Abstract
Interferon-gamma (IFN-gamma) is an attractive target for imaging active anti-tumor immunity due to its function in the T cell signaling axis. Here we test an IFN-gamma immuno-positron emission tomography (immunoPET) probe for its capacity to identify adaptive immunotherapy (ITx) response after HER2/neu vaccination in both spontaneous salivary and orthotopic neu+ mouse mammary tumors. IFN-gamma immunoPET detected elevated cytokine levels in situ post-vaccination, which inversely correlated with tumor growth rate, an indicator of response to therapy. In a model of induced T cell anergy where CD8 T cells infiltrate the tumor, but upregulate PD-1, IFN-gamma tracer uptake was equivalent to isotype control, illustrating a lack of anti-tumor T cell activity. The IFN-gamma immunoPET tracer detected IFN-gamma protein sequestered on the surface of tumor cells, likely in complex with the IFN-gamma receptor, which may explain imaging localization of this soluble factor in vivo. Collectively, we find that the activation status of cytotoxic T cells is annotated by IFN-gamma immunoPET, with reduced off-target binding to secondary lymphoid tissues compared to imaging total CD3+ tumor infiltrating lymphocytes. Targeting of soluble cytokines such as IFN-gamma by PET imaging may provide valuable non-invasive insight into the function of immune cells in situ.
Keywords: Interferon-γ, PET, Immunotherapy, HER2/Neu, DNA Vaccine
Introduction
During adaptive immunotherapy (ITx), activated T cells infiltrating the tumor are often the principal components of treatment providing a “search-and-destroy” mechanism through specific recognition of tumor-associated antigens (TAA)(1,2). Recent emerging tumor-targeted ITx strategies are met with positive and durable outcomes in a subset of patients, however many remain non-responsive, exposing a strong urgency for consistent methods to monitor therapeutic response in a timely manner(3). Peripheral immune monitoring assays are often restricted to one antigen, are non-standardized, and may not reflect the dynamic activity occurring within the tumor(4,5). Post-treatment biopsy can be used to evaluate tumor infiltrates(6), however tumor heterogeneity and general accessibility may impact the adequacy and/or feasibility of this approach(7). Image-guided focal analysis of intratumoral immune activity may eliminate these issues by providing non-invasive, real-time efficacy predictions in situ. To date, ITx positron emission tomography (PET) tracer development has focused on immune cell surface molecule detection, particularly against CD3(8), and CD8(9). Others have developed tracers targeting immune checkpoint molecules PD1/PD-L1(10–15) to help identify candidate patients for checkpoint blockade therapy. These probes are limited, however, as they do not mark functional downstream effector tumoricidal activity.
The cytokine interferon-γ (IFN-γ) is predominantly produced by activated Type 1 T helper (Th1)-skewed CD4 T cells, cytotoxic CD8 T cells (CTL), and both NK and NKT cells(16). Both Th1 and CTL contribute to antigen-specific tumor cell recognition and destruction, which is particularly advantageous in the context of immunotherapeutic approaches including checkpoint blockade, adoptive cell therapies, and vaccination(17–19). IFN-γ signaling contributes to tumor cell killing by a variety of mechanisms including upregulation of Fas/FasL and MHC molecules (20,21); however tumor expression of PD-L1 is also positively regulated by IFN-γ signaling, which ultimately serves as a feedback mechanism to quell immune activation.
The focus of our study underscores the development of a monoclonal antibody PET tracer targeting IFN-γ. We show that IFN-γ PET associates with response to ITx. Tumors treated with TAA DNA vaccination show increased IFN-γ detection coupled with with an influx of T cells. The level of IFN-γ uptake inversely correlates with tumor growth rate. Alternatively, in a model of induced T cell exhaustion, we find T cells infiltrate but fail to produce detectable IFN-γ as measured by PET imaging. We further demonstrated that IFN-γ PET provides consistent sensitivity for the detection of ITx response when compared to antigen-specific peripheral immune monitoring. Collectively, IFN-γ PET may serve as a non-invasive, comprehensive approach to the evaluation of tumor ITx.
Materials & Methods
Mice
Female BALB/c mice (6–8 week old) were purchased from Charles River Laboratories (Wilmington, MA). Heterozygous BALB/NeuT (NeuT) mice were bred in-house. NeuT male mice, which express a transforming rat neu, develop atypical ductal hyperplasia in 1–2 parotid glands by 6 weeks of age which progresses to multifocal acinic cell adenocarcinoma in situ at ~19 weeks of age(22). All animal procedures were approved by and performed in accordance with the regulation of Wayne State University Animal Investigation Committee.
Radiochemistry
All antibodies were obtained from eBioscience (San Diego, CA) whereas the non-specific IgG isotype controls were purchased from Jackson ImmunoResearch (Westgrove, PA) and Thermo Fisher (Waltham, MA) unless otherwise stated. Chelators were obtained from Macrocyclics (Plano, TX). Radioisotopes were obtained from 3D Imaging, LLC (Little Rock, AR). Synthesis of 89Zr-anti-CD3, 89Zr-anti-IFN-γ, and non-specific 89Zr-anti-IgG followed established protocols(23,24). Briefly, p-SCN-Bn-Desferrioxamine (DFO, Macrocyclics, Plano, TX) was incubated with 145–2C11 (anti-CD3ε, Armenian hamster, IgG, eBioscience, San Diego, CA), or AN-18 (anti-IFNγ, rat IgG1, kappa, eBioscience, San Diego, CA) at a ratio of 1:5 (Ab:DFO) in phosphate buffered saline at pH ~9 for 1 h at 37 °C and purified via centrifugation with molecular weight column filter (MWCO: 30 kDa, GE Vivaspin 500). Facile 89Zr-radiolabeling (3D Imaging, Little Rock, AR) of the 145–2C11-DFO or AN-18-DFO conjugates proceeded in a neutral pH environment in saline at room temperature after 1 h of incubation. Unbound 89Zr (t1/2 = 3.27 d) was removed via centrifugation with molecular weight column filters (MWCO: 30 kDa, GE Vivaspin 500). Labeling yields of >85% were achieved as monitored through radio-instant thin layer chromatography (iTLC, MiniScan, Eckert and Ziegler, Berlin, Germany) within 1 h. A radiochemical purity of >98% was obtained after filtration based on iTLC. Specific activities of 4.20 ± 0.12 mCi/mg for 89Zr-anti-IFN-γ and 3.98 ± 0.08 mCi/mg for 89Zr-anti-CD3 were achieved.
PET imaging
Tumor-bearing animals were injected intravenously (i.v.). with the radiolabeled antibody (~180–240 μCi/mouse, 42.8 – 57.1 μg in 150 μL sterile saline) in the lateral tail vein. Imaging was acquired from 4–72 h post-injection (p.i.). The animals were anesthetized with 3–5% isoflurane (Baxter Healthcare, Deerfield, IL) in air for induction, then lowered to 1.5–2% for maintenance. Images were acquired with a microPET R4 camera (Siemens Medical Solutions, USA formerly Concorde Microsystems). Manually drawn 3-dimensional volumes-of-interest (VOI) were selected to determine maximum and mean percent injected dose per gram (%ID/g) in various tissues. Images were decay-corrected to the time of injection and analyzed for tracer uptake using ASIpro VMTM software v. 6.3.3.0.
In vivo treatment with CpG-ODN and IFN-γ PET
IFN-γ was systemically induced in naïve BALB/c mice by intramuscular (i.m.) injection of 100 μg CpG-ODN (Integrated DNA Technologies, Coralville, IA). Within an hour of treatment, the mice were injected with 89Zr-anti-IFN-γ in the lateral tail vein (180–240 μCi/mouse, 49.9 ± 7.2 μg). PET imaging was conducted at 72 h p.i. Tracer uptake within the spleen and peripheral immune tissues were analyzed and compared against a control, untreated group.
Biodistribution and competitive inhibition blocking assay
Mice were injected i.v. in the lateral tail vein with 89Zr-anti-IFN-γ (20–30 μCi, 4.7–7.1 μg) in 100–150 μL saline followed by euthanasia via CO2 asphyxiation after 72 h p.i., the time point identified at which tumor-to-background is sufficient. For blocking studies, 80 μg of cold AN-18 was co-injected with the probe in a separate cohort of mice. Select organs were harvested post-sacrifice, weighed and measured for bound radioactivity with a gamma counter (Perkin Elmer 2480 Wizard 2). The %ID/g was calculated as the % of activity bound to the tissue normalized against total administered activity per gram of tissue weight.
In vitro 89Zr-anti-IFN-γ Internalization Assay
TUBO cells (4.5 × 105) were plated into 6 well plates in triplicate and allowed to adhere overnight. In separate wells, cells were either left untreated or incubated with recombinant IFN-γ (10 ng/mL) for 15 minutes before 3 PBS washes. 89Zr-anti-IFN-γ (150 ng, 0.63 nCi) was added and cells were incubated for 1 h at 37 °C. Following the incubation period, the media was collected and the cells were rinsed with PBS twice. Surface-bound activity was removed by washing with 100 mM acetic acid + 100 mM glycine (1:1, pH 3.5) at 4 °C. The cells were then lysed with 1 M NaOH. Each fraction was measured for activity using a gamma counter (Perkin Elmer). The %-internalized activity was calculated as the ratio lysate to total activity collectednormalized to cell number.
Tumor inoculation
The neu+ TUBO line cloned from a spontaneous mammary tumor in a female NeuT mouse was obtained through Dr. Guido Forni (U. Torino, Torino, Italy)(25). Mice were inoculated in the #4 mammary fat pad with 2.5 × 105 tumor cells. Tumor growth was monitored by palpation and caliper measurement. Tumor volume was calculated as v = (l × w2)/2.
DNA vaccination
The HER2/neu DNA vaccine consists of 20 μg of pGM-CSF (encoding murine GM-CSF) and 50 μg pE2TM (encoding the extracellular and transmembrane regions of human HER2) in 50 μL PBS, which is injected i.m. into each gastrocnemius followed immediately by application of electrode gel and square wave electroporation using a BTX830 (BTX Harvard Apparatus, Holliston, MA)(26). For NeuT vaccination, mice were depleted of Tregs by intraperitoneal (i.p.) injection of 500 μg anti-CD25 mAb PC61 10 days prior to the first vaccination.
Quantitative real-time PCR
Tumor tissue was snap frozen in liquid nitrogen. Total tumor RNA was collected by Trizol preparation (Thermo Fisher, Waltham, MA) after homogenization. cDNA was synthesized with ProtoScript II reverse transcriptase (New England Biolabs, MA). Real-time qPCR was conducted with iTaq Universal SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA) using 10 ng cDNA/well and 500 nM primers specific to the indicated gene (Life Tech, Carlsbad, CA). Primer sequences are found in Supplementary Table 1. Relative mRNA quantities are calculated by 2-ΔCT compared to GAPDH.
ELISA
Tumor tissue was homogenized in standard RIPA buffer with protease inhibitor cocktail (Sigma-Aldrich). Protein concentration was measured by BCA assay (ThermoFisher). High protein binding plates (ThermoFisher) were coated with 3 μg/mL anti-mouse-IFN-γ mAb clone AN-18 (eBioscience) in coating buffer (0.1 M Na2HPO4, pH to 9.0) and washed prior to addition of samples or standard curve using recombinant mouse IFN-γ (Peprotech, Rocky Hill, NJ) in duplicate. IFN-γ was detected with biotin-conjugated anti-mouse IFN-γ clone R4–6A2 (eBioscience), avidin-HRP (ThermoFisher), and TMB substrate (ThermoFisher).
Serum IgG measurement
Serum HER2- and neu-specific IgG were quantified by flow cytometry with a BD FACSCanto II flow cytometer (Becton Dickinson, Franklin Lakes, NJ), using HER2 over-expressing SKOV3 cells or neu transfected 3T3/NKB cells as previously described(27). Regression analysis was conducted using standard curves of anti-HER2 mAb TA-1 (Calbiochem, Burlington, MA) or anti-neu mAb 7.16.4 (Calbiochem, Burlington, MA).
IFN-γ ELISPOT
HER2- and neu-specific IFN-γ production was measured by ELISPOT assay as previously described(28). Recombinant HER2 or neu (10 μg/mL, Sino Biologicals, Beijing, China) were incubated with splenocytes for 48 h in round-bottom wells, followed by transfer to anti-IFN-γ coated (clone AN-18, eBioscience) ELISPOT plates (Millipore Sigma, Burlington, MA) for an additional 48 h. Spots were detected by biotinylated anti-IFN-γ (clone R4–6A2, eBioscience) and avidin-HRP (Becton Dickinson, Franklin Lakes, NJ), followed by enumeration with an ImmunoSpot analyzer (Cellular Technology Limited, Cleveland, OH). Results are expressed as spot forming units (SFU) per 106 cells.
Treatment with anti-neu mAb 7.16.4
Mice bearing TUBO tumors were injected i.p. 5 times every 3–4 days with sterile-filtered ascites containing 1 mg anti-neu mAb 7.16.4 diluted in PBS to a final volume of 300 μL.
Immunohistochemistry (IHC) and H&E Staining
IHC was performed using an anti-CD8 antibody (1:200, clone D4W2Z, Cell Signaling). After euthanasia, tumors were harvested, formalin fixed, and embedded in paraffin. 4 μm sections were cut using a Sakura Accu-Cut SRM microtome (Catalog#: SRM- 200 CV) and adhered onto positively charged slides (Histomax Plus, VWR). Slides were then incubated for 12 minutes at 65 °C and deparrafinized in washes of xylene and graded alcohols. For IHC, antigen retrieval was performed in PT module buffer (TA-250-PM4X, Fisher) and primary antibody incubation was performed for 1 h at room temperature in a humidified chamber. Secondary antibody incubations and DAB were performed following manufacturers’ protocols. CD8 T cell enumeration was conducted by a blinded board certified pathologist. Each tumor sample was screened for hotspots of CD8 lymphocytes using a Nikon Eclipse Ci microscope at 100× magnification. The number of CD8+ T lymphocytes were counted in three regions with the highest infiltration at 400× magnification with a 0.55 mm field diameter, and an average was calculated. For H&E staining, washed tissue sections were stained with hematoxylin (TA-125-MH, Fisher) for 5 minutes, rinsed with an acid wash for 1 minute followed by treatment with Shandon Bluing Reagent (Thermo Scientific) for 15 seconds. Eosin staining was applied to slides for 1 minute and slides were rinsed in 95% ethanol three times. Sections were then dehydrated using a series of graded alcohol and xylenes washes for mounting on slides with Permount (UN1294, Fisher). Images were taken with a Spot Idea camera using Spot 5.2 software (Spot, Sterling Heights, MI).
Tumor dissociation and flow cytometry
TUBO tumors from untreated BALB/c mice were dissociated using the GentleMACs dissociator and mouse tumor dissociation kit (Miltenyi, Germany) following the manufacturer protocol. Cells were stained with a combination of either: FITC-conjugated CD45 mAb (clone 30-F11), APC-conjugated TCRβ mAb (clone H57–597), and eFluor780-conjugated viability dye or FITC-conjugated CD45 mAb (clone 30-F11), PE-Cy7-conjugated CD8 mAb (clone 53–6.7), APC-conjugated PD-1 mAb (J43), and eFluor780-conjugated viability dye. All antibodies/dyes were purchased from eBIoscience (San Diego, CA). Samples were analyzed on a BD FACSCantoII flow cytometer (Becton Dickinson, Franklin Lakes, NJ) and samples were gated on the viable fraction.
Statistical analyses
Statistical analyses were conducted using GraphPad Prism 7.02. Data shown are presented as mean ± standard deviation. All tests use one-way ANOVA (estimating a different variance for each group) with Tukey’s post-test unless otherwise noted. Weekly tumor growth rate for each mouse was calculated by regression analysis of log tumor growth from day 14, when T cells become active after the initial vaccination, until the final measurement on day 38, versus time in weeks. The relationship between the estimated growth rate per week (slope from the regression analysis) for each mouse and the 89Zr-anti-IFN-γ tracer uptake measured at day 28 was assessed using Pearson’s correlation. *p<0.05, **p<0.01, ***p<0.001.
Results
89Zr-anti-IFN-γ PET tracer identifies localized IFN-γ production
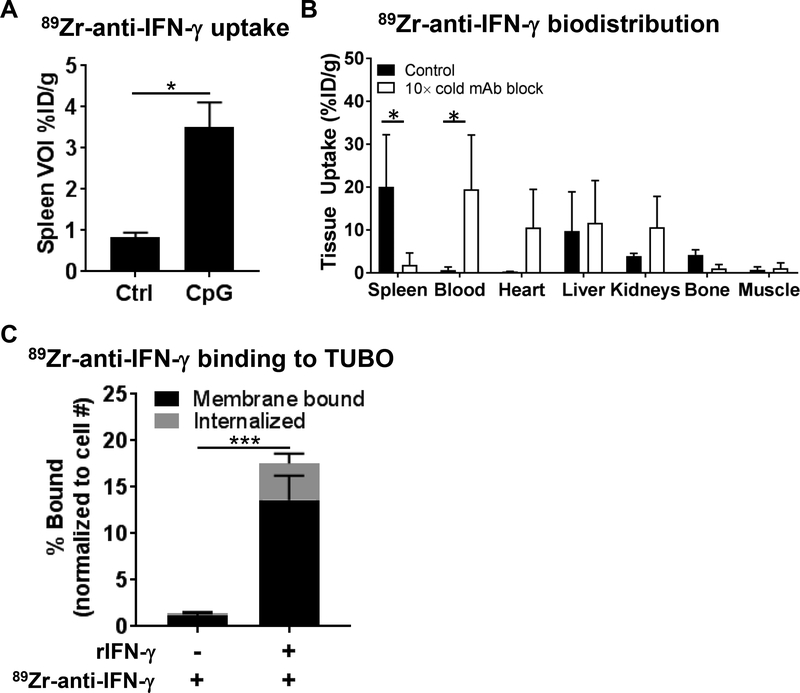
The rat mAb AN-18 to murine IFN-γ was labeled with 89Zr using desferrioxamine as the chelate (89Zr-anti-IFN-γ) in good yields and purities according to previously reported methods(23). In mice treated with CpG-ODN to stimulate IFN-γ, whole-body PET images were acquired at 72 h p.i., a time point identified to exhibit reliable tracer uptake in the tumor, with low liver and blood pool background (Supplementary Fig. 1–2). VOIs drawn on splenic tissues demonstrated higher tracer accumulation (3.50 ± 0.61% ID/g, n=3) in CpG-ODN-treated groups compared to untreated controls (Ctrl: 0.83 ± 0.12% ID/g, n=3) (Fig. 1A).
Figure 1. Validation of specificity of 89Zr-anti-IFN-γ.
A) BALB/c mice treated with CpG-ODN and imaged with the tracer 72 h p.i. displayed higher uptake in the spleen compared to control (Ctrl) untreated cohorts (n=3 each). B) Tissue distribution of 89Zr-anti-IFN-γ at 72 h p.i. demonstrated lower probe accumulation in the spleen upon competitive saturation with 10× cold AN-18 mAb (n=4 each). C) Binding of 89Zr-anti-IFN-γ receptor-localized IFN-γ was tested in vitro. TUBO cells were incubated with 89Zr-anti-IFN-γ alone (n=5), or with recombinant IFN-γ (rIFN-γ) and washed before addition of 89Zr-anti-IFN-γ (n=5). Activity was measured by a gamma counter and adjusted for cell count.
Tissue distribution of 89Zr-anti-IFN-γ at 72 h p.i. demonstrated 20.04 ± 12.2% ID/g uptake in the spleen (Fig. 1B, Supplementary Table 2). Uptake within the blood circulation (0.67 ± 0.69% ID/g), as well as tissues responsible for excretion such as the liver (9.77 ± 9.12% ID/g), and kidneys (3.93 ± 0.6% ID/g), were low. There was also low uptake in the bone and muscle. We further confirmed specificity through competitive binding experiment where we observed a decrease in spleen uptake (20.04 ± 12.20 vs. 1.88 ± 2.74% ID/g, n=4, p=0.0061) with 10× cold mAb blockade, consequently increasing non-specific tissue accumulation in the blood (19.46 ± 12.69% ID/g, p=0.0043), heart (10.57 ± 8.91, p=0.30), and liver (11.69 ± 9.82% ID/g, p=0.99). Notable differences in splenic uptake in the imaging and tissue distribution (10-fold lower mass) are due to “mass effects”, wherein a greater mass of protein administered potentially saturated receptor binding sites and rendered slower pharmacokinetics(29).
Since IFN-γ is a soluble protein, we sought to determine the mechanism of localized IFN-γ imaging. Plated TUBO tumor cells were exposed to IFN-γ and/or 89Zr-anti-IFN-γ tracer in quintuplicate followed by analysis of membrane binding and internalization (Fig. 1C). TUBO cells incubated with 89Zr-anti-IFN-γ alone show limited tracer surface binding (1.13 ± 0.28%) and internalization (0.29 ± 0.13%). When TUBO is pre-incubated with IFN-γ, enhanced 89Zr-anti-IFN-γ surface binding (13.62 ± 2.60%) and internalization (3.93 ± 1.07%) is observed (membrane: p=0.00039, internalized: p=0.0015). Detection of tracer binding to TUBO cells after IFN-γ exposure suggests localized imaging may be due to sequestration of IFN-γ on its receptor in vivo.
IFN-γ PET detects active anti-tumor immunity in situ in a syngeneic tumor model
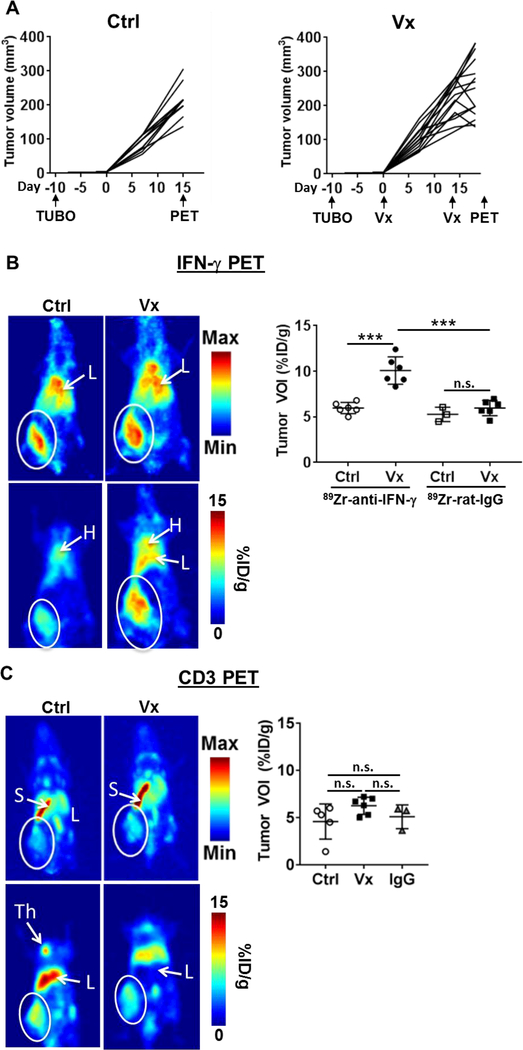
To test the capacity of 89Zr-anti-IFN-γ as a non-invasive measure of anti-tumor immune response, neu+ TUBO tumor bearing BALB/c mice were imaged after receiving two rounds of HER2/neu DNA vaccination as detailed in Figure 2A. This vaccine induces HER2-specific humoral and T cell responses and ~10% equivalent of cross-reactive anti-neu T cells without cross-reactive neu-specific antibody(26). Tumor volumes began to stabilize or regress within 1 week after the second vaccination compared to untreated TUBO-bearing mice (Fig. 2A). Control tumors were imaged a week earlier to match tumor volumes between control and vaccinated cohorts, and to prevent potential necrosis in the control tumors at the time of imaging. Mice were injected with 89Zr-anti-IFN-γ tracer for PET imaging (Fig. 2B and Supplementary Fig. 3A) at 72 h p.i. A nearly two-fold increase in tumor uptake was observed in vaccinated (Vx: 10.07 ± 1.50% ID/g, n=6) versus control mice (Ctrl: 5.97 ± 0.61% ID/g, n=6, p=0.0001). A 89Zr-labeled rat IgG isotype control tracer demonstrated similar tumor accumulation (72 h p.i.) in both untreated control (5.27 ± 0.79% ID/g) and vaccinated (5.93 ± 0.85% ID/g) mice. This suggests baseline intratumoral IFN-γ levels are low without treatment. The notable low accumulation of the isotype control tracer after vaccination supports the specificity of the IFN-γ tracer and suggests increased 89Zr-anti-IFN-γ uptake is not simply due vascular permeability and retention effects post-ITx.
Figure 2. PET evaluation of immunotherapy response in orthotopic TUBO mammary tumors.
A) Tumor volume was monitored in both untreated control (Ctrl, n=11, left) and vaccinated (Vx, n=12, right) tumors. TUBO cells were inoculated 10 days prior to the start of vaccinations, given on days 0 and 14. PET imaging was conducted on day 15 (Ctrl) and 21 (Vx). B) Representative whole body maximum intensity projections (MIP, top row) and planar (bottom row) images of control (left panels, n=6) and vaccinated (right panels, n=6) mice with 89Zr-anti-IFN-γ tracer (left). White circle = tumor, L = liver, H = heart, S = spleen, Th = Thymus. Tumor VOIs were measured for each mouse with a 89Zr labeled rat IgG isotype control included for each treatment group (n=3, untreated control; n=6, vaccinated control). C) MIP image (top panels) and planar sections (bottom panels) of 89Zr-anti-CD3 images in control (left, n=5) and vaccinated mice (middle, n=6). A non-specific 89Zr labeled Armenian hamster IgG isotype control was used to measure tumor VOI in a separate group of untreated mice (right, n = 3).
Detection of tumor infiltrating lymphocytes via CD3 immunoPET
Total T cell presence in the tumor microenvironment was assessed in separate groups of mice via immunoPET imaging of CD3+ tumor infiltrating lymphocytes using 89Zr-anti-CD3 (Fig. 2C and Supplementary Fig. 3B). Vaccinated tumors exhibited a modest, insignificant increase of CD3 tracer binding compared to control (Vx: 6.25 ± 0.37% ID/g, n=6 vs. Ctrl: 4.58 ± 0.83% ID/g, n=5, p=0.16). Both cohorts failed to demonstrate a significant change in uptake compared to Armenian hamster isotype control IgG (5.90 ± 1.26% ID/g, n=3, p=0.87 (Ctrl), p=0.49 (Vx)). Untreated TUBO tumors have endogenous T cell infiltrates as detected by flow cytometry upon dissociation (Supplementary Fig. 4A). However, CD3 immunoPET suboptimally detected these TILs in both untreated and vaccinated mice with measured VOIs similar to the non-specific IgG tumor accumulation. This may be due to excessive uptake by the spleen, a T cell-homing secondary lymphoid tissue (Ctrl: 17.06 ± 3.56% ID/g, Vx: 18.36 ± 1.49% ID/g, Supplementary Fig. 4B), which can act as a tracer “sink.” In contrast, limited splenic accumulation was observed with the IFN-γ PET probe (Ctrl: 3.58 ± 0.81% ID/g, p<0.0001, Vx: 4.97 ± 0.97% ID/g, p<0.0001).
Ex vivo validation via IHC, qPCR, and ELISA
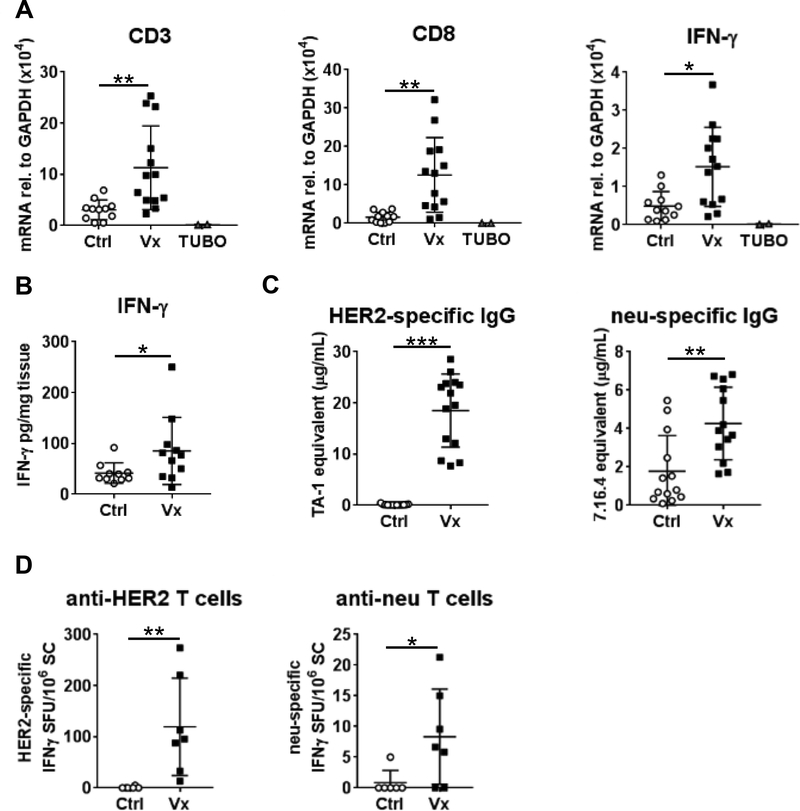
Upon completion of imaging, tissues were collected for ex vivo validation. Tumor tissue was assessed to verify CD3+ and CD8+ T cell presence, as well as expression of IFN-γ. Transcripts levels of CD3, CD8 and IFN-γ were increased in tumor tissue after vaccination (Fig. 3A, Ctrl: n=11, Vx: n=13), in concordance with the PET imaging data. Cultured TUBO cell cDNA is included as a negative control. CD3 and CD8 proteins were increased after treatment (Supplementary Fig. 5) and intratumoral IFN-γ protein was also confirmed and quantitated by ELISA (Fig. 3B). ELISA results showed higher total IFN-γ in Vx (n=11) versus Ctrl (n=10) TUBO tumors (Vx: 85.37 ± 65.89 vs. Ctrl: 41.69 ± 20.12 pg/mg tissue, p=0.043).
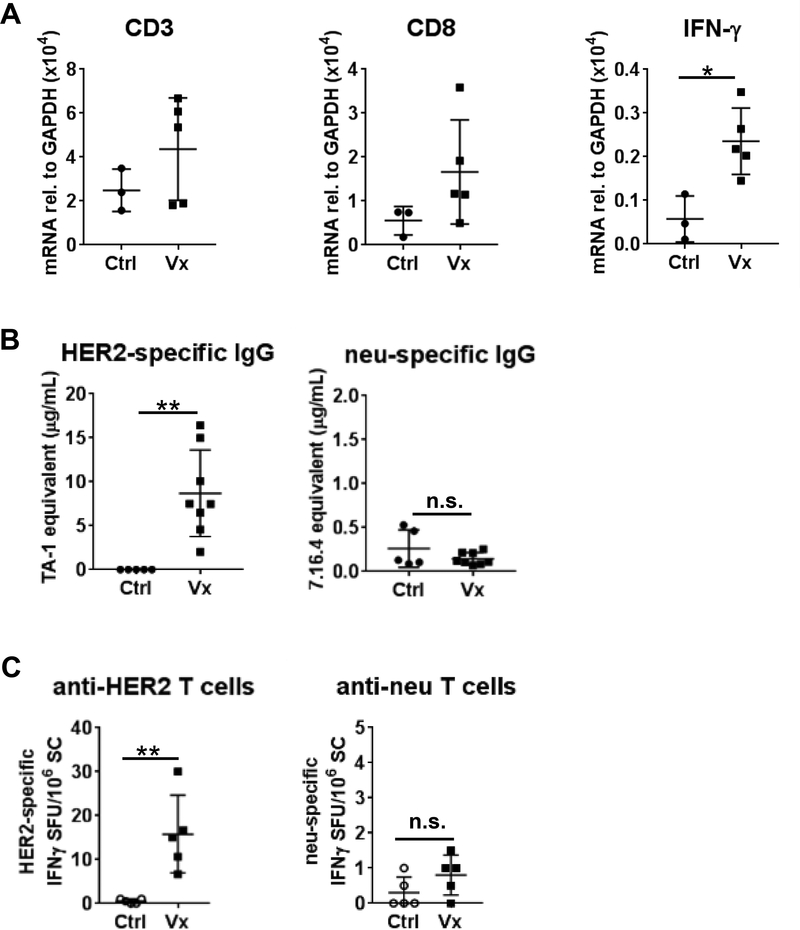
Figure 3. Ex Vivo validation of immunotherapy response in TUBO-bearing mice.
Tumors were removed after imaging and validated. A) Total RNA obtained from Ctrl (n=11) and Vx (n=13) tumor tissue was analyzed by qPCR with primers specific to CD3 (left), CD8 (middle), and IFN-γ (right). Cultured TUBO cells serve as control (n=2). B) IFN-γ ELISA was conducted with protein lysates of TUBO tumor segments of control Ctrl (n=10) and Vx (n=11) mice. C) HER2 and neu-specific IgG were measured in serum by flow cytometry (Ctrl: n=13, Vx: n=14). D) HER2 and neu-responsive T cells were measured by IFN-γ ELISPOT (Ctrl: n=6, Vx: n=7).
Peripheral vaccine-induced immunity was measured by HER2/neu-specific serum IgG (Fig. 3C) and splenic T cell responses (Fig. 3D). HER2-specific IgG was only detected in vaccinated mice (18.68 ± 7.40 μg/mL, n=14, p<0.0001). TUBO tumors constitutively express the cell surface oncogene neu, which is foreign in wild-type BALB/c mice. Neu-specific IgG is detected in unvaccinated control TUBO-bearing mice (1.58 ± 1.60 μg/mL, n=13), which is further increased in vaccinated animals (6.18 ± 7.34 μg/mL, n=14, p=0.0019). While the HER2 DNA vaccine itself does not induce anti-neu IgG(26), tumor cell killing likely enhanced immune activity to this foreign antigen. Detection of HER2-specific IFN-γ-producing T cells was restricted to vaccinated mice, similar to anti-HER2 IgG (119.40 ± 95.18/106 splenocytes (SC), n=7, vs. 0.83 ± 2.04/106 SC in untreated controls, n=6, p=0.0012). Peripheral anti-neu T cells were detected in all vaccinated animals (8.33 ± 7.75/106 SC) while only 1 of 4 untreated controls showed T cell responsiveness to neu (0.83 ± 2.04/106 SC, p=0.033). The absolute quantities of HER2 and neu-specific IgG and T cells were ~10-fold lower than similarly vaccinated non-tumor-bearing mice(26). This may be due to tumor-associated immune suppression by myeloid-derived suppressor cells or regulatory T cells (Tregs), which are reportedly increased in TUBO-bearing mice(26,30,31).
Detection of ITx response in a spontaneous tumor model
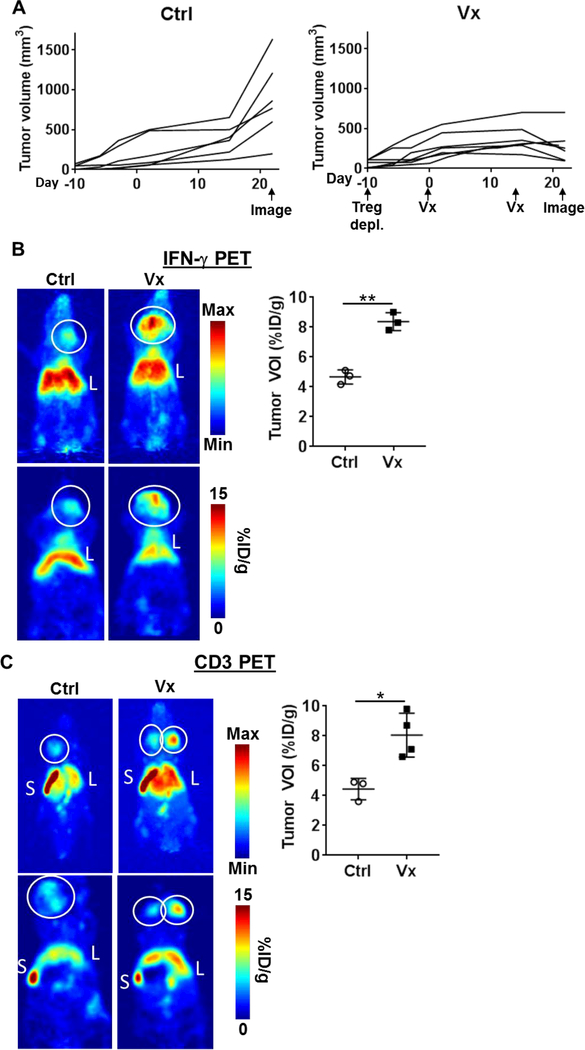
We next tested the capacity of IFN-γ PET imaging to detect anti-tumor immune activity in a spontaneous tumor setting. Neu transgenic (NeuT) mice are engineered to express a transforming rat neu under the mouse mammary tumor virus promoter(22), allowing immune system recognition of neu as a self-antigen(26). We conducted our studies in male NeuT mice, which develop 1–2 spontaneous neu+ salivary tumors between 30–40 weeks of age(22). Once tumors were palpable, Tregs were depleted using anti-CD25 mAb clone PC61 to enhance ITx response owing to the fact that NeuT mice are immune tolerant to rat neu(26,32); this was followed by two HER2/neu DNA vaccinations as detailed in Figure 3A. Vaccination of NeuT mice (n=7) controlled tumor growth rate compared to untreated (n=6) tumor-bearing NeuT mice (Fig. 4A, p=0.032). IFN-γ PET of vaccinated tumors displayed a nearly two-fold higher uptake of 89Zr-anti-IFN-γ (8.37 ± 0.35% ID/g, n=4) vs. control (4.63 ± 0.47% ID/g, n=3, p=0.001), indicating infiltration of functional anti-tumor T cells (Fig. 4B and Supplementary Fig. 6A). An examination of tumor infiltrates via CD3 PET (Fig. 4C and Supplementary Fig. 6B) revealed a similar trend (Vx: 8.05 ± 1.47% ID/g vs. Ctrl: 4.43 ± 0.72% ID/g, n=3 per group, p=0.012).
Figure 4. PET detection of anti-tumor immunity in spontaneous tumor-bearing NeuT mice.
A) Control, untreated mice (Ctrl: n=6) were imaged by PET after palpable tumors were permitted to grow 31 days. For vaccinated mice (Vx: n=7), upon detection of palpable spontaneous salivary tumors, regulatory T cells (Treg) were depleted 10 d prior to the first vaccination. Mice received two HER2/neu DNA vaccinations 14 d apart. PET imaging was conducted 7 days after the final vaccination. B) Representative whole body maximum intensity projections (MIP, top row) and planar (bottom row) images of control (left panels, n=3) and HER2/neu DNA-vaccinated (right panels, n=4) mice with 89Zr-anti-IFN-γ tracer (left). White circle = tumor, L = liver, S = spleen. Tumor VOIs were calculated for each mouse. C) Representative CD3 PET images of MIP (top) and planar sections (bottom) are shown for Ctrl (left) vs. Vx groups (right).
Validation of T cell infiltration and IFN-γ production was conducted by qPCR in tumor tissue samples (Fig. 5A). CD3 and CD8 detection showed a variable modest, insignificant increase after vaccination, while IFN-γ mRNA increased (Ctrl: n=3, Vx: n=5, p=0.036). Peripheral immune response to the vaccine was evaluated by measuring serum anti-HER2 and anti-neu IgG, as well as spleen-resident HER2- and neu-responsive IFN-γ-producing T cells. Tolerance to HER2/neu in NeuT mice was apparent with a comparatively lower ITx response vs. wild-type BALB/c mice bearing TUBO tumors in Figure 2. HER2-specific IgG was detected in vaccinated animals (Fig. 5B, Vx: 8.7 ± 4.9 μg/mL, n=8, p=0.0016 vs. Ctrl, n=5), while anti-neu IgG was negligible or absent in all samples tested. Despite increased intratumoral detection of IFN-γ in vaccinated NeuT mice by PET, peripheral T cell response to neu was low (Fig. 5C, 15.80 ± 8.84/106 SC, n=5) with HER2 vaccination, and was not significantly increased relative to untreated control (n=5, p=0.27). T cell response to the cognate vaccine antigen was evident in anti-HER2 T cells (15.80 ± 8.84/106 SC, n=5, p=0.0079). The ratio of anti-neu to anti-HER2 T cells detected was ~5.1% in NeuT mice, which is two-fold lower relative to that detected in non-immune tolerant BALB/c mice bearing TUBO (10.0%, Fig. 3D). These results support the hypothesis that peripheral immune monitoring may be an inadequate measure of anti-tumor immunity with tumor-responsive T cells preferentially localizing within the tumor, supporting the use of in situ analysis methods such as PET imaging.
Figure 5. Ex vivo validation of anti-tumor immunity in spontaneous tumor-bearing NeuT mice.
A) Total RNA was isolated from tumor tissue and qPCR analysis for CD3, CD8, and IFN-γ was conducted (Ctrl: n=3, Vx n=5) B) Serum HER2 (Ctrl, n=5; Vx, n=8) and neu-specific IgG (Ctrl, n=5; Vx n=8) was measured by flow cytometry. C) HER2 and neu-responsive T cells were measured by IFN-γ ELISPOT (n=5 each).
IFN-γ PET imaging is an indicator of immune activation status in situ
To test the capacity of 89Zr-anti-IFN-γ to predict treatment outcomes, BALB/c mice (n=11) bearing variably-sized TUBO tumors were treated with our HER2 vaccine as described previously, resulting in a range of growth slopes (Fig. 6A). 89Zr-anti-IFN-γ PET imaging was conducted two weeks after the final vaccination and tumor volume was monitored for an additional 10 days. Tumor-localized 89Zr-anti-IFN-γ tracer uptake inversely correlated with tumor growth rate (Fig. 6B and Supplementary Fig. 7, r=−0.64, 95% CI: (−0.90,−0.06); p=0.034), suggesting IFN-γ PET is an indicator of the effects of ITx on these tumors.
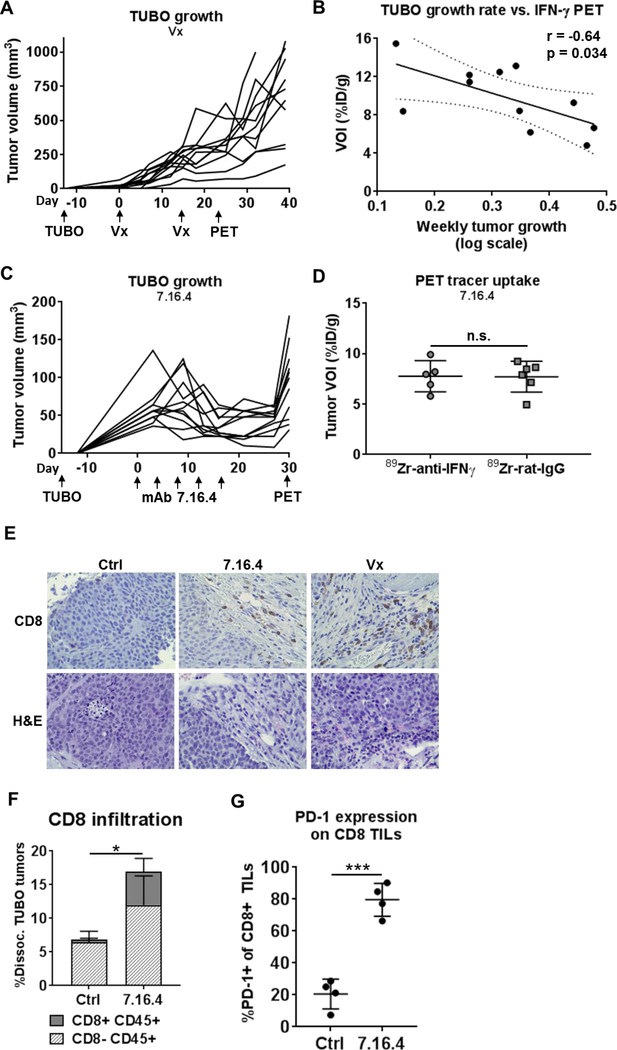
Figure 6. IFN-γ PET depicts response to ITx.
A) Tumor volume was monitored in TUBO-bearing vaccinated BALB/c mice (n=11). TUBO cells were inoculated 13 days prior to the start of vaccinations, to allow for variability in tumor volumes at treatment onset. Vaccines were given on days 0 and 14. PET imaging was conducted on day 28. B) Weekly tumor growth rate, calculated by regression analysis of log tumor growth, versus 89Zr-anti-IFN-γ tracer uptake is plotted for each mouse and evaluated by Pearson’s correlation. C) Tumor growth was monitored during passive immunotherapy with anti-neu mAb 7.16.4, given as 5 doses at 1.5 mg i.p. every 3–4 days as indicated beginning 15 days after tumor inoculation. 89Zr-anti-IFN-γ (n=5) or 89Zr-rat-IgG control (n=6) PET imaging was conducted 30 days after treatment onset. D) Tumor VOIs were calculated for 89Zr-anti-IFN-γ or 89Zr-rat-IgG tracers in 7.16.4 treated TUBO-bearing mice. E) Intratumoral localization of CD8 was analyzed by IHC on FFPE tissue (400×). H&E sections are included. F) Control and 7.16.4-treated tumors (n=4 each) were dissociated and analyzed for T cell infiltration by flow cytometry by staining for CD45 and CD8. G) PD-1 expression was analyzed by flow cytometry on CD8+ tumor infiltrates from (F).
We next evaluated IFN-γ PET in a setting where tumor-infiltrating T cells are present but have become exhausted. TUBO-bearing mice were treated with passive ITx, using mAb 7.16.4 to rat neu. This mAb has been shown to inhibit neu signaling in addition to initiating host anti-tumor immunity(33,34). Once tumors were established at ~50 mm3, 1 mg doses of 7.16.4 were given i.p. at 3–4 day intervals for a total of 5 treatments, which reduced and stabilized tumor growth (Fig. 6C). 89Zr-anti-IFN-γ (n=5) or control IgG (n=6) PET imaging was conducted on day 30 after treatment onset, at which time tumor growth had resumed. Tumor uptake of the IFN-γ tracer was indistinguishable from IgG control, suggesting a lack of immune activity (Fig. 6D). CD8 T cell infiltration was evaluated by IHC (Fig. 6E). A blinded pathologist’s enumeration of the three regions with highest infiltration were calculated, showing a 12-fold increase in CD8 T cells after 7.16.4 treatment versus control (Ctrl: 3 ± 1, 7.16.4: 36 ± 19). Vaccinated TUBO tumor had the largest detected CD8 infiltration (74 ± 25). Overall, CD8+ tumor infiltration was intermittent, with high-density regions scattered among areas with no detectable CD8+ TILs (Supplementary Fig. 8). We further validated CD8 T cell infiltration after 7.16.4 therapy by flow cytometry in a parallel cohort of treated and control mice (Fig. 6F, n=4 each). An overall increase in CD45+ infiltrates (Ctrl: 6.84 ± 1.85%, 7.16.4: 16.95 ± 5.88%, p=0.036) and CD8+ T cells (Ctrl: 0.41 ± 0.19%, 7.16.4: 4.96 ± 1.96%, p=0.018) was detected after mAb treatment. Interestingly, the majority of CD8+ TILs expressed the T cell exhaustion marker PD-1 (Fig. 6G, 79.7 ± 10.3%) compared to control tumors (20.5 ± 9.3%, p=0.0001). Collectively, these results suggest this treatment model promotes an inactive and exhausted CD8+ T cell status despite tumor infiltration, leading to reduced IFN-γ production, which was detected by 89Zr-anti-IFN-γ PET imaging.
Discussion
Several major drawbacks to the use of general T cell surface markers and immune checkpoint ligands for PET imaging can complicate the assessment of ITx response. T cells are densely present in normal secondary lymphoid tissues, such as the spleen, thymus and lymph nodes, which may hinder tumor-specific T cell imaging. Intratumoral detection of total CD3+ T cells and CD8+ cytotoxic lymphocytes has been shown to positively correlate with patient outcomes(35,36). However, the chronic inflammatory tumor microenvironment promotes checkpoint molecule expression, driving cytotoxic T cells into an exhausted state with diminished effector activity. Visualizing components of checkpoint signaling axes (e.g. PD1/PD-L1) can influence treatment decisions by selecting patients with higher likelihood of responding to checkpoint blockade(37). In general, these methods do not measure downstream effector function of cytotoxic T cells. Larimer et al. reported on the utility of a peptide-based imaging tracer specific for granzyme B, a cytotoxin released by activated CTL using a syngeneic colon cancer model(38). The tracer identified responders from non-responders after mono- or combinatorial anti-CTLA-4 and anti-PD1 targeted inhibition; however, it is unclear whether the peptide tracer (7.46 ± 2.24 μg per mouse) solicited inhibitory effects on the enzymatic activity of granzyme B. Nevertheless, the study substantiates our rationale that imaging effector molecules along the T cell signaling axis may provide a better readout of immune response to treatment.
To the best of our knowledge, this is the first study investigating the utility of immunoPET for detecting IFN-γ. In this study, we demonstrated the capabilities of IFN-γ PET to measure active anti-tumor immunity, providing a predictive tool for non-invasive in situ tumor evaluation. This approach is highly specific to the tumor compared to total T cell imaging due to the fact that IFN-γ is secreted by CTLs within the tumor. Imaging CD3, on the other hand, targets the general T cell population that are not only localized in the tumor, but also in other lymphoid tissues. We also find antibody-based tracers to immune cell surface molecules may create artifacts in the experimental system. Efforts to label CD8-specific full-length mAbs (clones 2.43 and non-depleting YTS-105.18) resulted in depletion of the target cell population and tracer accumulation in the kidneys with lack of secondary lymphoid tissue detection (data not shown) despite detection of CD8+ tumor infiltrates by flow cytometry and IHC (Fig. 6E-F). Anti-CD3 mAb clone 2C11 is routinely utilized for its pan-T cell receptor agonist activity, which may also potentially alter T cell function in vivo. Alternatively, it is conceivable that tracers to surface receptors may antagonize signaling, which could create off-target effects. Careful selection of antibody clones or construction of antibody fragment-based tracers like the CD8 diabody generated by Tavare et al.(39) or the VHH probe by Rashidian et al.(9) may alleviate some of these factors, but thorough quality control is necessary. Tracers targeting soluble cell products may also circumvent many of these problems.
A caveat to the detection of cytokines by PET imaging is the soluble nature of these proteins. We find localization of 89Zr-anti-IFN-γ in the spleen after CpG-ODN treatment and in the tumor after HER2/neu vaccination, suggesting the tracer is sequestered within the tissue. We tested the ability of TUBO tumor cells to retain IFN-γ:89Zr-anti-IFN-γ complexes in vitro and find maximal binding when TUBO cells are pre-incubated with IFN-γ, supporting the working hypothesis that localized imaging is due to detection of IFN-γ associated with its receptor.
Though our ex vivo validation showed a general trend in agreement with the PET imaging results, a direct correlation to tracer uptake is difficult to establish. These assays, similar to a biopsy, are sampling a fragment of a heterogeneous tumor, yielding opportunity for equivocal results. Further, our IHC analyses on TUBO tumors (Fig. 6E) show the tissue is non-uniform, with regions of variable CD8 T cell infiltration after ITx ranging from moderate density to a virtual absence. For these reasons, imaging tools like immunoPET are advantageous, bridging a clinical need by providing a more comprehensive view of the entire tumor microenvironment.
Our results showed 89Zr-anti-IFN-γ tracer uptake can be indicative of response to therapy in both cancer vaccination and TAA-specific mAb models. Importantly, in a tumor model recapitulating resistance to passive ITx, IFN-γ PET identified an inactive, dysfunctional immune system despite the presence of CD8+ T cell infiltrates. This discordance was likely due to elevated PD-1 expression as shown by flow cytometry, hindering CTLs from productive anti-tumor immunity. We further demonstrated that IFN-γ PET may be more sensitive for determining response to ITx when compared to peripheral immune evaluation, a point which should be evaluated further. IFN-γ PET can also serve as a universal non-invasive measurement of immune activity in situ for a variety of cancers with virtually any ITx modality, without the need for knowledge of specific antigens or cumbersome ex vivo antigen recall assays. In situ detection of IFN-γ may be a powerful tool for assessing intratumoral immune activation status, which could help distinguish between true tumor progression and pseudoprogression, where tumor volume swells as inflammatory cells infiltrate. Additionally, the utility of IFN-γ PET can potentially expand beyond cancer immune monitoring to include examination of localized inflammatory conditions such as injury, infection, or autoimmune disease. Taken together, these results support the development of IFN-γ PET tracers for clinical evaluation of tumor ITx.
Supplementary Material
Significance:
This study presents a novel approach to monitor therapeutic outcomes via interferon-gamma-targeted positron emission tomography (PET).
Acknowledgments
Financial Support: The authors gratefully acknowledge the Microscopy, Imaging and Cytometry Resources Core and the Biobanking and Correlative Sciences Core which are supported, in part, by the NIH Center grant P30 CA022453 to the Karmanos Cancer Institute at Wayne State University. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers R00 CA181492 (N.T. Viola-Villegas), R37 CA220482 (N.T. Viola-Villegas, H.M. Gibson), R01 CA76340 (W.-Z. Wei), NIH T32 CAA09531 (B.N. McKnight). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support was provided by Karmanos Cancer Institute Strategic Research Initiative Grant (H.M. Gibson, N.T. Viola-Villegas), and Wayne State University OVPR Grants Boost (H.M. Gibson, N.T. Viola-Villegas).
Footnotes
Conflict of interest: The authors declare no potential conflicts of interest.
References:
- 1.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: Harnessing the T cell response. Nat. Rev. Immunol. 2012. page 269–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kroemer G, Senovilla L, Galluzzi L, André F, Zitvogel L. Natural and therapy-induced immunosurveillance in breast cancer. Nat. Med. 2015. page 1128–38. [DOI] [PubMed] [Google Scholar]
- 3.Kohrt HE, Tumeh PC, Benson D, Bhardwaj N, Brody J, Formenti S, et al. Immunodynamics: A cancer immunotherapy trials network review of immune monitoring in immuno-oncology clinical trials. J. Immunother. Cancer. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoos A, Janetzki S, Britten CM. Advancing the field of cancer immunotherapy: MIATA consensus guidelines become available to improve data reporting and interpretation for T-cell immune monitoring. Oncoimmunology. 2012;1:1457–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan J, Hegde PS, Clynes R, Foukas PG, Harari A, Kleen TO, et al. Novel technologies and emerging biomarkers for personalized cancer immunotherapy. J. Immunother. Cancer. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen PL, Roh W, Reuben A, Cooper ZA, Spencer CN, Prieto PA, et al. Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov. 2016;6:827–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing. N Engl J Med [Internet]. 2012;366:883–92. Available from: http://www.nejm.org/doi/abs/10.1056/NEJMoa1113205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larimer BM, Wehrenberg-Klee E, Caraballo A, Mahmood U. Quantitative CD3 PET Imaging Predicts Tumor Growth Response to Anti-CTLA-4 Therapy. J Nucl Med [Internet]. 2016;57:1607–11. Available from: http://jnm.snmjournals.org/cgi/doi/10.2967/jnumed.116.173930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rashidian M, Ingram JR, Dougan M, Dongre A, Whang KA, LeGall C, et al. Predicting the response to CTLA-4 blockade by longitudinal noninvasive monitoring of CD8 T cells. J Exp Med [Internet]. 2017;214:2243–55. Available from: http://www.jem.org/lookup/doi/10.1084/jem.20161950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Natarajan A, Mayer AT, Xu L, Reeves RE, Gano J, Gambhir SS. Novel Radiotracer for ImmunoPET Imaging of PD-1 Checkpoint Expression on Tumor Infiltrating Lymphocytes. Bioconjug Chem. 2015; [DOI] [PubMed] [Google Scholar]
- 11.Truillet C, Oh HLJ, Yeo SP, Lee CY, Huynh LT, Wei J, et al. Imaging PD-L1 Expression with ImmunoPET. Bioconjug Chem. 2018;29:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole EL, Kim J, Donnelly DJ, Smith RA, Cohen D, Lafont V, et al. Radiosynthesis and preclinical PET evaluation of89Zr-nivolumab (BMS-936558) in healthy non-human primates. Bioorganic Med Chem. 2017;25:5407–14. [DOI] [PubMed] [Google Scholar]
- 13.England CG, Ehlerding EB, Hernandez R, Rekoske BT, Graves SA, Sun H, et al. Preclinical Pharmacokinetics and Biodistribution Studies of 89Zr-Labeled Pembrolizumab. J Nucl Med [Internet]. 2017;58:162–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27493273%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC5209640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kikuchi M, Clump DA, Srivastava RM, Sun L, Zeng D, Diaz-Perez JA, et al. Preclinical immunoPET/CT imaging using Zr-89-labeled anti-PD-L1 monoclonal antibody for assessing radiation-induced PD-L1 upregulation in head and neck cancer and melanoma. Oncoimmunology [Internet]. 2017. [cited 2018 Jun 12];6:e1329071 Available from: http://www.ncbi.nlm.nih.gov/pubmed/28811971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hettich M, Braun F, Bartholomä MD, Schirmbeck R, Niedermann G. High-resolution PET imaging with therapeutic antibody-based PD-1/PD-L1 checkpoint tracers. Theranostics. 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv. Immunol. 2007. [DOI] [PubMed] [Google Scholar]
- 17.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: A magic bullet? Science (80-.). 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li K, Baird M, Yang J, Jackson C, Ronchese F, Young S. Conditions for the generation of cytotoxic CD4+ Th cells that enhance CD8+ CTL-mediated tumor regression. Clin Transl Immunol. 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol. Immunother. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu X, Fu X, Plate J, Chong AS. IFN-y Induces Cell Growth Inhibition by Fas-mediated Apoptosis : Requirement of STATI Protein for Up-Regulation of Fas and FasL Expression1 Fas FasL ^. Cell. 1998 [PubMed] [Google Scholar]
- 21.Zaidi MR, Merlino G. The two faces of interferon-?? in cancer. Clin. Cancer Res. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diodoro MG, Carlo E Di, Zappacosta R, Iezzi M, Coletti A, Modesti A, et al. Salivary carcinoma in HER-2/neu transgenic male mice: An angiogenic switch is not required for tumor onset and progression. Int J Cancer. 2000 [PubMed] [Google Scholar]
- 23.Viola-Villegas NT, Sevak KK, Carlin SD, Doran MG, Evans HW, Bartlett DW, et al. Noninvasive imaging of PSMA in prostate tumors with89Zr-Labeled huJ591 engineered antibody fragments: The faster alternatives. Mol Pharm. 2014;11:3965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viola-Villegas NT, Rice SL, Carlin S, Wu X, Evans MJ, Sevak KK, et al. Applying PET to Broaden the Diagnostic Utility of the Clinically Validated CA19.9 Serum Biomarker for Oncology. J Nucl Med [Internet]. 2013;54:1876–82. Available from: http://jnm.snmjournals.org/cgi/doi/10.2967/jnumed.113.119867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rovero S, Amici A, Carlo ED, Bei R, Nanni P, Quaglino E, et al. DNA Vaccination Against Rat Her-2/Neu p185 More Effectively Inhibits Carcinogenesis Than Transplantable Carcinomas in Transgenic BALB/c Mice. J Immunol [Internet]. 2000;165:5133–42. Available from: http://www.jimmunol.org/cgi/doi/10.4049/jimmunol.165.9.5133 [DOI] [PubMed] [Google Scholar]
- 26.Jacob J, Radkevich O, Forni G, Zielinski J, Shim D, Jones RF, et al. Activity of DNA vaccines encoding self or heterologous Her-2/neu in Her-2 or neu transgenic mice. Cell Immunol. 2006 [DOI] [PubMed] [Google Scholar]
- 27.Gibson HM, Veenstra JJ, Jones R, Vaishampayan U, Sauerbrey M, Bepler G, et al. Induction of HER2 Immunity in Outbred Domestic Cats by DNA Electrovaccination. Cancer Immunol Res [Internet]. 2015. [cited 2018 Jun 12];3:777–86. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25711535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibson HM, Veenstra JJ, Jones R, Vaishampayan U, Sauerbrey M, Bepler G, et al. Induction of HER2 Immunity in Outbred Domestic Cats by DNA Electrovaccination. Cancer Immunol Res. 2015;3:777–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pandit-Taskar N, O’Donoghue JA, Durack JC, Lyashchenko SK, Cheal SM, Beylergil V, et al. A Phase I/II Study for Analytic Validation of 89Zr-J591 ImmunoPET as a Molecular Imaging Agent for Metastatic Prostate Cancer. Clin Cancer Res [Internet]. 2015;21:5277–85. Available from: http://clincancerres.aacrjournals.org/cgi/doi/10.1158/1078-0432.CCR-15-0552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linch SN, Kasiewicz MJ, McNamara MJ, Hilgart-Martiszus IF, Farhad M, Redmond WL. Combination OX40 agonism/CTLA-4 blockade with HER2 vaccination reverses T-cell anergy and promotes survival in tumor-bearing mice. Proc Natl Acad Sci. 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ambrosino E, Spadaro M, Iezzi M, Curcio C, Forni G, Musiani P, et al. Immunosurveillance of Erbb2 carcinogenesis in transgenic mice is concealed by a dominant regulatory T-cell self-tolerance. Cancer Res. 2006;66:7734–40. [DOI] [PubMed] [Google Scholar]
- 33.Whittington PJ, Piechocki MP, Heng HH, Jacob JB, Jones RF, Back JB, et al. DNA vaccination controls Her-2+ tumors that are refractory to targeted therapies. Cancer Res [Internet]. 2008. [cited 2018 Jun 12];68:7502–11. Available from: http://cancerres.aacrjournals.org/cgi/doi/10.1158/0008-5472.CAN-08-1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X, et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell [Internet]. 2010. [cited 2018 Jun 12];18:160–70. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1535610810002485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother cancer [Internet]. 2016. [cited 2018 Jun 13];4:59 Available from: http://jitc.biomedcentral.com/articles/10.1186/s40425-016-0165-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gooden MJM, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer [Internet]. 2011. [cited 2018 Jun 13];105:93–103. Available from: http://www.nature.com/articles/bjc2011189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gnjatic S, Bronte V, Brunet LR, Butler MO, Disis ML, Galon J, et al. Identifying baseline immune-related biomarkers to predict clinical outcome of immunotherapy. J. Immunother. Cancer. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larimer BM, Wehrenberg-Klee E, Dubois F, Mehta A, Kalomeris T, Flaherty K, et al. Granzyme B PET imaging as a predictive biomarker of immunotherapy response. Cancer Res. 2017;77:2318–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tavaré R, Escuin-Ordinas H, Mok S, McCracken MN, Zettlitz KA, Salazar FB, et al. An effective immuno-PET imaging method to monitor CD8-dependent responses to immunotherapy. Cancer Res. 2016;76:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.