Abstract
Aim:
To investigate, in participants with pain-related temporomandibular disorders (TMD), the association of long-term TMD pain intensity with baseline Health-Related Quality of Life (HRQoL) and jaw functional limitation.
Methods:
Of 513 cases with baseline pain-related TMD (masticatory muscle and/or temporomandibular joint pain), 273 were reevaluated after 8 years and 258 of these had follow-up Characteristic Pain Intensity (CPI) and complete baseline data for Jaw Functional Limitation Scale (JFLS) and HRQoL measured by Short Form-12’s Physical Component Summary (PCS) and Mental Component Summary (MCS). Secondary analyses of existing data quantified effects of primary (PCS, MCS) and secondary (JFLS) predictors on follow-up CPI using multivariable linear regression. Sensitivity analyses considered differences between participants included (n=258) and not included (n=255) using inverse probability weighting. Interactions of baseline predictors with age, sex and baseline CPI were evaluated using multivariable linear regression.
Results:
Baseline PCS, but not MCS or JFLS, was associated with follow-up CPI (p=.012). One standard deviation (SD=9.0) higher baseline PCS predicted an overall 3.2-point lower follow-up CPI (95%CI: −5.8, −0.7), adjusting for age, sex, MCS, JFLS, and baseline CPI. However, the effect of PCS wasn’t uniform: the association between PCS and follow-up CPI was statistically significant for participants with baseline CPI≥51.3/100, and clinically significant for participants with baseline CPI≥68.7/100. Adjustment for TMD treatments and sensitivity analyses had negligible effect.
Conclusions:
In participants with moderate-to-severe baseline TMD pain intensity, higher baseline physical HRQoL predicted lower TMD pain intensity at 8 years’ follow-up. PCS could contribute to a multifactorial long-term TMD pain prediction model.
Keywords: Temporomandibular Disorders, Facial Pain, Chronic Pain, Quality of Life, Epidemiology
Introduction
Temporomandibular disorders (TMD) affect the masticatory muscles, temporomandibular joints (TMJ), and associated structures1 Pain, the most frequent reason patients seek TMD treatment, affects approximately 10% of adults2 It been reported that most TMD cases tend to remit or present as recurrent pain episodes. Approximately 15% of patients who seek care progress to chronic TMD pain3, often defined as having pain for at least 34 to 6 months.5 Causal factors for persistent TMD pain are not clear, though psychosocial factors differ significantly in TMD cases compared to pain-free controls6–9 and are associated with chronic TMD pain.10–12 Previous studies suggest that biopsychosocial factors including poor general health can predict onset of chronic pain conditions such as widespread pain13 and musculoskeletal disorders14–18 including TMD.19–22
Health related quality of life (HRQoL) measures have been used to assess an individual’s functioning and disease burden for many chronic physical conditions including headaches,23 arthritis,24,25 back pain,16,24,25 and TMD.7,26,27 The 12-item Short Form Health Survey (SF-12),28,29 is a commonly used HRQoL questionnaire, derived from the 36-item version, SF-36.30 The SF-12 and SF-36 evaluate 8 health domains (physical functioning, role physical, bodily pain, general health, mental health, role emotional, social functioning, and vitality), yielding 2 summary scores: the Physical Component Summary (PCS) and the Mental Component Summary (MCS). SF-12 and SF-36 scores have been shown to differ significantly between healthy controls and patients with migraine,31,32 fibromyalgia,32 and TMD.26 The SF-12 score has been found to have a dose-response relationship with severity of various chronic health conditions.33 In a multivariable model, the SF-12 bodily pain and general health sub-scales were among the 11 best predictors for new-onset TMD out of 202 putative risk factors evaluated in a large cohort.34 To date, no studies have evaluated the capacity of the SF-12 scores to predict long-term pain intensity in TMD participants.
According to the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT),35 there are 2 broad areas of assessment for physical functioning and HRQoL: generic measures such as the SF-12, and disease-specific physical functioning measures such as the Jaw Functional Limitation Scale (JFLS)36. In a large case-control study, the JFLS score was significantly worse in TMD cases compared to pain-free controls.37
Although some factors have been identified as having cross-sectional and retrospective associations with TMD pain or as longitudinal risk factors for new-onset TMD, factors that can identify existing TMD cases who are at risk for long-term TMD pain are largely unknown. The present study addresses this gap and elucidates the effects of a few potential predictors of TMD pain intensity. A unique opportunity to address this hiatus was available in conducting secondary data analyses of a well-defined cohort of TMD pain cases from the multicenter Validation Project38 and its 8-year follow-up, the TMJ Impact Project.39 The present study’s aim was to investigate, in participants with pain-related TMD, the association of long-term TMD pain intensity with baseline HRQoL and jaw functional limitation. This aim was addressed by quantifying the effect of the baseline PCS, MCS and JFLS on follow-up TMD pain intensity measured by the Characteristic Pain Intensity (CPI).5 The authors hypothesized that baseline PCS and MCS would be negatively associated with long-term TMD pain intensity while baseline JFLS would be positively associated.
Methods
Study sample
At baseline, participants in the Validation Project were recruited at the University of Minnesota, University of Washington, and University at Buffalo between 2003 and 2006. Participants were either referred from local health care providers to each university-based TMD clinic, or responded to community advertisements. Institutional review board approval was obtained at each study site before study initiation; all participants provided informed consent. Enrollment was consecutive until two-thirds of the Validation Project’s target recruitment38 was achieved and thereafter participants with less common TMD diagnoses and of older age were selectively enrolled. Thus, the Validation Project participant population was a convenience sample of individuals 18 to 70 years old, consisting of healthy controls and clinical and community TMD cases with the full spectrum of TMD signs and symptoms but without significant non-TMD pain co-morbidities.
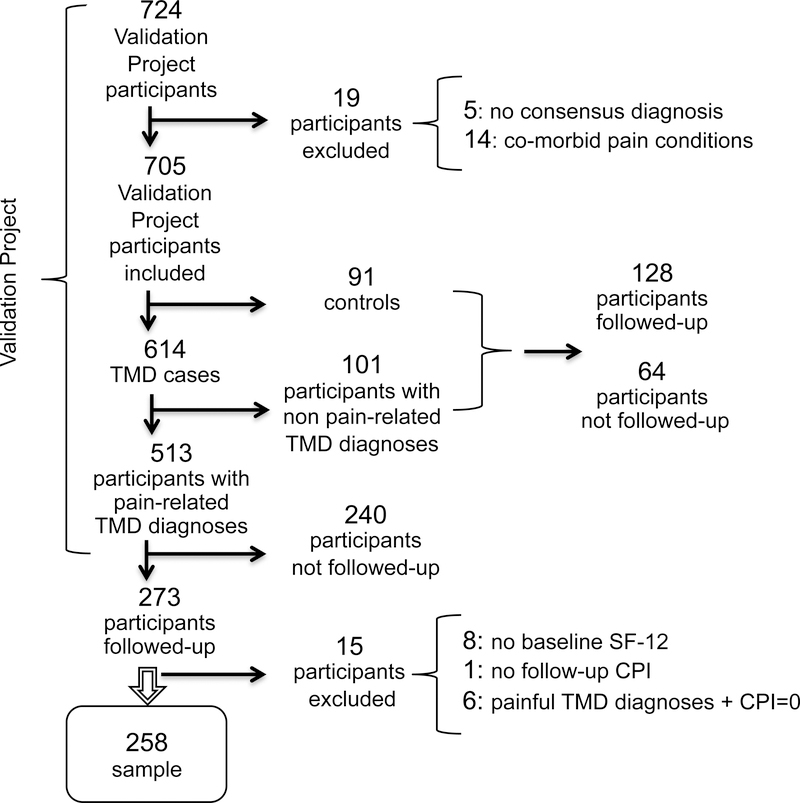
Figure 1 shows the flow of study participants from the baseline Validation Project to the follow-up TMJ Impact project and, ultimately, the subset of participants eligible for the present study’s secondary data analyses. The Validation Project included 513 potentially eligible participants who had a pain-related gold-standard TMD diagnosis (masticatory muscle and/or TMJ pain) at baseline. Approximately 8 years later, funding was approved for the TMJ Impact Project to follow-up 400 of the 705 Validation Project’s participants, the sampling frame being limited to those who had previously agreed to be contacted. Actual follow-up included 401 participants of whom 273 had pain-related TMD diagnoses at baseline and 128 had non-painful TMD diagnosis or were pain-free controls. Among the 304 Validation Project participants not followed-up in the TMJ Impact Project, 240 had pain-related TMD diagnoses and 64 had non-painful TMD diagnoses or were pain-free controls.
Figure 1:
CPI: Characteristic Pain Intensity
Inclusion in the present study required complete data for: (1) at least one baseline gold-standard pain-related TMD diagnosis (masticatory muscle and/or TMJ pain), (2) baseline CPI score greater than 0, (3) baseline SF-12 (PCS and MCS) scores, and (4) follow-up CPI score. Those with pain-related TMD diagnoses who were not included in this study (n=255) consisted of those not followed-up in the TMJ Impact Project (n=240) and those excluded from the present analysis (n=15) for the following reasons: missing data for baseline SF-12 (n=8), missing data for follow-up CPI (n=1), and those who had baseline pain-related TMD diagnoses with a concurrent CPI of 0 (n=6). Thus, the present study included 258 participants with TMD pain at baseline.
For the present study’s purpose, TMD pain at baseline was defined as the presence of at least one pain-related TMD diagnosis and a CPI score greater than 0. Long-term TMD pain was defined by its presence at both baseline and at follow-up, i.e., by evidence that conditions causing the TMD pain had remained unresolved for a longer time than would normally be expected.3,4 TMD pain between baseline and follow-up visits was not assessed; whether it was continuous or episodic was not known.
Clinical assessment and TMD diagnoses
Baseline pain-related TMD diagnoses were established by consensus between two clinical examiners at each study site after each independently assessed the participants using a comprehensive history and clinical examination protocol.38 The Validation Project’s complete methods and eligibility criteria have been previously reported.38 At follow-up, each participant was seen at the same study site by only one of the examiners. Pain-related TMD diagnoses at follow-up were algorithmically derived from history and exam data collected using the Axis I Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) diagnostic protocol.40 At all three study sites the examiners at baseline and at follow-up were the same TMD experts. Inter-rater reliability of examiners, assessed with kappa, was 0.83 for masticatory muscle pain and 0.85 for TMJ pain at baseline41 and 0.84 for masticatory muscle pain and 0.76 for TMJ pain at follow-up (unpublished data).
Outcome measure
This study’s primary outcome measure was a participant’s follow-up TMD pain intensity, measured by the CPI score from the Graded Chronic Pain Scale (GCPS).5 The CPI score ranges from 0 to 100 and is calculated from three items of self-reported TMD pain intensity: (a) pain at present time, (b) worst pain in the last 6 months and (c) average pain in the last 6 months. Each item consists of a 0–10 numerical rating scale (NRS) where 0 indicates “no pain” and 10 indicates “pain as bad as it could be”. The 3 scores are averaged and multiplied by 10 to obtain the CPI score.
Baseline predictors
Baseline questionnaires included the SF-12 version 228 and the JFLS-20 (JFLS).36,42 The SF-12 is a reliable and valid HRQoL questionnaire29,33,43 used in both clinical and research settings. The SF-12’s concurrent validity with the SF-36 has been established for PCS and MCS,29,33 and with the EuroQol EQ-5D, another HRQoL questionnaire.44 MCS and PCS summary scores range from 0 to 100 with a higher score indicating better quality of life. The JFLS consists of 20 items, each with a 0–10 NRS, where 0 indicates “no jaw limitation” and 10 “severe jaw limitation” for each specified activity. The JFLS has three subscales: mastication, vertical jaw mobility, and verbal and emotional expression; their average yields a global jaw functional limitation score on a 0–10 scale. The JFLS has been validated in TMD patients with reliability coefficients of 0.82 for persons and 0.99 for items.42
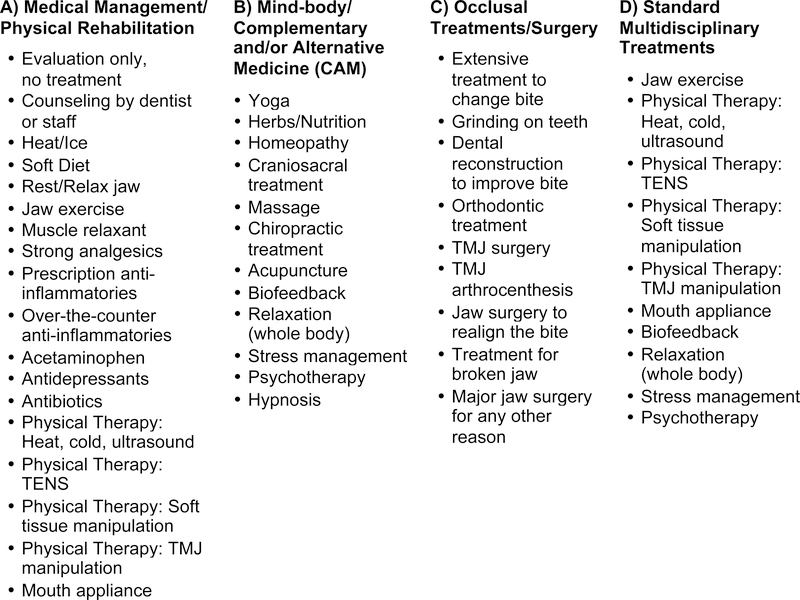
Participant self-report of TMD treatments during the follow-up period consisted of a questionnaire with 39 Yes/No items, which were clustered into three groups for analyses: A) Medical Management/Physical Rehabilitation, B) Mind-body/Complementary and Alternative Medicine, and C) Occlusal Treatments/Surgery. A fourth grouping, D) Standard Multidisciplinary Treatments, was formed from a subset of 10 of these items (Figure 2). Three definitions for TMD treatments received were considered as adjusters in the analyses: 1) any treatment (Yes/No); 2) any Group-A (Yes/No), any Group-B (Yes/No), any Group-C (Yes/No) treatments; and 3) any Group-D (Yes/No).
Figure 2:
Survey of Treatments Received - Items and Treatment Categories Used for Multivariable Analyses Adjustments
Statistical analyses
Linear regression was used to investigate baseline PCS, MCS, and JFLS scores as predictors of follow-up CPI in multivariable models adjusted for baseline characteristics: age, sex, TMD treatments received and baseline CPI. Linear regression coefficient estimates are often reported as the difference in outcome associated with a one-unit increase in a given predictor. Since the present study’s multiple study predictors were measured on different scales, the respective effects of one-unit increases were not directly comparable. Thus, for each predictor, the difference in the outcome associated with a one standard deviation (SD) increase in the predictor was computed. SD-standardization of coefficient estimates is often employed in epidemiology to facilitate direct comparison of the effect sizes of study predictors.
Additional analyses were implemented:
Since the analyzed subset of cases differed from excluded cases on average age and global JFLS score, the analyzed sample was differentially weighted to adjust for these differences using the inverse of each person’s probability of being an included case.45 These probabilities were estimated in a secondary logistic-regression analysis with the outcome “Yes” (included case) versus “No” (excluded case). Covariates for the weighting analysis were age and baseline JFLS.
Given that about one-half of TMD pain participants from the Validation Project were not included in the analysis in the interest of generalizing the present study’s findings to all 513 TMD pain cases in the Validation Project, the authors did a series of variant analyses adding to the main analysis interactions of the main effects with selected population characteristics: sex, age (continuous), or age (categorical).
To determine whether the association between baseline predictors and follow-up CPI differed according to baseline CPI, the authors also estimated and tested their interaction with baseline CPI.
Statistical significance was set at p<.05/2 = .025 for each of the two primary analyses, baseline PCS and MCS predicting follow-up CPI (i.e., Bonferroni correction). For all other tests, considered exploratory, statistical significance was defined as p<.05. All analyses were done using SAS (v. 9.3; SAS Institute, Cary, NC, USA). Graphs were plotted in R (http://www.r-project.org).
Results
Elapsed time between the baseline and follow-up examinations averaged 8.0 years (SD 0.7, range 6.3–10.0). Of the 258 participants with TMD pain at baseline who were included in the analysis, 186 (72%) were diagnosed with TMD pain at follow-up. Of these 258 participants, 88% were women, with a mean age of 38 years (SD 13, range 18–67). Table 1 presents characteristics of participants with and without follow-up pain-related TMD diagnoses. Considering all 258 participants, follow-up TMD pain intensity (CPI score) had an overall mean of 28.1 points (SD 19.9, range 0–86.7) on a 0–100 scale, an average of 22.1 points (43.8%) lower compared to baseline CPI scores (mean 50.2, SD 20.0 points). For the subset of participants with pain-related TMD diagnoses at follow-up, the mean follow-up CPI was 34.5 points (SD 18.0, range 6.7–86.7), that is, lower than baseline by an average of 15.7 points (31.3%). At follow-up some participants reported a CPI score greater than 0 for a 6-month reference time frame but were classified as not having a pain-related TMD diagnosis. This situation was clinically possible because the diagnostic criteria were based on pain in the last month; hence, Table 1 includes some participants with follow-up CPI>0 in the group without follow-up pain-related TMD diagnoses.
Table 1:
CPI: Characteristic Pain Intensity, PCS: Physical Component Summary, MCS: Mental Component Summary, JFLS: Jaw Functional Limitation Scale, SD: Standard Deviation.
| Variable | Category | Overall | Follow-up Pain-Related TMD Diagnoses | |
|---|---|---|---|---|
| Yes | No | |||
| n=258 | n=186 | n=72 | ||
| Sex n(%) | Male | 31 (12.0) | 18 (9.7) | 13 (18.1) |
| Female | 227 (88.0) | 168 (90.3) | 59 (81.9) | |
| Age(years) | Mean (SD) | 37.8 (13.0) | 38.4 (13.2) | 36.2 (12.7) |
| (Min, Max) | (18.0, 67.0) | (18.0, 67.0) | (18.0, 65.0) | |
| Baseline CPI 0–100 | Mean (SD) | 50.2 (20.0) | 51.0 (19.5) | 48.1 (21.4) |
| (Min, Max) | (6.7, 100.0) | (10.0, 93.3) | (6.7, 100.0) | |
| BaselineSF-12 PCS 0–100 | Mean (SD) | 50.6 (9.0) | 49.8 (9.5) | 52.6 (7.0) |
| (Min, Max) | (15.7, 65.4) | (24.1, 65.4) | (15.7, 64.5) | |
| Baseline SF-12MCS 0–100 | Mean (SD) | 49.6 (9.1) | 49.1 (9.21) | 50.8 (8.7) |
| (Min, Max) | (22.8, 67.3) | (22.8, 65.2) | (23.3, 67.3) | |
| BaselineTMD PainLocation n(%) | TMJ Pain only | 35 (13.6) | 26 (14.0) | 9 (12.5) |
| Muscle pain only | 3 (1.2) | 1 (0.5) | 2 (2.8) | |
| Both | 220 (85.3) | 159 (85.5) | 61 (84.7) | |
| Baseline JFLSGlobal Score 0–10 | Mean (SD) | 1.8 (1.4) | 1.9 (1.4) | 1.6 (1.5) |
| (Min, Max) | (0.0, 8.0) | (0.0, 6.5) | (0.00, 8.0) | |
| Follow-up CPI 0–100 | Mean (SD) | 28.1 (19.9) | 34.5 (18.0) | 11.6 (14.2) |
| (Min, Max) | (0.0, 86.7) | (6.7, 86.7) | (0.0, 66.7) | |
| Follow-upTMD PainLocation n(%) | None | 72 (27.9) | 0 | 72 (100.0) |
| TMJ Pain Only | 35 (13.6) | 35(18.8) | 0 | |
| Muscle Pain Only | 5 (1.9) | 5 (2.7) | 0 | |
| Both | 146 (56.6) | 146 (78.5) | 0 | |
Multivariable linear regression
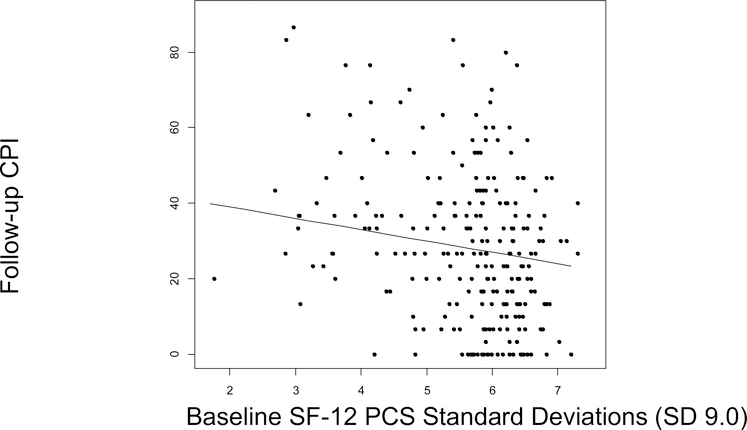
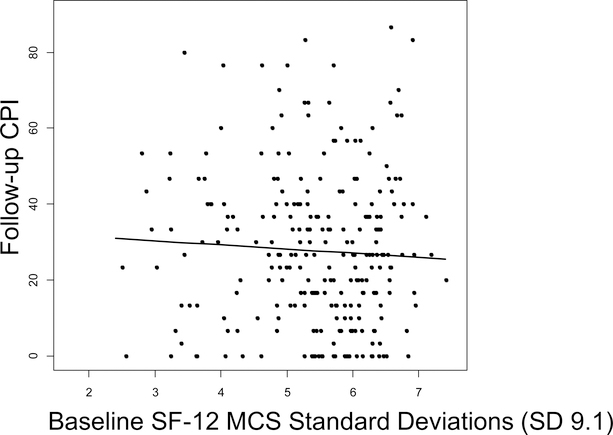
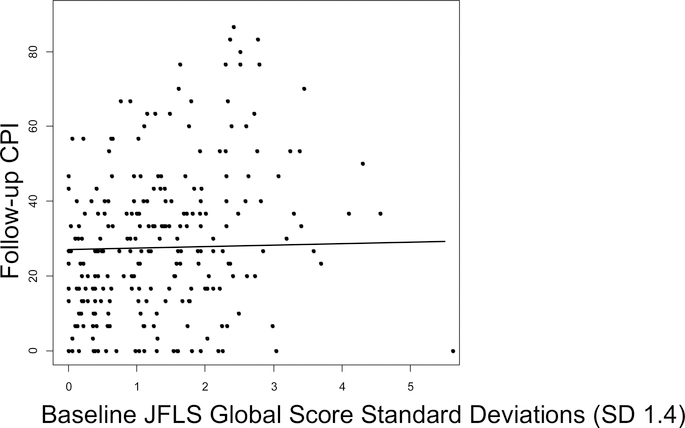
The multivariable linear regression model included all three baseline study predictors (PCS, MCS, and JFLS), as well as potential confounders: age, sex and baseline CPI (Table 2). One SD (9.0) higher baseline PCS predicted a 3.2-point (6.4%) lower follow-up CPI (95% Confidence Interval [CI] −5.8 to −0.7; p=.012), adjusting for age, sex, MCS, JFLS, and baseline CPI (Figure 3). One SD (9.1) increase in baseline MCS predicted a 1.2-point (2.4%) lower mean follow-up CPI score (95%CI −3.6 to 1.1, p=.30), adjusting for age, sex, PCS, JFLS and baseline CPI (Figure 4). One SD (1.4) higher baseline JFLS score predicted a 0.37-point (0.74%) higher mean follow-up CPI score (95%CI −2.2 to 3.0; p=.78), adjusting for age, sex, PCS, MCS, and baseline CPI (Figure 5). After adjusting further for treatments received, using each of the three ways of summarizing treatments received, each predictor maintained similar statistical significance and overall magnitude of its effect (Table 3).
Table 2:
SD-coefficient estimates represent β (regression line slope), interpreted as the difference in follow-up CPI associated with 1 SD higher baseline predictors when controlling for age and sex, the other baseline predictors in the model and baseline CPI. Please compare to Table 5 since the SF-12 PCS estimate was not uniform for all levels of baseline CPI.
CPI: Characteristic Pain Intensity, PCS: Physical Component Summary, MCS: Mental Component Summary, JFLS: Jaw Functional Limitation Scale, SD: Standard Deviation, CI: Confidence Interval.
| Baseline Predictor | SD-Coefficient Estimates | 95%CI | p-value |
|---|---|---|---|
| SF-12 PCS (SD 9.0) | −3.2 | (−5.8, −0.7) | .012 |
| SF-12 MCS (SD 9.1) | −1.2 | (−3.6, 1.1) | .30 |
| JFLS (SD 1.4) | 0.4 | (−2.2, 3.0) | .78 |
Figure 3:
1 SD (9.0) higher baseline PCS was associated with a 3.2-point lower follow-up CPI score (95%CI −5.5 to −0.6; p=.012), adjusting for age, sex, MCS, JFLS and baseline CPI. PCS: Physical Component Summary, CPI: Characteristic Pain Intensity, SD: Standard Deviation, CI: Confidence Interval.
Figure 4:
There was not a significant linear association between baseline MCS and follow-up CPI (−1.2, 95%CI −3.6 to 1.1; p=.30), adjusting for age, sex, PCS, JFLS and baseline CPI. MCS: Mental Component Summary, CPI: Characteristic Pain Intensity, SD: Standard Deviation, CI: Confidence Interval.
Figure 5:
There was not a significant linear association between baseline JFLS and follow-up CPI (−0.37, 95%CI −2.2 to 3.0; p=.78), adjusting for age, sex, PCS, MCS and baseline CPI. JFLS: Jaw Functional Limitation Scale, CPI: Characteristic Pain Intensity, SD: Standard Deviation, CI: Confidence Interval.
Table 3:
SD-coefficient estimates represent β (regression line slope), interpreted as the difference in follow-up CPI associated with 1 SD higher baseline predictors when controlling for age, sex, the other predictors in the model, baseline CPI, as well as for treatments received, with the latter defined in three different ways: 1) Any Treatment, 2) Any Group A, B or C Treatments, and 3) Any Group D Treatment. Group A: Medical Management/Physical Rehabilitation, Group B: Mind-body/Complementary and/or Alternative Medicine (CAM), Group C: Occlusal Treatments/Surgery, and Group D: Standard Multidisciplinary Treatments. CPI: Characteristic Pain Intensity, PCS: Physical Component Summary, MCS: Mental Component Summary, JFLS: Jaw Functional Limitation Scale, SD: Standard Deviation, CI: Confidence Interval.
| Baseline Predictor | Any Treatment | Any Group A, B, C | Any Group D | |||
|---|---|---|---|---|---|---|
| SD-Coefficient Estimates (95% CI) | p-value | SD-Coefficient Estimates (95%CI) | p-value | SD-Coefficient Estimates (95%CI) | p-value | |
| SF-12 PCS(SD 9.0) | −3.0 (−5.5, −0.6) | .014 | −3.6 (−6.0, −1.2) | .004 | −3.2 (−5.8, −0.7) | .012 |
| SF-12 MCS(SD 9.1) | −1.2 (−3.4, 1.1) | .31 | −0.81 (−3.0, 1.4) | .48 | −1.2 (−3.5, 1.2) | .34 |
| JFLS(SD 1.4) | 0.47 (−3.0, 2.1) | .72 | −0.31 (−2.8, 2.2) | .81 | 0.26 (−2.3, 2.9) | .84 |
Sensitivity analysis: potential bias due to partial follow-up
Compared to those cases who were not followed-up or who were excluded from the analysis, cases included in the analysis were on average 2.6 years older (included=37.8 years, 95%CI: 36.2 to 39.4; SD 13.0; not included=35.2, 95%CI: 33.6 to 36.8; SD 13.1, p=.028). Included cases had a 0.39-point lower average JFLS score at baseline (included=1.8 points, 95%CI: 1.7 to 2.0; SD 1.4; not included=2.2, 95%CI: 2.0 to 2.4; SD 1.5, p=.004). Although this baseline difference in JFLS was less than 5% of the range for JFLS (0.00 – 7.98), the statistical influence of this difference was investigated to rule out a systematic difference between cases included and not included in the analysis. A sensitivity analysis compared the unweighted linear regression estimates above with suitably weighted analyses in a series of variant regression models with and without adjusters (Table 4). Weighting created slight differences in the SD-standardized coefficient estimates for predicting follow-up CPI but the p-values for these estimates were similar. No other baseline characteristics (sex, CPI, PCS, MCS, or pain location) differed significantly between included and excluded cases.
Table 4:
SD-coefficient estimates represent β (regression line slope), interpreted as the difference in follow-up CPI associated with 1 SD higher baseline predictors. Weighted analyses were based on the inverse probability weighting method to address bias associated with loss to follow-up.
| Type of Analysis | Baseline Predictors | Unweighted SD-Coefficient Estimates (95%CI) | p-value | Weighted SD-Coefficient Estimates (95%CI) | p-value |
|---|---|---|---|---|---|
| Bivariate | SF-12 PCS (SD 9.0) | −6.0 (−8.3, −3.6) | <.001 | −6.1 (−8.5, −3.7) | <.001 |
| SF-12 MCS (SD 9.1) | −0.8 (−3.3, 1.6) | .51 | −0.5 (−3.0, 2.0) | .72 | |
| JFLS (SD 1.4) | 5.7 (3.3, 8.1) | <.001 | 5.1 (2.7, 7.4) | <.001 | |
| Adjusted for sex and age | SF-12 PCS (SD 9.0) | −4.9 (−7.3, −2.4) | <.001 | −5.2 (−7.8, −2.7) | <.001 |
| SF-12 MCS (SD 9.1) | −1.4 (−3.7. 1.0) | .26 | −1.0 (−3.4, 1.5) | .44 | |
| JFLS (SD 1.4) | 5.0 (2.6, 7.3) | <.001 | 4.5 (2.2, 6.8) | <.001 | |
| Adjusted for baseline PCS, MCS, JFLS, sex, age, baseline CPI and any treatment | SF-12 PCS (SD 9.0) | −3.0 (−5.5, −0.6) | .014 | −3.4 (−6.0, −0.9) | .009 |
| SF-12 MCS (SD 9.1) | −1.2 (−3.4, 1.1) | .31 | −1.1 (−3.4, 1.2) | .34 | |
| JFLS (SD 1.4) | 0.5 (−3.0, 2.1) | .72 | −0.7 (−3.2, 1.8) | .59 | |
Generalization of results to population subgroups
To assess the generalizability of the present study’s findings to population subgroups, the authors investigated potential interactions between baseline predictors and three principal population characteristics. Interactions of sex with the three predictors showed p-values ranging from .23 to .86. For age treated as a continuous measure, the range of p-values was .25 to .81. For the age category of 18 – 35 years, the p-value range was .11 to .48. Interactions by the category of 36 – 50 years showed p-values ranging from .29 to .91.
Differences in the association between baseline predictors and follow-up TMD pain intensity according to baseline TMD pain intensity
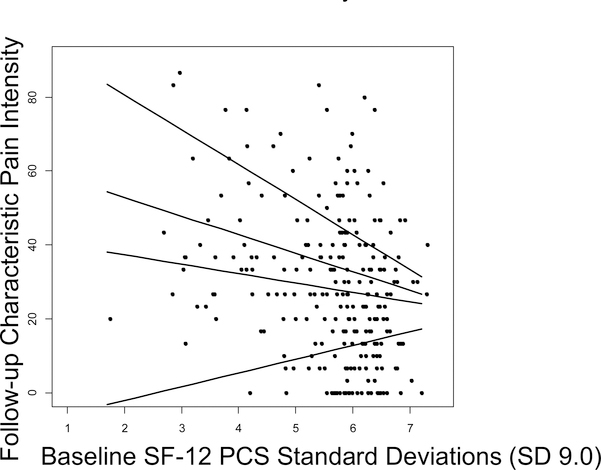
The multivariable linear regression estimate for the overall PCS effect size associated with follow-up CPI was an average 3.2-point lower follow-up CPI score for every one SD increase in baseline PCS. Although this estimate was statistically significant (p=.012), an analysis including an interaction of baseline CPI score with baseline PCS found that interaction statistically significant (p=.008), implying that the PCS effect size associated with follow-up CPI depended on baseline CPI. In particular, PCS predicted significantly larger differences in follow-up CPI in participants who had higher baseline CPI. For every SD (20.0) higher baseline CPI, the follow-up CPI predicted by one SD higher baseline PCS was 3.14 points lower (95%CI: 0.82 to 5.47). Using this analysis, Table 5 presents examples of the estimated effect of baseline PCS on follow-up CPI for four values of baseline CPI: (a) minimum observed; (b) maximum observed; (c) minimum baseline CPI for which the association between baseline PCS and follow-up CPI was statistically significant; and (d) minimum baseline CPI for which the association between baseline PCS and follow-up CPI was clinically significant (at least 10% change from mean baseline CPI, or 5.0 points; Figure 6). This table shows that the effect of PCS was statistically significant only for participants with baseline CPI>51.3, and was clinically significant only for participants with baseline CPI>68.7. Conversely, baseline PCS had no significant effect on follow-up CPI for about half of the participants, those with baseline CPI<51. The association of baseline MCS and JFLS with follow-up CPI did not depend on baseline CPI (p=.24 and p=.81 for the interactions, respectively).
Table 5:
SD-coefficient estimates represent β (regression line slope), interpreted as the difference in follow-up CPI associated with 1 SD (9.0) higher baseline PCS at different values of baseline CPI, controlling for age, sex, MCS and JFLS. CPI: Characteristic Pain Intensity, PCS: Physical Component Summary, MCS: Mental Component Summary, JFLS: Jaw Functional Limitation Scale, SD: Standard Deviation, CI: Confidence Interval.
| Baseline CPI | SD-Coefficient Estimates | 95%CI | p-value |
|---|---|---|---|
| Minimum(6.7) | 3.7 | (−2.6, 10.1) | .25 |
| Maximum(100) | −9.4 | (−15.2, −3.7) | .001 |
| StatisticallySignificance (51.3) | −2.6 | (−5.1, 0.0) | .050 |
| ClinicallySignificance(68.7) | −5.0 | (−7.9, −2.1) | .001 |
Figure 6:
Linear association between baseline PCS and follow-up CPI, adjusting for age, sex, MCS and JFLS at 4 values of baseline CPI (in ascending order of depicted lines): (a) minimum baseline CPI observed; (b) minimum baseline CPI for statistical significance of the association between baseline PCS and follow-up CPI; (c) minimum baseline CPI for clinical significance (at least 10% of mean baseline CPI, or 5.0 points) of the association between baseline PCS and follow-up CPI; and (d) maximum baseline CPI observed
Discussion
To the authors’ knowledge, this is the first study to report the effect size and clinical significance PCS, MCS, and JFLS as predictors of long-term TMD pain intensity in individuals with baseline TMD pain. The present study found that PCS had a statistically significant association with follow-up CPI, and this association was also clinically significant for participants with higher baseline TMD pain intensity. The differences in outcome predicted by MCS and JFLS were not statistically significant.
Clinical significance of differences in pain intensity
Statistically significant outcomes may not attain clinical significance. IMMPACT recommended that a decrease of 10–20% in self-reported pain intensity is minimally important, 30% or greater is moderately important, and 50% is substantially important improvement.47 Also, a difference of 2 points on a 0–10 NRS or a 30% change from baseline was considered clinically important to patients with osteoarthritis, fibromyalgia, chronic low-back pain, diabetic neuropathy, or post-herpetic neuralgia.48 In the present study, accepting the threshold of a 10–20% improvement for minimum clinical importance, PCS was a clinically significant predictor of follow-up CPI in the subset of participants reporting moderate-to-severe baseline TMD pain intensity (i.e., CPI>68.7).
After taking into consideration effect modification by baseline CPI, PCS could contribute to a multifactorial model for predicting long-term pain, along with other factors not considered in this study’s focused analyses. Such a comprehensive model could then be used to inform treatment and secondary prevention of long-term TMD pain.
Power and Sample Size
As a secondary data analysis, the present study had a fixed sample of 258 cases, determined by applying the inclusion and exclusion criteria to the existing cohort of the Validation38 and TMJ Impact projects.39 With statistically significant results for PCS, the statistical power was necessarily adequate. For JFLS and MCS, which did not have statistically significant associations with follow-up CPI, the respective confidence intervals did not include clinically significant changes (at least 10% change from mean baseline CPI, or 5.0 points).
Factors that may have reduced observed clinical significance of predictors
The long interval between baseline and follow-up data may partially account for the limited clinical significance of PCS and the absence of statistical significance of MCS and JFLS as predictors of long-term TMD pain intensity. The validated 6-month reference43,55 for the CPI questions was used because this was the period used in the original GCPS questionnaire5 and in the Validation Project.38 Also, considering the fluctuating nature of TMD pain, it is possible that pain status at follow-up may differ from other times between assessments. However, a 6-month reference period, compared to shorter intervals, is thought to provide a better estimate of pain in the long interval between baseline and follow-up.
Potential bias associated with partial follow-up
Of the 513 eligible participants with baseline TMD pain at the Validation Project, 273 participants were recalled for the TMJ Impact Project and 240 that were not followed-up. Another 15 participants were excluded due to inconsistent or missing data, yielding the sample of 258 participants. Comparing included (n=258) versus not included (n=255) participants, the statistically significant 2.6-year difference in average age was unlikely to have caused a clinically significant difference for the risk of long-term TMD pain, and the 0.39-point difference in JFLS score represented less than 5% of the baseline JFLS range of scores. Finally, the unchanged statistical significance of the study predictors in the sensitivity analysis gave the most compelling evidence that selective follow-up most likely did not introduce noteworthy bias.
Generalizability of study results
Participants in the Validation Project were enrolled according to the Standards for Reporting Diagnostic Accuracy (STARD)53 guidelines, which recommend using a sample of individuals with the target condition who are free of relevant co-morbidities when first validating diagnostic criteria. Thus, it is possible that results could have differed in samples with other medical and pain co-morbidities.
The Validation Project was designed to generalize its findings to TMD cases with the most common pain-related TMD diagnoses, TMJ soft tissue disorders (disc displacement) and TMJ hard tissue disorders (degenerative joint disease). With a large number of subjects recruited across the USA (NY, MN, WA), the generalizability of the Validation Project results has been demonstrated.41 No significant interactions (p>.1) were found between the three study predictors and three selected population characteristics, sex, age as a continuous measure, and age when the population was split into two categories. The authors conclude that these study results are generalizable to individuals having one or more pain-related TMD diagnoses, whether male or female, for ages in the range of this study.
Conclusions
In participants with moderate-to-severe baseline TMD pain intensity, higher baseline physical Health-Related Quality of Life, but not baseline mental Health-Related Quality of Life or baseline jaw functioning, was a statistically and clinically significant predictor of lower TMD pain intensity at 8-years’ follow-up. This secondary data analysis suggests PCS could contribute to a future multifactorial prediction model for long-term TMD pain for the purpose of improving clinical management and secondary prevention of ongoing TMD pain. Further research is needed to validate these findings.
Acknowledgments
Research reported in this publication was supported by the NIH/NIDCR: U01-DE013331, U01-DE019784, R90-DE023059 and the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank Dr. Donald R. Nixdorf and Dr. Estephan J. Moana-Filho for project guidance and critical review. The authors report no conflicts of interest related to this study.
Contributor Information
Flavia P. Kapos, Department of Oral Health Sciences, School of Dentistry, University of Washington; PhD Student, Department of Epidemiology, School of Public Health, University of Washington, 1959 NE Pacific St, Box 357475, Seattle, WA 98195, (206) 685-5059, kapos@uw.edu.
John O. Look, Division of TMD and Orofacial Pain, Department of Diagnostic and Biological Sciences, School of Dentistry, University of Minnesota, 515 Delaware St SE, 6-320 Moos Tower, Minneapolis, MN 55455.
Lei Zhang, Biostatistical Design and Analysis Center (BDAC), Clinical and Translational Science Institute, University of Minnesota, 717 Delaware Street SE, Second Floor, Minneapolis, MN 55414.
James S. Hodges, Division of Biostatistics, School of Public Health, University of Minnesota, 2221 University Ave SE, Suite 200, Minneapolis, MN 55414.
Eric L. Schiffman, Division of TMD and Orofacial Pain, Department of Diagnostic and Biological Sciences, School of Dentistry, University of Minnesota, 515 Delaware St SE, 6-320 Moos Tower, Minneapolis, MN 55455.
References
- 1.Okeson JP, de Kanter RJ. Temporomandibular disorders in the medical practice. J Fam Pract 1996;43:347–356. [PubMed] [Google Scholar]
- 2.LeResche L Epidemiology of temporomandibular disorders: Implications for the investigation of etiologic factors. Crit Rev Oral Biol Med 1997;8:291–305. [DOI] [PubMed] [Google Scholar]
- 3.NIDCR - National Institute of Dental and Craniofacial Research. Find Data by Topic: Facial Pain. Available at: http://www.nidcr.nih.gov/DataStatistics/FindDataByTopic/FacialPain/2014.
- 4.NIH Medline Plus. Chronic Pain: Symptoms, Diagnosis, & Treatment. Available at: https://www.nlm.nih.gov/medlineplus/magazine/issues/spring11/articles/spring11pg5-6.html. Accessed 10/21 2015.
- 5.Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain 1992;50:133–149. [DOI] [PubMed] [Google Scholar]
- 6.Quartana PJ, Buenaver LF, Edwards RR, Klick B, Haythornthwaite JA, Smith MT. Pain catastrophizing and salivary cortisol responses to laboratory pain testing in temporomandibular disorder and healthy participants. J Pain 2010;11:186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson J, Johansson M, Ryden A, Houltz E, Finizia C. The impact of trismus on healt-hrelated quality of life and mental health. Head Neck 2014. [DOI] [PubMed] [Google Scholar]
- 8.Dahlstrom L, Carlsson GE. Temporomandibular disorders and oral health-related quality of life. A systematic review. Acta Odontol Scand 2010;68:80–85. [DOI] [PubMed] [Google Scholar]
- 9.De Leeuw R, Bertoli E, Schmidt JE, Carlson CR. Prevalence of traumatic stressors in patients with temporomandibular disorders. J Oral Maxillofac Surg 2005;63:42–50. [DOI] [PubMed] [Google Scholar]
- 10.Epker J, Gatchel RJ. Coping profile differences in the biopsychosocial functioning of patients with temporomandibular disorder. Psychosom Med 2000;62:69–75. [DOI] [PubMed] [Google Scholar]
- 11.Zakrzewska JM. Multi-dimensionality of chronic pain of the oral cavity and face. J Headache Pain 2013;14:37–2377-14–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Velly AM, Look JO, Carlson C, Lenton PA, Kang W, Holcroft CA, Fricton JR. The effect of catastrophizing and depression on chronic pain--a prospective cohort study of temporomandibular muscle and joint pain disorders. Pain 2011;152:2377–2383. [DOI] [PubMed] [Google Scholar]
- 13.McBeth J, Macfarlane GJ, Benjamin S, Silman AJ. Features of somatization predict the onset of chronic widespread pain: Results of a large population-based study. Arthritis Rheum 2001;44:940–946. [DOI] [PubMed] [Google Scholar]
- 14.Lacey RJ, Lewis M, Sim J. Piecework, musculoskeletal pain and the impact of workplace psychosocial factors. Occup Med (Lond) 2007;57:430–437. [DOI] [PubMed] [Google Scholar]
- 15.Linton SJ. A review of psychological risk factors in back and neck pain. Spine (Phila Pa 1976) 2000;25:1148–1156. [DOI] [PubMed] [Google Scholar]
- 16.Melloh M, Elfering A, Egli Presland C, Roeder C, Barz T, Rolli Salathe C, Tamcan O, Mueller U, Theis JC. Identification of prognostic factors for chronicity in patients with low back pain: A review of screening instruments. Int Orthop 2009;33:301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walton DM, Carroll LJ, Kasch H, Sterling M, Verhagen AP, Macdermid JC, Gross A, Santaguida PL, Carlesso L, ICON. An overview of systematic reviews on prognostic factors in neck pain: Results from the international collaboration on neck pain (ICON) project. Open Orthop J 2013;7:494–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zakrzewska JM. Differential diagnosis of facial pain and guidelines for management. Br J Anaesth 2013;111:95–104. [DOI] [PubMed] [Google Scholar]
- 19.Sanders AE, Slade GD, Bair E, Fillingim RB, Knott C, Dubner R, Greenspan JD, Maixner W, Ohrbach R. General health status and incidence of first-onset temporomandibular disorder: The OPPERA prospective cohort study. J Pain 2013;14:T51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Diatchenko L, Dubner R, Bair E, Baraian C, Mack N, Slade GD, Maixner W. Psychological factors associated with development of TMD: The OPPERA prospective cohort study. J Pain 2013;14:T75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Dubner R, Bair E, Baraian C, Slade GD, Maixner W. Potential psychosocial risk factors for chronic TMD: Descriptive data and empirically identified domains from the OPPERA case-control study. J Pain 2011;12:T46–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aggarwal VR, Macfarlane GJ, Farragher TM, McBeth J. Risk factors for onset of chronic oro-facial pain--results of the north cheshire oro-facial pain prospective population study. Pain 2010;149:354–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lipton RB, Liberman JN, Kolodner KB, Bigal ME, Dowson A, Stewart WF. Migraine headache disability and health-related quality-of-life: A population-based case-control study from england. Cephalalgia 2003;23:441–450. [DOI] [PubMed] [Google Scholar]
- 24.Picavet HS, Hoeymans N. Health related quality of life in multiple musculoskeletal diseases: SF-36 and EQ-5D in the DMC3 study. Ann Rheum Dis 2004;63:723–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salaffi F, De Angelis R, Stancati A, Grassi W, MArche Pain, Prevalence INvestigation Group (MAPPING) study. Health-related quality of life in multiple musculoskeletal conditions: A cross-sectional population based epidemiological study. II. the MAPPING study. Clin Exp Rheumatol 2005;23:829–839. [PubMed] [Google Scholar]
- 26.Tjakkes GH, Reinders JJ, Tenvergert EM, Stegenga B. TMD pain: The effect on health related quality of life and the influence of pain duration. Health Qual Life Outcomes 2010;8:46–7525-8–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barros Vde M, Seraidarian PI, Cortes MI, de Paula LV. The impact of orofacial pain on the quality of life of patients with temporomandibular disorder. J Orofac Pain 2009;23:28–37. [PubMed] [Google Scholar]
- 28.Optum™. SF-12® Health Survey. Available at: https://www.optum.com/optumoutcomes/what-we-do/health-surveys/sf-12v2-health-survey.html.
- 29.Ware J Jr, Kosinski M, Keller SD. A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220–233. [DOI] [PubMed] [Google Scholar]
- 30.Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. conceptual framework and item selection. Med Care 1992;30:473–483. [PubMed] [Google Scholar]
- 31.Lipton RB, Hamelsky SW, Kolodner KB, Steiner TJ, Stewart WF. Migraine, quality of life, and depression: A population-based case-control study. Neurology 2000;55:629–635. [DOI] [PubMed] [Google Scholar]
- 32.Mas AJ, Carmona L, Valverde M, Ribas B, EPISER Study Group. Prevalence and impact of fibromyalgia on function and quality of life in individuals from the general population: Results from a nationwide study in spain. Clin Exp Rheumatol 2008;26:519–526. [PubMed] [Google Scholar]
- 33.Cheak-Zamora NC, Wyrwich KW, McBride TD. Reliability and validity of the SF-12v2 in the medical expenditure panel survey. Qual Life Res 2009;18:727–735. [DOI] [PubMed] [Google Scholar]
- 34.Bair E, Ohrbach R, Fillingim RB, Greenspan JD, Dubner R, Diatchenko L, Helgeson E, Knott C, Maixner W, Slade GD. Multivariable modeling of phenotypic risk factors for first-onset TMD: The OPPERA prospective cohort study. J Pain 2013;14:T102–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J, IMMPACT. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113:9–19. [DOI] [PubMed] [Google Scholar]
- 36.Ohrbach R, Larsson P, List T. The jaw functional limitation scale: Development, reliability, and validity of 8-item and 20-item versions. J Orofac Pain 2008;22:219–230. [PubMed] [Google Scholar]
- 37.Ohrbach R, Fillingim RB, Mulkey F, Gonzalez Y, Gordon S, Gremillion H, Lim PF, Ribeiro-Dasilva M, Greenspan JD, Knott C, Maixner W, Slade G. Clinical findings and pain symptoms as potential risk factors for chronic TMD: Descriptive data and empirically identified domains from the OPPERA case-control study. J Pain 2011;12:T27–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schiffman EL, Truelove EL, Ohrbach R, Anderson GC, John MT, List T, Look JO. The research diagnostic criteria for temporomandibular disorders. I: Overview and methodology for assessment of validity. J Orofac Pain 2010;24:7–24. [PMC free article] [PubMed] [Google Scholar]
- 39.Schiffman EL, Ahmad M, Hollender L, Kartha K, Ohrbach R, Truelove EL, Zhang L, Hodges JS, Sommers E, Anderson GC, Gonzalez YM, Guo X, Look JO. Longitudinal stability of common TMJ structural disorders. J Dent Res 2017;96:270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet JP, List T, Svensson P, Gonzalez Y, Lobbezoo F, Michelotti A, Brooks SL, Ceusters W, Drangsholt M, Ettlin D, Gaul C, Goldberg LJ, Haythornthwaite JA, Hollender L, Jensen R, John MT, De Laat A, de Leeuw R, Maixner W, van der Meulen M, Murray GM, Nixdorf DR, Palla S, Petersson A, Pionchon P, Smith B, Visscher CM, Zakrzewska J, Dworkin SF, International RDC/TMD Consortium Network, International association for Dental Research, Orofacial Pain Special Interest Group, International Association for the Study of Pain. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: Recommendations of the international RDC/TMD consortium network* and orofacial pain special interest groupdagger. J Oral Facial Pain Headache 2014;28:6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schiffman EL, Ohrbach R, Truelove EL, Tai F, Anderson GC, Pan W, Gonzalez YM, John MT, Sommers E, List T, Velly AM, Kang W, Look JO. The research diagnostic criteria for temporomandibular disorders. V: Methods used to establish and validate revised axis I diagnostic algorithms. J Orofac Pain 2010;24:63–78. [PMC free article] [PubMed] [Google Scholar]
- 42.Ohrbach R, Granger C, List T, Dworkin S. Preliminary development and validation of the jaw functional limitation scale. Community Dent Oral Epidemiol 2008;36:228–236. [DOI] [PubMed] [Google Scholar]
- 43.Ohrbach R, Turner JA, Sherman JJ, Mancl LA, Truelove EL, Schiffman EL, Dworkin SF. The research diagnostic criteria for temporomandibular disorders. IV: Evaluation of psychometric properties of the axis II measures. J Orofac Pain 2010;24:48–62. [PMC free article] [PubMed] [Google Scholar]
- 44.EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 45.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res 2013;22:278–295. [DOI] [PubMed] [Google Scholar]
- 46.Slade GD, Fillingim RB, Sanders AE, Bair E, Greenspan JD, Ohrbach R, Dubner R, Diatchenko L, Smith SB, Knott C, Maixner W. Summary of findings from the OPPERA prospective cohort study of incidence of first-onset temporomandibular disorder: Implications and future directions. J Pain 2013;14:T116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, Haythornthwaite JA, Jensen MP, Kerns RD, Ader DN, Brandenburg N, Burke LB, Cella D, Chandler J, Cowan P, Dimitrova R, Dionne R, Hertz S, Jadad AR, Katz NP, Kehlet H, Kramer LD, Manning DC, McCormick C, McDermott MP, McQuay HJ, Patel S, Porter L, Quessy S, Rappaport BA, Rauschkolb C, Revicki DA, Rothman M, Schmader KE, Stacey BR, Stauffer JW, von Stein T, White RE, Witter J, Zavisic S. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008;9:105–121. [DOI] [PubMed] [Google Scholar]
- 48.Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;94:149–158. [DOI] [PubMed] [Google Scholar]
- 49.Osoba D, King M. Meaningful differences In: Fayers P, Hays RD (eds). Assessing quality of life in clinical trials. New York, NY: Oxford Press, 2005:244–57. [Google Scholar]
- 50.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOM-AC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833–1840. [PubMed] [Google Scholar]
- 51.Roland M, Morris R. A study of the natural history of back pain. part I: Development of a reliable and sensitive measure of disability in low-back pain. Spine (Phila Pa 1976) 1983;8:141–144. [DOI] [PubMed] [Google Scholar]
- 52.Ohrbach R, Bair E, Fillingim RB, Gonzalez Y, Gordon SM, Lim PF, Ribeiro-Dasilva M, Diatchenko L, Dubner R, Greenspan JD, Knott C, Maixner W, Smith SB, Slade GD. Clinical orofacial characteristics associated with risk of first-onset TMD: The OPPERA prospective cohort study. J Pain 2013;14:T33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Moher D, Rennie D, de Vet HC, Lijmer JG, Standards for Reporting of Diagnostic Accuracy. The STARD statement for reporting studies of diagnostic accuracy: Explanation and elaboration. Ann Intern Med 2003;138:W1–12. [DOI] [PubMed] [Google Scholar]
- 54.Underwood MR, Barnett AG, Vickers MR. Evaluation of two time-specific back pain outcome measures. Spine (Phila Pa 1976) 1999;24:1104–1112. [DOI] [PubMed] [Google Scholar]
- 55.Von Korff M Assessment of chronic pain in epidemiological and health services research In: Turk DC, Melzack R (eds). Handbook of Pain Assessment. New York, NY: Guilford Press, 2011:455–71. [Google Scholar]