ABSTRACT
Background: Neck circumference is a predictor of cardiovascular disease (CVD) risk. However, detailed assessment of neck fat has not been explored, and the contribution from individual neck fat compartments to CVD risk is unknown.
Objective: The objective was to measure neck adipose tissue (NAT) compartments and examine relations with CVD risk markers, with the hypothesis that neck adipose tissue (NAT) accumulation preferentially involves specific compartments that contribute differently to metabolic risk.
Design: We retrospectively studied 303 subjects with successfully treated malignancies or benign etiologies [151 women, 152 men; mean (±SD) age: 55 ± 17 y; mean body mass index (BMI; in kg/m2): 28 ± 6] who underwent whole-body positron emission tomography/computed tomography. NAT was measured at the level of the C5 vertebral body, subdivided into posterior (NATpost), subcutaneous (NATsc), and perivertebral (NATperivert) compartments. Data on CVD risk factors (BMI, abdominal circumference, visceral and abdominal subcutaneous adipose tissue, blood pressure, serum lipids, and fasting plasma glucose) were collected. We compared NAT compartments across lean, overweight, and obese groups and performed multivariate regression models correlating NAT with CVD risk factors. Receiver operating characteristic curve and prevalence ratio analyses were performed to examine the association of NAT compartments with metabolic syndrome.
Results: NATpost and NATsc were more consistently associated with cardiometabolic risk, especially in women, correlating with visceral adipose tissue (P < 0.0001) and triglycerides (P < 0.001) and a nearly 1.5-fold increase in the prevalence ratio for metabolic syndrome after adjustment for age and BMI (P < 0.05). NATsc was most abundant in women, whereas intermuscular compartments (NATpost and NATperivert) were higher in men. In both sexes, NATpost and NATperivert showed the largest increment between lean and obese subjects.
Conclusions: Neck fat compartments expand differently with increasing adiposity, correlate with CVD risk factors, and are associated with metabolic syndrome, most notably NATpost and NATsc in women. Although neck circumference remains an important method to assess metabolic risk, cross-sectional NAT assessment provides further insight into fat accumulation in the neck. This trial was registered at clinicaltrials.gov as NCT02205021.
INTRODUCTION
Detailed quantification of fat depots allows improved assessment of metabolic risk compared with standard anthropometrics, such as BMI and waist circumference (1, 2). For example, waist circumference has limitations in distinguishing the contribution from visceral adipose tissue (VAT)4 and subcutaneous adipose tissue (SAT), which show strong and modest correlations to metabolic risk, respectively (1–5). Importantly, expansion of fat anatomically unrelated to the abdomen also plays a significant role in metabolic syndrome.
Fat accumulation in the neck—usually estimated by neck circumference—confers metabolic risk beyond VAT accumulation (2). Prior studies have shown that upper body fat is a major contributor to systemic free fatty acid (FFA) availability (6), suggesting it plays an important role in metabolic risk (2). Furthermore, neck circumference is an independent predictor of metabolic risk beyond BMI and waist circumference (2) and is positively associated with insulin resistance and VAT (7). In contrast to the well-defined abdominal VAT and SAT depots, to our knowledge, no prior studies have assessed neck fat compartments and their relation to cardiometabolic risk. It is unknown whether neck adipose expansion occurs globally or preferentially involves specific compartments. Furthermore, there are no data about whether certain neck fat compartments contribute differently to metabolic risk.
The purpose of our study was to perform compartmental measures of neck fat by cross-sectional imaging. We investigated sex and BMI differences in compartmental fat accumulation, examined associations with body composition and markers of cardiovascular disease (CVD) risk, and tested which fat compartments are associated with metabolic syndrome. We hypothesized that neck fat accumulation preferentially involves specific compartments, which in turn present distinct contributions to metabolic risk.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board of Partners Health Care Inc and complied with Health Insurance Portability and Accountability Act guidelines, with exemption status for individual informed consent (www.clinicaltrials.gov; NCT02205021). We performed a retrospective search of whole-body 18F-fluorodeoxy-glucose (FDG) positron emission tomography and computed tomography (PET/CT) examinations performed at our institution from August 2009 through October 2013. We consecutively selected imaging studies from adult subjects (>18 y of age), aiming to accrue an equal number of subjects within each BMI category as defined by the World Health Organization [lean, BMI (in kg/m2) <25; overweight, BMI 25 to <30; and obese, BMI ≥30]. Additional inclusion criteria comprised fasting glucose measured immediately before PET/CT, no history of malignancy at the time of image acquisition, no FDG-avid lesions to suggest malignancy in subjects with successfully treated malignancy, and FDG-PET/CT performed for benign etiologies. We excluded subjects with a history of neck cancer, radiation therapy, and/or surgery. We discarded blood pressure and serum lipids obtained beyond 12 mo relative to the PET/CT date. Use of antihypertensives, medication for type 2 diabetes, and lipid-lowering agents was recorded. Data regarding bone structure and brown adipose tissue were reported in a portion of study subjects (n = 35) (8), but no data on neck fat content and CVD risk factors have been reported previously.
PET/CT technique
Only the whole-body attenuation correction noncontrast CT component was used for this study. Briefly, all PET/CT studies were performed on an integrated scanner (Siemens or GE), with a 16- or 64-slice CT and a full-ring HI-REZ lutetium oxyorthosilicate PET. Examinations were performed after a 6-h fast, and blood glucose levels were measured on arrival. Attenuation correction CT obtained in the midexpiration phase without intravenous contrast was performed with the following parameters: slice thickness, 5 mm; table feed per rotation, 18 mm; time per table rotation, 0.5 s; tube voltage, 120 peak kilovoltage; tube current, 11 mA; and field of view, 20 cm.
Quantification of neck circumference and adipose tissue
We measured neck circumference and fat areas at the level of C5 for comparison with prior studies that used neck circumference as a predictor of metabolic risk (2, 7, 9). In these studies, the landmark for placement of measuring tape was the laryngeal prominence, typically located at the C5 vertebral body. We extracted an axial image parallel to the end plates of C5 from the whole-body CT data set by using the 3D multiplanar reconstruction tool in OsiriX software, version 5.8 (http://www.osirix-viewer.com/index.html).
With the use of the same software, we measured neck circumference on the CT image by freehand tracing the skin surface at C5. Areas of neck adipose tissue (NAT) were measured by using a thresholding tool set to include pixels between −50 and −250 Hounsfield units. The 3 dominant neck fat compartments were named after infrahyoid neck spaces (10, 11) and measured as follows (Figure 1):
FIGURE 1.
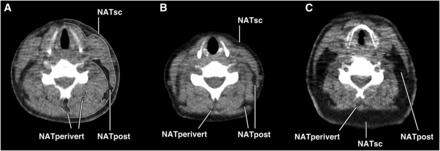
A: Anatomic compartments of the neck at the level of C5 as defined in this study. Tracings delimiting the NATsc, NATpost, and NATperivert compartments are shown. Adipose tissue area measurements were performed bilaterally, and the left-sided compartments are shown for illustration purposes. Neck circumference was determined by tracing the perimeter of the neck. B: NAT compartments in a lean woman without metabolic syndrome [age: 27 y; BMI (in kg/m2): 23]. Small amounts of NATpost, NATsc, and NATperivert are noted. C: NAT accumulation in an obese woman with metabolic syndrome (age: 61 y; BMI: 30). Fat expansion of NATpost and NATsc is seen, with scattered areas of NATperivert. NAT, neck adipose tissue; NATperivert, neck adipose tissue perivertebral compartment; NATpost, neck adipose tissue posterior compartment; NATsc, neck adipose tissue subcutaneous compartment.
Subcutaneous/superficial NAT (NATsc): between skin and deep cervical fascia.
Posterior cervical NAT (NATpost): between the sternocleidomastoid, scalene, and trapezius muscles, separated from the subcutaneous fat by the deep cervical fascia; if the fascia was not visualized, a curved line was traced following and connecting the surfaces of sternocleidomastoid and trapezius muscles, separating the NATsc from the NATpost.
Perivertebral NAT (NATperivert): fat interspersed between muscles surrounding the cervical vertebral body.
Quantification of abdominal circumference and VAT and SAT areas
A CT image at the level of L4 from the same whole-body PET/CT data set used for neck measures provided VAT and SAT areas and abdominal circumference measures. Fat attenuation thresholds were set at −50 to −250 Hounsfield units, and compartments were separated by semiautomated tracing with manual adjustments (TeraRecon Inc).
Cardiometabolic risk factor data
Systolic blood pressure (SBP) and diastolic blood pressure (DBP), triglycerides, and total, LDL, and HDL cholesterol were collected when available within 12 mo of PET/CT date. Fasting glucose obtained immediately before PET/CT scanning was recorded. The presence of metabolic syndrome was defined according to National Cholesterol Education Program criteria (NCEP Adult Treatment Panel III) (12). Metabolic syndrome was considered present if 3 or more parameters were found: abdominal circumference, >88 cm in women and >102 cm in men; fasting triglycerides, ≥150 mg/dL; fasting HDL cholesterol, <50 mg/dL in women and <40 mg/dL in men; blood pressure, ≥130/≥85 mm Hg; and fasting glucose, ≥110 mg/dL.
Statistical analyses
Statistical analyses were performed with JMP 11 (SAS Institute) and MedCalc (version 12.7). The Shapiro-Wilk test was used to determine normal distribution. All variables were log-transformed except DBP. The t test was performed to detect differences between female and male groups regarding all measured parameters. Multiple comparisons of neck measurements across BMI groups were performed by using the Tukey-Kramer method. We performed linear regression analyses between NAT quantification, body composition, and CVD risk parameters separately in female and male subjects. Standard least squares modeling was controlled for age and disease status (those with a benign etiology compared with subjects with successfully treated malignancies and no evidence of active disease or FDG-avid areas to suggest malignancy), with use of antihypertensives, medication for type 2 diabetes, and lipid-lowering agents added to the model when examining correlations with blood pressure, fasting plasma glucose, and serum lipids, respectively. Receiver operating characteristic (ROC) curve analyses of neck measurements were performed for sensitivity and specificity, AUC, and CIs, with threshold values to detect metabolic syndrome being those with the highest sensitivity and specificity. Finally, we performed logistic regression of dichotomous data for metabolic syndrome (present compared with absent) to determine ORs per SD increase in neck measures. Because of the high prevalence of metabolic syndrome in our cohort, logistic ORs were corrected to prevalence ratios (PRs) as previously described (13). P < 0.05 was considered significant and 0.05 ≤ P < 0.1 was defined as marginally significant. Missing data for cardiometabolic parameters were not imputed. Data are presented as means ± SDs unless otherwise noted.
RESULTS
Clinical characteristics of study subjects
A total of 343 subjects were identified. Forty subjects were excluded because of suboptimal quality of the PET/CT scan at C5 for measurement of NAT compartments (n = 12) or prior neck surgery, neck cancer, or radiation therapy (n = 28). After exclusions, our cohort comprised 303 subjects (151 women, 152 men) with a mean age of 55 ± 17 (range: 18–91) y and a mean BMI of 28 ± 6 (range: 16–47), with 101 subjects in each BMI category and no age difference between sexes in each category (P > 0.2). Lean men had a higher BMI than did lean women (P = 0.03) but with no differences in BMI between sexes in overweight and obese groups (P > 0.2).
Included subjects underwent PET/CT for benign etiologies (n = 94) or follow-up of successfully treated malignancies (n = 209) and had no evidence of active disease at imaging or FDG-avid areas to suggest malignancy. The mean interval between imaging and last treatment was 17 ± 23 (range: 1–120) mo. Blood pressure and serum lipid data >12 mo relative to PET/CT were discarded in 33 and 3 subjects, respectively. Mean intervals between PET/CT to blood pressure and serum lipids were 2 ± 2 (0–11) mo and 4 ± 4 (0–12) mo, respectively. Mean use was 40 ± 45 (0–222) mo (n = 93) for antihypertensives, 48 ± 53 (0–165) mo (n = 26) for medications for type 2 diabetes, and 37 ± 34 (0–135) mo (n = 60) for lipid-lowering agents. Additional data are outlined in Table 1.
TABLE 1.
Study sample characteristics1
| Female (n = 151) | Male (n = 152) | P value | |
| Age (y) | 56 ± 17 | 54 ± 17 | 0.3 |
| BMI (kg/m2) | 27 ± 6 | 28 ± 5 | 0.05 |
| NATpost (cm2) | 6 ± 6 | 9 ± 7 | <0.0001 |
| NATsc (cm2) | 26 ± 21 | 23 ± 16 | 0.08 |
| NATperivert (cm2) | 0.5 ± 0.9 | 1.1 ± 1.7 | <0.0001 |
| Neck circumference (cm) | 39 ± 7 | 44 ± 6 | <0.0001 |
| Abdominal circumference (cm) | 94 ± 14 | 97 ± 13 | 0.03 |
| VAT (cm2) | 106 ± 70 | 138 ± 78 | 0.0002 |
| SAT (cm2) | 268 ± 138 | 233 ± 117 | 0.02 |
| Fasting glucose (mg/dL) | 109 ± 21 | 115 ± 28 | 0.04 |
| SBP (mm Hg) | 126 ± 18 | 126 ± 15 | 0.9 |
| DBP (mm Hg) | 75 ± 9 | 76 ± 10 | 0.4 |
| Total cholesterol (mg/dL) | 204 ± 61 | 175 ± 43 | 0.003 |
| HDL cholesterol (mg/dL) | 61 ± 21 | 42 ± 16 | <0.0001 |
| LDL cholesterol (mg/dL) | 112 ± 50 | 95 ± 35 | 0.03 |
| Triglycerides (mg/dL) | 141 ± 105 | 176 ± 128 | 0.09 |
Values are means ± SDs. DBP, diastolic blood pressure; NATperivert, neck adipose tissue perivertebral compartment; NATpost, neck adipose tissue posterior compartment; NATsc, neck adipose tissue subcutaneous compartment; SAT, subcutaneous adipose tissue; SBP, systolic blood pressure; VAT, visceral adipose tissue.
Sex differences in neck fat accumulation
Sex differences in neck measurements are illustrated in Figure 2. Overweight and obese women had a significantly higher NATsc than did men (P < 0.02), despite being age and BMI matched. Conversely, overweight and obese men had significantly higher intermuscular adipose tissue (IMAT) depots (NATpost and NATperivert). NATperivert was higher in overweight (P = 0.04) and obese (P = 0.0003) men. NATpost (P = 0.04) was higher in obese men. Men had a higher neck circumference in all 3 BMI categories (P < 0.01).
FIGURE 2.
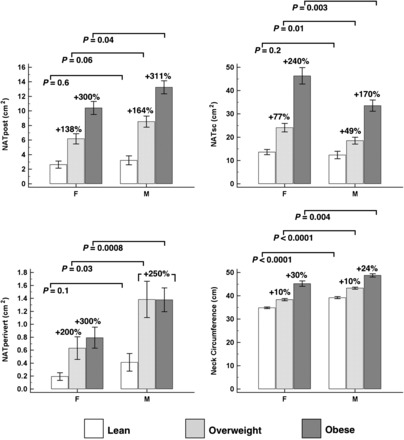
Mean (±SEM) sex differences in neck measures across BMI groups of lean, overweight, and obese subjects (n = 303 and n = 101 in each BMI group). P values compare men with women who have a similar BMI, by using the Tukey-Kramer method. Percentage differences are referenced to lean subjects in each sex. NATperivert, neck adipose tissue perivertebral compartment; NATpost, neck adipose tissue posterior compartment; NATsc, neck adipose tissue subcutaneous compartment.
Distinct patterns of compartmental fat accumulation with BMI increase
Patterns in neck fat accumulation with increasing adiposity are illustrated in Figure 2. In both sexes, all neck measures were significantly higher with increasing BMI category, with the exception of NATperivert, which showed no difference between overweight and obese subjects. This plateau-like pattern was more pronounced in male subjects.
In comparison of lean and obese subjects, NATpost and NATperivert showed the largest percentage differences: NATpost in lean compared with obese women showed a +300% difference (2.6 ± 3.9 compared with 10.4 ± 6.0 cm2), whereas in men, it was +311% (3.2 ± 3.7 compared with 13.2 ± 6.7 cm2). NATperivert in lean compared with obese women showed a +300% difference (0.2 ± 0.5 compared with 0.8 ± 1.1 cm2), whereas in men, it was +250% (0.4 ± 0.8 compared with 1.4 ± 1.4 cm2). Although lean women and men had similar NATsc, it was substantially higher between overweight and obese women. NATsc in lean compared with obese women showed a +240% difference (13.6 ± 9.2 compared with 46.3 ± 23.4 cm2), whereas in men, it was +170% (12.4 ± 9.9 compared with 33.5 ± 18.0 cm2). Neck circumference showed percentage differences of +30% (34.8 ± 2.9 compared with 45.2 ± 7.6 cm) in women and +24% (39.2 ± 2.9 compared with 48.7 ± 5.4 cm) in men when comparing lean with obese subjects.
Association of neck fat compartments with CVD risk factors
Multivariate correlations between neck measurements and cardiometabolic risk factors are outlined in Table 2 and Table 3. Overall, NATpost and NATsc were associated with CVD risk factors similar to or slightly stronger than neck circumference, with a greater magnitude of correlations with adipose measures. SBP, total cholesterol, and LDL cholesterol did not correlate with neck measures in either sex. VAT was associated with fasting glucose, HDL cholesterol, and triglycerides in women, but in men, VAT was associated with DBP and triglycerides.
TABLE 2.
Multivariate correlations between neck measurements and body composition, adjusted for age and disease status, by sex1
| NATpost | NATsc | NATperivert | Neck circumference | VAT | ||||||
| F (n = 149) | M (n = 152) | F (n = 151) | M (n = 152) | F (n = 121) | M (n = 141) | F (n = 151) | M (n = 152) | F (n = 151) | M (n = 152) | |
| Abdominal circumference | 0.72 | 0.71 | 0.75 | 0.71 | 0.57 | 0.60 | 0.69 | 0.71 | 0.85 | 0.85 |
| BMI | 0.73 | 0.73 | 0.76 | 0.66 | 0.58 | 0.56 | 0.75 | 0.70 | 0.82 | 0.80 |
| SAT | 0.71 | 0.77 | 0.75 | 0.71 | 0.55 | 0.58 | 0.63 | 0.64 | 0.79 | 0.77 |
| VAT | 0.82 | 0.74 | 0.80 | 0.73 | 0.66 | 0.65 | 0.70 | 0.61 | — | — |
All variables are log-transformed. Presented are r values for the model. All correlations, P < 0.0001. NATperivert, neck adipose tissue perivertebral compartment; NATpost, neck adipose tissue posterior compartment; NATsc, neck adipose tissue subcutaneous compartment; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
TABLE 3.
Multivariate correlations between neck measurements and cardiovascular disease risk factors, adjusted for age and disease status, by sex1
| NATpost | NATsc | NATperivert | Neck circumference | VAT | ||||||
| F | M | F | M | F | M | F | M | F | M | |
| Fasting glucose2 | 0.44** (149) | NS | 0.28* (151) | NS | 0.51* (121) | NS | 0.28* (151) | NS | 0.48* (151) | NS |
| DBP3 | NS | 0.45* (150) | 0.264 (149) | 0.37* (150) | NS | NS | NS | 0.30* (150) | NS | 0.55** (150) |
| HDL cholesterol5 | −0.49* (62) | NS | −0.48* (63) | NS | NS | NS | −0.37* (63) | NS | −0.61** (63) | NS |
| Triglycerides5 | 0.51** (64) | 0.474 (64) | 0.42* (65) | NS | NS | 0.52* (60) | NS | NS | 0.58** (65) | 0.56* (64) |
Values are r values for the model; number of subjects in parentheses. All neck measures and cardiovascular disease risk factors are log-transformed except DBP. Systolic blood pressure correlated with VAT only in men (r = 0.51, P < 0.05, n = 150). *P < 0.05, **P < 0.001. DBP, diastolic blood pressure; NATperivert, neck adipose tissue perivertebral compartment; NATpost, neck adipose tissue posterior compartment; NATsc, neck adipose tissue subcutaneous compartment; VAT, visceral adipose tissue.
Controlled for use of medication for type 2 diabetes.
Controlled for use of medication for hypertension.
Marginally significant (0.05 ≤ P < 0.1).
Controlled for use of medication for hyperlipidemia. Total cholesterol and LDL cholesterol did not significantly correlate with neck measures in either sex.
Association of neck fat compartments with metabolic syndrome
ROC curve analyses are detailed in Table 4. Data to determine metabolic syndrome were available in 206 subjects (104 women and 102 men). The prevalence of metabolic syndrome in these subjects was 50% (103/206; 50 women and 53 men), with women showing a higher ROC AUC for body composition measures to detect metabolic syndrome.
TABLE 4.
Results of ROC curve analyses to detect metabolic syndrome1
| Threshold | Sensitivity2 | Specificity2 | ROC AUC3 | |
| Female (n = 104) | ||||
| NATpost | >3.2 | 88 (76, 96) | 72 (58, 84) | 0.87 (0.78, 0.92) |
| NATsc | >19.0 | 86 (73, 94) | 74 (60, 85) | 0.83 (0.75, 0.90) |
| NATperivert | >0.1 | 78 (64, 89) | 78 (64, 88) | 0.86 (0.78, 0.92) |
| Neck circumference | >38.6 | 74 (60, 85) | 91 (80, 97) | 0.85 (0.76, 0.91) |
| VAT | >94.9 | 84 (71, 93) | 85 (73, 93) | 0.89 (0.82, 0.95) |
| Male (n = 102) | ||||
| NATpost | >7.2 | 85 (72, 93) | 76 (61, 87) | 0.81 (0.72, 0.88) |
| NATsc | >16.2 | 79 (66, 89) | 78 (63, 88) | 0.82 (0.73, 0.89) |
| NATperivert | >0.3 | 85 (72, 93) | 67 (53, 80) | 0.77 (0.68, 0.85) |
| Neck circumference | >43.6 | 74 (60, 85) | 80 (66, 90) | 0.79 (0.70, 0.86) |
| VAT | >121 | 83 (70, 92) | 82 (68, 91) | 0.86 (0.78, 0.92) |
Threshold values with highest sensitivity and specificity to detect metabolic syndrome are in centimeters for neck circumference and in centimeters squared for all other measures. All ROC curves, P < 0.001. NATperivert, neck adipose tissue perivertebral compartment; NATpost, neck adipose tissue posterior compartment; NATsc, neck adipose tissue subcutaneous compartment; ROC, receiver operating characteristic; VAT, visceral adipose tissue.
Values are percentages; 95% CIs in parentheses.
Values in parentheses are 95% CIs.
All neck measures were significantly higher in subjects with metabolic syndrome in both sexes (P < 0.001, data not shown). With control for BMI, all NAT measures were higher in women with metabolic syndrome (P < 0.05). In men with metabolic syndrome, only NATsc and NATperivert remained higher after BMI adjustment (P < 0.05), with higher NATpost being marginally significant (P = 0.09). Differences in neck circumference between subjects with and without metabolic syndrome were lost after BMI adjustment (both sexes, P > 0.1). With control for VAT, differences in all neck measures were lost (P > 0.1), except for persistent higher neck circumference in men with metabolic syndrome (P = 0.03).
The PR for metabolic syndrome per SD increase in neck measures and VAT is presented in Table 5. In both sexes, NATpost had the highest PR for metabolic syndrome of all neck measures in a multivariate model adjusting for age (P < 0.0001), but this persisted in women only after additional adjustment for BMI (P < 0.05). Although neck circumference had increased PR for metabolic syndrome (P < 0.0001), it lost significance in both sexes after BMI adjustment. All neck measures, when adjusted for age and VAT, did not show significant PR for metabolic syndrome.
TABLE 5.
Prevalence ratios (95% CIs) of metabolic syndrome on neck measures1
| NATpost | NATsc | NATperivert | Neck circumference | VAT | ||||||
| F (n = 103) | M (n = 102) | F (n = 104) | M (n = 102) | F (n = 81) | M (n = 95) | F (n = 104) | M (n = 116) | F (n = 104) | M (n = 116) | |
| Metabolic syndrome | 1.7*** (1.5, 1.9) | 1.8*** (1.5, 1.9) | 1.6*** (1.4, 1.8) | 1.6*** (1.4, 1.7) | 1.5*** (1.3, 1.7) | 1.5** (1.2, 1.7) | 1.6*** (1.4, 1.8) | 1.7*** (1.4, 1.8) | 1.8*** (1.6, 1.9) | 1.8*** (1.6, 1.9) |
| + BMI2 | 1.5* (1.2, 1.8) | NS | 1.4* (1.0, 1.6) | 1.4* (1.1, 1.6) | 1.4* (1.1, 1.6) | NS | NS | NS | 1.6* (1.3, 1.9) | 1.6* (1.2, 1.9) |
Values are the prevalence ratios for metabolic syndrome per SD increase in neck measure, obtained from corrected logistic odds ratios as described in Zhang and Yu (13). All variables are log-transformed and adjusted for log age. Neck measures are standardized to a mean of 0 and an SD of 1. *P < 0.05, **P < 0.001, ***P < 0.0001. NATperivert, neck adipose tissue perivertebral compartment; NATpost, neck adipose tissue posterior compartment; NATsc, neck adipose tissue subcutaneous compartment; VAT, visceral adipose tissue.
Multivariate model adjusted for log age and log BMI.
DISCUSSION
Our results provide insight into patterns of NAT accumulation and suggest specific compartments may have different biological behavior and associations to metabolic risk. First, we describe neck fat depots associated with VAT and with metabolic syndrome. Second, we show that intermuscular neck fat compartments have the greatest expansion with increasing adiposity. Third, NATperivert shows the highest increment between lean and overweight, NATsc has major accrual between overweight and obese, and NATpost expands more uniformly throughout the BMI range.
Although lower body obesity (fat accumulation caudal to the inguinal ligaments and iliac crests) is associated with lower levels of adverse metabolic outcomes (14), upper body fat, which includes VAT and abdominal SAT, has a more pathogenic profile. However, VAT is strongly associated with an adverse CVD risk profile, including insulin resistance, hypertension, and hypertriglyceridemia (1, 6, 14), whereas SAT has more modest associations with CVD risk factors (1, 15). Importantly, upper body subcutaneous fat accounts for most (>60%) of systemic FFA release under basal and insulin-suppressed conditions (6, 14). Therefore, in the context of upper body obesity, the subcutaneous fat depot contributes to the development of metabolic syndrome, because FFA concentrations are directly associated with insulin resistance, hepatic VLDL production, and endothelial dysfunction (7).
Neck fat accumulation is a proxy for upper body subcutaneous fat and is estimated by measuring neck circumference at the level of the laryngeal prominence (2, 7, 9). Prior studies established neck circumference as a simple and effective tool to estimate CVD risk, positively correlating with VAT, insulin resistance, and metabolic syndrome, especially in women (2, 7, 9, 16, 17). Nevertheless, detailed assessment of NAT expansion has not been explored. Two studies used CT to measure neck fat and explore its role in obstructive sleep apnea (18, 19) but did not individually measure compartments or correlate data to body composition and CVD risk factors.
Our study suggests fat accumulation occurs in 3 neck compartments, with NATpost and NATsc being more consistently associated with cardiometabolic risk, especially in women. NATpost is a neck space separated from subcutaneous fat by the deep cervical fascia and is located between the sternocleidomastoid, scalene, and trapezius muscles (Figure 1) (10, 11), comprising a distinct IMAT depot. NATpost had uniform increment across BMI categories and a 3-fold percent difference between lean and obese subjects. In women, NATpost was associated with fasting glucose and was the neck measure most strongly correlated with VAT, serum triglycerides, and HDL cholesterol. In both sexes, NATpost was higher in subjects with metabolic syndrome, which persisted after controlling for BMI. Although NATpost correlated with increased PR for metabolic syndrome in both sexes, this finding persisted only in women after age and BMI adjustments. After adjustments for age and VAT, NATpost was not associated with metabolic syndrome, suggesting it may not independently contribute to metabolic risk.
The majority of fat in overweight and obese women accumulated in the NATsc, being higher than in overweight and obese men. This was also seen comparing subjects with and without metabolic syndrome and is concordant with a large population-based study (4). Interestingly, the percent difference between lean and overweight NATsc was 70% higher in women than in men, underscoring a strong tendency for fat storage in this compartment in women with increasing adiposity. This phenomenon is partly explained by women having a 3-fold higher FFA disposal into upper body subcutaneous fat (20). In addition, our finding also suggests adipocytes in NATsc have the greatest storage capacity compared with other compartments, mostly in women. Similar to NATpost, NATsc correlated with cardiometabolic risk factors and metabolic syndrome, mostly in women. Notably, increased PR for metabolic syndrome linked to NATsc remained significant after age and BMI adjustments in both sexes, slightly lower to PR for metabolic syndrome linked to VAT. In fact, VAT had the strongest associations with cardiometabolic risk compared with all other body composition measures, underscoring its established role as a biomarker for cardiometabolic risk. In the neck, NATpost and NATsc may be proxies to VAT, combining a higher metabolic risk profile with the tendency for expansion as BMI increases. Therefore, although fat accumulates in distinct neck compartments, the most metabolically relevant may be NATpost and NATsc.
We found higher intermuscular fat content in obese (NATpost and NATperivert) and overweight (NATperivert) men, suggesting they tend to accumulate more IMAT with increasing adiposity. These sex-based differences in fat storage are supported by whole-body measures from another study, in which men had ~0.2 kg more IMAT than did women, and women had 38% more SAT than did men (21). One interesting finding of our study was the higher NATperivert comparing lean and overweight in each sex but no significant difference between overweight and obese. In contrast, other neck fat compartments, such as NATpost and NATsc, had positive differences between each BMI group. This plateau-shaped pattern of IMAT accumulation, to our knowledge, has not been described. Ectopic fat deposition occurs partly because of the inability of subcutaneous fat to store excess energy, being diverted to ectopic depots such as the liver, marrow, and skeletal muscle (22, 23). Our finding raises the hypothesis that in the neck, NATperivert has a relatively limited fat storage capacity, and excess fat may be diverted to other local depots, including NATpost (also an IMAT depot) and NATsc, which appear to carry a higher metabolic risk.
Our multivariate analyses between neck fat compartments and CVD risk factors are in line with prior studies using neck circumference as a marker of cardiometabolic risk. Neck circumference is correlated with many CVD risk factors, including BMI, VAT, SAT, blood pressure, insulin sensitivity by HOMA-IR, fasting glucose, HDL cholesterol, and triglycerides (2, 7, 9, 16). In our study, SBP, total cholesterol, and LDL cholesterol did not correlate with neck measures, and fasting glucose correlated with neck measures only in women. The lack of correlation of fasting glucose with neck measures in men may reflect study sample size but supports observations of neck circumference being more sensitive to cardiometabolic risk in women (2, 7).
A limitation of our study was its retrospective cross-sectional design. Our results may not be generalizable because they originate from subjects with successfully treated malignancies or benign etiologies, which have unknown effects on body composition. Nevertheless, many analyses remained significant after adjusting for disease status and BMI, with a mean of 17 mo between imaging and last treatment and measurements only from subjects with no active disease. Furthermore, dietary changes and medications used between imaging and cardiometabolic data collection could lead to misclassification of subjects regarding metabolic syndrome. Our study supports neck circumference as a useful metabolic risk indicator, although its association with metabolic syndrome was not BMI independent. Nevertheless, cross-sectional imaging is not a substitute for neck circumference in clinical management or risk stratification, and our results generate hypotheses regarding NAT accumulation that warrant further investigation.
In summary, by using cross-sectional imaging, we measured fat in 3 distinct neck compartments, showing different accumulation patterns in women and men. Neck fat compartments expand differently with increasing adiposity, correlate with CVD risk factors, and are associated with metabolic syndrome, most notably NATpost and NATsc in women.
Acknowledgments
The authors’ responsibilities were as follows—MT and CMG: designed the research; MT, CMG, ALO, SD, and DCA: conducted the research and analyzed data; MT and MAB: wrote the manuscript; and MT: had primary responsibility for the final content. All authors read and approved the final manuscript. No conflicts of interest were reported.
ABBREVIATIONS
- CT
computed tomography
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- FDG
18F-fluorodeoxy-glucose
- FFA
free fatty acid
- IMAT
intermuscular adipose tissue
- NAT
neck adipose tissue
- NATperivert
neck adipose tissue perivertebral compartment
- NATpost
neck adipose tissue posterior compartment
- NATsc
neck adipose tissue subcutaneous compartment
- PET
positron emission tomography
- PR
prevalence ratio
- ROC
receiver operating characteristic
- SAT
subcutaneous adipose tissue
- SBP
systolic blood pressure
- VAT
visceral adipose tissue
FOOTNOTES
Supported in part by Pilot and Feasibility Grant, Nutrition and Obesity Research Center at Harvard.
REFERENCES
- 1. Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu C-Y, Vasan RS, Murabito JM, Meigs JB, Cupples LA et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 2007;116:39–48. [DOI] [PubMed] [Google Scholar]
- 2. Preis SR, Massaro JM, Hoffmann U, D’Agostino RB, Levy D, Robins SJ, Meigs JB, Vasan RS, O’Donnell CJ, Fox CS.. Neck circumference as a novel measure of cardiometabolic risk: the Framingham Heart study. J Clin Endocrinol Metab 2010;95:3701–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ding J, Kritchevsky SB, Hsu F-C, Harris TB, Burke GL, Detrano RC, Szklo M, Criqui MH, Allison M, Ouyang P et al. Association between non-subcutaneous adiposity and calcified coronary plaque: a substudy of the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr 2008;88:645–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goodpaster BH, Krishnaswami S, Harris TB, Katsiaras A, Kritchevsky SB, Simonsick EM, Nevitt M, Holvoet P, Newman AB.. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med 2005;165:777–83. [DOI] [PubMed] [Google Scholar]
- 5. Neeland IJ, Ayers CR, Rohatgi AK, Turer AT, Berry JD, Das SR, Vega GL, Khera A, McGuire DK, Grundy SM et al. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity (Silver Spring) 2013;21:E439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD.. Splanchnic lipolysis in human obesity. J Clin Invest 2004;113:1582–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stabe C, Vasques ACJ, Lima MMO, Tambascia MA, Pareja JC, Yamanaka A, Geloneze B.. Neck circumference as a simple tool for identifying the metabolic syndrome and insulin resistance: results from the Brazilian Metabolic Syndrome Study. Clin Endocrinol (Oxf) 2013;78:874–81. [DOI] [PubMed] [Google Scholar]
- 8. Bredella MA, Gill CM, Rosen CJ, Klibanski A, Torriani M.. Positive effects of brown adipose tissue on femoral bone structure. Bone 2014;58:55–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fitch KV, Stanley TL, Looby SE, Rope AM, Grinspoon SK.. Relationship between neck circumference and cardiometabolic parameters in HIV-infected and non-HIV-infected adults. Diabetes Care 2011;34:1026–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parker GD, Harnsberger HR.. Radiologic evaluation of the normal and diseased posterior cervical space. AJR Am J Roentgenol 1991;157:161–5. [DOI] [PubMed] [Google Scholar]
- 11. Gupta S, Henningsen JA, Wallace MJ, Madoff DC, Morello FA, Ahrar K, Murthy R, Hicks ME, Frank A, Morello J.. Percutaneous biopsy of head and neck lesions with CT guidance: various approaches and relevant anatomic and technical considerations. Radiographics 2013;27:371–90. [DOI] [PubMed] [Google Scholar]
- 12. Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486–97. [DOI] [PubMed] [Google Scholar]
- 13. Zhang J, Yu KF.. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998;280:1690–1. [DOI] [PubMed] [Google Scholar]
- 14. Jensen MD.. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab 2008;93:S57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu J, Fox CS, Hickson DA, May WD, Hairston KG, Carr JJ, Taylor HA.. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. J Clin Endocrinol Metab 2010;95:5419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosenquist KJ, Massaro JM, Pencina KM, D’Agostino RB, Beiser A, O’Connor GT, O’Donnell CJ, Wolf PA, Polak JF, Seshadri S et al. Neck circumference, carotid wall intima-media thickness, and incident stroke. Diabetes Care 2013;36:e153–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ben-Noun L, Sohar E, Laor A.. Neck circumference as a simple screening measure for identifying overweight and obese patients. Obes Res 2001;9:470–7. [DOI] [PubMed] [Google Scholar]
- 18. Karimi M, Koranyi J, Franco C, Peker Y, Eder DN, Angelhed J-E, Lönn L, Grote L, Bengtsson B-A, Svensson J et al. Increased neck soft tissue mass and worsening of obstructive sleep apnea after growth hormone treatment in men with abdominal obesity. J Clin Sleep Med 2010;6:256–63. [PMC free article] [PubMed] [Google Scholar]
- 19. Shigeta Y, Enciso R, Ogawa T, Ikawa T, Clark GT.. Cervical CT derived neck fat tissue distribution differences in Japanese males and females and its effect on retroglossal and retropalatal airway volume. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008;106:275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koutsari C, Snozek CLH, Jensen MD.. Plasma NEFA storage in adipose tissue in the postprandial state: sex-related and regional differences. Diabetologia 2008;51:2041–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gallagher D, Kuznia P, Heshka S, Albu J, Heymsfield SB, Goodpaster B, Visser M, Harris TB.. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr 2005;81:903–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bredella MA, Gill CM, Gerweck AV, Landa MG, Kumar V, Daley SM, Torriani M, Miller KK.. Ectopic and serum lipid levels are positively associated with bone marrow fat in obesity. Radiology 2013;269:534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Després JP, Lemieux I.. Abdominal obesity and metabolic syndrome. Nature 2006;444:881–7. [DOI] [PubMed] [Google Scholar]


