Abstract
Intestinal fibrosis is one of the major complications of Crohn’s disease (CD) for which there are no effective pharmacological therapies. Vitamin D deficiency is common in CD, though it is not known whether this is a contributing factor to fibrosis, or simply a consequence of the disease itself. In CD, fibrosis is mediated mainly by activated intestinal myofibroblasts during remodeling of extracellular matrix in response to wound healing. We investigated the effects of CARD-024 (1-alpha-hydroxyvitamin D5), a vitamin D analog with minimal hypercalcemic effects, on the pro-fibrotic response of intestinal myofibroblasts to two fibrogenic stimuli: TGFβ stimulation and culture on a physiologically stiff matrix.
TGFβ stimulated a fibrogenic phenotype in Ccd-18co colonic myofibroblasts, characterized by an increase in actin stress fibers and mature focal adhesions, and increased αSMA protein expression, while CARD-024 repressed αSMA protein expression in a dose-dependent manner. Culture of colonic myofibroblasts on physiological high stiffness substrates induced morphological changes with increased actin stress fibers and focal adhesion staining, induction of αSMA protein expression, FAK phosphorylation, induction of fibrogenic genes, and repression of COX-2 and IL-1β. CARD-024 treatment repressed the stiffness-induced morphological features including stellate cell morphology and the maturation of focal adhesions. CARD-024 repressed the stiffness-mediated induction of αSMA protein expression, FAK phosphorylation, and MLCK and ET-1 gene expression. In addition, CARD-024 partially stimulated members of the COX-2/IL-1β inflammatory pathway.
In summary, CARD-024 attenuated the pro-fibrotic response of colonic myofibroblasts to high matrix stiffness, suggesting that vitamin D analogs such as CARD-024 may ameliorate intestinal fibrosis.
Keywords: Crohn’s disease, Vitamin D, CARD-024, fibrosis, myofibroblast, matrix stiffness
Introduction
Crohn’s disease (CD) is a chronic, progressive intestinal disorder characterized by cycles of intestinal inflammation and muscosal healing. Despite the advent of powerful anti-inflammatory therapies, 70% of patients ultimately develop fibrostenotic disease for which there are no effective pharmacologic therapies (Andres and Friedman, 1999; Cosnes et al., 2002; Loftus, 2004). Patients with CD have an increased prevalence of vitamin D deficiency, but it is not clear whether vitamin D deficiency is a contributing factor to fibrosis, or merely a disease consequence (Harries et al., 1985; Siffledeen et al., 2003). In other organ systems, including kidney, liver, lung, skin, and heart, vitamin D deficiency is associated with fibrosis (Li et al., 2005; Rahman et al., 2007; Ramirez et al., 2010; Tan et al., 2006; Weishaar et al., 1990; Zhang et al., 2011). Vitamin D analogs have been shown to reduce fibrosis in cell culture and animal models of cardiac, kidney, and renal fibrosis (Li et al., 2005; Mancuso et al., 2008; Tan et al., 2006; Zhang et al., 2010).
In CD, activated subepithelial myofibroblasts are the major contributor to intestinal fibrosis (Powell et al., 1999; Tomasek et al., 2002). In colonic myofibroblasts, TGFβ induces differentiation and a pro-fibrotic phenotype, characterized by stress fiber formation (Brenmoehl et al., 2009; Simmons et al., 2002) and induction of α-smooth muscle actin protein expression. In other myofibroblast lineages, including lung, heart, and kidney, extracellular matrix (ECM) stiffness alone stimulates a pro-fibrotic phenotype (Arora et al., 1999; Liu et al., 2010; Olsen et al., 2011), however the effect of matrix stiffness upon colonic myofibroblasts is unknown.
Though calcitriol (vitamin D) and vitamin D analogs have been clinically used for several diseases, including hyperparathyroidism, toxic hypercalcemic effects have been reported, underscoring the need for a vitamin D analog with minimal hypercalcemic effects. CARD-024 (1-alpha-hydroxyvitamin D5), a non-hypercalcemic vitamin D analog was identified as a potential anti-fibrotic drug. (Mehta et al., 2003) Based on superior efficacy and reduced hypercalemic effects of CADR-024 compared to other vitamin D analogs in a cardiac fibrosis model (Simpson, manuscript in preparation), in this study, we examine the effect of a non-hypercalcemic vitamin D analog, CARD-024 on the colonic myofibroblast fibrogenic response to TGFβ stimulation and to culture on physiologically high stiffness matrix.
Materials and Methods
Human recombinant TGFβ1 was obtained from R&D Systems (Minneapolis, MN). CARD-024 (1-alpha-hydroxyvitamin D5) was provided by R. Simpson (University of Michigan, Ann Arbor, MI) and dissolved in 100% ethanol. All chemicals were purchased from Sigma (Sigma-Aldrich, Saint Louis, MO), except where noted.
Cell culture
Colonic human myofibroblast Ccd-18co cells (CRL-1459 from ATCC) were cultured in alpha-MEM (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum and sub-cultured weekly. Cells were plated at 30–40% confluence. To stimulate a fibrotic phenotype, Ccd-18co cells were serum-starved for 24hr prior to treatment with 1 ng/ml TGFβ or 1 ng/ml TGFβ and increasing doses of CARD-024 (10 – 1000 nM) for 48 hr. Given ethanol was used to dilute CARD-024, ethanol was added to the untreated and TGFβ treated cells to a final concentration of 0.1%.
For stiffness experiments, low-passage number Ccd-18co cells were seeded at 1×105 cells/ml on 6-well plates containing collagen-coated acrylamide gels corresponding to soft (4.3 kPa, 0.02% bisacrylamide) or stiff (28.1 kPa, 0.16%) matrices. Cells were allowed to attach to the matrix for 4hr and then the gels were transferred to new wells to avoid paracrine signaling from cells attached to the plastic well bottom. For the CARD-024 stiffness experiments, cells were plated as described above and serum-starved overnight prior to treatment with 1000 nM of CARD-024 diluted in 100% ethanol for 24 hr. The 1000 nM CARD-024 dose was selected from the results of the TGFβ experiments. Since ethanol was used to dilute CARD-024, ethanol was added to untreated cells to a final concentration of 0.1%. Experiments were performed on early (4 to 7) passage cells. Cultures were routinely assayed for mycoplasma contamination.
Matrix stiffness gels
Collagen-coated polyacrylamide gels corresponding to physiological stiffnesses of 2.6 kPa to 28.1 kPa were generated using varying ratios of 40% acrylamide to 2% bisacrylamide (Bio-Rad, Hercules, CA) (Aplin and Hughes, 1981; Pelham and Wang, 1998). Specifically, an aqueous solution of 0.10% ammonium persulfate (Bio-Rad), 0.15% TEMED (Bio-Rad), 40% acrylamide solution was supplemented with 0.01%, 0.02%, 0.08%, or 0.16% bisacrylamide. The acrylamide gels were polymerized on a NaOH treated, amino-silanated (3-aminopropyltriethoxysilane), and gluteraldehyde treated 25mm round glass coverslip. 10ml of each acrylamide/bisacrylamide solution was filter-sterilized and pipetted unto a chloro-silanated glass surface treated with dichlorordimethylsilane (DCDMS) and a treated coverslip was inverted onto the acrylamide/biacrylamide solution. The acrylamide matrix was allowed to polymerize for 10 minutes between the two surfaces and transferred to a 6-well tissue culture plate containing sterile PBS. As determined by a series of stability experiments (data not shown), the acrylamide gels were stable for at least 1 month. For all experiments, gels were used within a month.
Prior to seeding with colonic myofibroblasts, the acrylamide gels were collagen-coated. 0.2 mg/ml of sulfo-SANPAH (Thermo Scientific, Rockford, IL) was added to each well containing the acrylamide gel-coated cover slip and UV crosslinked at a wavelength of 254 nm in a Stratagene UV crosslinker oven (Stratagene, La Jolla, CA) at a 5-inch distance from the UV source for 10 minutes. The gels were coated with 0.2 mg/ml of rat tail collagen I (BD Biosciences, Bedford, MA) overnight at 37° C with gentle agitation. Excess collagen was removed by several washes with sterile PBS. Gels were UV sterilized for 30 minutes prior to seeding with colonic myofibroblasts. Cells were serum-starved overnight prior to treatment with CARD-024. From the results of the TGFβ treatment experiments, 1000 nM CARD-024 was selected for all stiffness matrix experiments. Control cells received ethanol at the same final concentration (0.1%) to control for the ethanol concentration in CARD-024 solution.
Microelastometer measurements
Microelastometer measurements were determined for acrylamide/bisacrylamide gels ranging from 2.6 to 28.1 kPa bisacrylamide using a microelastometer (Micro-Elastometer, Artann Laboratories, West Trenton, NJ) (Egorov et al., 2008). For the microelastomer measurements, gels were synthesized without a NaOH-treated glass coverslip. For measurements, thicker gels were used than for cell plating since previous experiments determined a larger material height (substrate thickness) was needed to generate accurate and reproducible microelastomer measurements (data not shown).
By measuring vertical displacement of the gels, the stress (force per unit area) and strain (material compression in response to force) were determined by the microelastometer. Raw stress and strain values were plotted and the slope of the stress-strain curve was determined using the region between the first and second points of inflection to calculate the Young’s Modulus (kPa), an expression of substrate stiffness.
Protein isolation and Western blotting
Immunoblotting was utilized for the detection of α-smooth muscle actin. Ccd-18Co cells were washed in ice-cold PBS containing protease inhibitors (Roche, Indianapolis, IN), then lysed in ice-cold RIPA buffer (1% Igepal CA 630, 1% sodium deoxycholate, 0.1% SDS, 0.15 M NaCl, 2 mM EDTA, 50 mM NaF) with a 1:100 dilution of protease inhibitor cocktail III (Calbiochem, La Jolla, CA) and 2 mM sodium orthovanadate. Total protein was separated by SDS polyacrylamide gel electrophoresis (Bio-Rad,) with prestained protein markers loaded for molecular mass determination (Invitrogen, Carlsbad, CA), and transferred to PVDF membranes (Amersham Biosciences, Piscataway, NJ). Membranes were blocked in 5% milk/TBST solution for one hour at room temperature or overnight at 4°C. α-smooth muscle actin was detected by incubating the membrane overnight at 4°C with mouse anti-human monoclonal antibody (Sigma, St. Louis, MO) at 1: 5000 dilution in 5% milk/TBST. pFAK was detected using a rabbit polyclonal phosphospecific antibody against Tyr-397-FAK (Invitrogen) at 1:5000. As a loading control, a mouse antibody for GAPDH (Chemicon, Temecula, CA) was used. After washing, the membranes were incubated with the appropriate secondary antibody (anti-mouse IgG+ HRP or anti-rabbit IgG+HRP, Amersham, Piscataway, NJ) for one hour at room temperature and the signal was detected by the Pierce detection system (Pierce, Rockford, IL). Autoradiographs were scanned and quantitated using ImageJ analysis software (NIH, Bethesda, MD).
Microscopy
Cells were photographed using a Leica (Leica Microsystems Inc., Buffalo Grove, IL) DMIRB inverted microscope and photographed with an Olympus DP-30 camera (Center Valley, PA). Cell length was determined from photomicrographs using ImageJ (NIH, Bethesda, MD). Cell count was determined from a minimum of 3 representative photographs of a 100x magnification field.
Immunofluorescence
Expression of activated myofibroblast markers was analyzed by confocal immunofluorescence microscopy using the Olympus FluoViewTM FV500/IX system (Olympus America, Center Valley, PA) at the University of Michigan Microscopy and Image Analysis Laboratory. Ccd-18Co cells were seeded onto collagen-coated polyacrylamide gels (0.02% bis or 0.16% bis) attached to glass coverslips. After 24 hours, gels were rinsed with PBS, then were fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) in PBS for 5 minutes, followed by permeabilization with 0.2%Triton X-100 (LabChem, Pittsburgh, PA) for 15 minutes. Gels were rinsed 3 times with PBS, then were pre-blocked with SFX signal enhancer (Invitrogen, Carlsbad, CA), and blocked with 20% goat serum (Invitrogen, Carlsbad, CA). For visualization of focal adhesions, the gels were incubated for 2 hours at room temperature with mouse anti-vinculin primary antibody (Sigma, St. Louis, MO) at 1:250 in PBS/0.1% Triton X-100. The gels were rinsed three times with PBS/0.1% Triton-X100, followed by incubation with Alexa 555-conjugated goat anti-mouse secondary antibody (Molecular Probes, Eugene, OR) for 30 minutes, at room temperature, in PBS/0.1% Triton X-100. Gels were washed 6 times, followed by incubation with phalloidin (Sigma, St. Louis, MO) at 1:100, overnight, at 4 degrees C, for visualization of actin stress fibers. Cells were co-stained with 4, 6′ diamidino-2-phenylindole (DAPI), (Molecular Probes, Eugene, OR) to visualize nuclei. The gels were mounted with ProLong Gold mounting medium (Invitrogen, Carlsbad, CA) prior to imaging by confocal immunofluorescence microscopy.
Quantitative RT-PCR
RNA from Ccd-18co cells was extracted using the RNeasy kit (Qiagen, Valencia, CA). cDNA was generated by reverse transcription of 1 μg of total RNA using the Superscript First Strand RT kit (Invitrogen, Carlsbad, CA). Quantitative real-time PCR (qPCR) was performed for MLCK, IL-1β, PTGS2, ET-1, and GAPDH with the TaqMan gene expression assays (ABI, Foster City, CA) on a Bio-Rad iCycler real-time PCR system. Cycling conditions were 95°C 10 minutes, followed by 40 cycles of 95°C 15 seconds and 62°C 60 seconds. Gene expression was normalized to GAPDH as the endogenous control, and fold-changes (RQ) relative to untreated controls were calculated using the ΔΔCt-method (Livak and Schmittgen, 2001).
Statistical Analysis
Comparisons between multiple were analyzed with ANOVA, while pairwise comparisons of two groups were performed with Student’s t test.
Results
Dose response of colonic myofibroblasts to CARD-024
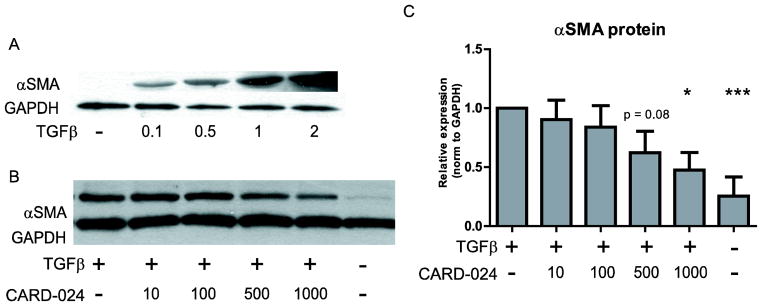
To induce a pro-fibrotic phenotype, human colonic myofibroblasts (Ccd-18co cells) were treated with increasing doses of TGFβ1 (0.1 – 2 ng/ml). Consistent with (Simmons et al., 2002), TGFβ induced a pro-fibrotic response as characterized by a dose-dependent increase in αSMA protein expression (Fig. 1A) and increased actin stress fiber and focal adhesion staining (data not shown).
Fig. 1. Effect of CARD-024 on TGFβ stimulated colonic myofibroblasts.
(A) Representative Western blot of αSMA protein expression in Ccd-18co cells stimulated with increasing doses of TGFβ (0.1 – 2 ng/ml). (B) Representative Western blot illustrating αSMA protein expression in response to treatment with TGFβ and increasing amounts of CARD-024 (10 – 1000 nM) compared to TGFβ treated or untreated cells. In (A) and (B) GAPDH expression is used as a protein loading control. (C) Quantification of αSMA protein expression as normalized to GAPDH in Ccd-18co cells treated with TGFβ and 10–1000 nM CARD-024. Results are from three independent experiments. (* p <0.05, *** p <0.001).
To determine whether CARD-024 represses fibrogenesis in colonic myofibroblasts, Ccd-18co cells were stimulated with TGFβ and treated with 10 – 1000 nM CARD-024 (Fig. 1B). In colonic myofibroblasts stimulated with TGFβ, increasing doses of CARD-024 attenuated αSMA protein expression in a dose-responsive manner. At the highest dose (1000 nM) CARD-024 attenuated αSMA protein expression 2-fold (p = 0.012) (Fig. 1C).
Development of collagen-coated polyacrylamide gels
Typical culture of lung myofibroblasts on rigid plastic substrates has profound effects on myofibroblast differentiation and activation (Hinz, 2010). In lung myofibroblasts, matrix stiffness induces changes in cell phenotype and function from a quiescent, non-proliferative phenotype to an activated, ECM-secreting phenotype (Hinz, 2010). Given that CARD-024 repressed αSMA protein expression on the rigid plastic matrix, we postulated that CARD-024 might have an anti-fibrotic effect on colonic myofibroblasts cultured on a more physiologically compliant substrate.
To determine the effect of matrix stiffness on colonic myofibroblast phenotype, collagen-coated polyacrylamide gels were generated as detailed in the Experimental Procedures. Varying the ratio of acrylamide to bisacrylamide from 0.01% to 0.16% produced substrates with stiffnesses from 2.6 to 28.1 kPa as determined by microelastometer measurements. Similar to published work in myofibroblasts from other tissues, Ccd-18co colonic myofibroblasts cultured on these matrices demonstrated a graded monotonic change in morphology with increasing matrix stiffness. On low stiffness substrates (2.6 and 4.3 kPa), cells exhibited an undifferentiated phenotype, characterized by a rounded appearance, with a few cells displaying small dendritic processes. However the dendritic processes were markedly truncated and less numerous compared to the typical myofibroblast morphology on the plastic substrate (Fig. 2). In contrast, cells cultured on the high stiffness substrates (15.6 and 28.1 kPa) exhibited a more differentiated morphology, with a characteristic stellate appearance and multiple, elongated dendritic processes resembling myofibroblasts cultured on plastic. Compared to cells cultured on the low matrix stiffness, myofibroblasts cultured on the high stiffness substrates had more actin stress fiber staining with mature focal adhesions apparent in the highest matrix stiffness (data not shown).
Fig. 2. Effect of increasing matrix stiffness on myofibroblast phenotype.
Brightfield photomicrographs of colonic myofibroblasts cultured on substrates of increasing matrix stiffness of 0.01% (2.6 kPa), 0.02% (4.3 kPa), 0.08% (15.6 kPa), and 0.16% (28.1 kPa). Myofibroblasts cultured on a standard plastic substrate are shown for comparison.
Effects of CARD-024 and substrate stiffness on myofibroblast morphology
To determine whether CARD-024 altered myofibroblast morphology, we analyzed the effect of CARD-024 treatment on colonic myofibroblasts cultured on low (4.3 kPa) and high (28.1 kPa) stiffness substrates. For the low stiffness substrate, 4.3 kPa gels were selected as the 2.6 kPa gels proved difficult to image by confocal microscopy.
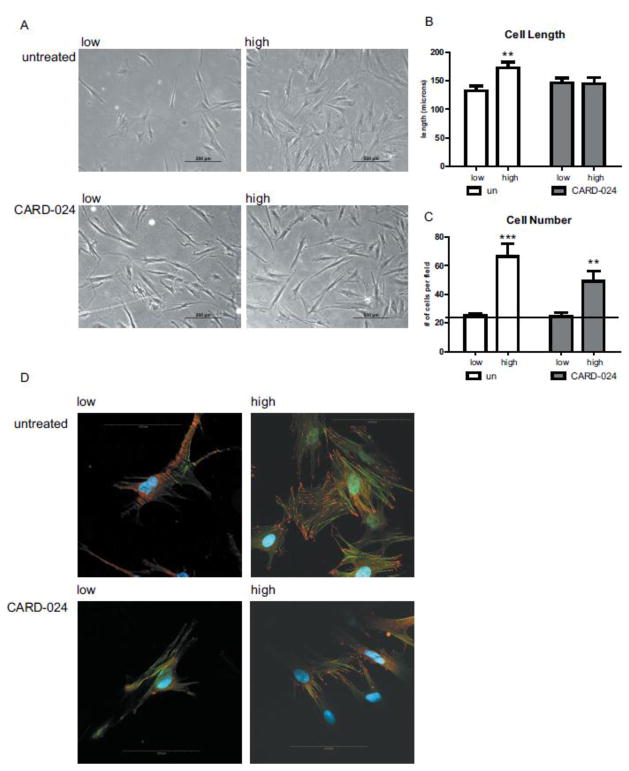
Ccd-18co cells cultured on the high stiffness (28.1 kPa) matrix developed a stellate morphology, with multiple dendritic processes compared to myofibroblasts grown on the low stiffness (4.3 kPa) matrix (Fig. 3B). Similar to human foreskin fibroblasts, which develop elongated dendritic processes in response to increase matrix stiffness(Jones and Ehrlich, 2011), colonic myofibroblasts on the stiff substrate had significantly elongated dendritic processes compared to myofibroblasts cultured on the soft substrate (173 um vs 133 um, p = 0.004)(Fig. 3C). High matrix stiffness also increased the number of cells >2.5-fold compared to the soft matrix (66.5 vs 25 cells/200x field, p = 0.0043) (Fig. 3D).
Fig. 3. Effect of CARD-024 and matrix substrate stiffness on cell morphology and growth in colonic myofibroblasts.
(A) Phase-contrast micrographs of untreated myofibroblasts (upper panel) on low and high matrix stiffness compared to myofibroblasts treated with 1000 nM CARD-024 at low and high stiffness (lower panel). 100x magnification. (B) Cell length of untreated (white bars) compared to CARD-024 treated cells (dark bars) on low or high stiffness matrices. (C) Cell density counts of untreated myofibroblasts (white bars) compared to CARD-024 (dark bars) on low or high stiffness matrices. The horizontal reference line denotes baseline (untreated) expression. Asterisks denote statistically significant comparisons between untreated/low stiffness and other experimental groups. ** p < 0.01, *** p < 0.001 (D) Confocal micrographs of myofibroblasts on low or high matrix substrates treated with 1000 nM CARD-024 compared to untreated cells using Alexa 555-conjugated vinculin (red) and phalloidin (green). Nuclei were visualized by DAPI (blue). The scale bar represents 100 microns.
Treatment with CARD-024 repressed the effects of matrix stiffness on cell morphology, with cell morphology on the high stiffness substrates in the presence of CARD-024 that was quite similar to the myofibroblasts on low stiffness substrates (Fig. 3B). Dendrite length was nearly identical (146 vs. 145 um, p = 0.95) between CARD-024 treated myofibroblasts on low compared to high stiffness matrices (Fig. 3C). While treatment with CARD-024 did not significantly decrease the number of myofibroblasts on the stiff substrate, a 50% reduction in cell number was observed compared to untreated cells on the stiff matrix, suggesting a non-significant trend toward decreased cell number with CARD-024 treatment.
Activated myofibroblasts are characterized by the development of actin stress fibers and focal adhesions (Hinz, 2010). Colonic myofibroblasts cultured on the soft substrate had fewer actin stress fibers and diffuse vinculin staining without organized focal adhesions.(Fig. 3D) In contrast, myofibroblasts cultured on the stiff matrix exhibited an activated morphology with increased numbers of actin stress fibers and mature, well-defined focal adhesions (Fig. 3D). On the stiff substrate, treatment with CARD-024 produced poorly organized immature focal adhesions with diffuse cytoplasmic staining, suggesting CARD-024 inhibited focal adhesion maturation (Fig. 3D).
Effect of CARD-024 and substrate stiffness on ECM protein and gene expression
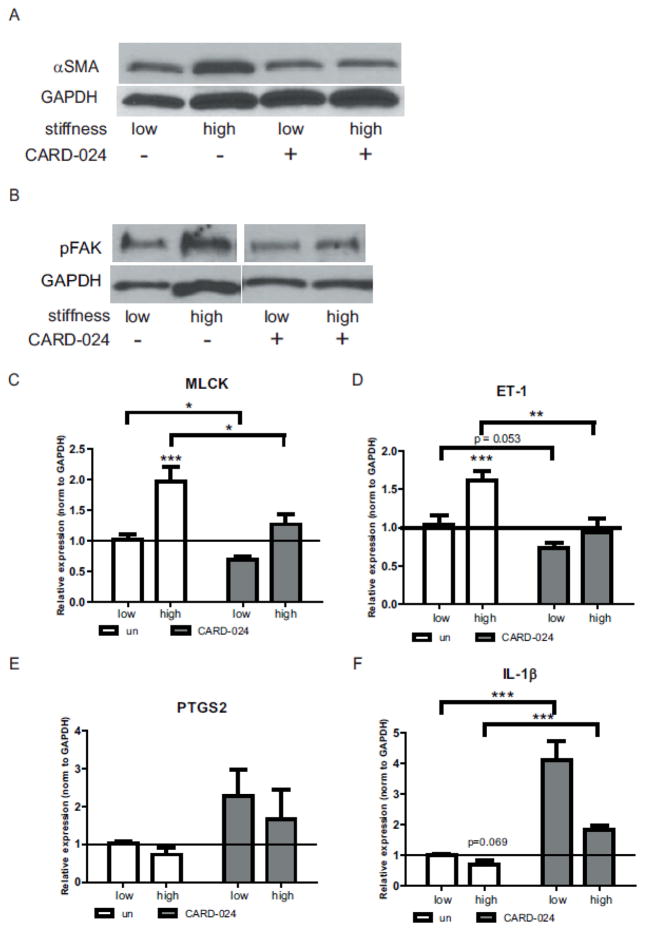
Similar to the pro-fibrotic effects of TGFβ stimulation, high matrix stiffness induced αSMA protein expression in colonic myofibroblasts (Fig. 4A). Treatment with CARD-024 repressed αSMA expression on the high stiffness substrate to levels indistinguishable from the low stiffness substrate. Myofibroblast differentiation and cytoskeletal reorganization is dependent upon a number of factors, including FAK (focal adhesion kinase) signaling (Brenmoehl et al., 2009). In colonic myofibroblasts, the high stiffness matrix induced phosphorylation of focal adhesion kinase (FAK), while CARD-024 inhibited FAK phosphorylation, suggesting CARD-024 affects pFAK signaling (Fig. 4B).
Fig. 4. Effect of CARD-024 and substrate stiffness ECM protein and gene expression.
(A) Representative Western of αSMA protein expression in untreated or CARD-024 treated colonic myofibroblasts on low or high stiffness substrates. (B) Representative Western of pFAK protein expression in untreated or CARD-024 treated colonic myofibroblasts on low or high stiffness substrates. In (A) and (B) GAPDH protein expression is used as a control for protein loading. (C–H) QRT-PCR expression of fibrogenic (MLCK (C), ET-1 (D)or COX-2 pathway (PTGS2 (E), IL-1β (F)) genes in colonic myofibroblasts cultured on low or high stiffness substrates treated with CARD-024 (dark bars) compared to untreated cells (white bars). Asterisks denote comparisons between untreated/low stiffness and other groups. The horizontal reference line denotes baseline (untreated/low stiffness) expression. Pairwise comparisons between groups are denoted with brackets. * p <0.05, ** p <0.01, *** p <0.001.
Actin stress fiber formation is regulated in part by myosin light chain kinase (MLCK) (Anderson et al., 2004). Given that CARD-024 repressed the development of actin stress fibers and mature focal adhesions, we examined the role of matrix stiffness and CARD-024 upon MLCK gene expression. MLCK was induced 2-fold (p = 0.004) in myofibroblasts on the high compared to a low stiffness substrate (Fig. 4C). Treatment with CARD-024 significantly repressed MLCK expression to levels comparable to untreated cells on the low stiffness substrate (Fig. 4C).
Similarly, the high stiffness substrate significantly induced ET-1 gene expression by 60% (p = 0.009) (Fig. 4D). Treatment with CARD-024 significantly repressed ET-1 expression in high stiffness conditions to levels indistinguishable from low stiffness conditions.
In pulmonary myofibroblasts, matrix stiffness suppresses an endogenous COX-2/PGE2 inhibitory pathway (Liu et al., 2010). While increased matrix stiffness attenuated but did not significantly repress PTGS2 (which encodes the COX-2 enzyme), treatment with CARD-024 induced PTGS2 at both low and high stiffness, suggesting CARD-024 may affect the COX-2/PGE2 pathway (Fig. 4E). In intestinal myofibroblasts, IL-1β stimulates COX-2 expression (Hinterleitner et al., 1996). Therefore we examined the effect of matrix stiffness and CARD-024 on IL-1β expression. High matrix stiffness attenuated IL-1β expression, though this did not achieve statistical significance (p = 0.069). Treatment with CARD-024 significantly induced IL-1β expression above low stiffness levels with a 4-fold induction at low stiffness (p <0.0001) and 1.8-fold induction at high stiffness (p = 0.002) (Fig. 4F).
Discussion
Crohn’s disease (CD) is characterized by cycles of intestinal inflammation (flares) and mucosal healing. While potent anti-inflammatory therapies reduce inflammation and disease symptoms, the need for anti-fibrotic therapies to prevent the inexorable development of fibrostenotic disease remains (Szabo et al., 2010). Intestinal fibrosis remains a significant serious complication of CD, often culminating in surgical intervention (Van Assche et al., 2010). In addition, patients with CD frequently have vitamin D deficiency, though whether a cause or a consequence of intestinal malabsorption remains open to debate (Harris et al., 2008; Siffledeen et al., 2003). In numerous other organ systems, vitamin D deficiency is associated with fibrosis. Treatment with vitamin D analogs reduces fibrosis both in cell culture and in animal models of fibrosis, suggesting that treatment with vitamin D analogs could reduce intestinal fibrosis (Li et al., 2005; Mancuso et al., 2008; Tan et al., 2006; Weishaar et al., 1990; Zhang et al., 2010).
In the intestine, subepithelial myofibroblasts contribute to intestinal wound healing in part by reconstituting the ECM while aberrant myofibroblast activation is postulated to produce fibrosis (Powell et al., 1999; Pucilowska et al., 2000). In CD, activated or dysregulated intestinal myofibroblasts are the major source of excessive ECM and subsequent fibrosis (Powell et al., 1999).
As seen in primary intestinal myofibroblasts (Brenmoehl et al., 2009; Simmons et al., 2002), TGFβ stimulation of human colonic myofibroblast Ccd-18co cells induced a pro-fibrotic phenotype, characterized by increased αSMA protein expression, actin stress fibers, and mature focal adhesions. Co-treatment with CARD-024, a vitamin D analog, reduced αSMA stimulation by TGFβ in a dose-dependent manner. CARD-024 treatment repressed the gene expression of endothelin-1 which is involved in myofibroblast differentiation and fibrosis (Guidry and Hook, 1991).
In the matrix stiffness model, human colonic myofibroblasts demonstrated morphological changes when cultured on collagen-coated polyacrylamide gels of increasing matrix stiffness. CARD-024 treatment attenuated morphological changes induced by high matrix substrates, including the organization of actin stress fibers, development of mature focal adhesions, and elongated dendritic processes. Actin stress fiber assembly is regulated by MLCK (Anderson et al., 2004). In colonic myofibroblasts, MLCK was transcriptionally induced by high matrix stiffness but repressed by CARD-024, suggesting that the morphological effects of CARD-024 occur through interruption of MLCK-mediated actin assembly.
Activated myofibroblasts are characterized by the expression of αSMA (Powell et al., 1999). Recent work in pulmonary, hepatic, and dermal myofibroblasts demonstrated that a stiff extracellular matrix induces a pro-fibrotic, activated phenotype characterized by increased αSMA expression (Jones and Ehrlich, 2011; Liu et al., 2010; Olsen et al., 2011). In colonic myofibroblasts, high matrix stiffness induced αSMA protein expression while treatment with CARD-024 blocked induction of αSMA protein expression.
Myofibroblast differentiation is (in part) dependent on FAK (focal adhesion kinase) signaling (Brenmoehl et al., 2009). FAK is a critical mediator of cytoskeletal responses including proliferation, migration, and adhesion. (Schaller, 2010) In addition, FAK mediates mechanosensing of the extracellular environment via an integrin signaling pathway (Assoian and Klein, 2008; Parsons, 2003). In colonic myofibroblasts, high matrix stiffness induced pFAK while CARD-024 repressed FAK activation suggesting that CARD-024 may inhibit pFAK signaling. Endothelin-1 (ET-1), a soluble fibrogenic peptide, has been shown to phosphorylate FAK in fibroblasts (Daher et al., 2008; Kennedy et al., 2008) while vitamin D antagonizes ET-1 stimulation in cardiac myocytes (Wu et al., 1996). In our stiffness model, ET-1 transcription is induced by high matrix stiffness and repressed by CARD-024, suggesting that vitamin D analogs may target the FAK/ET-1 pathway. However, CARD-024 treatment did not repress the expression of the classic fibrogenic genes col1A1 and Fn-1. Collagen expression is regulated in part by both extra- and intracellular calcium levels (Fitzgerald et al., 2006; Nakade et al., 2001). CARD-024 has minimal effects on calcium metabolism, therefore failure to repress collagen gene expression may due to the calcium-mediated regulation of collagen gene expression Vitamin D and its metabolites have been reported to repress or induce fibronectin expression, depending on the cell type (Ezzat and Asa, 2005; Ramirez et al., 2010). Fibronectin expression is necessary for cell adhesion and attenuation of fibronectin expression may reduce cellular responsiveness to vitamin D or its analogs (Ezzat and Asa, 2005).
Recently, the COX-2/PGE2 pathway has been described as mediating the pro-fibrotic response to matrix stiffness.(Liu et al., 2010). Though our work did not conclusively demonstrate that high matrix stiffness represses COX-2/PTGS2 in colonic myofibroblasts, a repressive trend was observed in high matrix stiffness while CARD-024 treatment showed a stimulatory trend in both low and high matrix stiffnesses. While the mechanism of matrix stiffness regulation of COX-2 is unknown, COX-2 expression in intestinal myofibroblasts is regulated by IL-1β (Hinterleitner et al., 1996). In colonic myofibroblasts cultured on low or high stiffness matrices, IL-1β is transcriptionally repressed by high stiffness. In macrophages, vitamin D (1,25-Dihydroxyvitamin D3 (1,25(OH)2D3), stimulates IL-1β in macrophages.(Lee et al., 2011) CARD-024 markedly induced IL-1β expression irrespective of matrix stiffness, suggesting CARD-024 may target an IL-1β/COX-2 pathway.
Intestinal fibrosis and vitamin D deficiency are two frequent complications of Crohn’s disease. The high proportion of CD patients ultimately requiring surgical intervention underscores the need for effective anti-fibrotic medical therapies. This study demonstrates that CARD-024, a vitamin D analog with minimal hypercalcemic effects, reduces the fibrogenic response of intestinal myofibroblasts. These results support monitoring and maintaining normal vitamin D levels in Crohn’s disease patients with a history of fibrostenotic disease. In the future, if prospective clinical trials demonstrate a benefit, it is possible that CARD-024 could be used to prevent recurrent strictures and surgery in patients with Crohn’s disease.
Acknowledgments
This work was support by a NIH-NIDDK grant K08DK080172 awarded to P.D.R.H and NIH-HIHLB grant HL074894 awarded to R.U.S.
Abbreviations
- CD
Crohn’s disease
- ECM
extracellular matrix
- TGFβ
transforming growth factor beta
- αSMA
alpha-smooth muscle actin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson S, DiCesare L, Tan I, Leung T, SundarRaj N. Rho-mediated assembly of stress fibers is differentially regulated in corneal fibroblasts and myofibroblasts. Exp Cell Res. 2004;298:574–83. doi: 10.1016/j.yexcr.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Andres PG, Friedman LS. Epidemiology and the natural course of inflammatory bowel disease. Gastroenterol Clin North Am. 1999;28:255–81. vii. doi: 10.1016/s0889-8553(05)70056-x. [DOI] [PubMed] [Google Scholar]
- Aplin JD, Hughes RC. Protein-derivatised glass coverslips for the study of cell-to substratum adhesion. Anal Biochem. 1981;113:144–8. doi: 10.1016/0003-2697(81)90057-9. [DOI] [PubMed] [Google Scholar]
- Arora PD, Narani N, McCulloch CA. The compliance of collagen gels regulates transforming growth factor-beta induction of alpha-smooth muscle actin in fibroblasts. Am J Pathol. 1999;154:871–82. doi: 10.1016/s0002-9440(10)65334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoian RK, Klein EA. Growth control by intracellular tension and extracellular stiffness. Trends Cell Biol. 2008;18:347–52. doi: 10.1016/j.tcb.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenmoehl J, Miller SN, Hofmann C, Vogl D, Falk W, Scholmerich J, Rogler G. Transforming growth factor-beta 1 induces intestinal myofibroblast differentiation and modulates their migration. World J Gastroenterol. 2009;15:1431–42. doi: 10.3748/wjg.15.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, Gendre JP. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis. 2002;8:244–50. doi: 10.1097/00054725-200207000-00002. [DOI] [PubMed] [Google Scholar]
- Daher Z, Noel J, Claing A. Endothelin-1 promotes migration of endothelial cells through the activation of ARF6 and the regulation of FAK activity. Cell Signal. 2008;20:2256–65. doi: 10.1016/j.cellsig.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Egorov V, Tsyuryupa S, Kanilo S, Kogit M, Sarvazyan A. Soft tissue elastometer. Med Eng Phys. 2008;30:206–12. doi: 10.1016/j.medengphy.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzat S, Asa SL. The molecular pathogenetic role of cell adhesion in endocrine neoplasia. J Clin Pathol. 2005;58:1121–5. doi: 10.1136/jcp.2004.024521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald JB, Jin M, Grodzinsky AJ. Shear and compression differentially regulate clusters of functionally related temporal transcription patterns in cartilage tissue. J Biol Chem. 2006;281:24095–103. doi: 10.1074/jbc.M510858200. [DOI] [PubMed] [Google Scholar]
- Guidry C, Hook M. Endothelins produced by endothelial cells promote collagen gel contraction by fibroblasts. J Cell Biol. 1991;115:873–80. doi: 10.1083/jcb.115.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harries AD, Brown R, Heatley RV, Williams LA, Woodhead S, Rhodes J. Vitamin D status in Crohn’s disease: association with nutrition and disease activity. Gut. 1985;26:1197–203. doi: 10.1136/gut.26.11.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PR, Wright SW, Serrano C, Riera F, Duarte I, Torres J, Pena A, Rollan A, Viviani P, Guiraldes E, Schmitz JM, Lorenz RG, Novak L, Smythies LE, Smith PD. Helicobacter pylori gastritis in children is associated with a regulatory T-cell response. Gastroenterology. 2008;134:491–9. doi: 10.1053/j.gastro.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Hinterleitner TA, Saada JI, Berschneider HM, Powell DW, Valentich JD. IL-1 stimulates intestinal myofibroblast COX gene expression and augments activation of Cl- secretion in T84 cells. Am J Physiol. 1996;271:C1262–8. doi: 10.1152/ajpcell.1996.271.4.C1262. [DOI] [PubMed] [Google Scholar]
- Hinz B. The myofibroblast: paradigm for a mechanically active cell. J Biomech. 2010;43:146–55. doi: 10.1016/j.jbiomech.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Jones C, Ehrlich HP. Fibroblast expression of alpha-smooth muscle actin, alpha2beta1 integrin and alphavbeta3 integrin: Influence of surface rigidity. Exp Mol Pathol. 2011;91:394–9. doi: 10.1016/j.yexmp.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy L, Shi-Wen X, Carter DE, Abraham DJ, Leask A. Fibroblast adhesion results in the induction of a matrix remodeling gene expression program. Matrix Biol. 2008;27:274–81. doi: 10.1016/j.matbio.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Lee BN, Kim TH, Jun JB, Yoo DH, Woo JH, Choi SJ, Lee YH, Song GG, Kim Y, Lee JY, Sohn J, Ji JD. Upregulation of interleukin-1beta production by 1,25-dihydroxyvitamin D(3) in activated human macrophages. Mol Biol Rep. 2011;38:2193–201. doi: 10.1007/s11033-010-0348-z. [DOI] [PubMed] [Google Scholar]
- Li Y, Spataro BC, Yang J, Dai C, Liu Y. 1,25-dihydroxyvitamin D inhibits renal interstitial myofibroblast activation by inducing hepatocyte growth factor expression. Kidney Int. 2005;68:1500–10. doi: 10.1111/j.1523-1755.2005.00562.x. [DOI] [PubMed] [Google Scholar]
- Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol. 2010;190:693–706. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–17. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- Mancuso P, Rahman A, Hershey SD, Dandu L, Nibbelink KA, Simpson RU. 1,25-Dihydroxyvitamin-D3 treatment reduces cardiac hypertrophy and left ventricular diameter in spontaneously hypertensive heart failure-prone (cp/+) rats independent of changes in serum leptin. J Cardiovasc Pharmacol. 2008;51:559–64. doi: 10.1097/FJC.0b013e3181761906. [DOI] [PubMed] [Google Scholar]
- Mehta RG, Hussain EA, Mehta RR, Das Gupta TK. Chemoprevention of mammary carcinogenesis by 1alpha-hydroxyvitamin D5, a synthetic analog of Vitamin D. Mutat Res. 2003;523–524:253–64. doi: 10.1016/s0027-5107(02)00341-x. [DOI] [PubMed] [Google Scholar]
- Nakade O, Takahashi K, Takuma T, Aoki T, Kaku T. Effect of extracellular calcium on the gene expression of bone morphogenetic protein-2 and -4 of normal human bone cells. J Bone Miner Metab. 2001;19:13–9. doi: 10.1007/s007740170055. [DOI] [PubMed] [Google Scholar]
- Olsen AL, Bloomer SA, Chan EP, Gaca MD, Georges PC, Sackey B, Uemura M, Janmey PA, Wells RG. Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am J Physiol Gastrointest Liver Physiol. 2011;301:G110–8. doi: 10.1152/ajpgi.00412.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–16. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- Pelham RJ, Jr, Wang YL. Cell locomotion and focal adhesions are regulated by the mechanical properties of the substrate. Biol Bull. 1998;194:348–9. doi: 10.2307/1543109. discussion 349–50. [DOI] [PubMed] [Google Scholar]
- Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol. 1999;277:C183–201. doi: 10.1152/ajpcell.1999.277.2.C183. [DOI] [PubMed] [Google Scholar]
- Pucilowska JB, Williams KL, Lund PK. Fibrogenesis. IV. Fibrosis and inflammatory bowel disease: cellular mediators and animal models. Am J Physiol Gastrointest Liver Physiol. 2000;279:G653–9. doi: 10.1152/ajpgi.2000.279.4.G653. [DOI] [PubMed] [Google Scholar]
- Rahman A, Hershey S, Ahmed S, Nibbelink K, Simpson RU. Heart extracellular matrix gene expression profile in the vitamin D receptor knockout mice. J Steroid Biochem Mol Biol. 2007;103:416–9. doi: 10.1016/j.jsbmb.2006.12.081. [DOI] [PubMed] [Google Scholar]
- Ramirez AM, Wongtrakool C, Welch T, Steinmeyer A, Zugel U, Roman J. Vitamin D inhibition of pro-fibrotic effects of transforming growth factor beta1 in lung fibroblasts and epithelial cells. J Steroid Biochem Mol Biol. 2010;118:142–50. doi: 10.1016/j.jsbmb.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD. Cellular functions of FAK kinases: insight into molecular mechanisms and novel functions. J Cell Sci. 2010;123:1007–13. doi: 10.1242/jcs.045112. [DOI] [PubMed] [Google Scholar]
- Siffledeen JS, Siminoski K, Steinhart H, Greenberg G, Fedorak RN. The frequency of vitamin D deficiency in adults with Crohn’s disease. Can J Gastroenterol. 2003;17:473–8. doi: 10.1155/2003/391308. [DOI] [PubMed] [Google Scholar]
- Simmons JG, Pucilowska JB, Keku TO, Lund PK. IGF-I and TGF-beta1 have distinct effects on phenotype and proliferation of intestinal fibroblasts. Am J Physiol Gastrointest Liver Physiol. 2002;283:G809–18. doi: 10.1152/ajpgi.00057.2002. [DOI] [PubMed] [Google Scholar]
- Szabo H, Fiorino G, Spinelli A, Rovida S, Repici A, Malesci AC, Danese S. Review article: anti-fibrotic agents for the treatment of Crohn’s disease - lessons learnt from other diseases. Aliment Pharmacol Ther. 2010;31:189–201. doi: 10.1111/j.1365-2036.2009.04171.x. [DOI] [PubMed] [Google Scholar]
- Tan X, Li Y, Liu Y. Paricalcitol attenuates renal interstitial fibrosis in obstructive nephropathy. J Am Soc Nephrol. 2006;17:3382–93. doi: 10.1681/ASN.2006050520. [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- Van Assche G, Vermeire S, Rutgeerts P. The potential for disease modification in Crohn’s disease. Nat Rev Gastroenterol Hepatol. 2010;7:79–85. doi: 10.1038/nrgastro.2009.220. [DOI] [PubMed] [Google Scholar]
- Weishaar RE, Kim SN, Saunders DE, Simpson RU. Involvement of vitamin D3 with cardiovascular function. III. Effects on physical and morphological properties. Am J Physiol. 1990;258:E134–42. doi: 10.1152/ajpendo.1990.258.1.E134. [DOI] [PubMed] [Google Scholar]
- Wu J, Garami M, Cheng T, Gardner DG. 1,25(OH)2 vitamin D3, and retinoic acid antagonize endothelin-stimulated hypertrophy of neonatal rat cardiac myocytes. J Clin Invest. 1996;97:1577–88. doi: 10.1172/JCI118582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GY, Cheng T, Luan Q, Liao T, Nie CL, Zheng X, Xie XG, Gao WY. Vitamin D: a novel therapeutic approach for keloid, an in vitro analysis. Br J Dermatol. 2011;164:729–37. doi: 10.1111/j.1365-2133.2010.10130.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kong J, Deb DK, Chang A, Li YC. Vitamin D receptor attenuates renal fibrosis by suppressing the renin-angiotensin system. J Am Soc Nephrol. 2010;21:966–73. doi: 10.1681/ASN.2009080872. [DOI] [PMC free article] [PubMed] [Google Scholar]