Summary
In a prospective cohort of 534 neonates with acute symptomatic seizures, 66% had incomplete response to the initial loading dose of antiseizure medication (ASM). Treatment response did not differ by gestational age, sex, medication, or dose. The risk of incomplete response was highest for seizures due to intracranial hemorrhage and lowest for hypoxic‐ischemic encephalopathy, although the difference was not significant after adjusting for high seizure burden and therapeutic hypothermia treatment. Future trial design may test ASMs in neonates with all acute symptomatic seizure etiologies and could target neonates with seizures refractory to an initial ASM.
Keywords: AED, antiepileptic drug, electroencephalogram, epilepsy, hypoxic-ischemic encephalopathy, neonatal encephalopathy, neonatal seizures, neurocritical care, seizure
1 |. INTRODUCTION
Acute symptomatic neonatal seizures are often refractory to first‐line antiseizure medications (ASMs). In the 1999 clinical trial of phenobarbital versus phenytoin, seizures recurred in >50% of neonates following the first loading dose of either medication, and neonates with higher seizure burden were less likely to respond.1 Little is known about additional characteristics associated with initial treatment failure, although we previously showed that preterm and term neonates were equally likely to have persistent seizures after a loading dose of phenobarbital2 and there was no difference in treatment response to initial doses of phenobarbital, phenytoin, or levetiracetam as the first‐line ASM.3
We hypothesized that treatment response to ASMs would not differ by seizure etiology and that treatment response would be dose dependent. Confirming these hypotheses provides important evidence for clinical care and data to inform future clinical trial design.
2 |. MATERIALS AND METHODS
This prospective, observational cohort study included neonates with acute symptomatic seizures due to hypoxicischemic encephalopathy (HIE), ischemic stroke (arterial or venous), or intracranial hemorrhage (ICH) treated at the nine sites of the Neonatal Seizure Registry (NSR). Each site has a level IV neonatal intensive care unit and follows the American Clinical Neurophysiology Society (ACNS) guidelines for continuous electroencephalography (cEEG) in neonates.4
Two prospective cohorts were merged: (1) NSR, a consecutive cohort of all neonates with seizures diagnosed clinically and/or with EEG confirmation enrolled January 2013 to November 2015 (waiver of consent); and (2) NSR‐II, a nonconsecutive cohort of neonates with acute symptomatic seizures diagnosed clinically and/or with EEG confirmation enrolled from July 2016 to March 2018 who survived the neonatal hospital admission (required written parental informed consent). The local institutional review board for every site approved the studies. Neonates from the initial NSR cohort were previously reported.2,3,5
Neonates were included if they received a loading dose of an ASM and adequate documentation regarding response to the loading dose was available. Neonates with events that were determined not to be seizures based on history, semiology, or cEEG were not enrolled. Inclusion of neonates with clinical events suspected to be seizures but without seizures confirmed on the study site EEG was at the discretion of the site investigator and considered if (1) events were treated with ASMs; and (2) the clinical history, event semiology, or referring hospital EEG supported the diagnosis of seizures. Study site investigators determined the primary seizure etiology based upon systematic medical record review. A neonate was considered to have incomplete response if electrographic seizures were documented >30 minutes after the initial load of medication was complete. The data collected could not be used to differentiate between incomplete and absent response to ASMs.
Seizures were defined as sudden, abnormal EEG events with repetitive and evolving pattern with minimum amplitude of 2 μV and duration ≥ 10 seconds and were not required to have a clinical correlate.6 Seizure exposure was extracted from cEEG reports at the study center and was categorized as follows: (1) high burden: status epilepticus, frequent recurrent seizures without status epilepticus, many (≥7) isolated seizures; and (2) low burden: <7 seizures.7
Seizure treatment, including ASM selection and determination of loading dose, was at the discretion of the provider. No study‐specific treatment guideline was provided, although seven of the nine sites had institutional guidelines, pathways, or workflows for seizure management. Initial EEG background was determined by the study site investigator based on the EEG report available for 253 neonates and was categorized as (1) normal or (2) abnormal (including burst suppression).
Descriptive statistics and results of t tests and chisquared tests are presented. Multivariate logistic regression was used to build an adjusted model (initial inclusion P ≤ 0.1, final model inclusion adjusted P < 0.1). EEG background was only available for a subset of participants and therefore was not included in the analysis. Confidence intervals (CIs) were calculated using pairwise comparison of 95% CIs. Analyses were completed using Stata 14 (StataCorp).
3 |. RESULTS
From January 2013 to March 2018, 534 neonates were enrolled (five neonates were excluded for unknown loading dose, one for unknown response, one for initial medication not known). EEG recording duration was a median of 72 (interquartile range = 45.7‐99.3) hours. Table 1 presents the patient characteristics. Overall, 354 neonates (66%) had an incomplete response to the initial loading dose of ASM. There was no significant difference in the response by treating institution (P = 0.2), although the range was broad (56%‐81%); 95% CIs suggested at most a difference of 52% between the highest and lowest centers with incomplete response.
TABLE 1.
Clinical characteristics of 534 prospectively enrolled infants with acute symptomatic neonatal seizures
| Total, N = 534 | |
|---|---|
| Male | 306 (57%) |
| Term | 455 (85%) |
| Seizure etiology | |
| Hypoxic-ischemic encephalopathy | 284 (53%) |
| Ischemic stroke | 142 (27%) |
| Intracranial hemorrhage | 108 (20%) |
| Initial ASM used as a loading dose | |
| Phenobarbital | 508 (95%) |
| Levetiracetam | 21 (4%) |
| Fosphenytoin | 5 (1%) |
Data are presented as n (%).
ASM, antiseizure medication.
3.1 |. Clinical risk factors for incomplete response
Incomplete response to the first loading dose of ASM was highest for neonates with seizures due to ICH (82/108 = 76%), as compared with HIE (175/284 = 62%) and stroke (97/142 = 68%, P = 0.02). There was no significant difference by sex (male 203/306 = 66% vs female 151/228 = 66%, P = 0.98).
When comparing gestational age (GA) at birth, incomplete response was highest for extremely preterm neonates (13/16 = 81% for extremely preterm [<28 weeks GA] vs 6/12 = 50% for very preterm [28 to <32 weeks GA] vs 30/51 = 59% for moderate/late preterm [32 to <37 weeks GA] vs 305/455 = 67% for term [≥37 weeks GA]); however, the differences were not significant (P = 0.2).
There was a higher chance of incomplete response to the initial dose of medication for neonates with higher electroencephalographic seizure exposure (status epilepticus, 97/100 = 96%; frequent recurrent seizures, 125/140 = 89%; ≥7 isolated seizures, 72/94 = 72%; <7 seizures, 60/198 = 30%; P < 0.0005), although it was unknown how many seizures occurred before versus after ASM initiation.
Among term neonates with HIE, those who were not treated with therapeutic hypothermia were more likely to have an incomplete response than those who were cooled (78/105 = 74% among those not treated with therapeutic hypothermia vs 77/143 = 54% among those who were, P < 0.001).
3.2 |. Treatment response by ASM
Phenobarbital was the initial loading medication for 95% of participants. Incomplete response to the initial loading dose of the ASMs was similar (phenobarbital, 336/508 = 66%; levetiracetam, 14/21 = 67%; fosphenytoin, 4/5 = 80%; P = 0.8); however, the number of neonates who received levetiracetam or fosphenytoin was very small. Difference in response by initial ASM was at most 49% using 95% CIs, with fosphenytoin performing worst.
There was no significant difference in loading dose measured in milligrams per kilogram for phenobarbital (mean loading dose was 19.9 ± 4.4 mg/kg with incomplete response vs 19.3 ± 3.2 mg/kg for neonates with complete response, P = 0.096). Similarly, there was no significant difference for levetiracetam (34.5 ± 18.0 mg/kg with incomplete response vs 24.5 ± 5.7 mg/kg with complete response, P = 0.2) and fosphenytoin (20.0 ± 4 mg/kg for neonates with incomplete response vs 20.0 mg/kg for the single neonate with complete response, P > 0.99; Figure 1); however, the numbers of neonates who received levetiracetam and fosphenytoin was very small.
FIGURE 1.
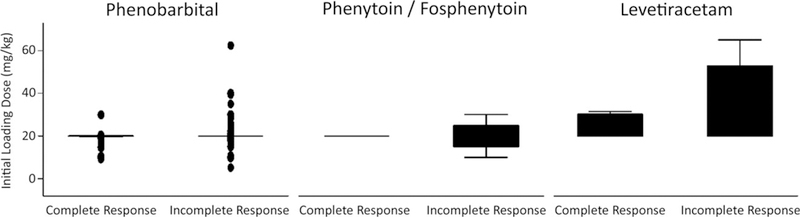
Response to antiseizure medication for 534 neonates with acute symptomatic seizures stratified by medication and dose. Response did not vary by medication (incomplete responses: phenobarbital, 336/508 = 66%; levetiracetam, 14/21 = 67%; fosphenytoin, 4/5 = 80%; P = 0.8)
3.3 |. Relationship between EEG background and treatment response
Among 253 neonates with cEEG background reports available for analysis, the risk of incomplete response was higher for those with abnormal interictal EEG (159/234 = 68%) as compared to those with normal interictal EEG (9/19 = 47%, relative risk = 1.4, 95% CI = 0.9‐2.3, P = 0.07).
3.4 |. Multivariate analysis
After adjusting for high seizure burden and therapeutic hypothermia, seizure etiology was no longer significantly associated with likelihood of response to ASM (ICH odds ratio [OR] = 1.5, 95% CI = 08‐2.6 and ischemic stroke OR = 0.8, 95% CI = 0.5‐1.5 when compared with HIE, P = 0.2), whereas high seizure burden (OR = 15.9, 95% CI = 10.1‐25.0, P < 0.0005) and therapeutic hypothermia (OR = 0.5, 95% CI = 0.3‐0.9, P = 0.01) remained very highly associated with ASM response.
4 |. DISCUSSION
In this large, prospective, multicenter study of neonates with acute symptomatic seizures due to the three most common neonatal seizure etiologies (HIE, ischemic stroke, or ICH), the rate of incomplete response to the first loading dose of ASM was very high (66%). Risk factors for incomplete response included lack of therapeutic hypothermia treatment and high electrographic seizure burden.
It has been 20 years since a trial of phenobarbital versus phenytoin for initial neonatal seizure management reported that standard loading doses control seizures in fewer than half of infants.1 Since then, more than 15 new ASMs have come to market for epilepsy management. Many new drugs are not suitable for critically ill neonates, yet even ASMs that may be appropriate (ie, drugs with intravenous formulations such as levetiracetam, lacosamide, and brivaracetam) have not been studied in neonates in published randomized and controlled clinical trials. Case reports and case series of levetiracetam have established pharmacokinetics8,9 and suggest that it is safe and may be effective for newborns with seizures.9–11 A clinical trial of phenobarbital versus levetiracetam is complete, but the results have not yet been published. Animal studies of bumetanide combined with phenobarbital as a rational synergistic therapy show some promise; however, a small clinical trial was inconclusive.12
Barriers to neonatal seizure treatment trials include relatively rare incidence of the condition; the need for rapid consent, enrollment, and randomization; lack of widespread cEEG availability; and uncertainty regarding optimal outcome measures. Clinical trials are needed not only to determine the most effective ASM and dose but also to examine treatment approaches. Such approaches include the value and optimal use of cEEG and determining whether speed of effective treatment influences seizure response or long‐term neurodevelopmental and epilepsy outcomes. Our results suggest that, in planning efficient neonatal clinical trials of ASMs, investigators could consider broad entry criteria (eg, including late preterm infants, as well as neonates with HIE, ischemic stroke, and ICH) but might need to stratify by therapeutic hypothermia treatment, pretreatment seizure burden, and initial EEG background. Our data underscore the need to test new medications in neonates with seizures refractory to an initial ASM, who are the majority of those with seizures, who usually have a higher seizure burden, and in whom improved treatment is more likely to have an impact on long‐term outcome.
Although we present a large, multicenter cohort, our data have limitations. First, our NSR‐II cohort excluded children who died during the neonatal admission and included nonconsecutive neonates. Therefore, it may be enriched for less severely affected infants. Second, incomplete response to ASM was defined as seizure recurrence >30 minutes after the initial loading dose. Although most treatment failures occur within the first few hours after a loading dose of ASMs, we cannot exclude that seizures recurred >24 hours after the loading dose given that study sites monitored neonates for a minimum of 24 hours after the last electroencephalographic seizure as per ACNS guidelines. Third, seizure exposure was measured using counts rather than other approaches (eg, percentage of the record comprised of seizures). Fourth, seizure exposure prior to the medication load was not universally available, so we cannot differentiate between seizures occurring before and after the initial loading dose; however, our findings are in keeping with the results of the Painter trial,1 which also showed a relationship between seizure burden and response to ASM. Fifth, the time from seizure recognition to treatment was not measured, and this timing might modify treatment response. Sixth, use of ASMs was not randomized, and therefore it is difficult to interpret any similarities or differences by ASM.
5 |. CONCLUSIONS
Incomplete response to ASM is expected for most neonates with acute symptomatic seizures who receive current standard treatment approaches, regardless of etiology, GA, initial ASM, and loading dose. This finding underscores the need for novel treatment approaches and suggests that future trials focused on ASM efficacy may be efficiently designed by stratification or exclusion of lower‐risk groups and could include all acute symptomatic etiologies of seizures, particularly those with seizures refractory to an initial ASM.
ACKNOWLEDGMENTS
The Pediatric Epilepsy Research Foundation and the Patient Centered Outcomes Research Institute supported this study but did not participate in design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. The authors thank Drs Donna Ferriero and Faye Silverstein for their contributions to the NSR, as well as the research assistants and parent partners at each study site.
Funding information
Patient-Centered Outcomes Research Institute, Grant/Award Number: CER-1507–31187; Pediatric Epilepsy Research Foundation, Grant/Award Number: A120625
Footnotes
DISCLOSURE OF CONFLICT OF INTEREST
J.S.S. receives royalties from UpToDate. C.J.W. has served as a paid consultant for Ceribell and Persyst. M.R.C. received research funding from Insys Development Company, paid to her Institution, for company sponsored trials; she has been a consultant for BioMarin Research and for GW Pharmaceuticals. R.A.S. is a consultant for the Epilepsy Study Consortium and receives honoraria from UpToDate for neonatal seizures topics. None of the other authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- 1.Painter MJ, Scher MS, Stein AD, et al. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. N Engl J Med 1999;341:485–9. [DOI] [PubMed] [Google Scholar]
- 2.Glass HC, Shellhaas RA, Tsuchida TN, et al. Seizures in preterm neonates: a multicenter observational cohort study. Pediatr Neurol 2017;72:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shellhaas RA, Chang T, Wusthoff CJ, et al. Treatment duration after acute symptomatic seizures in neonates: a multicenter cohort study. J Pediatr 2017;181:298–301.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shellhaas RA, Chang T, Tsuchida T, et al. The American clinical neurophysiology society’s guideline on continuous electroencephalography monitoring in neonates. J Clin Neurophysiol 2011;28:611–7. [DOI] [PubMed] [Google Scholar]
- 5.Glass HC, Shellhaas RA, Wusthoff CJ, et al. Contemporary profile of seizures in neonates: a prospective cohort study. J Pediatr 2016;174:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsuchida TN, Wusthoff CJ, Shellhaas RA, et al. American Clinical Neurophysiology Society standardized EEG terminology and categorization for the description of continuous EEG monitoring in neonates: report of the American Clinical Neurophysiology Society Critical Care Monitoring Committee. J Clin Neurophysiol 2013;30:161–73. [DOI] [PubMed] [Google Scholar]
- 7.Glass HC, Shellhaas RA, Wusthoff CJ, et al. Contemporary profile of seizures in neonates: a prospective cohort study. J Pediatr 2016;174:98–103.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merhar SL, Schibler KR, Sherwin CM, et al. Pharmacokinetics of levetiracetam in neonates with seizures. J Pediatr 2011;159:152–4.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharpe CM, Capparelli EV, Mower A, Farrell MJ, Soldin SJ, Haas RH. A seven‐day study of the pharmacokinetics of intravenous levetiracetam in neonates: marked changes in pharmacokinetics occur during the first week of life. Pediatr Res 2012;72:43–9. [DOI] [PubMed] [Google Scholar]
- 10.Abend NS, Gutierrez-Colina AM, Monk HM, Dlugos DJ, Clancy RR. Levetiracetam for treatment of neonatal seizures. J Child Neurol 2011;26:465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rakshasbhuvankar A, Rao S, Kohan R, Simmer K, Nagarajan L. Intravenous levetiracetam for treatment of neonatal seizures. J Child Neurol 2013;20:1165–7. [DOI] [PubMed] [Google Scholar]
- 12.Pressler RM, Boylan GB, Marlow N, et al. Bumetanide for the treatment of seizures in newborn babies with hypoxic ischaemic encephalopathy (NEMO): an open‐label, dose finding, and feasibility phase 1/2 trial. Lancet Neurol 2015;14:469–77. [DOI] [PubMed] [Google Scholar]


