Abstract
Objective.
Laryngotracheal stenosis (LTS) is resource-intensive disease. The cost-effectiveness of LTS treatments has not been adequately explored. We aimed to conduct a cost-effectiveness analysis comparing open reconstruction (cricotracheal/tracheal resection [CTR/TR]) with endoscopic dilation in the treatment of LTS.
Study Design.
Retrospective cohort.
Setting.
Tertiary referral center (2013–2017).
Subjects and Methods.
Thirty-four LTS patients were recruited. Annual costs were derived from the Department of Otolaryngology–Head and Neck Surgery, Johns Hopkins University. Cost-effectiveness analysis compared CTR/TR versus endoscopic dilation at a willingness-to-pay threshold of $50,000 per quality-adjusted life year (QALY) over 5- and 10-year time horizons. The incremental cost-effectiveness ratio (ICER) was calculated with deterministic analysis and tested for sensitivity with univariate and probabilistic sensitivity analysis.
Results.
Mean LTS costs were $4080.09 (SE, $569.29) annually for related health care visits. The major risk factor for increased cost was etiology of stenosis. As compared with idiopathic patients, patients with intubation-related stenosis had significantly higher annual costs ($5286.56 vs $2873.62, P = .03). The cost of CTR/TR was $8583.91 (SE, $2263.22). Over a 5-year time horizon, CTR/TR gained $896 per QALY over serial dilations and was cost-effective. Over a 10-year time horizon, CTR/TR dominated dilations with a lower cost and higher QALY.
Conclusion.
The cost of treatment for LTS is significant. Patients with intubation-related stenosis have significantly higher annual costs than do idiopathic patients. CTR/TR contributes significantly to cost in LTS but is cost-effective versus endoscopic dilations for appropriately selected patients over a 5- and 10-year horizon.
Keywords: cost, incremental cost-effectiveness ratio, cost-effectiveness, laryngotracheal stenosis, subglottic stenosis
Laryngotracheal stenosis (LTS) is defined by luminal narrowing at the level of the glottis, subglottis, or trachea.1 Recent studies support the concept that, rather than a homogeneous disease, LTS is a collection of heterogeneous diseases with a common physiologic endpoint.2 LTS can follow endotracheal intubation (iatrogenic LTS [iLTS]), be related to autoimmune diseases, or occur without a known antecedent event (idiopathic subglottic stenosis [iSGS]).2 While clinical consequences of both diseases include respiratory distress1 and dysphonia,3 the demographic characteristics,4,5 disease course,5 and management strategies6 in iLTS and iSGS differ significantly.
Understanding which treatments for LTS hold greater value to patients and providers is crucial to improving care. Modern clinical decision making requires consideration of costs in relation to expected benefits.7 To date, there have been no studies describing the cost-effectiveness of treatments associated with adult LTS. No investigators have identified patient- or provider-specific variables associated with increased cost. Understanding the costs associated with disease allows utilization of cost-effectiveness modeling, which can highlight surgical interventions that produce better outcomes at a lower cost.8
In this study, we aimed to conduct a cost-effectiveness analysis (CEA) comparing open reconstruction (cricotracheal/tracheal resection [CTR/TR]) with endoscopic dilation in the treatment of LTS. We hypothesize that CTR/TR is an expensive but cost-effective procedure at a US health care sector willingness-to-pay threshold of $50,000 per quality-adjusted life year (QALY).
Methods
Patient Recruitment
A retrospective cohort of 34 LTS patients who received treatment from the senior author (A.T.H.) between April 2013 and March 2017 were enrolled at Johns Hopkins Hospital. This study was approved by the JHMI Institutional Review Board (NA_00081469). Inclusion criteria included adults with a definitive diagnosis of LTS (either iLTS or iSGS). Patients were excluded if they received significant portions of their LTS care at other facilities, had glottic involvement, or had an autoimmune cause of LTS. In this cohort, all 17 iLTS patients had a significant history of prolonged intubation. All 17 iSGS patients had negative autoimmune serologies and no history of prolonged intubation.
Data Collection
Demographic and clinical background information was obtained from the electronic medical record. The Charlson Comorbidity Index (CCI) was calculated with the patient’s medical history, according to established criteria.9 All financial and health care utilization data were obtained from the accounting division of the Department of Otolaryngology–Head and Neck Surgery, Johns Hopkins University. Charges were converted to costs based on cost-to-charge ratios as defined for emergency, outpatient, and inpatient Current Procedural Terminology code charges incurred by each patient. Costs and encounters unrelated to the diagnosis of LTS were not included. Encounters were confirmed with the electronic medical record. “Procedures” were defined only as procedures that required anesthesia and operating room time. In this study, CTR/TR was offered only to patients who failed endoscopic management and did not desire a long-term tracheostomy. A successful outcome to CTR/TR was defined as a condition in which patients required no further treatment for their airway disease, including permanent decannulation from tracheostomy.10
Statistical Analysis
Annual health care costs (2017 US dollars) were calculated as total health care costs over the 4-year study period, divided by the total amount of time in care (study end date to date of first patient encounter). Comparisons were made between the iLTS and iSGS cohorts with t tests for continuous variables and chi-square tests for categorical variables. Average annual costs before and after CTR/TR for patients undergoing these procedures were compared with nonparametric equality-of-median tests. All statistical analyses were conducted in STATA 12 (StataCorp, College Station, Texas).
Cost-effectiveness Analysis
This analysis was conducted from a US health care sector perspective, which evaluated outcomes for LTS over 5- and 10-year time horizons. In this CEA, effectiveness was measured in units of QALYs, which takes morbidity associated with LTS into account. Due to a lack of availability of data on quality of life (QOL) for patients with LTS, QALY values for symptom relief (taken from QALYs associated with a forced expiratory volume >80% predicted) and stenosis/restenosis (taken from QALYs associated with a forced expiratory volume <30% predicted) were taken from a study of patients with chronic obstructive pulmonary disease (COPD).11 Health care costs were estimated from our study cohort. If one treatment cost less and resulted in a higher QALY, that treatment “dominated” the alternative. However, if one treatment cost more but had higher value outcomes, we calculated an incremental cost-effectiveness ratio (ICER): the ratio of difference in costs to the difference in QALYs. If the ICER was lower than the hypothesized conventional willingness-to-pay threshold, the treatment was considered be cost-effective.8
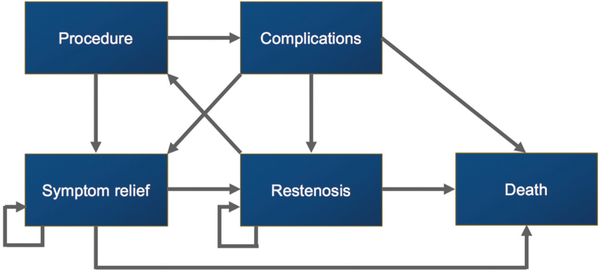
We utilized a Markov model in our analysis. Markov models assume that a patient is always in one of a finite number of discrete health states, and all events are represented as transitions from one state to another. Our health states (Figure 1) included complications from surgery, restenosis, symptom relief, and death. We used a cycle period of 1 month. Costs and QALYs were incorporated into the model as a mean value per state per cycle, and expected values were calculated by adding the costs and outcomes across each state and weighing these according to the time that the patient was expected to be in each state, with a 3% annual discount rate. Probabilities of transitioning between states were estimated with previous studies (see Supplemental Table S1, available in the online version of the article). Univariate and bayesian multivariate probabilistic sensitivity analyses applied distributions for each variable to characterize uncertainty on all parameters. Beta distributions represented probabilities and QALYs in the probabilistic sensitivity analysis, while gamma distributions were used for costs. The Markov model was then evaluated with 10,000 Monte Carlo simulations to produce distributions of possible outcome values.12
Figure 1.
Markov model. Patients are assumed to be in one of the discrete health states. “Procedure” represents cricotracheal/tracheal resection or endoscopic dilation. Transition probabilities between health states are summarized in Supplemental Table S1 (available in the online version of the article).
Results
Characteristics of Study Population
Table 1 displays the demographics and clinical histories of all 34 LTS patients included in this study. As compared with iLTS patients, iSGS patients were much more likely to be Caucasian (94% vs 47%, P < .01). iLTS patients had a much higher comorbidity index as compared with iSGS patients (1.82 vs 0.47, P = .05) and were more likely to have a history of CTR/TR and tracheostomy dependence at the time of their last follow-up (P < .01 for both).
Table 1.
Demographic and Clinical Background of Patients with Laryngotracheal Stenosis.
| Patients, n (%) |
||||
|---|---|---|---|---|
| Overall (N = 34) | Iatrogenic (n = 17) | Idiopathic (n = 17) | P Valuea | |
| Age,b y | 45.1 ± 2.2 | 41.9 ± 3.4 | 48.4 ± 2.6 | .14 |
| Sex | .15 | |||
| Male | 5 (15) | 4 (24) | 1 (5.9) | |
| Female | 29 (85) | 13 (76) | 16 (94) | |
| Race | <.01 | |||
| Caucasian | 24 (70) | 8 (47) | 16 (94) | |
| African American | 2 (5.9) | 2 (12) | 0 (0) | |
| Other | 8 (24) | 7 (41) | 1 (5.9) | |
| Charleston Comorbidity Indexb | 1.15 ± 0.35 | 1.82 ± 0.55 | 0.47 ± 0.36 | .05 |
| History of cricotracheal/tracheal resection | 7 (20) | 7 (41) | 0 (0) | <.01 |
| Tracheostomy dependence (at last follow-up) | 7 (20) | 7 (41) | 0 (0) | <.01 |
Bold indicates P <.05.
Mean ± SE.
Costs
Table 2 presents the health care costs and utilization for this cohort of 34 LTS patients. On average, LTS costs $4080.09 per year for health care visits related to airway disease. As compared with iSGS patients, iLTS patients had significantly higher average annual costs ($5286.56 vs $2873.62, P = .03). Specific unit costs are available in Supplemental Table S2 (available in the online version of the article), which shows that iLTS patients had higher costs per inpatient stay as compared with iSGS patients, although this was not significant (P = .07). On average, iLTS patients accessed more medical care than that of iSGS patients, with more total days in care and greater emergency department and inpatient visits (P < .01 for each).
Table 2.
Differences in Cost and Health Care Utilization between Iatrogenic and Idiopathic Laryngotracheal Stenosis Patients.
| Patient Group, Mean (SE) |
||||
|---|---|---|---|---|
| Overall (N = 34) | Iatrogenic (n = 17) | Idiopathic (n = 17) | P Valuea | |
| Annual costs, $ | 4080.09 (569.29) | 5286.56 (889.24) | 2873.62 (603.49) | .03 |
| Total, n | ||||
| Days in care | 18.62 (3.20) | 28.12 (5.28) | 9.12 (1.77) | <.01 |
| OR procedures | 2.94 (0.37) | 3.12 (0.49) | 2.76 (0.64) | .64 |
| ED visits | 0.32 (0.11) | 0.65 (0.19) | 0 (0) | <.01 |
| Outpatient visits | 8.73 (0.90) | 9.53 (1.15) | 7.94 (1.39) | .38 |
| Inpatient visits | 1.32 (0.27) | 2.12 (0.44) | 0.53 (0.17) | <.01 |
Abbreviations: ED, emergency department; OR, operating room.
Bold indicates P <.05.
Seven iLTS patients underwent CTR/TR. The average cost of a CTR/TR procedure was $8583.91 (SE, $2263.22). When comparing costs before and after the surgery in the same set of patients, we found that iLTS patients suffered significantly greater average costs ($14,973.03 [SE, $5516.56] vs $864.30 [SE $412.62], P = .02) before their CTR/TR versus after it. Table 3 shows the clinical outcomes of these 7 patients.
Table 3.
Clinical Outcomes of Patients Undergoing CTR/TR.
| No. | Resection Type | Complications | CCI | Cost, $ | Tracheostomy at Last Follow-up? | Discharged from Practice? |
|---|---|---|---|---|---|---|
| 1 | CTR | Tracheitis, tracheal bleed with obstructive clot | 2 | 16,544.17 | Yes | No |
| 2 | TR | None | 0 | 2681.46 | No | Yes |
| 3 | TR | None | 1 | 4248.14 | No | Yes |
| 4 | CTR | Unilateral vocal fold paresis, persistent dysphagia requiring PEG × 9 mo | 0 | 5630.68 | No | Yes |
| 5 | CTR | None | 1 | 7869.02 | No | Yes |
| 6 | CTR | None | 0 | 7057.84 | No | Yes |
| 7 | CTR | DVT, delirium | 6 | 16,056.09 | Yes | Deceaseda |
Abbreviations: CCI, Charlson Comorbidity Index; CTR, cricotracheal resection; DVT, deep vein thrombosis; PEG, percutaneous endoscopic gastrostomy; TR,tracheal resection.
Unrelated to airway.
Costs of CTR/TR and endoscopic dilation were calculated from our cohort and are displayed in Table 4. The cost of death was assumed to be $ 0. The monthly cost associated with “symptom relief” was the average monthly health care costs for the United States: $861.13 The monthly cost associated with “restenosis” or “complication” was taken as $861 plus the excess costs associated with LTS from Table 2: $4080 /12 months = $340 and $861 1 $340 = $1201.
Table 4.
Costs of Various Markov States.
| Cost, $ | Range for Sensitivity Analysis | |
|---|---|---|
| Procedure | ||
| Resection | 8584 | 6758–10,640 |
| Dilation | 1363 | 680–2059 |
| Restenosis/complicationsa | 1201 | 832–1569 |
| Symptom relief13,a | 861 | 547–1187 |
| Death | 0 | 0 |
Monthly cost.
Cost-effectiveness Analysis
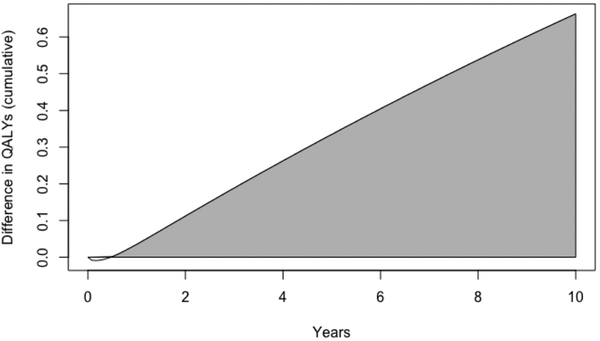
The results of the Markov model showed that over the 5-year time horizon, CTR/TR had a higher cost but greater QALY (Figure 2, Table 5), which resulted in a cost-effective result at our predetermined willingness-to-pay threshold. As compared with endoscopic dilations, CTR/TR had an ICER of $896 per QALY. When extended to a 10-year time horizon, CTR/TR had a lower cost and produced a greater QALY (Figure 2, Table 5), thus dominating the alternative of endoscopic dilation. The multivariate probabilistic sensitivity analysis also showed that CTR/TR was cost-effective in 91.6% of 5-year simulations and 93.5% of 10-year simulations (Supplemental Figure, available in the online version of the article). No model parameters individually swayed results of the study.
Figure 2.
Quality-adjusted life year (QALY) differences over time. Over a 10-year horizon, cricotracheal/tracheal resection led to many more quality-adjusted life years versus endoscopic dilations. The area under the curve represents the cumulative QALYs gained by cricotracheal/tracheal resection over endoscopic dilations over 10 years.
Table 5.
ICER Analysis of CTR/TR vs Endoscopic Dilations.
| 5-y Time Horizon |
10-y Time Horizon, $ |
|||
|---|---|---|---|---|
| Procedure | Cost, $ | QALYs | Cost, $ | QALYs |
| Resection | 55,466 | 3.785 | 95,529 | 6.988 |
| Dilation | 55,166 | 3.451 | 101,501 | 6.325 |
| Difference | 300 | 0.334 | −5972 | 0.663 |
| Resection vs dilation ICER | 896 per QALY | Resection dominates dilation | ||
Abbreviations: CTR, cricotracheal resection; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year; TR, tracheal resection.
Discussion
This is the first study to describe the health care costs of adult LTS patients. Our results demonstrate that at an average annual cost of $4080.09, LTS is a costly disease and poses a significant burden on the US health care system. These annual health care costs are comparable to those of other chronic medical diseases, such as diabetes mellitus (annual health care burden, ~$7900)14 or COPD (annual health care burden, $2700-$5900).15
When variables associated with higher health care cost were investigated, it was clear that iLTS patients had significantly higher costs (Table 2) and utilized health care more frequently as compared with iSGS patients, despite undergoing a similar number of operative airway procedures. Disparities in health care utilization likely contributed greatly to the large cost discrepancy between iLTS and iSGS patients. These cost differences distinguish the distinct disease processes and are another measure of the consequences of iLTS. The biggest driver of cost appears to be the time that patients spent in health care—specifically, time spent as inpatients with multiple hospitalizations. iLTS patients spent significantly more time on inpatient and ED visits as compared with iSGS patients. While not significant, our unit cost data (Supplemental Table S2, available in the online version of the article) also show that iLTS patients trend toward greater average costs per inpatient stay as compared with iSGS patients. Lesser-contributing factors included (1) the presence of greater comorbidities among iLTS patients,2 (2) the higher likelihood for iLTS patients to become tracheostomy dependent, and (3) the higher likelihood for iLTS patients to require more complex surgical procedures (ie, CTR/TR). It is likely that surgical procedures such as CTR/TR contribute to an increased length of stay (including intensive care unit stays) and longer exposure to health care, leading to increased costs. While we did not perform a regression analysis or show this causal relationship, our leading hypothesis is that the increased costs seen in iLTS is related to the increased likelihood to undergo CTR and the hospital costs associated with that procedure.
Similar to findings from a prior study,5 our results showed that iLTS patients have significantly more medical comorbidities as compared with their iSGS counterparts (CCI: 1.82 vs 0.47, P = .05). These comorbidities increase the complexity of medical care and likely increase overall costs. Additionally, endoscopic repair among iLTS patients can be challenging,16,17 as airway injury frequently includes the cartilaginous suprastructure of the trachea.2,6 Definitive treatment often demands aggressive surgical intervention such as CTR/TR.10 However, iSGS patients are usually otherwise healthy.4 Their stenosis exclusively involves the mucosal lamina propria, which responds well to endoscopic procedures.6,18–21 Consistent with the disease course, none of the iSGS patients in this study required tracheostomy or resection for airway management; however, 7 iLTS patients remained tracheostomy dependent at the conclusion of this study, and 7 iLTS patients underwent open surgical management.
Among the 7 iLTS patients who underwent CTR/TR, a higher CCI was anecdotally associated with higher health care costs and a higher likelihood of tracheostomy dependence (Table 3). Patient 1 ($16,544.17) had a history of myocardial infarction and heart failure, while patient 7 ($16,056.09) had COPD, diabetes, chronic kidney disease, and dementia. Unsurprisingly, these 2 patients were the only CTR/TR patients who had a tracheostomy in place at the study’s conclusion. These complications highlight the importance of patient selection in achieving a successful CTR/TR. In our subset of 7 iLTS patients undergoing CTR/ TR, 5 were discharged from practice in the months following CTR/TR. This is consistent with previous studies examining the long-term efficacy of CTR/TR among LTS patients, which has between 79% and 95% success rates with low rates of restenosis and improvements in patient QOL.10,22–25
The findings of our CEA suggest that CTR/TR is a cost-effective treatment for LTS at 5 years and the dominant treatment for LTS over a 10-year horizon. However, there are a few key points to consider when interpreting this model. First, no individual patient fits perfectly into the inputs that were utilized for this Markov model. All costs and probabilistic inputs were estimated from our cohort and the literature. Therefore, inputs for the model are limited to these retrospective studies. In our study, all 7 patients who were used to estimate CTR/TR costs were iLTS patients, but our cost inputs for dilation included both iLTS and iSGS patients. In the subset of iSGS patients with long dilation intervals, the conclusion that CTR/TR is more cost-effective than endoscopic dilation may not hold true. Second, despite the results of the CEA, CTR/TR is still a morbid procedure with a high complication rate, as indicated by our cohort (Table 3) and the literature (Supplemental Table S1, available in the online version of the article).11,26–31 Clinical judgment must be applied to each patient, and the risks of CTR/TR for an individual may outweigh its overall cost-effectiveness. Finally, in the United States, ICERs do not dictate health care or insurance policy. We do not comment on or advocate for any changes in overarching health care policy or insurance coverage. The simple conclusion to take away from these CEA results is that in a carefully selected group of patients (short dilation interval, long life expectancy, and low CCI), CTR/TR is more cost-effective than serial endoscopic dilations at the 5- and 10-year horizon.
A prior study explored the cost associated with endotracheal tube injuries, including iLTS, which amounted to an extra $1888 during the initial hospital stay and an average $11,025 after discharge.32 Additionally, in the pediatric population, mean total charges for subglottic stenosis were $53,787, with higher charges for children undergoing surgical intervention.33 Our study found a similar overall cost burden for adult patients with LTS. However, instead of using charges in our study, we converted charges into costs using charge-to-cost ratios. Health care charges represent the amount of money that a hospital requests in reimbursement, while costs reflect the actual resource consumption by the hospital. Charges can vary among institutions, while costs, which are unbiased by hospital-specific inflation rates, provide a more accurate proxy for understanding the true health care burden of disease.31,34
This retrospective cohort study is not without limitations. The only costs we were able to capture were associated with patients’ hospital and clinic visits relevant to their LTS at a single institution. Costs from clinical encounters related to other comorbidities and home nursing care were not captured through our data collection methods. As such, iLTS patients may actually have a higher cost burden than that reported in this study. We hope that future research into the cost of LTS may go beyond the tertiary care setting and explore these out-of-hospital costs and opportunity costs. Other limitations to this study include its retrospective nature and the possible inaccuracies in patient-recalled data. From a treatment paradigm perspective, we recognize that novel treatments in iSGS often include injection of intralesional steroids following endoscopic dilation, which may increase the interval between dilations and thus decrease overall long-term cost for dilation. In our cohort, only 1 patient received intralesional steroids and did not respond to them, so we did not factor this treatment into our CEA. Thus, our long-term cost estimates for endoscopic dilations may be higher than those for approaches combining endoscopic dilations with intralesional steroid injection. We also did not include patients in this study who had an autoimmune etiology for their LTS, as we had a small number of patients in our practice. We understand that these patients may have reduced costs due to their responsiveness to medical therapies, but we cannot comment on the cost-effectiveness of endoscopic dilation or CTR/TR in this subset of patients.
Limitations to all CEAs include incorporation of assumptions and extrapolations into the model. It is important to emphasize that CEAs are predictive financial models and not retrospective reviews of true costs accumulated over 5-and 10-year periods. As there are limited QOL data for patients with LTS, QOL data were extrapolated from patients with COPD. The extrapolation was made with the assumption that patients with symptom relief from the dyspnea of LTS experience a similar QOL as compared with patients with symptom relief from COPD and that patients with severe stenosis experience a QOL similar to that of patients with severe COPD. There is some evidence in the literature to support this, as multiple studies have shown that QOL questionnaires validated for COPD correlate with disease severity in LTS and may be used reliably among LTS patients.35,36 Furthermore, health care economists believe that utilities may be extrapolated across different disease conditions and similar severities.31 Finally, our sensitivity analysis shows that our model holds up against fairly wide ranges of the utility parameters. We acknowledge QOL data specific to LTS would provide greater accuracy our results, and as such data emerge in the literature, future studies will include them.
On the cost portion of the analysis, CTR/TR cost data were extracted from only 7 iLTS patients, but dilation cost data were extracted from 28 patients. Therefore, there may be differences in the accuracy of our cost estimates between CTR/TR and dilations. Our CTR/TR cost estimate may be an overestimate of the real costs, as our resection population had high CCIs. Additionally, some tracheostomy-associated costs in our study—specifically, home tracheostomy costs— were not included in our model, as these data were not available. While not having a “tracheostomy-dependent” state is a weakness of this study, the absence of those costs likely makes for a more conservative estimate of costs in iLTS and ultimately does not affect our conclusions of the CEA. To address these assumptions and estimations in cost and utility, our Monte Carlo simulation (Supplemental Figure, available in the online version of the article) demonstrates the consistency and precision of our eventual outcome despite large variations in input parameters.
Conclusions
LTS is a morbid disease with a high annual health care cost. iLTS is a more costly disease than iSGS. CTR/TR is an expensive procedure and a large contributing factor for costs in LTS. Using a Markov model to project 5- and 10-year ICERs showed that CTR/TR is more cost-effective than serial endoscopic dilations in a select population of patients. This includes those with a long life expectancy, short dilation interval, and few comorbid conditions.
Supplementary Material
Acknowledgments
Funding source: None.
Footnotes
Disclosures
Competing interests:
William V. Padula, Monument Analytics—principal, received income; Molnlycke Health Care—Scientific Advisory Board and Speakers Bureau, received income. Zachary Predmore, Analysis Group—salaried position from June to August 2018. Alexander T. Hillel, Olympus USA—consultant.
Sponsorships: None.
Supplemental Material
Additional supporting information is available in the online version of the article.
This article was presented at the annual Combined Otolaryngology Spring Meetings; April 18, 2018; National Harbor, Maryland.
The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health, Triological Society (TRIO), American College of Surgeons (ACS), or Patient Centered Outcomes Research Institute (PCORI).
References
- 1.Rosow DE, Barbarite E. Review of adult laryngotracheal stenosis: pathogenesis, management, and outcomes. Curr Opin Otolaryngol Head Neck Surg. 2016;24:489–493. [DOI] [PubMed] [Google Scholar]
- 2.Gelbard A, Francis DO, Sandulache VC, Simmons JC, Donovan DT, Ongkasuwan J. Causes and consequences of adult laryngotracheal stenosis. Laryngoscope. 2015;125:1137–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hillel AT, Karatayli-Ozgursoy S, Benke JR, et al. Voice quality in laryngotracheal stenosis: impact of dilation and level of stenosis. Ann Otol Rhinol Laryngol. 2015;124:413–418. [DOI] [PubMed] [Google Scholar]
- 4.Gelbard A, Donovan DT, Ongkasuwan J, et al. Disease homogeneity and treatment heterogeneity in idiopathic subglottic stenosis. Laryngoscope. 2016;126:1390–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gadkaree SK, Pandian V, Best S, et al. Laryngotracheal stenosis. Otolaryngol Head Neck Surg. 2017;156:321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis S, Earley M, Rosenfeld R, Silverman J. Systematic review for surgical treatment of adult and adolescent laryngotracheal stenosis. Laryngoscope. 2017;127:191–198. [DOI] [PubMed] [Google Scholar]
- 7.Weinstein MC, Stason WB. Foundations of cost-effectiveness analysis for health and medical practices. N Engl J Med. 1977; 296:716–721. [DOI] [PubMed] [Google Scholar]
- 8.Russell LB, Gold MR, Siegel JE, Daniels N, Weinstein MC.The role of cost-effectiveness analysis in health and medicine. JAMA. 1996;276:1172–1177. [PubMed] [Google Scholar]
- 9.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40: 373–383. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto K, Kojima F, Tomiyama K, Nakamura T, Hayashino Y. Meta-analysis of therapeutic procedures for acquired subglottic stenosis in adults. Ann Thorac Surg. 2011; 91:1747–1753. [DOI] [PubMed] [Google Scholar]
- 11.Ståhl E, Lindberg A, Jansson S-A, et al. Health-related quality of life is related to COPD disease severity. Health Qual Life Outcomes. 2005;3:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13:322–338. [DOI] [PubMed] [Google Scholar]
- 13.Keehan SP, Poisal JA, Cuckler GA, et al. National health expenditure projections, 2015–25: economy, prices, and aging expected to shape spending and enrollment. Health Aff (Millwood). 2016;35:1522–1531. [DOI] [PubMed] [Google Scholar]
- 14.American Diabetes Association. Economic costs of diabetes in the US in 2012. Diabetes Care. 2013;36:1033–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster TS, Miller JD, Marton JP, Caloyeras JP, Russell MW, Menzin J. Assessment of the economic burden of COPD in the US: a review and synthesis of the literature. COPD. 2006;3: 211–218. [DOI] [PubMed] [Google Scholar]
- 16.Kocdor P, Siegel ER, Suen JY, Richter G, Tulunay-Ugur OE. Comorbidities and factors associated with endoscopic surgical outcomes in adult laryngotracheal stenosis. Eur Arch Otorhinolaryngol. 2016;273:419–424. [DOI] [PubMed] [Google Scholar]
- 17.Parker NP, Bandyopadhyay D, Misono S, Goding GS Jr. Endoscopic cold incision, balloon dilation, mitomycin C application, and steroid injection for adult laryngotracheal stenosis. Laryngoscope. 2013;123:220–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shabani S, Hoffman MR, Brand WT, Dailey SH. Endoscopic management of idiopathic subglottic stenosis. Ann Otol Rhinol Laryngol. 2017;126:96–102. [DOI] [PubMed] [Google Scholar]
- 19.Nouraei SA, Sandhu GS. Outcome of a multimodality approach to the management of idiopathic subglottic stenosis. Laryngoscope. 2013;123:2474–2484. [DOI] [PubMed] [Google Scholar]
- 20.Menapace DC, Modest MC, Ekbom DC, Moore EJ, Edell ES, Kasperbauer JL. Idiopathic subglottic stenosis: long-term outcomes of open surgical techniques. Otolaryngol Head Neck Surg. 2017;156:906–911. [DOI] [PubMed] [Google Scholar]
- 21.Tanner K, Dromey C, Berardi ML, et al. Effects of voice-sparing cricotracheal resection on phonation in women. Laryngoscope. 2017;127:2085–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.George M, Lang F, Pasche P, Monnier P. Surgical management of laryngotracheal stenosis in adults. Eur Arch Otorhinolaryngol. 2005;262:609–615. [DOI] [PubMed] [Google Scholar]
- 23.Rubikas R, Matukaityte I, Jelisiejevas JJ, Rackauskas M. Surgical treatment of non-malignant laryngotracheal stenosis. Eur Arch Otorhinolaryngol. 2014;271:2481–2487. [DOI] [PubMed] [Google Scholar]
- 24.Tayfun MA, Eren E, Basoglu MS, Aslan H, Ozturkcan S, Katilmis H. Postintubation laryngotracheal stenosis: assessing the success of surgery. J Craniofac Surg. 2013;24:1716–1719. [DOI] [PubMed] [Google Scholar]
- 25.Deckard N, Yeh J, Soares DJ, Criddle M, Stachler R, Coticchia J. Utility of two-stage laryngotracheal reconstruction in the management of subglottic stenosis in adults. Ann Otol Rhinol Laryngol. 2013;122:322–329. [DOI] [PubMed] [Google Scholar]
- 26.Grillo HC, Donahue DM, Mathisen DJ, Wain JC, Wright CD.Postintubation tracheal stenosis: treatment and results. J Thorac Cardiovasc Surg. 1995;109:486–493. [DOI] [PubMed] [Google Scholar]
- 27.Herrington HC, Weber SM, Andersen PE. Modern management of laryngotracheal stenosis. Laryngoscope. 2006;116: 1553–1557. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Deaths and mortality. https://www.cdc.gov/nchs/fastats/deaths.htm. Published 2017. Accessed June 21, 2017.
- 29.Hseu AF, Benninger MS, Haffey TM, Lorenz R. Subglottic stenosis: a ten-year review of treatment outcomes. Laryngoscope. 2014;124:736–741. [DOI] [PubMed] [Google Scholar]
- 30.Gadkaree SK, Pandian V, Best S, et al. Laryngotracheal stenosis: risk factors for tracheostomy dependence and dilation interval. Otolaryngol Head Neck Surg. 2017;156:321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG. Cost effectiveness in health and medicine. 2nd ed. Oxford, UK: Oxford University Press; 2017. [Google Scholar]
- 32.Bhatti NI, Mohyuddin A, Reaven N, et al. Cost analysis of intubation-related tracheal injury using a national database. Otolaryngol Head Neck Surg. 2010;143:31–36. [DOI] [PubMed] [Google Scholar]
- 33.Shah RK, Lander L, Choi SS, Zalzal GH. Resource utilization in the management of subglottic stenosis. Otolaryngol Head Neck Surg. 2008;138:233–241. [DOI] [PubMed] [Google Scholar]
- 34.Finkler SA. The distinction between cost and charges. Ann Intern Med. 1982;96:102–109. [DOI] [PubMed] [Google Scholar]
- 35.Nouraei SA, Randhawa PS, Koury EF, et al. Validation of the Clinical COPD Questionnaire as a psychophysical outcome measure in adult laryngotracheal stenosis. Clin Otolaryngol. 2009;34:343–348. [DOI] [PubMed] [Google Scholar]
- 36.Naunheim MR, Paddle PM, Husain I, Wangchalabovorn P, Rosario D, Franco RA Jr. Quality-of-life metrics correlate with disease severity in idiopathic subglottic stenosis [published online March 7, 2018]. Laryngoscope. doi: 10.1002/lary. 26930. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.