ABSTRACT
Background: Inadequate caloric intake increases the risk of sepsis-induced complications. Metabolic changes during sepsis indicate that the availability of the amino acid l-arginine decreases. Availability of arginine may further decrease during reduced caloric intake, which thereby limits the adaptive response of arginine–nitric oxide metabolism during sepsis.
Objective: We tested the hypothesis that reduced caloric intake during endotoxemia, as an experimental model for sepsis, further reduces arginine availability.
Design: In a randomized trial, a 7-d reduced caloric intake feed regimen (RE; n = 9) was compared with a normal control feed regimen (CE; n = 9), before 24 h of endotoxemia, as a model for sepsis. Whole-body arginine–nitric oxide metabolism and protein metabolism were measured by using a stable-isotope infusion of [15N2]arginine, [13C-2H2]citrulline, [2H5]phenylalanine, and [2H2]tyrosine. Plasma pyruvate and lactate concentrations were determined by fully automated HPLC.
Results: Pre-endotoxin arginine appearance was significantly lower in the RE group than in the CE group (P = 0.002). During endotoxemia, arginine appearance increased in the CE animals but not in the RE animals (P = 0.04). In addition, nitric oxide production was significantly lower in the RE animals (P < 0.0001). Protein synthesis was significantly lower at the start of endotoxin infusion (P < 0.05) and remained lower during endotoxemia in the RE group than in the CE group (P < 0.001). The lactate:pyruvate ratio was not higher in the RE group than in the CE group before endotoxemia but increased significantly during endotoxemia in the RE group (P = 0.04).
Conclusion: A well-nourished condition before prolonged endotoxemia results in a better ability to adapt to endotoxin-induced metabolic deterioration of arginine–nitric oxide metabolism than does reduced caloric intake before endotoxemia.
INTRODUCTION
Malnourished patients who become septic have an increased risk of organ failure and death compared with normally fed individuals (1, 2). However, the pathologic processes underlying this observation are relatively unknown. Malnutrition, by a reduced food intake, is thought to be a risk factor for the occurrence of infectious complications, such as sepsis, because of the lack of protein supply (3). The presence of protein-caloric underfeeding increases the odds of developing nosocomial infection and sepsis almost 2.5 times and the odds of dying from sepsis-induced complications 4-fold above that seen in patients without malnourishment (4).
Reducing food intake induces a biphasic adaptive response in protein breakdown (PB), with an initial rise in the breakdown rate, releasing amino acids that support the enhanced gluconeogenic activity (5). As malnutrition progresses, gluconeogenesis and PB are suppressed in relation to the enhanced release of free fatty acids from fat tissue. However, during catabolic conditions, such as inflammation, the muscle PB rate remains high and the liver protein synthesis rate is stimulated (6). In addition, reduced food intake that is usually present during inflammatory states will result in a reduced supply of amino acids to the liver and consequently reduced plasma amino acid concentrations are observed.
The amino acid arginine has an important role in the transport, storage, and excretion of nitrogen during inflammation (7). Moreover, arginine is pivotal in metabolic functions because it serves as a precursor of urea, nitric oxide (NO), polyamines, proline, glutamate, creatine, and agmatine. NO plays an important role in cellular functions, such as neurotransmission (8), and immune defense (9) and organ perfusion (10). In humans, under conditions of sustained stress due to sepsis, arginine may become a conditionally essential amino acid because endogenous production cannot meet the elevated requirements (11). This mechanism may explain the observed 30% reduction in plasma arginine concentrations in severely septic patients compared with critically ill patients with moderate inflammation (12). Reduced food intake during inflammation will also reduce exogenous arginine intake, which could further contribute to the reduced arginine availability during sepsis.
Although arginine plays an important metabolic role in either malnutrition or inflammatory conditions (11, 13), no data are yet available on the role of arginine during conditions of reduced caloric intake combined with severe inflammation. Our extensively described endotoxemia model in pigs was used as a model for inflammation-related metabolism (6, 14–16). Therefore, we tested the hypothesis that reduced caloric intake during endotoxemia further reduces arginine availability as compared with the reduction in arginine availability observed during endotoxemia alone. To this end we investigated whole-body arginine metabolism, using stable-isotope techniques, in a porcine model of combined reduced caloric intake and endotoxemia, as model for sepsis.
MATERIALS AND METHODS
Animals
Female crossbred pigs (Yorkshire × Dutch Landrace, 20–25 kg body weight, age 8–10 wk) were individually housed, had free access to water, and received 1 kg regular pig feed (gross energy: 16.2 MJ/kg; dry matter: 88.5%; crude protein: 16%; crude fat: 2.9%; crude fiber: 8.5%; crude ash: 6.6%; nitrogen-free extracts: 54.9%; starch: 30.1%; sugar: 7.0%; Landbouwbelang, Roermond, Netherlands) daily in the morning, supporting a growth rate of 300 g body weight/day before the start of the protocol. The Animal Ethics Committee of the Maastricht University approved the study.
Surgical procedure
Animals were starved overnight and received pre- and intraoperative care as previously described (17). Briefly, after premedication, anesthesia was induced. During intubation, the pigs received bactericidal prophylaxis. During surgery, anesthesia was maintained with a mixture of nitrous oxide and water, and halothane and fluid supplementation were provided with intravenous fluids. By using a midline laparotomy, several catheters (80-cm long; internal diameter: 1.0 mm; outside diameter: 1.8 mm; Tygon, Westvaco, Cleveland, OH) were inserted in the blood vessels as previously reported (17). In brief, catheters for blood sampling were placed in the abdominal aorta (above the right renal vein). For infusion of isotopes and endotoxin, a catheter was implanted in the caval vein above the right renal vein. A thermodilution catheter (7F; Baxter Edwards Critical Care, Irvine, CA) was inserted to monitor cardiac output. Finally, a jejunostomy was made by transecting the jejunum 20–25 cm distal to the ligament of Treitz and making an end-to-side anastomosis 10 cm distal to the transected distal end. This jejunostomy was used to introduce a tonometry catheter. All catheters were tunneled through the abdominal wall and skin and sutured to the skin.
Postoperative care
Postoperative care was standardized as previously described (17). A canvas harness was fitted to each pig to protect the catheters and to allow easy handling of the animal. During the whole recovery period (14 d), the animals remained healthy without signs of infection.
Experimental protocol
One week after surgery, pigs were randomly assigned to a regimen of inadequate caloric intake by using a 7-d period of caloric restriction (n = 9) or a 7-d normal control feed regimen (n = 9) before endotoxemia as the control group (Figure 1). In the reduced caloric intake endotoxemia (RE) group, feed intake was 25% of that in the control endotoxemia (CE) group. The normal feed intake (1 kg/d) in the CE group supported a growth of 300 g body weight (bw)/d. In both groups, feed was withdrawn 12 h before endotoxin infusion.
FIGURE 1.
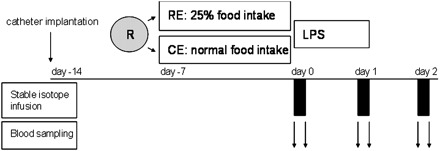
Illustration of the experimental design. After surgical catheter implantation (day −14), animals were randomized (R) on day 7 after surgery (day −7) to a reduced caloric intake (semistarvation) and endotoxin group (RE; n = 9) or to a normal control feed endotoxin (CE; n = 9) group for 7 d. Both groups subsequently received a lipopolysaccharide (LPS) endotoxin infusion over 24 h. Stable-isotope infusion and blood sampling were performed on days 0, 1, and 2 after the start of the endotoxin infusion.
The endotoxemia infusion started 14 d after surgery as previously described (17). The pigs were placed in a movable cage on the morning of the endotoxin infusion phase. After taking initial blood samples, all pigs received 3 μg · kg bw−1 · h−1 lipopolysaccharide endotoxin (LPS) from Escherichia coli (055:B5; Sigma Chemicals Co, St Louis, MO) dissolved in saline for 24 h via the inferior caval vein catheter. The endotoxin dose used makes this model a zero mortality model (6, 15). All pigs received 0.9% saline as a fluid support: 30 mL · kg bw−1· h−1 during the first 8 h and 20 mL · kg bw−1 · h−1 during the next 16 h of endotoxin infusion. During this 24-h endotoxin infusion and the next 24 h after the infusion, all pigs were kept starved. At the end of each experiment, the pigs were killed by using an intravenous dose of thiopental (Nesdonal; Rhône-Poulenc Pharma, Amstelveen, the Netherlands).
Infusion and sampling protocol
Stable-isotope studies were done at baseline to measure whole-body arginine-NO metabolism and were repeated at 24 h (at the end of the endotoxin infusion period) and at 48 h after the start of endotoxin infusion (24 h after stopping the endotoxin infusion). Before the start of the isotope infusions, background blood samples were collected. Two hours after the start of the primed-constant infusion of the isotopes through the inferior caval vein catheter, steady state conditions were obtained (18, 19) and blood was sampled. Prime and tracer infusion concentrations were based on the expected whole-body tracee appearance (20). l-[guanidino-15N2]Arginine (15N2-arginine; infusion: 6.4 μmol · kg−1 · h−1, prime: 3.2 μmol/kg), l-[ureido-13C; 5,5-2H2]citrulline (C1-D2-citrulline; infusion: 0.3 μmol · kg−1 · h−1, prime: 1.5 μmol/kg), l-[2H5]phenylalanine (D5-phenyalanine infusion: 1.9 μmol · kg−1 · h−1, prime: 1.0 μmol/kg), and l-[2H2]tyrosine (D2-tyrosine infusion: 1.9 μmol · kg−1 · h−1, prime: 1.0 μmol/kg) were purchased from Mass Trace (Woburn, MA). Blood was sampled from the catheters in the abdominal aorta before isotope infusions ceased.
Hemodynamics
Before and 4, 8, 24, and 48 h after the start of the endotoxin infusion, 3 boluses of 5 mL ice-cold saline each were administered to estimate the cardiac output by using the thermodilution technique with a cardiac output computer (9520A; Baxter Edwards Critical Care, Irvine, CA). At these time points, the mean arterial pressure (MAP) was measured in triplicate after “zeroing” the pressure transducer to the level of the heart. A tonometry catheter (14 F; Datex, Ohmeda, Finland) was inserted through the jejunostomy, with which intramucosal partial pressure carbon dioxide (pCO2) was measured by using the saline equilibration method. The pCO2 of saline was measured as for the other blood gasses (see Sample processing) and was corrected for the equilibration time. Tonometric measurements were measured 0, 4, 8, 24, and 48 h after the start of the endotoxin infusion.
Sample processing
Promptly after sampling, blood was distributed in prechilled, heparinized tubes (Sarstedt, Nümbrecht, Germany) on ice. For analysis of amino acid concentrations and stable-isotope enrichments, plasma was deproteinized by mixing 500 μL with 20 mg dry sulfosalicylic acid. All samples were frozen in liquid nitrogen and stored at −80°C until analyzed further.
Biochemical analysis
Concentrations and enrichments of amino acids were calculated as tracer-to-tracee ratios (TTRs) and were determined by a fully automated liquid chromatography–mass spectrometry system (Thermoquest LCQ, Veenendaal, Netherlands) after precolumn derivatization with o-phthaldialdehyde (21). Plasma pyruvate and lactate concentrations were determined by a fully automated HPLC (Pharmacia, Woerden, Netherlands), as described previously (22). For blood gas analysis, 0.2 mL blood was sealed airtight in heparinized 1-mL syringes and immediately analyzed on an automatic blood gas system (Acid Base Laboratory; Radiometer, Copenhagen, Denmark) and corrected for pig hemoglobin and core temperature of the animal. The core temperature of the pig was measured in the central venous blood by using the pulmonary artery floatation catheter.
Calculations
The whole-body rate of appearance (μmol · kg bw−1 · min−1) of arginine (RaARG) and citrulline (RaCIT) were derived from formula (1) by using the N2-arginine and the C1D2-citrulline isotope, respectively. The tracer infusion rate is represented by I and divided by TTRA, which is the tracer-to-tracee ratio in arterial plasma (15). TTR values were corrected for background values.
Equation 2 was used to calculate the rate of whole-body synthesis of NO (RaNO) based on the direct plasma isotope transfer from 15N2-arginine to 15N-citrulline.
where TTRA CIT1 and TTRA ARG2 represent the TTR of 15N-citrulline and 15N2-arginine in arterial plasma, respectively.
The whole-body rate of production or appearance of phenylalanine (RaPHE) and tyrosine (RaTYR) was also derived from Equation 1. The whole-body rate of production of phenylalanine was used as an indication of whole-body PB because this amino acid cannot be newly synthesized. The rate of whole-body hydroxylation of phenylalanine (RaPHE→TYR) was represented by Equation 3, in which the rate of [2H5]phenylalanine (PHE5) to [2H4]tyrosine (TYR4) is calculated and can be considered representative of net PB.
The whole-body rate of disappearance of D5-phenylalanine is a combination of the whole-body protein synthesis rate (PS) and RaPHE→TYR. PS can be calculated as indicated in Equation 4.
Statistics
The results are presented as means ± SDs. Levels of significance were set at P < 0.05. The statistical analysis was performed by using SPSS 15.0 (SPSS Inc, Chicago, IL). The data were analyzed by repeated-measures ANOVA with correction of the df by using the Greenhouse-Geisser estimates of sphericity when Mauchly’s test indicated that the assumption of sphericity had been violated, using time at 3 levels (repeated measures: t = 0, 24, and 48 h) as the within-factor and intervention (RE or CE group) as the between-factor. Where appropriate, the last observation carried forward principle and series mean were used to replace missing data. Error α values are reported in the tables and figures in which PT indicates the significance of repeated measures over time, PG the intervention group effects (± reduced caloric intake), and PT×G the interaction of repeated measures and intervention group (time × intervention). Post hoc analysis was performed to test for differences between groups at different time points.
RESULTS
Animals
Daily oral intake was intentionally reduced in the RE group to 250 ± 2 g/d during the 7 d before the endotoxin phase compared with 1000 ± 5 g/d in the control endotoxin (CE) group.
Before the phase of reduced caloric intake, weight (Figure 2) was 19.3 ± 2.2 kg bw in the CE group and 21.1 ± 2.1 kg bw in the RE group. During the semistarvation phase, weight increased significantly less in the RE group than in the CE group (1.5 ± 2.0 kg bw compared with 2.9 ± 1.0 kg bw; P < 0.05). Weights before endotoxemia were similar between groups, but at 48 h, 24 h after ending the 24-h endotoxin infusion period, body weight in the RE group was 4 kg lower than in the CE group (P = 0.04).
FIGURE 2.
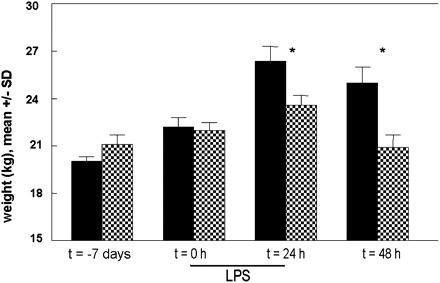
Mean (±SD) body weight during reduced caloric intake (semistarvation) and endotoxemia (RE; shaded bars) increased significantly less (P = 0.04) during the semistarvation period than during the control endotoxemia period (CE; solid bars). Both groups subsequently received a lipopolysaccharide (LPS) endotoxin infusion over 24 h. The number of animals per time group was 9 for both groups, except at 48 h in the RE group (n = 7); the last observation carried forward principle was used to replace missing data. Weight was significantly lower in the RE animals than in the CE animals 24 and 48 h after the start of the endotoxin infusion (*P < 0.05).
In total, 2 of 9 animals in the RE group (22%) and no animals in the control group died (P = 0.2). The lactate/pyruvate ratio (Table 1) was not higher in RE animals before endotoxemia, but increased significantly more in the RE group during and after the 24-h endotoxin infusion period than in the control animals (PT×G = 0.04).
TABLE 1.
Hemodynamic variables and plasma lactate and pyruvate concentrations during reduced caloric intake and endotoxemia (RE)1
| Time | P 2 | |||||||
| Variable and groups | 0 h | 4 h | 8 h | 24 h | 48 h | Group effect | Time effect | Group × time interaction |
| Temperature (°C) | 0.5 | 0.03 | 0.03 | |||||
| CE | 38.7 ± 0.5 (9)3 | 40.1 ± 1.1 (9) | 40.3 ± 0.7 (9) | 40.4 ± 1.0 (9) | 39.7 ± 1.04 (9) | |||
| RE | 38.7 ± 0.5 (9) | 40.5 ± 0.5 (9) | 40.4 ± 0.6 (9) | 39.7 ± 0.5 (7) | 39.0 ± 0.8 (7) | |||
| MAP (mm Hg) | 0.5 | 0.4 | 0.5 | |||||
| CE | 113 ± 54 (6) | 111 ± 9 (6) | 109 ± 8 (6) | 108 ± 84 (6) | 101 ± 9 (6) | |||
| RE | 104 ± 6 (6) | 101 ± 8 (6) | 105 ± 5 (6) | 96 ± 4 (6) | 106 ± 1 (6) | |||
| CI (mL · kgminus1 · minminus1) | 0.8 | <0.0001 | 0.002 | |||||
| CE | 159 ± 55 (8) | 183 ± 57 (8) | 200 ± 57 (8) | 190 ± 42 (6) | 165 ± 44 (6) | |||
| RE | 151 ± 27 (6) | 218 ± 57 (6) | 259 ± 23 (6) | 238 ± 16 (6) | 147 ± 12 (6) | |||
| pHa | 0.004 | <0.0001 | <0.0001 | |||||
| CE | 7.44 ± 0.04 (9) | 7.39 ± 0.04 (9) | 7.41 ± 0.034 (9) | 7.43 ± 0.03 (8) | 7.46 ± 0.03 (8) | |||
| RE | 7.46 ± 0.02 (9) | 7.39 ± 0.08 (9) | 7.36 ± 0.06 (9) | 7.45 ± 0.03 (6) | 7.47 ± 0.04 (6) | |||
| BE (mEq/L) | 0.4 | 0.006 | ||||||
| CE | 3.9 ± 2.3 (9) | −1.0 ± 3.6 (9) | −1.0 ± 1.44 (9) | 0.4 ± 3.8 (8) | 5.8 ± 3.0 (8) | 0.04 | ||
| RE | 4.0 ± 2.3 (9) | −1.7 ± 3.2 (9) | −4.8 ± 4.3 (9) | 2.0 ± 1.4 (6) | 6.2 ± 1.8 (6) | |||
| P mCO2 (kPa) | 0.2 | 0.02 | <0.0001 | |||||
| CE | 6.2 ± 0.6 (9) | 7.2 ± 1.3 (9) | 6.2 ± 0.24 (9) | 7.6 ± 1.4 (8) | 6.6 ± 1.64 (8) | |||
| RE | 5.8 ± 1.0 (9) | 7.3 ± 0.6 (9) | 6.9 ± 1.3 (9) | 8.3 ± 0.4 (6) | 8.8 ± 0.5 (6) | |||
| P m-aCO2 (kPa) | 0.2 | 0.002 | 0.002 | |||||
| CE | 0.6 ± 0.8 (9) | 1.7 ± 0.8 (9) | 2.1 ± 0.9 (9) | 1.3 ± 0.84 (8) | 0.9 ± 1.44 (8) | |||
| RE | 1.2 ± 1.4 (9) | 1.9 ± 0.7 (9) | 1.9 ± 0.1 (9) | 3.3 ± 0.5 (6) | 3.4 ± 0.4 (6) | |||
| Lactate (mmol/L) | 0.001 | 0.005 | 0.04 | |||||
| CE | 1.6 ± 1.0 (9) | ND | ND | 5.7 ± 6.9 (9) | 0.9 ± 0.3 (8) | |||
| RE | 1.9 ± 1.2 (9) | 5.3 ± 7.3 (6) | 0.8 ± 0.1 (6) | |||||
| Pyruvate (mmol/L) | 0.8 | 0.01 | 1.0 | |||||
| CE | 0.5 ± 0.6 (9) | ND | ND | 1.4 ± 2.5 (9) | 0.3 ± 0.5 (8) | |||
| RE | 0.2 ± 0.1 (9) | 0.3 ± 0.5 (6) | 0.1 ± 0.1 (6) | |||||
| L/P ratio | 0.2 | 0.3 | 0.2 | |||||
| CE | 9.1 ± 1.1 (9) | ND | ND | 7.5 ± 3.64 (9) | 9.8 ± 2.34 (8) | |||
| RE | 12.3 ± 1.0 (9) | 16.1 ± 1.4 (6) | 16.0 ± 1.8 (6) | |||||
ND, not determined; MAP, mean arterial pressure; CI, cardiac index; pHa, arterial pH; BE, base excess; PmCO2, intramucosal partial pressure carbon dioxide; Pm-aCO2, mucosal minus arterial PCO2; L/P, lactate/pyruvate; CE, control endotoxemia.
P for group, time, and interaction effects by repeated-measures ANOVA.
Mean ± SD; number of animals per time group in parentheses (all such values); n values <9 represent missing data (the last observation carried forward principle was used to replace missing data).
Significantly different from RE, P < 0.05.
Hemodynamics
MAP was lower before the endotoxin infusion and after the 24-h endotoxin infusion in the animals in the RE group (Table 1). The cardiac index increased significantly more in the RE group than in the CE group (PT×G = 0.002). The blood gas analysis indicated that a reduced caloric intake induced a significantly lower pH and base excess. In addition to the differences in systemic hemodynamic variables, tonometric data showed a significantly higher increase in PmCO2 (mucosal PCO2) and Pm-aCO2 (mucosal minus arterial PCO2) during endotoxemia when preceded by caloric intake reduction (Table 1).
Protein metabolism
Whole-body protein synthesis was significantly lower at the start of the endotoxin infusion and remained lower during and after endotoxemia in the RE group compared with the CE group (Figure 3A). PB (Figure 3B) was reduced in the RE group, but, because of a larger variance, was not reduced 48 h after endotoxemia. Whole-body phenylalanine hydroxylation (net PB) was significantly lower in the RE group before endotoxemia (P = 0.03) and during the total study period (PG = 0.04) (Figure 3C). Protein balance was similar between the 2 study groups, except after 48 h, during which net protein synthesis in the control group was present compared with a net PB in the RE group (Figure 3D).
FIGURE 3.
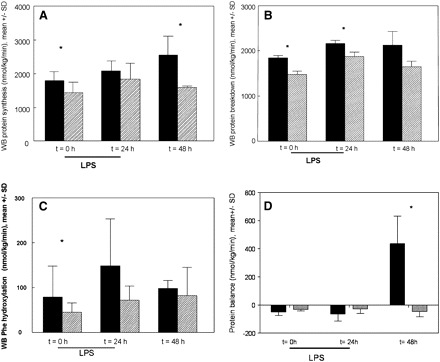
Mean (±SD) whole-body (WB) protein synthesis rate (A), WB protein breakdown (PB) rate (B), WB phenylalanine (Phe) hydroxylation rate (C), and WB protein balance (D) during reduced caloric intake (semistarvation) and endotoxemia (RE, shaded bars; n = 9) compared with control endotoxemia (CE, solid bars; n = 9). Both groups subsequently received a lipopolysaccharide (LPS) endotoxin infusion (3 μg · kg body weight−1 · h−1) over 24 h. The number of animals per time group was 9 for both groups, except at 48 h in the RE group (n = 7); the last observation carried forward principle was used to replace missing data. A: WB protein synthesis was significantly lower in the RE group than in the CE group. P for group effect < 0.0001, P for time effect = 0.02, P for interaction = 0.5 (repeated-measures ANOVA). *P for individual time points <0.05. B: WB PB was significantly lower in the RE group than in the CE group. P for group effect = 0.03, P for time effect = 0.1, P for interaction = 0.6 (repeated-measures ANOVA). *P for individual time points <0.05. C: WB Phe hydroxylation was significantly lower in the RE group than in the CE group. P for group effect = 0.04, P for time effect = 0.02, P for interaction = 0.3 (repeated-measures ANOVA). *P for individual time points <0.05. D: WB protein balance was not significantly different between the RE group and the CE group. P for group effect = 0.7, P for time effect = 0.5, P for interaction = 0.1 (repeated-measures ANOVA). *P for individual time points <0.05.
No differences in arterial amino acid plasma concentrations were found between the CE and RE groups before the start of the endotoxin infusion, except for ornithine, glutamate, asparagine, and isoleucine concentrations (Table 2). Glutamate and methionine concentrations were significantly lower in the RE group than in the CE group during endotoxemia.
TABLE 2.
Plasma amino acid concentrations during reduced caloric intake and endotoxemia (RE)1
| Time | P 2 | |||||
| Arterial amino acids and groups | 0 h | 24 h | 48 h | Group effect | Time effect | Group × time interaction |
| Arginine (μmol/L) | 0.002 | 0.7 | 1.0 | |||
| CE | 90 ± 4 (9) | 75 ± 22 (8) | 69 ± 1 (8) | |||
| RE | 82 ± 9 (9) | 65 ± 4 (6) | 67 ± 4 (6) | |||
| Citrulline (μmol/L) | <0.0001 | 0.8 | 0.006 | |||
| CE | 60 ± 11 (9) | 40 ± 8 (8) | 69 ± 143 (8) | |||
| RE | 51 ± 6 (9) | 34 ± 5 (6) | 39 ± 5 (6) | |||
| Ornithine (μmol/L) | 0.02 | 0.8 | 1.0 | |||
| CE | 57 ± 173 (9) | 49 ± 16 (8) | 45 ± 83 (8) | |||
| RE | 44 ± 1 (9) | 31 ± 7 (6) | 32 ± 4 (6) | |||
| Glutamine (μmol/L) | 0.4 | <0.0001 | 0.3 | |||
| CE | 327 ± 10 (9) | 203 ± 12 (8) | 270 ± 33 (8) | |||
| RE | 331 ± 21 (9) | 201 ± 6 (6) | 217 ± 15 (6) | |||
| Glutamate (μmol/L) | 0.01 | <0.0001 | 0.2 | |||
| CE | 256 ± 163 (9) | 116 ± 11 (8) | 114 ± 3 (8) | |||
| RE | 223 ± 10 (9) | 108 ± 2 (6) | 98 ± 6 (6) | |||
| Phenylalanine (μmol/L) | 0.06 | <0.0001 | 0.8 | |||
| CE | 62 ± 4 (9) | 81 ± 9 (8) | 93 ± 6 (8) | |||
| RE | 52 ± 5 (9) | 79 ± 6 (6) | 89 ± 8 (6) | |||
| Leucine (μmol/L) | 0.3 | <0.0001 | 1.0 | |||
| CE | 110 ± 6 (9) | 144 ± 14 (8) | 111 ± 83 (8) | |||
| RE | 109 ± 9 (9) | 130 ± 14 (6) | 142 ± 4 (6) | |||
| Alanine (μmol/L) | 0.7 | 0.001 | 0.3 | |||
| CE | 293 ± 22 (9) | 231 ± 49 (8) | 143 ± 173 (8) | |||
| RE | 327 ± 36 (9) | 232 ± 39 (6) | 91 ± 11 (6) | |||
| Valine (μmol/L) | 0.5 | 0.1 | ||||
| CE | 226 ± 7 (9) | 215 ± 12 (8) | 205 ± 73 (8) | 1.0 | ||
| RE | 228 ± 11 (9) | 228 ± 17 (6) | 244 ± 15 (6) | |||
| Glycine (mmol/L) | 0.5 | 0.2 | 0.7 | |||
| CE | 0.30 ± 0.06 (9) | 0.23 ± 0.09 (8) | 0.31 ± 0.11 (8) | |||
| RE | 0.27 ± 0.07 (9) | 0.27 ± 0.10 (6) | 0.40 ± 0.20 (6) | |||
| Asparagine (μmol/L) | 0.2 | 0.08 | 0.8 | |||
| CE | 29 ± 63 (9) | 23 ± 843 (8) | 24 ± 6 (8) | |||
| RE | 25 ± 3 (9) | 19 ± 3 (6) | 20 ± 2 (6) | |||
| Serine (μmol/L) | 0.2 | 0.02 | 0.3 | |||
| CE | 120 ± 31 (9) | 119 ± 253 (8) | 94 ± 183 (8) | |||
| RE | 105 ± 18 (9) | 83 ± 20 (6) | 67 ± 16 (6) | |||
| Threonine (μmol/L) | 0.8 | 0.03 | 0.9 | |||
| CE | 85 ± 24 (9) | 119 ± 29 (8) | 139 ± 66 (8) | |||
| RE | 78 ± 29 (9) | 135 ± 36 (6) | 140 ± 46 (6) | |||
| Histidine (μmol/L) | 0.5 | 0.3 | 0.4 | |||
| CE | 63 ± 4 (9) | 47 ± 13 (8) | 42 ± 11 (8) | |||
| RE | 46 ± 7 (9) | 43 ± 6 (6) | 41 ± 7 (6) | |||
| Taurine (μmol/L) | 0.6 | 0.7 | 0.7 | |||
| CE | 60 ± 22 (9) | 37 ± 24 (8) | 48 ± 37 (8) | |||
| RE | 52 ± 6 (9) | 51 ± 14 (6) | 49 ± 4 (6) | |||
| Tyrosine (μmol/L) | 0.2 | 0.2 | 0.5 | |||
| CE | 34 ± 10 (9) | 43 ± 16 (8) | 37 ± 14 (8) | |||
| RE | 33 ± 7 (9) | 34 ± 4 (6) | 31 ± 4 (6) | |||
| Methionine (μmol/L) | 0.04 | 0.4 | 0.2 | |||
| CE | 21 ± 6 (9) | 15 ± 7 (8) | 17 ± 43 (8) | |||
| RE | 19 ± 4 (9) | 20 ± 5 (6) | 23 ± 4 (6) | |||
| Isoleucine (μmol/L) | 0.8 | 0.01 | 0.4 | |||
| CE | 97 ± 173 (9) | 118 ± 21 (8) | 118 ± 22 (8) | |||
| RE | 80 ± 19 (9) | 112 ± 33 (6) | 141 ± 30 (6) | |||
| Tryptophan (μmol/L) | 0.5 | 0.3 | 0.02 | |||
| CE | 13 ± 9 (9) | 5 ± 2 (8) | 14 ± 63 (8) | |||
| RE | 12 ± 5 (9) | 6 ± 2 (6) | 6 ± 2 (6) | |||
All values are means ± SDs; number of animals per time group in parentheses; n values <9 represent missing data (the last observation carried forward principle was used to replace missing data). CE, control endotoxemia.
P for group, time, and interaction effects by repeated-measures ANOVA.
Significantly different from RE, P < 0.05.
Arginine metabolism
Pre-endotoxin whole-body arginine appearance was significantly lower in the RE group (Figure 4A). During endotoxemia, whole-body arginine appearance increased in the CE animals, but not in the RE animals (PT×G = 0.04). Compared with the CE animals, whole-body NO production was significantly lower in the RE animals (Figure 4B) (PG < 0.0001). Moreover, whole-body citrulline appearance was significantly lower in the RE group and remained lower than pre-endotoxin concentrations in this group (Figure 4C). Arginine de novo synthesis in the RE group was significantly lower at 0 h and remained significantly lower at 24 and 48 h compared with the CE group (Figure 4D) (PG < 0.0001).
FIGURE 4.
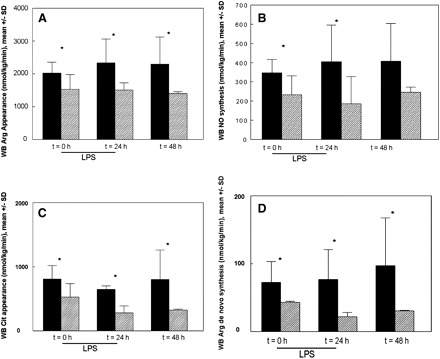
Mean (±SD) whole-body (WB) arginine (Arg) appearance rate (A), nitric oxide (NO) synthesis rate (B), WB citrulline (Cit) appearance rate (C), and WB arginine de novo synthesis rate (D) during reduced caloric intake (semistarvation) and endotoxemia (RE, shaded bars; n = 9) compared with control endotoxemia (CE, solid bars; n = 9). Both groups subsequently received a lipopolysaccharide (LPS) endotoxin infusion (3 μg · kg body weight−1 · h−1) over 24 h. The number of animals per time group was 9 for both groups, except at 48 h in the RE group (n = 7); the last observation carried forward principle was used to replace missing data. A: WB arginine appearance was significantly lower in the RE group than in the CE group. P for group effect = 0.002, P for time effect = 0.06, P for interaction = 0.04 (repeated-measures ANOVA). *P for individual time points <0.05. B: WB NO synthesis was significantly lower in the RE group than in the CE group. P for group effect = 0.02, P for time effect = 0.6, P for interaction = 0.6 (repeated-measures ANOVA). *P for individual time points <0.05. C: WB citrulline appearance was significantly lower in the RE group than in the CE group. P for group effect < 0.0001, P for time effect = 0.05, P for interaction = 0.5 (repeated-measures ANOVA). *P for individual time points <0.05. D: WB arginine de novo synthesis was significantly lower in the RE group than in the CE group. P for group effect < 0.0001, P for time effect = 0.5, P for interaction = 0.3 (repeated-measures ANOVA). *P for individual time points <0.05.
Arterial arginine concentrations (Table 2) before endotoxemia tended to be lower in the RE animals (P = 0.08), but were significantly lower during the whole 48-h period (PG = 0.002). Systemic concentrations of citrulline and ornithine were lower in the RE animals than in the CE animals during endotoxemia.
Missing data analysis
The mean percentage of missing data for all variables was 16% (range: 0–33%). Therefore, additionally, a statistical sensitivity analysis was performed by using the series mean method for replacing missing data and by using the crude data to detect the presence of possible bias (see Supplemental Tables 1–6 under “Supplemental data” in the online issue). Although 5 of 38 variables changed in significance, 1 one of these variables was related to the primary outcome variables (perfusion, arginine-NO metabolism). Compared with the last observation carried forward principle, the P group lactate/pyruvate ratio changed from being nonsignificantly to significantly different between the 2 groups during the series mean method.
DISCUSSION
The results from this study demonstrate that a well-nourished condition before endotoxemia results in a better compensation to endotoxin-induced metabolic deterioration than during inadequate caloric intake. During endotoxemia, whole-body arginine production increases, probably in relation to the stimulated PB. With preceding reduced caloric intake, arginine production is not stimulated, which leads to failure of the normal response with a reduction in citrulline production, arginine de novo synthesis, and whole-body NO production. This was related to an increased septic hemodynamic response with increased metabolic acidosis and decreased intestinal perfusion.
Nutritional depletion has been shown to be a major determinant of the development of postoperative complications (23, 24). The exposure of pigs to postoperative inadequate calorie intakes before the endotoxemic stimulus resulted in a decreased growth rate and decreased protein turnover.
The endotoxemic inflammatory phase represents the response of the animal to a septic insult. Reduced MAP, increased cardiac index, and decreased systemic vascular resistance are characteristic of our porcine endotoxemia model (15). Moreover, this model is characterized by metabolic acidosis, oliguria, and pulmonary edema (15). Therefore, the endotoxemic phase of this model is considered representative of human sepsis and organ dysfunction (6, 14).
Effects of caloric intake reduction
As far as we know, this is the first study to describe the effects of reduced calorie intakes on in vivo arginine de novo, plasma arginine, citrulline, and NO synthesis. The 7-d caloric restriction before endotoxemia showed that, using stable-isotope-infusion techniques, arginine and citrulline metabolism is compromised as illustrated by a significantly reduced whole-body arginine appearance rate and reduced NO production. Several pathways in arginine metabolism may be responsible for this reduced arginine appearance. Reduced citrulline availability may impair arginine de novo synthesis because citrulline appearance is decreased during malnutrition (25). This is indeed supported by the reduced citrulline appearance during caloric restriction before endotoxin infusion. Citrulline is predominantly produced by the conversion of arterial and luminal glutamine, glutamate, and proline through the glutamate-to-ornithine pathway in the gut, but also by NO synthesis and by the breakdown of asymmetrical dimethyl arginine (7). Decreased citrulline concentrations may be caused by decreased substrate availability in the gut (glutamine, glutamate) or by decreased formation as a result of a decreased gut function. This association between low citrulline concentrations and decreased gut function has been shown before (26). Indeed, in our pigs with restricted caloric supplementation, carbon dioxide production in the gut mucosa, an indicator of aerobic function, was increased. In addition, the concentrations of the precursor glutamine were not reduced in the intervention group. On the other hand, reduced glutamine production (from the muscle) with unchanged glutamine utilization may well explain these unchanged plasma glutamine concentrations, as has been shown in cachectic mice undergoing surgical stress (27).
Interestingly, arginine de novo synthesis was significantly reduced after 7 d of caloric restriction and was still reduced during and after endotoxemia. As during a period of reduced caloric intake, protein intake is diminished, arginine de novo synthesis becomes an important source of circulating arginine. Under normal healthy conditions, the magnitude of endogenous synthesis of arginine is sufficiently large to prohibit arginine to become an essential dietary amino acid (28). Lau et al (29) showed that arginine de novo synthesis is essentially independent of arginine intake. This poses the question of which mechanisms reduce arginine de novo synthesis during a period of reduced caloric intake. Depressed expression of argininosuccinate synthase and/or argininosuccinate lyase, converting citrulline back into arginine, may explain our finding of reduced arginine de novo synthesis (30). Although endotoxin can reduce the mRNA of these enzymes in the liver (31), other tissues showed an increased up-regulation of argininosuccinate synthase and argininosuccinate lyase enzymes during endotoxin infusion (32).
In addition, increased arginase activity during caloric restriction may be another cause for decreased arginine appearance. Experimental data indicated increased arginase activity during prolonged semistarvation (33), but studies in human malnutrition indicate decreased arginase activity in plasma (34). Unfortunately, we did not measure arginase activity directly, but the lower arginine/ornithine ratio in the pigs with a reduced caloric intake than in those normally fed may be an indication of increased arginase activity (35, 36).
Effect of endotoxemia after caloric intake reduction
Under endotoxemic conditions, protein catabolism becomes an important source of circulating arginine (15). In our study, whole-body arginine concentrations could not be maintained during endotoxemia (irrespective of previous starvation), despite this increased net PB (15). Several pathways of arginine metabolism are affected during endotoxemic conditions. The observation that both whole-body arginine and NO synthesis decrease during the combined caloric intake reduction and endotoxemia indicates that decreased arginine de novo synthesis becomes an important contributor to the arginine depletion. Because the conversion of citrulline to arginine requires 2 high-energy phosphate bonds and the donation of an amino group from aspartate per molecule of arginine produced, cellular energy balance or aspartate availability during reduced caloric intake and increased caloric requirements may become limiting for the de novo production of arginine. Previously, we showed in critically ill patients that a differentiation between moderate inflammation and severe inflammation/sepsis could be made based on reduced arginine de novo synthesis (12); arginine de novo synthesis was only reduced during severe sepsis and not in conditions of moderately severe inflammation (12). Shen et al (37) found that the arginine de novo pathway is essential to stimulating NO production by NOS-3 during endotoxemia. Interestingly, whole-body NO production was already decreased before the endotoxin infusion as a result of the reduced food intake and further decreased during endotoxemia. Without an endotoxin stimulus, NO production is mainly produced by NOS-3 activity (38) and reduced arginine availability may thereby compromise baseline NO production. During endotoxemia, NO is mainly produced by induced activity of NOS-2, whereas NOS-3 activity is reduced (39). In the absence of arginine, NO production was not stimulated during cytokine stimulation in endothelial cells (37). This effect was attenuated when citrulline was added to the medium and argininosuccinate inhibited conditions, which suggests an important role for the citrulline-arginine de novo–NO pathway also during the endotoxemic phase. The increased arginine de novo synthesis in the presence of low plasma arginine concentrations during endotoxemia alone is consistent with an increased demand for arginine that is not met by an increased production of citrulline (40).
There are indications that reduced arginine availability is detrimental during sepsis (3, 12, 41). This detrimental effect was shown in this study by the significantly increased lactate/pyruvate ratio, presence of mortality in the zero-mortality model during decreased caloric intake, and endotoxemia. A mechanism to explain the increased production of lactate compared with pyruvate is related to the reduced NO production leading to reduced organ perfusion (42). A lactate/pyruvate ratio >10 is considered an indication of increased anaerobic metabolism due to oxygen deficits (42). Whereas this was only present in the endotoxemic group at 24 h, an increased ratio >10 was present before endotoxemia in the group with reduced caloric intake and increased even further during the combined regimen. In addition, the reduction in caloric intake during the endotoxin infusion led to an increased septic hemodynamic profile with a decreased systemic pressure, an increased cardiac output, an increased metabolic acidosis, and disturbed regional perfusion as indicated by an increased pCO2 in the intestinal mucosa. Previously, increased arginine availability was associated with an improved splanchnic perfusion (43, 44).
In conclusion, we observed increased arginine production in our model of endotoxemia, most likely to compensate for the reduced protein intake and increased metabolic demand. During preceding reduced food intake, arginine production cannot be maintained, which has major consequences for the metabolic response to inflammation. Our study indicated that reduced citrulline production and arginine de novo synthesis are key pathways for the reduced arginine availability during sepsis, which is associated with reduced NO production. The exact causative relation needs further exploration.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—MP: study design, data collection, data analysis, and writing of the manuscript; MJB: study design, data collection, and review of the manuscript; YCL: review of the manuscript; and NED: study design, data analysis, and review of the manuscript. None of the authors had any financial or personal interest in any company or organization sponsoring the research, including advisory board affiliations. Current employment or companies had no influence on the study design, data analysis, and/or writing of the manuscript.
REFERENCES
- 1. Barr J, Hecht M, Flavin KE, Khorana A, Gould MK.. Outcomes in critically ill patients before and after the implementation of an evidence-based nutritional management protocol. Chest 2004;125:1446–57. [DOI] [PubMed] [Google Scholar]
- 2. Ing AF, Meakins JL, McLean AP, Christou NV.. Determinants of susceptibility to sepsis and mortality: malnutrition vs anergy. J Surg Res 1982;323:249–55. [DOI] [PubMed] [Google Scholar]
- 3. Milewski PJ, Threlfall CJ, Heath DF, Holbrook IB, Wilford K, Irving MH.. Intracellular free amino acids in undernourished patients with or without sepsis. Clin Sci (Lond) 1982;621:83–91. [DOI] [PubMed] [Google Scholar]
- 4. McClave SA, Mitoraj TE, Thielmeier KA, Greenburg RA.. Differentiating subtypes (hypoalbuminemic vs marasmic) of protein-calorie malnutrition: incidence and clinical significance in a university hospital setting. JPEN J Parenter Enteral Nutr 1992;16:337–42. [DOI] [PubMed] [Google Scholar]
- 5. Beisel WR.. Metabolic response to infection. Annu Rev Med 1975;26:9–20. [DOI] [PubMed] [Google Scholar]
- 6. Bruins MJ, Soeters PB, Deutz NE.. Endotoxemia affects organ protein metabolism differently during prolonged feeding in pigs. J Nutr 2000;13012:3003–13. [DOI] [PubMed] [Google Scholar]
- 7. Luiking YC, Poeze M, Ramsay G, Deutz NE.. The role of arginine in infection and sepsis. JPEN J Parenter Enteral Nutr 2005;29(suppl):S70–4. [DOI] [PubMed] [Google Scholar]
- 8. Wiesinger H.. Arginine metabolism and the synthesis of nitric oxide in the nervous system. Prog Neurobiol 2001;64:365–91. [DOI] [PubMed] [Google Scholar]
- 9. Cifone MG, Cironi L, Meccia MA, et al. Role of nitric oxide in cell-mediated tumor cytotoxicity. Adv Neuroimmunol 1995;5:443–61. [DOI] [PubMed] [Google Scholar]
- 10. Hsieh NK, Chang HR, Hu CT, Chen HI.. Effects of nitric oxide donor and nitric oxide synthase inhibitor on the resistance, exchange and capacitance functions of the canine intestinal vasculature. Vascul Pharmacol 2008;48:122–8. [DOI] [PubMed] [Google Scholar]
- 11. Luiking YC, Poeze M, Dejong CH, Ramsay G, Deutz NE.. Sepsis: an arginine deficiency state? Crit Care Med 2004;32:2135–45. [DOI] [PubMed] [Google Scholar]
- 12. Luiking YC, Poeze M, Ramsay G, Deutz NE.. Reduced citrulline production in sepsis is related to diminished de novo arginine and nitric oxide production. Am J Clin Nutr 2009;891:142–52. [DOI] [PubMed] [Google Scholar]
- 13. Argaman Z, Young VR, Noviski N, et al. Arginine and nitric oxide metabolism in critically ill septic pediatric patients. Crit Care Med 2003;31:591–7. [DOI] [PubMed] [Google Scholar]
- 14. Bruins MJ, Deutz NEP, Soeters PB.. Aspects of organ protein, amino acid and glucose metabolism in a porcine model of hypermetabolic sepsis. Clin Sci (Lond) 2003;104:127–41. [DOI] [PubMed] [Google Scholar]
- 15. Bruins MJ, Lamers WH, Meijer AJ, Soeters PB, Deutz NE.. In vivo measurement of nitric oxide production in porcine gut, liver and muscle during hyperdynamic endotoxaemia. Br J Pharmacol 2002;137:1225–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bruins MJ, Luiking YC, Soeters PB, Lamers WH, Akkermans LM, Deutz NE.. Effects of long-term intravenous and intragastric L-arginine intervention on jejunal motility and visceral nitric oxide production in the hyperdynamic compensated endotoxaemic pig. Neurogastroenterol Motil 2004;16:819–28. [DOI] [PubMed] [Google Scholar]
- 17. Ten Have GAM, Bost MCF, Suyk-Wierts JCAW, van den Bogaard AEJM, Deutz NEP.. Simultaneous measurement of metabolic flux in portally-drained viscera, liver, spleen, kidney and hindquarter in the conscious pig. Lab Anim 1996;30:347–58. [DOI] [PubMed] [Google Scholar]
- 18. Bruins MJ, Soeters PB, Lamers WH, Deutz NE.. L-arginine supplementation in pigs decreases liver protein turnover and increases hindquarter protein turnover both during and after endotoxemia. Am J Clin Nutr 2002;756:1031–44. [DOI] [PubMed] [Google Scholar]
- 19. Castillo L, Beaumier L, Ajami A, Young VR.. Whole body nitric oxide synthesis in healthy mean determined from [15N]arginine-to-[15N]citrulline labeling. Proc Natl Acad Sci USA 1996;93:11460–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wolfe RR.. Radioactive and stable isotope tracers in biomedicine. Principle and practice of kinetic analysis. 1st ed. New York, NY: Wiley-Liss, 1992:49–86. [Google Scholar]
- 21. van Eijk HM, Rooyakkers DR, Deutz NEP.. Rapid routine determination of amino acids in plasma by high-performance liquid chromatography with a 2-3 microns Spherisorb ODS II column. J Chromatogr 1993;6201:143–8. [DOI] [PubMed] [Google Scholar]
- 22. Dejong CH, Deutz NE, Soeters PB.. Renal ammonia and glutamine metabolism during liver insufficiency-induced hyperammonemia in the rat. J Clin Invest 1993;92:2834–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nespoli A, Gianotti L, Bovo G, Brivio F, Nespoli L, Totis M.. Impact of postoperative infections on survival in colon cancer patients. Surg Infect (Larchmt) 2006;7(suppl 2):S41–3. [DOI] [PubMed] [Google Scholar]
- 24. Gianotti L.. Nutrition and infections. Surg Infect (Larchmt) 2006;7(suppl 2):S29–32. [DOI] [PubMed] [Google Scholar]
- 25. Hallemeesch MM, Lamers WH, Deutz NE.. Reduced arginine availability and nitric oxide production. Clin Nutr 2002;21:273–9. [DOI] [PubMed] [Google Scholar]
- 26. Crenn P, De Truchis P, Neveux N, Galpérine T, Cynober L, Melchior JC.. Plasma citrulline is a biomarker of enterocyte mass and an indicator of parenteral nutrition in HIV-infected patients. Am J Clin Nutr (Epub ahead of print 8 July 2009). [DOI] [PubMed] [Google Scholar]
- 27. Vissers YL, von Meyenfeldt MF, Luiking YC, Dejong CH, Deutz NE.. Interorgan synthesis of arginine is down-regulated in tumor-bearing mice undergoing surgical trauma. Metabolism 2008;577:896–902.27. [DOI] [PubMed] [Google Scholar]
- 28. van de Poll MC, Soeters PB, Deutz NE, Fearon KC, Dejong CH.. Renal metabolism of amino acids: its role in interorgan amino acid exchange. Am J Clin Nutr 2004;79:185–97. [DOI] [PubMed] [Google Scholar]
- 29. Lau T, Owen W, Yu YM, et al. Arginine, citrulline, and nitric oxide metabolism in end-stage renal disease patients. J Clin Invest 2000;1059:1217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu G, Jaeger LA, Bazer FW, Rhoads JM.. Arginine deficiency in preterm infants: biochemical mechanisms and nutritional implications. J Nutr Biochem 2004;158:442–51. [DOI] [PubMed] [Google Scholar]
- 31. Tabuchi S, Gotoh T, Miyanaka K, Tomita K, Mori M.. Regulation of genes for inducible nitric oxide synthase and urea cycle enzymes in rat liver in endotoxin shock. Biochem Biophys Res Commun 2000;268:221–4. [DOI] [PubMed] [Google Scholar]
- 32. Nagasaki A, Gotoh T, Takeya M, et al. Coinduction of nitric oxide synthase, argininosuccinate synthetase, and argininosuccinate lyase in lipopolysaccharide-treated rats. RNA blot, immunoblot, and immunohistochemical analyses. J Biol Chem 1996;271:2658–62. [DOI] [PubMed] [Google Scholar]
- 33. Lenaerts K, Sokolović M, Bouwman FG, Lamers WH, Mariman EC, Renes J.. Starvation induces phase-specific changes in the proteome of mouse small intestine. J Proteome Res 2006;5:2113–22. [DOI] [PubMed] [Google Scholar]
- 34. Agarwal PK, Agarwal KN, Agarwal DK.. Biochemical changes in saliva of malnourished children. Am J Clin Nutr 1984;39:181–4. [DOI] [PubMed] [Google Scholar]
- 35. Morris CR, Poljakovic M, Lavrisha L, Machado L, Kuypers FA, Morris SM Jr.. Decreased arginine bioavailability and increased serum arginase activity in asthma. Am J Respir Crit Care Med 2004;170:148–53. [DOI] [PubMed] [Google Scholar]
- 36. Morris CR, Kato GJ, Poljakovic M, et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA 2005;294:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shen LJ, Beloussow K, Shen WC.. Accessibility of endothelial and inducible nitric oxide synthase to the intracellular citrulline-arginine regeneration pathway. Biochem Pharmacol 2005;69:97–104. [DOI] [PubMed] [Google Scholar]
- 38. Javeshghani D, Magder S.. Regional changes in constitutive nitric oxide synthase and the hemodynamic consequences of its inhibition in lipopolysaccharide-treated pigs. Shock 2001;16:232–8. [DOI] [PubMed] [Google Scholar]
- 39. Schwartz D, Mendonca M, Schwartz I, et al. Inhibition of constitutive nitric oxide synthase (NOS) by nitric oxide generated by inducible NOS after lipopolysaccharide administration provokes renal dysfunction in rats. J Clin Invest 1997;100:439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Soeters PB, van de Poll MC, van Gemert WG, Dejong CH.. Amino acid adequacy in pathophysiological states. J Nutr 2004;134(suppl):1575S–82S. [DOI] [PubMed] [Google Scholar]
- 41. Freund H, Atamian S, Holroyde J, Fischer JE.. Plasma amino acids as predictors of the severity and outcome of sepsis. Ann Surg 1979;190:571–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Landow L.. Splanchnic lactate production in cardiac surgery patients. Crit Care Med 1993;21(suppl 2):S84–91. [DOI] [PubMed] [Google Scholar]
- 43. Poeze M, Luiking YC, Deutz N.. Beneficial effects of L-arginine on nitric oxide metabolism and hepatosplanchnic perfusion during porcine endotoxemia. Intensive Care Med 2004;30:S122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Allman KG, Stoddart AP, Kennedy MM, Young JD.. L-arginine augments nitric oxide production and mesenteric blood flow in ovine endotoxemia. Am J Physiol 1996;271:H1296–301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


