Abstract
Conidiobolomycosis is a rare fungal infection that affects adults in tropical regions. We report a 42-year-old male patient who was referred to the Sulaiman Al Habib Hospital, Dubai, United Arab Emirates (UAE), in 2013 with excessive nasal bleeding and a suspected nasal tumour. He reported having briefly visited central India nine months previously. Computed tomography and magnetic resonance imaging showed a highly vascularised mass in the nasal cavity. However, after surgical excision, initial treatment with amphotericin B deoxycholate was unsuccessful and the disease progressed, leading to external and internal nasal deformation and necessitating further excision and facial reconstruction. Histopathological analysis of the second biopsy revealed Splendore-Hoeppli changes consistent with a fungal infection. Microbiological findings subsequently confirmed Conidiobolus coronatus. Subsequently, the patient was successfully treated with a combination of itraconazole and fluconazole. To the best of the authors’ knowledge, this is the first report of a case of rhinofacial conidiobolomycosis from the UAE.
Keywords: Nasal Obstruction, Conidiobolus, Zygomycosis, Antifungal Agents, Case Report, United Arab Emirates
First identified in mammals such as horses, dolphins and chimpanzees, Conidiobolus coronatus is an opportunistic pathogen which causes rhinofacial conidiobolomycosis in humans.1,2 Initially, cases were restricted to areas with tropical and subtropical climates; however, due to climate change and modern advances in global travel, the disease can now be found on almost every continent.1 The main symptoms of C. coronatus infections are facial deformation with extensive nasal blockage and bleeding due to the presence of subcutaneous granulomatous lesions.1,3,4 The fungus usually infects healthy male farm workers between 20–60 years old.1,2
In humans, C. coronatus infections likely occur due to inhalation of the fungal spores which imbed into the nasal mucosa; subsequently, as a result of enzymatic activity, they can penetrate into the subcutaneous area of the face as well as the nasal cavity and sinuses.1 Swelling inside the nasal cavity is usually localised to the lower turbinate and nasal mucosa.1,2 More fulminant progression has been reported in immunocompromised hosts, with invasion into the blood vessels; however, in otherwise healthy patients, the infection is non-fatal and localised to the subcutaneous and mucocutaneous tissues.2,5
Case Report
A 42-year-old male patient was referred to the Sulaiman Al Habib Hospital, Dubai, United Arab Emirates (UAE), in 2013 with excessive nasal bleeding and a suspected nasal tumour. Nine months previously, the patient had visited central India for a few days; subsequently, three months after returning to the UAE, he had noticed swelling and tenderness above his nasal dorsum, with progressive nasal blockage. Six months later, he developed recurrent epistaxis, resulting in admission to another healthcare facility. However, following failed conventional management, he was referred to the Sulaiman Al Habib Hospital.
Upon physical examination, there was thickening of the right nasal vestibule as well as occlusion of the nasal cavity due to highly vascularised granulomatous tissue growth involving the right inferior turbinate, the floor of the nasal cavity and the right side of the nasal septum. No airway could be established, despite the administration of xylometazoline as a decongestant. In addition, the left nasal cavity was narrowed due to a deviated septum. The nasal mucosa was intact and the nasal dorsum was slightly tender and widened. The patient was otherwise in good health with no evidence of underlying disease.
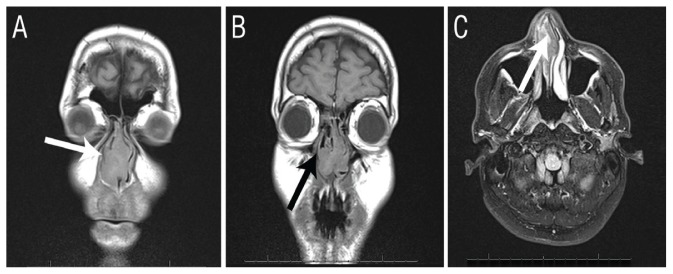
Computed tomography and magnetic resonance imaging scans of the nose and paranasal sinuses revealed a highly vascularised mass occupying the entire nasal cavity. The sinuses were intact without bony erosion or extension to the adjacent anatomical areas [Figure 1]. Under general anaesthesia, resectioning of the lower turbinate was performed and a biopsy of the mass and the involved mucosa was taken. Significant bleeding was encountered and controlled by laser vaporisation of the mucosa edges. A fresh frozen section examination of the biopsy suggested a fungal infection; however, a fungal culture did not yield any growth. The patient was therefore suspected of having aspergillosis.
Figure 1.
Imaging of a 42-year-old male patient with excessive nasal bleeding and a suspected nasal tumour. A & B: Computed tomography scans confirming the intact nasal bony wall (arrows). C: Magnetic resonance imaging showing increased T2 signals in the nasal cavity alone (arrow), without intracranial spread or extension to the sinuses.
Following extensive nasal debridement, the patient was treated with 2.5 mg/kg/day of intravenous amphotericin B deoxycholate and was monitored for all necessary blood parameters. However, after 10 days of treatment, the mass regrew rapidly. A further tissue biopsy and culture were performed, followed by a radical turbinectomy and excision of the mucosa from the floor of the nose together with the mid-portion of the cartilaginous nasal septum. The vestibular skin was also found to be affected and was removed [Figure 2]. The second biopsy was sent for histopathological and microbiological analysis.
Figure 2.

Endoscopic photograph of the nasal cavity of a 42-year-old male patient showing the spread of a highly vascularised mass to both the nasal mucosa and vestibular skin, indicating subcutaneous fat involvement.
NS = nasal mucosa; VS = vestibular skin.
A haematoxylin and eosin stain of the biopsy specimen showed eosinophilic deposits surrounded by hyphae (i.e. Splendore-Hoeppli phenomenon), while Gram staining showed broad sparsely septate hyphae. The biopsy specimen was cultured using both Sabouraud dextrose Agar (SDA) with chloramphenicol and cycloheximide and plain SDA and incubated at 30 °C and 37 °C. After five days of incubation at 30 °C, the plates with the plain SDA medium showed multiple flat, white, glabrous, slightly powdery-to-granular and radially furrowed colonies of mould with several peripheral satellite colonies [Figure 3]. As these features were highly indicative of a Conidiobolus species, the lid of the plate was secured with a thermoplastic self-sealing film to prevent contagion (Parafilm®, Bemis Co. Inc., Neenah, Wisconsin, USA). In addition, a lactophenol cotton blue stain preparation showed broad hyaline sparsely septate hyphae with a few spherical conidia with hair-like appendages (i.e. villae), also characteristic of a Zygomycetes species.
Figure 3.

Photograph of a nasal mass culture showing multiple, flat, white, glabrous, slightly powdery-to-granular and radially furrowed colonies of fungus with several peripheral satellite colonies.
The cultured fungus was sent for molecular identification and antifungal susceptibility testing at a reputable laboratory affiliated with the Postgraduate Institute of Medical Education & Research, Chandigarh, India. Molecular typing and an antifungal susceptibility profile was performed as per standard methods and the recommendations of the Clinical & Laboratory Standards Institute (CLSI).6–8 Briefly, the macrobroth was diluted according to M61 CLSI standards and a final concentration of 3.5 × 102 colony forming units/mL was used as the inoculum, before being incubated at 35 °C for 48 hours.8 Based on these findings, the fungus was identified to be C. coronatus.
As per the antifungal susceptibility profile, oral antifungal treatment was initiated with 200 mg/day of itraconazole combined with 100 mg/day of fluconazole [Table 1]. After six months of treatment, only a small granulomatous lesion remained which was subsequently removed under local anaesthesia. The nasal deformation resulting from the nostril retraction was corrected with a skin flap rotation procedure. The previously noted enlargement of the nasal dorsum reduced without the need for surgical intervention. Antifungal therapy was continued for a total of 18 months. There was no sign of disease recurrence at a three-year follow-up.
Table 1.
Antifungal susceptibility profile of a cultured fungus identified as Conidiobolus coronatus
| Antifungal agent | MIC in μg/mL |
|---|---|
| Amphotericin B deoxycholate | 2 |
| Voriconazole | >16 |
| Itraconazole | >16 |
| Posaconazole | >16* |
| Caspofungin | >64 |
| Anidulafungin | 8 |
| Micafungin | >8 |
MIC = minimal inhibitory concentration.
80% inhibition at 4 μg/mL.
Discussion
As in the current case, most published reports of rhinofacial C. coronatus infections have occurred in males with normal immune status who have recently travelled to a tropical region.5,9–11 Overall, very few cases of rhinofacial C. coronatus infections have been reported in the literature; as such, early identification of the organism remains challenging.1,3,4 To the best of the authors’ knowledge, the current case is the first report of a patient with rhinofacial conidiobolomycosis from the UAE. The diagnosis of a C. coronatus infection is based on a tissue biopsy in which eosinophilic deposits surrounding the hyphae can be seen (i.e. Splendore-Hoeppli phenomenon).1,2 However, physicians often face difficulties in obtaining a positive culture in order to demonstrate antimicrobial susceptibility; as such, the disease has a high morbidity and mortality rate.1,2,9,12
Currently, there is no standard recommended therapy for conidiobolomycosis cases. Generally, treatment ranges from conservative options to the radical resectioning of infected tissues, with approaches varying according to individual cases and local drug resistance patterns.1,4,13,14 In the current case, the initial culture was negative and histopathologically misdiagnosed as aspergillosis. Following disease progression and unsuccessful treatment with amphotericin B deoxycholate, the patient required extensive surgical debridement. At this point, a repeated culture confirmed a diagnosis of a C. coronatus infection and a detailed antifungal susceptibility profile was established. The patient was then successfully treated with prolonged combination therapy involving itraconazole and fluconazole.
Other researchers have reported similar success using potassium iodide, trimethoprim/sulfamethoxazole, amphotericin B, ketoconazole and itraconazole, either individually or in combination.1,3,15,16 Blumentrath et al. proposed a treatment strategy according to stages of disease progression; based on these classifications, the current case would be considered intermediate due to the incubation period and vestibular skin involvement and a surgical approach would not be recommended.17 However, the current patient had non-responsive bleeding and a blocked airway; as such, his quality of life immediately improved following surgical debridement.
Conclusion
Due to climate change and increasing global travel, rhinofacial C. coronatus infections are no longer restricted to tropical regions. However, successful management of such cases remains challenging due to the low incidence of the disease, difficulties in confirming the diagnosis and the lack of established treatment strategies. As highlighted by the current case, physicians should bear this fungal infection in mind when encountering nasal obstructions or a nasal tumour of uncertain aetiology. Early identification of the infection is crucial to reduce morbidity.
ACKNOWLEDGEMENTS
A preliminary version of this case report was presented at the International Meeting on Clinical Case Reports on 18–20 April 2016 in Dubai, UAE. An abstract of this presentation was subsequently published in the Journal of Clinical Case Reports in 2016, Volume 6, Issue S2, P. 40.
References
- 1.Isa-Isa R, Arenas R, Fernández RF, Isa M. Rhinofacial conidiobolomycosis (entomophthoramycosis) Clin Dermatol. 2012;30:409–12. doi: 10.1016/j.clindermatol.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 2.El-Shabrawi MH, Arnaout H, Madkour L, Kamal NM. Entomophthoromycosis: A challenging emerging disease. Mycoses. 2014;57:132–7. doi: 10.1111/myc.12248. [DOI] [PubMed] [Google Scholar]
- 3.Gupta M, Narang T, Kaur RJ, Manhas A, Saikia UN, Dogra S. A prospective case series evaluating efficacy and safety of combination of itraconazole and potassium iodide in rhinofacial conidiobolomycosis. Int J Dermatol. 2016;55:208–14. doi: 10.1111/ijd.12966. [DOI] [PubMed] [Google Scholar]
- 4.Bento DP, Tavares R, Martins Mda L, Faria N, Maduro AP, Araújo C, et al. Atypical presentation of entomophthoromycosis caused by Conidiobolus coronatus. Med Mycol. 2010;48:1099–104. doi: 10.3109/13693786.2010.497973. [DOI] [PubMed] [Google Scholar]
- 5.Youssef NM, Shaheen MA, Zu-El Fakkar N, Ghadiry G, El-Darouty M, Massoud MA, et al. A clinicopathological and mycological study of chronic rhinofacial zygomycosis (rhinoentomophthoromycosis) Egypt Dermatol Online J. 2005;1:4. [Google Scholar]
- 6.Baghela A, Thungapathra M, Shivaprakash MR, Chakrabarti A. Multilocus microsatellite typing for Rhizopus oryzae. J Med Microbiol. 2010;59:1449–55. doi: 10.1099/jmm.0.023002-0. [DOI] [PubMed] [Google Scholar]
- 7.Gil-Lamaignere C, Roilides E, Hacker J, Müller FM. Molecular typing for fungi: A critical review of the possibilities and limitations of currently and future methods. Clin Microbiol Infect. 2003;9:172–85. doi: 10.1046/j.1469-0691.2003.00649.x. [DOI] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. M61: Performance standards for antifungal susceptibility testing of filamentous fungi. 1st edition. [Accessed: Sep 2018]. From: https://clsi.org/standards/products/microbiology/documents/m61/
- 9.Gonzalez CE, Rinaldi MG, Sugar AM. Zygomycosis. Infect Dis Clin North Am. 2002;16:895–914. doi: 10.1016/S0891-5520(02)00037-5. [DOI] [PubMed] [Google Scholar]
- 10.Yang X, Li Y, Zhou X, Wang Y, Geng S, Liu H, et al. Rhinofacial conidiobolomycosis caused by Conidiobolus coronatus in a Chinese rice farmer. Mycoses. 2010;53:369–73. doi: 10.1111/j.1439-0507.2009.01716.x. [DOI] [PubMed] [Google Scholar]
- 11.Moncada DC, Montes M, Molina V, Velásquez JB, Gómez CI. Orofacial infection by Conidiobolus coronatus. Biomedica. 2016;36:15–22. doi: 10.7705/biomedica.v36i2.2806. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert EF, Khoury GH, Pore RS. Histopathological identification of Entomophthora phycomycosis: Deep mycotic infection in an infant. Arch Pathol. 1970;90:583–7. [PubMed] [Google Scholar]
- 13.Kimura M, Yaguchi T, Sutton DA, Fothergill AW, Thompson EH, Wickes BL. Disseminated human conidiobolomycosis due to Conidiobolus lamprauges. J Clin Microbiol. 2011;49:752–6. doi: 10.1128/JCM.01484-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer N, Ruef Ch, Ebnöther C, Bächli EB. Rhinofacial Conidiobolus coronatus infection presenting with nasal enlargement. Infection. 2008;36:594–6. doi: 10.1007/s15010-008-8056-5. [DOI] [PubMed] [Google Scholar]
- 15.Cherian LM, Varghese L, Panchatcharam BS, Parmar HV, Varghese GM. Nasal conidiobolomycosis: A successful treatment option for localized disease. J Postgrad Med. 2015;61:143–4. doi: 10.4103/0022-3859.153112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukhopadhyay D, Ghosh LM, Thammayya A, Sanyal M. Entomophthoromycosis caused by Conidobolus coronatus: Clinicomycological study of a case. Auris Nasus Larynx. 1995;22:139–42. doi: 10.1016/S0385-8146(12)80113-1. [DOI] [PubMed] [Google Scholar]
- 17.Blumentrath CG, Grobusch MP, Matsiégui PB, Pahlke F, Zoleko-Manego R, Nzenze-Aféne S, et al. Classification of rhinoentomophthoromycosis into atypical, early, intermediate, and late disease: A proposal. PLoS Negl Trop Dis. 2015;9:e0003984. doi: 10.1371/journal.pntd.0003984. [DOI] [PMC free article] [PubMed] [Google Scholar]