Abstract
Background.
Studies have demonstrated persistent changes in CNS cytokine gene expression following ethanol exposure. However, the low endogenous expression and short half-lives of cytokines in the CNS have made cytokine protein detection challenging. The goal of these studies was to establish parameters for use of large-molecule microdialysis and sensitive multiplexing technology for the simultaneous detection of brain cytokines, corticosterone, and ethanol concentrations in the awake behaving rat.
Methods.
Adult (P75+) male Sprague Dawley rats that were either naïve to ethanol (Experiment 1) or had a history of adolescent chronic intermittent ethanol (CIE; Experiment 2) were given an acute ethanol challenge during microdialysis. Experiment 1 examined brain ethanol concentrations, corticosterone, and a panel of neuroimmune analytes, including cytokines associated with innate and adaptive immunity. The natural timecourse of changes in these cytokines was compared to the effects of an acute 1.5 or 3.0 g/kg intraperitoneal ethanol challenge. In Experiment 2, rats with a history of adolescent CIE or controls exposed to vehicle were challenged with 3.0 g/kg intraperitoneal ethanol during microdialysis in adulthood, and a panel of cytokines was examined in parallel with brain ethanol concentrations and corticosterone.
Results.
The microdialysis procedure itself induced a cytokine-specific response that replicated across studies; specifically, a sequential elevation of IL-6, TNFα, and IL-10. Surprisingly, acute ethanol did not significantly alter this course of cytokine fluctuations in the hippocampus. However, a history of adolescent CIE showed drastic effects on multiple neuroimmune analytes when re-challenged with ethanol as adults. Rats with a history of adolescent ethanol displayed a severely blunted neuroimmune response in adulthood, evinced by suppressed IL-1β, IL-10, and TNFα.
Conclusions.
Together, these findings provide a methodological framework for assessment of cytokine release patterns, their modulation by ethanol, and the long-lasting changes to neuroimmune reactivity evoked by a history of adolescent CIE.
Keywords: Adolescence, CIE, ethanol, cytokine, microdialysis
Introduction.
Cytokines and chemokines have emerged as vital conduits of ethanol’s effects on the central nervous system [CNS] (for review, see Crews et al., 2006), endowed with the ability to both govern the progression of alcohol-induced pathology, as well as influence the development of alcohol use disorders (Vetreno and Crews, 2014). The cytokines Interleukin (IL)-6, IL-1β, and Tumor necrosis factor alpha (TNFα), are amongst the most well-described targets of alcohol’s action on immune signaling within the CNS (Whitman et al., 2013, Lippai et al., 2013, Kane et al., 2014, Pascual et al., 2015), though there are also others that are associated with peripheral immunity that influence CNS function, such as anti-inflammatory cytokines IL-10 and IL-4 (Marshall et al., 2017, Frank et al., 2018). These cytokines are affected by ethanol across a variety of exposure paradigms ranging from a single acute binge-like exposure (Doremus-Fitzwater et al., 2015, Suryanarayanan et al., 2016) to chronic administration (Knapp et al., 2016, Doremus-Fitzwater et al., 2014). The potential functional impact of such alterations can be wide-ranging. The effects of cytokines on CNS function and behavior have been repeatedly documented in modulating cognitive function, anxiety measures, locomotion, sickness behavior, and others (Murray et al., 2013, Harden et al., 2008, Chourbaji et al., 2006, Dinel et al., 2011, Donegan et al., 2014). The literature concerning the functional effects of cytokines on ethanol-related behaviors is comparatively scant, though behavioral validation of changes in neuroimmune-associated genes has emerged. For instance, IL-6, amongst other neuroimmune factors, has been shown to have effects on ethanol consumption (Blednov et al., 2012) and to a lesser extent, some intoxication-related behaviors (Barney et al., 2018). Thus, changes in cytokine expression can be a biomarker for the effects of alcohol, though further work is necessary to fully understand their effects in an in vivo rodent model.
Adolescence in particular is a time of vulnerability to the effects of alcohol. From a behavioral and epidemiological standpoint, an alarming percent of the adolescent population reports regular binge consumption of alcohol, with assessments indicating that young people between the ages of 12 and 20 consume 90% of their alcohol intake by binge drinking, which accounts for 11% of all the alcohol consumed in the United States (NIAAA, 2017).Studies have shown that such exposure during this developmental window can increase the risk of alcohol use disorders in adulthood (Grant and Dawson, 1997). From a neuroimmune perspective, this life stage is also associated with rapid brain development and an as-yet-immature neuroimmune system that is highly susceptible to modification by experiential events (Brenhouse and Schwarz, 2016). In particular, brain cytokine responses to acute ethanol administration, as well as stress and immune challenge, were severely blunted in adolescents as compared to adult counterparts (Girard-Joyal et al., 2015, Doremus-Fitzwater et al., 2015b). Recent work from our lab indicates that chronic intermittent ethanol exposure during this adolescent time period (adolescent CIE) resulted in a locking-in-like immune effect, wherein animals exposed to adolescent CIE displayed an impaired cytokine response in white blood cells when challenged with either LPS or acute restraint in adulthood (Vore et al., 2017). Given the emergent role for neuroimmune signaling in the development of alcohol addiction and alcohol-related brain pathology, it is of importance to expand our understanding of these locking-in-like effects and their level of contribution to the maladaptive drinking patterns induced by adolescent ethanol exposure.
There are multiple technical issues that complicate our understanding of the connection between ethanol-induced cytokine change and behavioral outcomes. Most studies to date have utilized gene expression effects as a sensitive and efficient method for detecting neuroimmune consequences of ethanol. A variety of unbiased network changes have been identified effectively with microarrays and RNAseq following ethanol consumption and administration models in rodents (e.g., Ferguson et al., 2014, Mulligan et al., 2011) as well as optimizing the use of tissue from deceased alcoholics (Blednov et al., 2005, Lewohl et al., 2000). This approach is ideally suited to identifying networks of change and pathways, and with additional targeted analyses using PCR, potential targets for therapeutic use can be identified efficiently. However, drugs like ethanol have a wide array of cellular consequences that can complicate conclusions. For instance, it has been noted that gene expression effects do not always translate to protein expression (Lewohl et al., 2004), and protein changes may even deviate in directionality from mRNA effects (Dodd and Lewohl, 1998). Ethanol also impairs Golgi Apparatus function and can have a multitude of effects on post-translational processing, as well (Powrozek and Olson, 2012): ethanol and its metabolites have been shown to interfere with protein synthesis (David et al., 1983, Lang et al., 2001, Hong-Brown Ly et al., 2006). Therefore, while gene expression remains a valuable tool, development of complementary assessments of protein to provide a more complete functional perspective on neuroimmune consequences of alcohol would be beneficial, especially if greater temporal resolution to capture dynamic changes across time post-ethanol were possible.
Assessment of cytokine proteins in the CNS has been a challenging task historically due to their low endogenous expression. Basal levels of cytokines such as IL-1β, IL-6, and TNFα, in the healthy adult brain have been reported in the range of 5–7, 1, and 200–250 pg/mg of protein across areas such as the hippocampus, hypothalamus, and pituitary, respectively (Agnello et al., 2000, Goujon et al., 1996, Tha et al., 2000). These low levels of expression pose an issue when it comes to standard approaches to protein detection, though even small changes in cytokine levels have been suggested to impact behavior (Arakawa et al., 2010, Hennessy et al., 2014). In addition to the low endogenous levels of these cytokines, a second challenge is that their macromolecule size (~20–60 kDa) and lipophobic character has precluded widespread study of their release patterns, with only a handful of studies to date reporting successful capture using procedures such as large-molecule microdialysis (Bhattacharya et al., 2013).
Finally, an additional difficulty in the analysis of brain cytokines changes is that of time resolution and specificity. As previously shown in our work, acute ethanol challenge led to time- and cytokine-specific changes across the timecourse of intoxication and withdrawal, suggesting that ethanol effects on cytokines are phase-specific. Notably, most studies examining cytokine expression after ethanol exposure focus on 24 hr after the final exposure, after ethanol has cleared. Yet, an acute binge-like ethanol challenge resulted in increased IL-6 gene expression during ethanol intoxication and suppression of IL-1β and TNFα, whereas clearance (withdrawal) of ethanol saw these effects reversed within 12–18 hours of ethanol administration (Gano et al., 2016a, Doremus-Fitzwater et al., 2014a, Doremus-Fitzwater et al., 2015a). Additionally, the expression of IL-6, but not other cytokines, was strongly influenced by prior ethanol exposure (Doremus-Fitzwater et al., 2014b), the schedule of ethanol exposure (Gano et al., 2016a), and recent stress history (Doremus-Fitzwater et al., 2018). Thus, the time-dependent effects of ethanol on a multitude of cytokines suggest there would be great value in the ability to assess continuous cytokine protein changes following ethanol exposure in awake, behaving rats.
The goal of the present studies was to establish a working procedure to enable the capture and detection of cytokines from the CNS of awake, behaving rats, while under the influence of ethanol. In these experiments, we utilized large-molecule microdialysis for continuous sampling of the extracellular space in the hippocampus, combined with highly sensitive multiplexing technology for cytokine protein detection. The latter allowed simultaneous measurement of 12 immune analytes from the same small-volume sample, allowing for excellent time resolution across an initial period of habituation, as well as throughout ethanol intoxication and clearance. Additionally, we measured ethanol concentrations and brain corticosterone levels from the same sample to further enrich our understanding of functional interactions among ethanol, cytokines, and hypothalamic-pituitary-adrenal function. It has been shown that while acute ethanol challenge results in a robust release of corticosterone, chronic ethanol engenders a blunted corticosterone response to future challenges (Spencer and McEwen, 1990). The hippocampus was selected as the target brain area of interest due both to prior data having shown its sensitivity to the cytokine-inducing effects of ethanol (Gano et al., 2016a,b), as well as its vulnerability to the effects of adolescent alcohol exposure (Risher et al., 2015). In the first experiment, a dose-response approach was used to analyze cytokine concentrations following either a saline injection, or an intraperitoneal (i.p.) administration of 1.5 or 3.0 g/kg ethanol. In Experiment 2, we assessed the cytokine response to the same high-dose ethanol challenge (3.0 g/kg i.p.) in rats with a history of adolescent CIE.
Materials and methods.
Subjects.
For Experiment 1, adult male Sprague Dawley rats (280–320 g) were ordered from Harlan (Frederick, MD) and allowed 2 weeks to acclimate to the colony (22±1°C with 12:12 light–dark cycle, lights on 0700) prior to experimental manipulation. To prevent the confounding stress effects of early-life shipping, rats for Experiment 2 (also male Sprague Dawley) were bred in our colony at Binghamton University as described elsewhere (Vore et al., 2017), weaned at P21, and housed in pairs with non-littermates. To control for litter effects, no more than 1 pup from each litter was assigned to a given experimental condition. Rats in both experiments were pair-housed in standard Plexiglas cages with ad libitum access to food and water and separated into individual housing after cannulation surgery. In both experiments, rats were handled (3–5 min) for 2 days prior to surgery, and again prior to experimentation. The procedures were approved by the Institutional Animal Care and Use Committee at Binghamton University and rats were treated in accordance with Public Health Service (PHS) policy.
Adolescent chronic intermittent ethanol exposure procedure (adolCIE; Experiment 2).
Rats were exposed to the adolescent chronic intermittent ethanol paradigm previously employed in our published work (Vore et al., 2017), an adapted and extended exposure paradigm based on that used by Dr. Toni Pak (e.g., Torcaso et al., 2017). Chronic exposure was initiated on P33–35 and consisted of a total of 4 cycles of ethanol exposure through P53–55. Each cycle comprised three days ON, followed by 2 days OFF. For every ON day, animals were weighed approximately one hour prior to intubation. Intubations consisted of either 4.0 g/kg ethanol or equivolumetric vehicle (tap water), delivered intragastrically (i.g.) via gavage. All intubations were completed before 1200 each day. No intubations were administered on OFF days. Following 4 cycles (totaling 12 ethanol/vehicle exposures across 20 days), rats then remained undisturbed in their home cages until adulthood (P75–80).
Adult acute ethanol challenge.
For the drug challenge during microdialysis testing, ethanol was administered intraperitoneally (i.p.) at the dose of 1.5 or 3.0 g/kg (20% ethanol v/v) and was mixed fresh daily using sterile physiological saline (0.9%, Teknova, Hollister, CA). This saline was also used for vehicle injections.
Adult cannulation surgery.
All rats underwent stereotaxic surgery for unilateral hippocampal cannulations at P75–80. Immediately prior to surgery, rats were given 0.05 mg/kg Buprenorphine (Reckitt Benckiser Healthcare Ltd, Hull, England) as analgesic and anesthetized with isoflurane (1–4% in oxygen). Guide cannulae (EICOM, CA) were implanted dorsal to the hippocampus (from Bregma: AP −5.28, ML +4.84, DV −3.30). Cannulae were secured with dental acrylic (Butler Schein, Ohio) and anchored to the skull with 2–3 screws adjacent to the cannula shaft. The cannulae were positioned such that the hippocampus itself remained intact until probe insertion. All rats were given 6–10 days of recovery prior to microdialysis testing.
Adult microdialysis procedure.
The morning of testing, rats were weighed immediately after lights-on and were transferred to a separate procedural room where they were placed in a standard microdialysis bowl with pine shavings, food pellets and water available for the duration of experimentation. The probe (EICOM, CA; PEP-6–04; 6.0 mm shaft, 4.0 mm probe membrane, 1,000 kDa pore size cutoff, atmospheric bleed-off vent) was activated and inserted per manufacturer’s instructions. In order to allow for large cytokine protein capture, the pressure-canceling AtmosLM (EICOM, CA) system was used, utilizing both an infusion syringe pump (CMA Microdialysis, Sweden; cat no. CMA400; flow rate 1 μl/min) as well as a specialized peristaltic roller pump (EICOM, CA; ERP-10, cat no. 3361; setting of 0022) to reduce pressure build-up and leakage, and thereby allow for the use of large membrane pore size without escalating intracranial pressure. During probe insertion, rats were not anesthetized and were briefly wrapped in a towel to restrain limbs and stabilize the head. Prior to the insertion of an activated probe, a dummy probe was briefly inserted so as to puncture the glial scarring around the previously implanted cannula. The procedure, starting from towel-wrapping and ending with the animal being released into the bowl with an activated and secured probe, took under 30 seconds. All samples were collected on ice in 1 h time bins; the time of offset between the sample diffusing across the probe membrane and entering the collection tube was 58 minutes. Upon completion of each hour, samples were aliquoted into separate tubes for measurement of either corticosterone and ethanol concentrations, or protein analysis, so that the number of freeze-thaw cycles could be minimized for all measures. Once distributed, samples were immediately transferred to storage at −80°C until the time of assay. After the conclusion of microdialysis testing, brains were harvested for cannula verification.
The manufacturers’ instructions for the use of the AtmosLM system suggest waiting 3 h after lowering the probe prior to collecting data. As we were able to detect consistent and replicable cytokine-specific patterns of change in this 3 h period, we have reported and analyzed these data here, as well, recognizing of course that homeostatic diffusion may not have fully equilibrated in these early samples. Additionally, we included two more habituation hours following this time period prior to ethanol or saline injection (collectively, samples H1 through H5 in these data sets) which served as additional baseline samples prior to any experimental manipulation as other microdialysis studies report. Most analytes of interest showed stable expression by the third hour after probe insertion, allowing us to use H4/5 as “baseline” samples whenever post hoc testing indicated the need for comparing against a control time point.
Artificial cerebrospinal fluid (aCSF).
Stock solutions of aCSF were mixed, stored at 4°C and used no more than one week from the date of preparation using the following recipe: 147 mM NaCl, 2.8 mM KCl, 1.2 mM CaCl2, 1.2 mM MgCl2, pH adjusted to 7.40 ± 0.02 using hydrochloric acid and sodium hydroxide, and was sterile filtered. On the morning of each experimental day, an aliquot of aCSF for that particular day was filtered a second time using a 0.2 μm sterile filter (VWR, cat# 28143–310) and supplemented with Bovine Serum Albumin (BSA; 0.15% w/v in aCSF; Sigma Aldrich, MO).
Dialysate ethanol concentrations (DECs).
Ethanol concentrations were determined in 5-μl aliquots using an Analox AM-1 alcohol analyzer (Analox Instruments, Lunenburg, MA). The machine was calibrated every 15 samples using the appropriate (50, 100, or 200) mg% Analox standard, with output recorded in milligram per deciliter. The lower limit of detection was 8.5 (± 10.2 range for noise) mg/dl, as evidenced by background measurements obtained from vehicle-injected rats never exposed to ethanol in Experiment 1 (the average of all vehicle-injected samples was 8.5 mg/dL, the ± 10.2 represents a window of 2 standard deviations as in Armbruster & Pry, 2008).
Dialysate corticosterone (CORT; Experiment 2).
Concentrations of corticosterone (CORT) were determined using a commercially available EIA kit (Cat No: ADI-901–097; Enzo Life Sciences, Farmingdale, NY) according to manufacturer’s instructions, with the exception that samples were heat-inactivated to denature endogenous corticosteroid binding globulin (CBG) by immersion in 75°C water for 60 min (Buck et al., 2011). The CORT assay had a sensitivity of 27.0 pg/ml, an inter-assay variability of 4.1%, and an intra-assay variability of 1.6%.
Dialysate cytokine multiplex.
Dialysate samples were assayed on the first thaw using magnetic bead-based multiplex procedures in accordance with the manufacturer’s instructions (12-plex, BioRad MagPix, catalog # 171-K1002M). This multiplex assay simultaneously detected 12 targets of interest: Interleukin (IL)-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, granulocyte macrophage colony-stimulating factor (GM-CSF), interferon (IFN)-γ, and tumor necrosis factor (TNF)-α.
Cannula placement confirmation.
After the conclusion of microdialysis, rats were euthanized, and brains were collected for cannula verification. Brains were post-fixed in paraformaldehyde and cryopreserved in sucrose solution. Brains were then flash frozen and stored at −80°C until cannula verification, for which a freezing cryostat was used to collect 20 μm slices for Cresyl Violet staining (Experiment 1). In Experiment 2, cannula placement was recorded, and probe tracts are shown in Figure 1 B and D, respectively.
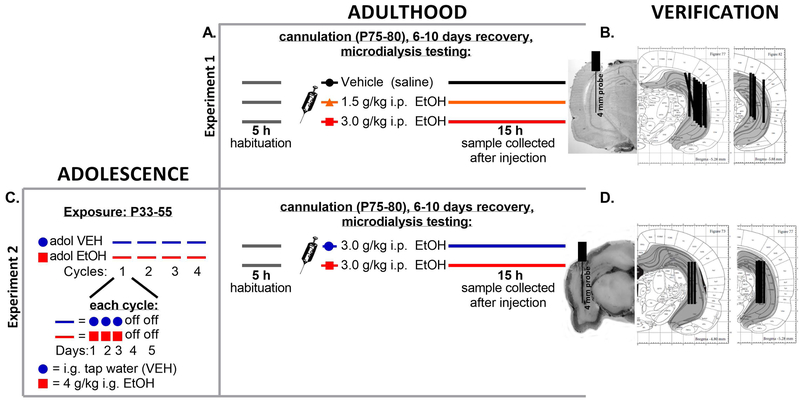
Figure 1.
This figure displays the experimental timelines and cannula verifications for Experiments 1 and 2. (A) The adult microdialysis manipulation for Experiment 1 is shown diagrammatically, showing the time line of microdialysis testing for 5 hours prior to injection, and the samples collected for 15 hours after injection of saline vehicle, 1.5 g/kg intraperitoneal (i.p.) ethanol, or 3.0 g/kg i.p. ethanol. (B) Cannula verification schematics for Experiment 1 are shown to the right of the experimental schedule. (C) The adolescent chronic intermittent ethanol exposure is shown on the left for rats in Experiment 2, followed by the adult microdialysis manipulation on the right depicting the time line of testing before and after both groups were challenged with 3.0 g/kg i.p. ethanol. (D) Cannula verification for Experiment 2 is shown to the right of the experimental manipulation.
Notes on data analyses.
Dialysate cytokine data were acquired using a standard curve in accordance with manufacturers’ instructions. A lowest limit of quantification (LLOQ) was determined using the MP Manager software for each analyte using a proprietary algorithm and is represented in all cytokine graphs with a horizontal dotted line. In order to preserve the integrity of the repeated measures design, samples extrapolated slightly below the standard curve were included in the analyses, and those that were below the possibility of extrapolation by the software were designated as zero concentration samples and also analyzed in these studies (similar to Bodnar et al., 2018). The total number of undetectable samples is reported in Supplemental Table 1.
Data were analyzed with Statistica software using an appropriate analysis of variance (ANOVA) design, and various post hoc approaches were utilized dependent on the specific data as described for individual experiments below. As these data sets varied greatly in size and format, a variety of post hoc approaches were necessary; for instance, for dialysate ethanol concentrations, the key point was to determine points of intoxication and clearance to enable subsequent cytokine analysis – therefore, Dunnett’s test was used to determine differences from designated control groups (the H4/5 time points in both experiments). In contrast, for cytokine dialysate outcomes we utilized Tukey’s post hoc test. The details of each test are described within the relevant results sections. An α-level of 0.05 was used as the criterion for significance for all effects.
Data management.
During the multiplex cytokine analyses for Experiments 1 and 2, some samples were missing. We did not use mean substitution to replace these values, but instead chose to analyze the data by pooling all samples into equal five-hour Epochs based on dialysate ethanol concentrations (DECs). The Epochs were as follows: 5 hours before ethanol or vehicle injection, 5 hours after ethanol or vehicle injection showing high levels of DECs in all groups that received ethanol injections, 5 hours after that showing lower levels of DECs that were yet higher than the baseline in the 3.0 g/kg group in both experiments, and the 5 final hours of testing during which DECs were at baseline-like levels for all ethanol-injected groups. In Experiment 1, the Epochs are titled 1, 2, 3, and 4. In Experiment 2, as both groups received an equivalent ethanol challenge, the Epochs are titled pre-ethanol (Pre-E), early intoxication (Early-I), late intoxication (Late-I), and clearance (Clear), respectively. We chose this approach as it allowed us to: 1) minimize the amount of data adjustment that needed to be done for statistical analysis, thus increasing the transparency and accuracy of our findings, 2) minimized the number of post hoc comparisons made after significant interactions, thereby decreasing the probability of making a Type I error, and 3) helped parse the data into clear categories as it pertained to levels of intoxication. We show the data here in both line format to display a detailed time course of extracellular protein, ethanol and CORT, as well as binned into 5-hr Epochs for statistical analysis and summary of findings.
For the analysis of DECs in Experiment 1, some samples with insufficient volume due to technical limitations (the occasional need to adjust tubing across a time course of this magnitude – 20 hours - resulted in small amounts of sample loss that was necessary to keep the timing of collection relative to the rat’s experience consistent) prevented DEC assessment; mean substitution was used for replacement of these data (3 time points for one animal in 1.5 g/kg EtOH group; 5 time points for another animal in 1.5 g/kg EtOH group; 1 time point for another animal in 1.5 g/kg group; and 1 time point for one animal in the 3.0 g/kg group). In Experiment 2, no samples were affected.
Experiment 1 methods.
This study examined the effects of an acute systemic ethanol challenge on extracellular cytokine concentrations in the hippocampus using a panel of neuroimmune analytes in adult male rats (N = 23; n = 7–8 per group). The study was performed with drug condition as a between-subjects variable with 3 levels (Drug: Vehicle [VEH], 1.5 g/kg EtOH, 3.0 g/kg EtOH) and time as a repeated measure (20 Time points: 5 h of habituation, 15 h of post-injection) design. Adult rats naïve to alcohol received hippocampal cannulations and, after a period of recovery (6–10 days), microdialysis testing commenced. After insertion of the probe and initiation of the microdialysis procedure, all rats were given 5 h acclimation/habituation, during which samples were collected for analysis. At this point, rats received an injection of either saline or one of the two ethanol doses, and samples were collected for another 15 h in order to assess the natural timecourse of cytokine changes as well as the cytokine response to ethanol across intoxication and clearance (for a diagrammatic experimental timeline, see Figure 1A; for cannula tract verification, Figure 1B).
Experiment 2 methods.
Having examined the effects of acute ethanol exposure on extracellular cytokines in the hippocampus, this Experiment determined the effects of adolescent chronic intermittent ethanol exposure (adolCIE) on the cytokine response to a similar acute adult ethanol challenge (3 g/kg i.p. ethanol). Rats (N = 11; n = 5–6 per group) were exposed to adolCIE, generating an adolCIE group that underwent chronic exposure to ethanol across adolescence, and an adolVEH group that received repeated intubations of vehicle across this period of time. These animals were allowed to mature to adulthood, yielding a period of abstinence from ethanol of 20–30 days, after which they underwent cannulation surgery and microdialysis at the same approximate age as rats in Experiment 1. On the day of microdialysis, 5 h of baseline sample was collected, following which both groups received an acute 3.0 g/kg i.p. ethanol challenge. Sample collection commenced for 15 h after injection, as in Experiment 1. Because acute ethanol did not significantly influence cytokine profiles in Experiment 1, all rats received ethanol as an adult challenge. As such, the design of this experiment was a two-group between-subjects experiment (adolCIE: adolVEH vs adolEtOH) with repeated measures (20 Time points: 5 h of habituation [H1–5], 15 h of post-injection [1–15]). For a diagrammatic experimental timeline, see Figure 1C, and Figure 1D for cannula tract verification.
Results
Experiment 1
Dialysate ethanol concentrations (DECs).
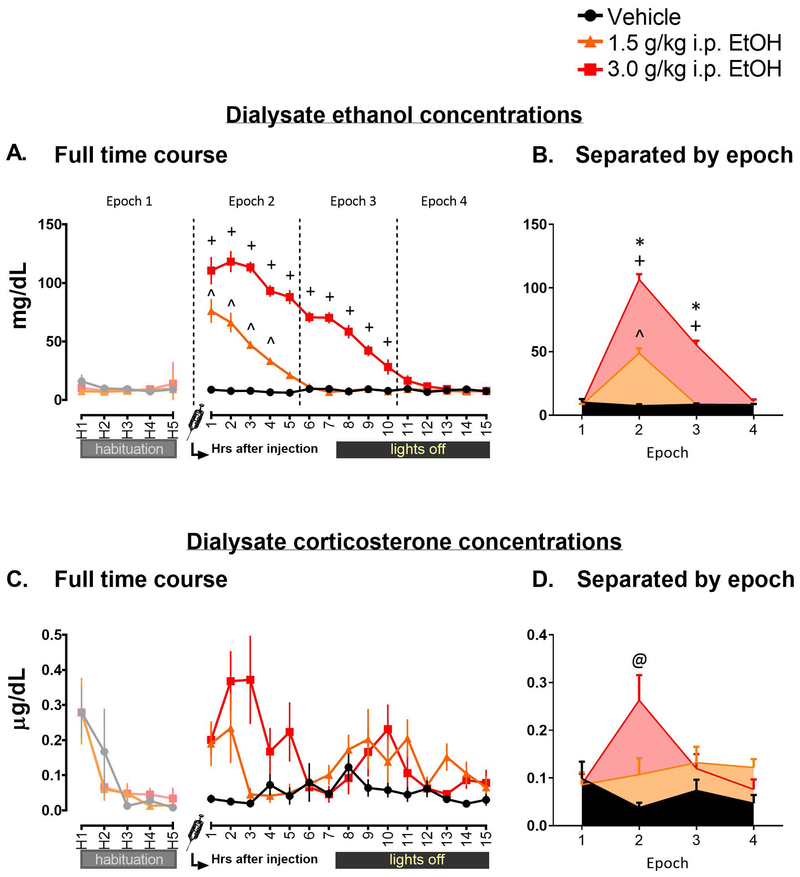
DECs were assayed for all groups across the time course (Figure 2A). After ensuring that no Vehicle animals had aberrant ethanol levels and determining the lower detection limit of the Analox for these data by analyzing samples from rats never exposed to ethanol, the 1.5 and 3.0 g/kg EtOH groups were analyzed separately using a mixed 2 (Drug: 1.5 g/kg EtOH, 3.0 g/kg EtOH) x 20 (Time points: 5 h of habituation, 15 h of post-injection) ANOVA. There were significant effects of Drug (F1, 13 = 162.32, p < 0.0001), Time (F19, 247 = 131.07, p < 0.0001), and a significant interaction (F19, 247 = 28.21, p < 0.0001). Tukey’s post hoc testing for the main effect of Drug indicated that the 1.5 and 3.0 groups differed from one another, with higher levels seen in the 3.0 group overall (p < 0.0001). In order to determine points of clearance for each group, post hoc testing on the interaction effect was performed using Dunnett’s test against the H5 time point for each group; the 1.5 g/kg EtOH group showed statistically elevated DEC levels for 4 h after injection (all p < 0.01), and the 3.0 group showed elevated levels for 10 h after injection (all p < 0.0001). Additionally, these data were also analyzed using the epoch approach (Figure 2B) using a mixed 2 (Drug: 1.5 g/kg EtOH, 3.0 g/kg EtOH) x 4 (Epochs: 1, 2, 3, 4) ANOVA, revealing an interaction (F3, 39 = 88.22, p < 0.0001). Subsequent Tukey’s post hoc tests indicated that in the 3.0 group, DECs were elevated above baseline in the 2nd and 3rd Epochs, whereas in the 1.5 group, DECs were elevated only in the 2nd Epoch. Additionally, the 3.0 group showed elevation significantly above the 1.5 group at both Epoch 2 and 3 (all p < 0.001).
Figure 2.
This figure displays the dialysate ethanol concentrations (2A for line graph and B for Epoch graph) across the microdialysis time course in rats before and following an injection with either saline (Vehicle group) or 1.5 or 3.0 g/kg intraperitoneal ethanol (data shown in mg/dL). Carets (^) indicate Time points or Epochs at which ethanol concentrations in the 1.5 g/kg ethanol group were elevated significantly above baseline (H5 for line graph, Epoch 1 for Epoch graph), whereas plus signs (+) indicate the same in the 3.0 g/kg ethanol group. Additionally, in the Epoch graph, an asterisk (*) is used to denote Epochs at which there was a significant elevation in the 3.0 group above that of 1.5. Figures 2C and 2C display the dialysate corticosterone concentrations in line and epoch format in μg/dL. An at symbol (@) is used to indicate the Epoch at which the 3.0 group showed significant elevation above all other Epochs within the group as well as in comparison to Epoch 2 of the 1.5 group.
Dialysate corticosterone (CORT) concentrations.
Dialysate CORT (Figure 2C and D) was analyzed using a mixed 3 (Drug: Vehicle [VEH], 1.5 g/kg EtOH, 3.0 g/kg EtOH) x 4 (Epochs: 1, 2, 3, 4) ANOVA. There was a main effect of Drug (F2, 20 = 6.44, p < 0.01), with Tukey’s post hoc test indicating that the 3.0 group showed significant elevation above the VEH but not 1.5 group (p < 0.01). Additionally, a significant interaction was observed (F6, 60 = 4.12, p < .01). Further probing with Tukey’s post hoc test revealed that while the VEH and 1.5 g remained stable across Epochs, the 3.0 group showed elevation at Epoch 2 as compared to its own baseline Epoch 1 (p < 0.01), as well as in comparison with the other groups at this time (p < 0.01).
Dialysate cytokine concentrations.
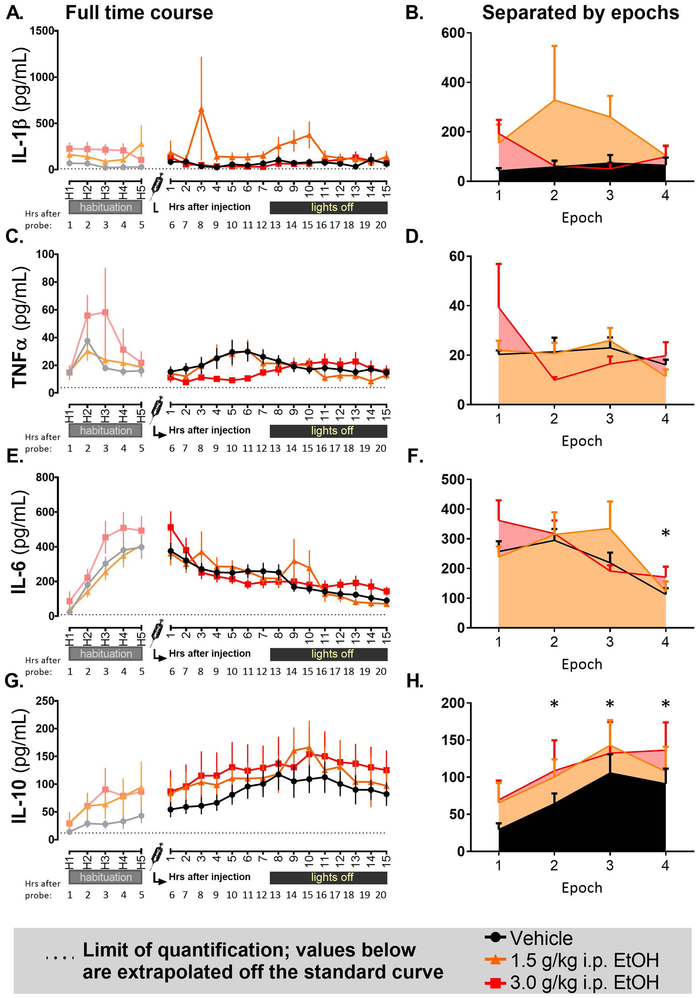
Out of the 12 assayed analytes, four were detectable across the time course, whereas 8 were extrapolated largely below the standard curve or were undetectable. Cytokine concentrations of detectable analytes (IL-1β, TNFα, IL-6, and IL-10) across the timecourse were analyzed using mixed 3 (Drug: Vehicle [VEH], 1.5 g/kg EtOH, 3.0 g/kg EtOH) x 4 (Epoch: 1, 2, 3, 4) ANOVAs.
IL-β (Figures 3A and B) showed a trend for an effect of Drug (F2, 20 = 2.99, p = 0.073) and no other effects. There were trends for effects of Epoch and Drug/Epoch interaction (F3, 60 = 2.48, p = 0.069 and F6, 60 = 1.92, p = 0.093, respectively) for TNFα expression (Figure 3C and D). Epoch analysis of IL-6 (Figure 3E and F) revealed a main effect of Epoch (F3, 60 = 12.08, p < 0.0001), with Tukey’s post hoc test indicating that the 4th Epoch showed suppression relative to all other Epochs (p < 0.01). Additionally, there was a trend for an interaction between Drug and Epoch (F6, 60 = 2.12, p = 0.064). IL-10 (Figure 3G and H) showed a main effect of Epoch (F3, 60 = 11.59, p < 0.0001), with progressive elevation observed across time. Tukey’s post hoc test indicated that Epochs 2, 3, and 4 all showed elevation above Epoch 1 (all p < 0.001).
Figure 3.
This figure displays pg/mL concentrations of cytokine levels across the microdialysis time course for (3A, B) Interleukin-1β, (3C, D) Tumor necrosis factor α, (3E, F) Interleukin-6, and (3G, H) Interleukin-10. Where significant effects of Epoch were observed, asterisks (*) indicate Epochs at which all groups showed elevation above baseline (Epoch 1). The dotted horizontal line represents the lowest limit of quantification in the MagPix assay used to acquire the data. Data at or below the dotted line are extrapolated beyond the standard curve.
Other analytes examined (IFNγ, IL-2, IL-4, IL-5, IL-12p70, IL-1α, GM-CSF, IL-13) were largely at or below the threshold of detection/extrapolation.
Experiment 2.
Body weights.
Body weight were collected across the adolescent exposure, before surgery, and a week after surgery on the day of microdialysis. Weights were analyzed using a 2 (adolCIE: adolVEH vs. adolEtOH) x 7 (Age at weight) ANOVA. There was a significant interaction (F7, 63 = 2.97, p < 0.01). Tukey’s post hoc test indicated that though the adolCIE group weighed slightly less than the adolVEH across all the repeated cycles of alcohol exposure and into adulthood, this difference was not significant: both groups gained significant amounts of weight at each time of weight collection as compared to the last (p < 0.05) and showed full weight recovery after surgery by the time of microdialysis testing (see Table 1).
Table 1. Weights across adolescence and adulthood in Experiment 2.
Weights (g) across Experiment 2 for rats that received either adolescent ethanol exposure (adolEtOH) or vehicle controls (adolVEH).
| Weight before Experiment (P34-35) |
After Cycle 1 (P39-40) |
After Cycle 2 (P44-45) |
After Cycle 3 (P48-49) |
End of Cycle 4 (P53-54) |
Before Surgery (P75) |
Day of testing (P85) |
|
|---|---|---|---|---|---|---|---|
| adolVEH | 132 ± 3 | 170 ± 4 | 206 ± 5 | 240 ± 7 | 255 ± 6 | 353 ± 13 | 372 ± 12 |
| adolEtOH | 139 ± 3 | 173 ± 4 | 205 ± 5 | 234 ± 5 | 246 ± 6 | 334 ± 6 | 356 ± 9 |
Dialysate ethanol concentrations (DECs).
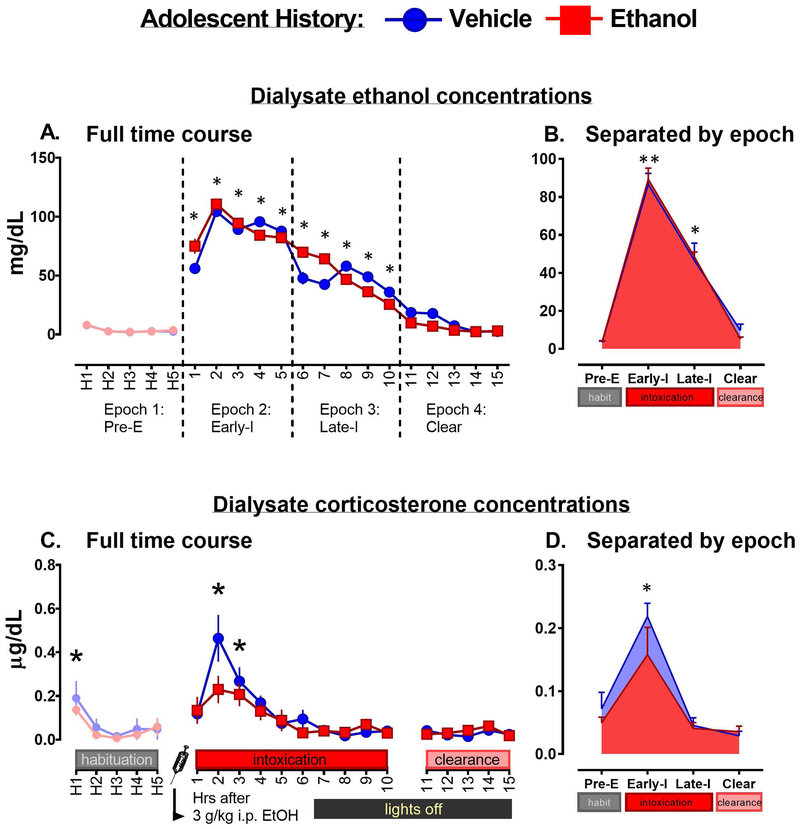
DECs were analyzed using a mixed-subject 2 (adolCIE: adolVEH vs. adolEtOH) x 20 (Time point: 5 h of habituation [H1–5], 15 h after 3.0 g/kg Ethanol injection [1–15]) ANOVA (Figure 4A) – both groups were analyzed as both were injected with ethanol during the microdialysis testing. There was no main effect of adolCIE on DECs. There was a main effect of Time point (F 19, 171 = 50.00, p < 0.0001). Post hoc testing was performed using Dunnett’s test, in which all time points were first compared to levels seen in the control Time point H5. Given these criteria, the Time points of significant DECs elevation were Time points 1–9 (p < 0.0001) and 10 (p < 0.01) h after injection, replicating the findings from Experiment 1 which also showed clearance from this dose after 10 h post-injection. DECs were used to split the time points into four Epochs for subsequent analyses (Figure 4B) as described in the general methods. When thus analyzed using a 2 (adolCIE: VEH vs. EtOH) x 4 (Epoch: Pre-E, Early-I, Late-I, Clear) mixed subjects design, there was a main effect of Epoch (F3, 27 =207.12, p < 0.0001), with elevation of DECs observed in both Early-I and Late-I as compared to Pre-E and Clear (all p < 0.0001), as well as a significant difference between higher levels seen in Early-I and lower levels observed in Late-I (p < 0.0001).
Figure 4.
Dialysate ethanol concentrations (4A for a line graph, 4B for epoch layout) following a 3 g/kg i.p. ethanol injection in rats with a history of adolescent vehicle intubation (blue) or adolescent ethanol (red). Dialysate corticosterone concentrations are displayed below (4C for line graph, 4D for epoch layout). For a main effect of Time point in Figures 4A and C, an asterisk (*) is used to indicate a significant difference in corticosterone and DECs from baseline (H5), for both adolVEH and adolEtOH history subjects. In Figures 4B and D, an asterisk signifies that the Epoch is different from Pre-E; a double asterisk denotes a difference from all other Epochs presented.
Dialysate corticosterone (CORT) concentrations.
Dialysate CORT was analyzed using a mixed-subject 2 (adolCIE: adolVEH vs. adolEtOH) x 20 (Time point: H1–5, and 1–15 h after injection) ANOVA (Figure 4C). There was a main effect of Time point (F19, 171 = 12.274, p < 0.0001). Post hoc testing was performed using Dunnett’s test, in which all time points were compared to H4 and H5 as controls. Only Time points that were significantly different from both of these controls were designated as having significantly elevated levels of CORT. Given these criteria, the Time points of significant CORT elevation were H1 (p < 0.05), 2 (two hours after injection; p < 0.0001), and 3 (three hours after injection; p < 0.0001). Corticosterone was also assessed in Epochs (Figure 4D) based on DECs using a 2 (adolCIE: adolVEH vs. adolEtOH) x 4 (Epoch: Pre-E, Early-I, Late-I, Clear) mixed subjects design. There was a main effect of Epoch (F3, 27 = 28.73, p < 0.0001), with Tukey’s post hoc test indicating significantly higher levels seen in Early-I than in any other Epoch (all p < 0.0001). Based on a priori hypotheses indicating that CORT levels would be highest during peak ethanol concentrations and that a history of chronic ethanol would affect the CORT response to challenge, independent t-tests were performed for data collected in the first three hours after ethanol injection. Two hours after injection, there was a trend for suppressed CORT levels in the adolEtOH history group as compared to adolVEH (t9 = 1.97, p = 0.08).
Dialysate cytokine concentrations.
As in Experiment 1, the same four analytes (IL-1β, TNFα, IL-6, and IL-10) were detectable in the dialysate and are reported here, whereas 8 other immune proteins were not in range. Detectable cytokines were analyzed based on Epoch using 2 (adolCIE: adolVEH vs adolEtOH) x 4 (Epoch: Pre-E, Early-I, Late-I, and Clear) mixed subjects ANOVAs, with post hoc testing performed using Tukey’s test. For full presentation, data are shown both in line format as well as Epoch format in Figure 5.
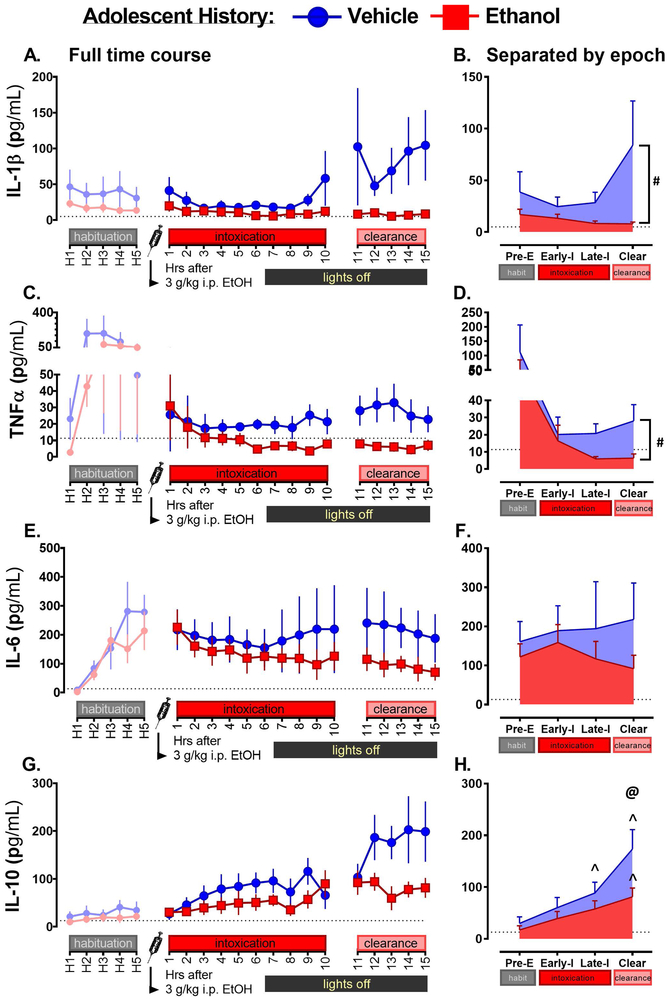
Figure 5.
Cytokine outcomes following a 3 g/kg i.p. ethanol injection in rats with a history of adolescent vehicle intubation (blue) and adolescent ethanol (red). Data are shown in line graph format as well as separated into four epochs (Pre Ethanol/Pre-E, Early Intoxication/Early-I, Late Intoxication/Late-I, and Clearance/Clear) in pg/mL, performed for Interleukin-1β (5A for line graph, 5B for epoch version), Interleukin-6 (5C for line graph, 5D for epoch version), Tumor necrosis factor α (5E for line graph, 5F for epoch version), and Interleukin-10 (5G for line graph, 5H for epoch version). For a main effect of Group, a pound sign (#) is used to indicate a significant difference between adolescent drug groups (adolVEH [Vehicle] vs. adolCIE [Ethanol]). In the case of an interaction between adolescent drug treatment and Epoch, symbols are used as follows: differences between Vehicle and Ethanol groups at a specific Epoch are indicated with an “at” symbol (@), whereas points at which an individual group showed an Epoch difference from its own Pre-E Epoch are indicated with a caret (^).
A main effect of adolCIE was observed for IL-1β (F1,9 = 6.316, p < 0.05; Figure 5A, B). Post hoc testing revealed that there was a significant difference (p <0.05) in cytokine concentration between adolVEH history rats and adolEtOH history rats, with suppressed IL-1β levels seen in rats with a history of ethanol.
There was a main effect of adolCIE (F1, 9 = 6.00, p < 0.05) observed for TNFα (Figures 5C and D), with adolCIE rats showing suppressed levels as compared to adolVEH. No effects were found for IL-6 (Figure 5E, F).
Finally, IL-10 showed a main effect of Epoch (F3,27 = 18.925 p < 0.001), a trend for adolCIE (F1, 9 = 3.608 p = 0.089), and a significant interaction between adolCIE and Epoch (F3,27 = 3.184 p < 0.05; Figure 5G, H). Post hoc testing for the main effect of Epoch revealed that the Pre-E Epoch differed significantly from both the Late-I Epoch (p < 0.01) and Clear (p < 0.0001). The Clear Epoch also differed significantly from the Early-I Epoch (p < 0.0001) and the Late-I Epoch (p < 0.01). Post hoc testing for the interaction between adolCIE and Epoch revealed that adolEtOH rats differed significantly (p <0.01) from the adolVEH group in the Clearance Epoch. In addition, the adolVEH group showed that the Pre-E Epoch differed significantly (p < 0.05) from both the Late-I Epoch, and the Clear Epoch (p < 0.0001). The Clear Epoch showed peak IL-10 for the adolVEH group and differed significantly from the first two Epochs (p < 0.0001) and the Late-I Epoch, as well (p < 0.001). The adolEtOH group showed a significant difference between the Pre-E Epoch and Late-I (p <0.05), and Clear (p < 0.01). As in the adolVEH group, the Clear Epoch served as the peak for IL-10 in the adolEtOH group, though it was not significantly higher than the Late-I Epoch. In summary, IL-10 levels rose steadily across time following ethanol challenge but were significantly suppressed in rats with a history of adolescent ethanol and failed to reach levels observed in vehicle history animals.
Discussion
Alcohol exposure has been shown to have a profound and time-dependent influence on many aspects of neuroimmune function, with important implications for both addictive processes and alcohol-related brain pathology. The over-arching goal of the present studies, therefore, was to develop a strategy for assessing the influence of both acute (Experiment 1) and prolonged developmental (Experiment 2) effects of ethanol exposure on cytokine release patterns in the CNS. The experiments described here establish a technical approach of using large-molecule microdialysis paired with multiplexing technology to detect extracellular cytokine fluctuations in the CNS of the awake, behaving rat. In addition to being the first studies in which large-molecule microdialysis was combined with multiplex arrays, an important contribution of this work is the establishment of a normative range of extracellular cytokines in the awake, behaving rat. These data provide the background required for using this technique to assess time-sensitive changes in central inflammatory mediators and begin to probe the CNS cytokine response to an acute ethanol challenge and its modulation by chronic ethanol history. Additionally, our findings with this approach demonstrate for the first time that adolescent binge-like ethanol exposure had a much more profound influence on extracellular cytokines than acute ethanol exposure in adulthood per se.
A major strength of the microdialysis and multiplexing approach utilized here was the ability to assess an unbiased cohort of cytokines as well as ethanol concentrations in the same samples. Although we elected to utilize a 12-plex in these initial studies, we have also utilized this same approach with commercially available 23-plex kits that include a broader array of growth factors and cytokines with success, though it should be noted that cost of multiplexing goes up astronomically when doing so. Thus, the possibilities of combining large-molecule microdialysis with multiplex approaches are great. Replicating across both experiments, brain ethanol concentrations following the high-dose injection of 3 g/kg i.p. reached peak levels (~125 mg/dl) by the second hour after injection and were cleared after 10 hours past injection. The low dose (1.5 g/kg i.p.) in Experiment 1 showed about half the brain ethanol concentrations (~75 mg/dl) of the high dose and was cleared after 4 hours past injection. In other studies where ethanol concentrations were examined during microdialysis, total values were lower than those found in the periphery at the same time, even when corrected for probe efficiency (Ferraro et al., 1990). When ethanol concentrations in dialysate were compared with whole tissue content, prior studies have reported that raw values obtained from in vivo microdialysis were a fraction of brain tissue ethanol concentrations (Schier et al., 2012, Robinson et al., 2002). Though we did not assess probe efficiency here, the raw values detected were sufficient to perform the analyses that were the focus of this work, similar to what has been done previously (Schier et al., 2012). Ideally, it would be advantageous to measure both blood and dialysate ethanol concentrations in the same animals. However, that was not possible in the present study out of concern for how blood sampling procedures might impact cytokine responses in brain. Future studies will be required to better test such associations between blood and dialysate concentrations.
In Experiment 1, we detected a wide dynamic range of cytokine changes following probe insertion. From these data, we can conclude that an acute moderate dose of 1.5 g/kg i.p. ethanol did not induce significant changes in cytokine levels in the naïve rat at the protein level. We also did not see effects of the larger 3.0 g/kg dose, though due to unexpected, minor differences observed in baseline for some of the analytes it may be premature to draw conclusions about ethanol effects at this dose. Unfortunately, we have no explanation for these minor baseline differences at this time, as the effect was (i) not dependent on a single outlier; (ii) could not be explained by an order effect in sample collection or assay (i.e., all groups were counter-balanced across days of experimental data collection); and (iii) rats had no differential history prior to ethanol injection. Regardless, even when data were analyzed as percent of baseline, similar results were obtained, and conclusions remain the same (data not shown). With that said, Experiment 2 closely recapitulated the findings of Experiment 1 as far as baseline levels of cytokines observed at the start of testing and patterns of response following probe insertion, except for minor differences in the baseline of TNFα that relate to adolescent CIE. Overall, we were somewhat surprised at the lack of effect of acute ethanol on extracellular cytokine concentrations.
In contrast, Experiment 2 demonstrated that adolescent CIE resulted in a blunted neuroimmune response to ethanol in adulthood that was cytokine-specific. Animals with an adolescent history of ethanol showed suppressed levels of IL-1β, TNFα, and IL-10 following an acute 3.0 g/kg i.p. ethanol challenge as compared to the controls who received vehicle intubations in adolescence. It is important to note that the acute adult ethanol challenge followed a prolonged period of abstinence (20–30 days). In prior studies, adolescent CIE led to substantially reduced cytokine gene induction after an in vivo LPS challenge in circulating immune cells (Vore et al., 2017). Thus, the pattern of changes in extracellular cytokines in the CNS observed here are consistent with what we previously observed after adolescent ethanol exposure, despite a completely different population of cells and a distinct approach for capture and detection of cytokine changes. One limitation of this experiment is that we did not include separate groups of rats receiving vehicle as adults, so we cannot rule out the possibility that the suppressed cytokine responses in the adolescent CIE group might reflect an interaction between adolescent and adult ethanol challenges. However, the overall lack of an effect of acute ethanol in adulthood (Experiment 1) and the similarity to what was observed in circulating immune cells (Vore et al., 2017) suggests that the effect is most likely attributable to adolescent CIE (and not the adult ethanol challenge). Future studies will be necessary to further clarify this point.
Although the mechanisms underlying the effect of adolescent CIE remain unclear, at least two possible interpretations of the effect of adolescent CIE on adult cytokine reactivity are possible. First, adolescent CIE may produce a persistent state of immunosuppression, evidenced by reduced cytokine expression in both circulating immune cells and in response to probe insertion into the hippocampus. This interpretation is consistent with prior studies showing that ethanol reduced TLR4 expression and sensitivity to TLR4-dependent ligands (Telles et al., 2017). Second, because adolescents naturally show reduced cytokine expression relative to adult subjects (Doremus-Fitzwater et al., 2015b, Girard-Joyal et al., 2015), the present findings may represent a “locking-in”-like effect where adolescent-typical responses are retained into adulthood (Spear and Swartzwelder, 2014). Both hypotheses are interesting and potentially transformative for our understanding of early developmental exposure effects on overall neuroimmune function. Furthermore, the functional implications of these changes will need to be addressed. The hippocampus is a brain region that has been shown to be particularly vulnerable to locking-in-like effects; findings have revealed that adolescent ethanol exposure enacted long-lasting structural damage in the hippocampus which contributed to functional abnormalities (Risher et al., 2015). Nevertheless, an important control that needs to be conducted is to assess whether adolescence represents a specific developmental period of vulnerability by testing whether adult rats exposed to an equivalent regimen of CIE display the same effect as adolescents or are resistant to such CIE effects. Those and other studies are already ongoing in our lab.
While these initial foundational studies have focused exclusively on male subjects, it will be necessary to expand our understanding of these effects across sex. While no sex differences have been shown in the neuroimmune gene expression response to acute alcohol challenge as far as IL-1β, TNFα, and IL-6 in the hippocampus (Gano et al., 2016b; Gano et al, in prep), there are more notable differences that emerge as ethanol exposure becomes chronic. For instance, it has been shown sex differences in the neuroimmune responses to chronic ethanol may be particular to the phase of intoxication (specifically emerging in withdrawal) and would therefore be ideally suited to examination using this novel approach (Alfonso-Loeches et al., 2013; Hashimoto & Wiren, 2008; Wilhelm Clare et al., 2015).
Perhaps as telling as the significant findings of these Experiments are what may be perceived as the null results. The unbiased multiplex assay probed a range of cytokine factors, some of which are associated with innate immunity and typically found in the CNS (i.e. IL-1β, IL-6, and TNFα), as well as those typically associated with acquired immunity (i.e., derived from T-cells or B-cells). We have reported these outcomes as well as our significant findings in order to best characterize the use of this approach and to lay a foundation for normative ranges of extracellular cytokines in the CNS. In both Experiments, levels of cytokines associated with acquired immunity (and in particular T-cell function) were largely unaffected by probe insertion as well as ethanol administration. This effect is largely to be expected given that T-Cell and B-cell activity in the CNS is not typically observed under normal conditions, and when present, often portend significant autoimmune-related problems (e.g. Grossman and Miller, 2010). Thus, the low or undetectable levels of cytokines more typically associated with acquired immunity were both predicted a priori and an important confirmation of our approach. Thus, future studies employing microdialysis with multiplex arrays could be tailored to focus more specifically on neuroimmune signaling molecules more typically associated with innate immunity.
In both Experiments, we also assessed brain corticosterone levels across the microdialysis time course. Overall, while ambient concentrations of CORT in dialysates were lower than some prior studies (as reviewed in Spencer and Deak, 2017), other literature has shown the same levels acquired during hippocampal microdialysis as we show here (Barrientos et al., 2015, Qian et al., 2012). Although the reason for this difference is not clear, it can be noted that the microdialysis procedure (small vs large pore size) and corticosterone measurement approaches (RIA vs EIA) were quite different across studies, perhaps accounting for differing corticosterone concentrations. Consistent with this, no significant escalation in CORT concentrations were observed around lights off, though modest transient increases were observed in Experiment 1. Nevertheless, in all groups, brief increases in CORT were observed during the first measured time point (H1), likely due to the handling and probe manipulations at the start of testing. A second peak was observed shortly after ethanol injection in both studies in animals that were given the high 3.0 g/kg ethanol challenge. Rats with a history of adolescent CIE showed a trend for lower and more variable CORT levels during this peak, indicating a modest level of HPA dysfunction lingering after adolescent exposure. This is in agreement with data indicating that chronic ethanol exposure disrupted corticosterone rhythms, resulting in a suppressed corticosterone response to further ethanol challenge (Spencer and McEwen, 1990). It should be noted that prior to the point of acute ethanol challenge during the microdialysis testing in adulthood, the adolescent CIE rats underwent a period of abstinence from ethanol of 20–30 days. This is a remarkably long-lasting effect of ethanol exposure that indicates substantial plasticity in HPA axis sensitivity after adolescent CIE. Interestingly, our prior work showed that the same adolescent CIE procedure did not impact plasma CORT responses in male rats when challenged with LPS as adults, whereas female rats with a history of adolescent CIE displayed enhanced CORT responses to LPS. These intriguing effects require further studies to delineate the mechanisms that might be at play.
In addition to examining the effects of ethanol exposure on extracellular cytokines in the hippocampus, these data also contribute greater understanding of the effects of invasive neuroscience procedures on cytokine levels in surrounding tissue. That is, microdialysis requires two consecutive procedures that evoke a tissue-damage response: implantation of the cannula during surgery, as well as probe insertion on the day of testing. Though several cytokines were unaffected by probe insertion, there was a distinctive pattern of changes observed in Experiment 1 and replicated again in Experiment 2 that warrants further comment. The issue of tissue damage inherent to cannulation surgery has been raised previously with a technique similar to that used here and yielding similar conclusions (Vasicek et al., 2013), but empirical studies on this topic are limited. Our findings report somewhat higher baseline levels of response than those shown by Vasicek and colleagues, yet we report less variability across rats and provide a longer time course for a more detailed examination of cytokine changes following probe insertion. These differences may be explained by slight variation in the equipment used and the parameters of our microdialysis paradigms, yet, importantly, report similar extracellular cytokine concentrations following insertion of the probe 7 days after cannulation. In the two Experiments reported here, there was a brief increase in TNFα shortly after probe insertion, followed by an elevation of IL-6, and succeeded by a sustained elevation of IL-10 that lasted up through the twentieth hour of testing. These results are in concordance with literature on traumatic brain injury (TBI), indicating that TNFα and IL-6 play a strong role in the early response to penetrating CNS damage such as that induced by the insertion of the probe (Ghirnikar et al., 1998). Work from laboratories specializing in the interaction between TBI and ethanol has shown that acute intoxication can prolong the cytokine response to a mild TBI (Teng and Molina, 2014); though our data did not show a similar effect, the parameters for these studies were quite different from those utilized here. However, accumulating evidence shows that the neuroimmune response is very sensitive to the relative timing and sequence of exposure to TBI versus ethanol (Janis et al., 1998, Goodman et al., 2013), so perhaps the approach described here can be used to probe these effects, capitalizing on the advantage of excellent temporal resolution in future studies. Furthermore, cytokines are able to influence other CNS neurotransmitters such as GABA (Suryanarayanan et al., 2016), glutamate (reviewed in Miller et al., 2009), dopamine (reviewed in Felger and Miller, 2012, Miller et al., 2013), and others, which may contribute to downstream effects of probe- or ethanol-induced alterations in cytokines. These data highlight the importance of considering the neuroimmune environment in its totality, including potential collateral damage incurred due to standard neuroscience approaches required for mechanistic studies.
In sum, the studies reported here establish working parameters for the assessment of extracellular cytokine concentrations in the awake, behaving rat that offer the advantages of (i) repeated sampling across the entire cycle of ethanol intoxication and withdrawal; and (ii) the capability of combining large-molecule microdialysis with multiplexing approaches. These studies also report the surprising outcome that extracellular concentrations of cytokines were largely unaffected by acute ethanol challenge, yet (iv) adolescent CIE led to substantially reduced extracellular cytokines in rats with a history of adolescent CIE. These findings may have important implications for understanding ethanol-neuroimmune interactions, and provide compelling evidence for the influence of repeated, binge-like exposures to ethanol during adolescence on subsequent immune (Vore et al., 2017) and neuroimmune (Experiment 2) reactivity.
Supplementary Material
Highlights.
Large molecule microdialysis was used with multiplexing to assess brain cytokines
Acute ethanol had no effect on extracellular cytokines in hippocampus
Adolescent CIE severely blunted cytokine responses to ethanol in adulthood
Adolescents were highly sensitive to long-lasting effects of ethanol on CNS cytokines
Acknowledgements:
Supported by the Developmental Exposure Alcohol Research Center (DEARC; P50AA017823) and the Center for Development and Behavioral Neuroscience at Binghamton University. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the above stated funding agencies. The authors have no conflicts of interest to declare.
References.
- AGNELLO D, VILLA P & GHEZZI P 2000. Increased tumor necrosis factor and interleukin-6 production in the central nervous system of interleukin-10-deficient mice. Brain Research, 869, 241–243. [DOI] [PubMed] [Google Scholar]
- ALFONSO-LOACHES S, PASCUAL M, & GUERRI C 2013. Gender differences in alcohol-induced neurotoxicity and brain damage. Toxicology, 311(1), 27–34. [DOI] [PubMed] [Google Scholar]
- ARAKAWA H, ARAKAWA K & DEAK T 2010. Sickness-related odor communication signals as determinants of social behavior in rat: A role for inflammatory processes. Hormones and Behavior, 57, 330–341. [DOI] [PubMed] [Google Scholar]
- ARMBRUSTER DA, PRY T 2008. Limit of blank, limit of detection, and limit of quantification. Clinical Biochemical Review, 29(1). [PMC free article] [PubMed] [Google Scholar]
- BARNEY T, VORE A, GANO A, MONDELLO J & DEAK T 2018. Assessment of Interleukin-6 Signaling Effects on Behavioral Changes Associated with Acute Alcohol Intoxication in adult male rats. Alcohol (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRIENTOS RM, THOMPSON VM, KITT MM, AMAT J, HALE MW, FRANK MG, CRYSDALE NY, STAMPER CE, HENNESSEY PA, WATKINS LR, SPENCER RL, LOWRY CA & MAIER SF 2015. Greater glucocorticoid receptor activation in hippocampus of aged rats sensitizes microglia. Neurobiology of Aging, 36, 1483–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BHATTACHARYA A, WANG Q, AO H, SHOBLOCK JR, LORD B, ALUISIO L, FRASER I, NEPOMUCENO D, NEFF RA, WELTY N, LOVENBERG TW, BONAVENTURE P, WICKENDEN AD & LETAVIC MA 2013. Pharmacological characterization of a novel centrally permeable P2X7 receptor antagonist: JNJ-47965567. British Journal of Pharmacology, 170, 624–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLEDNOV YA, BERGESON SE, WALKER D, FERREIRA VMM, KUZIEL WA & HARRIS RA 2005. Perturbation of chemokine networks by gene deletion alters the reinforcing actions of ethanol. Behavioural Brain Research, 165, 110–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLEDNOV YA, PONOMAREV I, GEIL C, BERGESON S, KOOB GF & HARRIS RA 2012. Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addiction Biology, 17, 108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BODNAR TS, RAINEKI C, WERTELECKI W, YEVTUSHOK L, PLOTKA L, ZYMAK-ZAKUTNYA N, HONERKAMP-SMITH G, WELLS A, ROLLAND M, WOODWARD TS, COLES CD, KABLE JA, CHAMBERS CD & WEINBERG J 2018. Altered maternal immune networks are associated with adverse child neurodevelopment: Impact of alcohol consumption during pregnancy. Brain, Behavior, and Immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRENHOUSE HC & SCHWARZ JM 2016. Immunoadolescence: Neuroimmune development and adolescent behavior. Neuroscience & Biobehavioral Reviews, 70, 288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUCK HM, HUESTON CM, BISHOP C & DEAK T 2011. Enhancement of the hypothalamic–pituitary–adrenal axis but not cytokine responses to stress challenges imposed during withdrawal from acute alcohol exposure in Sprague–Dawley rats. Psychopharmacology, 218, 203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOURBAJI S, URANI A, INTA I, SANCHIS-SEGURA C, BRANDWEIN C, ZINK M, SCHWANINGER M & GASS P 2006. IL-6 knockout mice exhibit resistance to stress-induced development of depression-like behaviors. Neurobiology of Disease, 23, 587–594. [DOI] [PubMed] [Google Scholar]
- CREWS FT, BECHARA R, BROWN LA, GUIDOT DM, MANDREKAR P, OAK S, QIN L, SZABO G, WHEELER M & ZOU J 2006. Cytokines and alcohol. Alcoholism, Clinical And Experimental Research, 30, 720–730. [DOI] [PubMed] [Google Scholar]
- DAVID TE, FSICHER I & MOLDAVE K 1983. Studies on the Effect of Ethanol on Eukaryotic Protein Synthesis in Vitro. The Journal of Biological Chemistry, 258, 7702–6. [PubMed] [Google Scholar]
- DINEL A-L, ANDRÉ C, AUBERT A, FERREIRA G, LAYÉ S & CASTANON N 2011. Cognitive and Emotional Alterations Are Related to Hippocampal Inflammation in a Mouse Model of Metabolic Syndrome. PLoS ONE, 6, e24325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODD PR & LEWOHL JM 1998. Cell Death Mediated by Amino Acid Transmitter Receptors in Human Alcoholic Brain Damage: Conflicts in the Evidencea. Annals of the New York Academy of Sciences, 844, 50–58. [DOI] [PubMed] [Google Scholar]
- DONEGAN JJ, GIROTTI M, WEINBERG MS & MORILAK DA 2014. A Novel Role for Brain Interleukin-6: Facilitation of Cognitive Flexibility in Rat Orbitofrontal Cortex. The Journal of Neuroscience, 34, 953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOREMUS-FITZWATER TL, BUCK HM, BORDNER K, RICHEY L, JONES ME & DEAK T 2014. Intoxication- and withdrawal-dependent expression of central and peripheral cytokines following initial ethanol exposure. Alcoholism, Clinical And Experimental Research, 38, 2186–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOREMUS-FITZWATER TL, GANO A, PANICCIA JE & DEAK T 2015. Male adolescent rats display blunted cytokine responses in the CNS after acute ethanol or lipopolysaccharide exposure. Physiology & behavior, 148, 131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOREMUS-FITZWATER TL, PANICCIA JE, GANO A, VORE AS & DEAK T 2018. Differential effects of acute versus chronic stress on ethanol sensitivity: Evidence for interactions on both behavioral and neuroimmune outcomes. Brain, Behavior, and Immunity, 70, 141–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELGER JC & MILLER AH 2012. Cytokine effects on the basal ganglia and dopamine function: The subcortical source of inflammatory malaise. Frontiers in Neuroendocrinology, 33, 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERGUSON LB, MOST D, BLEDNOV YA & HARRIS RA 2014. PPAR agonists regulate brain gene expression: Relationship to their effects on ethanol consumption. Neuropharmacology, 86, 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERRARO TN, WEYERS P, CARROZZA DP & VOGEL WH 1990. Continuous monitoring of brain ethanol levels by intracerebral microdialysis. Alcohol, 7, 129–132. [DOI] [PubMed] [Google Scholar]
- FRANK MG, FONKEN LK, DOLZANI SD, ANNIS JL, SIEBLER PH, SCHMIDT D, WATKINS LR, MAIER SF & LOWRY CA 2018. Immunization with Mycobacterium vaccae induces an anti-inflammatory milieu in the CNS: Attenuation of stress-induced microglial priming, alarmins and anxiety-like behavior. Brain, Behavior, and Immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GANO A, DOREMUS-FITZWATER TL & DEAK T 2016a. Sustained alterations in neuroimmune gene expression after daily, but not intermittent, alcohol exposure. Brain research, 1646, 62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GANO A, DOREMUS-FITZWATER TL & DEAK T 2016b. A cross-sectional comparison of ethanol-related cytokine expression in the hippocampus of young and aged Fischer 344 rats. Neurobiology of Aging, 54, 40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GANO A, MONDELLO JE, DOREMUS-FITZWATER TL, & DEAK T in prep Cytokine-specific plasticity in the central neuroimmune response to ethanol. [Google Scholar]
- GHIRNIKAR RS, LEE YL & ENG LF 1998. Inflammation in Traumatic Brain Injury: Role of Cytokines and Chemokines. Neurochemical Research, 23, 329–340. [DOI] [PubMed] [Google Scholar]
- GIRARD-JOYAL O, FARAGHER A, BRADLEY K, KANE L, HRYCYK L & ISMAIL N 2015. Age and sex differences in c-Fos expression and serum corticosterone concentration following LPS treatment. Neuroscience, 305, 293–301. [DOI] [PubMed] [Google Scholar]
- GOODMAN MD, MAKLEY AT, CAMPION EM, FRIEND LAW, LENTSCH AB & PRITTS TA 2013. Preinjury alcohol exposure attenuates the neuroinflammatory response to traumatic brain injury. Journal of Surgical Research, 184, 1053–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOUJON E, PARNET P, LAYÉ S, COMBE C & DANTZER R 1996. Adrenalectomy enhances pro-inflammatory cytokines gene expression, in the spleen, pituitary and brain of mice in response to lipopolysaccharide. Molecular Brain Research, 36, 53–62. [DOI] [PubMed] [Google Scholar]
- GRANT B & DAWSON D 1997. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse, 9, 103–10. [DOI] [PubMed] [Google Scholar]
- GROSSMAN I & MILLER A 2010. Multiple sclerosis pharmacogenetics: personalized approach towards tailored therapeutics. The EPMA Journal, 1, 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDEN LM, PLESSIS ID, POOLE S & LABURN HP 2008. Interleukin (IL)-6 and IL-1β act synergistically within the brain to induce sickness behavior and fever in rats. Brain, Behavior, and Immunity, 22, 838–849. [DOI] [PubMed] [Google Scholar]
- HASHIMOTO JG, WIREN KM 2008. Neurotoxic consequences of chronic alcohol withdrawal: expression profiling revelas importance of gender over withdrawal severity. Neuropsychopharmacology, 33(5), 1084–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENNESSY MB, DEAK T & SCHIML PA 2014. Sociality and sickness: Have cytokines evolved to serve social functions beyond times of pathogen exposure? Brain, Behavior, and Immunity, 37, 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HONG-BROWN LY Q, FROST ROBERT A & LANG CHARLES H 2006. Alcohol Impairs Protein Synthesis and Degradation in Cultured Skeletal Muscle Cells. Alcoholism: Clinical and Experimental Research, 25, 1373–1382. [PubMed] [Google Scholar]
- JANIS LS, HOANE MR, CONDE D, FULOP Z & STEIN DG 1998. Acute Ethanol Administration Reduces the Cognitive Deficits Associated With Traumatic Brain Injury in Rats. Journal of Neurotrauma, 15, 105–115. [DOI] [PubMed] [Google Scholar]
- KANE CJM, PHELAN KD, DOUGLAS JC, WAGONER G, JOHNSON JW, XU J, PHELAN PS & DREW PD 2014. Effects of Ethanol on Immune Response in the Brain: Region Specific Changes in Adolescent versus Adult Mice. Alcoholism, clinical and experimental research, 38, 384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNAPP DJ, HARPER KM, WHITMAN BA, ZIMOMRA Z & BREESE GR 2016. Stress and Withdrawal from Chronic Ethanol Induce Selective Changes in Neuroimmune mRNAs in Differing Brain Sites. Brain Sciences, 6, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANG CH, KIMBALL SR, FROST RA & VARY TC 2001. Alcohol myopathy: impairment of protein synthesis and translation initiation. The International Journal of Biochemistry & Cell Biology, 33, 457–473. [DOI] [PubMed] [Google Scholar]
- LEWOHL JM, VAN DYK DD, CRAFT GE, INNES DJ, MAYFIELD RD, COBON G, HARRIS RA & DODD PR 2004. The Application of Proteomics to the Human Alcoholic Brain. Annals of the New York Academy of Sciences, 1025, 14–26. [DOI] [PubMed] [Google Scholar]
- LEWOHL JM, WANG L, MILES MF, ZHANG L, DODD PR & HARRIS RA 2000. Gene Expression in Human Alcoholism: Microarray Analysis of Frontal Cortex. Alcoholism: Clinical and Experimental Research, 24, 1873–1882. [PubMed] [Google Scholar]
- LIPPAI D, BALA S, PETRASEK J, CSAK T, LEVIN I, KURT-JONES EA & SZABO G 2013. Alcohol-induced IL-1β in the brain is mediated by NLRP3/ASC inflammasome activation that amplifies neuroinflammation. Journal of Leukocyte Biology, 94, 171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSHALL SA, MCKNIGHT KH, BLOSE AK, LYSLE DT & THIELE TE 2017. Modulation of Binge-like Ethanol Consumption by IL-10 Signaling in the Basolateral Amygdala. Journal of Neuroimmune Pharmacology, 12, 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER AH, HAROON E, RAISON CL & FELGER JC 2013. Cytokine Targets in the Brain: Impact on Neurotransmitters and Neurocircuits. Depression and anxiety, 30, 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER AH, MALETIC V & RAISON CL 2009. Inflammation and Its Discontents: The Role of Cytokines in the Pathophysiology of Major Depression. Biological psychiatry, 65, 732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLIGAN MK, RHODES JS, CRABBE JC, MAYFIELD RD, HARRIS RA & PONOMAREV I 2011. Molecular profiles of drinking alcohol to intoxication in C57BL/6J mice. Alcoholism, Clinical And Experimental Research, 35, 659–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURRAY CL, OBIANG P, BANNERMAN D & CUNNINGHAM C 2013. Endogenous IL-1 in Cognitive Function and Anxiety: A Study in IL-1RI(−/−) Mice. PLoS ONE, 8, e78385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NATIONAL INSTITUTE ON ALCOHOL ABUSE AND ALCOHOLISM (NIAAA). 2017. Underage Drinking [Online]. Available: https://pubs.niaaa.nih.gov/publications/UnderageDrinking/UnderageFact.htm [Accessed 7/12/2018].
- PASCUAL M, BALIÑO P, ARAGÓN CMG & GUERRI C 2015. Cytokines and chemokines as biomarkers of ethanol-induced neuroinflammation and anxiety-related behavior: Role of TLR4 and TLR2. Neuropharmacology, 89, 352–359. [DOI] [PubMed] [Google Scholar]
- POWROZEK TA & OLSON EC 2012. Ethanol-induced disruption of Golgi apparatus morphology, primary neurite number and cellular orientation in developing cortical neurons. Alcohol, 46, 619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QIAN X, DROSTE SK, LIGHTMAN SL, REUL JMHM & LINTHORST ACE 2012. Circadian and Ultradian Rhythms of Free Glucocorticoid Hormone Are Highly Synchronized between the Blood, the Subcutaneous Tissue, and the Brain. Endocrinology, 153, 4346–4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RISHER M-L, FLEMING RL, RISHER C, MILLER KM, KLEIN RC, WILLS T, ACHESON SK, MOORE SD, WILSON WA, EROGLU C & SWARTZWELDER HS 2015. Adolescent Intermittent Alcohol Exposure: Persistence of Structural and Functional Hippocampal Abnormalities into Adulthood. Alcoholism, clinical and experimental research, 39, 989–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBINSON DONITA L, LARA JENNIFER A, BRUNNER LANE J & GONZALES RUEBEN A 2002. Quantification of Ethanol Concentrations in the Extracellular Fluid of the Rat Brain. Journal of Neurochemistry, 75, 1685–1693. [DOI] [PubMed] [Google Scholar]
- SCHIER CJ, MANGIERI RA, DILLY GA & GONZALES RA 2012. Microdialysis of Ethanol During Operant Ethanol Self-administration and Ethanol Determination by Gas Chromatography. Journal of Visualized Experiments : JoVE, 4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPEAR LP & SWARTZWELDER HS 2014. Adolescent alcohol exposure and persistence of adolescent-typical phenotypes into adulthood: A mini-review. Neuroscience & Biobehavioral Reviews, 45, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPENCER RL & DEAK T 2017. A users guide to HPA axis research. Physiology & Behavior, 178, 43–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPENCER RL & MCEWEN BS 1990. Adaptation of the Hypothalamic-Pituitary-Adrenal Axis to Chronic Ethanol Stress. Neuroendocrinology, 52, 481–489. [DOI] [PubMed] [Google Scholar]
- SURYANARAYANAN A, CARTER JM, LANDIN JD, MORROW AL, WERNER DF & SPIGELMAN I 2016. Role of interleukin-10 (IL-10) in regulation of GABAergic transmission and acute response to ethanol. Neuropharmacology, 107, 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TELLES TMBB, DE OLIVEIRA BMT, LOMBA LA, LEITE-AVALCA MG, CORREIA D & ZAMPRONIO AR 2017. Effects of Binge-Like Ethanol Exposure During Adolescence on the Febrile Response in Rats. Alcoholism, Clinical And Experimental Research, 41, 507–515. [DOI] [PubMed] [Google Scholar]
- TENG SX & MOLINA PE 2014. Acute Alcohol Intoxication Prolongs Neuroinflammation without Exacerbating Neurobehavioral Dysfunction following Mild Traumatic Brain Injury. Journal of Neurotrauma, 31, 378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THA KK, OKUMA Y, MIYAZAKI H, MURAYAMA T, UEHARA T, HATAKEYAMA R, HAYASHI Y & NOMURA Y 2000. Changes in expressions of proinflammatory cytokines IL-1β, TNF-α and IL-6 in the brain of senescence accelerated mouse (SAM) P8. Brain Research, 885, 25–31. [DOI] [PubMed] [Google Scholar]
- TORCASO A, ASIMES A, MEAGHER M & PAK TR 2017. Adolescent binge alcohol exposure increases risk assessment behaviors in male Wistar rats after exposure to an acute psychological stressor in adulthood. Psychoneuroendocrinology, 76, 154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VASICEK TW, JACKSON MR, POSENO TM & STENKEN JA 2013. In Vivo Microdialysis Sampling of Cytokines from Rat Hippocampus: Comparison of Cannula Implantation Procedures. ACS Chemical Neuroscience, 4, 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VETRENO R & CREWS FT 2014. Current hypotheses on the mechanisms of alcoholism. Handbook of Clinical Neurology, 125, 466–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VORE AS, DOREMUS-FITZWATER TL, GANO A, DEAK T 2018. Adolescent ethanol exposure leads to stimulus-specific changes in cytokine reactivity and hypothalamic-pituitary-adrenal axis sensitivity in adulthood. Frontiers Behavioral Neuroscience, 11, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITMAN BA, KNAPP DJ, WERNER DF, CREWS FT & BREESE GR 2013. The Cytokine-mRNA Increase Induced by Withdrawal from Chronic Ethanol in the Sterile Environment of Brain is mediated by CRF and HMGB1 Release. Alcoholism, clinical and experimental research, 37, 10.1111/acer.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILHELM CJ, HASHIMOTO JG, ROBERTS ML, BLOOM SH, ANDREW MR, WIREN KM 2015. Astrocyte dysfunction induced by alcohol in females but not males. Brain Pathology, 26(4), 433–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.