Abstract
The Hedgehog (Hh) family of secreted proteins governs a wide variety of processes during embryonic development and adult tissue homeostasis. Here we review the current understanding of the molecular and cellular basis of Hh morphogen gradient formation and signal transduction, and the multifaceted roles of Hh signaling in development and tumorigenesis. We discuss how the Hh pathway has diverged during evolution and how it integrates with other signaling pathways to control cell growth and patterning.
Introduction
hh was initially discovered by Christiane Nüsslein-Volhard and Eric Wieschaus nearly thirty years ago as a “segment-polarity” gene that controls Drosophila embryonic cuticle pattern (Nüsslein-Volhard and Wieschaus, 1980). Since the molecular cloning of hh and the discovery of its vertebrate counterparts in the early 1990s, enormous progress has been made in revealing the role of Hh signaling in development and disease as well as the molecular underpinning of the Hh signaling cascade. We now know that Hh signaling is not only important in fruit flies to pattern their embryonic cuticles and adult appendages but vital for diverse aspects of animal development and essential in humans to regulate cell fate and number in their brains and spinal cords, pattern their limbs and internal organs, and even control their body height (Ingham and McMahon, 2001; Weedon et al., 2008). Recent studies have also implicated Hh signaling as essential for stem cell maintenance (Beachy et al., 2004). Not surprisingly, malfunction of Hh signaling contributes to numerous human disorders including birth defects, such as Gorlin syndrome and Greg cephalopolysyndactyly syndrome, and cancer including basal cell carcinoma and medulloblastoma (McMahon et al., 2003; Nieuwenhuis and Hui, 2005).
A hallmark of Hh signaling is its ability to act over a long range and control distinct cell fates as a function of Hh concentration, raising important questions of how Hh gradients are generated and maintained during development, and how different thresholds of Hh are transduced to elicit distinct developmental outcomes. In addition to signaling strength, signaling duration is also important for shaping developmental outcomes, raising questions of how the responses to Hh signaling change over time and how the signals are terminated.
Here, we review the multifaceted roles of Hh signaling in pattern formation and cell growth control. We focus on a current understanding of the molecular and cellular mechanisms that govern the formation and transduction of Hh morphogen gradients. We also discuss how the Hh pathway has diverged during evolution and how it integrates with other signaling pathways to control cell growth, survival, and differentiation.
Hh signaling in embryonic development: differential roles of GliA and GliR
Hh exerts its biological influence through a signaling cascade that culminates in an alteration of the balance between activator and repressor forms of the Gli family of Zinc finger transcription factors (GliA and GliR; Fig. 1A). The Hh reception system consists of a twelve-span transmembrane protein Patched (Ptc) as the Hh receptor and a seven-span transmembrane protein Smoothened (Smo) as the obligatory signal transducer across the plasma membrane. In the absence of Hh, Ptc blocks Smo activity, full-length Gli proteins are proteolytically processed to generate C-terminally truncated GliR that actively represses a subset of Hh target genes. Hh binding to Ptc unleashes Smo activity, which blocks GliR production and promotes GliA activation. Whereas Drosophila has only one hh gene and one Gli homolog, Cubitus interruptus (Ci), vertebrate Hh signal transduction involves both multiple ligands -- Sonic hedgehog (Shh), Indian hedgehog (Ihh), and Desert hedgehog (Dhh) -- and multiple Gli transcription factors (Gli1, Gli2, and Gli3) (Ingham and McMahon, 2001). The vertebrate GliR function is largely derived from Gli3 while the primary GliA activity is largely contributed by Gli2. Gli1 is a transcriptional target of Hh signaling and acts as a transcriptional activator to reinforce GliA function (see below and figure 3 for more detail).
Figure 1. The morphogen and mitogen role of Hh.
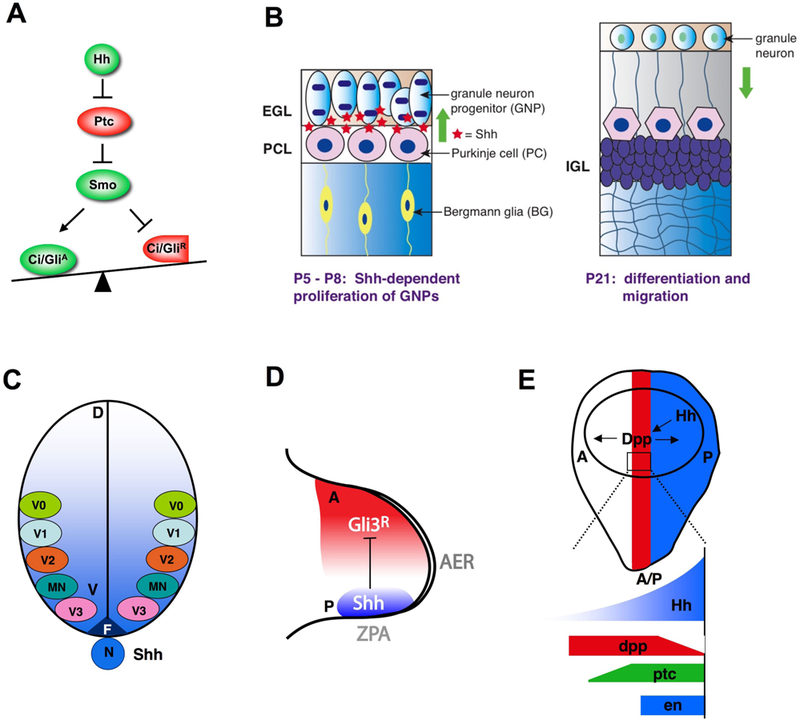
(A) A simplified Hh signaling pathway.
(B) In cerebellar development, Shh secreted by Purkinje cells controls the proliferation of granule neuron progenitors in the external germinal cell layer (EGL).
(C) In developing neural tube, Shh emanates from notochord (N) and floor-plate (F) to form a ventral (V) to dorsal (D) concentration gradient that directs the formation of distinct pools of neural progenitor cells at different positions along the D/V axis. Increasing levels of Shh progressively specify progenitors giving rise to neuronal subtypes of more ventral characters. MN: motor neuron; V0-V3: V0-V3 interneurons.
(D) In vertebrate limb bud, Shh produced by the ZPA propagates anteriorly to form a posterior to anterior concentration gradient that patterns the limb.
(E) In Drosophila wing imaginal disc, Hh produced by posterior (P) compartment cells (blue) acts as a local morphogen, inducing gene expression in the neighboring anterior (A) compartment cells (red). Low levels of Hh suffice to activate dpp while higher levels of Hh are required to activate ptc, and peak levels of Hh are required to activate en.
Figure 3. Regulation of Smo trafficking and conformation.
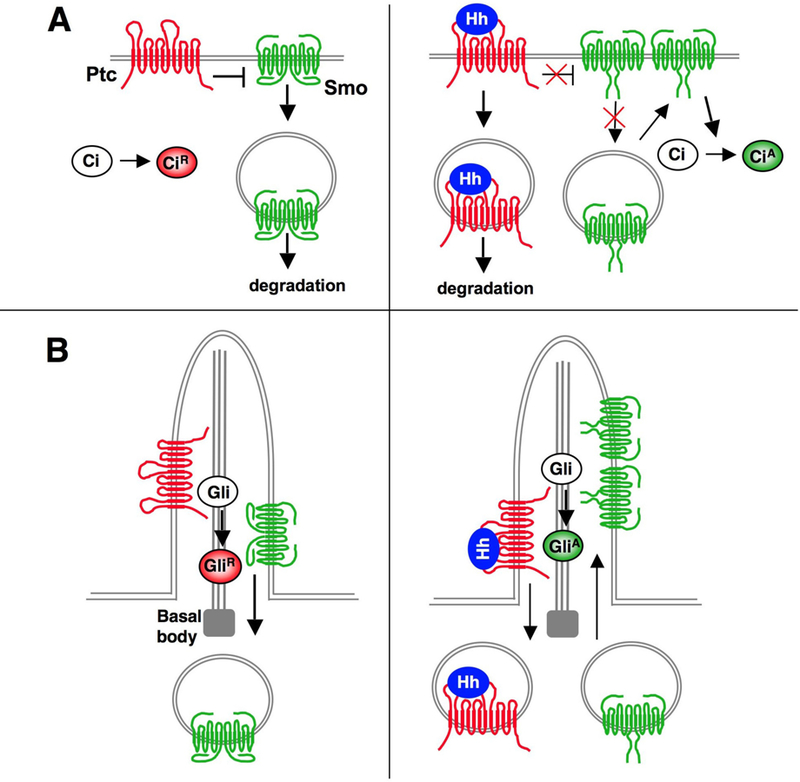
(A) In Drosophila, the absence of Hh allows Ptc to inhibit Smo phosphorylation, which promotes Smo endocytosis and degradation. Binding of Hh to Ptc stimulates Smo hyperphosphorylation, which increases Smo cell surface expression by inhibiting endocytosis and/or promoting recycling. In addition, phosphorylation of Smo promotes a conformational change leading to oligomerization of Smo C-tails
(B) In mammals, Ptc resides in the primary cilium and prevents Smo ciliary accumulation in the absence of Hh. Hh binding to Ptc promotes the exit of Ptc from the cilium and allows Smo to accumulate in the cilium. Hh also induces conformational change and clustering of Smo C-tails.
Through the analysis of Gli2 and Gli3 mutant mice, an unexpectedly complex and differential utilization of GliA and GliR function in various Hh-dependent processes during embryonic development was uncovered. For example, Shh is a mitogen and promotes cell proliferation in many embryonic and adult tissues. In the embryonic epidermis, GliA function is the principal effector of Shh-dependent cell proliferation (Mill et al., 2003) and overexpression of Gli1 or Gli2 can induce spontaneous skin tumorigenesis (Grachtchouk et al., 2000; Nilsson et al., 2000); however, loss of Gli3 function has minimal effects on hair follicle development and is unable to restore cell proliferation in Shh−/− skin (Mill et al., 2005). In many other tissues, GliA function is not essential for Shh-dependent cell proliferation and Shh appears to function primarily by preventing Gli3/GliR action (see for example Hu et al., 2006). Interestingly, there is evidence that Gli3R can block Wnt signaling by binding β-catenin and inhibiting its transcriptional activator activity (Ulloa et al., 2007). This mechanism may account for many contexts, such as the vertebrate neural tube, in which Hh and Wnt signaling promote cell proliferation in a cooperative or interdependent manner. However, a genome-wide in silico study has predicted a large number of mammalian enhancers harboring both Gli and Tcf binding sites (Hallikas et al., 2006), suggesting that shared enhancer elements may also represent a common mechanism integrating Hh and Wnt signaling. Thus, depending on the developmental context, Shh signaling regulates cell proliferation through a variety of mechanisms involving GliA activity or GliR inhibition.
In addition to regulating cell proliferation, Shh signaling also plays a critical role in the survival as well as patterning of neural progenitors. It promotes survival and proliferation of neural progenitor cells in the ventral spinal cord by inhibiting Gli3 (Litingtung and Chiang, 2000). However, Shh signaling variously utilizes GliA or GliR to control the expression of distinct sets of homeodomain proteins in different progenitor cell populations (Wijgerde et al., 2002). Similarly, GliA and GliR affect different sets of targets during Shh-induced sclerotome development (Buttitta et al., 2003). How GliA and GliR regulate the expression of different sets of target genes is not known since they bind to similar consensus sequences and genomic regions (Hallikas et al., 2006; Vokes et al., 2007; Vokes et al., 2008). It is very likely that other signaling pathways or transcription factors may influence the expression of Gli target genes. In addition, Gli proteins such as Gli3 may also bind target promoters indirectly through other DNA binding proteins (Vokes et al., 2008). Thus, characterization of Gli target genes at the genomic scale coupled to in-depth analysis of selected Gli targets, as well as identification of Gli interacting cofactors and other transcription factors that cooperate with Gli at specific target promoters, will be necessary to define sets of genes that are differentially regulated by GliA and GliR, and to understand the mechanisms by which these transcription factors act.
Hh signaling in tissue homeostasis and tumorigenesis
The Hh pathway has been implicated in the maintenance of stem or progenitor cells in many adult tissues, including the epithelia of many internal organs and brain (Beachy et al., 2004). Consistent with this, Hh signaling is critical for regeneration of the pulmonary epithelium (Watkins et al., 2003), prostate epithelium (Karhadkar et al., 2004), and exocrine pancreas (Fendrich et al., 2008). Importantly, abnormal Hh pathway activation in some of these tissues is also associated with tumorigenesis. Mutations in Hh pathway components, including Ptc1 and Smo, leading to pathway activation have been linked to basal cell carcinoma and medulloblastoma (Wicking et al., 1999). One hallmark of these tumors is constitutive pathway activation in the absence of Hh ligand. In contrast, ligand-dependent pathway activation is important for growth and/or survival of a wide variety of cancers, including gastrointestinal tumors, prostate cancer, hematological malignancies, and gliomas (Beachy et al., 2004; Lindemann, 2008; Ruiz i Altaba et al., 2007). These tumors generally do not harbor mutations of Hh pathway components and their growth can be effectively suppressed by various pathway inhibitors, such as Hh-neutralizing antibodies or Smo antagonist. These findings lead to the model that Hh ligands produced by these tumors and/or their stromal environment maintain stem cells in the tumor in an undifferentiated, proliferative state. A recent report provided an alternative interpretation of these observations and highlighted the importance of Hh signaling in promoting the tumor microenvironment (Yauch et al., 2008). In mouse xenograft models, Hh pathway activation was shown to be required in the stromal cells for supporting tumor growth; Hh ligands secreted by the tumor functions in a paracrine manner instead of directly acting on the tumor itself and, through yet to be determined mechanisms, pathway activation in the stromal microenvironment promotes tumor growth. Thus, Hh can influence tumor formation and/or growth through both cell autonomous and non-autonomous mechanisms.
Hh signaling and hematopoietic stem cells
Hh signaling plays a conserved regulatory role in the formation and/or maintenance of adult hematopoietic stem cells (HSC) in both Drosophila and vertebrates. In the absence of Hh signaling, Drosophila blood progenitor cells in the lymph gland are depleted due to premature differentiation (Mandal et al., 2007), definitive adult blood cells do not form in zebrafish embryos (Gering and Patient, 2005), and activation of hematopoiesis and vasculogenesis is inhibited in early postimplantation mouse embryos (Dyer et al., 2001). In adult mice, Ihh is expressed in bone marrow stroma, while Shh expression is found in lymph node and spleen stroma. Consistent with a role for these stroma-derived Hh signals in HSC homeostasis, Ihh overexpression in stromal cells promotes hematopoietic regeneration after bone marrow transplantation (Kobune et al., 2008) and Hh pathway activation induces cycling and expansion of primitive hematopoietic cells (Trowbridge et al., 2006). Furthermore, growth of the hematopoietic neoplasms, including multiple myeloma, lymphoma, and chronic myeloid leukemia, could be inhibited by Smo antagonist (Dierks et al., 2007; Peacock et al., 2007). Importantly, specific deletion of Smo in Bcr-Abl-positive chronic myeloid leukemic stem cells completely abolished tumor viability upon transplantation indicating that Hh signaling in the leukemic stem cell population is indeed essential for its maintenance (Dierks et al., 2008). Taken together, these observations suggest a critical role for stroma-derived Hh signals in the survival and expansion of cancer stem cells in hematologic malignancies.
Hh signaling and neural stem cells
The Hh pathway has been extensively analyzed in neural stem and progenitor cells (Ahn and Joyner, 2005; Balordi and Fishell, 2007; Dahmane et al., 2001; Lai et al., 2003; Machold et al., 2003; Palma et al., 2005; Wechsler-Reya and Scott, 1999). Besides its multifaceted roles in the specification, proliferation and differentiation of neural precursors during embryogenesis, Hh signaling is required for the maintenance of Hh-responsive Gli1+ quiescent neural stem cells in the adult brain (Ahn and Joyner, 2005; Balordi and Fishell, 2007; Palma et al., 2005). During cerebellar development, Shh secreted by the Purkinje cells promotes rapid proliferation of granule cell precursors in the external granular layer (Fig. 1B) (Wechsler-Reya and Scott, 1999), and Ptc1 mutations are commonly found in both familial and sporadic medulloblastomas. Studies of Ptc1 heterozygous Gorlin syndrome patients, as well as analogous mutant mice, have strongly suggested that Hh pathway activation is critical for the transformation of granule cell precursors (Goodrich et al., 1997). However, it seems likely that the ability of the pathway to act in this fashion is nonetheless dependent on cell type-specific determinants. For example, although the Hh pathway is activated in low- and high-grade human gliomas, and Hh signaling positively regulates the self-renewal of glioma cancer stem cells (Clement et al., 2007; Ehtesham et al., 2007), Gorlin syndrome patients do not develop gliomas. Consistent with this, two recent studies have demonstrated that Hh pathway activation in mouse neural stem cells or restricted neural progenitors induces only medulloblastoma, not glioma (Schuller et al., 2008; Yang et al., 2008). To understand how deregulated Hh signaling leads to medulloblastoma and participates in other tumors, it will be necessary to have a detailed understanding of how Hh target genes are regulated in different contexts, to confer the type of specificity discussed above. The cell autonomous determinants that control Gli action in the nucleus only represent a small fraction of the range of mechanisms that control the Hh response. For example, in many developmental contexts, there is considerable regulation at the level of production, dissemination, and presentation of Hh ligands.
Hh morphogen gradients and Ci/Gli activity gradients
Hh proteins are locally produced by many organizing tissues, including posterior compartment cells of Drosophila wing disc, vertebrate notochord and floor plate, and the zone of polarizing activity (ZPA) of developing vertebrate limb (Fig. 1C–1E). Secreted Hh proteins can move many cell diameters from their source of production and often control developmental outcomes in a concentration-dependent manner (Ingham and McMahon, 2001). For example, during ventral spinal cord patterning, Shh forms a ventral to dorsal gradient with different concentrations specifying distinct pools of neural progenitors (Fig. 1C), and there is evidence that Shh exerts its graded influence through Gli activity gradients (Stamataki et al., 2005).
In Drosophila wing development, graded responses to the Hh morphogen are mediated by differential regulation of CiR and CiA. CiR blocks the expression of a subset of Hh target genes, such as dpp, which encodes a TGFβ/BMP family of secreted protein essential for the growth and patterning of the entire wing, while the expression of other Hh target genes, such as ptc and en, is mediated by CiA (Methot and Basler, 1999). Accumulation of full-length Ci and expression of dpp are responsive to lower levels of Hh signaling and are observed in more anterior cells than activation of ptc and en, which requires higher levels of Hh (Fig. 1E); thus partial loss of Hh affects ptc and en expression but not dpp expression or Ci accumulation (Strigini and Cohen, 1997), suggesting that lower levels of Hh are needed to block Ci processing than to stimulate CiA. However, the regulation of CiR or CiA is unlikely to be a simple on/off switch but may occur in a graded fashion, and there is evidence for graded control of Hh signaling at multiple steps along the pathway including Smo and Ci (Jia et al., 2004; Smelkinson et al., 2007).
In vertebrate limb, early studies suggested that the Shh morphogenetic gradient is translated into an anterior-posterior GliR gradient through regulating Gli3 processing (Wang et al., 2000a). However, recent genetic studies have suggested that a Gli3R gradient is not absolutely required for Shh-dependent patterning of most digits; except for an extra digit 1, digit formation is grossly normal in mice containing one copy of an unprocessed form of Gli3 with multiple PKA sites mutated (Gli3P1−4) and another copy of truncated Gli3 (Gli3∆699, which lacks the C-terminal half and thus functions as a constitutive repressor)(Wang et al., 2007). It remains possible that a Gli3A gradient, contributed by Gli3P1−4, mediates the graded responses to Shh in these mice. It is also worth noting that, while Gli1 and Gli2 are dispensable for digit patterning (Park et al., 2000), they may contribute to limb development in Gli3P1−4/∆699 mice.
There are additional reasons to question whether a traditional morphogen gradient, like that seen in the fly wing or vertebrate neural tube, patterns the mammalian limb. For example, lineage analysis of Shh-expressing ZPA cells (Harfe et al., 2004) as well as recent mutant studies (Scherz et al., 2007; Zhu et al., 2008) indicate that autocrine Shh signaling (i.e. signaling in the ZPA cells, which give rise to all of digits 4 and 5 and part of digit 3) in the developing limb bud may be more important than previously thought. More importantly, increasing evidence suggests that developmental outcomes are controlled not only by the absolute concentration of Hh but also by the duration of exposure to Hh (Dessaud et al., 2007; Harfe et al., 2004; Scherz et al., 2007). In ventral neural tubes, genes regulated by high levels of Shh require longer exposure time to be turned on and thus are expressed at later stages of development than genes regulated by low levels of Shh (Dessaud et al., 2007; Jeong and McMahon, 2005; Stamataki et al., 2005). When exposed to different concentrations of Shh, neural plate explants initially have similar levels of Gli activity but exhibit diminishing levels of Gli activity over time at a rate that is inversely proportional to initial Shh concentration. This cell autonomous adaptation to Shh signaling is largely due to Shh-induced upregulation of Ptc1 (Dessaud et al., 2007), a conserved feedback mechanism that also shapes the Hh gradient (Briscoe et al., 2001; Chen and Struhl, 1996; Jeong and McMahon, 2005). Thus, continuous exposure to high levels of Shh sufficient to overcome accumulated Ptc is required to sustain high levels of Gli activity for long period of time, which is required for activation of high threshold Hh-responsive genes (Dessaud et al., 2007).
Hh gradient formation
The range of Hh signaling varies depending on the developmental context. As such, sophisticated molecular mechanisms have evolved to control the biosynthesis, secretion, and propagation of Hh ligands and to ensure the formation of tissue-appropriate activity gradients. In addition, the final shape of the Hh activity gradients is further regulated by feedback mechanisms.
During biosynthesis, the full-length Hh precursor undergoes autocleavage to release an N-terminal fragment (HhN) with a cholesterol moiety covalently linked to its C-terminus (Fig. 2)(Porter et al., 1996). Cholesterol modification is essential for the formation of a steep gradient by increasing the affinity of Hh for cell membranes and restricting its free dispersal (Burke et al., 1999; Li et al., 2006). HhN is further palmitoylated near its N-terminus by the acyltransferase Skinny Hedgehog (Ski/Skn)(Chamoun et al., 2001). Subsequent release of lipidated Hh into the extracellular space requires Dispatched (Disp), a transmembrane protein that is structurally related to Ptc (Burke et al., 1999). Using a biologically active GFP-tagged form of Shh, a recent study revealed a dynamic gradient of Shh::GFP protein in the ventral neural tube (Chamberlain et al., 2008). The distribution of Shh::GFP was modified by Smo and Skn mutations, indicating that both target field response and ligand lipidation regulate the Shh gradient. In Drosophila, lipoproteins have been implicated in transporting lipid-modified morphogens including Hh and Wnt/Wg (Panakova et al., 2005). Overall, a picture is emerging in which dual lipid modifications regulate the formation of large multimeric Hh complexes, and cellular regulation of these larger particles controls Hh movement over long distances (Guerrero and Chiang, 2007).
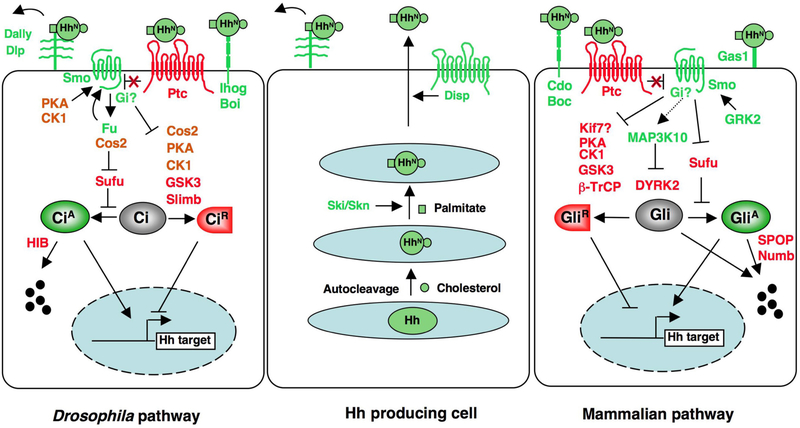
Figure 2. Sending and transducing the Hh signal.
In Hh-producing cells, full-length Hh is auto-catalytically cleaved to generate an N-terminal fragment (HhN) modified by cholesterol. HhN is palmitoylated by Ski/Skn. Secretion of dual lipid modified Hh is mediated by Disp. Movement of Hh requires the HSPGs, Dally and Dlp, in Drosophila. Hh signal reception is facilitated by Ihog/Boi in Drosophila and Cdo/Boc/Gas1 in mammals. In the absence of Hh, Ptc blocks Smo activation and full-length Ci/Gli (Gli2 and Gli3) is phosphorylated by multiple kinases and subsequently targeted to ubiquitin/proteasome-mediated proteolysis through SCFSlimb/β-TRCP to generate a truncated repressor form (CiR/GliR). In Drosophila, efficient phosphorylation of Ci requires the kinesin like protein Cos2, which acts as a molecular scaffold to bridge Ci and its kinases. Hh binding to Ptc blocks its inhibition of Smo. In Drosophila, this triggers Smo phosphorylation by PKA and CKI, leading to the cell surface accumulation and activation of Smo. Smo then recruits Cos2-Fu to activate Fu and dissociates Cos2-Ci-kinase complexes to inhibit Ci phosphorylation and processing. Furthermore, high levels of Hh stimulate CiA via Fu-mediated antagonism of Sufu. Hh signaling induces the expression of nuclear HIB that targets CiA for degradation. Fu-Cos2 is also involved in a feedback regulation of Smo phosphorylation. In mammalian systems, Fu homolog is not required for Hh signaling and the involvement of Cos2 homologs, such as Kif7, is uncertain. Sufu is the major Gli inhibitor. DYRK2 phosphorylates Gli2 and targets it for degradation whereas MAP3K10 activates Gli2 in part by inhibiting DYKR2. Smo phosphorylation requires GRK2. Gαi has been implicated in Hh signaling downstream of Smo in both Drosophila and vertebrates.
A related theme in the regulation of Hh protein distribution is based on known interactions with heparan sulfate proteoglycans (HSPGs). HSPGs are extracellular matrix proteins implicated in cell signaling mainly from genetic studies in Drosophila (Lin, 2004). Mutations that affect either the Drosophila EXT family of glycosyltransferases required for the biosynthesis of HSPGs or two members of the glypican subfamily of HSPGs, Dally and Dally-like (Dlp), impede Hh spread and reduce signaling range (Lin, 2004). HSPGs are not specific for Hh signaling but also regulate other signaling molecules including Wg/Wnt and Dpp/TGF-β (Lin, 2004). How do HSPGs engage individual signaling molecules? In Drosophila, a secreted protein homologous to the human Wnt inhibitory factor, Shifted (Shf), is important for Hh spreading and long range signaling in wing imaginal discs (Glise et al., 2005; Gorfinkiel et al., 2005). Shf interacts with both Hh and HSPGs, and may facilitate Hh binding to HSPGs. In the absence of Shf or HSPGs, cell surface Hh diminishes, suggesting that Shf and HSPGs cooperate to retain and stabilize Hh on the cell surface.
How HSPGs facilitate Hh movement is not understood but may involve dynamic Hh/HSPGs interactions. A recent study using optical imaging of fluorescence-tagged HhN expressed in Drosophila wing discs or cultured cells visualized nanoscale oligomers that form visible clusters with HSPGs (Vyas et al., 2008). Mutating a conserved lysine residue predicted to mediate electrostatic interaction at the interface between two HhN monomers affected HhN oligomerization, HhN/HSPG interaction, and higher-order clustering. Strikingly, this HhN variant failed to signal over a distance but retained normal autocrine signaling activity (Vyas et al., 2008). Thus, oligomerization, HSPG binding, and long-range signaling are all tightly correlated.
Modulation of Hh reception
In addition to regulating Hh spread, HSPGs can modulate Hh signaling intensity in a cell-autonomous fashion (Lum et al., 2003a; Gallet et al., 2008). The GPI linkage of Dlp promotes apical internalization and its subsequent targeting to the basolateral compartment of the epithelium, which appears to be essential for Dlp to enhance Hh signaling (Gallet et al., 2008). The role of HSPGs in vertebrate Hh signaling remains largely unexplored but a recent study reported that a member of the glypican subfamily of HSPGs, GPC3, is a negative regulator of Hh signaling (Capurro et al., 2008). GPC3 competes with Ptc for Hh binding and promotes Hh endocytosis and degradation, thereby reducing the cell surface Hh available for Ptc binding. The role of GPC3 in Hh signaling mirrors that of Hip1, a membrane bound glycoprotein that acts as a negative regulator of Hh signaling by competing with Ptc for Hh binding (Chuang and McMahon, 1999). Hip1 is induced by Hh and forms a negative feedback loop to restrict Hh signaling (Chuang et al., 2003; Jeong and McMahon, 2005). It remains to be determined whether GPC3 is also regulated by Hh and whether other mammalian glypicans are involved in Hh signaling.
Several recent studies revealed that Hh reception is modulated by additional Hh-binding proteins, including the Ihog/Cdo family of immunoglobin/fibronectin repeat containing proteins and the GPI anchored membrane bound protein Gas1 (Fig. 3) (Allen et al., 2007; Tenzen et al., 2006; Yao et al., 2006; Zhang et al., 2006b). Genetic studies in Drosophila and mice suggest that Ihog, Cdo, and Boc positively regulate Hh signaling (Tenzen et al., 2006; Yao et al., 2006; Zhang et al., 2006b), and Cdo can cooperate with Gas1 in doing so (Allen et al., 2007). The Ihog/Cdo family proteins bind Hh through fibronectin domains (Tenzen et al., 2006; Yao et al., 2006), and Ihog can enhance Hh binding to Ptc (Yao et al., 2006), suggesting that the Ihog/Cdo family members may function as Hh coreceptors to facilitate Hh signal reception.
Patched: an unusual receptor
Unlike other signaling pathways where receptors act as signal transducers to activate the pathways upon ligand binding, Ptc functions as a pathway inhibitor, blocking pathway activation in the absence of Hh. Ptc is therefore the focus of considerable interest and mystery. It is homologous to the RND family of prokaryotic proton-driven transporters, and several conserved features of RND-like transporters are essential for catalytic Ptc function in Smo inhibition, raising the interesting possibility that Ptc transports an endogenous small molecule Smo agonist or antagonist across membranes (Taipale et al., 2002). Indeed, a number of natural and synthetic small molecules can inhibit or activate Hh pathway at the level of Smo (Chen et al., 2002). In cultured cells, Ptc induces the secretion of Pro-Vitamin D3, and both Pro-Vitamin D3 and Vitamin D3 inhibit Hh signaling at high concentrations (Bijlsma et al., 2006), although regulation of a diffusible small molecule Smo inhibitor is incompatible with genetic studies suggesting that the function of Ptc is strictly cell-autonomous (Briscoe et al., 2001). Two recent studies identified oxysterols, which lie downstream of Vitamin D3 in the cholesterol biosynthetic pathway, as positive regulators of Hh signaling that act at a level upstream of Smo (Corcoran and Scott, 2006; Dwyer et al., 2007). It remains to be determined whether oxysterols or related molecules function as physiological Smo regulators and whether their subcellular distributions are controlled by Ptc.
Smoothened activation: phosphorylation, conformation, and trafficking
In Drosophila, Smo is phosphorylated and stabilized in response to Hh stimulation or Ptc inhibition (Denef et al., 2000). Studies of the relevant kinases (PKA and CKI) and phosphorylation sites in Smo C-terminal cytoplasmic tail (C-tail) indicate that phosphorylation represents an essential step in Smo activation (Jia et al., 2004). Further analysis of Smo C-tail suggested that these phosphorylation events counteract autoinhibition imposed by adjacent clusters of Arg residues (Zhao et al., 2007). Phosphorylation at individual clusters only neutralized adjacent Arg motifs, leading to incremental changes in Smo activity and providing a plausible mechanism for graded Smo activation. Smo belongs to the G protein coupled receptor (GPCR) superfamily and may share features characteristic of GPCRs, including dimerization/oligomerization and ligand-induced conformational change. Indeed, there is evidence that antagonistic interactions between the Arg clusters and Hh-induced phosphorylation regulate the conformation states and oligomerization of Smo C-tails, a key step in signal transduction (Zhao et al., 2007). Although the functionally relevant motifs within the Smo C-tail are not conserved between Drosophila and vertebrates, mammalian Smo may be regulated in an analogous manner by a long stretch of conserved basic residues (Zhao et al., 2007). Since mammalian Smo is phosphorylated via the G-protein coupled receptor kinase GRK2, which positively regulates Hh signaling (Chen et al., 2004; Meloni et al., 2006), GRK2 and perhaps other kinases may substitute for PKA and CK1 to regulate Smo conformation in vertebrates.
In Drosophila, Ptc restricts Smo cell surface expression by promoting Smo endocytosis and degradation, whereas Hh induces opposite changes in Ptc and Smo subcellular distribution with Smo accumulating on the cell surface and Ptc entering the cytoplasm (Fig. 3A) (Denef et al., 2000; Jia et al., 2004; Zhu et al., 2003). How Hh and Ptc reciprocally regulate Smo trafficking is not clear but it is mediated, at least in part, by Smo phosphorylation, as phosphorylation-deficient Smo variants fail to accumulate on the cell surface in response to Hh (Jia et al., 2004). Conversely, phospho-mimicking or Arg cluster mutations lead to constitutive cell surface accumulation of Smo, suggesting that Hh-induced phosphorylation may enhance Smo cell surface expression by antagonizing Arg-mediated endocytosis and degradation (Zhao et al., 2007).
A similar reciprocal trafficking relationship has been observed for mammalian Ptc1 and Smo but this occurs in the primary cilium, a microtubule-based cell surface protrusion present in most, if not all, mammalian cells (Fig. 3B). Recent studies have implicated primary cilia as cellular organelles associated with mammalian Hh signaling (Garcia-Garcia et al., 2005). Mutations in several components of the intraflagellar transport (IFT) machinery required for the establishment and/or maintenance of cilia and flagella affect Hh signaling in several developmental contexts, including neural tube patterning, limb development, and adult neural stem cell formation (Han et al., 2008; Haycraft et al., 2005; Huangfu et al., 2003; Liu et al., 2005; May et al., 2005). In addition, all the major Hh pathway components, including Ptc1, Smo, and Gli proteins, at least partially localize to cilia (Corbit et al., 2005; Haycraft et al., 2005; May et al., 2005; Rohatgi et al., 2007). In the absence of Hh, Ptc localizes to cilia and prevents Smo from accumulating in the cilia; binding of Hh to Ptc triggers reciprocal trafficking of Ptc and Smo, with Ptc moving out of and Smo accumulating in the cilia (Fig. 5B) (Rohatgi et al., 2007). Ciliary localization of Smo correlates with Hh pathway activation: both an oncogenic Smo mutation and Smo agonists, such as SAG and oxysterols, promoted accumulation of Smo in the cilia (Corbit et al., 2005; Rohatgi et al., 2007). In addition to enrich Hh signaling components, cilia may function as a signaling center to orchestrate dynamic and ordered interactions among Hh signaling components, leading to the production of GliA or GliR. In support of this notion, defects in cilia architecture can differentially affect Gli transcription factors (Caspary et al., 2007).
How does Ptc restrict Smo ciliary accumulation? Smo may constantly move in and out of the cilia by binding to anterograde and retrograde IFT motors; Ptc and Hh signaling may tip this balance by modulating Smo/IFT motor interactions. In support of this model, Smo is constitutively enriched in the cilia of mouse embryonic fibroblast (MEF) cells defective in retrograde IFT (Ocbina and Anderson, 2008). Furthermore, β-arrestins, which normally bind and regulate GPCRs, promote Smo ciliary localization by mediating its association with the anterograde IFT motor kinesin-II in response to Hh (Kovacs et al., 2008). As GRK2 promotes Smo phosphorylation and its association with β-arrestin 2 (Chen et al., 2004), it is tempting to speculate that Hh-induced phosphorylation through GRK2 promotes the loading of Smo onto the kinesin-II motor via recruiting β-arrestins, leading to accelerated transport of Smo into the cilia. In this regard, Ptc may restrict Smo ciliary localization by preventing its phosphorylation. However, some mutations affecting cilia formation result in diverse and unexpected effects on Hh signaling (Caspary et al., 2007), suggesting that the relationship between cilia and Hh signaling is unlikely to be limited to a simple requirement for anterograde IFT of Smo and other Hh signaling components.
Intracellular signal transduction
The intracellular signal relay systems for Drosophila and mammalian Hh pathways have diverged but both culminate at the activation of the latent Ci/Gli transcription factor (Fig. 2). Ci/Gli activity is regulated by multiple mechanisms including phosphorylation, proteolysis, cytoplasmic/nuclear shuttling, and protein-protein interactions. PKA, GSK3, and CK1 sequentially phosphorylate multiple clusters of sites in the C-terminal region of Ci/Gli, resulting in the recruitment of Slimb/β-TRCP, the F-box subunit of an SCF E3 ubiquitin ligase, SCFSlimb/β-TRCP (Jiang, 2006; Smelkinson et al., 2007). Ubiquitination by SCFSlimb/β-TRCP targets Ci/Gli for proteasome-mediated degradation that selectively removes its C-terminal half, resulting in a truncated form that contains the Zn finger DNA binding domain and the N-terminal region thought to recruit a corepressor(s). How the proteasome selectively degrades the C-terminal half of Ci/Gli is not fully understood but a domain (PDD for processing determinant domain) situated between the Slimb/β-TRCP binding domain and the Zn-finger DNA binding domain in Ci/Gli appears to be critical (Pan and Wang, 2007)(Wang and Price, 2008). Deletion of this domain from Ci renders complete degradation of Ci (Smelkinson et al., 2007). The PDD in Gli3 acts more effectively in promoting partial degradation than the PDD in Gli2, which explains in part why Gli3 is processed more efficiently than Gli2 (Pan and Wang, 2007). How PDD domains halt complete degradation by the proteasome remains to be determined. One scenario is that they may direct the proteasome to cleave Ci/Gli internally and that the tightly folded Zn-finger domain of Ci/Gli3 can escape from degradation after the internal cleavage (Jiang, 2006).
The requirement of extensive phosphorylation for SCFSlimb/β-TRCP recruitment and Ci/Gli processing raises the question of how efficient Ci/Gli phosphorylation is achieved. In Drosophila, Ci processing depends on the kinesin-like protein Costal-2 (Cos2) that acts as a molecular scaffold to bring Ci and its kinases together, leading to efficient phosphorylation of Ci (Zhang et al., 2005). Using live cell imaging, a recent study has revealed that Cos2 can move along microtubules (Farzan et al., 2008). A point mutation in the putative ATP binding site of Cos2 motor domain abolished its motility as well as its ability to promote Ci processing. However, it is not clear whether the motor activity of Cos2 is required for Ci phosphorylation or for its subsequent targeting to the ubiquination/proteasome machinery. In zebrafish, the kinesin protein Kif7 has been identified as a putative Cos2 homolog (Tay et al., 2005). Kif7 can physically interact with Gli1 but whether it plays a role in Gli phosphorylation is unknown. In contrast, overexpression and knockdown of mammalian Cos2 homologs, including Kif7 and Kif27, in cultured cells did not perturb Hh signaling activity (Varjosalo et al., 2006). Thus, whether Gli proteins are regulated by a Cos2 homolog in mammals remains an unresolved issue. It has been suggested that the scaffolding role of Cos2 in Drosophila could correspond to that of primary cilia in mammals since the production of GliR is compromised in mice with defects in the formation and/or function of primary cilia (Haycraft et al., 2005; Huangfu and Anderson, 2005; Liu et al., 2005). As proteasomes are enriched at centrosomes that give rise to the basal body underneath the primary cilia (Wigley et al., 1999), Gli proteins might be phosphorylated at primary cilia and then targeted to the centrosome-associated proteasomes for proteolysis.
In addition to Slimb/β-TRCP-mediated proteolysis, Ci/Gli is also regulated by mechanisms that control its subcellular localization and transcriptional activity. In Drosophila, Ci forms a complex with Cos2 and the Ser/Thr kinase Fused (Fu) that impedes Ci nuclear translocation through microtubule tethering and by masking the Ci nuclear localization signals (Wang et al., 2000b; Wang and Jiang, 2004; Wang and Holmgren, 2000). The activity of full-length Ci is further blocked by stoichiometric binding of Sufu (Ohlmeyer and Kalderon, 1998), which appears to be the predominant mechanism for restricting Ci activity when Ci proteolysis is blocked (Smelkinson et al., 2007). The mechanism by which Sufu inhibits Ci remains poorly understood but may involve regulating Ci subcellular localization and transcriptional activity in the nucleus (Methot and Basler, 2000; Wang et al., 2000b). A striking difference between Drosophila and mammalian Hh signal transduction is the role of Sufu. In Drosophila, loss of Sufu does not elicit ectopic Hh signaling, likely due to the presence of multiple parallel inhibitory mechanisms. In mammals, however, loss of Sufu leads to ectopic Hh pathway activation similar to loss of Ptc1 (Svard et al., 2006; Varjosalo et al., 2006), indicating that Sufu is the major intracellular inhibitor of Gli activity. Sufu may have assumed a more important inhibitory function in the mammalian Hh pathway due to the existence of multiple GliA forms. To inhibit GliA function, Sufu could impede Gli nuclear localization or suppress Gli activity by recruiting a corepressor complex (Cheng and Bishop, 2002). Furthermore, Sufu may have adopted new functions during evolution. As Sufu is also found in the primary cilia (Haycraft et al., 2005), it will be interesting to examine whether Sufu plays a role in Gli processing and/or degradation.
How is Hh signal relayed from Smo to Ci/Gli? Smo is related to GPCRs; however, evidence for the involvemnt of trimeric G proteins in Hh signaling has been obscured. While several studies using pharmacological inhibitors and overexpression implied that Smo might couple to several G proteins (DeCamp et al., 2000; Kasai et al., 2004; Riobo et al., 2006b), others disputed such coupling or its biological significance (Murone et al., 1999; Low et al., 2008). A recent study provided genetic evidence that Gαi participates in Hh signaling likely downstream of Smo in Drosophila (Ogden et al., 2008). Severe or near null Gαi mutants were viable but exhibited reduced wing size, a phenotype indicative of reduced dpp expression (Ogden et al., 2008). Indeed, Gαi mutations diminished dpp expression in a cell autonomous fashion whereas a constitutively active Gαi caused ectopic dpp expression (Ogden et al., 2008). Furthermore, Hh reduced the basal level of cAMP through Smo and Gαi in cl8 cells, implying that Hh may regulate PKA activity by modulating the intracellular cAMP concentration (Ogden et al., 2008). However, early studies demonstrated that low levels of a constitutively active, cAMP-independent PKA can substitute for the endogenous PKA to confer normal Hh signal transduction, suggesting that Hh regulates Ci through a cAMP-independent mechanism (Li et al., 1995; Jiang and Struhl, 1995). In addition, a global regulation of PKA activity through reducing cAMP concentration is not compatible with the observation that Hh induces increased Smo phosphorylation by PKA (Jia et al., 2004). Therefore, it remains to be determined to what extent the observed downregulation of cAMP level contributes to Hh signaling and whether Gαi modulates Hh signaling activity through other effectors. On the other hand, it has been well documented that Smo directly interacts with the intracellular signaling complexes containing Cos2, Fu, and Ci through its C-tail, and such interaction mediates Hh signal transduction (Jia and Jiang, 2006). Binding of Smo to Cos2 may inhibit its ability to promote Ci phosphorylation and processing as treating with Hh-conditoned medium or overexpressing a membrane tethered Smo C-tail attenuated Cos2-Ci-kinase complex formation in cultured cells (Zhang et al., 2005).
The association of the Cos2-Fu-Ci complex with Smo is dynamic. Recent studies have revealed that Fu and Cos2 engage in a positive feedback to promote Smo phosphorylation in addition to their traditional roles as signal transducers acting downstream of Smo (Claret et al., 2007; Liu et al., 2007). Cos2, Fu, and Ci can be coimmunoprecipitated with Smo, and Cos2 colocalizes with Smo in the absence of Hh (Lum et al., 2003b; Ruel et al., 2003), implying constitutive association of Cos2-Fu-Ci with Smo in quiescent cells. Two regions of SmoC have been implicated in Cos2 binding: a membrane proximal region between aa651–686 that appears to bind Cos2 directly (Lum et al., 2003b), and a C-terminal region between aa818–1035 that is essential for Smo activity in vivo (Jia et al., 2003), although direct association of this region with Cos2 has not been established. It is possible that Cos2 interacts with the membrane proximal region in the absence of Hh and such interaction could interfere with Smo phosphorylation. Upon Hh stimulation, Fu-mediated phosphorylation dissociates Cos2 from the membrane proximal region to permit Smo hyperphosphorylation (Liu et al., 2007), and activated Smo adopts an open conformation and may re-associate with Cos2-Fu through its exposed C-terminal domain. In addition, Hh-induced oligomerization of Smo C-tails may facilitate clustering of Cos2-Fu complexes, leading to phosphorylation and activation of the Fu kinase (Zhao et al., 2007).
While the zebrafish Fu homolog play an analogous role in Hh signaling (Wolff et al., 2003), knockout of mouse Fu homolog did not produce any Hh-related phenotypes (Merchant et al., 2005; Chen et al., 2005). On the other hand, several protein kinases, including DYRK2, MAP3K10, and Cdc2l1 have been shown to influence Gli activity (Varjosalo et al., 2008; Evangelista et al., 2008). DYRK2 can directly phosphorylate Gli2, which targets Gli2 for proteasome-mediated degradation, and MAP3K10 activates Gli2 indirectly by modulating DYRK2 and possibly other Hh pathway components (Varjosalo et al., 2008). Cdc2l1 interacts with Sufu to relieve its inhibition on Gli (Evangelista et al., 2008). In addition, several recent studies suggest that PI3-AKT, RAS-MEK1, and PKC-δ pathways can modulate Hh signaling by regulating the activity and/or nuclear localization of Gli proteins and integration of these oncogenic pathways with Hh signaling may promote tumorigenesis (Pasca di Magliano et al., 2006; Riobo et al., 2006a; Stecca et al., 2007).
Signal termination
Hh signaling utilizes a variety of feedback mechanisms to control the amplitude and duration of its pathway activity. In addition to the aforementioned mechanisms operating at the level of signal reception, such as upregulation of Ptc and Hip, mechanisms acting at the level of Ci/Gli have also been identified. In Drosophila embryos and wing imaginal discs, Hh induces the expression of hib (also called rdx), which encodes a BTB/MATH domain-containing protein that acts as the substrate recognition component of a Cul3-based E3 ubiquitin ligase complex (Kent et al., 2006; Zhang et al., 2006a). HIB binds and degrades the active form of Ci, forming a negative feedback loop to tune down Hh signaling activity (Zhang et al., 2006a). Interestingly, HIB is also highly expressed in differentiating cells posterior to the morphogenetic furrow in eye imaginal discs where HIB acts together with Cul3 to degrade Ci, thereby limiting the duration of Hh signaling (Zhang et al., 2006a; Kent et al., 2006). Failure to restrict Hh signaling in differentiating cells, as seen in hib or cul3 mutant clones, leads to excessive cell proliferation and disruption in cell patterning (Ou et al., 2007; (Zhang et al., 2006a; Kent et al., 2006). Homologous BTB/MATH proteins, such as SPOP, exist in mammals; however, their roles in development and tumorigenesis await further study. Regardless, it is likely that multiple analogous signal-terminating mechanisms exist. For example, during cerebellar development, granule neuron progenitors (GNPs) proliferate in response to Shh (Wechsler-Reya and Scott, 1999), but as they differentiate, GNPs upregulate Numb, which then acts in conjunction with the E3 ubiquitin ligase Itch to target Gli1 for ubiquitination and degradation (Di Marcotullio et al., 2006). Malfunction of such mechanisms may cause excessive progenitor cell proliferation, leading to tumor formation. Indeed, Numb expression is downregulated in GNP-derived cancer cells and forced expression of Numb in medulloblastoma cells induces growth arrest and neural differentiation (Di Marcotullio et al., 2006).
Conclusion and future prospects
The past decade has witnessed an explosion of information regarding the multifaceted roles of Hh signaling in development and disease as well as the molecular and cellular mechanisms underlying Hh signal production and transduction. However, important questions regarding many aspects of Hh signaling mechanism as well as its physiological and pathological roles remain to be answered. For example, the precise mechanisms of Hh gradient formation are still not well understood. The biochemical activity of Ptc has not been elucidated, thus how Ptc regulates Smo phosphorylation, conformation, and trafficking remained to be determined. The requirement of the primary cilium for the production of both GliA and GliR in mammals raises important questions about how this unique cellular organelle perceives and transduces Hh signals. The mammalian Hh signaling mechanism immediately downstream of Smo is still poorly defined and important gaps may exist between Smo and Gli proteins. The G protein pathway(s) that modulates Hh signaling activity remains to be defined. Sufu plays a conserved role in restricting CiA/GliA activity but the precise mechanism remains unknown. Neither is it clear how Hh alleviates Sufu inhibition. Perhaps not all the Hh responses are mediated by Gli proteins, as has been suggested for the regulation of axon guidance by Shh (Okada et al., 2006) and Shh-mediated cell migration (Lipinski et al., 2008). On the other hand, evidence for non-canonical activation of Gli by other signaling pathways has emerged (Riobo et al., 2006a; Stecca et al., 2007), although the role of such regulation during development has remained largely unexplored. The diverse biological effects of Hh signaling likely rely on tissue specific factors as well as other spatially and temporally regulated signals, many of which remain unidentified. How cells integrate different concentrations, durations, and combinations of multiple signals to orchestrate gene expression programs that execute distinct cellular behaviors also remains a challenge for future studies. Answers to these and other questions will not only provide important insights into the fundamental problems in developmental biology such as how positional information is generated and interpreted to control cell behavior but may also lead to new strategies for the diagnosis and therapeutic treatment of cancer.
Acknowledgments
We thank Drs. Keith Wharton, Brian Ciruna, and Stephane Angers, and members of the Jiang and Hui laboratories for comments. We apologize to colleagues whose original researches on many aspects of Hh signalling were not cited due to space limitations. J. J. is supported by grants from NIH, Leukemia and Lymphoma Society Scholar Program, and Welch Foundation, and C. C. H. is supported by National Cancer Institute of Canada.
References
- Ahn S, and Joyner AL (2005). In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature 437, 894–897. [DOI] [PubMed] [Google Scholar]
- Allen BL, Tenzen T, and McMahon AP (2007). The Hedgehog-binding proteins Gas1 and Cdo cooperate to positively regulate Shh signaling during mouse development. Genes & development 21, 1244–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balordi F, and Fishell G (2007). Hedgehog signaling in the subventricular zone is required for both the maintenance of stem cells and the migration of newborn neurons. J Neurosci 27, 5936–5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachy PA, Karhadkar SS, and Berman DM (2004). Tissue repair and stem cell renewal in carcinogenesis. Nature 432, 324–331. [DOI] [PubMed] [Google Scholar]
- Bijlsma MF, Spek CA, Zivkovic D, van de Water S, Rezaee F, and Peppelenbosch MP (2006). Repression of smoothened by patched-dependent (pro-)vitamin D3 secretion. PLoS biology 4, e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J, Chen Y, Jessell TM, and Struhl G (2001). A hedgehog-insensitive form of patched provides evidence for direct long-range morphogen activity of sonic hedgehog in the neural tube. Molecular cell 7, 1279–1291. [DOI] [PubMed] [Google Scholar]
- Burke R, Nellen D, Bellotto M, Hafen E, Senti KA, Dickson BJ, and Basler K (1999). Dispatched, a novel sterol-sensing domain protein dedicated to the release of cholesterol-modified hedgehog from signaling cells. Cell 99, 803–815. [DOI] [PubMed] [Google Scholar]
- Buttitta L, Mo R, Hui CC, and Fan CM (2003). Interplays of Gli2 and Gli3 and their requirement in mediating Shh-dependent sclerotome induction. Development 130, 6233–6243. [DOI] [PubMed] [Google Scholar]
- Capurro MI, Xu P, Shi W, Li F, Jia A, and Filmus J (2008). Glypican-3 inhibits Hedgehog signaling during development by competing with patched for Hedgehog binding. Developmental cell 14, 700–711. [DOI] [PubMed] [Google Scholar]
- Caspary T, Larkins CE, and Anderson KV (2007). The graded response to Sonic Hedgehog depends on cilia architecture. Developmental cell 12, 767–778. [DOI] [PubMed] [Google Scholar]
- Chamberlain CE, Jeong J, Guo C, Allen BL, and McMahon AP (2008). Notochord-derived Shh concentrates in close association with the apically positioned basal body in neural target cells and forms a dynamic gradient during neural patterning. Development (Cambridge, England) 135, 1097–1106. [DOI] [PubMed] [Google Scholar]
- Chamoun Z, Mann RK, Nellen D, von Kessler DP, Bellotto M, Beachy PA, and Basler K (2001). Skinny hedgehog, an acyltransferase required for palmitoylation and activity of the hedgehog signal. Science (New York, N.Y) 293, 2080–2084. [DOI] [PubMed] [Google Scholar]
- Chen JK, Taipale J, Young KE, Maiti T, and Beachy PA (2002). Small molecule modulation of Smoothened activity. Proceedings of the National Academy of Sciences of the United States of America 99, 14071–14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MH, Gao N, Kawakami T, and Chuang PT (2005). Mice deficient in the fused homolog do not exhibit phenotypes indicative of perturbed hedgehog signaling during embryonic development. Molecular and cellular biology 25, 7042–7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Ren XR, Nelson CD, Barak LS, Chen JK, Beachy PA, de Sauvage F, and Lefkowitz RJ (2004). Activity-dependent internalization of smoothened mediated by beta-arrestin 2 and GRK2. Science (New York, N.Y) 306, 2257–2260. [DOI] [PubMed] [Google Scholar]
- Chen Y, and Struhl G (1996). Dual roles for patched in sequestering and transducing Hedgehog. Cell 87, 553–563. [DOI] [PubMed] [Google Scholar]
- Cheng SY, and Bishop JM (2002). Suppressor of Fused represses Gli-mediated transcription by recruiting the SAP18-mSin3 corepressor complex. Proceedings of the National Academy of Sciences of the United States of America 99, 5442–5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang PT, Kawcak T, and McMahon AP (2003). Feedback control of mammalian Hedgehog signaling by the Hedgehog-binding protein, Hip1, modulates Fgf signaling during branching morphogenesis of the lung. Genes & development 17, 342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang PT, and McMahon AP (1999). Vertebrate Hedgehog signalling modulated by induction of a Hedgehog-binding protein. Nature 397, 617–621. [DOI] [PubMed] [Google Scholar]
- Claret S, Sanial M, and Plessis A (2007). Evidence for a novel feedback loop in the Hedgehog pathway involving Smoothened and Fused. Curr Biol 17, 1326–1333. [DOI] [PubMed] [Google Scholar]
- Clement V, Sanchez P, de Tribolet N, Radovanovic I, and Ruiz i Altaba A (2007). HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol 17, 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, and Reiter JF (2005). Vertebrate Smoothened functions at the primary cilium. Nature 437, 1018–1021. [DOI] [PubMed] [Google Scholar]
- Corcoran RB, and Scott MP (2006). Oxysterols stimulate Sonic hedgehog signal transduction and proliferation of medulloblastoma cells. Proceedings of the National Academy of Sciences of the United States of America 103, 8408–8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmane N, Sanchez P, Gitton Y, Palma V, Sun T, Beyna M, Weiner H, and Ruiz i Altaba A (2001). The Sonic Hedgehog-Gli pathway regulates dorsal brain growth and tumorigenesis. Development 128, 5201–5212. [DOI] [PubMed] [Google Scholar]
- DeCamp DL, Thompson TM, de Sauvage FJ, and Lerner MR (2000). Smoothened activates Galphai-mediated signaling in frog melanophores. The Journal of biological chemistry 275, 26322–26327. [DOI] [PubMed] [Google Scholar]
- Denef N, Neubuser D, Perez L, and Cohen SM (2000). Hedgehog induces opposite changes in turnover and subcellular localization of patched and smoothened. Cell 102, 521–531. [DOI] [PubMed] [Google Scholar]
- Dessaud E, Yang LL, Hill K, Cox B, Ulloa F, Ribeiro A, Mynett A, Novitch BG, and Briscoe J (2007). Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature 450, 717–720. [DOI] [PubMed] [Google Scholar]
- Di Marcotullio L, Ferretti E, Greco A, De Smaele E, Po A, Sico MA, Alimandi M, Giannini G, Maroder M, Screpanti I, et al. (2006). Numb is a suppressor of Hedgehog signalling and targets Gli1 for Itch-dependent ubiquitination. Nature cell biology 8, 1415–1423. [DOI] [PubMed] [Google Scholar]
- Dierks C, Beigi R, Guo GR, Zirlik K, Stegert MR, Manley P, Trussell C, Schmitt-Graeff A, Landwerlin K, Veelken H, et al. (2008). Expansion of Bcr-Abl-positive leukemic stem cells is dependent on Hedgehog pathway activation. Cancer cell 14, 238–249. [DOI] [PubMed] [Google Scholar]
- Dierks C, Grbic J, Zirlik K, Beigi R, Englund NP, Guo GR, Veelken H, Engelhardt M, Mertelsmann R, Kelleher JF, et al. (2007). Essential role of stromally induced hedgehog signaling in B-cell malignancies. Nat Med 13, 944–951. [DOI] [PubMed] [Google Scholar]
- Dwyer JR, Sever N, Carlson M, Nelson SF, Beachy PA, and Parhami F (2007). Oxysterols are novel activators of the hedgehog signaling pathway in pluripotent mesenchymal cells. The Journal of biological chemistry 282, 8959–8968. [DOI] [PubMed] [Google Scholar]
- Dyer MA, Farrington SM, Mohn D, Munday JR, and Baron MH (2001). Indian hedgehog activates hematopoiesis and vasculogenesis and can respecify prospective neurectodermal cell fate in the mouse embryo. Development 128, 1717–1730. [DOI] [PubMed] [Google Scholar]
- Ehtesham M, Sarangi A, Valadez JG, Chanthaphaychith S, Becher MW, Abel TW, Thompson RC, and Cooper MK (2007). Ligand-dependent activation of the hedgehog pathway in glioma progenitor cells. Oncogene 26, 5752–5761. [DOI] [PubMed] [Google Scholar]
- Evangelista M, Lim TY, Lee J, Parker L, Ashique A, Peterson AS, Ye W, Davis DP, and de Sauvage FJ (2008). Kinome siRNA screen identifies regulators of ciliogenesis and hedgehog signal transduction. Science signaling 1, ra7. [DOI] [PubMed] [Google Scholar]
- Farzan SF, Ascano M Jr., Ogden SK, Sanial M, Brigui A, Plessis A, and Robbins DJ (2008). Costal2 Functions as a Kinesin-like Protein in the Hedgehog Signal Transduction Pathway. Curr Biol 18, 1215–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendrich V, Esni F, Garay MV, Feldmann G, Habbe N, Jensen JN, Dor Y, Stoffers D, Jensen J, Leach SD, et al. (2008). Hedgehog signaling is required for effective regeneration of exocrine pancreas. Gastroenterology 135, 621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallet A, Staccini-Lavenant L, and Therond PP (2008). Cellular trafficking of the glypican Dally-like is required for full-strength Hedgehog signaling and wingless transcytosis. Developmental cell 14, 712–725. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia MJ, Eggenschwiler JT, Caspary T, Alcorn HL, Wyler MR, Huangfu D, Rakeman AS, Lee JD, Feinberg EH, Timmer JR, et al. (2005). Analysis of mouse embryonic patterning and morphogenesis by forward genetics. Proceedings of the National Academy of Sciences of the United States of America 102, 5913–5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gering M, and Patient R (2005). Hedgehog signaling is required for adult blood stem cell formation in zebrafish embryos. Dev Cell 8, 389–400. [DOI] [PubMed] [Google Scholar]
- Glise B, Miller CA, Crozatier M, Halbisen MA, Wise S, Olson DJ, Vincent A, and Blair SS (2005). Shifted, the Drosophila ortholog of Wnt inhibitory factor-1, controls the distribution and movement of Hedgehog. Developmental cell 8, 255–266. [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Milenkovic L, Higgins KM, and Scott MP (1997). Altered neural cell fates and medulloblastoma in mouse patched mutants. Science (New York, N.Y) 277, 1109–1113. [DOI] [PubMed] [Google Scholar]
- Gorfinkiel N, Sierra J, Callejo A, Ibanez C, and Guerrero I (2005). The Drosophila ortholog of the human Wnt inhibitor factor Shifted controls the diffusion of lipid-modified Hedgehog. Developmental cell 8, 241–253. [DOI] [PubMed] [Google Scholar]
- Grachtchouk M, Mo R, Yu S, Zhang X, Sasaki H, Hui CC, and Dlugosz AA (2000). Basal cell carcinomas in mice overexpressing Gli2 in skin. Nat Genet 24, 216–217. [DOI] [PubMed] [Google Scholar]
- Guerrero I, and Chiang C (2007). A conserved mechanism of Hedgehog gradient formation by lipid modifications. Trends Cell Biol 17, 1–5. [DOI] [PubMed] [Google Scholar]
- Hallikas O, Palin K, Sinjushina N, Rautiainen R, Partanen J, Ukkonen E, and Taipale J (2006). Genome-wide prediction of mammalian enhancers based on analysis of transcription-factor binding affinity. Cell 124, 47–59. [DOI] [PubMed] [Google Scholar]
- Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo JM, Aguilar A, Schneider-Maunoury S, and Alvarez-Buylla A (2008). Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nature neuroscience 11, 277–284. [DOI] [PubMed] [Google Scholar]
- Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, and Tabin CJ (2004). Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell 118, 517–528. [DOI] [PubMed] [Google Scholar]
- Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, and Yoder BK (2005). Gli2 and gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet 1, e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu MC, Mo R, Bhella S, Wilson CW, Chuang PT, Hui CC, and Rosenblum ND (2006). GLI3-dependent transcriptional repression of Gli1, Gli2 and kidney patterning genes disrupts renal morphogenesis. Development 133, 569–578. [DOI] [PubMed] [Google Scholar]
- Huangfu D, and Anderson KV (2005). Cilia and Hedgehog responsiveness in the mouse. Proceedings of the National Academy of Sciences of the United States of America 102, 11325–11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, and Anderson KV (2003). Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426, 83–87. [DOI] [PubMed] [Google Scholar]
- Ingham PW, and McMahon AP (2001). Hedgehog signaling in animal development: paradigms and principles. Genes & development 15, 3059–3087. [DOI] [PubMed] [Google Scholar]
- Jeong J, and McMahon AP (2005). Growth and pattern of the mammalian neural tube are governed by partially overlapping feedback activities of the hedgehog antagonists patched 1 and Hhip1. Development (Cambridge, England) 132, 143–154. [DOI] [PubMed] [Google Scholar]
- Jia J, and Jiang J (2006). Decoding the Hedgehog signal in animal development. Cell Mol Life Sci 63, 1249–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Tong C, and Jiang J (2003). Smoothened transduces Hedgehog signal by physically interacting with Costal2/Fused complex through its C-terminal tail. Genes & development 17, 2709–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Tong C, Wang B, Luo L, and Jiang J (2004). Hedgehog Signalling Activity of Smoothened Requires Phosphorylation by Protein Kinase A and Casein Kinase I. Nature 432, 1045–1050. [DOI] [PubMed] [Google Scholar]
- Jiang J (2006). Regulation of Hh/Gli signaling by dual ubiquitin pathways. Cell cycle (Georgetown, Tex 5, 2457–2463. [DOI] [PubMed] [Google Scholar]
- Jiang J, and Struhl G (1995). Protein kinase A and Hedgehog signalling in Drosophila limb development. Cell 80, 563–572. [DOI] [PubMed] [Google Scholar]
- Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, Maitra A, Isaacs JT, Berman DM, and Beachy PA (2004). Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature 431, 707–712. [DOI] [PubMed] [Google Scholar]
- Kasai K, Takahashi M, Osumi N, Sinnarajah S, Takeo T, Ikeda H, Kehrl JH, Itoh G, and Arnheiter H (2004). The G12 family of heterotrimeric G proteins and Rho GTPase mediate Sonic hedgehog signalling. Genes Cells 9, 49–58. [DOI] [PubMed] [Google Scholar]
- Kent D, Bush EW, and Hooper JE (2006). Roadkill attenuates Hedgehog responses through degradation of Cubitus interruptus. Development (Cambridge, England) 133, 2001–2010. [DOI] [PubMed] [Google Scholar]
- Kobune M, Kato J, Kawano Y, Sasaki K, Uchida H, Takada K, Takahashi S, Takimoto R, and Niitsu Y (2008). Adenoviral vector-mediated transfer of the Indian hedgehog gene modulates lymphomyelopoiesis in vivo. Stem Cells 26, 534–542. [DOI] [PubMed] [Google Scholar]
- Kovacs JJ, Whalen EJ, Liu R, Xiao K, Kim J, Chen M, Wang J, Chen W, and Lefkowitz RJ (2008). Beta-arrestin-mediated localization of smoothened to the primary cilium. Science (New York, N.Y 320, 1777–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai K, Kaspar BK, Gage FH, and Schaffer DV (2003). Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci 6, 21–27. [DOI] [PubMed] [Google Scholar]
- Li W, Ohlmeyer JT, Lane ME, and Kalderon D (1995). Function of protein kinase A in hedghehog signal transduction and Drosophila imaginal disc development. Cell 80, 553–562. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang H, Litingtung Y, and Chiang C (2006). Cholesterol modification restricts the spread of Shh gradient in the limb bud. Proceedings of the National Academy of Sciences of the United States of America 103, 6548–6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X (2004). Functions of heparan sulfate proteoglycans in cell signaling during development. Development (Cambridge, England) 131, 6009–6021. [DOI] [PubMed] [Google Scholar]
- Lindemann RK (2008). Stroma-initiated hedgehog signaling takes center stage in B-cell lymphoma. Cancer research 68, 961–964. [DOI] [PubMed] [Google Scholar]
- Litingtung Y, and Chiang C (2000). Specification of ventral neuron types is mediated by an antagonistic interaction between Shh and Gli3. Nat Neurosci 3, 979–985. [DOI] [PubMed] [Google Scholar]
- Liu A, Wang B, and Niswander LA (2005). Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development (Cambridge, England) 132, 3103–3111. [DOI] [PubMed] [Google Scholar]
- Liu Y, Cao X, Jiang J, and Jia J (2007). Fused-Costal2 protein complex regulates Hedgehog-induced Smo phosphorylation and cell-surface accumulation. Genes & development 21, 1949–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low WC, Wang C, Pan Y, Huang XY, Chen JK, and Wang B (2008). The decoupling of Smoothened from Galphai proteins has little effect on Gli3 protein processing and Hedgehog-regulated chick neural tube patterning. Developmental biology 321, 188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum L, Yao S, Mozer B, Rovescalli A, Von Kessler D, Nirenberg M, and Beachy PA (2003a). Identification of Hedgehog pathway components by RNAi in Drosophila cultured cells. Science (New York, N.Y 299, 2039–2045. [DOI] [PubMed] [Google Scholar]
- Lum L, Zhang C, Oh S, Mann RK, von Kessler DP, Taipale J, Weis-Garcia F, Gong R, Wang B, and Beachy PA (2003b). Hedgehog signal transduction via Smoothened association with a cytoplasmic complex scaffolded by the atypical kinesin, Costal-2. Molecular cell 12, 1261–1274. [DOI] [PubMed] [Google Scholar]
- Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S, Corbin JG, Gritli-Linde A, Dellovade T, Porter JA, Rubin LL, et al. (2003). Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron 39, 937–950. [DOI] [PubMed] [Google Scholar]
- Mandal L, Martinez-Agosto JA, Evans CJ, Hartenstein V, and Banerjee U (2007). A Hedgehog- and Antennapedia-dependent niche maintains Drosophila haematopoietic precursors. Nature 446, 320–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May SR, Ashique AM, Karlen M, Wang B, Shen Y, Zarbalis K, Reiter J, Ericson J, and Peterson AS (2005). Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Developmental biology 287, 378–389. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Ingham PW, and Tabin CJ (2003). Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol 53, 1–114. [DOI] [PubMed] [Google Scholar]
- Meloni AR, Fralish GB, Kelly P, Salahpour A, Chen JK, Wechsler-Reya RJ, Lefkowitz RJ, and Caron MG (2006). Smoothened signal transduction is promoted by G protein-coupled receptor kinase 2. Molecular and cellular biology 26, 7550–7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant M, Evangelista M, Luoh SM, Frantz GD, Chalasani S, Carano RA, van Hoy M, Ramirez J, Ogasawara AK, McFarland LM, et al. (2005). Loss of the serine/threonine kinase fused results in postnatal growth defects and lethality due to progressive hydrocephalus. Molecular and cellular biology 25, 7054–7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Methot N, and Basler K (1999). Hedgehog controls limb development by regulating the activities of distinct transcriptional activator and repressor forms of Cubitus interruptus. Cell 96, 819–831. [DOI] [PubMed] [Google Scholar]
- Methot N, and Basler K (2000). Suppressor of Fused opposes Hedgehog signal transduction by impeding nuclear accumulation of the activator form of Cubitus interruptus. Development (Cambridge, England) 127, 4001–4010. [DOI] [PubMed] [Google Scholar]
- Mill P, Mo R, Fu H, Grachtchouk M, Kim PC, Dlugosz AA, and Hui CC (2003). Sonic hedgehog-dependent activation of Gli2 is essential for embryonic hair follicle development. Genes Dev 17, 282–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mill P, Mo R, Hu MC, Dagnino L, Rosenblum ND, and Hui CC (2005). Shh controls epithelial proliferation via independent pathways that converge on N-Myc. Dev Cell 9, 293–303. [DOI] [PubMed] [Google Scholar]
- Murone M, Rosenthal A, and de Sauvage FJ (1999). Sonic hedgehog signaling by the patched-smoothened receptor complex. Curr Biol 9, 76–84. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis E, and Hui CC (2005). Hedgehog signaling and congenital malformations. Clin Genet 67, 193–208. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Unden AB, Krause D, Malmqwist U, Raza K, Zaphiropoulos PG, and Toftgard R (2000). Induction of basal cell carcinomas and trichoepitheliomas in mice overexpressing GLI −1. Proc Natl Acad Sci U S A 97, 3438–3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüsslein-Volhard C, and Wieschaus E (1980). Mutations affecting segment number and polarity in Drosophila. Nature 287, 795–801. [DOI] [PubMed] [Google Scholar]
- Ocbina PJ, and Anderson KV (2008). Intraflagellar transport, cilia, and mammalian Hedgehog signaling: analysis in mouse embryonic fibroblasts. Dev Dyn 237, 2030–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden SK, Fei DL, Schilling NS, Ahmed YF, Hwa J, and Robbins DJ (2008). G protein Galpha(i) functions immediately downstream of Smoothened in Hedgehog signalling. Nature [DOI] [PMC free article] [PubMed]
- Ohlmeyer JT, and Kalderon D (1998). Hedgehog stimulates maturation of Cubitus interruptus into a labile transcriptional activator. Nature 396, 749–753. [DOI] [PubMed] [Google Scholar]
- Okada A, Charron F, Morin S, Shin DS, Wong K, Fabre PJ, Tessier-Lavigne M, and McConnell SK (2006). Boc is a receptor for sonic hedgehog in the guidance of commissural axons. Nature 444, 369–373. [DOI] [PubMed] [Google Scholar]
- Ou CY, Wang CH, Jiang J, and Chien CT (2007). Suppression of Hedgehog signaling by Cul3 ligases in proliferation control of retinal precursors. Developmental biology 308, 106–119. [DOI] [PubMed] [Google Scholar]
- Palma V, Lim DA, Dahmane N, Sanchez P, Brionne TC, Herzberg CD, Gitton Y, Carleton A, Alvarez-Buylla A, and Ruiz i Altaba A (2005). Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development 132, 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, and Wang B (2007). A novel protein-processing domain in Gli2 and Gli3 differentially blocks complete protein degradation by the proteasome. The Journal of biological chemistry 282, 10846–10852. [DOI] [PubMed] [Google Scholar]
- Panakova D, Sprong H, Marois E, Thiele C, and Eaton S (2005). Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature 435, 58–65. [DOI] [PubMed] [Google Scholar]
- Park HL, Bai C, Platt KA, Matise MP, Beeghly A, Hui CC, Nakashima M, and Joyner AL (2000). Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development 127, 1593–1605. [DOI] [PubMed] [Google Scholar]
- Pasca di Magliano M, Sekine S, Ermilov A, Ferris J, Dlugosz AA, and Hebrok M (2006). Hedgehog/Ras interactions regulate early stages of pancreatic cancer. Genes & development 20, 3161–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock CD, Wang Q, Gesell GS, Corcoran-Schwartz IM, Jones E, Kim J, Devereux WL, Rhodes JT, Huff CA, Beachy PA, et al. (2007). Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proc Natl Acad Sci U S A 104, 4048–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JA, Young KE, and Beachy PA (1996). Cholesterol modification of Hedgehog signaling proteins in animal development. Science (New York, N.Y 274, 255–258. [DOI] [PubMed] [Google Scholar]
- Riobo NA, Lu K, Ai X, Haines GM, and Emerson CP Jr. (2006a). Phosphoinositide 3-kinase and Akt are essential for Sonic Hedgehog signaling. Proceedings of the National Academy of Sciences of the United States of America 103, 4505–4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riobo NA, Saucy B, Dilizio C, and Manning DR (2006b). Activation of heterotrimeric G proteins by Smoothened. Proceedings of the National Academy of Sciences of the United States of America 103, 12607–12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R, Milenkovic L, and Scott MP (2007). Patched1 regulates hedgehog signaling at the primary cilium. Science (New York, N.Y 317, 372–376. [DOI] [PubMed] [Google Scholar]
- Ruel L, Rodriguez R, Gallet A, Lavenant-Staccini L, and Therond PP (2003). Stability and association of Smoothened, Costal2 and Fused with Cubitus interruptus are regulated by Hedgehog. Nature cell biology 5, 907–913. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A, Mas C, and Stecca B (2007). The Gli code: an information nexus regulating cell fate, stemness and cancer. Trends Cell Biol 17, 438–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherz PJ, McGlinn E, Nissim S, and Tabin CJ (2007). Extended exposure to Sonic hedgehog is required for patterning the posterior digits of the vertebrate limb. Dev Biol 308, 343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller U, Heine VM, Mao J, Kho AT, Dillon AK, Han YG, Huillard E, Sun T, Ligon AH, Qian Y, et al. (2008). Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma. Cancer Cell 14, 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smelkinson MG, Zhou Q, and Kalderon D (2007). Regulation of Ci-SCFSlimb binding, Ci proteolysis, and hedgehog pathway activity by Ci phosphorylation. Developmental cell 13, 481–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamataki D, Ulloa F, Tsoni SV, Mynett A, and Briscoe J (2005). A gradient of Gli activity mediates graded Sonic Hedgehog signaling in the neural tube. Genes & development 19, 626–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecca B, Mas C, Clement V, Zbinden M, Correa R, Piguet V, Beermann F, and Ruiz IAA (2007). Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proceedings of the National Academy of Sciences of the United States of America 104, 5895–5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigini M, and Cohen SM (1997). A Hedgehog activity gradient contributes to AP axial patterning of the Drosophila wing. Development (Cambridge, England) 124, 4697–4705. [DOI] [PubMed] [Google Scholar]
- Svard J, Heby-Henricson K, Persson-Lek M, Rozell B, Lauth M, Bergstrom A, Ericson J, Toftgard R, and Teglund S (2006). Genetic elimination of Suppressor of fused reveals an essential repressor function in the mammalian Hedgehog signaling pathway. Developmental cell 10, 187–197. [DOI] [PubMed] [Google Scholar]
- Taipale J, Cooper MK, Maiti T, and Beachy PA (2002). Patched acts catalytically to suppress the activity of Smoothened. Nature 418, 892–897. [DOI] [PubMed] [Google Scholar]
- Tay SY, Ingham PW, and Roy S (2005). A homologue of the Drosophila kinesin-like protein Costal2 regulates Hedgehog signal transduction in the vertebrate embryo. Development (Cambridge, England) 132, 625–634. [DOI] [PubMed] [Google Scholar]
- Tenzen T, Allen BL, Cole F, Kang JS, Krauss RS, and McMahon AP (2006). The cell surface membrane proteins Cdo and Boc are components and targets of the Hedgehog signaling pathway and feedback network in mice. Developmental cell 10, 647–656. [DOI] [PubMed] [Google Scholar]
- Trowbridge JJ, Scott MP, and Bhatia M (2006). Hedgehog modulates cell cycle regulators in stem cells to control hematopoietic regeneration. Proc Natl Acad Sci U S A 103, 14134–14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa F, Itasaki N, and Briscoe J (2007). Inhibitory Gli3 activity negatively regulates Wnt/beta-catenin signaling. Curr Biol 17, 545–550. [DOI] [PubMed] [Google Scholar]
- Varjosalo M, Bjorklund M, Cheng F, Syvanen H, Kivioja T, Kilpinen S, Sun Z, Kallioniemi O, Stunnenberg HG, He WW, et al. (2008). Application of active and kinase-deficient kinome collection for identification of kinases regulating hedgehog signaling. Cell 133, 537–548. [DOI] [PubMed] [Google Scholar]
- Varjosalo M, Li SP, and Taipale J (2006). Divergence of hedgehog signal transduction mechanism between Drosophila and mammals. Developmental cell 10, 177–186. [DOI] [PubMed] [Google Scholar]
- Vokes SA, Ji H, McCuine S, Tenzen T, Giles S, Zhong S, Longabaugh WJ, Davidson EH, Wong WH, and McMahon AP (2007). Genomic characterization of Gli-activator targets in sonic hedgehog-mediated neural patterning. Development 134, 1977–1989. [DOI] [PubMed] [Google Scholar]
- Vokes SA, Ji H, Wong WH, and McMahon AP (2008). A genome-scale analysis of the cis-regulatory circuitry underlying sonic hedgehog-mediated patterning of the mammalian limb. Genes & development 22, 2651–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas N, Goswami D, Manonmani A, Sharma P, Ranganath HA, VijayRaghavan K, Shashidhara LS, Sowdhamini R, and Mayor S (2008). Nanoscale organization of hedgehog is essential for long-range signaling. Cell 133, 1214–1227. [DOI] [PubMed] [Google Scholar]
- Wang B, Fallon JF, and Beachy PA (2000a). Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell 100, 423–434. [DOI] [PubMed] [Google Scholar]
- Wang C, Ruther U, and Wang B (2007). The Shh-independent activator function of the full-length Gli3 protein and its role in vertebrate limb digit patterning. Dev Biol 305, 460–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Amanai K, Wang B, and Jiang J (2000b). Interactions with Costal2 and suppressor of fused regulate nuclear translocation and activity of cubitus interruptus. Genes & development 14, 2893–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, and Jiang J (2004). Multiple Cos2/Ci interactions regulate Ci subcellular localization through microtubule dependent and independent mechanisms. Developmental biology 268, 493–505. [DOI] [PubMed] [Google Scholar]
- Wang QT, and Holmgren RA (2000). Nuclear import of cubitus interruptus is regulated by hedgehog via a mechanism distinct from Ci stabilization and Ci activation. Development (Cambridge, England) 127, 3131–3139. [DOI] [PubMed] [Google Scholar]
- Wang Y, and Price MA (2008). A unique protection signal in Cubitus interruptus prevents its complete proteasomal degradation. Molecular and cellular biology 28, 5555–5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins DN, Berman DM, Burkholder SG, Wang B, Beachy PA, and Baylin SB (2003). Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature 422, 313–317. [DOI] [PubMed] [Google Scholar]
- Wechsler-Reya RJ, and Scott MP (1999). Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron 22, 103–114. [DOI] [PubMed] [Google Scholar]
- Weedon MN, Lango H, Lindgren CM, Wallace C, Evans DM, Mangino M, Freathy RM, Perry JR, Stevens S, Hall AS, et al. (2008). Genome-wide association analysis identifies 20 loci that influence adult height. Nature genetics 40, 575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicking C, Smyth I, and Bale A (1999). The hedgehog signalling pathway in tumorigenesis and development. Oncogene 18, 7844–7851. [DOI] [PubMed] [Google Scholar]
- Wigley WC, Fabunmi RP, Lee MG, Marino CR, Muallem S, DeMartino GN, and Thomas PJ (1999). Dynamic association of proteasomal machinery with the centrosome. The Journal of cell biology 145, 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijgerde M, McMahon JA, Rule M, and McMahon AP (2002). A direct requirement for Hedgehog signaling for normal specification of all ventral progenitor domains in the presumptive mammalian spinal cord. Genes Dev 16, 2849–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff C, Roy S, and Ingham PW (2003). Multiple muscle cell identities induced by distinct levels and timing of hedgehog activity in the zebrafish embryo. Curr Biol 13, 1169–1181. [DOI] [PubMed] [Google Scholar]
- Yang ZJ, Ellis T, Markant SL, Read TA, Kessler JD, Bourboulas M, Schuller U, Machold R, Fishell G, Rowitch DH, et al. (2008). Medulloblastoma can be initiated by deletion of Patched in lineage-restricted progenitors or stem cells. Cancer Cell 14, 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Lum L, and Beachy P (2006). The ihog cell-surface proteins bind Hedgehog and mediate pathway activation. Cell 125, 343–357. [DOI] [PubMed] [Google Scholar]
- Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, Marshall D, Fu L, Januario T, Kallop D, et al. (2008). A paracrine requirement for hedgehog signalling in cancer. Nature 455, 406–410. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Zhang L, Wang B, Ou CY, Chien CT, and Jiang J (2006a). A hedgehog-induced BTB protein modulates hedgehog signaling by degrading Ci/Gli transcription factor. Developmental cell 10, 719–729. [DOI] [PubMed] [Google Scholar]
- Zhang W, Kang JS, Cole F, Yi MJ, and Krauss RS (2006b). Cdo functions at multiple points in the Sonic Hedgehog pathway, and Cdo-deficient mice accurately model human holoprosencephaly. Developmental cell 10, 657–665. [DOI] [PubMed] [Google Scholar]
- Zhang W, Zhao Y, Tong C, Wang G, Wang B, Jia J, and Jiang J (2005). Hedgehog-regulated costal2-kinase complexes control phosphorylation and proteolytic processing of cubitus interruptus. Developmental cell 8, 267–278. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Tong C, and Jiang J (2007). Hedgehog regulates smoothened activity by inducing a conformational switch. Nature 450, 252–258. [DOI] [PubMed] [Google Scholar]
- Zhu AJ, Zheng L, Suyama K, and Scott MP (2003). Altered localization of Drosophila Smoothened protein activates Hedgehog signal transduction. Genes & development 17, 1240–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Nakamura E, Nguyen MT, Bao X, Akiyama H, and Mackem S (2008). Uncoupling Sonic hedgehog control of pattern and expansion of the developing limb bud. Dev Cell 14, 624–632. [DOI] [PMC free article] [PubMed] [Google Scholar]