Abstract
Introduction
Reduced concentrations of placental protein 13 (PP13) during the first trimester of human pregnancy are associated with elevated risk for the subsequent development of preeclampsia, which is one of the deadliest obstetrical complications of pregnancy. Previous studies by our group have shown that PP13 lowers blood pressure in pregnant rats, increases the size and weight of pups and placentas, and induces vasodilation of resistance arteries through endothelial signaling pathways involving endothelial nitric oxid synthase and prostaglandin.
Methods
In the present study, the effect of PP13 was investigated in nonpregnant female Sprague Dawley rats (n=27). Osmotic pumps were surgically implanted subcutaneously that released a constant dose of PP13 or saline over 7 days. Most animals were sacrificed 6 days after the end of PP13 release (on day 13), while some were sacrificed immediately at the end of day 7 (the last PP13 release day), to compare the short- and long-term impact of PP13 on vessels’ growth and size.
Results
The uterine vessels were significantly expanded in the group exposed to recombinant PP13 (rPP13) compared to the control (saline) group. Both veins and arteries were significantly expanded by rPP13 with a more pronounced effect after 13 days compared to the corresponding vessels after 7 days. Furthermore, the long-term effect of treatment by rPP13 was more pronounced in the veins compared to the corresponding arteries. The effect of a PP13 variant with a histidine-tag (His-PP13) remained the same between 7 and 13 days.
Conclusion
In conclusion, PP13 might play a key role in the expansive remodeling of the uterine vessels, reflecting what would happen if the rat was pregnant, preparing the uterine vascu-lature for the increase in uteroplacental blood flow, which is necessary for normal pregnancy. We suggest that PP13 could act by NO signaling pathways, a hypothesis that requires future study.
Keywords: preeclampsia, placenta, biomarkers, LGALS13, hypertension, pregnancy, rat vascular system, galectins, eNOS, slow release
Introduction
During mammalian pregnancy, the uterine circulation undergoes significant remodeling to enable an increased blood flow, which is necessary for the development and outcome of a healthy pregnancy.1 The structural adaptation of the uteroplacental vasculature in pregnancy is reflected by a reduced systemic and uterine vascular resistance, especially during the first trimester, to ensure the appropriate blood supply for a successful fetal growth.2
Placental protein 13 (PP13) was identified as a strong modifier for uterine vasculature expansion in a pregnant rat model.3 PP13 is a member of the placenta-specific galectins (LGALS13) that is found only in human beings and some primates (anthropoid apes).4 Like all the galectins, it binds to various glycoproteins and glycolipids through their sugar residues, eg, on the outer surface of the placental syncytiotrophoblast. It also binds to sugar residues of white blood cells through its carbohydrate recognition domain (CRD), creating a carbohydrate tethering effect.4 This pattern of galectin binding is thus considered to occur via a nonclassical receptor entity. A recent study has identified PP13 on the surface and within the placental syncy-tiotrophoblast-derived extracellular vesicles (both exosomes and microvesicles) that deliver their content to the maternal circulation.5 Several studies have reported that low PP13 levels in the first trimester of human pregnancy may be associated with an increased risk for the subsequent development of preeclampsia, which is a life-threatening pregnancy complication occurring in 2%–5% of all pregnancies worldwide that is characterized by a new onset of hypertension and proteinuria from the midst of pregnancy in previously non-symptomatic women.6,7 PP13 was identified as a possible biomarker for predicting this pregnancy complication that is associated with a failure of the maternal vascular system to undergo the required changes to accommodate pregnancy. Accordingly, the shortage of PP13 during the early stages of pregnancy may be related to impaired vascular adaptation to pregnancy.
Our team has previously shown that a slow continuous release of PP13 into the subcutaneous area in pregnant rats induces hypotension and dilation of the uterine vasculature. When PP13 is allowed to flow through isolated arteries from pregnant and nonpregnant rats, it induces dilation of these resistance arteries through endothelial signaling involving the pathways of endothelial nitric oxid synthase (eNOS) and prosta-glandin.3,8 PP13 is also implied in venous dilation, and the closer the veins are to the placenta, the greater effect was observed.3
The purpose of this study was to evaluate whether the effect of PP13 on uterine blood vessels is related to their distance from the uterine endometrium. Specifically, we were interested to 1) study in vivo effects of PP13 on the uterine vascular system as a whole and compare its effect on uterine veins and arteries; 2) evaluate the type of the most affected uterine vessels comparing resistance (small) vessels to conductive (large) vessels; and 3) assess whether the PP13 effect lasts longer than the administration of the protein. The analysis took into consideration presence of PP13 in blood is between 6 and 12 hours following a single injection, although binding of PP13 to body tissues, including white and the red blood cells, as well as ABO antigen system may extend the time PP13 may be found in the circulation.9,10
Materials and methods
PP13
Recombinant PP13 (rPP13) was made using two variants of the PP13 with (His-PP13) and without (rPP13) histidine tag. The His-tag was localized at the N-terminus of the PP13 primary sequence. Both variants were cloned and expressed in Escherichia coli. The rPP13 was harvested from the inclusion bodies and solubilized and refolded in the presence of urea, cysteine, and protease inhibitors. The refolded protein was subsequently purified by ion exchange chromatography as previously described.11
The His-PP13 variant was initially purified by NiNTA affinity purification followed by ion exchange chromatography as previously described.12
The molecular weight and the purity of the proteins were determined by SDS-PAGE (15.6 and 18.2 kDa for rPP13 and His-PP13, respectively), and the purity was verified by HPLC and immunoblots with PP13-specific monoclonal antibodies. The concentration was determined using a PP13 ELISA kit as previously described.12–14
Animals and ethical approvals
The animal experiments were approved by the National Laboratory Review Board of Iceland (no 2015-02-01) and by the Institutional Animal Care and Use Committee (IACUC) at the University of Vermont, Burlington, VT, USA. All studies were carried out in accordance with the European Communities Council Directive (86/609/EEC) or the US National Institutes of Health (NIH) Guidelines for the care and use of laboratory animals. All efforts were undertaken according to the “3R principles” (www.nc3rs.org.uk) to reduce the number of animals used in this study and optimize experimental protocols for obtaining maximum data from each tested animal. Part of the experiments (13 day study) was carried out at an authorized animal facility at ArticLAS ehf (Reykjavik, Iceland), and part of them (the 7-day study) was carried out at the College of Medicine, University of Vermont, Burlington, VT, USA.
We used two groups of animals: 1) 18 female nonpreg-nant Sprague Dawley rats (aged 11 weeks) were purchased from Taconic bioscience (Ejby, Denmark) and studied in Iceland; 2) 11 female nonpregnant Sprague Dawley rats (aged 12–14 weeks) were purchased from Charles River Laboratories (St Constant, QC, Canada) and studied in Vermont. The animals were acclimatized for 3–14 days prior to the start of the experiments. Food and water were provided ad libitum, and animals were kept under 12/12-hour light/dark cycle.
Drug administration and osmotic pump implantation
All animals were subcutaneously surgically implanted with osmotic pumps, which were inserted into the periscapular region.
The slow release osmotic mini-pumps (Alzet, Model 2 ML1) with 2 mL capacity were loaded with 127 ng of rPP13 (n=10) or of His-PP13 (n=8), releasing 10 µL/h or 0.625 ng protein/h and emptying their content over 7 days. The dosage chosen corresponds to the concentration of the protein in maternal blood in the first trimester of pregnancy.5 Control animals received similar osmotic pumps filled with saline solution (n=9).
The short-term study consisted of three treatment groups with three to four animals per group. The animals were euthanized 7 days after surgery (active protein effect). The long-term study consisted of six animals per group, of which two of the His-tag PP13 group died during the pump implantation surgery. Animals were euthanized 13 days after the pump implantation surgery (extended effect). The 13-day timing was chosen to mimic the study performed in pregnant rats, in which the osmotic pump implantation took place at day 8 of pregnancy (trophoblast invasion time in rats15), and rats were sacrificed at day 21.
Diameter evaluation of vessels
Extended protein effect (13 days)
All rat uteri were dissected, one uterine horn was photographed on a petri dish and the other was placed into buffered formalin solution for 48 hours, and then transferred to PBS pH 7.4. Formalin-fixed parts of the uterine horns were paraffin embedded and sliced to 4 µm thick sections before staining. The sections were stained by H&E dye and visualized and analyzed using Nanozoomer Digital Pathology software.
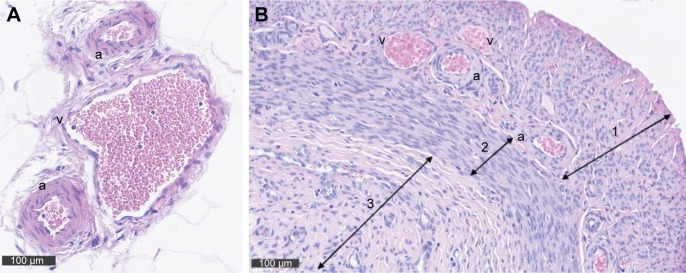
To determine the vessel type, first vessels in the uterine mesometrium were measured (main uterine vein [MUV], main uterine artery [MUA], and arcuate vessels [arcuate uterine artery and vein {AUA and AUV, respectively}]; Figure 1A), and then myometrial vessels (radial uterine vein and artery [RUV and RUA, respectively]) were assessed (Figure 1B).
Figure 1.
(A) Cross sections of the uterus showing the veins (v) and arteries (a) within the mesometrium stained with H&E. Corresponding to the two uterine horns there are two main utero-ovarian arteries and veins that are positioned in the mesometrium parallel to each uterine horn. Arcuate vessels run from the main vessels and form loops that are also positioned in the mesometrium. (B) At the same magnification, a cross section through the uterus provides a view on a fraction of the uterus showing the myometrial vessels (radial, a, v) connecting the arcuate vessels to the uterine wall. Arrow 1 marks the myometrium, and arrow 2 indicates the endometrium.
Vessel sizes were analyzed using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA) that calculated the area of each vessel’s lumen and converted it to vessel diameter based on the following equation:
where D is the diameter and A is the area.
Altogether, the analysis included for each animal was as follows: 2–3 MUAs and MUVs, 10–15 AUVs and AUAs, and 15–25 RUAs and RUVs, depending on the size of the sample.
Active protein effect (7 days)
All fresh uteri were pinned in petri dishes that were filled with relaxing solution (10 mM HEPES saline at 4°C containing 100 µM papaverine and 1 µM diltiazem mixture). All vessel diameters were measured directly through a calibrated stereomicroscope (Carl Zeiss Meditec AG, Jena, Germany) to evaluate vascular diameter between groups. In this group, MUVs and MUAs were measured on both uterine horns of each animal at three sites, the ovarian end, in the middle of the uterine horn and at the cervical end (n=24, n=24, and n=18, for the PP13, His-PP13, and the saline groups, respectively; Table 1). The radial and smaller uterine arteries and veins were not included in this analysis since they were too small for the resolution of the stereomicroscope.
Table 1.
Vessel diameters measured after 7 days
| Type of vessels | rPP13 | His-PP13 | Saline solution | p-value |
|---|---|---|---|---|
| N MUV (µm) | 24 301.3±39.6a (31%) | 24 302.0±97.0 (11%) | 18 273.3±5.8 | 0.0172 |
| N MUA (µm) | 24 151.9±15.5b (29%) | 24 128.8±23.2a (16%) | 18 107.5±32.0 | <0.01 |
| N RUV (µm) | 79 184.2±25.6a (29%) | 76 177.3±52.7a (27%) | 65 130.1±25.4 | <0.01 |
Notes: Diameters of uterine veins and arteries from nonpregnant rats treated with rPP13, His-PP13, or saline solution (control) released for 7 days from subcutaneous pumps. The values in brackets show the ratio of size increase compared to the control. Data are reported as mean ± SD. N is the number of vessels. Small letters right to the numbers are the Mann–Whitney values for the direction of significant differences between the vessels of the same type among the treatment groups.16
Significantly higher than all other groups.
Significantly lower than a but significantly higher than control group.
Abbreviations: MUA, main uterine artery; MUV, main uterine vein; RUV, radial uterine vein.
Statistical analyses
The data groups were compared by Mann–Whitney test to evaluate the significance of differences between the study groups: rPP13, His-PP13, and saline solution (control).16 All values listed in Tables 1 and 2 are reported as mean ± SD, with p-values <0.05 considered significant.
Table 2.
Vessel diameter measured after 13 days
| Type of vessels | rPP13 | His-PP13 | Saline solution | p-value |
|---|---|---|---|---|
| Veins | ||||
| N MUV (µm) | 17 442.9±64.4a,* (42%) | 9 315.7±47.7b (18%) | 14 258.3±40.5 | <0.001 |
| N AUV (µm) | 76 157.2±24.6a (29%) | 33 116.6±20.3 (4%) | 83 112.3±11 | <0.001 |
| N RUV (µm) | 94 191.7±41.2a (28%) | 76 125.7±24c (−9%) | 83 137.1±23.3b | <0.001 |
| Arteries | ||||
| N MUA (µm) | 8 143.0±24.9 (4%) | 5 146.8±20.7 (6%) | 9 137.6±28.0 | 0.3011 |
| N AUA (µm) | 42 88.5±13.1a (11%) | 44 78.1±12.7 (−0.5%) | 57 78.5±6.2 | 0.0044 |
| N RUA (µm) | 92 49.1±7.3a (46%) | 57 39.1±4.8b (6%) | 82 38.7±2.7 | <0.001 |
Notes: Diameters of nonpregnant rat uterine veins (top part) and arteries (bottom part) treated with rPP13, His-PP13, or the control saline group, measured 13 days after pump insertion (ie, 6 days post 7 days of active pump release). The values in brackets show the ratio of size increase compared to the control group. Data are reported as mean ± SD. N is the number of vessels. The superscript letters are Mann–Whitney indication for the direction of the significant differences for vessels of the same type among the treatment groups:
significantly higher than all other groups;
significantly lower than all groups.16
A significant difference (p<0.05) of the same vessel’s types between 13 days (this table) compared to 7 days (Table 1).
Abbreviations: AUA, arcuate uterine artery; AUV, arcuate uterine vein; MUA, main uterine artery; MUV, main uterine vein; RUA, radial uterine artery; RUV, radial uterine vein.
Results
General
There were no differences among the nonpregnant animal groups in terms of weight gain or blood pressure. The latter was different from previous studies in pregnant rats, where blood pressure was reduced during the active stage of PP13 release.10
Vascular expansion
Images of the effect of PP13 on the vascular system of the uterine horns of three representative animals are depicted at 13 days (eg, consisted of 7 days of active PP13 release and the following 6 days) as shown in Figure 2. The clear expansion of the uterine vessels in the rPP13 group (Figure 2, bottom) is depicted compared to the His-PP13 (middle) and the saline solution (top) groups.
Figure 2.
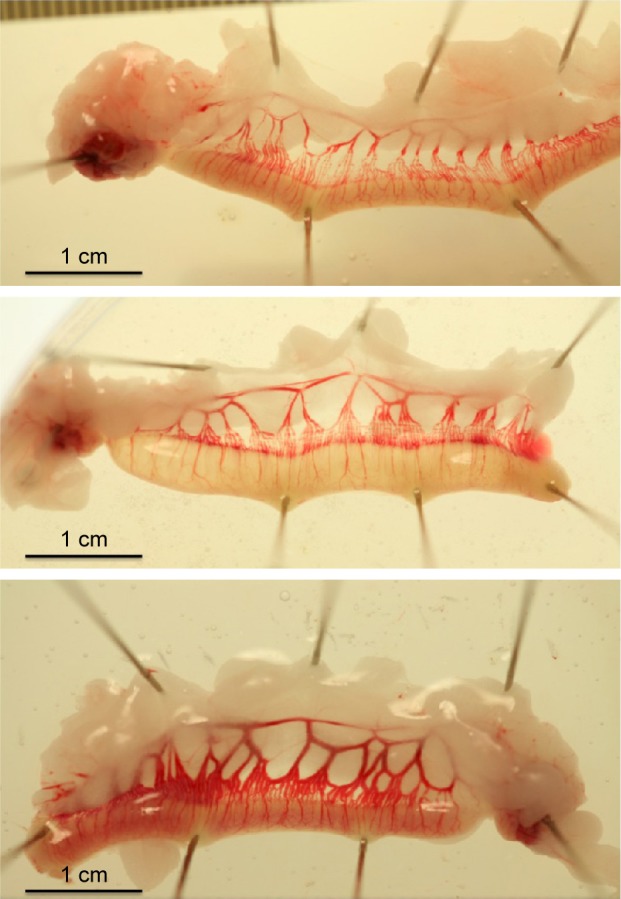
Uterine horns freshly dissected from non-pregnant rats at 13 days (six days after seven-day exposure to reagent release from the inserted osmotic pumps). Top: saline treated animal; middle: His-PP13 treated animal; bottom: rPP13 treated animal.
Short-term vascular expansion (7 days)
After 7 days of active release of rPP13, His-PP13, and saline solution, there was a significant expansion of the MUAs and MUVs and of the RUVs by rPP13 compared to saline solution (Table 1). The table also depicts a tendency toward vessel expansion by His-PP13, but the effect was significant only on the RUVs.
Long-term vascular expansion (13 days)
Veins
The analysis of uterine vein diameters for the main vein (MUV; Figure 3A, p<0.05), arcuate vein (AUV; Figure 3B, p<0.01), and RUV (Figure 3C, p<0.01) revealed that exposure to rPP13 is accompanied with a significant dilation of all three venous types compared to the saline solution, as summarized in Table 2. The vessels of the His-PP13 group also showed significant expansion of the MUV, but not of the AUV and RUV (Table 2 [veins]).
Figure 3.
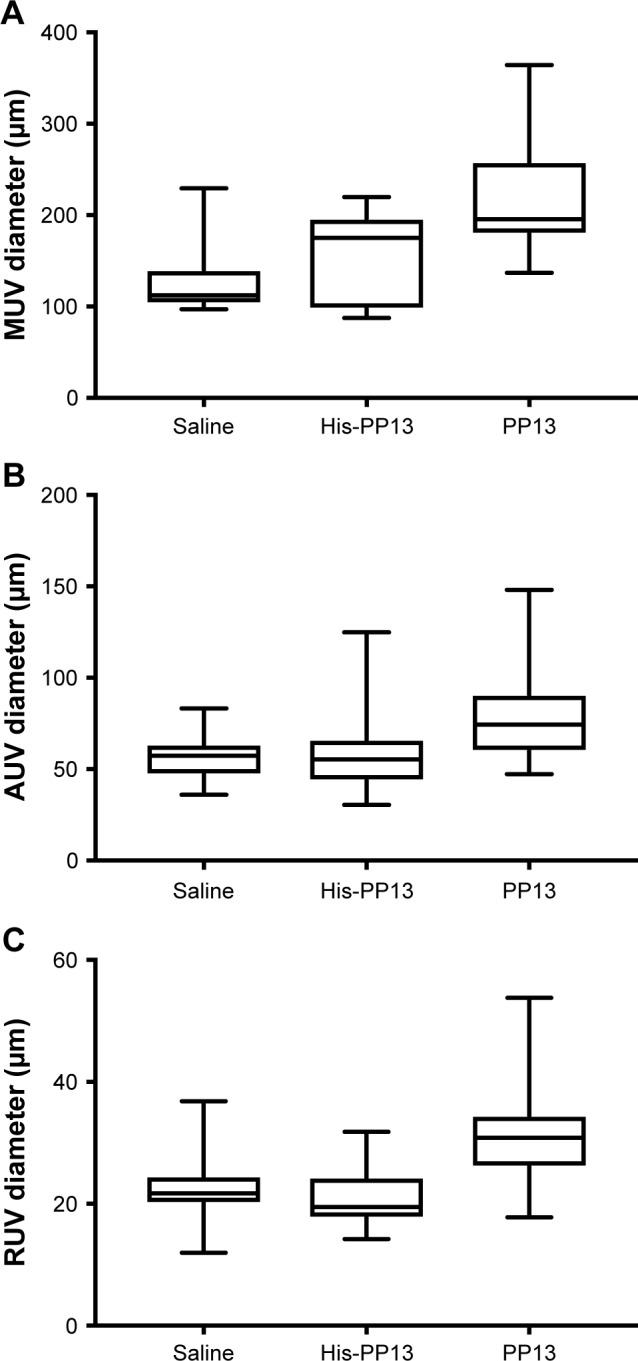
Long-term effects of rPP13 and His-PP13 on the diameters of uterine veins: MUV, AUV, and RUV. Data are reported as median ± IQR.
Abbreviations: AUV, arcuate uterine vein; MUV, main uterine vein; RUV, radial uterine vein.
Arteries
The analysis of diameter change was performed on the main (MUA; Figure 4A), arcuate (AUA; Figure 4B), and radial (RUA; Figure 4C) arteries. It revealed that there was no significant expansion of the MUA caused by either rPP13 or His-PP13 (Table 2 [arteries]). However, a significant difference was observed in both AUA and RUA (p<0.05) for the rPP13 treated group, compared to the saline solution (Table 2 [arteries]). In contrast, there were no significant changes in uterine arteries following His-PP13 administration, compared to controls (Table 2 [arteries]).
Figure 4.
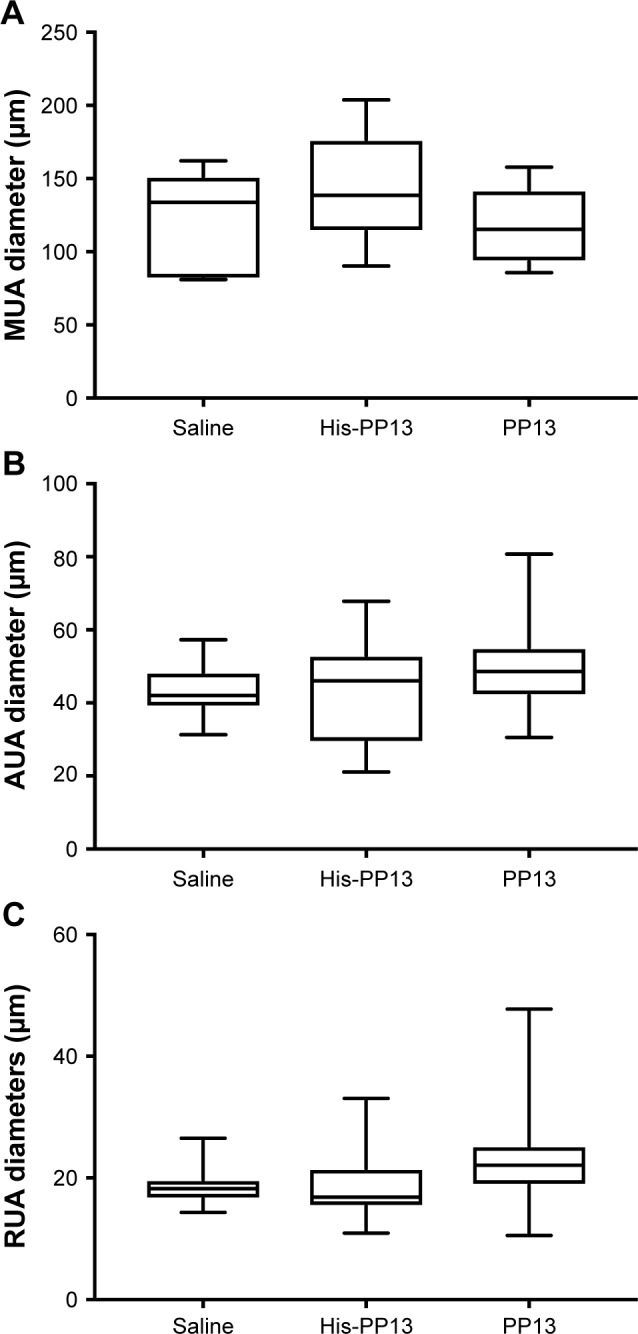
Long-term effects of rPP13 and His-PP13 on the diameters of uterine arteries: MUA, AUA, and RUA. Data are reported as median ± IQR.
Abbreviations: AUA, arcuate uterine artery; MUA, main uterine artery; RUA, radial uterine artery.
Comparison of long- and short-term effects
The long- and short-term effects of the PP13 variants on the vessel diameters were compared between the tested time points (7 and 13 days).
The comparison of rPP13 effects on the MUV showed that the venous diameter further increased from the end of protein release (7 days) to 6 days later (p=0.0159), while there was no further significant expansion for His-PP13 (p=0.6857).
The diameter of the RUVs and of the MUA did not further change between 7 and 13 days, keeping the differences between the groups unchanged (compare Tables 1 and 2).
Discussion
This study revealed the expansion of the uterine arteries and veins at various levels in nonpregnant rats. The analysis of the uterine arteries and veins was carried out immediately at the end of the PP13 release at days 7 and 13 (6 days after the pumps were emptied of their PP13 content). The major findings of this study are as follows: 1) rPP13 dilates both the uterine arteries and veins during the active polypeptide release (7 days) and also at 6 days after the pumps were empty (13 days group). The polypeptide effect appears greater in the extended time group (ie, a larger extension by rPP13 at 13 days compared to 7 days). 2) His-PP13 was found to be an effective short-term vasodilator of both arteries and veins, but its long-term effect is limited. 3) For the veins – the larger the vein (MUV) the greater was the long-term dilation effect. Arterial dilation appears to behave in the opposite direction – with a larger extension of the radial arteries than the extension of the arcuate arteries and MUAs after 13 days.
Maternal uterine vascular adaptations in pregnancy are among the most important physiological events occurring during pregnancy to enable the increase in blood flow to supply sufficient oxygen and nutrients to the fetal–placental unit. If altered or impaired, it might trigger complications related to placental insufficiency such as in the early-onset preeclampsia and fetal growth restriction. Previous studies have shown that PP13 can be detected in the maternal circulation of human pregnancies from the 5th week of gestation. Thus, it is reasonable to hypothesize that PP13 may have a role in vascular remodeling of uterine vessels in a very early stage right from the beginning of pregnancy.17
The expansion of uterine arteries and veins by PP13 has to be evaluated in the context of the uterine vascular changes during pregnancy. During human pregnancy, cardiac output and maternal blood volume increase by approximately 40% and heart rate increases by approximately 30%. Uterine vascular remodeling is part of a large vascular change that takes place in pregnancy, where the circulatory blood flow directed through the uterus increases significantly, and the vascular resistance drops significantly, resulting in the decrease in blood pressure.18,19 In experimental animals such as rats, the changes in uteroplacental blood flow during pregnancy are greater than in human beings, reaching almost a 70-fold increase, compared to the nonpregnant state.2,20 In this respect, the significant arterial and venous expansion in nonpregnant rats by PP13 appears to be an important component that explains the physiological adaptation of the vascular system during pregnancy.
In the past, the major research emphasis was on arterial remodeling, their expansion by the migrating trophoblasts causing the expansion and remodeling of the spiral arteries in human pregnancy. A much lower attention was given to the remodeling of the vein that is necessary to complete the circle of blood flow through the uterus during pregnancy. However, more recent studies have pointed out that both the uterine arteries and the veins undergo changes and remodeling during pregnancy21,22 to allow for the increase in uterine blood flow. Yet, the changes in the venous side of the circulation are much less investigated. Gyselaers et al23,24 showed in a series of studies that venous hemodynamic changes in several organs during gestation and that the failure of such changes are associated with pregnancy complications such as preeclampsia.23,24 Interestingly, the vasodilating effect of PP13 measured in this study suggests that PP13 is a potent mediator not only of the arteries but also of venous expansion.
Our findings emphasize the involvement of PP13, a placenta-specific galectin, expressed by the placental syncytiotrophoblast, in uterine blood vessel remodeling in pregnancy. In addition, previous studies have shown that PP13 turns the decidua immune tolerant to the invading trophoblast during pregnancy.4,25 Kliman et al25 showed strong PP13 staining around small uterine veins. In that study, performed in sections of women placentas during the first trimester after social termination, the presence of PP13 around the veins was discussed in the context of rendering the decidua immune tolerant to the migrating trophoblasts. The vein accumulation of PP13 was proposed to cause a divergence of the immune response away from the arteries to allow spiral arteries remodeling by the migrating tropho-blasts, thereby enabling the deep placentation.4,25–27 In the current study, we discovered the very strong direct effect of PP13 on uterine venous expansion. Therefore, PP13 appears to have a dual effect: immunologically, it turns the decidua immune tolerant to the pregnancy, and physiologically it remodels both arteries and veins thus increasing blood flow to the pregnancy and contributing to the major vascular changes in pregnancy. Thus, this study suggests that the shortage of PP13 in the first trimester may lead to impaired vascular remodeling by a lack of vessel expansion and increased immune rejection in pregnancy.
Studies performed in the third trimester of pregnancy have shown an increased release of necrotic vesicles from the villous trophoblast into the maternal blood, bringing large amounts of PP13 to the circulation near the time of the outbreak of hypertension in preeclampsia and during the development of all other clinical symptoms.17 Dys-regulated villous trophoblast differentiation contributes to elevated PP13 release at the time of clinical symptoms of preeclampsia. The role of such elevated levels of PP13 at the time of the pathology is not fully explored. In view of the effect of PP13 on arterial and venous expansion that was identified in this study, a third trimester increase in maternal blood PP13 may be interpreted as a body rescue mechanism to fight hypertension by enhancing vasodilation to decrease peripheral resistance.3,17 This assumption warrants further studies.
In our previous in vitro studies on isolated rat arteries, we demonstrated that the endothelial layer of the arteries in both pregnant and nonpregnant rats is mediating the PP13 effects. The PP13-induced vasodilation of the resistance arteries involves the endothelium-derived relaxing factors, eNOS and prostaglandins.8 In addition, to the former in vivo findings in pregnant rats, we have demonstrated arterial expansion caused by PP13.3 Here, we did a thorough quantitative comparison of arterial and venous expansion in vivo in nonpregnant rats. It emphasizes that the underlying PP13-associated vascular expansion is general and not dependent on pregnancy.
The comparison of the long-term PP13 effects on arteries and veins revealed a much larger (double) expansion of veins by 39%–86%, compared to a lower expansion of the corresponding arteries by 19%–46%, showing that the uterine vessel dilation effect associated with pregnancy is higher in veins than in arteries. A thorough in vitro analysis of the PP13 effect on isolated veins is now conducted by our group. The different vasodilation effects of PP13 on arteries and veins as was determined in this study could be the result of: 1) a relative difference in the time and amount of PP13 they are exposed to (small veins are most potent in absorbing polypeptides from the inter-peritoneal flow compared to arteries), and thus PP13 that was absorbed by the veins might be further diluted; 2) arteries and veins may have different sensitivity to PP13. Although the polypeptide does not have a known “receptor” in the classical definition, it is acting through the eNOS and prostaglandin 2 pathways, and the density of these systems may be different between arteries and veins. Additional in vitro studies may shed light on this aspect.
Our study also shows the central role of veins in the circulating blood volume changes. The blood return to the heart is regulated by venous reflex-induced constriction and creates negative pressure gradients in the circulation. This is important in the case of blood volume expansion, like in pregnancy, where the excess blood volume is stored in the venous compartment.23,24,28 Our study implies that PP13 might be a key molecule or a major signaling molecule by which the placenta can regulate hemodynamic changes in the reproductive maternal organs during pregnancy either accelerating blood release from the placenta through the expanded uterine veins and/or by increasing the supply of oxygen and nutrients via the expanded uterine arteries. It is interesting that the rat radial arteries (corresponding to human spiral arteries) appear to be more affected by PP13 given their much closer proximity to the placenta.17,29,30 Further studies are required to analyze the underlying pathways involved in the venous expansion by PP13. However, there are some indications that the effect could be mediated by the NO signaling pathway. It has been found that endothelial vasodilatory responsiveness in pregnancy increases and that this expansion appears to be primarily mediated by an NO-dependent mechanism.1,6,8 Interestingly, this effect appears to last for a prolonged time frame that could be measured 6 days after emptying the pumps releasing PP13.
Our group is currently conducting an additional set of experiments, where the impact of adding the NO inhibitor N-nitro-L-arginine methyl ester is tested in vivo to evaluate its potential impact on vasodilation following the slow release of PP13 from implanted pumps. Preliminary data indicated that indeed NO blockers could decrease the vasodilation produced by slow PP13 release in vivo, but a thorough analysis is still warranted.
This study examined the arterial and venous expansion immediately after the full release of PP13 from the subcutaneous inserted pumps and 6 days after the release had stopped. We observed that the release of rPP13 and His-PP13 actively expanded the diameters of both veins and arteries. The measured effect of rPP13 at day 7 is less for MUV, than 6 days later, showing that the rPP13 is still working on the vessels, although the active polypeptide delivery had stopped. Therefore, it seems that in these vessels the expansion is primed and is continuously acting without the requirement of the permanent release of PP13. The extended effect may be derived from a very high affinity of PP13 to certain binding sites in these vessels that lasts beyond its active release. Alternatively, it can be attributed to the features of the underlying pathways, which may be independent of the active presence of rPP13. Our pharmacokinetic studies revealed that the half-life of rPP13 is 6–13 hours.10 The pharmacoki-netic studies also indicated a slow release of low amount of PP13 from body tissues within 24 hours or more. Overall, it appears that the long-term effect measured here reflects a PP13-induced vasodilation that is maintained throughout the period of the polypeptide release from the pumps and beyond and primes vessels for additional structural expansion that is manifested for a longer period. His-PP13, on the other hand, has the polyhistidine sequence attached to the N-terminus, which prohibits PP13 dimerization, has been already shown to be less stable in the body fluids, tends to precipitate, and becomes rapidly inactive (compared to rPP13 that forms stable dimers), corresponding to its lower and shorter lasting effects.
There are few limitations to this study: 1) the effect of PP13 on vessel diameter examined when sacrificed after 7 days was conducted when the vessels were left in relaxing solution and reached maximum relaxation whereas at 13 days they were fixed after a shorter period. To compare the groups, the size factor between saline-treated groups was used to normalize the size of the groups; 2) at the end of the treatment (both 7 and 13 days), the rats were euthanized without taking the estrous cycle phase into consideration; 3) also, we know that the life span of the His-PP13 (a monomer) is shorter than the life span of rPP13, which tends to form stable dimers. A previous study has shown that the rPP13 dimer (as well as the native PP13 in vivo) is more stable in solution, whereas the His-PP13 forms monomers or long oligomers that precipitate in solution.12 Thus, it appears reasonable to assume that the lower impact of His-PP13 compared to the rPP13 may be due to the faster removal of His-PP13 from blood. Interestingly, we have previously been able to show that a single subcutaneous application of PP13 takes over 24 hours to be cleared from blood.10 However, an additional direct monitoring of the clearance of PP13 from blood after a continuous infusion is required. Further studies are also required to examine the PP13-NO pathway in veins, which could be implied from the current study.
Another study limitation was related to our focus on the uterus. A thorough analysis of the PP13 effect on other organs is ahead of us. It was relatively surprising that the vasodilation of arteries and veins in the nonpregnant animals was not accompanied by a reduced blood pressure. Drobnjak et al10 showed that the effect of PP13 on vasodilation in pregnant animals is much larger in the uterine vascular system (approximately 48% dilation) compared to mesenteric vessels (approximately 20% dilation). Unlike pregnant rats where the blood flow through the uterus accounts for 30% of the total blood flow, in nonpregnant rats only a very small fraction of the blood flows through the uterus. Thus, it may not contribute much to the entire systemic blood pressure in nonpregnant animals. Further evaluation of this point is required.
Conclusion
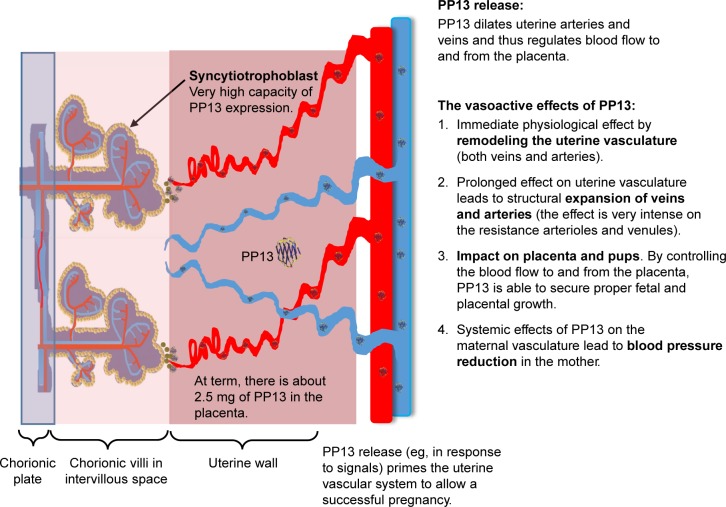
The comprehensive impact of PP13 on the uterine vascular system is shown in Figure 5. In a very early stage of pregnancy (week 5), PP13 is expressed by the placental syncytiotrophoblast in very high amount (reaching 2.5 mg in the term placenta). The release of PP13 dilates arteries and veins and thereby regulates the flow to and from the feto-placental unit. The vasoactive effect of PP13: 1) generates an immediate effect on both veins and arteries; 2) generates a longer effect, which is subsequently developed (more intense in the most resistant arterioles and venules) leading to a structural expansion of the veins and the arteries; and 3) by redirecting the blood flow to the uterus, PP13 is able to secure selective growth of the fetus and the placenta. It may also generate a systemic effect on the maternal vasculature, thereby causing a reduced maternal blood pressure, at least during pregnancy. A potential cross talk between the arteries and the veins should be further investigated. Based on the proposed model, PP13 might be a potential candidate for the treatment of preeclampsia cases associated with impaired vascular remodeling, where the vasodilatory effect of the protein can overcome the lack of arterial expansion given the long-term effect identified here. This prolonged impact may be attributed to high protein affinity to the vessel walls that contributes to the long-term effect. Altogether, this study shows that PP13 may be an effective preconditioning modifier of vascular remodeling of the rat uterine vasculature. Its effect in human beings remains to be evaluated.
Figure 5.
Integrated effect of PP13 on the vascular system. A schematic description integrating the impact of PP13 on the vascular system that shows how PP13 could influence the vascular system and prime it to be ready for its increased role in pregnancy.
Acknowledgments
The authors would like to thank Hy-Laboratories Ltd. for providing PP13 for this study through support provided by the European Union through ASPRE project (#601852). This study was mainly sponsored by Hananja ehf and Icelandic Research Fund (Rannís), grant no 163403-052. The funders had no influence on study design or the analysis of the data.
Footnotes
Disclosure
Hamutal Meiri and Sveinbjörn Gizurarson hold a patent for the potential therapeutic use of PP13 in pregnancy complications. Hamutal Meiri is the CEO and Chairman of TeleMarpe Ltd. and is a consultant of Hy Laboratories Ltd. The authors report no other conflicts of interest in this work.
References
- 1.Mandala M, Osol G. Physiological remodelling of the maternal uterine circulation during pregnancy. Basic Clin Pharmacol Toxicol. 2012;110(1):12–18. doi: 10.1111/j.1742-7843.2011.00793.x. [DOI] [PubMed] [Google Scholar]
- 2.Osol G, Mandala M. Maternal uterine vascular remodeling during pregnancy. Physiology. 2009;24:58–71. doi: 10.1152/physiol.00033.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gizurarson S, Sigurdardottir ER, Meiri H, et al. Placental protein 13 administration to pregnant rats lowers blood pressure and augments fetal growth and venous remodeling. Fetal Diagn Ther. 2015 Aug 28; doi: 10.1159/000381914. Epub. [DOI] [PubMed] [Google Scholar]
- 4.Than NG, Romero R, Goodman M, et al. A primate subfamily of galec-tins expressed at the maternal-fetal interface that promote immune cell death. Proc Natl Acad Sci U S A. 2009;106(24):9731–9736. doi: 10.1073/pnas.0903568106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huppertz B, Meiri H, Gizurarson S, Osol G, Sammar M. Placental protein 13 (PP13): a new biological target shifting individualized risk assessment to personalized drug design combating pre-eclampsia. Hum Reprod Update. 2013;19(4):391–405. doi: 10.1093/humupd/dmt003. [DOI] [PubMed] [Google Scholar]
- 6.Naljayan MV, Karumanchi SA. New developments in the pathogenesis of preeclampsia. Adv Chronic Kidney Dis. 2013;20(3):265–270. doi: 10.1053/j.ackd.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365(9461):785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 8.Drobnjak T, Gizurarson S, Gokina NI, et al. Placental protein 13 (PP13)-induced vasodilation of resistance arteries from pregnant and nonpregnant rats occurs via endothelial-signaling pathways. Hyper-tens Pregnancy. 2017;36(2):186–195. doi: 10.1080/10641955.2017.1295052. [DOI] [PubMed] [Google Scholar]
- 9.Than NG, Romero R, Meiri H, et al. PP13, maternal ABO blood groups and the risk assessment of pregnancy complications. PLoS One. 2011;6(7):e21564. doi: 10.1371/journal.pone.0021564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drobnjak T, Meiri H, Mandala M, Huppertz B, Gizurarson S. Pharma-cokinetics of placental protein 13 after intravenous and subcutaneous administration in rabbits. Drug Des Devel Ther. 2018;12:1977–1983. doi: 10.2147/DDDT.S167926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maymon R, Trahtenherts A, Svirsky R, et al. Developing a new algorithm for first and second trimester preeclampsia screening in twin pregnancies. Hypertens Pregnancy. 2017;36(1):108–115. doi: 10.1080/10641955.2016.1242605. [DOI] [PubMed] [Google Scholar]
- 12.Sammar M, Nisamblatt S, Gonen R, et al. The role of the carbohydrate recognition domain of placental protein 13 (PP13) in pregnancy evaluated with recombinant PP13 and the delT(221) PP13 variant. PLoS One. 2014;9(7):e102832. doi: 10.1371/journal.pone.0102832. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Than NG, Sumegi B, Than GN, Berente Z, Bohn H. Isolation and sequence analysis of a cDNA encoding human placental tissue protein 13 (PP13), a new lysophospholipase, homologue of human eosino-phil Charcot-Leyden crystal protein. Placenta. 1999;20(8):703–710. doi: 10.1053/plac.1999.0436. [DOI] [PubMed] [Google Scholar]
- 14.Visegrady B, Than NG, Kilar F, Sumegi B, Than GN, Bohn H. Homology modelling and molecular dynamics studies of human placental tissue protein 13 (galectin-13) Protein Eng. 2001;14(11):875–880. doi: 10.1093/protein/14.11.875. [DOI] [PubMed] [Google Scholar]
- 15.Soares MJ, Chakraborty D, Rumi MAK, Konno T, Renaud SJ. Rat placentation: an experimental model for investigating the hemochorial maternal-fetal interface. Placenta. 2012;33(4):233–243. doi: 10.1016/j.placenta.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamada C. Statistical analysis for toxicity studies. J Toxicol Pathol. 2018;31(1):15–22. doi: 10.1293/tox.2017-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huppertz B, Sammar M, Chefetz I, Neumaier-Wagner P, Bartz C, Meiri H. Longitudinal determination of serum placental protein 13 during development of preeclampsia. Fetal Diagn Ther. 2008;24(3):230–236. doi: 10.1159/000151344. [DOI] [PubMed] [Google Scholar]
- 18.Moore LG, McCullough RE, Weil JV. Increased HVR in pregnancy: relationship to hormonal and metabolic changes. J Appl Physiol (1985) 1987;62(1):158–163. doi: 10.1152/jappl.1987.62.1.158. [DOI] [PubMed] [Google Scholar]
- 19.Palmer SK, Zamudio S, Coffin C, Parker S, Stamm E, Moore LG. Quantitative estimation of human uterine artery blood flow and pelvic blood flow redistribution in pregnancy. Obstet Gynecol. 1992;80(6):1000–1006. [PubMed] [Google Scholar]
- 20.Page KL, Celia G, Leddy G, Taatjes DJ, Osol G. Structural remodeling of rat uterine veins in pregnancy. Am J Obstet Gynecol. 2002;187(6):1647–1652. doi: 10.1067/mob.2002.127599. [DOI] [PubMed] [Google Scholar]
- 21.Moser G, Huppertz B. Implantation and extravillous trophoblast invasion: from rare archival specimens to modern biobanking. Placenta. 2017;56:19–26. doi: 10.1016/j.placenta.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moser G, Weiss G, Sundl M, et al. Extravillous trophoblasts invade more than uterine arteries: evidence for the invasion of uterine veins. Histochem Cell Biol. 2017;147(3):353–366. doi: 10.1007/s00418-016-1509-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gyselaers W, Mullens W, Tomsin K, Mesens T, Peeters L. Role of dysfunctional maternal venous hemodynamics in the pathophysiology of pre-eclampsia: a review. Ultrasound Obstet Gynecol. 2011;38(2):123–129. doi: 10.1002/uog.9061. [DOI] [PubMed] [Google Scholar]
- 24.Gyselaers W, Tomsin K, Staelens A, Mesens T, Oben J, Molenberghs G. Maternal venous hemodynamics in gestational hypertension and pre-eclampsia. BMC Pregnancy Childbirth. 2014;14:212. doi: 10.1186/1471-2393-14-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kliman HJ, Sammar M, Grimpel YI, et al. Placental protein 13 and decidual zones of necrosis: an immunologic diversion that may be linked to preeclampsia. Reprod Sci. 2012;19(1):16–30. doi: 10.1177/1933719111424445. [DOI] [PubMed] [Google Scholar]
- 26.Sammar M, Dragovic R, Meiri H, et al. Reduced placental protein 13 (PP13) in placental derived syncytiotrophoblast extracellular vesicles in preeclampsia – A novel tool to study the impaired cargo transmission of the placenta to the maternal organs. Placenta. 2018;66:17–25. doi: 10.1016/j.placenta.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 27.Than NG, Romero R, Balogh A, et al. Galectins: double-edged Swords in the cross-roads of pregnancy complications and female reproductive tract inflammation and neoplasia. J Pathol Transl Med. 2015;49(3):181–208. doi: 10.4132/jptm.2015.02.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gyselaers W, Mesens T, Tomsin K, Peeters L. Doppler assessment of maternal central venous hemodynamics in uncomplicated pregnancy: a comprehensive review. Facts Views Vis Obgyn. 2009;1(3):171–181. [PMC free article] [PubMed] [Google Scholar]
- 29.Odibo AO, Zhong Y, Goetzinger KR, et al. First-trimester placental protein 13, PAPP-A, uterine artery Doppler and maternal characteristics in the prediction of pre-eclampsia. Placenta. 2011;32(8):598–602. doi: 10.1016/j.placenta.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicolaides KH, Bindra R, Turan OM, et al. A novel approach to first-trimester screening for early pre-eclampsia combining serum PP-13 and Doppler ultrasound. Ultrasound Obstet Gynecol. 2006;27(1):13–17. doi: 10.1002/uog.2686. [DOI] [PubMed] [Google Scholar]