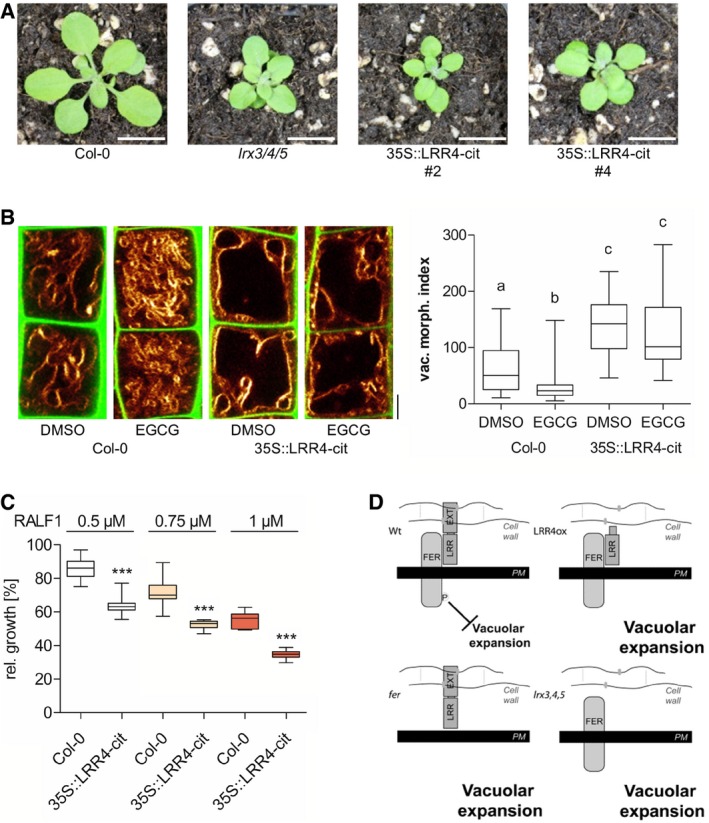
- Rosette phenotype of 3‐week‐old Col‐0, lrx3/4/5 and 35S::LRR4‐cit.
- Representative images and quantification of vacuolar morphology of late meristematic cells of Col‐0 and 35S::LRR4‐cit. PI (green) and MDY‐64 (yellow) staining depict cell wall and vacuolar membrane, respectively. Col‐0 (n = 36) and 35S::LRR4‐cit (n = 32–36) seedlings were treated with DMSO or 50 μM EGCG for 22 h on solid medium. Kruskal–Wallis test followed by Dunn's multiple comparison test (b: P < 0.05, c: P < 0.001).
- Relative root length of Col‐0 (n = 11–13) and 35S::LRR4‐cit (n = 11–14) after 3 days of RALF1 treatment. Statistical analyses were performed for each concentration using Student's t‐test (***P < 0.001).
- Schematic model depicting the FER‐ and LRX‐dependent cell wall sensing mechanism, which limits vacuolar expansion (WT) in late meristematic cells. In contrast, dysfunctional cell wall perception in lrx3 lrx4 lrx5, fer‐4 and 35S::LRR4‐cit triggers premature vacuolar expansion.
The expression of LRR4 largely phenocopied the lrx3 lrx4 lrx5 triple mutant, but, intriguingly, the p35S::LRR4‐citrine expressing roots were not slightly resistant (as seen for the triple mutant), but hypersensitive to RALF1 treatments when compared to its respective wild‐type control (Fig 7C). This finding illustrates again that LRXs indeed seem to modulate RALF sensitivity, but also strongly suggests that the function of LRX in FER‐dependent cell wall sensing is at least partially distinct from its impact on FER‐dependent perception of the RALF1 peptide.
Discussion
Vacuoles are essential for plant development (Rojo et al, 2001), and interference with vacuolar function evokes severe cell expansion and developmental defects (Schumacher et al, 1999; Li et al, 2005). The size of vacuoles, moreover, correlates with individual cell size in plant cell cultures (Owens & Poole, 1979) as well as in the root tip of Arabidopsis (Berger et al, 1998; Löfke et al, 2013), proposing a role in cell size determination (Löfke et al, 2015). Here, we further support these previous assumptions and show that the increase in vacuolar volume allows for rapid cellular elongation with relatively little de novo production of cytosolic content. Accordingly, we define the vacuolar size as a suitable parameter to quantify an important intracellular process, marking the regulation of cell expansion. Using this read‐out, our data suggest that a FER‐dependent cell wall sensing mechanism impacts on intracellular processes, including the expansion of the vacuole. FER has been previously proposed to be required for cell wall sensing (Shih et al, 2014), but molecular interactors involved in the underlying mechanism were largely unknown. We used the vacuolar size as a quantitative, cellular trait to study FER‐dependent mechanisms and show that interaction of extracellular LRX with FER contributes to extracellular sensing (Fig 7D). We propose that this LRX‐ and FER‐dependent module integrates the cell wall status with intracellular growth processes, such as the expansion of the vacuole. It remains to be seen precisely how LRX/FER signalling at the cell surface leads to the modulation of vacuolar size. FER‐dependent phosphorylation acts as a signalling relay (Shih et al, 2014; Du et al, 2016; Haruta et al, 2018) and modulates, among others, the Rho of plants (ROP) guanine nucleotide exchange factor 1 (GEF1; Duan et al, 2010). ROP GEF1 in turn activates RAC/ROP GTPases (Rho‐related molecular switches in plants; Duan et al, 2010), which are known regulators of actin dynamics. Notably, the actin cytoskeleton surrounds the vacuole and contributes to the regulation of vacuolar size (Scheuring et al, 2016). This hypothetical link could in principle connect the LRX/FER‐dependent, extracellular sensing mechanism with the intracellular control of vacuolar expansion.
Our work proposes LRX as a physical link between the plasma membrane‐localised FER on one side (via the LRR domain) and the cell wall (via the EXT domain) on the other side (Fig 7D). It is currently unknown which cell wall component is recognised by the EXT domain. The PME inhibitor EGCG induces smaller vacuoles in a FER‐ and LRX‐dependent manner. Accordingly, it is tempting to speculate that pectin may play also a role in this LRX/FER‐dependent process.
On the other hand, not only FER, but also THE1, binds to RALF peptides, proposing conserved interaction partners for CrRLK1Ls (Gonneau et al, 2018). It is similarly likely that LRX proteins also interact with a variety, if not all, CrRLK1Ls. It remains, however, unclear precisely how FER and other CrRLK1Ls interact with RALFs and/or LRX proteins. Future studies are required to elucidate whether they form a trimeric complex or rather binding of two proteins excludes interaction with the third. Moreover, recent work on salt‐induced cell wall damages proposes that FER binds also to polygalacturonic acid, the backbone of pectin (Feng et al, 2018). Whereas animal and bacterial malectin domains indeed display carbohydrate‐interaction surfaces, recent work questions that the malectin domains of plant CrRLK1L directly bind to oligosaccharides (Moussu et al, 2018). Instead, the crystal structure of the tandem malectin domains of CrRLK1Ls proposes a protein–protein interface (Moussu et al, 2018). Based on our work, we assume that LRXs binds via the LRR domain to FER and bridge FER via the EXT domain on its other side with cell wall components, such as pectin. However, further research is needed to assess this assumption and to reveal how a presumable binding of the EXT domain to pectin (or other cell wall components) may impact on the interaction of LRX and FER.
Both LRX and FER proteins are essential for constraining the vacuole, suggesting that the LRX‐FER interaction is required for its signalling to the underlying cell. FER has a RALF‐dependent scaffold function during immune responses (Stegmann et al, 2017) and pollen expressed LRX function in RALF4/19‐dependent regulation of pollen tube's cell wall integrity (Mecchia et al, 2017). Our data reveal that LRR4 overexpression largely resembles the morphological and cellular phenotype of lrx3 lrx4 lrx5 mutants. In contrast, the lrx triple mutant and LRR4 overexpression are partially resistant and hypersensitive to RALF1, respectively. This finding proposes that the roles of LRX in FER‐dependent cell wall and RALF sensing are at least partially distinct. Accordingly, we conclude that LRX and FER form distinct complexes, allowing them to integrate very diverse processes.
Here, we propose that the LRX/FER‐dependent feedback mechanism aligns the cell wall status with the intracellular expansion of the vacuole. This mechanism, consequently, ensures the spatial and temporal coordination of cell wall acidification/loosening with the increase in vacuolar size, which ultimately effects cytosol homeostasis during rapid cell expansion.
Materials and Methods
Plant material and growth conditions
Most experiments were carried out in A. thaliana (Col‐0 ecotype). The following plant lines were described previously: fer‐4 (Shih et al, 2014), fer‐2 (Deslauriers & Larsen, 2010), lrx3/lrx4/lrx5 (Draeger et al, 2015), pUBQ10::VAMP711‐YFP (Geldner et al, 2009), pER8::GFP‐SAUR19 (Spartz et al, 2014), pHusion (Gjetting et al, 2012), pFER::FERKR‐GFP (in fer‐4; Shih et al, 2014), pFER::FER‐GFP (in fer‐4; Shih et al, 2014) and 35S::LRR4‐citrine (Fabrice et al, 2018). Seeds were stratified at 4°C for 2 days in the dark and were grown on vertically orientated ½ Murashige and Skoog (MS) medium plates containing 1% sucrose under a long‐day regime (16‐h light/8‐h dark) at 20–22°C.
Chemicals and RALF1
All chemicals were dissolved in dimethyl sulfoxide (DMSO) and applied in solid or liquid ½ MS medium. MDY‐64 was obtained from Life Technologies (CA, USA), β‐estradiol, propidium iodide (PI), 2′,7′‐Bis(2‐carboxyethyl)‐5(6)‐carboxyfluorescein acetoxymethyl ester (BCECF‐AM) from Sigma (MO, USA), and fusicoccin (FC) and epigallocatechin gallate (EGCG) from Cayman Chemical (MI, USA). The RALF1 peptide (mature RALF1, amino acid sequence: ATTKYISYQSLKRNSVPCSRRGASYYNCQNGAQANPYSRGCSKIARCRS) was obtained from Peptron (KOR).
Phenotype analysis
Vacuolar morphology index and occupancy were quantified in 6‐day‐old seedlings. Confocal images were analysed using ImageJ (vacuolar morphology index) or processed using Imaris (vacuolar occupancy of cells). To calculate the vacuolar morphology index, the longest and widest distance of the biggest luminal structure was measured and multiplied (Löfke et al, 2015). The atrichoblast cells were quantified before the onset of elongation (late meristematic). To depict this region, the first cell being twice as long as wide was considered as the onset of elongation. Starting from this cell, the next cell towards the meristem was excluded (as it usually shows either partial elongation and/or already substantial vacuolar expansion), and vacuoles of the subsequent 4 cells were quantified as described previously (Scheuring et al, 2016). For the analysis of occupancy, 1 cell in this region was used. Vacuolar shape/size was quantified in at least 8 roots (unless stated otherwise in the figure legend). MDY‐64 and BCECF staining was performed as described previously (Scheuring et al, 2015). Plant rosette phenotype evaluation was performed 3 weeks after germination.
For the analysis of root growth under salt stress conditions, 5‐day‐old seedlings were transferred to ½ MS plates supplemented with 100 mM NaCl and grown for another 9 days. Plates were scanned and root length assessed using ImageJ.
Cell length analysis
Six‐day‐old seedlings were used to quantify atrichoblast cell length. The region of analysis was similar to the quantification of vacuolar morphology index (see above). The distance covering four cells was measured per root and divided by four, resulting in the average cell length per root.
RALF1 and EGCG root length assay
Three‐day‐old seedlings (n = 10–12) were transferred for another 3 days to 3 ml liquid ½ MS medium containing 1 μM RALF1 or 25 μM EGCG or the appropriate amount of solvent (water or DMSO, respectively). The seedlings were then placed on solid MS plates prior to scanning. Root length was analysed using ImageJ.
Apoplastic pH visualisation in root cells
Five‐day‐old seedlings were treated for 10 min in liquid ½ MS medium supplemented with 1 μM RALF1 or the appropriate amount of solvent (water) or 6‐day‐old seedlings were treated in liquid ½ MS medium supplemented with 5 μM FC or DMSO for the indicated time. Subsequently, the seedlings were transferred to a block of solid ½ MS medium containing 1 mM of 8‐hydroxypyrene‐1,3,6‐trisulfonic acid trisodium salt (HPTS; Sigma‐Aldrich). This block was then mounted on a microscope slide and instantly used for imaging. Image processing was performed as described in Barbez et al (2017). Four transversal cell walls before the onset of elongation were quantified per root, and these values were averaged per root.
Agar penetration assay
Sixty millilitres of ½ MS medium containing 2% of plant agar was poured into square Petri dishes (12 × 12 cm). Approximately 2 cm of medium at the top of the plate was removed with a scalpel, and small notches were generated on the surface using a toothpick (enabling root penetration into the medium). Subsequently, a single seed was placed in each notch.
3‐D reconstruction of vacuoles
Imaris 8.4.0 was used for the reconstruction of cell and vacuole volumes. Based on the PI channel, every 3rd slice of the z‐stack was utilised to define the cell borders using the isoline, magic wand or manual (distance) drawing functions in the manual surface creation tool. After creating the surface corresponding to the entire cell, a masked channel (based on BCECF) was generated by setting the voxels outside the surface to 0. Subsequently, a second surface (based on the masked BCECF channel) was generated automatically with the smooth option checked. The obtained surface was visually compared to the underlying BCECF channel, and, if necessary, the surface was fitted to the underlying signal by adjusting the absolute intensity threshold slider. Finally, volumes of both surfaces were extracted from the statistics window.
Confocal microscopy
For image acquisition, a Leica TCS SP5 (DM6000 CS) confocal laser‐scanning microscope, equipped with a Leica HCX PL APO CS 63 × 1.20 water‐immersion objective, was used. MDY‐64 was excited at 458 nm (fluorescence emission: 465–550 nm), GFP and BCECF at 488 nm (fluorescence emission: 500–550 nm), YFP at 514 nm (fluorescence emission: 525–578 nm) and PI at 561 nm (fluorescence emission: 644–753 nm). Roots were mounted in PI solution (0.02 mg/ml) to counterstain cell walls. Z‐stacks were recorded with a step size of 420 nm. On average, 36 slices in z‐direction were captured, resulting in an average thickness of approximately 15 μm. The argon laser power was set to 30%, the AOTF for the 488 nm laser line was set to 2%, and the HyD gain was set to 300 (BCECF channel). The AOTF for the 561 nm laser line was set to 20%, and the PMT gain was set to 900 (PI channel). The pinhole was set to 111.6 μm. HPTS was excited at 405 nm (protonated form) with 4% laser line intensity and at 458 nm (deprotonated version) with the AOTF set to 100%, and the argon laser power was set to 60%. The gain was set to 799. HPTS images were acquired in sequential scan mode with the detection window set to 499–546 nm.
Co‐immunoprecipitation assay
For pulldown and co‐IP analysis of FER and LRR4, Agrobacteria containing pFER::FER‐GFP (Escobar‐Restrepo et al, 2007) and/or p35S::LRR4‐HA (Fabrice et al, 2018) were infiltrated into Nicotiana benthamiana leaves. After 48 h of infiltration, the tobacco leaves were excised and grinded in liquid nitrogen. The tissue powder was re‐suspended in extraction buffer [50 mM HEPES‐KOH (pH 7.6), 150 mM NaCl, 1 mM DTT, 1 mM PMSF, protease inhibitor and 1% NP‐40]. The suspension was incubated on ice for 30 min and then centrifuged at 17,000 g for 20 min at 4°C. The supernatant obtained was then incubated with GFP‐trap agarose beads or anti‐HA agarose beads for 3–4 h at 4°C on a rotating shaker. After incubation, the beads were washed three times with the extraction buffer containing 0.1% NP‐40 and boiled in SDS–PAGE loading buffer for 15 min at 75°C. The immunoprecipitates were then run on a 10% SDS–PAGE and transferred to nitrocellulose membrane to perform Western blotting.
RALF1 and NtermFER construct
For RALF1_2FLAG overexpression, the full‐length coding sequence was amplified using the primers RALF1oE_F GGTACCATGGACAAGTCCTTTACTC and RALF1oE_R CTGCAGAACTCCTGCAACGAGCA. The fragment was cloned into pJET1.2 (Thermo Scientific). A correct clone was cut with PstI and XbaI and fused with a PstI‐2FLAG‐stop XbaI fragment. The resulting RALF1_2FLAG was cut with KpnI and XbaI and cloned into pART7 vector (Gleave, 1992), using the same enzymes. The resulting 35S:RALF1_2FLAG construct was cut out by NotI and cloned into the binary vector pBART (Stintzi & Browse, 2000).
For overexpression of the Nterm FER extracellular domain, the coding sequence was amplified with the primers FER_ECD_F CTCGAGATGAAGATCACAGAGGGAC and FER_ECD_R CTGCAGGCCGTCTGAGAAGCACTG, cloned into pJET1.2 (Thermo Scientific). A correct clone was cut with XhoI as well as PstI and cloned into pART7 (Gleave, 1992) containing a 2FLAG coding sequence with a PstI site at the 5′ end, cut with XhoI and PstI.
Yeast two hybrid
For the yeast two‐hybrid experiment, the coding sequence of the LRR domain of LRX4 was amplified using the primers y2hs_LRR4_F GGATCCAAGCTCTTGATAACCGGAAG and y2h_LRR4_R CTCGAGCTATCCACAATCCACCGAAGGCCG and cloned into pJET1.2 (Thermo Scientific). A correct clone was cut with BamHI and XhoI and cloned into pGBKT7 cut with BamHI and SalI. The extracellular domain coding sequence of FER (NtermFER) was amplified using the primers Y2H_FER_F CATGAATTCCGTATATGGATCTCCGAT and Y2H_FER_R ATGCCCGGGTCCGCCGTCTGAGAAGCAC and cloned into pJET1.2 (Thermo Scientific). A correct clone was cut with Eco RI and XmaI and cloned into pGADT7 cut with Eco RI and XmaI. Transformation of the yeast strain PJ69‐4A (James et al, 1996) was done following standard procedures and quadruple drop‐out medium lacking Leu, Trp, His and Ade were used to screen for positive interactions.
Author contributions
KD, CR and JK‐V designed research. KD conducted most experiments; SG, AH and CR performed pulldown and yeast experiments; MIF established multiple mutants. KD, SG, AH, MIF, CR and JK‐V analysed data. KD and JK‐V wrote and all co‐authors commented on the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Movie EV1
Movie EV2
Movie EV3
Movie EV4
Source Data for Appendix
Review Process File
Source Data for Figure 1
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 4
Source Data for Figure 5
Source Data for Figure 6
Source Data for Figure 7
Acknowledgements
We are grateful to Y. Belkhadir, A.T. Fuglsang, N. Geldner, B. Gray, P.B. Larsen, C. Luschnig, G. Monshausen and C. Zipfel for sharing published material; Elke Barbez for critical reading of the manuscript; Jit Thacker for help with preparing the manuscript; and the BOKU‐VIBT Imaging Centre for access and expertise. This work was supported by the Austrian Academy of Sciences (ÖAW) (DOC fellowship to K.D.), Vienna Research Group (VRG) programme of the Vienna Science and Technology Fund (WWTF), the Austrian Science Fund (FWF) (Projects: P26568‐B16 and P26591‐B16), the European Research Council (ERC) (Starting Grant 639478‐AuxinER) (to J.K‐V.), and the Swiss National Science Foundation (SNF) (31003A_166577/1, to C.R.).
The EMBO Journal (2019) 38: e100353 30850388
References
- Barbez E, Dünser K, Gaidora A, Lendl T, Busch W (2017) Auxin steers root cell expansion via apoplastic pH regulation in Arabidopsis thaliana . Proc Natl Acad Sci USA 114: E4884–E4893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumberger N, Steiner M, Ryser U, Keller B, Ringli C (2003a) Synergistic interaction of the two paralogous Arabidopsis genes LRX1 and LRX2 in cell wall formation during root hair development. Plant J 35: 71–81 [DOI] [PubMed] [Google Scholar]
- Baumberger N, Doesseger B, Guyot R, Diet A, Parsons RL, Clark MA, Simmons MP, Bedinger P, Goff SA, Ringli C, Keller B (2003b) Whole‐genome comparison of leucine‐rich repeat extensins in Arabidopsis and rice. A conserved family of cell wall proteins form a vegetative and a reproductive clade. Plant Physiol 131: 1313–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger F, Hung CY, Dolan L, Schiefelbein J (1998) Control of cell division in the root epidermis of Arabidopsis thaliana . Dev Biol 194: 235–245 [DOI] [PubMed] [Google Scholar]
- Braidwood L, Breuer C, Sugimoto K (2013) My body is a cage: mechanisms and modulation of plant cell growth. New Phytol 201: 388–402 [DOI] [PubMed] [Google Scholar]
- Deslauriers SD, Larsen PB (2010) FERONIA is a key modulator of brassinosteroid and ethylene responsiveness in Arabidopsis hypocotyls. Mol Plant 3: 626–640 [DOI] [PubMed] [Google Scholar]
- Draeger C, Fabrice TN, Gineau E, Mouille G, Kuhn BM, Moller I, Abdou MT, Frey B, Pauly M, Bacic A, Ringli C (2015) Arabidopsis leucine‐rich repeat extensin (LRX) proteins modify cell wall composition and influence plant growth. BMC Plant Biol 15: 155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C, Li X, Chen J, Chen W, Li B, Li C, Wang L, Li J, Zhao X, Lin J, Liu X (2016) Receptor kinase complex transmits RALF peptide signal to inhibit root growth in Arabidopsis . Proc Natl Acad Sci USA 113: E8326–E8334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Q, Kita D, Li C, Cheung AY, Wu HM (2010) FERONIA receptor‐like kinase regulates RHO GTPase signaling of root hair development. Proc Natl Acad Sci USA 107: 17821–17826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar‐Restrepo JM, Huck N, Kessler S, Gagliardini V, Gheyselinck J, Yang WC, Grossniklaus U (2007) The FERONIA receptor‐like kinase mediates male‐female interactions during pollen tube reception. Science 317: 656–660 [DOI] [PubMed] [Google Scholar]
- Fabrice TN, Vogler H, Draeger C, Munglani G, Gupta S, Herger AG, Knox P, Grossniklaus U, Ringli C (2018) LRX Proteins play a crucial role in pollen grain and pollen tube cell wall development. Plant Physiol 176: 1981–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendrych M, Leung J, Friml J (2016) TIR1/AFB‐Aux/IAA auxin perception mediates rapid cell wall acidification and growth of Arabidopsis hypocotyls. Elife 5: e19048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Kita D, Peaucelle A, Cartwright HN, Doan V, Duan Q, Liu MC, Maman J, Steinhorst L, Schmitz‐Thom I, Yvon R, Kudla J, Wu HM, Cheung AY, Dinenny JR (2018) The FERONIA receptor kinase maintains cell‐wall integrity during salt stress through Ca2+ signaling. Curr Biol 28: 666–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldner N, Dénervaud‐Tendon V, Hyman DL, Mayer U, Stierhof YD, Chory J (2009) Rapid, combinatorial analysis of membrane compartments in intact plants with a multicolor marker set. Plant J 59: 169–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjetting SK, Ytting CK, Schulz A, Fuglsang AT (2012) Live imaging of intra‐and extracellular pH in plants using pHusion, a novel genetically encoded biosensor. J Exp Bot 63: 3207–3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleave AP (1992) A versatile binary vector system with a T‐DNA organisational structure conducive to efficient integration of cloned DNA into the plant genome. Plant Mol Biol 20: 1203–1207 [DOI] [PubMed] [Google Scholar]
- Gonneau M, Desprez T, Martin M, Doblas VG, Bacete L, Miart F, Sormani R, Hématy K, Renou J, Landrein B, Murphy E (2018) Receptor kinase THESEUS1 is a rapid alkalinization factor 34 receptor in Arabidopsis . Curr Biol 28: 2452–2458 [DOI] [PubMed] [Google Scholar]
- Haruta M, Sabat G, Stecker K, Minkoff BB, Sussman MR (2014) A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science 343: 408–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruta M, Gaddameedi V, Burch H, Fernandez D, Sussman MR (2018) Comparison of the effects of a kinase dead mutation of FERONIA on ovule fertilization and root growth of Arabidopsis . FEBS Lett 592: 2395–2402 [DOI] [PubMed] [Google Scholar]
- Hématy K, Sado PE, Van Tuinen A, Rochange S, Desnos T, Balzergue S, Pelletier S, Renou JP, Höfte H (2007) A receptor‐like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Curr Biol 17: 922–931 [DOI] [PubMed] [Google Scholar]
- Höfte H (2015) The yin and yang of cell wall integrity control: brassinosteroid and FERONIA signaling. Plant Cell Physiol 56: 224–231 [DOI] [PubMed] [Google Scholar]
- Humphrey TV, Bonetta DT, Goring DR (2007) Sentinels at the wall: cell wall receptors and sensors. New Phytol 176: 7–21 [DOI] [PubMed] [Google Scholar]
- James P, Halladay J, Craig EA (1996) Genomic libraries and a host strain designed for highly efficient two‐hybrid selection in yeast. Genetics 144: 1425–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KC, Selzer T, Shahar C, Udi Y, Tworowski D, Sagi I (2008) Inhibition of pectin methyl esterase activity by green tea catechins. Phytochemistry 69: 2586–2592 [DOI] [PubMed] [Google Scholar]
- Li J, Yang H, Peer WA, Richter G, Blakeslee J, Bandyopadhyay A, Titapiwantakun B, Undurraga S, Khodakovskaya M, Richards EL, Krizek B (2005) Arabidopsis H+‐PPase AVP1 regulates auxin‐mediated organ development. Science 310: 121–125 [DOI] [PubMed] [Google Scholar]
- Li C, Wu HM, Cheung AY (2016) FERONIA and her pals: functions and mechanisms. Plant Physiol 171: 2379–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löfke C, Dünser K, Kleine‐Vehn J (2013) Epidermal patterning genes impose non‐cell autonomous cell size determination and have additional roles in root meristem size control. J Integr Plant Biol 55: 864–875 [DOI] [PubMed] [Google Scholar]
- Löfke C, Dünser K, Scheuring D, Kleine‐Vehn J (2015) Auxin regulates SNARE‐dependent vacuolar morphology restricting cell size. Elife 4: e05868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marre E (1979) Fusicoccin: a tool in plant physiol. Annu Rev Plant Phys 30: 273–288 [Google Scholar]
- Mecchia MA, Santos‐Fernandez G, Dus NN, Somoza SC, Boisson‐Dernier A, Gagliardini V, Martínez‐Bernardini A, Fabrice TN, Ringli C, Muschietti JP, Grossniklaus U (2017) RALF4/19 peptides interact with LRX proteins to control pollen tube growth in Arabidopsis . Science 358: 1600–1603 [DOI] [PubMed] [Google Scholar]
- Moussu S, Augustin S, Roman AO, Broyart C, Santiago J (2018) Crystal structures of two tandem malectin‐like receptor kinases involved in plant reproduction. Acta Crystallogr D Struct Biol 74: 671–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens T, Poole RJ (1979) Regulation of cytoplasmic and vacuolar volumes by plant cells in suspension culture. Plant Physiol 64: 900–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringli C (2010) The hydroxyproline‐rich glycoprotein domain of the Arabidopsis LRX1 requires Tyr for function but not for insolubilization in the cell wall. Plant J 63: 662–669 [DOI] [PubMed] [Google Scholar]
- Rojo E, Gillmor CS, Kovaleva V, Somerville CR, Raikhel NV (2001) VACUOLELESS1 is an essential gene required for vacuole formation and morphogenesis in Arabidopsis . Dev Cell 1: 303–310 [DOI] [PubMed] [Google Scholar]
- Scheuring D, Schöller M, Kleine‐Vehn J, Löfke C (2015) Vacuolar staining methods in plant cells In Plant cell expansion, Estevez JM. (ed.), pp 83–92. New York, NY: Humana Press; [DOI] [PubMed] [Google Scholar]
- Scheuring D, Löfke C, Krüger F, Kittelmann M, Eisa A, Hughes L, Smith RS, Hawes C, Schumacher K, Kleine‐Vehn J (2016) Actin‐dependent vacuolar occupancy of the cell determines auxin‐induced growth repression. Proc Natl Acad Sci USA 113: 452–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher K, Vafeados D, McCarthy M, Sze H, Wilkins T, Chory J (1999) The Arabidopsis det3 mutant reveals a central role for the vacuolar H+–ATPase in plant growth and development. Genes Dev 13: 3259–3270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih HW, Miller ND, DaI C, Spalding EP, Monshausen GB (2014) The receptor‐like kinase FERONIA is required for mechanical signal transduction in Arabidopsis seedlings. Curr Biol 24: 1887–1892 [DOI] [PubMed] [Google Scholar]
- Spartz AK, Ren H, Park MY, Grandt KN, Lee SH, Murphy AS, Sussman MR, Overvoorde PJ, Gray WM (2014) SAUR inhibition of PP2C‐D phosphatases activates plasma membrane H+‐ATPases to promote cell expansion in Arabidopsis . Plant Cell 26: 2129–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spartz AK, Lor VS, Ren H, Olszewski NE, Miller ND, Wu G, Spalding EP, Gray WM (2017) Constitutive expression of the auxin‐related AtSAUR19 protein confers auxin‐independent hypocotyl elongation. Plant Physiol 173: 1453–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmann M, Monaghan J, Smakowska‐Luzan E, Rovenich H, Lehner A, Holton N, Belkhadir Y, Zipfel C (2017) The receptor kinase FER is a RALF‐regulated scaffold controlling plant immune signaling. Science 355: 287–289 [DOI] [PubMed] [Google Scholar]
- Stintzi A, Browse J (2000) The Arabidopsis male‐sterile mutant, opr3, lacks the 12‐oxophytodienoic acid reductase required for jasmonate synthesis. Proc Natl Acad Sci USA 97: 10625–10630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S, Mravec J, Greiner S, Mouille G, Höfte H (2012) Plant cell wall homeostasis is mediated by brassinosteroid feedback signaling. Curr Biol 22: 1732–1737 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Movie EV1
Movie EV2
Movie EV3
Movie EV4
Source Data for Appendix
Review Process File
Source Data for Figure 1
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 4
Source Data for Figure 5
Source Data for Figure 6
Source Data for Figure 7


