Abstract
Type 2 diabetes mellitus (T2DM) is a disease associated with a number of metabolic disturbances, including protein metabolism. In the present study, blood samples were obtained from Bahraini subjects, including 6 patients with T2DM and 6 age- and sex-matched, non-diabetic, healthy controls. Depleted and non-depleted sera were prepared from the collected blood, and the global protein expression changes were evaluated by liquid chromatography tandem mass spectrometry. Only significantly and markedly differentially-expressed proteins (P<0.05, analysis of variance; maximum fold change ≥1.5) were considered as candidate proteins for informatics analysis. Accordingly, a total of 62 proteins were identified to be differentially expressed in T2DM, compared with control subjects, and they were grouped functionally into 16 classes of proteins. The largest class was that of the immune-associated proteins. Additionally, ~25 of these proteins (40%) had previously been associated with DM; however, the association of the other 37 proteins with T2DM was a novel observation. The majority of the identified proteins were upregulated in T2DM. The identified proteins could be involved in the pathogenesis of the disease or serve as disease biomarkers. Further validation of the identified proteins in a large study cohort is required, in order to fully access their potential clinical usefulness.
Keywords: type 2 diabetes mellitus, proteomics, liquid chromatography tandem mass spectrometry, differential expression, Bahrain
Introduction
Diabetes mellitus (DM), a chronic metabolic disorder with multiple etiologies, is characterized by hyperglycemia and other metabolic abnormalities attributed to defects in insulin secretion, actions on target tissues or both (1). The global report on diabetes in 2016, reported that type 2 DM (T2DM) affects millions of people globally, including the Middle East (2); in the latter, a high prevalence has been identified in Bahrain, although no recent data has been published (3). In 2015, the International Diabetes Federation estimates indicate that the states of the Gulf Co-operation Council have the highest T2DM rates at a global level (4).
The aim of the present study was to elaborate on the metabolic changes, particularly the blood proteome in T2DM. Proteomics and bioinformatics are complementary molecular tools that have revolutionized biomedical research (5). The robustness of proteomics is enhanced by the continuous upgrading and development of liquid chromatography tandem mass spectrometry (LC-MS/MS) with built-in statistical and graphical software (6,7). Such tools are of the utmost importance for understanding the natural history of multifactorial complex disorders, including cancer, autoimmune diseases and DM. The heterogeneous nature of T2DM (8) makes it a suitable subject for proteomic analysis. However, the applications of proteomics in DM studies to date have fallen short of expectations, as revealed from a literature search. In the present study, LC-MS/MS was used to examine the protein profile of T2DM using serum samples from Bahraini subjects.
The T2DM pathogenesis involves complex interactions between genetic and environmental attributes (8). Insulin resistance is the hallmark of T2DM, although a number of individuals may have normal, decreased or elevated insulin levels (9). Generally, a metabolic malfunction is rarely attributed to a single molecule or etiology, and it is more frequently the combined input of numerous altered molecules, largely proteins. The majority of individuals suffering from T2DM have the polygenic heterogeneous variants with multiple genes involved but in different combinations, resulting in different T2DM subsets (8). Therefore, studies that use 'omics' analysis platforms to assess the protein profile changes in T2DM may identify clinically beneficial panels of protein biomarkers. Generally, 'omics' platforms allow for the investigation of more complex interactions of biological systems (10). Proteomics and peptidomics have a number of advantages over genomics, as they investigate a global protein profile that accounts for >2,000,000 proteins, compared with the undetermined number of genes (expected to be 20-100,000) (11). The analysis of such a large number of data and variables presents a challenge for 'omics' platforms. Quantitative expression proteomics profiling is expected to advance the understanding of the pathophysiological mechanisms of T2DM, and may generate results demonstrating variations in numerous individual proteins, particularly in communities with a high prevalence of T2DM, such as Bahraini.
T2DM is a systemic disease affecting multiple organs, including skin, liver, kidney and eye (12). Since all tissues come into contact with blood, proteins secreted or leaking from different tissues are reflected in the blood protein profile. The blood is therefore the ideal for proteomic analysis in T2DM. The information regarding these proteins may result in an improved approach for disease prediction and diagnosis, as well as an improved understanding of the disease pathology, and thus a more effective treatment (13). Previously, proteomics profiling of serum and plasma has become a focus of growing interest, and the characterization of their protein content has enabled the discovery of an increasing number of reliable markers indicative of the disease (14). In the present study, label-free quantitative protein expression profiling was used for the discovery of proteins that may potentially serve as biomarkers for T2DM.
Patients and methods
Study subjects
The peripheral blood samples used in the present study were selected from a larger number of samples collected from Bahraini subjects with T2DM (cases) and healthy individuals (controls) in the period between January and February 2015 for the study of T2DM biomarkers. The samples selected for this study were obtained from 12 subjects, including 6 with T2DM (3 males and 3 females) and 6 age-, sex- and ethnic background-matched, healthy, non-diabetic subjects (3 males and 3 females) (Table I).
Table I.
Clinical, biochemical and demographic characteristics of the study subjects.
| Serial no. | Sample ID | Clinical diagnosis | Sex | Age (years) | Glucose (mmol/l) | HbA1c (%) | LDLC (mmol/l) | HDLC (mmol/l) | TGs (mmol/l) | T CHOL (mmol/l) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M1 | T2DM | F | 42 | 6.3 | 49 | 3.6 | 1.3 | 1.8 | 3.6 |
| 2 | S4 | Control | F | 42 | 5.7 | ND | 3.3 | 1.5 | 1.2 | 5.3 |
| 3 | M18 | T2DM | F | 41 | 7.2 | 30 | 3.3 | 1 | 1.7 | 5.1 |
| 4 | S8 | Control | F | 41 | 5.4 | ND | 2.5 | 1.7 | 1 | 4.7 |
| 5 | ES12 | T2DM | F | 47 | 7.4 | 71 | 4.4 | 1 | 1.6 | 6.1 |
| 6 | SR | Control | F | 47 | 4.9 | ND | 3.73 | 1.6 | 0.8 | 5.7 |
| 7 | ES27 | T2DM | M | 42 | 7.3 | 53 | 3.6 | 0.9 | 1.4 | 5.1 |
| 8 | S52 | Control | M | 42 | 5.1 | ND | 2.5 | 0.98 | 1.4 | 4.1 |
| 9 | ES29 | T2DM | M | 41 | 6.9 | 44 | 1.8 | 0.9 | 2.8 | 4 |
| 10 | S15 | Control | M | 41 | 4.9 | ND | 3.1 | 1.2 | 0.4 | 4.5 |
| 11 | S75 | T2DM | M | 47 | 12.1 | 89 | 4.4 | 1 | 1.5 | 6.1 |
| 12 | SK | Control | M | 47 | 5.5 | ND | 3.2 | 0.9 | 1.7 | 4.3 |
LDLC, low density lipoprotein cholesterol; HbA1c, glycated hemoglobin; HDLC, high density lipoprotein cholesterol; TGs, triglycerides; T CHOL, total cholesterol; ND, not done; T2DM, type 2 diabetes mellitus; M, male; F, female.
The patients with T2DM were clinically diagnosed according to the World Health Organization criteria (15). The inclusion criteria for cases were: Male or female Bahraini subjects with confirmed T2DM. The exclusion criteria were: Individuals with other metabolic, cancerous or chronic disease, chronic infections, other major diseases, alcoholism/smoking, non-Bahraini subjects or T1DM. The cases were selected randomly from patients reporting to Salmanyia Medical Complex (SMC; Manama, Bahrain). The healthy controls were recruited by an email advertisement sent to the members of College of Health Sciences of Bahrain University (Manama, Bahrain) and extended to their families and friends. The exclusion criteria for the controls were: DM and fasting blood glucose levels of >5.2 mmol/l, in addition to the exclusion criteria used for cases. All study participants provided their verbal informed consent prior to blood collection. The study design was in compliance with the terms of the Declaration of Helsinki and was approved by the Research and Ethics Committees of Arabian Gulf University and SMC.
Sample collection
Fasting blood samples were collected from all study subjects after 10-12 h of overnight fasting. Subsequently, ~10 ml venous blood samples were collected from peripheral vessels into a heparinized collection tube for plasma and into separation gel tubes (both from Thermo Fisher Scientific, Inc., Waltham, MA, USA) for serum collection. All tubes were gently mixed and centrifuged using a UNIVERSAL 320 (Andreas Hettich GmbH & Co.KG, Tuttlingen, Germany) (10 min at 3,000 × g at 25°C) for serum separation. The plasma was separated using Ficoll gradient centrifugation (10 min at 3,000 × g at 25°C). Serum was used for lipid analysis and stored as aliquots at −80°C for later use in protein profiling. Plasma was used for fasting glucose concentration measurements and stored as aliquots at −80°C for other experiments.
Biochemical tests
Blood glucose and glycated hemoglobin (HbA1c) determi- nation
Blood glucose levels were analyzed using Clinical Analyzer Roche COBAS Integra® 800 (Roche Applied Science, Rotkreuz, Switzerland). The results were expressed using SI units (mmol/l).
The assay for HbA1c measurement involved the use of four reagents: Total Hb, HbA1c R1 antibody and HbA1c R2 agglutinate reagents, and Hb denaturant (Beckman Coulter, Inc., Brea, CA, USA). The ratio of HbA1c to total Hb was expressed as a percentage, HbA1c %.
Serum lipid profile
The lipid profile parameters, including total cholesterol, triglyceride, and low-and high-density lipo-protein cholesterol, were measured at the SMC Biochemistry Laboratory using Clinical Analyzer Roche COBAS Integra 800 (Roche Applied Science).
Protein profiling using LC-MS/MS
Protein depletion and serum preparation
To overcome the serum protein complexity, human serum albumin (HSA) and the major subclasses of γ globulin (IgG) were removed from the serum. Pierce Albumin/IgG Removal kit (Thermo Fisher Scientific, Inc.) was used and the depletion protocol was followed according to the manufacturer's protocols. The filtrate containing the sample depleted from HSA and IgG subclasses was considered a depleted sample, and the unprocessed serum a non-depleted sample. The proteins were precipitated using 1 M NaCl. The depleted samples were concentrated using Amicon® Ultra-0.5 Centrifugal Filter Devices (EMD Millipore, Billerica, MA, USA). The non-depleted samples were first desalted with 1 M NaCl and then concentrated similar to the depleted samples.
Protein determination
The Bradford method (16) was used to determine the exact quantity of proteins in the serum, in order to calculate the amount of sample required for protein analysis, a prerequisite for LC-MS/MS analysis. Briefly, the assay was performed in a 96-well micro-titer plate. The stock solution used for this assay contained bovine serum albumin (BSA; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) as Stock I, 1:10 (v/v) RapiGest™ SF (Waters Corporation, Milford, MA, USA) as Stock II and 1:100 (v/v) RapiGest SF as blank solution. A set of 8 diluted BSA standards (0.50, 0.85, 1.40, 2.40, 4.00, 7.00, 11.80 and 20.00 µg/100 µl) in a final volume of 50 ml with Stock 1 were prepared. The absorbance for the samples was measured at 590 nm using a spectrophotometer (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The estimated protein concentration for each sample was determined using a standard curve.
All serum samples were diluted at a 1:1 ratio with 0.1% RapiGest SF prior to protein determination assay, in order to achieve a protein concentration for the diluted samples within the working range of the standards (0.5-20 µg/µl). The RapiGest SF solution was prepared from 50 mM ammonium bicarbonate (AmBic), bezamidine and 20 mM phenylmethylsulfonyl fluoride (all from Sigma-Aldrich; Merck KGaA). The diluted samples were stored at −80°C prior to protein analysis.
In-solution protein digestion prior to LC-MS/MS
For LC-MS/MS analysis, the non-depleted and depleted sera were diluted at a 1:1 ratio with 0.1% RapiGest SF and study samples were pooled together. Accordingly, the duplicates of the 12 sera samples (12 depleted and 12 non-depleted) were each pooled into 4 sets of samples (T2DM males/females and control males/females). The total load for each pooled sample used for analysis was 100 µg in a final volume of 25 µl. The volume of each sample to be included in the pooled sample was calculated from the protein concentration in each sample. The final volume was achieved by adding 0.1% RapiGest SF in 50 mM AmBic solution. The proteins in the pooled samples were denatured at 80°C for 15 min in a Thermo-mixer R (Eppendorf, Hamburg, Germany) at a speed of 8 × g. The denatured proteins were reduced by 100 mM dithiothreitol at 60°C for 30 min and then alkylated by 200 mM iodoacet-amide (both from Sigma-Aldrich; Merck KGaA) at room temperature for 40 min in the dark. Finally, the proteins were digested using Trypsin at a 1:50 ratio (Promega Corporation, Madison, WI, USA). The samples were incubated at 37°C overnight in an Eppendorf Thermo-mixer R (8 × g). Subsequently, the digestion was stopped by acidification (12 M HCl at 37°C for 15 min) and centrifugation (15,682 × g at 4°C for 10 min). The samples were diluted at a 1:1 ratio with the dilution solution (1% acetonitrile, 0.1% formic acid and 99% H2O LC/MS grade). The protein digest was then analyzed using Synapt G2 MS (Waters Corporation).
Protein identification by LC-MS/MS
Label-free quantitative expression protein profiling was performed using 1-dimensional Nano Acquity liquid chromatography coupled with LC-MS/MS on a Synapt G2 instrument (Waters Corporation). The instrument settings for electrospray ionization MS analysis were optimized on the tune page, as previously described (17,18). Briefly, detectors set up was adjusted using 0.5 ng/µl leucine-enkephalin (556.277 Da). Mass (m/z) calibration was achieved on a separate infusion of 500 fmol Glu-fibrinopeptide B (785.843 Da), using the Mass Lynx 4.1 SCN870. The setting of other parameters was as follows: Capillary voltage 3.5 kV, sample cone 35 V, extraction cone 4 V, source temperature 85°C, cone gas 10 l/h, Nano flow gas 0.6 bar and purge gas 600 l/h. All analyses were conducted on Trizaic Nano source (Waters Corporation).
A total of 3 µg protein digest was loaded on LC-MS/MS column and all samples were spiked with yeast alcohol dehydrogenase (Sigma-Aldrich; Merck KGaA) as an internal standard to the digests, to provide 200 fmol per injection for absolute quantitation. All the analyzed samples were processed using the Acquity sample manager with mobile phase consisting of A1 (99% water, 0.1% formic acid and 1% acetonitrile) and B1 (100% acetonitrile and 0.1% formic acid) solutions with a sample flow rate of 0.500 µl/min. Data independent acquisition (DIA) and ion mobility separation experiments were performed, and data was acquired over a range of m/z 50-2,000 Da with a scan time of 1 sec, at a ramped transfer collision energy of 25-50 V with a total acquisition time of 120 min in DIA analysis. All peptides within a defined m/z range were subjected to fragmentation, resulting in accurate protein quantification.
The samples were analyzed in duplicate and data were acquired using Mass Lynx software (version 4.1, SCN870; Waters Corporation) operated in resolution and positive polarity modes. The acquired MS data were background-subtracted, smoothed and deisotoped at a medium threshold. Progenesis QI V2.0 (QIfp) for proteomics (Nonlinear Dynamics; Waters Corporation) was used for automated data processing and database searching. The generated peptide masses were searched against UniProt protein sequence database using the Progenesis QI V2.0 (QIfp) for proteomics, for protein identification and quantification (Nonlinear Dynamics; Waters Corporation).
Data analysis and informatics
The comparisons were made between the mean ± standard error of the mean values of the readings of each subset of samples, such as the depleted samples of T2DM males and female vs. the controls. The Progenesis QI V2.0/TransOmics Informatics (Waters Corporation) software was used to process and search the data using the principle of the search algorithm, as previously described (19,20). The data were filtered to show only statistically significant [P<0.05; analysis of variance (ANOVA) and post hoc Tukey's test] changes in protein concentration with maximum fold change (MFC) ≥1.5. Normalized label-free quantification was achieved to plot principal component analysis against data split into two groups (data not shown). Hierarchical Cluster Analysis of the expression profiles of the test samples between all (males and females) controls and T2DM subjects was conducted and Bray Curtis Correlation distance metric and an average linkage clustering method from the J-Express Pro V1.1 software (java.sun.com) was used to generate dendrograms. P≤0.05 was considered to indicate a statistically significant difference. For classification of the identified proteins, the UniProt database (https://www.uniprot.org/) and PANTHER Class Information (http://www.pantherdb.org/panther/category.do?categoryAcc=GO:0030234) were the bioinformatics tools used. PubMed (https://www.ncbi.nlm.nih.gov/pubmed) was used for searching the literature for the association of each of the identified proteins with DM (any type) and insulin resistance, if no publication was located, the association of that protein with any other disorder was searched for.
Results
Total and dif ferentially-expressed proteins in non-depleted and depleted sera
LC-MS/MS identified ~1,400,000 proteins/peptides in non-depleted sera and ~2,400,000 in depleted sera from healthy non-diabetic subjects (controls) and patients with T2DM (cases). From the non-depleted samples, 117 proteins exhibited significantly different expression changes between cases and controls (P<0.05; ANOVA). Of the 117 identified differentially-expressed proteins, only 43 exhibited a significantly marked difference (MFC ≥1.5, P<0.05) between the 2 groups. By contrast, the levels of 168 proteins in the depleted samples were significantly different between patients and controls (P<0.05), and of the 168, only 23 proteins differed markedly (MFG ≥1.5). However, 4 of the proteins with MFC ≥1.5 and P<0.05 were detected in both depleted and non-deleted data-sets (data not shown).
Markedly differentially-expressed proteins in T2DM in the analysis of non-depleted sera samples
As detailed in Table II, of the 43 proteins with MFG ≥1.5 and P<0.05 in non-depleted samples, 29 were upregulated and 14 downregulated in T2DM, compared with control samples. The most upregulated proteins (P<0.001) were α-1-acid glycoprotein 2, Ig μ chain C region, Ig γ-3 chain C region, thrombin light chain, and heparin cofactor 2, while the most downregulated proteins were Ig γ-4 chain C region, and ribulose-5-phosphate-3-epimerase isoform CRA_a.
Table II.
List of significantly and notably upregulated and downregulated proteins in T2DM relative to control subjects, identified by liquid chromatography tandem mass spectrometry analysis using non-depleted serum samples.
| A, Upregulated proteins in T2DM
| ||||||
|---|---|---|---|---|---|---|
| Serial no. | Accession | Description | Peptide count | Unique peptides | P-value (ANOVA) | MFC |
| 1 | P19652 | α-1-acid glycoprotein 2 | 3 | 2 | 0.0001 | 4.190 |
| 2 | A0A087WYJ9 | Ig μ chain C region | 17 | 1 | 0.0005 | 2.387 |
| 3 | A0A087WXL8 | Ig γ-3 chain C region | 13 | 6 | 0.0005 | 1.848 |
| 4 | E9PIT3 | Thrombin light chain | 5 | 4 | 0.0008 | 2.346 |
| 5 | P05546 | Heparin cofactor 2 | 10 | 10 | 0.0012 | 2.229 |
| 6 | A0A087WZW8 | Protein IGKV3-11 | 9 | 1 | 0.0015 | 1.655 |
| 7 | A0A087WTX5 | Ig κ chain C region | 9 | 1 | 0.0015 | 1.655 |
| 8 | P02743 | Serum amyloid P-component | 5 | 4 | 0.0022 | 1.566 |
| 9 | H0Y7Z1 | Ugl-Y3 (fragment) | 16 | 1 | 0.0030 | 2.644 |
| 10 | P01859 | Ig γ-2 chain C region | 11 | 4 | 0.0031 | 1.725 |
| 11 | P25311 | Zinc-α-2-glycoprotein | 2 | 2 | 0.0033 | 1.531 |
| 12 | A0A087WV47 | Ig γ-1 chain C region | 18 | 1 | 0.0043 | 1.928 |
| 13 | P00450 | Ceruloplasmin | 17 | 17 | 0.0071 | 1.620 |
| 14 | O75970 | Multiple PDZ domain protein | 3 | 3 | 0.0077 | 1.931 |
| 15 | P00739-2 | Isoform 2 of Haptoglobin-related protein | 7 | 2 | 0.0088 | 1.883 |
| 16 | P07357 | Complement component C8 α chain | 4 | 4 | 0.0088 | 2.060 |
| 17 | P04004 | Vitronectin | 9 | 8 | 0.0093 | 2.280 |
| 18 | P0CG05 | Ig λ-2 chain C regions | 5 | 1 | 0.0096 | 1.771 |
| 19 | Q5JSZ5 | Protein PRRC2B | 3 | 3 | 0.0117 | 3.432 |
| 20 | E7ERK6 | Clusterin β chain (fragment) | 1 | 1 | 0.0120 | 1.728 |
| 21 | P01876 | Ig α-1 chain C region | 15 | 5 | 0.0136 | 2.099 |
| 22 | P00747 | Plasminogen | 35 | 32 | 0.0179 | 1.736 |
| 23 | O75636-2 | Isoform 2 of Ficolin-3 | 1 | 1 | 0.0220 | 1.812 |
| 24 | P01877 | Ig α-2 chain C region | 12 | 1 | 0.0231 | 1.947 |
| 25 | S4R471 | Protein AMBP (fragment) | 3 | 3 | 0.0251 | 1.712 |
| 26 | Q5T985 | Inter-α-trypsin inhibitor heavy chain H2 | 18 | 18 | 0.0307 | 1.590 |
| 27 | B4E1Z4 | Uncharacterized protein | 30 | 5 | 0.0343 | 1.590 |
| 28 | P36955 | Pigment epithelium-derived factor | 6 | 6 | 0.0431 | 2.204 |
| 29 | A0A087X1V9 | Protein IGKV2-28 | 2 | 2 | 0.0491 | 1.796 |
|
B, Downregulated proteins in T2DM | ||||||
| Serial no. | Accession | Description | Peptide count | Unique peptides | P-value (ANOVA) | MFC |
|
| ||||||
| 30 | P01861 | Ig γ-4 chain C region | 8 | 3 | 0.0002 | 2.360 |
| 31 | C9J9T0 | Ribulose-5-phosphate-3-epimerase, isoform CRA_a | 1 | 1 | 0.0007 | 2.914 |
| 32 | Q96LX7-3 | Isoform 4 of Coiled-coil domain-containing protein 17 | 2 | 1 | 0.0013 | 2.842 |
| 33 | Q9HAS0 | Protein Njmu-R1 | 2 | 2 | 0.0053 | 1.615 |
| 34 | P19827 | Inter-α-trypsin inhibitor heavy chain H1 | 10 | 8 | 0.0080 | 1.649 |
| 35 | P01593 | Ig κ chain V-I region AG | 3 | 3 | 0.0095 | 2.375 |
| 36 | H7C520 | Procollagen C-endopeptidase enhancer 2 (fragment) | 2 | 2 | 0.0102 | 2.180 |
| 37 | O60733-2 | Isoform SH-iPLA2 of 85/88 kDa calcium-independent phospholipase A2 | 2 | 2 | 0.0111 | 1.564 |
| 38 | P78524 | Suppression of tumorigenicity 5 protein | 1 | 1 | 0.0220 | 3.031 |
| 39 | V9GYJ8 | Apolipoprotein C-II | 1 | 1 | 0.0270 | 4.480 |
| 40 | P02647 | Apolipoprotein A-I | 19 | 19 | 0.0283 | 1.644 |
| 41 | P69892 | Hemoglobin subunit γ-2 | 2 | 1 | 0.0363 | 2.617 |
| 42 | H0Y9E5 | Intraflagellar transport protein 122 homolog (fragment) | 1 | 1 | 0.0462 | 2.198 |
| 43 | P68871 | Hemoglobin subunit β | 4 | 3 | 0.0476 | 1.825 |
P<0.05 was considered to indicate a statistically significant difference; MFC ≥1.5 was considered to indicate a notable change. ANOVA and MFC were calculated using TransOmics software (Progenesis QI 2.0). T2DM, type 2 diabetes mellitus; ANOVA, analysis of variance; MFC, maximum fold change.
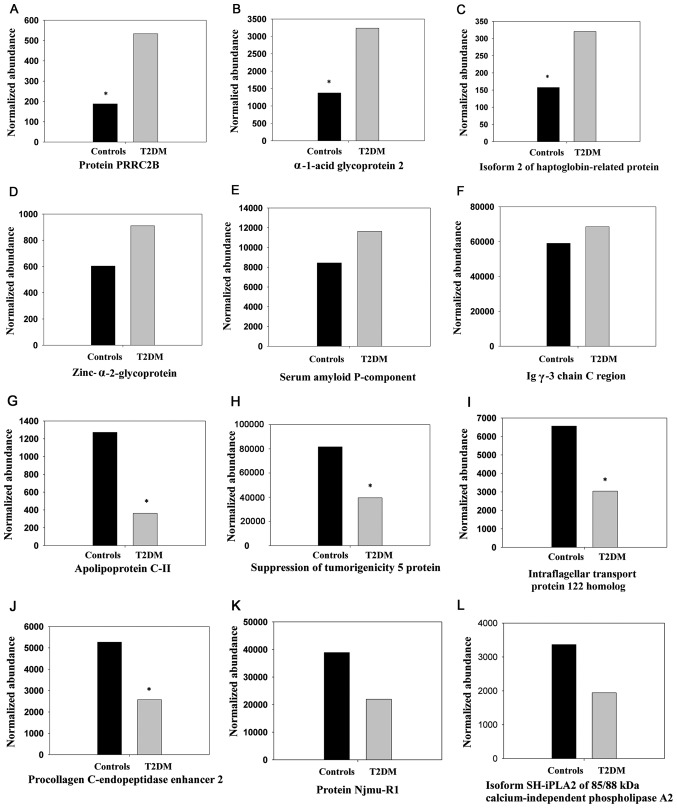
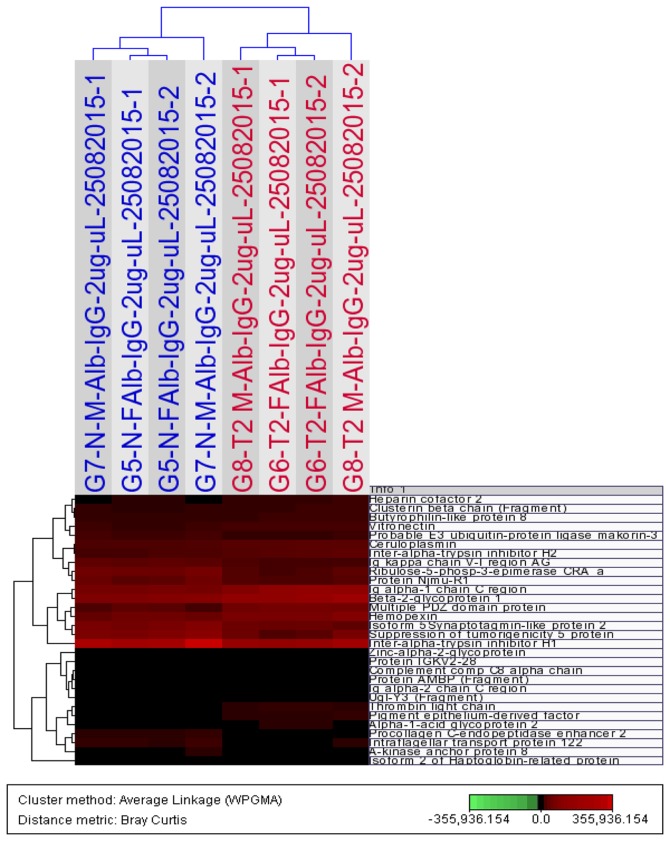
Details on the expression changes, and protein characteristics and descriptions are presented in Table II. The peptide count range of the upregulated proteins was 1-35 peptides [mean ± standard deviation (SD), 10.1±8.2 peptides; median, 9.0 peptides; 25-75%, 3.0-16.3 peptides] and the unique peptide range was 1-32 peptides (mean ± SD, 4.8±6.4 peptides; median, 3.0 peptides; 25-75%, 1.0-5.0 peptides). The peptide count range of the downregulated proteins was 1-19 peptides (mean ± SD, 4.7±5.4 peptides; median, 2.0 peptides; 25-75%, 1.3-7.0 peptides), and the unique peptides range was 1-19 peptides (mean, 3.6±4.7 peptides; median, 2.0 peptides; 25-75%, 1.0-3.0 peptides). The expression changes, upregulation, including proline rich coiled-coil 2B and α-1-acid glycoprotein 2, and downregulation, including apolipoprotein C-II and suppression of tumorigenicity 5 protein, of a number of these proteins between subjects with T2DM and non-diabetic subjects (P<0.05, ANOVA) are depicted in Fig. 1A-F and G-L, respectively. As depicted in Fig. 2, the expression levels of 30 differentially-expressed proteins (P<0.05, ANOVA; MFC ≥1.5), including heparin cofactor 2, Ig α-1 chain C region, zinc-α-2-glycoprotein and inter-α-trypsin inhibitor heavy chain H1, among all 4 study groups, (T2DM males/females and control males/females) were subjected to Hierarchical Cluster Analysis, which separates the samples into two distinct clusters, cases and controls,
Figure 1.
Histograms of the normalized abundance of selected differentially-expressed proteins (highest and lowest) identified in non-depleted serum samples from patients with T2DM (males and females together) and normal control subjects (males and females together). The expression changes of (A-F) upregulated and (G-L) downregulated proteins in T2DM, compared with the control, are indicated. The differences between T2DM subjects and controls for the upregu-lated proteins, including (A) protein PRRC2B (P=0.012), (B) α-1-acid glycoprotein 2 (P<0.001) and (C) isoform 2 of Haptoglobin-related protein (P=0.009), and downregulated proteins, including (G) apolipoprotein C-II (P=0.027), (H) suppression of tumorigenicity 5 protein (P=0.022), (I) intraflagellar transport protein 122 homolog (fragment) (P=0.046), and (J) procollagen C-endopeptidase enhancer 2 (fragment) (P=0.01), were significant (*P<0.05), according to analysis of variance. The other proteins D-F, K and L were comparable between the cases and the controls. The proteins were identified using label-free quantified liquid chromatography tandem mass spectrometry on Synapt G2 analysis. The expression levels of (A-L) all proteins were significantly different between T2DM subjects and controls when males and females compared separately. T2DM, type 2 diabetes mellitus.
Figure 2.
Hierarchical Cluster Analysis of the expression profiles of non-depleted serum samples using 30 proteins that differ significantly (P<0.05, analysis of variance; maximum fold change ≥1.5) between all controls (males and females; blue) subjects and all subjects with type 2 diabetes mellitus (red). The dendrogram was generated using the Bray Curtis Correlation distance metric and an average linkage clustering method from the J-Express Pro V1.1 software (java.sun.com).
Markedly differentially expressed proteins in T2DM in the analysis of depleted sera samples
Using the depleted sera, 23/168 proteins were markedly differentially expressed, including 15 up- and 8 downregulated proteins in T2DM, compared with control samples. These changes were considered significant based on the ANOVA results (P<0.05; Table III). Among the most upregulated proteins in the depleted samples from T2DM subjects (P<0.001) were vitronectin, Hb subunit δ and apolipoprotein B-100. Additionally, the most downregulated protein in T2DM was CD5 antigen-like protein (P=0.0087).
Table III.
List of significantly and markedly upregulated and downregulated proteins in T2DM relative to control subjects, identified by liquid chromatography tandem mass spectrometry analysis using depleted serum samples.
| A, Upregulated proteins in T2DM
| ||||||
|---|---|---|---|---|---|---|
| Serial no. | Accession | Description | Peptide count | Unique peptides | P-value (ANOVA) | MFC |
| 1 | P04004 | Vitronectin | 2 | 1 | 0.0005 | 2.912 |
| 2 | P02042 | Hemoglobin subunit δ | 8 | 2 | 0.0005 | 2.051 |
| 3 | A8MUN2 | Apolipoprotein B-100 | 16 | 16 | 0.00089 | 1.518 |
| 4 | C9J7Z6 | Eukaryotic translation initiation factor 4E type 3 (fragment) | 3 | 3 | 0.00148 | 2.030 |
| 5 | A0A087 | Multiple epidermal growth factor-like domains protein 11 (fragment) | 1 | 1 | 0.00169 | 1.830 |
| 6 | P69905 | Hemoglobin subunit α | 9 | 3 | 0.0030 | 2.040 |
| 7 | Q08380 | Galectin-3-binding protein | 4 | 4 | 0.00861 | 1.698 |
| 8 | Q30KQ8 | β-defensin 112 | 1 | 1 | 0.01019 | 1.782 |
| 9 | D6RA88 | Myosin light chain 5 | 1 | 1 | 0.0132 | 2.193 |
| 10 | P02766 | Transthyretin | 6 | 5 | 0.0175 | 3.053 |
| 11 | Q9Y4G8 | Rap guanine nucleotide exchange factor 2 | 4 | 4 | 0.0187 | 1.553 |
| 12 | Q96J01 | THO complex subunit 3 | 3 | 3 | 0.02369 | 1.662 |
| 13 | O95342 | Bile salt export pump | 4 | 4 | 0.0391 | 1.627 |
| 14 | O15397 | Importin-8 | 1 | 1 | 0.0437 | 1.555 |
| 15 | Q5VU13 | V-set and immunoglobulin domain-containing protein 8 | 2 | 2 | 0.04537 | 2.090 |
|
| ||||||
| B, Downregulated proteins in T2DM | ||||||
|
| ||||||
| Serial no. | Accession | Description | Peptide count | Unique peptides | P-value (ANOVA) | MFC |
|
| ||||||
| 16 | O43866 | CD5 antigen-like | 6 | 6 | 0.0087 | 2.849 |
| 17 | A0A087WXL8 | Ig γ-3 chain C region | 17 | 6 | 0.0102 | 2.638 |
| 18 | P05155 | Plasma protease C1 inhibitor | 3 | 3 | 0.0179 | 2.921 |
| 19 | P01859 | Ig γ-2 chain C region | 14 | 6 | 0.0218 | 1.547 |
| 20 | H3BTQ6 | TATA box-binding protein-associated factor RNA polymerase I subunit C | 5 | 2 | 0.0229 | 1.636 |
| 21 | Q5TAQ9 | DDB1- and CUL4-associated factor 8 | 5 | 5 | 0.02439 | 1.512 |
| 22 | P01861 | Ig γ-4 chain C region | 7 | 2 | 0.0289 | 5.884 |
| 23 | Q8N196 | Homeobox protein SIX5 | 1 | 1 | 0.0413 | 1.577 |
P<0.05 was considered to indicate a statistically significant difference; MFC ≥1.5 was considered to indicate a notable change. ANOVA and MFC were calculated using TransOmics software (Progenesis QI 2.0). T2DM, type 2 diabetes mellitus; ANOVA, analysis of variance; MFC, maximum fold change.
Details on expression changes, and protein characteristics and descriptions are presented in Table III. The peptide count range of the upregulated proteins was 1-16 peptides (mean ± SD, 4.3±4.1 peptides; median, 3 peptides; 25-75%, 1.3-5.5 peptides) and the unique peptides range was 1-16 peptides (mean ± SD, 3.4±3.7 peptides; median, 3 peptides; 25-75%, 1.0-4.0 peptides). The peptide count range of the downregulated proteins was 1-16 peptides (mean ± SD, 4.7±5.4 peptides; median, 2.0 peptides; 25-75%, 1.3-7.0 peptides), and the unique peptides range was 1-6 peptides (mean ± SD, 3.9±2.1 peptides; median, 4.0 peptides; 25-75%, 2.0-6.0 peptides).
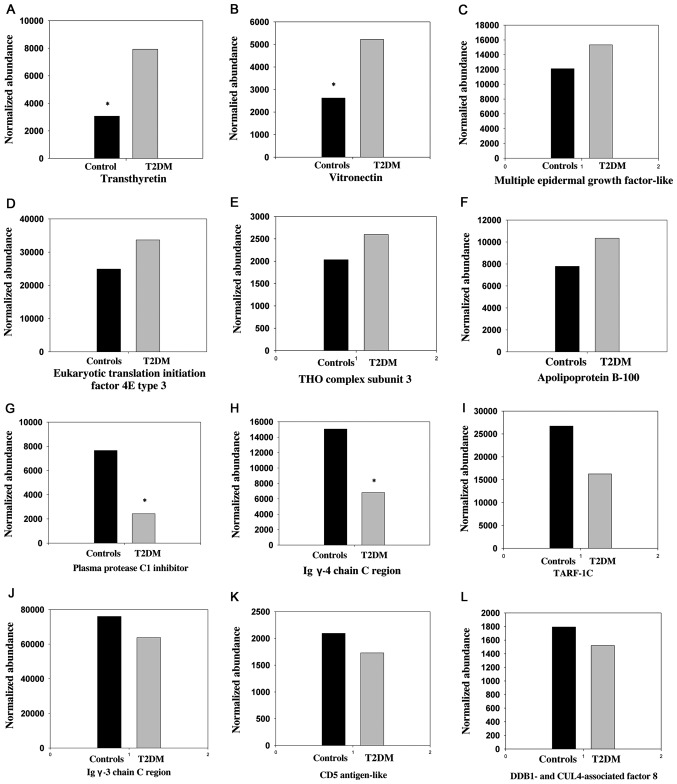
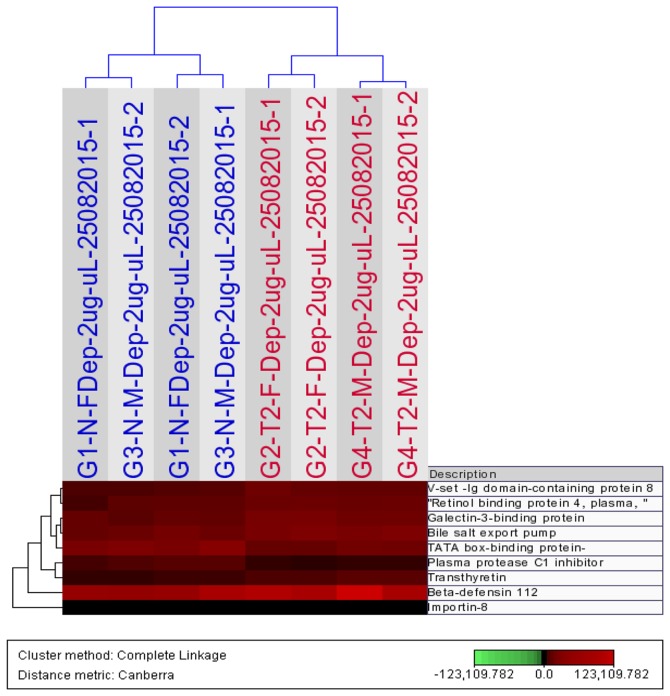
The difference in expression levels (up- and downregulation) of a number of these proteins between subjects with T2DM and control subjects, are depicted in Fig. 3A-F and G-L, respectively. The expression levels of 9 differentially-expressed proteins (P<0.05, ANOVA; MFC ≥1.5), including importin-8 and plasma protease C1 inhibitor, among all 4 study groups, (T2DM males/females and control males/females) was subjected to Hierarchical Cluster Analysis, which separates the samples into two distinct clusters, cases and controls, as depicted in Fig. 4.
Figure 3.
Histograms of the normalized abundance of selected differentially-expressed proteins (highest and lowest) identified in depleted serum samples from patients with T2DM (males and females together) and normal control subjects (males and females together). The expression changes of (A-F) upregulated and (G-L) downregulated proteins in T2DM, compared with the control, are shown. The differences between T2DM subjects and controls for the upregulated proteins, including (A) transthyretin (P=0.018) and (B) vitronectin (P<0.001), and downregulated proteins, including (G) plasma protease C1 inhibitor (P=0.018) and (H) Ig γ-4 chain C region (P=0.029), were significant (*P<0.05), according to analysis of variance. Expression levels of the other proteins (C-F) and (I-L) were not significantly different between cases and controls. The proteins were identified using label-free quantified liquid chromatography tandem mass spectrometry on Synapt G2 analysis. The expression levels of (A-L) all proteins were significantly different between T2DM subjects and controls when males and females compared separately. T2DM, type 2 diabetes mellitus.
Figure 4.
Hierarchical Cluster Analysis of the expression profiles of depleted serum samples using 9 proteins that differ significantly (P<0.05, analysis of variance; maximum fold change ≥1.5) between all controls (males and females; blue) subjects and all subjects with type 2 diabetes mellitus (red). The dendrogram was generated using the Bray Curtis Correlation distance metric and an average linkage clustering method from the J-Express Pro V1.1 software (java.sun.com).
Functional classification of the differentially-expressed proteins
The UniProt database, being one of the reliable sources (21) for the biological and/or molecular functions of the identified differentially-expressed proteins, was searched. The 62 identified proteins could be categorized into multiple sub-classes. The largest class was that of immune system-associated proteins, consisting of 18 proteins, which included 13 different types of Igs [11 Ig chain C regions (6 γ, 2 α, 1 κ, 1 λ and 1 μ), 2 Ig chain V regions (1 Ig κ chain V-I region AG and 1 Protein IGKV2-28)], and 5 were proteins with variable immune functions (Table IV). The majority (83%, 15/18) of the proteins in this class were upregulated in T2DM, compared with the control samples. The other classes were enzyme regulators (6 proteins), Hb chains and Hb-associated proteins (5 proteins), catalytic proteins (4 proteins) and cell adhesion proteins (4 proteins). Additional classes of proteins identified included signal transduction (4 proteins), transport (3 proteins), lipid metabolism and apolipoproteins (3 proteins), blood coagulation (2 proteins) and regulatory (2 proteins) classes. In the remaining classes of proteins, including cytoskeletal, secretory, RNA-binding and spermatogenesis, only 1 protein was identified in each class. Additionally, classes of 7 proteins were not identified. However, functionally, a number of proteins could belong to more than one class
Table IV.
Functional classification of the differentially-expressed proteins, identified by liquid chromatography tandem mass spectrometry, in T2DM.
| No. | Class of proteins | No. of proteins | Upregulation in T2DM | Downregulation in T2DM |
|---|---|---|---|---|
| 1 | Immunity (largely antibodies) | 18a | 15 | 3 |
| 2 | Undefined class | 7 | 3 | 4 |
| 3 | Enzyme regulator | 6 | 4 | 2 |
| 4 | Hemoglobin chains and hemoglobin binding | 5 | 3 | 2 |
| 5 | Cell adhesion | 4 | 4 | 0 |
| 6 | Catalytic: enzymes | 4 | 2 | 2 |
| 7 | Signal transduction | 4 | 2 | 2 |
| 8 | Lipid metabolism and transport | 3 | 2 | 1 |
| 9 | Transport | 3 | 3 | 0 |
| 10 | Blood coagulation | 2 | 2 | 0 |
| 11 | Regulatory | 2 | 2 | 0 |
| 12 | Transcription factor | 2 | 1 | 1 |
| 13 | Cytoskeletal protein | 1 | 1 | 0 |
| 14 | RNA binding | 1 | 1 | 0 |
| 15 | Secretory | 1 | 1 | 0 |
| 16 | Spermatogensis | 1 | 0 | 1 |
| Total | 62 | 46 | 18 |
Two proteins were both up- and downregulated, but in a different set of sera (depleted and non-depleted). Additionally, a number of proteins belong to multiple classes. T2DM, type 2 diabetes mellitus.
Association of the identified proteins with DM and development of T2DM protein panel
A literature search on PubMed was performed to determine the association between the 62 identified proteins and DM. It was determined that 25 proteins have been previously reported to be associated with DM. Of these proteins, 8 (apolipoprotein A-I, plasminogen, pigment epithelium-derived factor, serum amyloid P-component, apolipoprotein B-100, ceruloplasmin, transthyretin and α-1-acid glycoprotein 28) had previously been thoroughly investigated in DM (each reported in >300 articles at the time of the search). Furthermore, 3 proteins (vitronectin, myosin light chain 5 and apolipoprotein C-II) had been frequently investigated in DM (100-300 articles), and 6 proteins (Hb subunit α, heparin cofactor 2, zinc-α-2-glycoprotein, Ig κ chain C region, multiple PDZ domain protein and Rap guanine nucleotide exchange factor 2) had been less investigated. Finally, 8 proteins, including protein AMBP, complement component C8 α chain, CD5 antigen-like, Ig λ-2 chain C regions, clusterin β chain, eukaryotic translation initiation factor 4E type 3, suppression of tumorigenicity 5 protein and bile salt export pump, had been infrequently reported in association with DM (<5 articles). The PubMed search did not reveal any study for DM with the remaining 37/62 proteins (Tables II and III). These 37 proteins are thus a potential 'novel T2DM-protein-panel' that requires further verification.
Discussion
DM is a classic metabolic disorder, during which the metabolism is frequently disturbed and metabolic homeostasis is disarranged, due to lack or reduction of the insulin action (22). Insulin is a major regulator of metabolism in all macromolecules, carbohydrates, lipids and proteins (23). By contrast, insulin secretion is regulated by a number of metabolites, including ATP/ADP and NAD/NADH ratios, blood glucose and amino acids (24). We expected that a number of proteins are involved in DM pathological changes and their consequences.
In the present study, depleted and non-depleted sera samples were analyzed, in order to evaluate the abundance of serum proteins in T2DM. An increased number of differentially-expressed proteins was identified in depleted sera samples, compared with non-depleted sera samples (163 vs. 117, respectively). This is not unexpected, due to the major plasma proteins (albumin and Igs) being known to mask detection of minor plasma proteins (25). Unexpectedly, the proteins whose levels of expression markedly differed between T2DM and control samples has an increased number in the non-depleted sera samples, compared with the depleted sera samples. (43 vs. 23, respectively). The identification of a larger number of markedly differentially-expressed proteins in T2DM in non-depleted sera may indicate that the majority of protein markers of T2DM are not minor plasma proteins, regardless of their physiological/pathological significance. However, only 4 proteins were identified in both datasets of sera samples. Consequently, the 62 proteins that were markedly differentially-expressed in either of the 2 sets of sera samples are subsequently referred to as the 'identified proteins'.
The majority of the identified proteins were upregulated (71%) in T2DM subjects, compared with the controls; whereas, the remaining proteins were downregulated. Using UniProt and PANTHER Class Information, the identified proteins were roughly categorized into 16 protein classes (Table IV).
The most notable observation was that the immune class of proteins was highly represented and markedly upregulated (15/18 proteins) in T2DM. These proteins mostly include the Ig chain C regions (γ, α, κ, λ and μ). However, 2 Ig variable regions, Ig κ chain V-I region AG and Protein IGKV2-28, were also identified and the latter was demonstrated to be upregulated. These 2 proteins had been previously reported to be associated with systemic lupus erythematous, an autoimmune disease (26). The role of immunity in T2DM is bimodal; since immunity could contribute to the pathology of T2DM (27) and, by contrast, the suppression of immunity is one of the major consequences of T2DM (28). Other immune proteins, including isoform 2 of Ficolin-3, β-defensin 112, Complement component C8 α chain and Serum amyloid P-component, were also upregulated in T2DM. Among the downregulated immune proteins were CD5 antigen-like, Ig γ-4 chain C region and Ig κ chain V-I region AG.
Out of the identified Ig chains, only 2 had been previously reported in relation to DM: The Ig κ chain C region, which was demonstrated to be upregulated in T2DM (29); and Ig λ-2 chain C regions, which has been indicated to be a site for methylglyoxal modification that associates hyperglycemia to T2DM complications (30). Of the other identified immune-associated proteins, CD5 antigen-like was reported to be associated with atherosclerosis in T2DM (31), the serum amyloid P-component was associated with progression of retinopathy in T2DM (32) and complement component C8 α chain has been indicated as a biomarker for maturity-onset diabetes of the young types of DM (33). However, the remaining 13 immune-related proteins have not been previously reported in association with DM, based on the literature PubMed search.
Among the identified potential biomarker proteins, 6 were enzyme regulators, 4 of which (inter-α-trypsin inhibitor heavy chain H1, plasma protease C1 inhibitor, thrombin light chain and inter-α-trypsin inhibitor heavy chain H2) have not been previously reported in association with DM. Regarding the remaining 2 proteins, the protein AMBP was reported as a urinary biomarker for diabetic nephropathy (34), while the pigment epithelium-derived factor was determined to be associated with T2DM in females with gestational diabetes (35). Both proteins were determined to be upregulated in T2DM samples in the present study, compared with the control. By contrast, the 4 enzymes cDNA FLJ55673 (similar to complement factor B), multiple epidermal growth factor-like domains protein 11, ribulose-5-phosphate-3-epimerase isoform CRA_a and isoform SH-iPLA2 of 85/88 kDa calcium-independent phospholipase A2, have not been previously reported in association with DM. In the present study, cDNA FLJ55673 and multiple epidermal growth factor-like domains protein 11 were upregulated, and ribulose-5-phosphate-3-epimerase isoform CRA_a and isoform SH-iPLA2, were downregulated in T2DM.
Furthermore, 2 Hb chains (Hb subunits α and δ), and a Hb carrier (isoform 2 of haptoglobin-related protein) were upregulated, while 2 Hb chains (Hb subunits β and γ-2) were downregulated in T2DM, compared with the control. Of the above Hb-associated proteins, only Hb subunit α had been previously indicated as a biomarker for T2DM (36).
The cell adhesion proteins were another class of proteins identified in the present study. Vitronectin, multiple PDZ domain protein, galectin-3-binding protein and zinc-α-2-glycoprotein were all determined to be upregulated. Vitronectin was reported to be associated with the risk of metabolic syndrome and T2DM (37) and zinc-α-2-glycoprotein was demonstrated to be associated with insulin resistance and T2DM (38), while the multiple PDZ domain protein was reported to be associated with DM (39). However, the galectin-3-binding protein has not been reported in association with DM previously.
Among the identified differentially-expressed proteins in T2DM were 4 signal transduction proteins, including importin-8, rap guanine nucleotide exchange factor 2, DDB1- and CUL4-associated factor 8 and suppression of tumorigenicity 5 protein. The former 2 proteins were upregulated and the latter 2 were downregulated. The suppression of tumorigenicity 5 protein and rap guanine nucleotide exchange factor 2 had been previously associated with pancreatic islets cells function (40,41), while the other 2 proteins were not reported with DM.
In the present study, ceruloplasmin, transthyretin and α-1-acid glycoprotein 2, classified as transporters proteins, were all highly expressed in T2DM, compared with the control. This observation was consistent with previously reported data revealing the association between these proteins and DM (42-44). Similarly, 3 proteins involved in lipid metabolism and transport, including apolipoproteins B-100, C-II and A-I, that were differentially-expressed in the present study (the former one was upregulated, while the latter 2 were downregulated), had previously been associated with DM and its complications (45-47).
The 2 blood coagulation-associated proteins identified in the present study, plasminogen and heparin cofactor 2, were upregulated in T2DM; however, both proteins had previously been demonstrated to be associated with DM (48,49). The 2 identified regulatory proteins, V-set and Ig domain-containing protein 8, and eukaryotic translation initiation factor 4E type 3 (eIF4E type 3) (both upregulated), had not been previously reported as DM-associated proteins. However, eIF4E type 3 is involved in renal insulin-induced protein synthesis (50).
Other identified proteins in the present study were as follows: Myosin light chain 5 (upregulated), which belongs to the cytoskeletal class of proteins and may be involved in endothelial tissue dysfunctions in DM (51); bile salt export pump, a secretory protein, which was highly expressed in T2DM samples and has been considered to be involved in the hepatotoxicity of troglitazone, an anti-diabetes agent (52); and THO complex subunit 3, an RNA-binding protein (53), which was upregulated in the present study, but had not been previously associated with DM.
Classes of 7 proteins were not identified: TATA box-binding protein-associated factor RNA polymerase I subunit C, intraflagellar transport protein 122 homolog, isoform 4 of coiled-coil domain-containing protein 17, procollagen C-endopeptidase enhancer 2, Ugl-Y3, protein IGKV3-11 and clusterin β chain. These proteins had also not been previously described in association with DM and their observed expression changes in T2DM in the present study was a novel observation. However, clusterin gene polymorphism had previously been determined to be associated with T2DM (54).
Finally, to validate the observations of LC-MS/MS analysis, 2 of the identified proteins were analyzed by ELISA in a relatively larger sample size from the same setting. Additionally, the results confirmed the differential expression of these proteins in T2DM (data not published). Notably, in the future, the remaining 35 identified novel proteins will be evaluated using a large sample size from the region.
In conclusion, in the present study, 62 markedly differentially-expressed proteins were identified by LC-MS/MS in Bahraini patients with T2DM. These proteins could be grouped functionally into 16 classes, the largest being that of immune-associated proteins. The majority of the identified proteins were upregulated in T2DM. The fact that 25 of the identified proteins have been previously implicated with DM is a validation of the present data. The remaining 37 proteins need to be validated in larger samples in order to access their potential clinical usefulness in DM; however, their association with T2DM in the present study is a novel finding. A detailed investigation of each of the 37 proteins may provide insight into the understanding of the pathogenesis and pathophysiology of T2DM and insulin resistance, since each of these proteins have a known physiological function. Finally, the global blood protein profile in T2DM, as revealed by LC-MS/MS proteomic analysis, demonstrated increased protein synthesis, including immune proteins.
Acknowledgments
The authors would like to express their gratitude to the clinicians in SMC, and Arad, Al-Dair and Sheikh Salman Health Centers (Muharraq, Bahrain). Additionally, there is special thanks for Dr Jaffar Abbas (Arad Health Center, Muharraq, Bahrain), who assisted in patients' recruitment, data collection and biochemical analysis. A sincere appreciation is extended to the staff of Proteomics Unit - King Faisal Specialist Hospital and Research Center (Riyadh, Saudi Arabia) for assistance in the proteomics work.
Funding
No funding received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
RAA, AA and HAG developed the research idea and study design. RAA collected the samples. RAA, AA and ZS conducted the laboratory work. RAA, AA, AAAA and HAG conducted the statistical analysis. RAA, AA and HAG drafted the manuscript and all authors contributed to the final version of the manuscript.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were conducted in accordance with the ethical standards of Arabian Gulf University (Manama, Bahrain) and the Research Committee of SMC, and with the 1964 Helsinki Declaration and its later amendments. Verbal informed consent was obtained from all study subjects.
Patient consent for publication
The patients consented for publication.
Competing interests
All authors declare that they have no competing interests.
References
- 1.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(Suppl 1):S62–S67. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . Global Report on Diabetes. World Health Organization; Geneva, Swizerland: 2016. http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf. [Google Scholar]
- 3.Al-Mahroos F. Diabetes mellitus in the Arabian peninsula. Ann Saudi Med. 2000;20:111–112. doi: 10.5144/0256-4947.2000.111. [DOI] [PubMed] [Google Scholar]
- 4.International Diabetes Federation . IDF Diabetes Atlas. 7th edition. IDF; Brussels, Belgium: 2015. [Google Scholar]
- 5.Van JA, Scholey JW, Konvalinka A. Insights into Diabetic Kidney Disease Using Urinary Proteomics and Bioinformatics. J Am Soc Nephrol. 2017;28:1050–1061. doi: 10.1681/ASN.2016091018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cottrell JS. Protein identification using MS/MS data. J Proteomics. 2011;74:1842–1851. doi: 10.1016/j.jprot.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Bukowiecka-Matusiak M, Chmielewska-Kassassir M, Szczesna D, Wozniak LA. Metabolomic insight into lipid and protein profile in diabetes using mass spectrometry. Mini Rev Med Chem. 2016;16:1167–1174. doi: 10.2174/1389557516666160722133534. [DOI] [PubMed] [Google Scholar]
- 8.Doria A, Patti ME, Kahn CR. The emerging genetic architecture of type 2 diabetes. Cell Metab. 2008;8:186–200. doi: 10.1016/j.cmet.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahn CR. Banting Lecture. Insulin action, diabetogenes, and the cause of type II diabetes. Diabetes. 1994;43:1066–1084. doi: 10.2337/diab.43.8.1066. [DOI] [PubMed] [Google Scholar]
- 10.Knox SS. From 'omics' to complex disease: A systems biology approach to gene-environment interactions in cancer. Cancer Cell Int. 2010;10:11. doi: 10.1186/1475-2867-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ponomarenko EA, Poverennaya EV, Ilgisonis EV, Pyatnitskiy MA, Kopylov AT, Zgoda VG, Lisitsa AV, Archakov AI. The size of the human proteome: The width and depth. Int J Anal Chem. 2016;2016:7436849. doi: 10.1155/2016/7436849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeFronzo RA, Ferrannini E, Zimmet P, George KM, Alberti M, editors. International Textbook of Diabetes Mellitus. 4th edition. John Wiley and Sons; New York, NY: 2015. [DOI] [Google Scholar]
- 13.Anderson NL, Anderson NG. The human plasma proteome: History, character, and diagnostic prospects. Mol Cell Proteomics. 2002;1:845–867. doi: 10.1074/mcp.R200007-MCP200. [DOI] [PubMed] [Google Scholar]
- 14.Anderson NL, Anderson NG, Pearson TW, Borchers CH, Paulovich AG, Patterson SD, Gillette M, Aebersold R, Carr SA. A human proteome detection and quantitation project. Mol Cell Proteomics. 2009;8:883–886. doi: 10.1074/mcp.R800015-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organisation . Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: Report of a WHO/IDF consultation. World Health Organisation; Geneva, Swizerland: 2002. https://www.idf.org/webdata/docs/WHO_IDF_definition_diagnosis_of_diabetes.pdf. [Google Scholar]
- 16.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 17.Alaiya A, Fox J, Bobis S, Matic G, Shinwari Z, Barhoush E, Márquez M, Nilsson S, Holmberg AR. Proteomic analysis of soft tissue tumor implants treated with a novel polybisphosphonate. Cancer Genomics Proteomics. 2014;11:39–49. [PubMed] [Google Scholar]
- 18.Alaiya A, Assad L, Alkhafaji D, Shinwari Z, Almana H, Shoukri M, Alkorbi L, Ibrahim HG, Abdelsalam MS, Skolnik E, et al. Proteomic analysis of Class IV lupus nephritis. Nephrol Dial Transplant. 2015;30:62–70. doi: 10.1093/ndt/gfu215. [DOI] [PubMed] [Google Scholar]
- 19.Li GZ, Vissers JP, Silva JC, Golick D, Gorenstein MV, Geromanos SJ. Database searching and accounting of multiplexed precursor and product ion spectra from the data independent analysis of simple and complex peptide mixtures. Proteomics. 2009;9:1696–1719. doi: 10.1002/pmic.200800564. [DOI] [PubMed] [Google Scholar]
- 20.Alaiya AA, Aljurf M, Shinwari Z, Almohareb F, Malhan H, Alzahrani H, Owaidah T, Fox J, Alsharif F, Mohamed SY, et al. Protein signatures as potential surrogate biomarkers for stratifi-cation and prediction of treatment response in chronic myeloid leukemia patients. Int J Oncol. 2016;49:913–933. doi: 10.3892/ijo.2016.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Apweiler R, Bairoch A, Wu CH, Barker WC, Boeckmann B, Ferro S, Gasteiger E, Huang H, Lopez R, Magrane M, et al. UniProt: The universal protein knowledgebase. Nucleic Acids Res. 2004;32:D115–D119. doi: 10.1093/nar/gkh131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weir GC, Leahy JL, editors. Joslin's Diabetes Mellitus. 13th edition. Lea and Febiger; Philadelphia, PA: 1994. Pathogenesis of non-insulin- dependent (type II) diabetes mellitus; pp. 240–264. [Google Scholar]
- 23.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;13:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 24.Meglasson MD, Matschinsky FM. Pancreatic islet glucose metabolism and regulation of insulin secretion. Diabetes Metab Rev. 1986;2:163–214. doi: 10.1002/dmr.5610020301. [DOI] [PubMed] [Google Scholar]
- 25.Gersten DM, Khirabadi BS, Kurian P, Ledley RS, Mahany T, Ramey ER, Ramwell PW. Albumin obscures sex differences in blood protein patterns of rats and humans. Biochem J. 1980;191:869–872. doi: 10.1042/bj1910869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al Kindi MA, Chataway TK, Gilada GA, Jackson MW, Goldblatt FM, Walker JG, Colella AD, Gordon TP. Serum SmD autoantibody proteomes are clonally restricted and share variable-region peptides. J Autoimmun. 2015;57:77–81. doi: 10.1016/j.jaut.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Brooks-Worrell B, Palmer JP. Immunology in the Clinic Review Series; focus on metabolic diseases: development of islet autoimmune disease in type 2 diabetes patients: potential sequelae of chronic inflammation. Clin Exp Immunol. 2012;167:40–46. doi: 10.1111/j.1365-2249.2011.04501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM) FEMS Immunol Med Microbiol. 1999;26:259–265. doi: 10.1111/j.1574-695X.1999.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 29.Murri M, Insenser M, Bernal-Lopez MR, Perez-Martinez P, Escobar-Morreale HF, Tinahones FJ. Proteomic analysis of visceral adipose tissue in pre-obese patients with type 2 diabetes. Mol Cell Endocrinol. 2013;376:99–106. doi: 10.1016/j.mce.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Kimzey MJ, Kinsky OR, Yassine HN, Tsaprailis G, Stump CS, Monks TJ, Lau SS. Site specific modification of the human plasma proteome by methylglyoxal. Toxicol Appl Pharmacol. 2015;289:155–162. doi: 10.1016/j.taap.2015.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lepedda AJ, Lobina O, Rocchiccioli S, Nieddu G, Ucciferri N, De Muro P, Idini M, Nguyen HQ, Guarino A, Spirito R, Formato M. Identification of differentially expressed plasma proteins in atherosclerotic patients with type 2 diabetes. J Diabetes Complications. 2016;30:880–886. doi: 10.1016/j.jdiacomp.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Yang HS, Woo JE, Lee SJ, Park SH, Woo JM. Elevated plasma pentraxin 3 levels are associated with development and progression of diabetic retinopathy in Korean patients with type 2 diabetes mellitus. Invest Ophthalmol Vis Sci. 2014;55:5989–5997. doi: 10.1167/iovs.14-14864. [DOI] [PubMed] [Google Scholar]
- 33.Karlsson E, Shaat N, Groop L. Can complement factors 5 and 8 and transthyretin be used as biomarkers for MODY 1 (HNF4A-MODY) and MODY 3 (HNF1A-MODY)? Diabet Med. 2008;25:788–791. doi: 10.1111/j.1464-5491.2008.02467.x. [DOI] [PubMed] [Google Scholar]
- 34.Zubiri I, Posada-Ayala M, Sanz-Maroto A, Calvo E, Martin-Lorenzo M, Gonzalez-Calero L, de la Cuesta F, Lopez JA, Fernandez-Fernandez B, Ortiz A, et al. Diabetic nephropathy induces changes in the proteome of human urinary exosomes as revealed by label-free comparative analysis. J Proteomics. 2014;96:92–102. doi: 10.1016/j.jprot.2013.10.037. [DOI] [PubMed] [Google Scholar]
- 35.Li TH, Qiu CJ, Yu XJ, Liu DD, Zhou PF, Wu L. Increased serum pigment epithelium-derived factor in women with gestational diabetes is associated with type 2 diabetes. Int J Endocrinol. 2015;2015:346938. doi: 10.1155/2015/346938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolberg JA, Gerwien RW, Watkins SM, Wuestehube LJ, Urdea M. Biomarkers in Type 2 diabetes: Improving risk stratification with the PreDx® Diabetes Risk Score. Expert Rev Mol Diagn. 2011;11:775–792. doi: 10.1586/erm.11.63. [DOI] [PubMed] [Google Scholar]
- 37.Alessi MC, Nicaud V, Scroyen I, Lange C, Saut N, Fumeron F, Marre M, Lantieri O, Fontaine-Bisson B, Juhan-Vague I, et al. DESIR Study Group Association of vitronectin and plasminogen activator inhibitor-1 levels with the risk of metabolic syndrome and type 2 diabetes mellitus. Results from the D.E.S.I.R. prospective cohort. Thromb Haemost. 2011;106:416–422. doi: 10.1160/TH11-03-0179. [DOI] [PubMed] [Google Scholar]
- 38.Yang M, Liu R, Li S, Luo Y, Zhang Y, Zhang L, Liu D, Wang Y, Xiong Z, Boden G, et al. Zinc-α2-glycoprotein is associated with insulin resistance in humans and is regulated by hyperglycemia, hyperinsulinemia, or liraglutide administration: Cross-sectional and interventional studies in normal subjects, insulin-resistant subjects, and subjects with newly diagnosed diabetes. Diabetes Care. 2013;36:1074–1082. doi: 10.2337/dc12-0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JH, Volinic JL, Banz C, Yao KM, Thomas MK. Interactions with p300 enhance transcriptional activation by the PDZ-domain coactivator Bridge-1. J Endocrinol. 2005;187:283–292. doi: 10.1677/joe.1.06305. [DOI] [PubMed] [Google Scholar]
- 40.Gupta RK, Gao N, Gorski RK, White P, Hardy OT, Rafiq K, Brestelli JE, Chen G, Stoeckert CJ, Jr, Kaestner KH. Expansion of adult beta-cell mass in response to increased metabolic demand is dependent on HNF-4alpha. Genes Dev. 2007;21:756–69. doi: 10.1101/gad.1535507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly P, Bailey CL, Fueger PT, Newgard CB, Casey PJ, Kimple ME. Rap1 promotes multiple pancreatic islet cell functions and signals through mammalian target of rapamycin complex 1 to enhance proliferation. J Biol Chem. 2010;285:15777–15785. doi: 10.1074/jbc.M109.069112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Christiansen MS, Iversen K, Larsen CT, Goetze JP, Hommel E, Mølvig J, Pedersen BK, Magid E, Feldt-Rasmussen B. Increased urinary orosomucoid excretion: A proposed marker for inflammation and endothelial dysfunction in patients with type 2 diabetes. Scand J Clin Lab Invest. 2009;69:272–281. doi: 10.1080/00365510802531100. [DOI] [PubMed] [Google Scholar]
- 43.Pullakhandam R, Palika R, Ghosh S, Reddy GB. Contrasting effects of type 2 and type 1 diabetes on plasma RBP4 levels: The significance of transthyretin. IUBMB Life. 2012;64:975–982. doi: 10.1002/iub.1096. [DOI] [PubMed] [Google Scholar]
- 44.Gluhovschi C, Gluhovschi G, Petrica L, Timar R, Velciov S, Ionita I, Kaycsa A, Timar B. Urinary Biomarkers in the Assessment of Early Diabetic Nephropathy. J Diabetes Res. 2016;2016:4626125. doi: 10.1155/2016/4626125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watts GF, Ooi EM, Chan DC. Therapeutic regulation of apoB100 metabolism in insulin resistance in vivo. Pharmacol Ther. 2009;123:281–291. doi: 10.1016/j.pharmthera.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 46.Kei AA, Filippatos TD, Tsimihodimos V, Elisaf MS. A review of the role of apolipoprotein C-II in lipoprotein metabolism and cardiovascular disease. Metabolism. 2012;61:906–921. doi: 10.1016/j.metabol.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 47.Rye KA, Barter PJ, Cochran BJ. Apolipoprotein A-I interactions with insulin secretion and production. Curr Opin Lipidol. 2016;27:8–13. doi: 10.1097/MOL.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 48.Yarmolinsky J, Bordin Barbieri N, Weinmann T, Ziegelmann PK, Duncan BB, Inês Schmidt M. Plasminogen activator inhibitor-1 and type 2 diabetes: a systematic review and meta-analysis of observational studies. Sci Rep. 2016;6:17714. doi: 10.1038/srep17714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hasegawa Y, Ishigaki Y. Heparin Cofactor II: A novel plausible link of obesity and diabetes with thrombosis. J Atheroscler Thromb. 2017;24:1202–1203. doi: 10.5551/jat.ED084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhandari BK, Feliers D, Duraisamy S, Stewart JL, Gingras AC, Abboud HE, Choudhury GG, Sonenberg N, Kasinath BS. Insulin regulation of protein translation repressor 4E-BP1 an eIF4E-binding protein, in renal epithelial cells. Kidney Int. 2001;59:866–875. doi: 10.1046/j.1523-1755.2001.059003866.x. [DOI] [PubMed] [Google Scholar]
- 51.Rigor RR, Shen Q, Pivetti CD, Wu MH, Yuan SY. Myosin light chain kinase signaling in endothelial barrier dysfunction. Med Res Rev. 2013;33:911–933. doi: 10.1002/med.21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Funk C, Ponelle C, Scheuermann G, Pantze M. Cholestatic potential of troglitazone as a possible factor contributing to troglitazone-induced hepatotoxicity: In vivo and in vitro interaction at the canalicular bile salt export pump (Bsep) in the rat. Mol Pharmacol. 2001;59:627–635. doi: 10.1124/mol.59.3.627. [DOI] [PubMed] [Google Scholar]
- 53.Strässer K, Masuda S, Mason P, Pfannstiel J, Oppizzi M, Rodriguez-Navarro S, Rondón AG, Aguilera A, Struhl K, Reed R, Hurt E. TREX is a conserved complex coupling transcription with messenger RNA export. Nature. 2002;417:304–308. doi: 10.1038/nature746. [DOI] [PubMed] [Google Scholar]
- 54.Daimon M, Oizumi T, Karasawa S, Kaino W, Takase K, Tada K, Jimbu Y, Wada K, Kameda W, Susa S, et al. Association of the clusterin gene polymorphisms with type 2 diabetes mellitus. Metabolism. 2011;60:815–822. doi: 10.1016/j.metabol.2010.07.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.