Abstract
Messenger RNA (mRNA) has become a promising class of drugs for diverse therapeutic applications in the past few years. A series of clinical trials are ongoing or will be initiated in the near future for the treatment of a variety of diseases. Currently, mRNA-based therapeutics mainly focuses on ex vivo transfection and local administration in clinical studies. Efficient and safe delivery of therapeutically relevant mRNAs remains one of the major challenges for their broad applications in humans. Thus, effective delivery systems are urgently needed to overcome this limitation. In recent years, numerous nanoscale biomaterials have been constructed for mRNA delivery in order to protect mRNA from extracellular degradation and facilitate endosomal escape after cellular uptake. Nanoscale platforms have expanded the feasibility of mRNA-based therapeutics, and enabled its potential applications to protein replacement therapy, cancer immunotherapy, therapeutic vaccines, regenerative medicine, and genome editing. This review focuses on recent advances, challenges, and future directions in nanoscale platforms designed for mRNA delivery, including lipid and lipid-derived nanoparticles, polymer-based nanoparticles, protein derivatives mRNA complexes, and other types of nanomaterials.
Keywords: delivery, messenger RNA, nanoparticles
1 |. INTRODUCTION
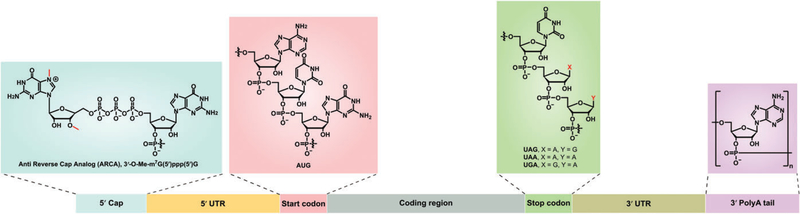
mRNA serves as an essential macromolecule in the central dogma for carrying genetic information from nucleus to cytoplasm, thereby expressing functional proteins (Sharp, 2009). Aberrant alterations in the expression level of proteins lead to numerous congenital and acquired diseases like genetic disorders and cancers (Sahin, Kariko, & Tureci, 2014). Thus, it is critical to maintain a normal level of mRNAs in the cytoplasm. Structurally, mRNA is a single-stranded RNA transcribed from a DNA template. The mature eukaryotic mRNA typically consists of a 5ʹ-methylguanosine (m7G) cap, 5ʹ-untranslated region (UTR), coding region starting with AUG codon, 3ʹ-UTR, and a polyadenylated A (poly(A)) tail (Midoux & Pichon, 2015; Weissman, 2015; Figure 1). The Kozak sequence in the 5ʹ-UTR is involved in the recognition by the ribosome to initiate the translation process. The 3ʹ-UTR is another important component in regulating mRNA translation and stability, which may contain miRNA binding sites. Likewise, the poly(A) tail is also a critical component for mRNA translation and degradation. When the poly(A) tail is fewer than 12 adenosine residues, mRNA is degraded from the 5ʹ cap structure (Midoux & Pichon, 2015; Yamamoto, Kormann, Rosenecker, & Rudolph, 2009). Hence, the stability and translation efficiency of exogenous mRNA can be enhanced by a number of methods such as UTR manipulation, codon optimization, chemical modification, and elongation of poly(A) tail of mRNA (Grabbe et al., 2016; Loomis, Kirschman, Bhosle, Bellamkonda, & Santangelo, 2016; Presnyak et al., 2015; Yamamoto et al., 2009). In addition, high performance liquid chromatography or fast protein liquid chromatography purified- and chemically modified-mRNA reduces different types of immune response in vivo (Granot & Peer, 2017; Karikó, Muramatsu, Ludwig, & Weissman, 2011). Currently, in vitro transcription using T7, SP6, or T3 RNA polymerase is a common method to produce a large amount of mRNAs (Sahin et al., 2014; Weissman, 2015).
FIGURE 1.
Structure of in vitro transcribed mRNA. Typically, mRNA consists of seven units including 5ʹ cap (ARCA, a representative cap), 5ʹ UTR, start codon (AUG), coding region, stop codon, 3ʹ UTR, and 3ʹ poly(A) tail
The concept of using exogenous mRNA to express protein can be traced back to 1978, when the first attempt was to deliver rabbit globin mRNA using liposomes to mouse lymphocytes and yield functional proteins (Dimitriadis, 1978). Over the next decade, however, mRNA was not applied as a therapeutic agent due to several concerns such as instability, poor cell penetration, immunogenicity as well as high cost of production (Granot & Peer, 2017; Stanton & Murphy-Benenato, 2017). This situation has been gradually changing over the past several years because of the accumulated knowledge of nucleic acid chemistry and the steady decline in production costs of mRNAs (Devoldere, Dewitte, De Smedt, & Remaut, 2016; Grabbe et al., 2016; Li, Luo, & Dong, 2016; Pascolo, 2017). Since 1990s, mRNA has been applied as potential immunotherapy agents. The early clinical trials were to transfect dendritic cells (DCs) with mRNA encoding tumor-specific antigens in order to stimulate cytotoxic T lymphocyte against cancers such as metastatic prostate tumors (Gilboa & Vieweg, 2004). In the last decade, applications have expanded to address numerous diseases and conditions because mRNA-based therapeutics possesses several advantages: (a) mRNA does not modify the genes of the host, which avoids genotoxicity. (b) mRNA can be delivered in a relatively controlled manner to regulate transfection efficiency and duration of the protein expression. (c) mRNA delivery does not require nuclear localization or transcription. In addition, mRNAs are particularly suitable for transient protein expression such as genome editing to minimize off-target effects (Sergeeva, Koteliansky, & Zatsepin, 2016). Moreover, exogenous mRNA is not regulated as a genetically modified organism by the Food and Drug Administration (FDA, Midoux & Pichon, 2015). Most recently, mRNA-based therapeutics benefited greatly from the rapid progress of delivery methods (Guan & Rosenecker, 2017; Hajj & Whitehead, 2017; Islam et al., 2015; Kauffman, Webber, & Anderson, 2016; Meng et al., 2017; Stanton & Murphy-Benenato, 2017) All these efforts have made mRNA a potential class of new therapeutic agents (Grabbe et al., 2016; Kauffman et al., 2016; Sahin et al., 2014; Vallazza et al., 2015), and have enabled mRNA-based therapeutics to be used in the fields of preclinical and clinical studies for treating genetic disorders, cancers, infectious diseases, cardiovascular diseases, and others (Hajj & Whitehead, 2017; Meng et al., 2017; Sahin et al., 2014; Sergeeva et al., 2016; Vallazza et al., 2015; Wang et al., 2017). In this review, we mainly focus on nanoscale platforms that were recently developed to deliver mRNA, and discuss their therapeutic applications in more detail.
2 |. THE PATH FROM EXOGENOUS mRNA TO FUNCTIONAL PROTEINS
Exogenous mRNAs offer the flexibility and ability to encode desired proteins and achieve their biological functions in vivo (Sahin et al., 2014). Nevertheless, to realize this process, delivery vehicles have a substantial influence on tissue biodistribution, cellular uptake, as well as endosome escape. As nonviral delivery systems, nanoparticles provide versatile platforms for exogenous mRNA expression in vivo. During the delivery process, nanoparticles protect mRNA from RNase degradation, increase their cellular uptake, and facilitate endosome escape, thereby expressing functional proteins in the cytosol (Figure 2). These attributes make it feasible to address technical challenges for in vivo delivery of mRNA for patients.
FIGURE 2.
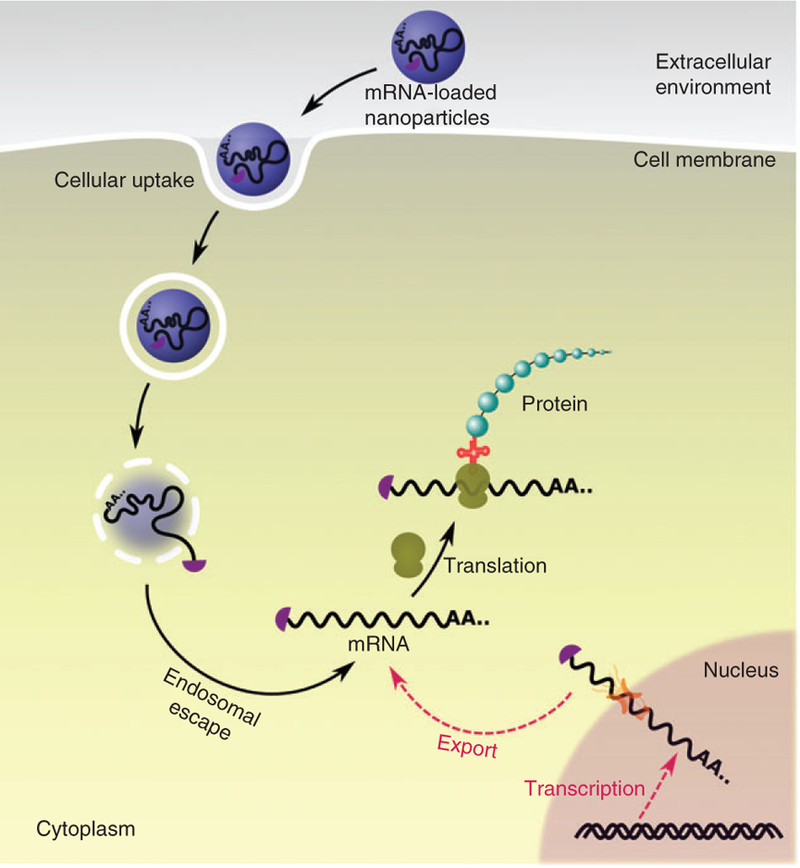
Schematic diagram of intracellular mRNA delivery. Endogenous mRNA is transcribed from DNA, processed, and exported to cytoplasm for translation (red pathway). Alternatively, in vitro transcribed mRNA can be introduced into cytoplasm by nanoscale platforms (black pathway). Using exogenous mRNAs, functional proteins can be produced in the cytoplasm
2.1 |. Pharmacokinetics
Administration routes of mRNA-encapsulated nanoparticles led to significantly different organ distribution in mice (Pardi et al., 2015). For example, a recent study compared six administration routes (intradermal, intramuscular, subcutaneous, intravenous, intraperitoneal, and intratracheal routes) of lipid-luciferase mRNA nanoparticles in mice (Pardi et al., 2015). Intravenous injection produced very strong bioluminescent signal in the liver with a half-life of 6.8 hr, while intradermal and intramuscular injections displayed a much longer duration (over 20 hr) at the site of injections (Pardi et al., 2015). Intratracheal administration gave protein production for several days at a dose of 5 μg mRNA per mouse. Therefore, it may be critical to determine the appropriate administration routes for different therapeutic applications.
In 2017, Sedic et al. (2017) reported a safety evaluation of lipid nanoparticles loaded with human erythropoietin (hEPO) mRNA in both Sprague-Dawley rats and cynomolgus monkeys, which established its pharmacology, pharmacokinetics, and safety profile. Both rats and monkeys were administered intravenously one or two doses per week for the course of 2 weeks at the dose of 0.03, 0.1, 0.3 mg/kg. After the first intravenous dose, plasma concentration of hEPO reached its maximal levels at approximately 6 hr in both animal species. The maximum serum concentration (Cmax) of hEPO (0.3 mg/kg, one dose per week for 2 weeks) was 2,340 and 253 ng/mL in rats and monkeys, respectively. In rats, area under the curve of hEPO-mRNA was in a greater than dose-proportional manner, while the dose-proportional manner that was observed in monkeys. Repeat administration of mRNA in lipid-based nanoparticles (LNPs) at doses as high as 0.3 mg/kg per dose (total of four doses) induced mild increase of alanine aminotransferase and aspartate aminotransferase, and caused a reversible inflammatory response in rats. These results provide useful information including pharmacokinetics and pharmacodynamics properties of LNPs/mRNA for future clinic trials (Sedic et al., 2017).
2.2 |. Cellular trafficking
Once distributed in organs, nanoparticles undergo a complex process of cellular uptake termed as endocytosis, which involves multiple steps (Sahay, Alakhova, & Kabanov, 2010). First, nanoparticles interact with cell members through various mechanisms including clathrin-dependent and clathrin-independent pathways (Sahay et al., 2010). Numerous surface proteins enable the occurrence of endocytosis such as clathrin, Arf6, flotillin, Cdc42, RhoA, and many others (Sahay et al., 2010). Particle properties including particle size, shape, material composition, and surface charge play a major role in determining the mechanism for cellular uptake (Sahay et al., 2010). In a lot of cases, more than one pathway is activated (Sahay et al., 2010).
After the endocytosis process, cell forms membrane-bound vesicles with nanoparticles inside called endosome (Sahay et al., 2010). In order to be translated, mRNA must be released from endosome to cytosol. Therefore, endosome escape is another barrier for mRNA delivery. Using LNPs, Patel et al. (2017) recently investigated the particle trafficking from early endosome, late endosome, to lysosome. Their results showed that late endosome/lysosome formation is essential for mRNA delivery into cytosol, because lysosomes provided a hub to modulate mammalian target of rapamycin (mTOR) signaling, thereby controlling mRNA translation (Patel et al., 2017). To improve LNP-mediated intracellular mRNA delivery, they next screened 212 lipid-like molecules such as prostaglandins, isoprostanes, thromboxanes, leukotrienes, lipoxins, and polyunsaturated fatty acids. They found that MK-571, a clinically approved leukotriene D4 antagonist, increased mRNA delivery over threefold in vitro and twofold in vivo when incorporated into commercial available lipoplex and proprietary LNPs, respectively (Patel et al., 2017). Once entering the cytoplasm, translated proteins can be detected within 1 h after transfection (Hecker, 2016). mRNA is finally degraded through various mechanisms with a half-life from minutes to hours, depending on multiple factors of the specific mRNA (Chen, Ezzeddine, & Shyu, 2008; Schoenberg & Maquat, 2012).
3 |. NANOSCALE DELIVERY PLATFORMS
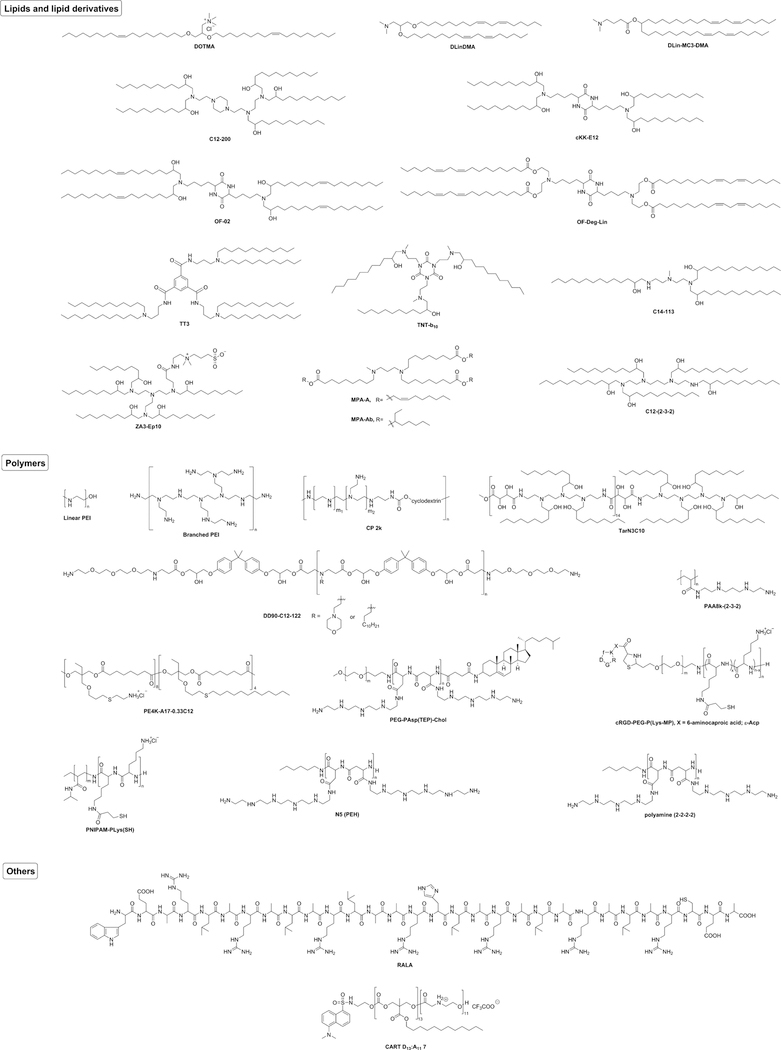
In this section, we mainly describe recent advances in nanoparticle-mediated mRNA delivery platforms. Figure 3 shows representative structures of the key components for particle formulation used for mRNA delivery in vivo. Table 1 summarizes delivery platforms, particle compositions, mRNA cargos, routes of administration, and corresponding references.
FIGURE 3.
Representative chemical structures of the main component of nanoparticles used for mRNA delivery in vivo
TABLE 1.
Synthetic nonviral mRNA delivery systems for in vivo administration
| Delivery platform | Composition | Payload mRNA | Route of administration | Reference |
|---|---|---|---|---|
| Lipid-based vehicles | DOTMA, DOPE | eGFP, Luc, HA, OVA, gp70 | i.v. | Kranz et al. (2016) |
| DlinDMA, DSPC, cholesterol, DMG-PEG2000 | Luc, SaRNA encoding RSV-F | i.m. | Geall et al. (2012) | |
| DLin-MC3-DMA, DSPC, cholesterol, DMG-PEG2000 | EPO, Frataxin | i.v., intrathecal | Sedic et al. (2017), Nabhan et al. (2016) | |
| C12–200, DOPE, cholesterol, DMG-PEG2000 | EPO, FIX, Cas9 | i.v. | Kauffman et al. (2015), DeRosa et al. (2016), Yin et al. (2016) | |
| cKK-E12, DOPE, cholesterol, DMG-PEG2000 | Luc, TRP2, gp100, OVA, β-gal, co-delivery of Cas9 mRNA and sgRNA targeting Pcsk9 gene | s.c., i.v. | Oberli et al. (2017), Yin et al. (2017) | |
| OF-02, DOPE, cholesterol, DMG-PEG2000 | EPO | i.v. | Fenton et al. (2016) | |
| OF-Deg-Lin, DOPE, cholesterol, DMG-PEG2000 | Luc | i.v. | Fenton et al. (2017) | |
| TT3, DOPE, cholesterol, DMG-PEG2000 | Luc, FIX, Cas9 | i.v. | Li et al. (2015), Li and Dong (2017), Luo et al. (2017), Jiang et al. (2017) | |
| LNP-INT01, DSPC, cholesterol, DMG-PEG2000 | Co-delivery of Cas9 mRNA and sgRNA targeting Ttr gene | i.v. | Finn et al. (2018) | |
| TNT-b10, DOPE, cholesterol, DMG-PEG2000 | Luc | i.v. | Li, Luo, Deng, et al. (2016) | |
| C14–113, DSPC, cholesterol, DMG-PEG2000 | eGFP | Intramyocardial | Turnbull et al. (2016) | |
| ZA3-Ep10, cholesterol, PEG-lipid | Co-delivery of Cas9 mRNA and sgRNA targeting LoxP gene | i.v. | Miller et al. (2017) | |
| MPA-A (or MPA-Ab), DOPE, cholesterol, DMG-PEG2000 | Luc, Cas9 | Intratumoral, i.v. | Zhang et al. (2017) | |
| C12-(2–3-2), DPPC, cholesterol, DMPE-PEG2000 | Luc, ACE2 | i.v. | Schrom et al. (2017) | |
| C12-(2–3-2), DOPE, cholesterol, DMPE-PEG2000 | Luc | i.v. | Jarzebinska et al. (2016) | |
| Polymer-based vehicles | CP 2k | HIV gp120 | i.n. | Li et al. (2016) |
| TarN3C10, DSPC, cholesterol, DMG-PEG2000 | EPO | i.v. | Dong et al. (2016) | |
| DD90-C12–122, 14:0 PEG2000 PE | Luc | i.v. | Kaczmarek et al. (2016) | |
| PAA8k-(2–3-2) | Luc | i.v. | Jarzebinska et al. (2016) | |
| PE4K-A17–0.33C12, Pluronic F127 | Luc | i.v. | Yan, Xiong, Zhang, Cheng, and Siegwart (2017) | |
| N5 (PEH), recombinant, human eIF4E | Luc | i.v. | Li, Wang, et al. (2017) | |
| Polyamine (2–2-2–2), PABP | Luc | i.v. | Li, He, et al. (2017) | |
| PEG-PAsp(TEP)-Chol | sFlt-1 | i.v. | Uchida, Kinoh, et al. (2016) | |
| cRGD-PEG-P(Lys-MP), PNIPAM-PLys(SH) | Luc, GFP | i.v. | Chen et al. (2017) | |
| Other vehicles | Squalene, DOTAP, sorbitan trioleate, polysorbate 80 | Clade C envelope glycoprotein | i.m. | Bogers et al. (2014) |
| RALA | OVA | i.d. | Udhayakumar et al. (2017) | |
| CART D13:A11 7 | eGFP, Luc | i.m. | McKinlaya et al. (2017) |
Note. ACE2 = angiotensin converting enzyme 2; Cas9 = clustered regularly interspaced short palindromic repeats (CRISPR) associated protein 9; DOPE = 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; DSPC = 1,2-distearoyl- sn-glycero-3-phosphocholine; eGFP = enhanced green fluorescence protein; eIF4E = eukaryotic initiation factor 4E; EPO = erythropoietin; FIX = factor FIX; HA = influenza virus hemagglutinin; i.d. = intradermal; i.m. = intramuscular; i.n. = intranasal; i.p. = intraperitoneal; i.t. = intratracheal; i.v. = intravenous; Luc = luciferase; OVA = ovalbumin; PABP = poly(A) binding proteins; RSV-F = respiratory syncytial virus fusion glycoprotein; saRNA = self-amplifying mRNA; s.c. = subcutaneous; sFlt-1 = anti-angiogenic protein.
3.1 |. Lipid and lipid-derived nanoparticles for mRNA delivery
Lipid and lipid-derived nanoparticles represent a large category of delivery systems. Using lipids with different molecular structures, researchers are able to formulate distinct delivery vehicles including “liposomes, solid lipid nanoparticles, oily suspensions, submicron lipid emulsions, lipid implants, lipid microbubbles, inverse lipid micelles, cochliar liposomes, lipid microtubules and lipid microcylinders” as stated by Pisal, Kosloski, and Balu-Iyer (2010). Since late 1970s, liposomes have been applied to encapsulate mRNA. For example, Dimitriadis (1978) formulated large unilamellar liposomes to deliver mRNA encoding rabbit globin into mouse lymphocytes ex vivo. In 1987, Felgner et al. (1987) synthesized N-[1-(2, 3-dioleoyloxy)propyl]-N,N,N-trimethylammonium chloride (DOTMA) and complexed it with 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) and DNA in order to transfect cells. Using this formulation, Malone, Felgner, and Verma (1989) developed cationic liposomes and effectively delivered luciferase mRNA into human, rat, mouse, Xenopus, and Drosophila cells. Subsequently, combination of DOTMA and DOPE (1:1 M ratio) resulted in Lipofectin, a widely used transfection reagent (Colosimo et al., 1999). In 1993, Martinon et al. (1993) developed liposomes to entrap mRNA encoding influenza antigen and induced virus-specific cytotoxic T lymphocytes in mice.
Recently, Lee et al. (2015) incorporated polyarginine-fused heart-targeting peptide into Lipofectamine and showed an effective delivery of mRNA, which induced direct cardiac reprogramming towards cardiomyocyte cells, as evidenced by increased expression of cardiomyocyte markers. In 2016, Kranz et al. (2016) explored the ratio of DOTMA/DOPE to mRNA and identified an optimal ratio, 1.3:2. Particle size of this lipid/mRNA complex was approximately 300 nm measured by Nicomp 380 ZLS. After injected intravenously, this complex showed systemic delivery into DCs and induced effector and memory T-cell responses. In this study, they also found that the ratio of lipid:RNA substantially varied surface charge, thereby leading to protein expression in different organs (Kranz et al., 2016). LNPs with excess negative charges were mainly taken up by the spleen. Currently, a phase I clinical trial is ongoing for the treatment of advanced melanoma using tetravalent RNA-lipoplex cancer vaccine encoding four tumor-associated antigens (NY-ESO-1, MAGE-A3, tyrosinase, and TPTE; Kranz et al., 2016). Preliminary human data supported the findings seen in mouse tumor models (Kranz et al., 2016). Meanwhile, Habrant et al. (2016) synthesized tobramycin-derived lipids using a biodegradable diester linker. A lead lipid was formulated with DOPE at a 1:1 M ratio and showed effective delivery of mRNA, siRNA and DNA in rat and human cell lines (Habrant et al., 2016). Their recently developed paromomycin-based cationic lipids were also used for delivery of these nucleic acid cargos (Colombani et al., 2017). In 2017, Michel et al. (2017) developed cationic liposomes using 3β-[N-(Nʹ,Nʹ-dimethylaminoethane) carbamoyl] (DC-Cholesterol) and DOPE. These liposomes were able to deliver mRNA encoding alpha-1-antitrypsin and express functional proteins in A549 cells, a human lung cancer cell line. The researchers also compared different storage conditions: room temperature, 4, 20, and 80 °C. For this type of liposomes, 4 °C was the optimal storage temperature (Michel et al., 2017). In the same year, Verbeke et al. (2017) reported co-delivery of mRNA and TLR agonists using 1,2-dioleoyloxy-3-trimethylammonium propane chloride (DOTAP) and cholesterol formulated lipid nanoparticles. In addition, Dharmalingam et al. (2017) synthesized a series of cationic lipids derived from fatty acyl chains of palmstearin and Satyal et al. (2017) produced pyridinium pseudogemini surfactants, both of which exhibited delivery of GFP mRNA in human cell lines.
In addition to these lipid/mRNA complexes, researchers explored additional formulation components that consist of cationic/ionizable lipids, helper lipids, cholesterol, and polyethylene glycol (PEG) lipids (Cullis & Hope, 2017; Lin, Chen, Zhang, & Zheng, 2014). Cationic or ionizable lipids condense negatively charged mRNA through electrostatic effects and facilitate endosomal escape of particles after cellular uptake (Kedmi, Ben-Arie, & Peer, 2010; Oberli et al., 2017). In order to further stabilize the particles and improve in vivo delivery efficiency, helper lipids, cholesterol, and PEGylated lipids are incorporated in the formulations (Cheng & Lee, 2016). DSPC (1,2-distearoyl-sn-glycero-3-phosphocholine), DOPE, POPE (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine) are widely used helper lipids. Cholesterol not only enhances particle stability but also promotes membrane fusion (Cheng & Lee, 2016). In addition, PEGylated lipids on the surface of particles prevent particle aggregation, decrease nonspecific interactions with serum proteins, and bypass the reticuloendothelial system uptake (Kolate et al., 2014). In 2012, Geall et al. (2012) used a combination of the following components: 1,2-dilinoleyloxy-3-dimethylaminopropane (DLinDMA), DSPC, cholesterol, and PEG2k-DMG to deliver self-amplifying RNA (saRNA, also termed as self-amplifying mRNA, SAM) as therapeutic vaccines. The commonly used saRNA is derived from alphavirus genome, which retains the alphavirus genes encoding replication components and replaces the structural protein genes with those encoding antigens of interest (Bogers et al., 2014; Brito et al., 2015; Ljungberg & Liljeström, 2014; Midoux & Pichon, 2015; Ulmer & Geall, 2016). After intramuscular injection, the lipid nanoparticles (LNP/saRNA) induced Respiratory Syncytial Virus Fusion Glycoprotien-specific antibody and T-cell responses in both mice and rats (Geall et al., 2012). In 2016, Nabhan et al. (2016) applied the DLinDMA derivative, DLin-MC3-DMA, to deliver frataxin mRNA. After intravenous administration, the half-life of frataxin mRNA encapsulated in lipid nanoparticles exceeded 1 week. Furthermore, intrathecal administration specifically yielded functional frataxin protein in dorsal root ganglia (Nabhan et al., 2016). In the same year, Badieyan et al. (2016) developed lipid nanoparticles to load mRNA encoding human bone morphogenetic protein 2. The nanoparticle/mRNA solution was then dropped onto collagen disks. The resulting collagen sponge not only exhibited long-term stability (stable for a half year at room temperature) and high transfection capacity, but also induced osteogenic differentiation in vitro and bone regeneration in vivo (Badieyan et al., 2016). In early 2017, Pardi et al. reported lipid nanoparticles to intradermally deliver mRNA encoding the premembrane and envelope (prM-E) glycoproteins of Zika virus (ZIKV) and protect both mice and non-human primates against ZIKV challenges (Pardi, Hogan, et al., 2017). Using a similar formulation, they intravenously injected mRNA encoding broadly neutralizing anti-HIV antibody in humanized mice. Consequently, the translated antibody protected those mice from HIV-1 challenge (Pardi, Secreto, et al., 2017).
Traditionally, lipids such as phospholipids are composed of a hydrophilic head and two lipid tails. In order to explore the broad chemical space of lipids, Anderson and Langer synthesized over one thousands of lipid derivatives (also termed as lipid-like molecules or lipidoids) using a combinatorial approach (Akinc et al., 2008). These lipids consist of multiple hydrophilic groups and several lipid tails, which were widely used for siRNA delivery (Akinc et al., 2008; Alabi et al., 2013; Dong, Eltoukhy, et al., 2014; Dong, Love, et al., 2014; Whitehead et al., 2014; Zhang et al., 2013). Based on C12–200 (Love et al., 2010), Kauffman et al. (2015) optimized its formulation using Design of Experiment methodologies and increased sevenfold of erythropoietin expression compared to the original formulation. Later on, this nanomaterial was used to deliver mRNAs encoding erythropoietin (DeRosa et al., 2016; Kauffman et al., 2015), factor IX (DeRosa et al., 2016), and SpCas9 (Yin et al., 2016). Through the same combinatorial method, cKK-E12, a lipopeptide nanoparticle was identified as a lead material for siRNA delivery (Dong, Love, et al., 2014). Subsequently, this material was optimized for diverse mRNA delivery such as ovalbumin mRNA for cancer immunotherapy and Cas9 mRNA delivery for genome editing (Oberli et al., 2017; Yin et al., 2017). In addition, structural modification of cKK-E12 lipid tails afforded OF-02, producing twofold higher erythropoietin than cKK-E12 (Fenton et al., 2016). By altering the local structure of OF-02 from 1,2-amino-alcohol to ester linkage, OF-Deg-Lin showed high distribution in the spleen, specifically in B-cell population (Fenton et al., 2017).
In the past several years, more and more new lipid derivatives have been developed. For example, Li et al. (2015) synthesized N1,N3,N5-tris(2-aminoethyl)benzene-1,3,5-tricarboxamide (TT) derivatives. Through an orthogonal experiment design, they identified TT3 lipid-like nanoparticles (LLNs), which were capable of delivering mRNA encoding human factor IX (hFIX) and restoring factor IX to normal levels in factor IX-deficient mice (Li et al., 2015; Li & Dong, 2017). Later on, Ramaswamy et al. (2017) delivered hFIX as well using proprietary lipid nanoparticles. In addition, TT3 LLNs were able to co-deliver multiple payloads. For example, both mRNA and MRI contrast agents can be encapsulated (Luo et al., 2017). Also, Jiang et al. (2017) further optimized TT3 LLNs to load both Cas9 mRNA and single-guide RNA (sgRNA), which displayed effective gene editing of HBV DNA and the proprotein convertase subtilisin/kexin type 9 (pcsk9) gene. Most recently, Finn et al. (2018) reported that lipid nanoparticles were able to co-deliver Cas9 mRNA and chemically modified sgRNA as well, which reduced target protein expression over 1 year. In 2016, Li et al. explored the effects of local structural transformation on mRNA delivery by exchanging the positions of hydroxyl and amino groups in a lipid-like compound reported previously (Dong, Eltoukhy, et al., 2014; Li, Luo, Deng, et al., 2016). They found that a minor structural alteration elevated over 10-fold more efficient than that of original counterpart in Hep3B cells, a human hepatoma cell line (Li, Luo, Deng, et al., 2016). In the same year, Turnbull et al. (2016) performed a direct intramyocardial injection of C14–113 lipid nanoparticles carrying chemically modified GFP mRNA. Ex vivo fluorescence imaging indicated that more than 90% green fluorescence was present at the heart 20 hr after injection of formulation (35 μg/kg). The translatable capacity of such formulations for myocardial delivery of mRNA was further confirmed with myocardial or intracoronary injection of a single low dose of formulation (3.6 μg/kg) in the pig (Turnbull et al., 2016). In late 2016, Miller et al. synthesized zwitterionic amino lipids and applied them for co-delivery of Cas9 mRNA and single guide RNA. One lead material, ZA3-Ep10 nanoparticles, elicited apparent gene editing in the liver, kidneys, and lungs in the engineered mice (Miller et al., 2017). To deliver Cas9 mRNA, Zhang et al. (2017) produced a series of amino-ester nanomaterials. The biodegradable amino-ester bond can be tuned to control the rate of biodegradability. Two materials MPA-A and MPA-Ab encapsulating Cas9 mRNA showed cleavage of target gene GFP in vivo (Zhang et al., 2017). In the same year, Schrom et al. (2017) tuned ionizable lipids and their formulation, which can target liver and lung, respectively. Using these formulations, angiotensin-converting enzyme 2 mRNA was delivered to the liver or lung, thus providing a potential therapeutic approach for the treatment of liver and lung fibrosis (Schrom et al., 2017).
3.2 |. Polymer-based nanoparticles for mRNA delivery
Polymeric nanoparticles are another class of nanoscale platforms for mRNA delivery. Previously, a wide variety of polymers were designed and synthesized including polyamines, polypeptides, diblock polymers, and triblock polymers. In this section, we will describe representative polymers for mRNA delivery. Among these polymers, polyethylenimine (PEI) is a widely used cationic polymer for nucleic acids delivery (Boussif et al., 1995). PEI is a group of linear or branched polyethylenimine polymers with strong affinity to nucleic acids and proton sponge effect for facilitating endosomal escape (Akinc, Thomas, Klibanov, & Langer, 2005; Demoulins et al., 2016). In 2016, Demoulins packaged saRNA encoding influenza virus hemagglutinin and nucleocapsid using linear or histidinylated PEI. These PEI/RNA nanoparticles were then injected subcutaneously in mice, inducing both humoral and cellular immune responses (Demoulins et al., 2016). PEI dendrimer was also reported for delivery of saRNA (Chahal et al., 2016, 2017). Chahal et al. installed lipid tails on PEI dendrimer and formulated saRNA together with a PEGylated lipid. The polymeric nanoparticles generated strong immunity in mice against H1N1 influenza, toxoplasma gondii, Ebola virus, and Zika virus (Chahal et al., 2016, 2017). In the same year, Zhao et al. reported that PEI-2k conjugated with stearic acid formed micelles with HIV-1 gag mRNA. After subcutaneous injection of the polymer micelles, they detected a significant level of HIV1-gag specific antibody (Zhao, Li, Zhang, Gong, & Sun, 2015). The same lab also attached cyclodextrin to PEI-2k via a carbamate linker and complexed it with mRNA encoding HIV-1 gp120. Through intranasal delivery of this mRNA vaccine, both nasal epithelium and nasal-associated lymphoid tissue displayed dramatic uptake, consequently leading to anti-HIV immune responses in mice (Li, Zhao, Fu, et al., 2016). Since PEI with low molecular weight (<2 kDa) exhibited less cytotoxicity, Chiper, Tounsi, Kole, Kichler, and Zuber (2017) grafted salicylamide with PEI1.8k using an amide linker. This modified PEI was used to deliver diverse DNA and RNA (Chiper et al., 2017). In addition to chemical conjugation, Choi et al. fabricated a complex of graphene oxide (GO)-polyethylenimine (PEI). The GO-PEI complexes were able to deliver mRNAs encoding four reprogramming transcription factors (Oct4, Sox2, Klf4, and c-Myc) and generated induced pluripotent stem cells from adipose tissue-derived fibroblasts without repetitive daily transfection (Choi et al., 2016). Bire and Huang used jetPEI, a linear PEI derivative to deliver mRNA encoding piggyBac transposase and insulin-like growth factor-1 (IGF1), respectively (Bire et al., 2013; Huang et al., 2015).
Poly(glycoamidoamine) brushes produced by ring opening reactions between poly(glycoamidoamine) and epoxides, were also applied to systemic delivery of mRNA in mice (Dong et al., 2016). Structure–activity relationship analysis indicated that increasing the number of amino groups improved the transfection efficiency, while shortening the length of alkyl tail generally prompted mRNA delivery. Additionally, the tartarate moiety displayed better activity than galactarate and glucarate moieties. When formulated with erythropoietin mRNA, the top-performing TarN3C10 nanoparticles produce 1,080 ng/mL of erythropoietin at a dose of 0.3 mg/kg, which was three orders of magnitude higher than free mRNA. Furthermore, histological analysis and biochemistry examination indicated that these nanoparticles were well-tolerated at their efficacious dose (Dong et al., 2016). In the same year, Kaczmarek et al. synthesized a series of poly(β-amino ester) (PBAE) with different capping amines. One polymer PEGylated DD90-C12–122 displayed apparent delivery into mouse lungs (Kaczmarek et al., 2016). In another study, after conjugation with oligoalkylamine, poly(acrylic acid) (PAA8k) was reported as a mRNA delivery system (Jarzebinska et al., 2016). Structure–activity relationship analysis suggested that PAA8k bearing an alkylamine chain length exhibited buffering capacity from pH 6.2 to 6.5. Intravenous injection of PAA8k-(2–3-2) formulated luciferase mRNA into mice led to predominant luminescence signal in the liver than PAA8k-(2–3-2) and PAA8k-(3–3-3) formulations (Jarzebinska et al., 2016). Similar phenomenon was observed for C12 lipid coupled 2–3-2 oligoalkylamine nanoparticles [C12-(2–3-2) LNPs] (Jarzebinska et al., 2016). In order to make biodegradable polymers, Yan et al. (2017) synthesized a library of functional polyesters. Formulated with 5% Pluronic F127 and luciferase mRNA, one polymer named PE4K-A17–0.33C12 showed high luminescence signal in mouse lungs after intravenous injection (Yan et al., 2017). Nuhn et al. co-polymerized tri(ethylene glycol) methyl ether methacrylate (MEO3MA) and pentafluorophenyl methacrylate (PFPMA), affording P(MEO3MA)-b-P(PFPMA). After that, they introduced disulfide bond and produced P(MEO3MA)-b-P(H2N-Cys-MA). This functionalized block copolymer showed effective mRNA delivery in fibroblast and macrophage cell lines (Nuhn, Kaps, Diken, Schuppan, & Zentel, 2016). Triblock copolymers were also developed for mRNA delivery by Cheng, Convertine, Stayton, and Bryers (2012). These copolymers, produced by reversible addition-fragmentation chain transfer polymerization, were composed of a cationic diethylaminoethyl methacrylate (DMAEMA), a poly(ethylene glycol) methyl ether methacrylate (PEGMA), and a copolymer of DEAEMA and BMA (DEAEMA-co-BMA). The mRNA polyplexes revealed the greatest stability and transfection efficiency in macrophages and DCs when the PEGMA moiety located in the center of the copolymer chain. What’s more, polyplexes treated DCs exhibited high levels of in vitro activation of antigen-specific T-cell (Cheng et al., 2012).
Previous studies also investigated a number of polypeptides for mRNA delivery. For example, Crowley, Poliskey, Baumhover, and Rice (2015) installed acridine and PEG5k on the polylysine backbone peptide. The formulated luciferase mRNA polyplexes extended mRNA degradation time and led to a high luminescence signal in the liver after hydrodynamic injection in mice (Crowley et al., 2015). In 2016, Aini et al. (2016) used a peptide-based polyethylene glycol-polyamino acid block copolymer, PEG-PAsp(DET), to prepare nanomicelles with runt-related transcription factor 1 mRNA. After locally injected into mouse knee joints, the nanomicelles significantly suppressed osteoarthritis progression in mice (Aini et al., 2016). When the number of the side chain aminoethylene repeat in PEG-PAsp(TET) was changed from 3 to 2, PAsp(DET) block copolymer was obtained, and was used for delivery of Bcl-2 mRNA to treat apoptosis-associated diseases (Matsui, Uchida, Ishii, Itaka, & Kataoka, 2015). PEG-PAsp(TEP) is also a PEGylated polyaspartamides block copolymer which possesses similar building blocks as PEG-PAsp(TET). When formulated with mRNA, cholesterol-modified PEG-PAsp(TEP) was assembled into nanomicelles (Uchida, Kinoh, et al., 2016). Conjugation of cholesterol to the copolymer resulted in enhanced nuclease resistance for the encapsulated mRNA in the blood circulation and delivered mRNA in the tumor. Systemic administration of nanomicelles loaded with mRNA encoding an anti-angiogenic protein sFlt-1 into a xenograft mouse model of pancreatic cancer exhibited strong antitumor effect via inhibiting angiogenesis (Uchida, Kinoh, et al., 2016). Interestingly, Uchida et al. indicated that different polyamines may affect mRNA translation via the preservative binding of eIF4E to the cap structure of mRNA (Uchida, Itaka, et al., 2016).
In order to improve accumulation of mRNA at the tumor site, Chen et al. (2017) prepared a targeted and stable polymeric architecture. This nanoformulation was composed of poly(N-isopropylacrylamide) (PNIPAM)-PLys and cRGD-poly(ethylene glycol) (PEG)-polylysine (PLys), in which two thiols was crosslinked through a disulfide linkage. The former contributed to prolonged half-life via shielding mRNA from nucleases, while the latter had the potential to carry mRNA to mouse tumors (Chen et al., 2017). In addition, Lallana et al. (2017) engineered chitosan/hyaluronic acid (HA) nanoparticles to target HA receptors such as CD44, which is overexpressed in a number of tumors. These nanoparticles showed a pH-dependent behavior with optimal performance around pH 6.4. This feature is especially useful for targeting the tumoral environment (Lallana et al., 2017).
3.3 |. Protein derivatives mRNA complexes
Protamine is a FDA approved arginine-rich protein and used as heparin antagonist and insulin delivery system (Sorgi, Bhattacharya, & Huang, 1997). In 2000, Hoerr, Obst, Rammensee, and Jung (2000) used protamine to condense mRNA encoding for β-galactosidase (β-gal). After vaccinated with this protamine/RNA complex, mice produced β-gal-specific cytotoxic T lymphocytes and antibody production. Their later studies suggested that the protamine/RNA complex activated DCs via the MyD88 pathways and might interact with toll-like receptor 1, 7, or 8 (Scheel et al., 2005). In 2009, Weide et al. (2009) reported the results from a phase 1/2 clinical trial in metastatic melanoma patients using a direction injection of protamine/ mRNA encoding tumor-associated antigens (Melan-A, Tyrosinase, gp100, Mage-A1, Mage-A3, and Survivin). After intradermal injection, patients were well-tolerated at the administered dose (640 mg total mRNA content and 128 mg protamine). Clinical response was observed in 1 of 7 patients with measurable disease and need additional clinical investigation in the future (Weide et al., 2009). Currently, using this platform, a phase I clinical trial is ongoing as a prophylactic vaccine for Rabies virus (Pardi, Hogan, Porter, & Weissman, 2018).
Several studies also covered the protamine/mRNA complex with lipids and polymers to improve stability and tumor accumulation (Palama, Cortese, D’Amone, & Gigli, 2015; Wang et al., 2013). For example, Wang et al. (2013) formulated liposome-protamine-RNA (LPR) and delivered mRNA encoding herpes simplex virus 1-thymidine kinase (HSV1-tk). The LPR significantly suppressed tumor growth in a xenograft mouse model with human lung cancer (Wang et al., 2013). In addition, poly(ε-caprolactone) was used to load protamine/mRNA complex, resulting in the formation of pH-responsive nanoparticles. These nanoparticles with a core-shell architecture effectively delivered mRNA in three cell lines in a pH-dependent manner (Palama et al., 2015).
Coat proteins derived from viruses were also used for mRNA delivery (Jekhmane et al., 2017; Li et al., 2014; Sun, Sun, Zhao, & Gao, 2016). Li et al. (2014) assembled the recombinant coat protein of bacteriophage MS2 with mRNA encoding granulocyte macrophage colony-stimulating factor. The formulated virus-like particles induced strong antigen-specific cytotoxic T-lymphocyte and balanced Th1/Th2 responses, protecting mice against prostate cancer (Li et al., 2014). Sun et al. (2016) developed bacteriophage PP7 coat protein containing low molecular weight protamine, which complexed with GFP mRNA and expressed GFP protein in a mouse cell line. Jekhmane et al. designed C-S10-K12, an artificial viral coat protein, consisting of three parts: a hydrophilic random coil polypeptide (a C domain contains 400 amino acids), a oligopeptide (GAGAGAGQ)10 (S10), and an oligolysine (K12) (Jekhmane et al., 2017). After adding mRNA molecules, C-S10-K12 formed rod-shaped virus-like particles, which were able to moderately transfect cells with a negligible toxicity in the cells tested (Jekhmane et al., 2017).
3.4 |. Other type of nanoparticles for mRNA delivery
In addition to the platforms mentioned above, researchers also investigated other types of nanoparticles for mRNA delivery such as gold nanoparticles, polymer-lipid hybrid nanoparticles, and peptide complex. Gold nanoparticles (AuNP) thiolated with a short DNA oligonucleotide enabled complementary binding with specific sequences on mRNA (Chan et al., 2017; Chan, Chao, & Kah, 2018; Yeom et al., 2013). The hybrid region can be the 5ʹ- or 3ʹ-UTR, or poly(A) tail. Yeom et al. hybridized DNA oligonucleotide on AuNP with 5ʹ-UTR before the Kozak sequence of BAX (BCL-2-associated X-protein) mRNA. The AuNP/mRNA not only induced cell apoptosis in vitro but also significantly inhibited the growth of xenograft tumors in mice following subcutaneous injection (Yeom et al., 2013). In another two studies, functionalized AuNP containing complementary DNA oligonucleotide to 3ʹ-UTR or poly(A) tail of mRNA elevated translation level up to 2.2-fold in the cell lysate (Chan et al., 2017, 2018). In 2011, Su, Fricke, Kavanagh, and Irvine (2011) reported polymer-lipid hybrid nanoparticles to deliver GFP and luciferase mRNAs. They constructed core/shell structured nanoparticles with PBAE core and lipid bilayer shell (DOPC, DOTAP, and PEG2k-DSPE), which expressed proteins both in vitro and in vivo (Su et al., 2011). Arginine-rich peptide comprising amphipathic RALA motif was designed as a mRNA carrier that displayed acidic pH-dependent membrane disruptive ability (Udhayakumar et al., 2017). In comparison with nonamphipathic cationic RGSG and RRRR motifs, RALA showed superior properties in mRNA complexation, cellular uptake and delivery efficiency in DCs and elicited cytolytic T-cell responses in vivo (Udhayakumar et al., 2017). McKinlaya et al. (2017) incorporated lipid chains and cationic groups on oligo(carbonate-b-α-amino ester) that binds mRNA via electrostatic interactions. These nanoparticles showed luminescence signal after intramuscular or intravenous injections (McKinlaya et al., 2017). Bogers et al. (2014) developed a cationic nanoemulsion using squalene, DOTAP, sorbitan trioleate, and polysorbate 80 in order to facilitate binding of self-amplifying mRNA. Packing synthetic self-amplifying mRNA encoded HIV Type 1 envelope into such nanoemulsion led to more antigen-specific antibody and T-cell immune responses than those induced by traditional viral replicon particle vaccines in rhesus macaques. Moreover, no notable adverse effects were observed in the animals tested (Bogers et al., 2014).
Aside from delivery of mRNA alone, Li et al. explored the approach to preassemble the translation components, 7-methylguanosine (m7G)-capped mRNA and eukaryotic initiation factor 4E (eIF4E) protein, and form ribonucleoproteins (RNPs) (Li, Wang, et al., 2017). Then, they loaded the RNPs into polyamines nanoparticles, resulting in up to 70-fold increase of protein expression at a mass ratio of 1:5 (mRNA/eIF4E) in different cell types tested (Li, Wang, et al., 2017). The side chain of polyamine was found to be important to regulate the assembly between mRNA and eIF4E. The nanoplexes containing five nitrogen atoms at the side chain of polyamine not only effectively activated cytotoxic CD8 T-cells ex vivo but also elevated protein expression in the lungs of mice (Li, Wang, et al., 2017). Alternatively, incorporation of poly(A) binding proteins into polyamines nanoparticles is another approach to enhance mRNA delivery (Li, He, et al., 2017). Interestingly, in this case, polyamines nanoparticles with four nitrogen atoms at the side chain revealed the highest transfection efficiency (Li, He, et al., 2017). In addition, through rolling circle replication reaction, in vitro transcribed mRNA can be self-assembled into nanoscale particles (mRNA-NPs) (Kim, Park, & Lee, 2015). After mixing with TransIT-X2, a commercially available transfection agent, GFP protein was translated in a human cell line (Kim et al., 2015).
4 |. CONCLUSION AND FUTURE PERSPECTIVES
Currently, over 10 clinical trials of mRNA-based therapy are ongoing or will be initiated for diverse therapeutic applications such as infectious diseases and cancers (Hajj & Whitehead, 2017; Sahin et al., 2014). Although significant advances have been made in the past decade, several issues still hinder the broad applications of mRNA-based therapies. Regarding mRNA itself, important aspects including translation efficiency, half-life, and immunogenicity of mRNA need further understanding of relevant biological pathways. Meanwhile, new approaches to engineer mRNA including its sequences, secondary structures, and interactions with relevant proteins in cells, are in urgent demand. In addition, mRNA, a large biomolecule, involves multiple components during the in vitro transcription production process. In both academia and industry, mRNA purity and impurities produced in the process should be carefully characterized and analyzed. A standard quality control of mRNA may need to be established in order to ensure the findings in preclinical and clinical studies.
In this review, we described a number of delivery platforms for mRNA delivery, several of which are being applied in clinical trials. Prior clinical data from these mRNA therapies and other types of RNA medicines provide a large amount of insightful information in order to design and develop new mRNA delivery systems in the future. Because mRNA possesses great potential for various therapeutic applications, its delivery platform needs to match with the indication. For example, a system favor for liver delivery may not be proper for cancer therapy. Therefore, future design of these delivery platforms may consider the following features: (a) targeting of specific organs and cell populations, (b) high delivery efficiency, (c) biodegradable and biocompatible, (d) appropriate duration of pharmacological effects, and (e) standard quality control. Combing the optimal therapeutically relevant mRNA and a proper delivery system, we anticipate that mRNA-based therapeutics will make significant contributions to human health in the near future.
ACKNOWLEDGMENTS
Y.D. acknowledges support from Early Career Investigator Award from the Bayer Hemophilia Awards Program, Research Awards from the National PKU Alliance, New Investigator Grant from the American Association of Pharmaceutical Scientists (AAPS) Foundation, Maximizing Investigators’ Research Award 1R35GM119679 from the National Institute of General Medical Sciences as well as the start-up fund from the College of Pharmacy at the Ohio State University.
Funding information
College of Pharmacy Ohio State University; National Institute of General Medical Sciences; American Association of Pharmaceutical Scientists; National PKU Alliance; Bayer Hemophilia Awards Program
Footnotes
CONFLICT OF INTEREST
The authors have declared no conflicts of interest for this article.
RELATED WIREs ARTICLES
Recombinant messenger RNA technology and its application in cancer immunotherapy, transcript replacement therapies, pluripotent stem cell induction, and beyond
REFERENCES
- Aini H, Itaka K, Fujisawa A, Uchida H, Uchida S, Fukushima S, … Ohba S (2016). Messenger RNA delivery of a cartilage-anabolic transcription factor as a disease-modifying strategy for osteoarthritis treatment. Scientific Reports, 6, 18743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinc A, Thomas M, Klibanov AM, & Langer R (2005). Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. Journal of Gene Medicine, 7, 657–663. [DOI] [PubMed] [Google Scholar]
- Akinc A, Zumbuehl A, Goldberg M, Leshchiner ES, Busini V, Hossain N, … Anderson DG (2008). A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nature Biotechnology, 26, 561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alabi CA, Love KT, Sahay G, Yin H, Luly KM, Langer R, & Anderson DG (2013). Multiparametric approach for the evaluation of lipid nanoparticles for siRNA delivery. Proceedings of the National Academy of Sciences of the United States of America, 110, 12881–12886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badieyan ZS, Berezhanskyy T, Utzinger M, Aneja MK, Emrich D, Erben R, … Plank C (2016). Transcript-activated collagen matrix as sustained mRNA delivery system for bone regeneration. Journal of Controlled Release, 239, 137–148. [DOI] [PubMed] [Google Scholar]
- Bire S, Gosset D, Jégot G, Midoux P, Pichon C, & Rouleux-Bonnin F (2013). Exogenous mRNA delivery and bioavailability in gene transfer mediated by piggyBac transposition. BMC Biotechnology, 13, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogers WM, Oostermeijer H, Mooij P, Koopman G, Verschoor EJ, Davis D, … Barnett SW (2014). Potent immune responses in rhesus macaques induced by nonviral delivery of a self-amplifying RNA vaccine expressing HIV type 1 envelope with a cationic nanoemulsion. Journal of Infectious Diseases, 211, 947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, & Behr J (1995). A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proceedings of the National Academy of Sciences of the United States of America, 92, 7297–7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito LA, Kommareddy S, Maione D, Uematsu Y, Giovani C, Berlanda Scorza F., … Geall AJ (2015). Self-amplifying mRNA vaccines. Advances in Genetics, 89, 179–233. [DOI] [PubMed] [Google Scholar]
- Chahal JS, Fang T, Woodham AW, Khan OF, Ling J, Anderson DG, & Ploegh HL (2017). An RNA nanoparticle vaccine against Zika virus elicits antibody and CD8+ T cell responses in a mouse model. Scientific Reports, 7, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahal JS, Khan OF, Cooper CL, McPartlan JS, Tsosie JK, Tilley LD, … Anderson DG (2016). Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and toxoplasma gondii challenges with a single dose. Proceedings of the National Academy of Sciences, 113, E4133–E4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KP, Chao SH, & Kah JCY (2018). Universal mRNA translation enhancement with gold nanoparticles conjugated to oligonucleotides with a poly(T) sequence. ACS Applied Materials & Interfaces, 10, 5203–5212. [DOI] [PubMed] [Google Scholar]
- Chan KP, Gao Y, Goh JX, Susanti D, Yeo EL, Chao SH, & Kah JC (2017). Exploiting the protein corona from cell lysate on DNA functionalized gold nanoparticles for enhanced mRNA translation. ACS Applied Materials & Interfaces, 9, 10408–10417. [DOI] [PubMed] [Google Scholar]
- Chen CYA, Ezzeddine N, & Shyu AB (2008). Messenger RNA half-life measurements in mammalian cells. Methods in Enzymology, 448, 335–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Qi R, Chen X, Yang X, Wu S, Xiao H, & Dong W (2017). A targeted and stable polymeric nanoformulation enhances systemic delivery of mRNA to tumors. Molecular Therapy, 25, 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Convertine AJ, Stayton PS, & Bryers JD (2012). Multifunctional triblock copolymers for intracellular messenger RNA delivery. Biomaterials, 33, 6868–6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, & Lee RJ (2016). The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery. Advanced Drug Delivery Reviews, 99, 129–137. [DOI] [PubMed] [Google Scholar]
- Chiper M, Tounsi N, Kole R, Kichler A, & Zuber G (2017). Self-aggregating 1.8 kDa polyethylenimines with dissolution switch at endosomal acidic pH are delivery carriers for plasmid DNA, mRNA, siRNA and exon-skipping oligonucleotides. Journal of Controlled Release, 246, 60–70. [DOI] [PubMed] [Google Scholar]
- Choi HY, Lee TJ, Yang GM, Oh J, Won J, Han J, … Cho SG (2016). Efficient mRNA delivery with graphene oxide-polyethylenimine for generation of footprint-free human induced pluripotent stem cells. Journal of Controlled Release, 235, 222–235. [DOI] [PubMed] [Google Scholar]
- Colombani T, Peuziat P, Dallet L, Haudebourg T, Mevel M, Berchel M, … Pitard B (2017). Self-assembling complexes between binary mixtures of lipids with different linkers and nucleic acids promote universal mRNA, DNA and siRNA delivery. Journal of Controlled Release, 249, 131–142. [DOI] [PubMed] [Google Scholar]
- Colosimo A, Serafino A, Sangiuolo F, Di Sario S, Bruscia E, Amicucci P, … Mossa G (1999). Gene transfection efficiency of tracheal epithelial cells by DC-chol-DOPE/DNA complexes. Biochimica et Biophysica Acta, 1419, 186–194. [DOI] [PubMed] [Google Scholar]
- Crowley ST, Poliskey JA, Baumhover NJ, & Rice KG (2015). Efficient expression of stabilized mRNA PEG-peptide polyplexes in liver. Gene Therapy, 22, 993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullis PR, & Hope MJ (2017). Lipid nanoparticle systems for enabling gene therapies. Molecular Therapy, 25, 1467–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demoulins T, Milona P, Englezou PC, Ebensen T, Schulze K, Suter R, … KC MC (2016). Polyethylenimine-based polyplex delivery of self-replicating RNA vaccines. Nanomedicine, 12, 711–722. [DOI] [PubMed] [Google Scholar]
- DeRosa F, Guild B, Karve S, Smith L, Love K, Dorkin JR, … Heartlein MW (2016). Therapeutic efficacy in a hemophilia B model using a biosynthetic mRNA liver depot system. Gene Therapy, 23, 699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoldere J, Dewitte H, De Smedt SC, & Remaut K (2016). Evading innate immunity in nonviral mRNA delivery: Don’t shoot the messenger. Drug Discovery Today, 21, 11–25. [DOI] [PubMed] [Google Scholar]
- Dharmalingam P, Rachamalla HKR, Lohchania B, Bandlamudi B, Thangavel S, Murugesan MK, … Marepally S (2017). Green transfection: Cationic lipid nanocarrier system derivatized from vegetable fat, palmstearin enhances nucleic acid transfections. ACS Omega, 2, 7892–7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriadis GJ (1978). Translation of rabbit globin mRNA introduced by liposomes into mouse lymphocytes. Nature, 274, 923–924. [DOI] [PubMed] [Google Scholar]
- Dong Y, Dorkin JR, Wang W, Chang PH, Webber MJ, Tang BC, … Anderson DG (2016). Poly(glycoamidoamine) brushes formulated nanomaterials for systemic siRNA and mRNA delivery in vivo. Nano Letters, 16, 842–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Eltoukhy AA, Alabi CA, Khan OF, Veiseh O, Dorkin JR, … Anderson DG (2014). Lipid-like nanomaterials for simultaneous gene expression and silencing in vivo. Advanced Healthcare Materials, 3, 1392–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Love KT, Dorkin JR, Sirirungruang S, Zhang Y, Chen D, … Anderson DG (2014). Lipopeptide nanoparticles for potent and selective siRNA delivery in rodents and nonhuman primates. Proceedings of the National Academy of Sciences of the United States of America, 111, 3955–3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, … Danielsen M (1987). Lipofection: A highly efficient, lipid-mediated DNA-transfection procedure. Proceedings of the National Academy of Sciences of the United States of America, 84, 4713–4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton OS, Kauffman KJ, Kaczmarek JC, McClellan RL, Jhunjhunwala S, Tibbitt MW, … Anderson DG (2017). Synthesis and biological evaluation of ionizable lipid materials for the in vivo delivery of messenger RNA to b lymphocytes. Advanced Materials, 29, 1606944. [DOI] [PubMed] [Google Scholar]
- Fenton OS, Kauffman KJ, McClellan RL, Appel EA, Dorkin JR, Tibbitt MW, … Anderson DG (2016). Bioinspired alkenyl amino alcohol ionizable lipid materials for highly potent in vivo mRNA delivery. Advanced Materials, 28, 2939–2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn JD, Smith AR, Patel MC, Shaw L, Youniss MR, van Heteren J, … Morrissey DV (2018). A single administration of CRISPR/Cas9 lipid nanoparticles achieves robust and persistent in vivo genome editing. Cell Reports, 22, 2227–2235. [DOI] [PubMed] [Google Scholar]
- Geall AJ, Verma A, Otten GR, Shaw CA, Hekele A, Banerjee K, et al. (2012). Nonviral delivery of self-amplifying RNA vaccines. Proceedings of the National Academy of Sciences of the United States of America, 104, 14604–14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa E, & Vieweg J (2004). Cancer immunotherapy with mRNA-transfected dendritic cells. Immunological Reviews, 199, 251–263. [DOI] [PubMed] [Google Scholar]
- Grabbe S, Haas H, Diken M, Kranz LM, Langguth P, & Sahin U (2016). Translating nanoparticulate-personalized cancer vaccines into clinical applications: Case study with RNA-lipoplexes for the treatment of melanoma. Nanomedicine (London, England), 11, 2723–2734. [DOI] [PubMed] [Google Scholar]
- Granot Y, & Peer D (2017). Delivering the right message: Challenges and opportunities in lipid nanoparticles-mediated modified mRNA therapeutics—An innate immune system standpoint. Seminars in Immunology, 34, 68–77. [DOI] [PubMed] [Google Scholar]
- Guan S, & Rosenecker J (2017). Nanotechnologies in delivery of mRNA therapeutics using nonviral vector-based delivery systems. Gene Therapy, 24, 133–143. [DOI] [PubMed] [Google Scholar]
- Habrant D, Peuziat P, Colombani T, Dallet L, Gehin J, Goudeau E, … Pitard B (2016). Design of ionizable lipids to overcome the limiting step of endosomal escape: Application in the intracellular delivery of mRNA, DNA, and siRNA. Journal of Medicinal Chemistry, 59, 3046–3062. [DOI] [PubMed] [Google Scholar]
- Hajj KA, & Whitehead KA (2017). Tools for translation: Non-viral materials for therapeutic mRNA delivery. Nature Reviews Materials, 2, 17056. [Google Scholar]
- Hecker JG (2016). Non-viral, lipid-mediated DNA and mRNA gene therapy of the central nervous system (CNS): Chemical-based transfection. Methods in Molecular Biology, 1382, 307–324. [DOI] [PubMed] [Google Scholar]
- Hoerr I, Obst R, Rammensee HG, & Jung G (2000). In vivo application of RNA leads to induction of specific cytotoxic T lymphocytes and antibodies. European Journal of Immunology, 30, 1–7. [DOI] [PubMed] [Google Scholar]
- Huang CL, Leblond AL, Turner EC, Kumar AH, Martin K, Whelan D, … Caplice NM (2015). Synthetic chemically modified mRNA-based delivery of cytoprotective factor promotes early cardiomyocyte survival post-acute myocardial infarction. Molecular Pharmaceutics, 12, 991–996. [DOI] [PubMed] [Google Scholar]
- Islam MA, Reesor EK, Xu Y, Zope HR, Zetter BR, & Shi J (2015). Biomaterials for mRNA delivery. Biomaterials Science, 3, 1519–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarzebinska A, Pasewald T, Lambrecht J, Mykhaylyk O, Kummerling L, Beck P, … Dohmen C (2016). A single methylene group in oligoalkylamine-based cationic polymers and lipids promotes enhanced mRNA delivery. Angewandte Chemie, 55, 9591–9595. [DOI] [PubMed] [Google Scholar]
- Jekhmane S, de Haas R, Paulino da Silva Filho O., van Asbeck AH, Favretto ME, Hernandez Garcia A., … de Vries R (2017). Virus-like particles of mRNA with artificial minimal coat proteins: Particle formation, stability, and transfection efficiency. Nucleic Acid Therapeutics, 27, 159–167. [DOI] [PubMed] [Google Scholar]
- Jiang C, Mei M, Li B, Zhu X, Zu W, Tian Y, … Tan X (2017). A non-viral CRISPR/Cas9 delivery system for therapeutically targeting HBV DNA and pcsk9 in vivo. Cell Research, 27, 440–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek JC, Patel AK, Kauffman KJ, Fenton OS, Webber MJ, Heartlein MW, … Anderson DG (2016). Polymer-lipid nanoparticles for systemic delivery of mRNA to the lungs. Angewandte Chemie, 55, 13808–13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karikó K, Muramatsu H, Ludwig J, & Weissman D (2011). Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Research, 39, e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman KJ, Dorkin JR, Yang JH, Heartlein MW, DeRosa F, Mir FF, … Anderson DG (2015). Optimization of lipid nanoparticle formulations for mRNA delivery in vivo with fractional factorial and definitive screening designs. Nano Letters, 15, 7300–7306. [DOI] [PubMed] [Google Scholar]
- Kauffman KJ, Webber MJ, & Anderson DG (2016). Materials for non-viral intracellular delivery of messenger RNA therapeutics. Journal of Controlled Release, 240, 227–234. [DOI] [PubMed] [Google Scholar]
- Kedmi R, Ben-Arie N, & Peer D (2010). The systemic toxicity of positively charged lipid nanoparticles and the role of toll-like receptor 4 in immune activation. Biomaterials, 31, 6867–6875. [DOI] [PubMed] [Google Scholar]
- Kim H, Park Y, & Lee JB (2015). Self-assembled messenger RNA nanoparticles (mRNA-NPs) for efficient gene expression. Scientific Reports, 5, 12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolate A, Baradia D, Patil S, Vhora I, Kore G, & Misra A (2014). PEG—a versatile conjugating ligand for drugs and drug delivery systems. Journal of Controlled Release, 192, 67–81. [DOI] [PubMed] [Google Scholar]
- Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC, … Sahin U (2016). Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature, 534, 396–401. [DOI] [PubMed] [Google Scholar]
- Lallana E, Rios de la Rosa J. M., Tirella A, Pelliccia M, Gennari A, Stratford IJ, … Tirelli N (2017). Chitosan/hyaluronic acid nanoparticles: Rational design revisited for RNA delivery. Molecular Pharmaceutics, 14, 2422–2436. [DOI] [PubMed] [Google Scholar]
- Lee K, Yu P, Lingampalli N, Kim HJ, Tang R, & Murthy N (2015). Peptide-enhanced mRNA transfection in cultured mouse cardiac fibroblasts and direct reprogramming towards cardiomyocyte-like cells. International Journal of Nanomedicine, 10, 1841–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, & Dong Y (2017). Preparation and optimization of lipid-like nanoparticles for mRNA delivery. Methods in Molecular Biology, 1632, 207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Luo X, Deng B, Giancola JB, McComb DW, Schmittgen TD, & Dong Y (2016). Effects of local structural transformation of lipid-like compounds on delivery of messenger RNA. Scientific Reports, 6, 22137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Luo X, Deng B, Wang J, McComb DW, Shi Y, … Dong Y (2015). An orthogonal array optimization of lipid-like nanoparticles for mRNA delivery in vivo. Nano Letters, 15, 8099–8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Luo X, & Dong Y (2016). Effects of chemically modified messenger RNA on protein expression. Bioconjugate Chemistry, 27, 849–853. [DOI] [PubMed] [Google Scholar]
- Li J, He Y, Wang W, Wu C, Hong C, & Hammond PT (2017). Polyamine-mediated stoichiometric assembly of ribonucleoproteins for enhanced mRNA delivery. Angewandte Chemie, 56, 13709–13712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Sun Y, Jia T, Zhang R, Zhang K, & Wang L (2014). Messenger RNA vaccine based on recombinant MS2 virus-like particles against prostate cancer. International Journal of Cancer, 134, 1683–1694. [DOI] [PubMed] [Google Scholar]
- Li J, Wang W, He Y, Li Y, Yan EZ, Zhang K, … Hammond PT (2017). Structurally programmed assembly of translation initiation nanoplex for superior mRNA delivery. ACS Nano, 11, 2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Zhao M, Fu Y, Li Y, Gong T, Zhang Z, & Sun X (2016). Enhanced intranasal delivery of mRNA vaccine by overcoming the nasal epithelial barrier via intra- and paracellular pathways. Journal of Controlled Release, 228, 9–19. [DOI] [PubMed] [Google Scholar]
- Lin Q, Chen J, Zhang Z, & Zheng G (2014). Lipid-based nanoparticles in the systemic delivery of siRNA. Nanomedicine, 9, 105–120. [DOI] [PubMed] [Google Scholar]
- Ljungberg K, & Liljeström P (2014). Self-replicating alphavirus RNA vaccines. Expert Review of Vaccines, 14, 177–194. [DOI] [PubMed] [Google Scholar]
- Loomis KH, Kirschman JL, Bhosle S, Bellamkonda RV, & Santangelo PJ (2016). Strategies for modulating innate immune activation and protein production of in vitro transcribed mRNAs. Journal of Materials Chemistry B, 4, 1619–1632. [DOI] [PubMed] [Google Scholar]
- Love KT, Mahon KP, Levins CG, Whitehead KA, Querbes W, Dorkin JR, … Anderson DG (2010). Lipid-like materials for low-dose, in vivo gene silencing. Proceedings of the National Academy of Sciences, 107, 1864–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Li B, Zhang X, Zhao W, Bratasz A, Deng B, … Dong Y (2017). Dual-functional lipid-like nanoparticles for delivery of mRNA and MRI contrast agents. Nanoscale, 9, 1575–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone RW, Felgner PL, & Verma IM (1989). Cationic liposome-mediated RNA transfection. Proceedings of the National Academy of Sciences of the United States of America, 86, 6077–6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Krishnan S, Lenzen G, Magné R, Gomard E, Guillet JG, … Meulien P (1993). Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. European Journal of Immunology, 23, 1719–1722. [DOI] [PubMed] [Google Scholar]
- Matsui A, Uchida S, Ishii T, Itaka K, & Kataoka K (2015). Messenger RNA-based therapeutics for the treatment of apoptosis-associated diseases. Scientific Reports, 5, 15810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinlaya CJ, Vargas JR, Blake TR, Hardy JW, Kanada M, Contag CH, … Waymouth RM (2017). Charge-altering releasable transporters (CARTs) for the delivery and release of mRNA in living animals. Proceedings of the National Academy of Sciences of the United States of America, 114, E448–E456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Z, O’Keeffe-Ahern J, Lyu J, Pierucci L, Zhou D, & Wang W (2017). A new developing class of gene delivery: Messenger RNA-based therapeutics. Biomaterials Science, 5, 2381–2392. [DOI] [PubMed] [Google Scholar]
- Michel T, Luft D, Abraham MK, Reinhardt S, Salinas Medina ML, Kurz J, … Krajewski S (2017). Cationic nanoliposomes meet mRNA: Efficient delivery of modified mRNA using hemocompatible and stable vectors for therapeutic applications. Molecular Therapy. Nucleic Acids, 8, 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midoux P, & Pichon C (2015). Lipid-based mRNA vaccine delivery systems. Expert Review of Vaccines, 14, 221–234. [DOI] [PubMed] [Google Scholar]
- Miller JB, Zhang S, Kos P, Xiong H, Zhou K, Perelman SS, … Siegwart DJ (2017). Non-viral CRISPR/Cas gene editing in vitro and in vivo enabled by synthetic nanoparticle co-delivery of cas9 mRNA and sgRNA. Angewandte Chemie, 56, 1059–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabhan JF, Wood KM, Rao VP, Morin J, Bhamidipaty S, LaBranche TP, … Guild BC (2016). Intrathecal delivery of frataxin mRNA encapsulated in lipid nanoparticles to dorsal root ganglia as a potential therapeutic for Friedreich’s ataxia. Scientific Reports, 6, 20019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuhn L, Kaps L, Diken M, Schuppan D, & Zentel R (2016). Reductive decationizable block copolymers for stimuli-responsive mRNA delivery. Macromolecular Rapid Communications, 37, 924–933. [DOI] [PubMed] [Google Scholar]
- Oberli MA, Reichmuth AM, Dorkin JR, Mitchell MJ, Fenton OS, Jaklenec A, … Blankschtein D (2017). Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Letters, 17, 1326–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palama IE, Cortese B, D’Amone S, & Gigli G (2015). mRNA delivery using non-viral PCL nanoparticles. Biomaterials Science, 3, 144–151. [DOI] [PubMed] [Google Scholar]
- Pardi N, Hogan MJ, Pelc RS, Muramatsu H, Andersen H, DeMaso CR, … Weissman D(2017). Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature, 543, 248–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardi N, Hogan MJ, Porter FW, & Weissman D (2018). mRNA vaccines—a new era in vaccinology. Nature Reviews. Drug Discovery, 17, 261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardi N, Secreto AJ, Shan X, Debonera F, Glover J, Yi Y, … Weissman D (2017). Administration of nucleoside-modified mRNA encoding broadly neutralizing antibody protects humanized mice from HIV-1 challenge. Nature Communications, 8, 14630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardi N, Tuyishime S, Muramatsu H, Kariko K, Mui BL, Tam YK, … Weissman D (2015). Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. Journal of Controlled Release, 217, 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascolo S (2017). Messenger RNA the inexpensive biopharmaceutical. Journal of Multidisciplinary Engineering Science and Technology, 4, 6937–6941. [Google Scholar]
- Patel S, Ashwanikumar N, Robinson E, DuRoss A, Sun C, Murphy-Benenato KE, … Sahay G (2017). Boosting intracellular delivery of lipid nanoparticle-encapsulated mRNA. Nano Letters, 17, 5711–5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisal DS, Kosloski MP, & Balu-Iyer SV (2010). Delivery of therapeutic proteins. Journal of Pharmaceutical Sciences, 99, 2557–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presnyak V, Alhusaini N, Chen YH, Martin S, Morris N, Kline N, … Coller J (2015). Codon optimality is a major determinant of mRNA stability. Cell, 160, 1111–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy S, Tonnu N, Tachikawa K, Limphong P, Vega JB, Karmali PP, … Verma IM (2017). Systemic delivery of factor IX messenger RNA for protein replacement therapy. Proceedings of the National Academy of Sciences of the United States of America, 114, E1941–E1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay G, Alakhova DY, & Kabanov AV (2010). Endocytosis of nanomedicines. Journal of Controlled Release, 145, 182–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin U, Kariko K, & Tureci O (2014). mRNA-based therapeutics—developing a new class of drugs. Nature Reviews. Drug Discovery, 13, 759–780. [DOI] [PubMed] [Google Scholar]
- Satyal U, Draghici B, Dragic LL, Zhang Q, Norris KW, Madesh M, … Ilies MA (2017). Interfacially engineered pyridinium pseudogemini surfactants as versatile and efficient supramolecular delivery systems for DNA, siRNA, and mRNA. ACS Applied Materials & Interfaces, 9, 29481–29495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel B, Teufel R, Probst J, Carralot JP, Geginat J, Radsak M, … Pascolo S (2005). Toll-like receptor-dependent activation of several human blood cell types by protamine-condensed mRNA. European Journal of Immunology, 35, 1557–1566. [DOI] [PubMed] [Google Scholar]
- Schoenberg DR, & Maquat LE (2012). Regulation of cytoplasmic mRNA decay. Nature Reviews. Genetics, 13, 246–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrom E, Huber M, Aneja M, Dohmen C, Emrich D, Geiger J, … Kubisch-Dohmen R (2017). Translation of angiotensin-converting enzyme 2 upon liver-and lung-targeted delivery of optimized chemically modified mRNA. Molecular Therapy. Nucleic Acids, 7, 350–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedic M, Senn JJ, Lynn A, Laska M, Smith M, Platz SJ, … Smith PF (2017). Safety evaluation of lipid nanoparticle-formulated modified mRNA in the Sprague-Dawley rat and cynomolgus monkey. Veterinary Pathology, 55, 341–354. [DOI] [PubMed] [Google Scholar]
- Sergeeva OV, Koteliansky VE, & Zatsepin TS (2016). mRNA-based therapeutics—advances and perspectives. Biochemistry (Moscow), 81, 709–722. [DOI] [PubMed] [Google Scholar]
- Sharp PA (2009). The centrality of RNA. Cell, 136, 577–580. [DOI] [PubMed] [Google Scholar]
- Sorgi FL, Bhattacharya S, & Huang L (1997). Protamine sulfate enhances lipid-mediated gene transfer. Gene Therapy, 4, 961–968. [DOI] [PubMed] [Google Scholar]
- Stanton MG, & Murphy-Benenato KE (2017). Messenger RNA as a novel therapeutic approach. Topics in Medicinal Chemistry, 27, 237–253. [Google Scholar]
- Su X, Fricke J, Kavanagh DG, & Irvine DJ (2011). In vitro and in vivo mRNA delivery using lipid-enveloped pH-responsive polymer nanoparticles. Molecular Pharmaceutics, 8, 774–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Sun Y, Zhao R, & Gao K (2016). Intracellular delivery of messenger RNA by recombinant PP7 virus-like particles carrying low molecular weight protamine. BMC Biotechnology, 16, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull IC, Eltoukhy AA, Fish KM, Nonnenmacher M, Ishikawa K, Chen J, … Costa KD (2016). Myocardial delivery of lipid nanoparticle carrying modRNA induces rapid and transient expression. Molecular Therapy, 24, 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida H, Itaka K, Uchida S, Ishii T, Suma T, Miyata K, … Kataoka K (2016). Synthetic polyamines to regulate mRNA translation through the preservative binding of eukaryotic initiation factor 4E to the cap structure. Journal of the American Chemical Society, 138, 1478–1481. [DOI] [PubMed] [Google Scholar]
- Uchida S, Kinoh H, Ishii T, Matsui A, Tockary TA, Takeda KM, … Kataoka K (2016). Systemic delivery of messenger RNA for the treatment of pancreatic cancer using polyplex nanomicelles with a cholesterol moiety. Biomaterials, 82, 221–228. [DOI] [PubMed] [Google Scholar]
- Udhayakumar VK, De Beuckelaer A, McCaffrey J, McCrudden CM, Kirschman JL, Vanover D, … De Koker S (2017). Arginine-rich peptide-based mRNA nanocomplexes efficiently instigate cytotoxic t cell immunity dependent on the amphipathic organization of the peptide. Advanced Healthcare Materials, 6, 1601412. [DOI] [PubMed] [Google Scholar]
- Ulmer JB, & Geall AJ (2016). Recent innovations in mRNA vaccines. Current Opinion in Immunology, 41, 18–22. [DOI] [PubMed] [Google Scholar]
- Vallazza B, Petri S, Poleganov MA, Eberle F, Kuhn AN, & Sahin U (2015). Recombinant messenger RNA technology and its application in cancer immunotherapy, transcript replacement therapies, pluripotent stem cell induction, and beyond. WIREs RNA, 6, 471–499. [DOI] [PubMed] [Google Scholar]
- Verbeke R, Lentacker I, Wayteck L, Breckpot K, Van Bockstal M, Descamps B, … Dewitte H (2017). Co-delivery of nucleoside-modified mRNA and TLR agonists for cancer immunotherapy: Restoring the immunogenicity of immunosilent mRNA. Journal of Controlled Release, 266, 287–300. [DOI] [PubMed] [Google Scholar]
- Wang H-X, Li M, Lee CM, Chakraborty S, Kim H-W, Bao G, & Leong KW (2017). CRISPR/Cas9-based genome editing for disease modeling and therapy: Challenges and opportunities for nonviral delivery. Chemical Reviews, 117, 9874–9906. [DOI] [PubMed] [Google Scholar]
- Wang Y, Su HH, Yang Y, Hu Y, Zhang L, Blancafort P, & Huang L (2013). Systemic delivery of modified mRNA encoding herpes simplex virus 1 thymidine kinase for targeted cancer gene therapy. Molecular Therapy, 21, 358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weide B, Pascolo S, Scheel B, Derhovanessian E, Pflugfelder A, Eigentler TK, … Garbe C (2009). Direct injection of protamine-protected mRNA: Results of a phase 1/2 vaccination trial in metastatic melanoma patients. Journal of Immunotherapy, 32, 498–507. [DOI] [PubMed] [Google Scholar]
- Weissman D (2015). mRNA transcript therapy. Expert Review of Vaccines, 14, 265–281. [DOI] [PubMed] [Google Scholar]
- Whitehead KA, Dorkin JR, Vegas AJ, Chang PH, Veiseh O, Matthews J, … Anderson DG (2014). Degradable lipid nanoparticles with predictable in vivo siRNA delivery activity. Nature Communications, 5, 4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A, Kormann M, Rosenecker J, & Rudolph C (2009). Current prospects for mRNA gene delivery. European Journal of Pharmaceutics and Biopharmaceutics, 71, 484–489. [DOI] [PubMed] [Google Scholar]
- Yan Y, Xiong H, Zhang X, Cheng Q, & Siegwart DJ (2017). Systemic mRNA delivery to the lungs by functional polyester-based carriers. Biomacromolecules, 18, 4307–4315. [DOI] [PubMed] [Google Scholar]
- Yeom JH, Ryou SM, Won M, Park M, Bae J, & Lee K (2013). Inhibition of xenograft tumor growth by gold nanoparticle-DNA oligonucleotide conjugates-assisted delivery of BAX mRNA. PLoS One, 8, e75369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Song CQ, Dorkin JR, Zhu LJ, Li Y, Wu Q, … Anderson DG (2016). Therapeutic genome editing by combined viral and non-viral delivery of CRISPR system components in vivo. Nature Biotechnology, 34, 328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Song CQ, Suresh S, Wu Q, Walsh S, Rhym LH, … Anderson DG (2017). Structure-guided chemical modification of guide RNA enables potent non-viral in vivo genome editing. Nature Biotechnology, 35, 1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Li B, Luo X, Zhao W, Jiang J, Zhang C, … Dong Y (2017). Biodegradable amino-ester nanomaterials for Cas9 mRNA delivery in vitro and in vivo. ACS Applied Materials & Interfaces, 9, 25481–25487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Pelet JM, Heller DA, Dong Y, Chen D, Gu Z, … Anderson DG (2013). Lipid-modified aminoglycoside derivatives for in vivo siRNA delivery. Advanced Materials, 25, 4641–4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Li M, Zhang Z, Gong T, & Sun X (2015). Induction of HIV-1 gag specific immune responses by cationic micelles mediated delivery of gag mRNA. Drug Delivery, 23, 2596–2607. [DOI] [PubMed] [Google Scholar]