Abstract
Background:
Genomic analysis may reveal both primary and secondary findings with direct relevance to the health of probands’ biological relatives. Researchers question their obligations to return findings not only to participants but also to family members. Given the social value of privacy protection, should researchers offer a proband’s results to family members, including after the proband’s death?
Methods:
Preferences were elicited using interviews and a survey. Respondents included probands from two pancreatic cancer research resources, plus biological and non-biological family members. Hypothetical scenarios based on actual research findings from the two cancer research resources were presented; participants were asked return of results preferences and justifications. Interview transcripts were coded and analyzed; survey data were analyzed descriptively.
Results:
51 individuals (17 probands, 21 biological relatives, 13 spouses/partners) were interviewed. Subsequently, a mailed survey was returned by 464 probands, 1,040 biological family members, and 399 spouses/partners. This analysis highlights the interviews, augmented by survey findings. Probands and family members attribute great predictive power and life-saving potential to genomic information. A majority hold that a proband’s genomic results relevant to family members’ health ought to be offered. While informants endorse each individual’s choice whether to learn results, most express a strong moral responsibility to know and to share, particularly with the younger generation. Most have few concerns about sharing genetic information within the family; rather, their concerns focus on the health consequences of not sharing.
Conclusions:
Although additional studies in diverse populations are needed, policies governing return of genomic results should consider how families understand genomic data, how they value confidentiality within the family, and whether they endorse an ethics of sharing. A focus on respect for individual privacy—without attention to how the broad social and cultural context shapes preferences within families—cannot be the sole foundation of policy.
Keywords: Return of results, incidental findings, genomics, family communication, ethics of disclosure
“Information is to your disease what insulin is for diabetes…” (Interviewee 34, daughter of proband)
“After death, a family should be entitled to [results from their deceased relative]… If they inherited the gene, they ought to inherit the data.” (Interviewee 27, husband of proband)
“We have a moral obligation to give people the information they need to be well.” (Interviewee 16, wife of proband)
INTRODUCTION
Return of primary results and incidental or secondary findings (RoR)1 to participants in genomic research is now common. Multiple groups have published empirical data and normative analyses to ground recommended best practices (Bredenoord and van Delden 2012; Green et al. 2013; Hallowell et al. 2013; Jarvik et al. 2014; Knoppers et al. 2013; Radecki Breitkopf et al. 2015, 2018; Shkedi-Rafid et al. 2014; Wolf et al. 2008; Wolf et al. 2012; Wolf et al. 2015). However, few studies have addressed return of a research participant’s—referred to here as the proband—genetic information to family members (Chan et al. 2012; Fernandez et al. 2014; Presidential Commission for the Study of Bioethical Issues 2013; Wolf et al. 2015). This is a question of increasing importance. A proband’s genetic results may have risk implications for biologically related family members who may carry the same genetic variants. Even family members who are not genetically related, such as the proband’s spouse/partner, may be interested in the proband’s genetic research results, as disease risk variants may be transmitted to their joint children. Because genomic information will increasingly be derived from the proband’s stored biospecimens, family interest in results may occur after the proband’s death.
Federal laws, state laws, and bioethics policies generally deem genetic information subject, at minimum, to the same protections as other personal health information, with the individual deciding whether to share it with others.2 As a rule, the confidentiality of an individual’s health information is to be protected, including unauthorized transmittal to family members, in life and after death.3 However, prioritizing this confidentiality can be ethically problematic, especially when genetic variants with high clinical actionability are discovered.
This raises new and challenging policy issues: under what circumstances should researchers offer a proband’s genetic research results to family members? Which results should be shared, with whom, and upon whose initiation? Who should decide this? What about the research results of a deceased proband? How should policy balance protection of the individual’s privacy and control of his or her genetic information with the potential for the family to benefit from learning the genomic findings?
To inform policy about these questions, we conducted a mixed-methods study to elicit the RoR preferences of probands and a sample of biological and non-biological family members of probands. The qualitative component of the study—based on in-depth interviews—informed the development of a survey that was mailed to living probands with pancreatic cancer and family members of living and deceased probands with the disease (Radecki Breitkopf et al. 2015). Together these studies constituted the empirical research component of a five-year project funded by the National Institutes of Health (NIH) on RoR to family, including after the proband’s death. Participants were drawn from two Mayo Clinic pancreatic disease research resources, a biobank (that included affected probands with and without a family history of the disease) and an associated pancreatic cancer family registry. The project also included development of normative recommendations by a multidisciplinary working group of national experts (Wolf et al. 2015); publication of a symposium journal issue collecting this and other papers (Wolf, Koenig, and Petersen 2015); and implementation of a pilot study of RoR to family members, the results of which are being analyzed.
Our study builds on preference studies of RoR to research participants, patients, their relatives, and the public (Bennette et al. 2013; Bollinger et al. 2012; Dheensa, Fenwick, and Lucassen 2016; Facio et al. 2013; Fernandez et al. 2014; Graves et al. 2015; Husedzinovic et al. 2015; Johns et al. 2014; Johns et al. 2017; Mackley et al. 2017; Ploug and Holm 2017). These studies show strong patient and public preferences for RoR, including results from deceased probands (Allen et al. 2014; Amendola et al. 2015; Bollinger et al. 2012; Fernandez et al. 2014; Meulenkamp et al. 2010; Murphy et al. 2008). Some have found that people want the return not only of clinically significant and medically actionable results, as recommended by a number of authors (Berg, Khoury, and Evans 2011; Wolf et al. 2012), but also of genetic results about conditions that cannot be treated or prevented (Bennette et al. 2013; Berg, Khoury, and Evans 2011; Facio et al. 2013; Fernandez et al. 2014; Wolf et al. 2012).
While useful, these studies have limitations. Parker and others have cautioned that many preference surveys rely on hypothetical questions or scenarios and document only what people prefer in the abstract, rather than when faced with actual choices about results (Beskow and Burke 2010; Clayton and Kelly 2013; Parker 2012). In contrast, our study elicits the preferences of actual research participants and family members. Further, we explore the underlying reasoning, justifications, and context of these preferences. While the scenarios of RoR presented are hypothetical, the context is realistic, since biobank participants and family members face the possibility of RoR, and the scenarios developed for this study are based on actual incidental findings in the Mayo Clinic pancreas biobank.
Most studies consider lay preferences out of context, as reflections of each individual’s choice. Our analysis is based on a different foundational assumption: that preferences are not simply idiosyncratic, autonomous statements of individual choice. Rather, they are also grounded in cultural meanings and institutions that shape each person’s moral intuitions and sense of what is most at stake. Preferences are grounded in the everyday context of people’s lives and in their experience of health and illness. They form part of what anthropologist Arthur Kleinman calls “local moral worlds” (Kleinman 1997). Preferences are not fixed, but fluid and emerging.
When genetic information is involved, the family is necessarily implicated. Individual preferences with no consideration of the family cannot be a sufficient guide to offering results. This, in turn, highlights the ways that family members handle shared information (Atkinson, Featherstone, and Gregory 2013; Hallowell et al. 2003). British anthropologist Monica Konrad terms this “local moralities of information disclosure” (Konrad 2003, 339; 2005, 109). Following Konrad, we consider the way families approach the disclosure of genetic information as a “family ethics of disclosure.” Together with descriptions and analyses of research participants’ individual approaches to communication within the family, we also consider collective ways and ethics of disclosing or not disclosing genetic information within the family.
Preferences are shaped within historical, social, cultural, and economic contexts, which are fundamental to policy making but often eclipsed by a focus on individual stakeholders’ views and opinions. Recently, an interdisciplinary group of scholars studying the ethical, legal, and social implications (ELSI) of translational genomics research concluded that our conceptualization of policy must be broadened (Burke et al. 2015). They argue that, “the background social narratives that inform patient, family and public decision making about the use of genomic information are also important in directing genomic translation” (Burke et al. 2015, 2). Similarly, analysis of social and cultural influences can reveal “key moral commitments, beliefs, and practices that shape the reception of genomic technologies by potential users” (Burke et al. 2015, 2).
We report key findings from our qualitative interviews, augmented by relevant survey findings, of the preferences and moral reasoning of 51 pancreatic cancer probands and family members of probands identified via a Mayo Clinic pancreas biobank and a family registry. Although our study relies on the responses of individuals and did not study family units, the interviews and subsequent survey focused on eliciting participants’ views of RoR to the family. In the discussion, we consider these findings in terms of a family ethics of disclosure, linking them to a broader social and cultural context.
Rationale: a mixed-methods study based in a Mayo Clinic pancreatic cancer biobank and a family registry
The research reported here grew out of earlier studies of the genetics of pancreatic cancer conducted at two research resources created at Mayo Clinic, in Rochester, Minn. (Couzin-Frankel 2014). Headed by co-author Gloria Petersen, the Mayo Clinic genetic epidemiology team identified pathogenic mutations in disease-causing genes in some probands. These mutations included gene variants conferring risk of hereditary breast and ovarian cancer (BRCA2), risk of malignant melanoma (CDKN2A/p16), and carrier status for cystic fibrosis (CFTR). Initially, these variants were not known to convey risk of pancreatic cancer, and thus our ethics project considered them to be incidental or secondary findings, i.e., unrelated to pancreatic cancer. However, with further study, these variants are now understood to increase risk of pancreatic cancer (Chaffee et al. 2017; McWilliams et al. 2010; Shindo et al. 2017; Zhen et al. 2015; Chaffee et al. 2018).
When Dr. Petersen’s study began, neither primary nor secondary research findings were returned to study participants. Novel findings linked to pancreatic cancer were not returned because of the lethality of pancreatic cancer. Results were not relevant to the participants’ treatment and had not been validated in a CLIA-certified laboratory. Return of secondary research findings was not addressed in the original research protocols. Researchers recognized, however, that variants in genes such as BRCA and CFTR have known health and reproductive implications. These pathogenic variants are traditionally offered to patients seeking clinical care. Their discovery after the probands’ death thus raised the question of whether such secondary results should be shared with surviving relatives, a question about which there was much ethical uncertainty.
METHODS
Sampling and recruitment procedures for the interview study
Interview and survey protocols were approved by the Institutional Review Boards at the Mayo Clinic and at the University of California, San Francisco. As noted, all participants were recruited from the Mayo Clinic Biobank of unselected patients with pancreatic cancer and the family registry of kindreds (familial pancreatic cancer probands and their family members) (McWilliams et al. 2009; Petersen et al. 2006). Both resources include next-of-kin and family contact information. In the family registry, some family members were themselves invited to complete registry questionnaires or provide blood samples.
Potential interviewees were selected from both research resources, in order to include individuals from families with and without a strong family history of pancreatic cancer. We sampled independently from three categories: 1) probands with pancreatic cancer (both those surviving longer than one year and those diagnosed within the prior year); 2) first-degree blood relatives of probands (siblings, parents, children); and 3) “social kin” of probands (spouses or partners). No effort was made to sample individuals from the same family.
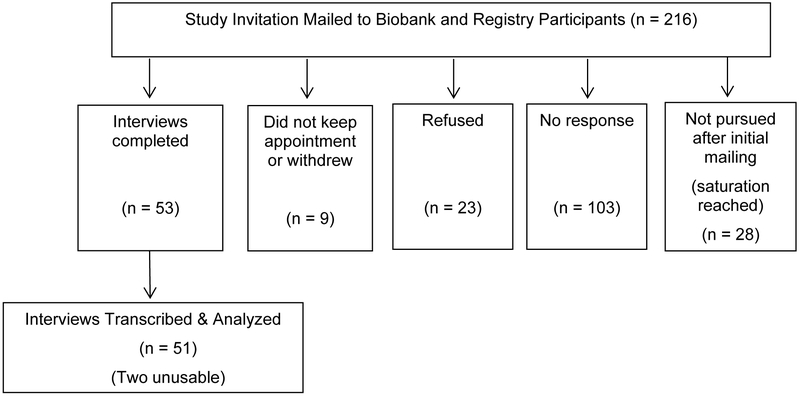
Eligible participants were contacted via a letter signed by the director of the two research resources (GP) (see Figure 1). Those who responded positively received a mailed consent form. Those who gave consent were contacted to set up a telephone interview with a team member (BK, DG, CRB, WP, JE). All study materials clarified that Mayo Clinic was not contacting them because new results had been discovered, although it was stated that such results could be available in the future. Rather, we invited them to participate in a study aimed at learning “what patients and family members of people with pancreatic cancer think about genetic research and how to share genetic research results within the family.”
Figure 1.
Interview study enrollment and completion diagram.
Developing the interview guides4
The research team developed semi-structured interview guides to explore both the range of participants’ preferences about RoR to family members and the values and moral reasoning behind them. We first developed a guide for a living proband with pancreatic cancer and then adapted it for use with family, one version for blood relatives and another for social kin. The guides were structured yet open, aiming for a conversational tone. All guides underwent iterative testing with members of a pancreatic cancer patient advocacy group in order to optimize clarity and order of the questions. A second round of pilot testing was conducted using actual interviewees. These six interview responses are included in the final sample of 51 and are identified by the prefix “pilot.”
To provide background information, we sent in advance a brief description of the three unexpected findings to be discussed (BRCA2 [breast/ovarian cancer], CDKN2A/p16 [melanoma], and CFTR [cystic fibrosis carrier]).5 Discussion of the materials enabled the interviewer to gauge the genetic literacy of the participant and frame questions accordingly.6
The interview guide’s prologue explained the goals of the research project, described the possibility of “unexpected findings”7 in genetic research, and explained the primary purpose of the interview questions: to hear what pancreatic cancer patients and family members think about the return of such findings to family members. The first interview question was as follows: “Could you tell me the story of your experience [for proband] or your family’s experience [for a relative] with pancreatic cancer?” When possible, we inquired about actual past and current actions and experiences, particularly about family communication, in order to ground the interviewee’s answers in present and past behavior.
The guide next covered the following topics: family structure and family communication; concerns about cancer risk; anticipated personal and familial communication of unexpected research results; benefits and harms of knowing genetic information; “factors that matter” in determining whether results should be communicated; practical preferences for communication of unexpected findings; genetic privacy and discrimination; and policy considerations. To stimulate participants to reflect on the possibility of family conflict about RoR, we included the following hypothetical scenario about the Jones family, summarized below:
Mrs. Jones died of pancreatic cancer before the research team discovered that she carried a mutation in BRCA2. The Joneses have two daughters, aged 20 and 23. When the researcher offered Mr. Jones—who was the “legal next-of-kin”—his wife’s genetic results, he said, “No, I don’t want to know, and please don’t tell my daughters.” He said that talking about his wife’s cancer is too painful for the family.
Throughout, we carefully distinguished between “offering” results vs. simply “returning” or “disclosing” results.8
Interview data analysis
We used standard methods of qualitative data analysis, including anthropological interpretation of meanings in the traditions of “thick description” and interpretive phenomenology, together with techniques based on grounded theory (Benner 1994; Geertz 1973; Strauss and Corbin 1990). A sub-group of the interview team conducted the analysis. To facilitate data retrieval, the transcripts were managed in QSR International’s NVivo 10 software. Team members BK and DG developed an initial list of thematic categories. Some themes reflected the topics in the interview guides while others were inductively derived. The code book was piloted, refined, and finalized by six members of the study team, resulting in 19 broad coding categories.9 Two team members (BK and DG) and two coders (coauthor JE and KH [named in the acknowledgements]) independently coded two transcripts to verify the coding. Once any differences were discussed and reconciled, the two coders each coded half of the 51 interviews.
Analysis team members prepared memos summarizing the material for each of the 19 codes.10 In addition, the two leaders of the analysis team (BK and DG) adapted for these interviews a two-page interview summary template that they had used in other studies; this included the demographic information of each interviewee, his/her answers to the most important research questions and any illustrative quotes. These interview summaries were written by the coder who had not previously read and coded the interview; discrepancies in interpretation were identified and resolved. Both the memos that summarized the responses to each code—and the interview summaries of the responses of each interviewee—were used in the data analysis, including determination of frequencies.
Survey
Interview data informed survey development to ensure the survey was an informed reflection of participants’ lived experiences. A detailed description of the survey sample and methods can be found elsewhere (Radecki Breitkopf et al. 2015).11 In brief, we mailed surveys to 3,311 individuals recruited from the two Mayo Clinic pancreatic cancer research resources used to create the interview sample, including probands and family members (blood relatives and spouse/partners).12 An expanded and slightly altered version of the hypothetical Jones family scenario was used, with the proband renamed “Pat” (purposefully gender-neutral), a spouse, and two adult, biologically-related children.
RESULTS
The interview study
Telephone interviews were completed with 51 individuals from the pancreatic cancer research biobank and family registry. The interviews averaged 60 minutes in length (range: 50 –120), were audio recorded, transcribed, validated for accuracy, and de-identified. Given the semi-structured format of the interviews, not all questions were asked of, or answered by, all the informants.
Table 1 describes the demographic characteristics of the interview sample. Respondents reflected a balance between familial (57%) and sporadic (43%) pancreatic cancer. 13 The probands’ age distribution reflects the average age of diagnosis with pancreatic cancer. The demographics of the sample population reflect the midwestern patient population of Mayo Clinic in Rochester, Minn.
Table 1.
Demographic characteristics of the participants* in the interview study (N = 51).
| No. | (%) | Range | |
|---|---|---|---|
| Median Age of sample, years | 66 | 44 - 83 | |
| Median Age of Probands | 67 | 51 - 83 | |
| Median Age of Siblings | 71 | 61 - 80 | |
| Median Age of Children | 55 | 44 - 80 | |
| Median Age of Spouse/Partners | 68 | 47 - 78 | |
| Sex | |||
| Male | 24 | (47) | |
| Female | 27 | (53) | |
| Race/Ethnicity | |||
| White, non-Hispanic/Latino | 50 | (98) | |
| Black, non-Hispanic/Latino | 1 | (2) | |
| Type of Participant | |||
| Proband | 17 | (33) | |
| Long-term survivor (>1y) | 13 | (76) | |
| Recently diagnosed (<1y) | 4 | (24) | |
| Non-proband | 34 | (67) | |
| Biological kin | 23 | (67) | |
| Sibling | 8 | (34) | |
| Child | 15 | (65) | |
| Non-biological kin (Spouses) | 13 | (38) | |
| Family registry participants | 29 | (57) | |
| Sporadic cancer biobank participants | 22 | (43) | |
| Highest Level of Education | |||
| High school graduate | 2 | (4) | |
| Some college or technical school | 2 | (4) | |
| College graduate | 9 | (18) | |
| Post-graduate | 38 | (75) | |
| Occupation | |||
| Business & Financial | 12 | (24) | |
| Education | 8 | (17) | |
| Healthcare | 7 | (14) | |
| Management | 7 | (14) | |
| Social Services | 4 | (8) | |
| Engineering & Construction | 6 | (12) | |
| Office & Administrative Support | 2 | (4) | |
| Retired (occupation not given) | 2 | (4) | |
| Other | 3 | (6) | |
| Religious Affiliation | |||
| Protestant | 29 | (58) | |
| Catholic | 15 | (30) | |
| None/Atheist | 3 | (6) | |
| Other | 3 | (6) | |
| Missing | 1 | (2) |
Potential interview participants were selected from Mayo Clinic research databases containing up-to-date demographic and vital status information on probands and relatives. Through mailed invitations, we recruited a balanced random sample of male/female, adults from four categories: proband within one year of diagnosis, proband beyond one year of diagnosis, blood relative, and non-biological relative (spouse/partner). We did not contact family of probands who had died during the prior 8 weeks.
All participants had experience with pancreatic cancer, either their own diagnosis or that of a family member. Pancreatic cancer is difficult to detect early, is rapidly fatal, and has a low six-month survival rate. With few exceptions, the experience of pancreatic cancer was described as powerful and difficult, affecting all family members. Several participants recounted unexplained symptoms that lasted months and up to a year before obtaining a correct diagnosis. All but one person reported having living relatives; most were married and had adult children. Many described communication within their families—in general and about health—as open; all but two reported open sharing of the diagnosis of pancreatic cancer within the family.
Most respondents were concerned about developing pancreatic cancer; family history of cancer was the most common source of worry (n=23). Several had already taken measures to address their concern. Some had experience with or were interested in genetic testing or genetic counseling (n=19); most indicated they were “information seekers,” albeit to different degrees, and spoke with some fluency about genetic disorders. Confidence in medicine, research, and Mayo Clinic specifically were particularly evident. Nearly half also expressed faith in God and or prayer; 88% self-identified as affiliated with a Christian religion.
The survey sample
Of the 3,311 surveys mailed to cancer biobank participants and family members, 1,903 were returned, for an overall response rate of 57.5% (Radecki Breitkopf et al. 2015). The survey data included in this analysis reflect the responses of 464 affected probands, 1,040 blood relatives, and 399 spouse/partners (Radecki Breitkopf et al. 2018). Survey respondents shared many characteristics with interviewees, such as age, education, and race/ethnicity (See Table 2 for demographics of the survey sample).
Table 2.
Demographic characteristics of survey respondents (N=1903; Response Rate 57.5%). †
| Characteristic | All Respondents n=1903 n (%) |
Proband/Affected n=464 n (%) |
Blood Relatives n=1040 n (%) |
Spouse/Partner n=399 n (%) |
|---|---|---|---|---|
| Sex | ||||
| Male | 707 (37.2) | 231 (49.8) | 339 (32.6) | 137 (34.3) |
| Female | 1196 (62.8) | 233 (50.2) | 701 (67.4) | 262 (65.7) |
| Age, years | ||||
| Mean (SD) | 63.6 (12.8) | 66.4 (11.3) | 60.3 (13.4) | 69.1 (10.4) |
| Range | 23-99 | 29-94 | 23-99 | 38-94 |
| Median | 64 | 66 | 61 | 70 |
| Race | ||||
| White | 1841 (98.1) | 444 (97.4) | 1012 (98.4) | 385 (98.0) |
| Black/African American | 8 (0.4) | 4 (0.9) | 4 (0.4) | 0 (0) |
| Asian | 10 (0.5) | 3 (0.7) | 4 (0.4) | 3 (0.8) |
| Hawaiian/Pacific Islander | 2 (0.1) | 0 (0) | 0 (0) | 2 (0.5) |
| American Indian/AK Native | 10 (0.5) | 3 (0.7) | 5 (0.5) | 2 (0.5) |
| Other | 6 (0.3) | 2 (0.4) | 3 (0.3) | 1 (0.3) |
| Missing | 26 | 8 | 12 | 6 |
| Hispanic Ethnicity | ||||
| Yes | 18 (1.0) | 6 (1.3) | 8 (0.8) | 4 (1.0) |
| No | 1854 (99.0) | 446 (98.7) | 1020 (99.2) | 388 (99.0) |
| Missing | 31 | 12 | 12 | 7 |
| Marital status | ||||
| Married/Life partner | 1279 (68.5) | 377 (83.2) | 797 (78.0) | 105 (26.9) |
| Separated/Divorced | 115 (6.2) | 37 (8.2) | 78 (7.6) | 0 (0) |
| Widowed | 395 (21.2) | 27 (6.0) | 82 (8.0) | 286 (73.1) |
| Single/never married | 77 (4.1) | 12 (2.6) | 65 (6.4) | 0 (0) |
| Missing | 37 | 11 | 18 | 8 |
| Education | ||||
| High school or less | 350 (18.6) | 111 (24.4) | 157 (15.2) | 82 (20.9) |
| 2 year college/technical school | 627 (33.4) | 140 (30.8) | 343 (33.3) | 144 (36.6) |
| 4 year college or greater | 900 (47.9) | 203 (44.7) | 530 (51.5) | 167 (42.5) |
| Missing | 26 | 10 | 10 | 6 |
| Employment | ||||
| Not employed | 142 (7.6) | 62 (13.7) | 67 (6.5) | 13 (3.3) |
| Employed | 833 (44.6) | 141 (31.2) | 577 (56.2) | 115 (29.4) |
| Retired | 894 (47.8) | 249 (55.1) | 382 (37.2) | 263 (67.3) |
| Missing | 34 | 12 | 14 | 8 |
| Health insurance coverage | ||||
| No | 31 (1.7) | 3 (0.7) | 22 (2.1) | 6 (1.5) |
| Yes (private, employer, public) | 1844 (98.3) | 451 (99.3) | 1006 (97.9) | 387 (98.5) |
| Missing | 28 | 10 | 12 | 6 |
The demographic table for the full sample (including controls) has been published in Radecki Breitkopf et al. 2015. The demographic table for the sample minus controls has been published in Radecki Breitkopf et al. 2018.
Results: Preferences and justifications for RoR
We first present a summary of respondents’ preferences for RoR. Next we explore participants’ reasoning by posing the following questions: 14
What is at stake in knowing results?
What kinds of results are important to know?
Are there harms in knowing?
Who is entitled to the results? Based on what criteria?
Is there a moral responsibility to know, to share, to keep private?
Who should control a person’s genetic data while living and once deceased?
In what ways do research participants and family members value a right to choose?
How should communication of genetic information be handled in the family?
Where relevant, interview results are presented in parallel with complementary data from the survey sample. The following abbreviations are used: I = interviewer, P = participant, M or F = male or female, numbers included in quote descriptors are study identifiers.
Family disclosure preferences: Should an individual’s results be communicated to his/her relatives (see Tables 3 & 9)?
Table 3.
Preferences for communication of genetic research results, including unexpected results, to proband and family members: Interview and survey responses.
| Topic | Interview responses | Survey responses | ||
|---|---|---|---|---|
| Percent (n) |
Statement | Percent Agreed* (n) |
Survey Statement | |
| Personal preference to be offered genetic results of: one’s own sample: |
94% (47/50) | Would want to be offered unexpected results found in own sample | 97% (1825/1889) |
“I would want to know about [medically useful] genetic research results in my sample.” |
| family’s sample: | Equivalent item not included in interview | 96% (1803/1887) |
“I would want to know about [medically useful] genetic research results discovered in my parent’s sample.” | |
| Intention to share results with family: | 92% (47/51) |
Would share results with family | 94% (1782/1887) |
“I would be OK with sharing my [medically useful] genetic research results with blood relatives who wanted to know them.” |
| Researchers’ duty to offer proband’s unexpected results: to proband him/herself: |
96% (49/51) |
Proband’s unexpected results should be offered to the proband | 99% (1859/1885) |
“Researchers should offer Pat the information about the BRCA2 mutation discovered in Pat’s blood sample.” |
| to the family: | 98% (48/50) |
Proband’s unexpected results should be offered to family | Equivalent item not included in survey | |
Percent of total “strongly agree” plus “agree” responses over total non-missing responses
Table 9.
Contrasting views on the role of living and deceased probands’ preferences about communication of results to family members: Examples of interview responses (I = Interviewer; P = Participant).
|
Living Proband |
Share results only after asking permission of proband or personal representative (n=34) |
|
I:
“So the research subject should control whether the information is offered to the family?” P: “Correct… And if the law said you can’t find out [family member’s] information, then you can either fight the law… or you can say, ‘Okay, that’s fine, if I can’t know that, I’ll go get myself tested anyway’” (Interviewee 05, Proband M). | |
| “The participant should furnish names … of people that he or she would want told” (Interviewee 42, Proband M). | |
|
I: “… imagine that Mayo Clinic researchers did find the BRCA2 mutation when your brother's sample was tested. Would you want your brother's information offered to you? P: “I would, yes …but I don’t want to be the guy pushing [him] into something…. He is the one that is suffering from the problem and I would want him to make the decision as to whether or not he wants to share that with me or any of his other brothers or sisters” (Interviewee 07, brother of Proband). | |
| Do not ask the proband’s consent to share results after death and/or do not promise to withhold them (n=3) | |
| “The participant should be informed, ‘We will honor your wishes until you are dead, but after that, the data goes…to the rest of your family’” (Interviewee 27, husband of Proband). | |
| Honor proband’s preferences while alive, but share results once proband has deceased (n=3) | |
| “…I think the initial patient is the one that … needs to be a little more protected…if the patient doesn’t want the information released while they are still alive, I think that, unfortunately, you have to respect [that]…Once they are gone, once they die, then I don’t think you really need to protect them anymore” (Interviewee 36, daughter of Proband). | |
| Response Missing (n=4) | |
|
Deceased
Proband |
Share results only with permission of proband or personal representative (n=11) |
| “I think it [the genetic result] should be kept private and let the legal heir be the gatekeeper” (Interviewee 09, Proband M). | |
| “It should be private unless that person, while they are alive, grants permission” (Interviewee 37, wife of Proband). | |
| OK to share results if the proband’s wishes are unknown (n=16) | |
| “…I don’t think it should be ‘Mrs. Jones’ or my husband who has to give permission… I would have no problem without his explicit permission to tell the family if there was something that…came to be known later after his death. I wouldn’t feel like I would be disrespectful to him or anything like that” (Interviewee 15, wife of Proband). | |
| OK to share results, regardless of the proband’s stated wishes (n=17) | |
| “After they are dead, it [the information] should be available, regardless…After death, a family…if they inherited the gene, they ought to inherit the data” (Interviewee 27, husband of Proband). | |
| Response Missing (n=6) |
The clear majority of participants in both interview and survey studies strongly agreed that: 1) they would want to be offered their own results (Interview: 94%; Survey: 97%) and those of family members (Survey: 96%), 2) they would share their results with family members (Interview: 92%; Survey: 94%), 3) researchers should offer probands their unexpected genetic results (Interview: 96%; Survey: 99%), and 4) probands’ unexpected genetic results, including those obtained posthumously, should be offered to family members (Interview: 98%; see Table 3). Only three interviewees opposed communication of a proband’s results to family members and only one expressed that view unconditionally. A large majority of both interview and survey participants thought that researchers have a duty to offer genetic results to probands. Most interviewees (34/49, 69%) felt that a living proband’s results should be shared only after asking permission of the proband or personal representative; once the proband was deceased, about a third of interviewees thought it was acceptable to share the results regardless of the proband’s stated wishes (17/50, 34%), roughly a third thought sharing was acceptable if the proband’s wishes were unknown (16/50, 32%), and nearly a quarter thought that results should be shared only with permission from the proband or personal representative (11/50, 22%; see Table 9).
What is at stake in knowing results?
Nearly everyone indicated that much is at stake in learning their own or a family member’s genetic results:
You know we are talking about a gene mutation … this information needs to go somewhere. You know, it can’t just sit in a file cabinet in the Mayo Clinic. It needs to be told…. there is a responsibility there, it needs to be revealed. (emphasis added, Interviewee 47, daughter of proband)
Genetic information was described by interviewees as powerful, predictive, and potentially very useful; 77.9% of survey respondents endorsed (agreed or strongly agreed) the statement: “genetic knowledge is power.”
Interviewees offered many reasons to disclose and learn genetic results (Table 4). Anticipated benefits to their own and their family’s health dominated; many believed that knowing would enable earlier diagnosis or prevention. Popular maxims, such as, the earlier the better, the more information the better, the more surveillance the better, received frequent mention.
Table 4.
Reasons given for wanting to receive unexpected genetic research results: Examples of interview responses (n reflects number of participants mentioning).
| 1. Clinical utility, health maintenance (n=17) |
|
Increase possibility of preventing or minimizing disease through early detection and diagnosis (n=10) • “Being on guard and knowing what to look for--early detection, early detection, early detection” (Interviewee 34, daughter of Proband). |
|
Presumed utility for treatment now or in the future (n=7) • “I think if the Mayo Clinic has information about a specific family member, they could provide a better … opportunity, perhaps, to be cured” (Pilot Interviewee 03, wife of Proband). • “…because the future could bring better treatments and cures, even if there is ‘nothing to be done’ today” (Interviewee 54, son of Proband). |
| 2. Personal utility and meaning (n=14) |
|
Allows for preparation and planning (n=6) • “….If I knew what was coming ahead then I would make sure I write down that my mother had pancreatic cancer and all of that for my daughter to know…” (Interviewee 30, daughter of Proband). |
|
Allows for informed reproductive decisions (n=2) • “Yes I would want results shared, because there is something that you could control by not having children…” (Interviewee 9, wife of Proband). |
|
Knowing is power, knowing is better (n=6) • “Knowledge is powerful and to withhold knowledge is to withhold power concerning your own body” (Interviewee 43, Proband M). |
Early diagnosis was particularly important to the interview participants. Pancreatic cancer is difficult to diagnose; some interviewees linked the importance of early diagnosis explicitly to their own experience of missed symptoms or misdiagnosis. Many thought that knowing about a familial genetic mutation would help them and the physicians to “keep an eye open,” “know what to look for,” “raise a flag,” and allow one to be more “aware” and more “attuned to an odd symptom.”
I: If an [unexpected] genetic finding was offered to you, do you think you would want to hear it?
P: Absolutely! … Because of the way pancreas cancer is. You are so unlikely to detect it in time to save yourself. The only real way to save yourself is to… be really vigilant…. (emphasis added, Interviewee 25, sister of proband)
Many assumed that a genetic finding could motivate people to be more diligent in their preventive practices, to adopt healthier lifestyles, or to improve their chances for better treatment for a range of diseases.
Even when presented with situations where no specific health benefit was believed possible, many informants saw great personal utility and meaning in knowing their own or their family members’ genetic information. Learning this information would allow them to know and plan for their future: “Get your will done, get your house in order” (Interviewee 52, Proband M). And looking ahead, knowing what to expect, how to prepare—even if a condition is unwanted and unalterable—was highly valued and important for living a good life.
Just the idea that I have so much time left is appealing to me. (Interviewee 12, wife of proband)
“How are you going to plan your life if you don’t know [your genetic information]?” (Interviewee 21, Proband F)
Most interviewees implicitly assumed that learning results would help with planning the future. In the interviews, many spoke openly and in a matter-of-fact way about how useful genetic information would be for planning and preparing for the end of life, an eventuality many seemed to accept. They would take out long-term care insurance and make arrangements so they wouldn’t be a burden on others.
… If I had [the results of a disease of cognitive decline] in advance, I can get ready…, I could do some preparation. I could get my family prepared… so it wouldn’t come as a shock to them. (Interviewee 12, wife of proband)
Many interviewees discussed the result of a genetic test for a hypothetical disease condition, such as an “untreatable condition,” as if it was a clear diagnosis of a disease. They viewed predictions of disease risk as reflecting a known future reality, seeing genetic information not as a probability but as a clear eventuality: a credible prophecy. By contrast, genetic information about cystic fibrosis was considered potentially useful for family members by only a few respondents. Most felt it was too late to be relevant to them and did not see any potential relevance for their children.
Beyond the perceived potential of genetic information to help reduce risk and aid planning, some described genetic results more generally as useful “data” to put into the “equation” of one’s health and life.
More data is good data…. anything you can add into the equation I think is going to give you a better shot at prolonging your life or heading off any problems.” (Interviewee 52, Proband M)
So I want to have whatever results from anything with my disease process be made known to them [my children] if they prefer….Knowledge is…power. Knowledge … for me is a great benefit. …You can choose not to act on it, but knowing is important. (Pilot Interviewee 02, Proband F)
In contrast to not knowing, for a small number of interviewees knowing was described as a sign of maturity, an ability to face reality and an important way of living life. One interviewee was told by her mother:
‘Learn as much as you can’….That has always been our philosophy on everything, you know: Don’t hide behind anything…don’t brush anything underneath the rug. (Interviewee 30, daughter of proband)
Most were critical of Mr. Jones’s attempts to “protect” his daughters because it was too painful to talk about their mother’s cancer:
Well, I don’t buy that! Death is part of life! And we all experience it. Sure it is painful, but you don’t turn your back on a train coming down the track. So yeah, I think it would be foolish not to tell his kids. (Interviewee 33, son of proband)
What kinds of results are important to know?
Genetic information is heterogeneous, raising an important policy question: which results should be offered? We asked, “What factors matter in whether you think genetic research results, including unexpected results, should be offered or not?” We asked about several characteristics of results, including preventability, treatability, level of uncertainty, regulatory approval, and results revealing conditions leading to cognitive decline. Of these, uncertainty was the only characteristic that participants said would influence their preferences about RoR: 58% of those interviewed would not want results “from a new test that was not well understood” (see Table 5).15 Ten interviewees noted the need for the test and results to be analytically and clinically valid. However, a majority of survey respondents indicated that results could be offered from a genetic test that had not been approved by a regulatory agency such as the Food and Drug Administration (Radecki Breitkopf et al. 2015).
Table 5.
Interview responses: “What kinds of genetic results, if any, do you think researchers should offer to research participant’s family members?”
| Result characteristics |
Percent (n) |
Interview response example1 |
|---|---|---|
| Preventability of condition | 95% (38/40) |
Preventability does not matter:
“Knowing that they have a fatal disease…maybe they take a cruise or something…” (Interviewee 13, wife of Proband). |
| Treatability of condition | 7.5% (3/40) |
Treatability does matter:
“If there’s nothing to be done, there’s no sense in putting stress on people” (Interviewee 09, Proband M). |
| 92.5% (37/40) |
Treatability does not matter:
“There are a lot of life decisions that could be made even if not treatable” (Interviewee 16, wife of Proband). |
|
| Whether condition involves cognitive decline | 2% (2/48) |
Cognitive decline does matter: “Oh, that’s a hard one. What’s the point? [Knowing] won’t make anybody’s life any better” (Interviewee 28, wife of Proband). |
| 98% (46/48) |
Cognitive decline does not matter: “…Just so that they know what is going on…what is going to happen…and how to proceed with it…I think I would want to be in the know about everything, I guess” (Interviewee 55, Proband M). |
|
| Uncertainty of results | 58% (28/48) |
Uncertainty does matter: Should reach a certain threshold of certainty to be offered: “There is a number at which the percentage accuracy of a test tips it towards worthy of knowing: around 75%” (Pilot Interviewee 06, husband of Proband). |
| 42% (20/48) |
Uncertainty does not matter: “Yeah, it’s better to be safe than sorry. If you are not certain, at least that raises a flag…” (Interviewee 33, son of Proband). “Anything that can change your health, you should know about it. Can always add a question mark to the test” (Interviewee 20, Proband M). |
|
| Whether genetic test is approved by a regulatory body, such as FDA | 25% (10/40) |
Regulatory approval does matter: “Would have been studied enough to find a link between the disease and the mutation” (Interviewee 40, Proband M) |
| 75% (30/40) |
Regulatory approval does not matter: Time constraints: “It takes a million years to go through the FDA. Would hope things would be offered before then” (Pilot Interviewee 02, Proband F). Distrust of regulatory agencies (e.g., FDA): “FDA decisions are political and don’t carry a lot of weight with me” (Interviewee 06, brother of Proband). |
Interview responses were coded as “mattered” or “did not matter.” See Online Supplemental Material for copy of Interview Guides.
Are there harms in knowing?
As noted above, only one respondent from the interview sample unambiguously opposed learning and sharing his results. However, nearly half of those who supported disclosure in general also expressed qualifications and concerns about offering and knowing results. The burden of a negative emotional impact, leading to unnecessary worry and destructive fear, was by far the most frequent justification (noted by nine). Three informants considered such worry and fear to be serious, like an illness. One noted, “Fear is the worst disease you can have, over anything…” (Interviewee 03, Proband M). This proband, in fact, opposed being told of any unexpected results, preferring to devote all his energy to the present disease, not to future possibilities:
So in my situation I know I had pancreatic cancer. I don’t want to know what the specifics are, I don’t want to know what other things I could get or have the possibility of getting. I want to deal with what I know, and what I know is that I had pancreatic cancer. Okay? So…if I were a researcher, I would never release information that would or could open up, for instance…that I had another possibility of getting other forms of cancer, okay? I don’t need that in my head right now. (emphasis added, Interviewee 03, Proband M)
One woman preferred living life “the way it was intended” and not knowing until something happened:
…We can make ourselves sick from waiting for something to happen, or we can live our life the way it was intended until it happens, you know, until we find out that there is a problem…. And I think that I prefer … to not know… I don’t want to sit and fret over something that may never happen to me. (Interviewee 35, sister of proband)
A few expressed concern that more information would overburden family members.
…It may be a burden on the family if you tell them, ‘Oh, by the way, in addition to that [serious cancer], then we have also got this other [thing] that we are dropping on top of you.’ (Interviewee 23, husband of proband)
The proband who most opposed learning his own results also emphatically opposed sharing his results with family members.
I would not want my family members to know [that] because I had pancreatic cancer they may be more susceptible to other forms of disease…. Let’s cross that bridge when we get to it… Never report it to family members! …Absolutely! …Sharing [genetic] information with family members … kind of opens Pandora’s Box. That …I just wouldn’t do.
…Let’s not borrow trouble in the future! (Interviewee 03, Proband M)
The few (three) in favor of non-disclosure were not alone in their concern that learning and sharing of genetic information would be emotionally difficult. However, disclosure advocates reasoned that the benefit of knowing was worth the cost. One informant became tearful when discussing the hypothetical Mr. Jones withholding Mrs. Jones’ genetic results from his daughters because of Mr. Jones’ anguish over his wife’s suffering:
So yeah, I don’t like talking about all this stuff either, and it makes me cry, and makes me relive the thoughts and the pain. But…there is something good that can come of it and I think the daughters are entitled to that. (Interviewee 28, wife of proband)
Few respondents noted the potential harms of returning uncertain or clinically questionable results, or issues that concern health professionals most, such as excess testing, false positives, and unnecessary treatment. Few spoke of a potential negative impact on family dynamics and none noted the danger of revealing misattributed paternity. Only 8.6% of survey respondents agreed with the statement: “Learning genetic research results can only lead to worry.”
Who is entitled to the results? Based on what criteria?
When asked, “To whom does genetic information belong?”, many voiced the conventional view that it belongs to the proband him- or herself. However, we also heard, sometimes from the same individuals, that genomic data belong not only to the proband, but to other family members as well (see Table 6). The idea of familial ownership was also supported in the survey; over half (62.6 %) endorsed the statement, “Genetic information belongs to all blood relatives, not just the person who gave the blood sample.” When reasoning about who should be offered results, interviewees proposed two interrelated criteria for deciding who is entitled to results: first, “People have a right to know if it can affect them [their health]” (emphasis added, Interviewee 38, sister of proband), and second, “If they share genetics” (Interviewee 05, Proband M). When asked directly whether they thought Mr. Jones’s daughters “had a right to information that might affect their health,” 49 of 51 interview participants said “yes.” (Discussed in more detail below.)
Table 6.
To whom does genetic information belong? Interview responses.
| Genetic information is familial: examples of responses |
|---|
| “I really think that family members need [to be informed about genetic information]… immediate family members…have a right to know…. The genetic code isn’t just his [the proband], it is also the daughters’” (Interviewee 12, wife of Proband). |
| “This is not legal information, this is genetic information. So the genetic information needs to [go] to the next of blood kin…Genetic information is not just one person's information. It’s passed on. So this is their [the daughters’] information, too. So, genetically, they’re coded together so this becomes the property of the daughters now—not the husband” (Pilot Interviewee 02, Proband F). |
| “Information needs to follow the gene line… the direct family tree” (Interviewee 47, daughter of Proband). |
| “The next-of-kin genetic tree…should be notified. I think it is their genetic right to know” (Interviewee 05, Proband M). |
Is there a moral responsibility to know, to share, to keep private?
A number of interviewees felt an obligation to learn their genetic information out of a sense of family duty. Several noted that parents have a responsibility to be informed for the sake of their children. Being a good parent demands being informed: “You have a responsibility to know” (Interviewee 38, sister of proband); “You should know [the results]…it’s about giving your kids a chance” (Interviewee 33, son of proband).
This sense of responsibility to know results was joined with an obligation “to tell” on the part of the parent: “Passing on information is a moral responsibility of a parent” (Interviewee 47, daughter of proband). This moral responsibility extended to probands, family members, legal next-of-kin, researchers, clinicians, and occasionally health care institutions. Informants justified their positions by emphasizing the presumed benefit of genomic knowledge to health. A few informants felt that given the life-saving potential of information, it is “immoral” not to tell. Others considered sharing genetic information to be a gift, but not an obligation.
The term “privacy” was used infrequently when referring to communication of results within the family. Only 7.4% of survey respondents would be concerned if their biological family members learned their results. Similarly, only 4.4% would want their genetic results to be kept private, even after their death. Finally, only 7.5% would not want their blood relatives to know about their genetic results (see Table 7). Echoed by interview responses (Table 8), these data suggest that participants expect that genetic information should and would be shared within the family, and that information should not be kept private.
Table 7.
Preferences about privacy and communication of genetic information inside and outside the family: Survey responses.
| Survey Question | Percent (n) |
|---|---|
| “How concerned would you be if your biological family members learned your genetic research results?” | 7.4% (139/1740) concerned* |
| “How concerned would you be if an employer learned your or your biological family member’s genetic research results?” | 77.6% (1450/1869) concerned |
| “How concerned would you be if your health insurance company learned your or your biological family member’s genetic research results?” | 81.1% (1521/1875) concerned |
| “I would NOT want my blood relatives to know about my genetic results [assuming they were medically useful]” (emphasis in the original). | 7.5% (142/1886) agreed** |
| “I would want my genetic results [assuming they were medically useful] to be kept PRIVATE, even after my death” (emphasis in the original). | 4.4% (82/1885) agreed |
“Concerned” combined “extremely concerned” and “quite concerned” responses on a 4-point scale that also included “slightly concerned,” and “not at all concerned.”
“Agreed” combined “strongly agree” and “agree” responses on a 5-point scale that also included “neither agree nor disagree,” “disagree,” and “strongly disagree.”
Table 8.
Benefit of genetic information to others overrides individual privacy: Examples of interview responses.
| “It [genetic information] is a very private personal thing, but… I still think that there is a responsibility that we tell them—the gene line of the kids” (Interviewee 47, daughter of Proband). |
| “If it is a disease that does not have a genetic influence, then that is different. But if it is something that other generations are going to have to be concerned about,…then I think that it's not so important to keep it so private….Once they die,…I don’t really think you need to protect [their results]” (Interviewee 36, daughter of Proband). |
| “I think if people are genetically linked to that person, and knowing this information would benefit them, would provide them a better life, then I think keeping it private is wrong…Privacy should be maintained, except with those who are genetically linked” (Interviewee 53, son of Proband). |
| “Maybe don’t offer [results of an unpreventable disease] unless the disease will be carried in generations to come. Then it would be important for the family to know” (Interviewee 29, Proband F). |
. . . I think it is like a moral responsibility to let the siblings and the family know that they could have this, but it is still their choice, they can do what they want with that information, but… Yeah, to me, it comes under more of a moral responsibility. (Interviewee 47, daughter of Proband)
Only 29.8% of the survey sample agreed that “Pat should be able to keep information about the BRCA2 mutation private from others in the family.” In contrast, concern for confidentiality was directed outside the family circle. Many interviewees indicated they would be concerned if their results were communicated to insurance companies, employers, and for some, physicians (Table 7).
I am not real keen on having it shared outside of the family. (Interviewee 05, Proband M).
I would not want my health insurance company to know it. Or, I guess any of my insurers to know it… I don’t want the government to know it. I don’t want anyone to know it that could impact my future care… I don’t want anybody that has…control over my financial future to know about it…. (Interviewee 09, Proband M)
These findings are consistent with many participants’ priorities. When asked on the survey to choose “the most important factor to consider in returning genetic research results,” two-thirds indicated it was “whether blood relatives will benefit,” while one-third chose “the wishes of the person who provided the sample” (Radecki Breitkopf et al. 2015).
Who should control a person’s genetic data while living and once deceased?
When questioned directly, half the interviewees thought a living proband should control his or her genomic information. When asked how communication of a proband’s findings should be handled, the most common recommendation was to ask the participant’s preferences when first entering a biobank or registry. They affirmed this position while at the same time noting the burdens placed on an ill proband. A few disagreed, arguing that confidentiality should not be promised from the start.
… I know… there are people who don’t want their families to know … and so then that is a tough question…. First of all, if you discussed that with a patient who had the cancer, but then if they said, “I don’t want anybody else to know,” then I probably think that you should tell them, “Well,…we feel it is necessary [to let your family know]….” (Interviewee 29, Proband F)
As noted above, whether a proband is living or deceased influenced participants’ views about sharing genetic information within the family (Table 9). A quarter of interviewees thought that sharing results from a deceased proband required permission from the proband, if alive, or the proband’s personal representative. Nearly a third thought that it was acceptable to share results if the proband’s wishes were unknown, and nearly a third thought it was acceptable to share results regardless of the proband’s stated wishes. This was echoed in the survey, where only 32.2% agreed that if Pat had specifically requested results not be shared, “information about the BRCA2 mutation should NOT be offered to Pat’s blood relatives.” Thus, over two-thirds of survey respondents did not agree with honoring Pat’s expressed desires when they involved withholding information from family members.
Such views were justified in several ways: “A dead person is no longer a person and therefore has no rights…” (Interviewee 51, son of proband), or, the deceased “no longer needs protection” (Interviewee 36, daughter of proband). For others, the unique familial nature of genetic information entered into their calculus of valuing or devaluing privacy; these qualities impose a unique responsibility to share information (see Tables 8 and 9).
In what ways do research participants and family members value a right to choose?
In the interviews, nearly all the probands and family members endorsed the practice of researchers’ offering results to research participants and their families. An important justification for that position, mentioned explicitly by 14 interviewees, was the right of research participants and their families to choose whether to receive results. Having the option to choose, in and of itself, was strongly favored.
[Knowing] should be an option…That is my big thing…We have to have an option of, “do you want it [the test result]?” (Interviewee 46, wife of proband)
Control over one’s life means having control over information to inform life choices:
There is a moral obligation to give me…control over that information so I can make a decision about how I choose to have children and what risk I put them in. (Pilot Interviewee 02, Proband F)
Everybody has the right to make a decision on their life and what knowledge they have. (Interviewee 32, daughter of proband)
Unsurprisingly, interviewees expressed a strong aversion to limiting access to information. Not even a father has the right to limit his daughters’ access to genetic results:
I: Who—Mr. Jones or his daughters—do you think should have priority in case of a conflict?
P: The daughters…. Because it is an individual right…Why would the father be given that right over somebody else’s life? It just doesn’t make sense to me. (emphasis added, Interviewee 12, wife of proband)
P: They [the daughters] are the ones that have ultimate control over their lives. (Interviewee 09, Proband M)
Several interviewees considered Mr. Jones’s not allowing his daughters access to their mother’s results to be “selfish,” even “immoral” (Interviewee 29, Proband F). A proband’s wife considered not offering information to others in the family to be patronizing:
If you assume that the people that you are sharing information with are big boys and girls, and it is up to them to take care of their situation, then let them know. (Interviewee 12, wife of proband)
A few even equated withholding information to causing death: “Why do you have the right to kill them [children] by withholding information?” (Interviewee 34, daughter of proband). Another felt a parent does not have the right to refrain from informing one’s children of a genetic condition:
I don’t think that I would have a right to keep my children from knowing if they needed to know there was a health condition in their genetic background. (Interviewee 16, wife of proband)
How should communication of genetic information be handled in the family?
Interviewees valued openness of communication:
Well, we try to bring everything out in the open. We really haven’t hidden things in our family, so … that’s why I brought my [pancreatic cancer] diagnosis out in the open and told everybody what I had, and…that it could possibly be genetic.… I just, you know, think that is the right way to do things like this.” (emphasis added, Interviewee 42, Proband M)
Notwithstanding their uniform belief in the benefits of genetic information, many participants specified that they would communicate genetic information only if their relatives wanted it. Probands and family members have the responsibility to offer the information, not to convey the information without asking or to dictate that others receive it:
… it is not for me to say what other people should or should not do. If it were my son, if it were me, I would want to know.” (Interviewee 53, son of proband)
Interviewees gave numerous examples of which family members would — or would not — want to know this kind of genetic information, and who could — or could not — manage it:
I: “How important is it to honor the wishes of people who do not want to hear any news about their genes?
P: Well, I don’t know. I’m thinking I have five kids. So two don’t want to know and three do. It would be different for each person.” (Interviewee 15, wife of proband)
Some family members communicated openly to each other, others did not:
We talked a little bit about my younger sister. I don’t talk too much to my older sister, but my younger sister, she talks to my older sister…. I don’t talk to my daughter a whole lot, but I talk to my son quite often, quite a bit. I feel as though they [my children] have a right to know…. (Interviewee 20, Proband M)
Other interviewees emphasized the importance of knowing who to tell and who not to tell. For example, when asked to whom researchers should give genetic information, one proband’s sister answered:
That is… a really excellent question, because healthcare providers are not going to know… [who the] family members are. But I can guarantee you that taking, for example, my family, if information had ever been filtered through the oldest child, my sister, nobody would have ever gotten it. She would have spilled coffee on it and accidentally lit it on fire with her cigarette or something! [Laughter] You know what I mean? (Interviewee 25, sister of proband)
Interviewees also emphasized the importance of how to tell, such as being “open but careful with health information so as not to scare family members” (Interviewee 41, daughter of proband).
Differences within the family were not described as serious problems or the basis of family conflict but as a known fact of family life.
I: …as a general policy, what do you think researchers should do if they discover a serious genetic finding in a research participant who is no longer living?
P: I think if it is something that is a strong possibility… I think the blood relatives should know…. “Would people do that [let them know]?” I don’t know. I know I would do it, I know one of my sisters would do it. I know my brother would not – because he says, “Well, they can figure it out on their own,” or “I don’t want to tell them that they are going to die, you know, in five years.” But I would say, “Hey, this is what I found that’s with me, you might want to be tested so you are prepared so you don’t have to go through what I am going through….” (Interviewee 30, daughter of proband)
Only when prompted by our scenarios did interviewees use the language of rights in responding to the case example of Mr. Jones—who withheld information from his daughters. A rights-based discourse was not used when speaking about communication within their own families or about specific individuals. Rather, they focused on family member preferences and idiosyncrasies, not conflicts in which the interests of one family member superseded one’s own or another’s “right” to have genetic information. While nearly all participants referenced family members who did not want to know genetic information, most did not invoke a rights-based language when discussing disclosure to individual family members. However, when we specifically asked about policies governing the “right not to know,” the spontaneous reaction was often silence or confusion; many did not understand the formal concept even though they clearly supported the idea. Furthermore, no one supported the practice of not offering genetic information to other family members in order to protect their own right not to know.
DISCUSSION
The cultural and moral logic of disclosure of genetic information to relatives
This mixed-methods study found that most research participants attribute great importance to genetic information and endorse offering a proband’s genetic research results to family members. Contrary to policy practices that privilege the values of individual privacy and control, most participants did not view sharing an individual’s genetic research results within the family as ethically problematic, even among family members assumed to have divergent views or desires. Rather, many considered genetic relatives to be entitled to this information, even questioning whether it is acceptable to keep genetic results from family members.
How should we understand these preferences? What moral claims are embedded in them, and upon what are they based? Preferences, we assume, are not simply idiosyncratic, autonomous choices made by individuals, but are also grounded in cultural meanings and practices based in a particular social and historical context. Two cultural and moral logics were dominant in the majority of accounts: 1) family ethics of disclosure of genetic information, and 2) approaches to health, genetic information, and preemptive medicine.16
Family ethics of disclosure of genetic information
It is notable how many interviewees spontaneously described established and unique ways that communication of health-related information was handled among family members. Each specific family ethics of disclosure was built on individual and family narratives; they also reflected shared understandings of genetics, health, family genealogies, and varying degrees of respect for the values of autonomy and responsibility.
A distinct theme in the support for the sharing of a proband’s genetic research results with family members was that family members are entitled to the information. This sense of entitlement was based on two other justifications: the shared nature of genetic material and the right to choose.
Many justified their endorsement of sharing genetic information among family members by noting that genes are shared. They considered genes and genetic information to be familial, not the exclusive property of the individual in whom a gene is first identified. As relatives are also affected by these shared genes, the information belongs to genetically-linked family members. In this way, genes are viewed as simultaneously individual and familial. This understanding meshes poorly with a view that only autonomous individuals should control their genetic information.17
The second justification given for relatives’ entitlement to a proband’s genetic information builds on a standard claim to autonomy: the right of each person to have control over his or her health and life. Given that information is fundamental to health, autonomy means having the option to choose what information to receive and what not to receive. Choice depends on having access to the information. Restrictions to that choice are negatively judged. For example, most felt that Mr. Jones should not withhold clinically meaningful genetic information from his adult daughters because they should have ultimate control over their lives. This claim of autonomy and control is one basis of relatives’ genetic right to know.
Finally, probands and relatives express a strong sense of moral responsibility to learn and share their genetic information, especially for the sake of the younger generation. This strong ethics of family sharing is consistent with a relational approach to ethics, described as an “ethics of care” and more recently as “relational autonomy” (Dheensa, Fenwick and Lucassen 2016; Gilbar and Miola 2015; Hallowell et al. 2003; Weaver 2016; Wertz and Fletcher 1991). The perceived value of genetic knowledge is seen as justification for overriding the stated wishes of a deceased relative. Whereas policy may recommend generally sharing probands’ results only with their permission, our interviewees were split on this while the proband was alive, and most of our interviewees believed that once the proband was deceased, results should be shared with or without permission.
Approaches to health, genetic information, and preemptive medicine
The second moral logic expressed in our interviewees’ preferences reflects the primacy of health as a core value. As many social theorists have noted, taking care of one’s own and one’s family’s health has become a moral duty, an end in itself rather than a means to a good life (Clarke et al. 2010, 179; Crawford 1980; Dumit 2012; Lupton 1995). Being informed and informing are fundamental ways of preserving and maximizing health.
This is a future-oriented understanding of health, health not as a feature of current well-being, but rather a promise. This view of health is based upon the need for constant attention to assessing risk and engaging in risk reduction. The “earlier the better” logic expressed by many interviewees echoes the fundamental premise of predictive, preemptive medicine.
Genetic information is regarded as a particularly valuable, truthful, and life-saving kind of predictive information. This should not surprise. In the United States, the gene is a cultural icon equated with progress and control (Nelkin and Lindee 1995). Celebrations of the concept of “precision genomics” provide the most contemporary example of that hope (Juengst, Flatt, and Settersten 2012). Most of our interviewees assumed that knowing one’s genetic risk allows for useful action. Even if the disease is not preventable, curable, or treatable, knowing your genetic risk can allow you to plan and prepare, if only for the end of life. The philosopher Ian Hacking called this “the taming of chance,” the conversion of an uncertain future into a predictable one through the language of probability and statistics (Hacking 1990). In fact, participants often discussed possible genetic mutations as if they were the disease itself, a common dilemma in preventive medicine (Aronowitz 2015).
Clearly, the value and veracity attributed to genetic information by our interviewees is excessive and sometimes inaccurate. In this they are not alone. Like other studies that report claims that “any information is valuable,” or that “all knowledge is good,” some interviewees expressed a desire for information qua information (Beskow et al. 2011; Facio et al. 2013).
To call these “false beliefs” that must be corrected through education, as some suggest, ignores the many exaggerated promises in popular media, academic medical center promotional materials, and health agency proclamations that undoubtedly shape public expectations. In fact, the tremendous value attributed to knowing genetic information is embedded in social institutions, in ways of living, and in how we understand health (Burke et al. 2015). Given this cultural grounding, people express enthusiasm for being offered, and learning, their own and their relatives’ genetic research results. In this context, sharing information at the expense of individual privacy is endorsed.
The bold promises of preemptive medicine obscure a dark side not voiced by our study participants: the possibility of over-diagnosis and over-treatment (Boenink and van der Burg 2010; Esserman, Thompson, and Reid 2013). Eclipsed are new ambiguities created in the quest for certainty, including years of living in a “pre-ill” state (Boenink and van der Burg 2010; Dumit 2012; Konrad 2005; Lock 1998; Press, Fishman, and Koenig 2000; Timmermans and Buchbinder 2013). Most participants had heard little of the impact genetic results can have on the family, or of how emotionally difficult informing one’s family of a genetic mutation can be (Arribas-Ayllon, Featherstone, and Atkinson 2011; Atkinson, Featherstone, and Gregory 2013). Studies showing the downside of genetic information call into question the cultural narratives that support the heightened hopes and exaggerated expectations attached to genomic information: Is earlier always better? Is knowledge always empowering? Does more knowledge inevitably lead to more certainty? Bringing these less obvious and less positive implications of genomic information to public light and debate could temper unrealistic expectations about the value of genomic information.
Limitations
Both components of our mixed methods study share the same limitations. The survey limitations are described in detail elsewhere (Radecki Breitkopf et al. 2015, 2018). As noted above, participants were not offered actual results, although they were selected from bona fide research participants who provided DNA for genetic research and are potential recipients of such results. Both samples were derived from a pancreatic cancer research biobank and family registry focused on a single disease and affiliated with an academic medical center known for leading-edge clinical care and research. The shared experience of pancreatic cancer, a rapidly fatal disease that is difficult to diagnose, dominated individual and family experience and often surfaced even when discussing less serious diseases. These powerful experiences with illness and death may have contributed to strong support for sharing information within the family, rather than protecting an abstract notion of privacy.
Individuals who participate in family research registries may value information, and the promise of research, more highly than others. In addition, the demands of participation in a family registry may have selected for higher functioning and more intact families, including those who value open communication.
Because the research team conducting interviews was identified with the institution providing cancer care, response candor may have been limited. Our use of the term “genetic research results” in our interview guides may have conveyed or reinforced an understanding of results as valuable, definitive, and true.
Finally, both samples were primarily white, educated, and insured, a population often invited into research, and hence offered genomic results. Our respondents’ optimism, expectations for control over health and life, and sense of the value of medical information may reflect the privilege of social and economic security. Our findings have the potential to contribute to genomic policy making, but require replication in other populations.
Conclusions
Results from a single study cannot form the foundation of broad-based policy change about if, when, and how researchers should offer a participant’s genetic research results to family members. But in spite of its limitations, our mixed methods study points the way to possible reform, providing important data about how probands and family members view the sharing of results.
Given our participants’ commitment to research, their embrace of genetics, and their proactive approach to health, we expected to find a full embrace of conventional research ethics practices based on respect for autonomy and personal privacy. Indeed, we did find strong, autonomy-based claims about individuals’ control over their own health and life, accompanied by a desire to choose what to know or not know. We also heard respect for the living probands’ autonomy and privacy.
But we also encountered a family ethics of disclosure based on shared genetics and focused on the welfare of family members. Many interviewees and respondents endorsed a familial approach to disclosure of genetic information—in which genetic information is thought to belong not only to the individual, but also to those who could share genes and thus share risk. We heard the view that within the family, blood relatives are entitled to information relevant to their health; because shared genes can affect health, relatives are entitled to information about those genes.
Current law and bioethics policies are grounded in a commitment to the privacy and confidentiality of each individual’s health information, but permit family access to the information under some circumstances. Almost all participants in our study stated clearly and consistently that privacy protection within the family was not a concern. Instead, most participants in this mixed-methods study voiced a strong moral responsibility to inform and be informed, a responsibility to share results in which the good of the family takes precedence over the welfare, privacy, and sovereignty of a single individual.
By contrast, sharing information outside the family circle did cause concern. Participants described a desire to draw the protective line of privacy between the family and those “outside” the family. To date, policy deliberations have paid too little attention to that divide. In a genomic era, such discussions are vital.
What often loomed larger than concern for individual privacy and autonomy was the primacy of the value of health and the significance of genetic information. Future health and life were deemed at stake for these participants—not just the health of an individual proband, but the health of future generations. Benefit to one’s own or a family member’s health was the most important criterion for distinguishing a morally sanctioned vs. an inappropriate disclosure of results. When genetic information is seen to have so much potential value for the family, withholding it—as keeping information private entails—was considered selfish and even unethical by many of our informants.
Our results illustrate the importance of an approach to return of results policy that considers not only the individual but also the family, as others have called for (Dheensa et al. 2015; Ho 2009; Koenig 2001; McGuire, Caulfield, and Cho 2008; Nelson 1997). Of course families differ; minority opinions expressed by study participants must be recognized and protected. There is an urgent need for research with more economically and culturally diverse populations, in order to bring to the fore alternative approaches to handling genomic information within the family.
Empirical bioethics research, such as our mixed-methods study, has the potential to inform policy. Ignoring the family constitutes a form of implicit or hidden policy in itself, limiting the options people are given and influencing the choices they can make (Bergner et al. 2014; Kaufman 2015). A robust understanding of how individuals and family members think and act in the face of rapidly expanding genomic information provides an essential background to policy makers charged with shaping return of results practices.
Acknowledgments
The authors thank the Mayo Clinic biobank participants and family members who graciously agreed to be interviewed and complete surveys. We also thank the RAPPORT pancreatic cancer advocacy group for helping with pilot design. We are grateful to the following for assistance with manuscript preparation and data analysis: Katherine Humeniuk, Krista Sigurdson, Jaennika Aniag, Lindsay Forbes, Matthew Norstad, and Jeremy Michelson.
Funding
Preparation of this article was supported by the National Cancer Institute (NCI) and National Human Genomic Research Institute (NHGRI) grant R01 CA154517 (G. Petersen, Koenig, Wolf, PIs). The PIs participated in the Clinical Sequencing Exploratory Research (CSER) Consortium supported by NHGRI and NCI. Additional support was provided by the NHGRI Center of Excellence in ELSI Research grant P20 HG007243 (Koenig, Somkin, PIs). The Mayo Clinic pancreatic cancer patient biobank and family research registry are supported by P50 CA102701 and R01 CA97075 (G. Petersen, PI). All views expressed are those of the authors and do not necessarily reflect the views of NIH or the CSER Consortium.
Footnotes
Supplemental data
The data-collection instruments for this article can be accessed at the University of Minnesota’s website for this research project. (https://consortium.umn.edu/research/disclosing-genomic-incidental-findings-cancer-biobank-elsi-experiment).
Conflicts of interest
The authors declare no financial relationships with any organizations that might have an interest in the submitted work in the previous three years and no other relationships or activities that could appear to have influenced the submitted work.
ETHICAL APPROVAL: This study was approved by the institutional review boards at the University of California, San Francisco, and the Mayo Clinic, Rochester, Minn.
While our research focused on the secondary findings discovered in biobanking, we found that participants do not make a clear distinction between primary and secondary research findings. Thus we include both types of findings in our analysis of offering results to family members.
Our analysis of the legal and regulatory privacy practices focuses on the United States. In the United Kingdom, family-oriented privacy, sharing, and consent protocols have been recommended in clinical genetic practice since 2011 (Lucassen and Hall 2012).
The HIPAA privacy law has several exceptions that allow a pathway for information sharing with family members. For example, HIPAA allows return of information to the physician of a family member who needs it for health purposes (Wolf et al. 2015). Some state laws also allow avenues for family member access.
See online supplemental materials for the Interview Guides (z.umn.edu/gifd1interviewguide).
Initially, the association of these genetic variants with pancreatic cancer risk was hypothesized but not yet known. Thus we first thought of the variants as incidental findings. In reality, the line between primary and secondary findings is hard to draw.
See online supplemental materials for the educational materials (z.umn.edu/gifd2edumats).
We chose the neutral, lay term of “unexpected” finding, rather than “secondary” or “incidental,” in order to avoid confusion.
We are using the term “offering” results, in contrast to “returning” results, to make clear that those who receive an offer are not obligated to receive the information proffered. We are also not referring to the distinction between passive vs. active disclosure by a researcher, i.e., the researcher’s obligations when a proband or family member requests information vs. the researcher’s obligations to proactively contact participants about research findings, a key distinction highlighted in our policy recommendations (Wolf et al. 2015).
See online supplemental materials for Code Book. (z.umn.edu/gifd3codebook)
The memos included a general overview and summary of the material in the code, a description of the range of responses, and important and illustrative quotes. Drafts were circulated and discussed among team members on weekly or bi-weekly conference calls and then finalized. When possible, a table of the verbatim responses to a question by each participant was prepared, some of which permitted analysis of frequencies.
See online supplemental materials for the Survey Instrument. (z.umn.edu/gifd4survey)
The survey was mailed to a total of 6,103 individuals; the analysis presented here excludes 2,792 controls because those controls were “healthy” individuals attending a general medical exam at Mayo Clinic. As noted above, probands and family members were selected independently: no effort was made to recruit complete families.
A “familial” case has other known family members with pancreatic cancer; a “sporadic” case has none. Often these categories are congruent with a registry or biobank designation. However, these categories are not stable; some start out labeled sporadic but later become familial as more is known. Also, we discovered that how probands and family members identified themselves did not necessarily coincide with the biobank designation. For example, some participants in the family registry did not conceive of their cancer as “familial.”
These questions derived initially from concerns raised by biobank and cancer registry investigators at the time, from the literature, and from the experience of the authors. They were further derived inductively through analysis of the study data, in part guided by the concept of “family ethics of disclosure.”
It should be noted, however, that many said that preventability or treatability of a condition did not matter and that genetic results should be offered nonetheless.
The discourse of “preemptive” medicine (averting disease) as one of the 4 “Ps” was put forth by former NIH Director Elias Zerhouni; he endorsed a translational research paradigm leading to a medical system that is “predictive, personalized, preemptive, and participatory” (Zerhouni 2007).
Some may disagree with this interpretation. Law, ethics, and policy do recognize the need to balance the privacy of the proband with the concerns of the family in some circumstances. Consider, for example, the debate since the early 1990s about the duty to warn family members. In this paper, we argue that privacy policies privileging the individual take on a form of cultural dominance because of the ubiquitous clinical presence of HIPAA privacy practices. The exceptions are much less well known and available.
Works Cited
- Allen NL, Karlson EW, Malspeis S, Lu B, Seidman CE, and Lehmann LS. 2014. “Biobank participants’ preferences for disclosure of genetic research results: Perspectives from the OurGenes, OurHealth, OurCommunity project.” Mayo Clinic Proceedings 89 (6):738–46. doi: 10.1016/j.mayocp.2014.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amendola LM, Horike-Pyne M, Trinidad SB, Fullerton SM, Evans BJ, Burke W, and Jarvik GP. 2015. “Patients’ choice for return of exome sequencing results to relatives in the event of their death.” Journal of Law, Medicine & Ethics 43 (3):476–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronowitz R 2015. Risky medicine: Our quest to cure fear and uncertainty. Chicago: University of Chicago Press. [Google Scholar]
- Arribas-Ayllon M, Featherstone K, and Atkinson P. 2011. “The practical ethics of genetic responsibility: Non-disclosure and the autonomy of affect.” Social Theory & Health 9 (1):3–23. [Google Scholar]
- Atkinson P, Featherstone K, and Gregory M. 2013. “Kinscapes, timescapes and genescapes: Families living with genetic risk.” Sociology of Health and Illness 35 (8):1227–41. doi: 10.1111/1467-9566.12034. [DOI] [PubMed] [Google Scholar]
- Benner PE 1994. Interpretive phenomenology: Embodiment, caring and ethics in health and illness. Thousand Oaks, CA: Sage Publications. [Google Scholar]
- Bennette CS, Trinidad SB, Fullerton SM, Patrick D, Amendola L, Burke W, Hisama FM, Jarvik GP, Regier DA, and Veenstra DL. 2013. “Return of incidental findings in genomic medicine: Measuring what patients value--development of an instrument to measure preferences for information from next-generation testing.” Genetics in Medicine 15 (11):873–81. doi: 10.1038/gim.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg JS, Khoury MJ, and Evans JP. 2011. “Deploying whole genome sequencing in clinical practice and public health: Meeting the challenge one bin at a time.” Genetics in Medicine 13 (6):499–504. doi: 10.1097/GIM.0b013e318220aaba. [DOI] [PubMed] [Google Scholar]
- Bergner AL, Bollinger J, Raraigh KS, Tichnell C, Murray B, Blout CL, Telegrafi AB, and James CA. 2014. “Informed consent for exome sequencing research in families with genetic disease: The emerging issue of incidental findings.” American Journal of Medical Genetics 164A (11):2745–52. doi: 10.1002/ajmg.a.36706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beskow LM, and Burke W. 2010. “Offering individual genetic research results: Context matters.” Science Translational Medicine 2 (38):38cm20–38cm20. doi: 10.1126/scitranslmed.3000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beskow LM, Namey EE, Cadigan RJ, Brazg T, Crouch J, Henderson GE, Michie M, Nelson DK, Tabor HK, and Wilfond BS. 2011. “Research participants’ perspectives on genotype-driven research recruitment.” Journal of Empirical Research on Human Research Ethics 6 (4):3–20. doi: 10.1525/jer.2011.6.4.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boenink M, and van der Burg S. 2010. “Informed decision making about predictive DNA tests: Arguments for more public visibility of personal deliberations about the good life.” Medicine, Health Care, and Philosophy 13 (2):127–38. doi: 10.1007/s11019-009-9227-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger JM, Scott J, Dvoskin R, and Kaufman D. 2012. “Public preferences regarding the return of individual genetic research results: Findings from a qualitative focus group study.” Genetics in Medicine 14 (4):451–7. doi: 10.1038/gim.2011.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredenoord AL, and van Delden JJM. 2012. “Disclosing individual genetic research results to deceased participants’ relatives by means of a qualified disclosure policy.” American Journal of Bioethics 12 (10):10–12. doi: 10.1080/15265161.2012.699145. [DOI] [PubMed] [Google Scholar]
- Burke W, Appelbaum P, Dame L, Marshall P, Press N, Pyeritz R, Sharp R, and Juengst E. 2015. “The translational potential of research on the ethical, legal, and social implications of genomics.” Genetics in Medicine 17 (1):12–20. doi: 10.1038/gim.2014.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffee KG, Oberg AL, McWilliams RR, Majithia N, Allen BA, Kidd J, Singh N, Hartman AR, Wenstrup RJ, and Petersen GM. 2017. “Prevalence of germ-line mutations in cancer genes among pancreatic cancer patients with a positive family history.” Genetics in Medicine. doi: 10.1038/gim.2017.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffee KG, Oberg AL, McWilliams RR, Majithia N, Allen BA, Kidd J, Singh N, Hartman AR, Wenstrup RJ, and Petersen GM. 2018. “Prevalence of germ-line mutations in cancer genes among pancreatic cancer patients with a positive family history.” Genetics in Medicine 20 (1):119–127. doi: 10.1038/gim.2017.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan B, Facio FM, Eidem H, Hull SC, Biesecker LG, and Berkman BE. 2012. “Genomic inheritances: Disclosing individual research results from whole-exome sequencing to deceased participants’ relatives.” American Journal of Bioethics 12 (10):1–8. doi: 10.1080/15265161.2012.699138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A, Mamo L, Fosket RJ, Fishman JR, and Shim JK. 2010. Biomedicalization : Technoscience, health, and illness in the U.S. Durham, NC: Duke University Press. [Google Scholar]
- Clayton EW, and Kelly SE. 2013. “Let us ask better questions.” Genetics in Medicine 15 (11):871–872. doi: 10.1038/gim.2013.68. [DOI] [PubMed] [Google Scholar]
- Couzin-Frankel J 2014. “Divulging DNA secrets of dead stirs debate.” Science 343 (6169):356–357. doi: 10.1126/science.343.6169.356. [DOI] [PubMed] [Google Scholar]
- Crawford R 1980. “Healthism and the medicalization of everyday life.” International Journal of Health Services 10 (3):365–88. [DOI] [PubMed] [Google Scholar]
- Dheensa S, Fenwick A, and Lucassen A. 2016. “‘Is this knowledge mine and nobody else’s? I don’t feel that.’ Patient views about consent, confidentiality and information-sharing in genetic medicine.” Journal of Medical Ethics 42 (3):174–79. doi: 10.1136/medethics-2015-102781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dheensa S, Fenwick A, Shkedi-Rafid S, Crawford G, and Lucassen A. 2015. “Health-care professionals’ responsibility to patients’ relatives in genetic medicine: A systematic review and synthesis of empirical research.” Genetics in Medicine. doi: 10.1038/gim.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumit J 2012. Drugs for life : How pharmaceutical companies define our health. Durham, N.C.: Duke University Press. [Google Scholar]
- Esserman LJ, Thompson IM Jr., and Reid B. 2013. “Overdiagnosis and overtreatment in cancer: An opportunity for improvement.” Journal of the American Medical Association 310 (8):797–98. doi: 10.1001/jama.2013.108415. [DOI] [PubMed] [Google Scholar]
- Facio FM, Eidem H, Fisher T, Brooks S, Linn A, Kaphingst KA, Biesecker LG, and Biesecker BB. 2013. “Intentions to receive individual results from whole-genome sequencing among participants in the ClinSeq study.” European Journal of Human Genetics 21 (3):261–65. doi: 10.1038/ejhg.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez CV, Bouffet E, Malkin D, Jabado N, O’Connell C, Avard D, Knoppers BM, Ferguson M, Boycott KM, Sorensen PH, Orr AC, Robitaille JM, and McMaster CR. 2014. “Attitudes of parents toward the return of targeted and incidental genomic research findings in children.” Genetics in Medicine 16 (8):633–40. doi: 10.1038/gim.2013.201. [DOI] [PubMed] [Google Scholar]
- Geertz C 1973. The interpretation of cultures: Selected essays. London: Fontana. [Google Scholar]
- Gilbar R, and Miola J. 2015. “One size fits all? On patient autonomy, medical decision-making, and the impact of culture.” Medical Law Review 23 (3):375–99. doi: 10.1093/medlaw/fwu032. [DOI] [PubMed] [Google Scholar]
- Graves KD, Sinicrope PS, McCormick JB, Zhou Y, Vadaparampil ST, and Lindor NM. 2015. “Public perceptions of disease severity but not actionability correlate with interest in receiving genomic results: nonalignment with current trends in practice.” Public Health Genomics 18 (3):173–83. doi: 10.1159/000375479. [DOI] [PubMed] [Google Scholar]
- Green RC, Berg JS, Grody WW, Kalia SS, Korf BR, Martin CL, McGuire AL, Nussbaum RL, O’Daniel JM, Ormond KE, Rehm HL, Watson MS, Williams MS, and Biesecker LG. 2013. “ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing.” Genetics in Medicine 15 (7):565–74. doi: 10.1038/gim.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacking I 1990. The Taming of Chance. Cambridge: Cambridge University Press. [Google Scholar]
- Hallowell N, Alsop K, Gleeson M, Crook A, Plunkett L, Bowtell D, Mitchell G, Group Australian Ovarian Cancer Study, and Young MA. 2013. “The responses of research participants and their next of kin to receiving feedback of genetic test results following participation in the Australian Ovarian Cancer Study.” Genetics in Medicine 15 (6):458–65. doi: 10.1038/gim.2012.154. [DOI] [PubMed] [Google Scholar]
- Hallowell N, Foster C, Eeles R, Ardern-Jones A, Murday V, and Watson M. 2003. “Balancing autonomy and responsibility: The ethics of generating and disclosing genetic information.” Journal of Medical Ethics 29 (2):74–79; discussion 80–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho A 2009. “‘They just don’t get it!’ When family disagrees with expert opinion.” Journal of Medical Ethics 35 (8):497–501. doi: 10.1136/jme.2008.028555. [DOI] [PubMed] [Google Scholar]
- Husedzinovic A, Ose D, Schickhardt C, Frohling S, and Winkler EC. 2015. “Stakeholders’ perspectives on biobank-based genomic research: Systematic review of the literature.” European Journal of Human Genetics 23 (12):1607–14. doi: 10.1038/ejhg.2015.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvik GP, Amendola LM, Berg JS, Brothers K, Clayton EW, Chung W, Evans BJ, Evans JP, Fullerton SM, Gallego CJ, Garrison NA, Gray SW, Holm IA, Kullo IJ, Lehmann LS, McCarty C, Prows CA, Rehm HL, Sharp RR, Salama J, Sanderson S, Van Driest SL, Williams MS, Wolf SM, Wolf WA, eMerge Act-R. O. R. Committee, CERC Committee, CSER Act-ROR Working Group, and Burke W. 2014. “Return of genomic results to research participants: The floor, the ceiling, and the choices in between.” American Journal of Human Genetics 94 (6):818–26. doi: 10.1016/j.ajhg.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns AL, McKay SH, Humphris JL, Pinese M, Chantrill LA, Mead RS, Tucker K, Andrews L, Goodwin A, Leonard C, High HA, Nones K, Patch AM, Merrett ND, Pavlakis N, Kassahn KS, Samra JS, Miller DK, Chang DK, Pajic M, Australian Pancreatic Cancer Genome Initiative, Pearson JV, Grimmond SM, Waddell N, Zeps N, Gill AJ, and Biankin AV. 2017. “Lost in translation: Returning germline genetic results in genome-scale cancer research.” Genome Medicine 9 (1):41. doi: 10.1186/s13073-017-0430-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns AL, Miller DK, Simpson SH, Gill AJ, Kassahn KS, Humphris JL, Samra JS, Tucker K, Andrews L, Chang DK, Waddell N, Pajic M, Pearson JV, Grimmond SM, Biankin AV, and Zeps N. 2014. “Returning individual research results for genome sequences of pancreatic cancer.” Genome Medicine 6 (5):42. doi: 10.1186/gm558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juengst ET, Flatt MA, and Settersten RA Jr. 2012. “Personalized genomic medicine and the rhetoric of empowerment.” Hastings Center Report 42 (5):34–40. doi: 10.1002/hast.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman SR 2015. Ordinary medicine: Extraordinary treatments, longer lives, and where to draw the line. Durham, NC: Duke University Press. [Google Scholar]
- Kleinman A 1997. Writing at the margin: Discourse between anthropology and medicine. Berkeley: University of California Press. [Google Scholar]
- Knoppers BM, Deschênes M, Ma’n HZ, and Tassé AM. 2013. “Population studies: Return of research results and incidental findings policy statement.” European Journal of Human Genetics 21 (3):245–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig BA 2001. “Why not grant primacy to the family?” American Journal of Bioethics 1 (3):33–34. doi: 10.1162/152651601750417937. [DOI] [PubMed] [Google Scholar]
- Konrad M 2003. “From secrets of life to the life of secrets: Tracing genetic knowledge as genealogical ethics in biomedical britain.” Journal of the Royal Anthropological Institute 9 (2):339–58. doi: 10.1111/1467-9655.00153. [DOI] [Google Scholar]
- Konrad M 2005. Narrating the new predictive genetics : Ethics, ethnography, and science. Cambridge: Cambridge University Press. [Google Scholar]
- Lock M 1998. “Breast cancer: Reading the omens.” Anthropology Today 14 (4):7–16. doi: 10.2307/2783351. [DOI] [Google Scholar]
- Lucassen A, and Hall A. 2012. “Consent and confidentiality in clinical genetic practice: Guidance on genetic testing and sharing genetic information.” Clinical Medicine 12 (1):5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupton D 1995. The imperative of health: Public health and the regulated body. London: SAGE Publications. [Google Scholar]
- Mackley MP, Fletcher B, Parker M, Watkins H, and Ormondroyd E. 2017. “Stakeholder views on secondary findings in whole-genome and whole-exome sequencing: A systematic review of quantitative and qualitative studies.” Genetics in Medicine 19 (3):283–93. doi: 10.1038/gim.2016.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire AL, Caulfield T, and Cho MK. 2008. “Research ethics and the challenge of whole-genome sequencing.” Nature Reviews Genetics 9 (2):152–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliams RR, Bamlet WR, de Andrade M, Rider DN, Cunningham JM, and Petersen GM. 2009. “Nucleotide excision repair pathway polymorphisms and pancreatic cancer risk: evidence for role of MMS19L” Cancer Epidemiology, Biomarkers & Prevention 18 (4):1295–302. doi: 10.1158/1055-9965.epi-08-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliams RR, Petersen GM, Rabe KG, Holtegaard LM, Lynch PJ, Bishop MD, and Highsmith WE Jr. 2010. “Cystic fibrosis transmembrane conductance regulator (CFTR) gene mutations and risk for pancreatic adenocarcinoma.” Cancer 116 (1):203–9. doi: 10.1002/cncr.24697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulenkamp TM, Gevers SK, Bovenberg JA, Koppelman GH, van Hylckama Vlieg A, and Smets EM. 2010. “Communication of biobanks’ research results: What do (potential) participants want?” American Journal of Medical Genetics 152A (10):2482–92. doi: 10.1002/ajmg.a.33617. [DOI] [PubMed] [Google Scholar]
- Murphy J, Scott J, Kaufman D, Geller G, LeRoy L, and Hudson K. 2008. “Public expectations for return of results from large-cohort genetic research.” American Journal of Bioethics 8 (11):36–43. doi: 10.1080/15265160802513093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelkin D, and S Lindee M. 1995. The DNA mystique: The gene as a cultural icon. New York: W. H. Freeman. [Google Scholar]
- Nelson AM 1997. Improving patient satisfaction now: How to earn patient and payer loyalty. Gaithersburg, MD: Aspen Publishers. [Google Scholar]
- Parker LS 2012. “Returning individual research results: What role should people’s preferences play?” Minnesota Journal of Law, Science & Technology 13 (2):449–84. [Google Scholar]
- Petersen GM, de Andrade M, Goggins M, Hruban RH, Bondy M, Korczak JF, Gallinger S, Lynch HT, Syngal S, Rabe KG, Seminara D, and Klein AP. 2006. “Pancreatic cancer genetic epidemiology consortium.” Cancer, Epidemiology, Biomarkers & Prevention 15 (4):704–10. doi: 10.1158/1055-9965.EPI-05-0734. [DOI] [PubMed] [Google Scholar]
- Ploug T, and Holm S. 2017. “Clinical genome sequencing and population preferences for information about ‘incidental’ findings: From medically actionable genes (MAGs) to patient actionable genes (PAGs).” PLoS One 12 (7):e0179935. doi: 10.1371/journal.pone.0179935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presidential Commission for the Study of Bioethical Issues. 2013. Anticipate and communicate: Ethical management of incidental and secondary findings in the clinical, research, and direct-to-consumer contexts. Washington, DC. [DOI] [PubMed] [Google Scholar]
- Press N, Fishman JR, and Koenig BA. 2000. “Collective fear, individualized risk: The social and cultural context of genetic testing for breast cancer.” Nursing Ethics 7 (3):237–49. doi: 10.1177/096973300000700306. [DOI] [PubMed] [Google Scholar]
- Radecki Breitkopf C, Petersen GM, Wolf SM, Chaffee KG, Robinson ME, Gordon DR, Lindor NM, and Koenig BA. 2015. “Preferences regarding return of genomic results to relatives of research participants, including after participant death: empirical results from a cancer biobank.” Journal of Law, Medicine & Ethics 43 (3):464–75. doi: 10.1111/jlme.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radecki Breitkopf C, Petersen GM, Wolf SM, Chaffee KG, Robinson ME, Gordon DR, Lindor NM, and Koenig BA. 2018. “Attitudes toward return of genetic research results to relatives, including after death: Comparison of cancer probands, blood relatives, and spouse/partners.” Journal of Empirical Research on Human Research Ethics 13 (3):295–304. doi: 10.1177/1556264618769165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo K, Yu J, Suenaga M, Fesharakizadeh S, Cho C, Macgregor-Das A, Siddiqui A, Witmer PD, Tamura K, Song TJ, Navarro Almario JA, Brant A, Borges M, Ford M, Barkley T, He J, Weiss MJ, Wolfgang CL, Roberts NJ, Hruban RH, Klein AP, and Goggins M. 2017. “Deleterious germline mutations in patients with apparently sporadic pancreatic adenocarcinoma.” Journal of Clinical Oncology:JCO2017723502. doi: 10.1200/JCO.2017.72.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shkedi-Rafid S, Dheensa S, Crawford G, Fenwick A, and Lucassen A. 2014. “Defining and managing incidental findings in genetic and genomic practice.” J Med Genet 51 (11):715–23. doi: 10.1136/jmedgenet-2014-102435. [DOI] [PubMed] [Google Scholar]
- Strauss AL, and Corbin JM. 1990. Basics of qualitative research: Grounded theory procedures and techniques. Newbury Park, CA: Sage Publications. [Google Scholar]
- Timmermans S, and Buchbinder M. 2013. Saving babies? The consequences of newborn genetic screening, fieldwork encounters and discoveries. Chicago: University of Chicago Press. [Google Scholar]
- Weaver M 2016. “The double helix: Applying an ethic of care to the duty to warn genetic relatives of genetic information.” Bioethics 30 (3):181–87. doi: 10.1111/bioe.12176. [DOI] [PubMed] [Google Scholar]
- Wertz DC, and Fletcher JC. 1991. “Privacy and disclosure in medical genetics examined in an ethics of care.” Bioethics 5 (3):212–32. [DOI] [PubMed] [Google Scholar]
- Wolf SM, Branum R, Koenig BA, Petersen GM, Berry SA, Beskow LM, Daly MB, Fernandez CV, Green RC, LeRoy BS, Lindor NM, O’Rourke PP, Radecki Breitkopf C, Rothstein MA, Van Ness B, and Wilfond BS. 2015. “Returning a research participant’s genomic results to relatives: Analysis and recommendations.” Journal of Law, Medicine & Ethics 43 (3):440–63. doi: 10.1111/jlme.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SM, Crock BN, Van Ness B, Lawrenz F, Kahn JP, Beskow LM, Cho MK, Christman MF, Green RC, Hall R, Illes J, Keane M, Knoppers BM, Koenig BA, Kohane IS, Leroy B, Maschke KJ, McGeveran W, Ossorio P, Parker LS, Petersen GM, Richardson HS, Scott JA, Terry SF, Wilfond BS, and Wolf WA. 2012. “Managing incidental findings and research results in genomic research involving biobanks and archived data sets.” Genetics in Medicine 14 (4):361–84. doi: 10.1038/gim.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SM, Koenig BA, and Petersen GM, eds. 2015. “Symposium: Should we offer genomic research results to a participant’s family, including after the participant’s death?” Journal of Law, Medicine & Ethics 43 (3):437–666. [Google Scholar]
- Wolf SM, Lawrenz FP, Nelson CA, Kahn JP, Cho MK, Wright Clayton E, Fletcher JG, Georgieff MK, Hammerschmidt D, and Hudson K. 2008. “Managing incidental findings in human subjects research: Analysis and recommendations.” Journal of Law, Medicine & Ethics 36 (2):219–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerhouni EA 2007. “Translational research: Moving discovery to practice.” Clinical Pharmacology & Therapeutics 81 (1):126–28. doi: 10.1038/sj.clpt.6100029. [DOI] [PubMed] [Google Scholar]
- Zhen DB, Rabe KG, Gallinger S, Syngal S, Schwartz AG, Goggins MG, Hruban RH, Cote ML, McWilliams RR, Roberts NJ, Cannon-Albright LA, Li D, Moyes K, Wenstrup RJ, Hartman AR, Seminara D, Klein AP, and Petersen GM. 2015. “BRCA1, BRCA2, PALB2, and CDKN2A mutations in familial pancreatic cancer: A PACGENE study.” Genetics in Medicine 17 (7):569–77. doi: 10.1038/gim.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]