Abstract
Background:
The emergence of vancomycin-resistant Staphylococcus aureus (VRSA) poses significant challenges for antibiotic therapy. We characterized the epidemiology of methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE) co-colonization that may facilitate resistance transfer and VRSA emergence among nursing facility (NF) patients.
Methods:
We cultured newly admitted patient hands, nares, oropharynx, groin, perianal region, plus wounds and device insertion sites if applicable upon enrollment, at day 14, day 30, and monthly thereafter for up to 6 months. Demographic, comorbidity, and antimicrobial use data was collected. Functional status was assessed at each visit using the Lawton & Brody Physical Self Maintenance Scale. Multinomial logistic regression was performed using a generalized linear mixed effect model to determine factors predictive of co-colonization.
Results:
508 patients were enrolled with an average follow-up time of 28.5 days. Prevalence of MRSA/VRE cocolonization, MRSA alone, and VRE alone were 8.7%, 8.9%, and 23.4% respectively. Independent predictors of co-colonization included indwelling device use [OR=5.5 (2.2-13.7)], antibiotic use within the previous 30 days [OR=2.5 (1.4-4.2)], diabetes [OR=1.9 (1.0-3.8)], and the presence of open wounds [OR=1.9 (1.0-3.6)].
Conclusions:
High rates of VRE are driving co-colonization with MRSA in these nursing facilities. Indwelling device use, recent antibiotic use, diabetes, and open wounds predicted patient co-colonization.
Keywords: MRSA, VRE, Nursing Homes, Infection Control
Introduction
Infections caused by methicillin-resistant Staphylococcus aureus (MRSA) are a significant cause of morbidity and mortality in healthcare settings.1, 2 Due to their inherent resistance to beta-lactams and oftentimes additional resistance to other classes of antibiotics including macrolides, fluoroquinolones, and aminoglycosides, therapeutic options are limited.3 Traditionally, glycopeptides such as vancomycin have been the antibiotic treatment of choice for MRSA infections, but in recent years the emergence of glycopeptide resistance has been increasing resulting in treatment failure and worse clinical outcomes.3-5 Importantly, vancomycin-resistant Staphylococcus aureus (VRSA) strains (MIC ≥16μg/mL) have been discovered with 14 cases reported since 2002 in the United States.6, 7 While the emergence of VRSA has been slow, the continued development of glycopeptide resistance among MRSA is concerning and presents unique treatment challenges as infections with these organisms require utilization of alternative, and oftentimes suboptimal, treatment regimens.3
Studies have demonstrated that VRSA results when MRSA acquires a vanA gene through genetic conjugation with vancomycin-resistant Enterococci (VRE) that impart vancomycin resistance via alteration of the peptidoglycan structure of the cell wall.8-10 In the majority of VRSA cases, VR E. faecalis has been the vanA donor vs other VRE species, and in order to facilitate conjugation, both bacterial species must be in close proximity to each other by co-colonizing a single site of a patient’s body.9,10 While patterns of MRSA and VRE co-colonization have been studied in hospitalized patients,9, 11-13 other healthcare settings may present unique environments for the dissemination of resistance to occur. Specifically, nursing facilities (NFs) are known reservoirs for multi-drug resistant organisms (MDROs) with an estimated one-third of all NF patients being colonized with one or more MDROs.3, 14 Patients are admitted to these facilities for rehabilitation following discharge from acute-care hospitals, and have high rates of bacterial colonization due to several factors including impaired functional status, widespread antibiotic use, and frequent long term use of indwelling devices such as urinary catheters and feeding tubes.15-19 One study in 2011 analyzed colonization patterns of MRSA and VRE among NF patients who had indwelling devices and identified decreased functional status and the presence of wounds to be significant risk factors for MRSA and VRE co-colonization.20 While an important first step, it is difficult to generalize these findings as those with indwelling devices represent a specific high risk population that make up a fraction of all NF patients.21-23
Given the possibility of resistance transfer between MRSA and VRE, our primary objective was to investigate the epidemiology of MRSA and VRE co-colonization among a predominantly short-stay, post-acute NF population regardless of indwelling device use. Specifically, we were interested in defining risk factors for MRSA and VRE co-colonization that may lead to better identification of high-risk patients and guide the implementation of targeted intervention practices. We hypothesized that decreased functional status would predict co-colonization in this population.
Methods
Study Population and Design
This study analyzed microbial culture data collected as part of a larger prospective cohort study, which aimed to identify patterns of MDRO colonization and dissemination between newly admitted NF patients, healthcare workers, and the environment.24 The parent study enrolled newly admitted patients from 6 NFs in Southeast Michigan between November 2013 and May 2016 and was approved by the University of Michigan Institutional Review Board. For this analysis, we considered patients who were enrolled into the study within 7 days of arriving at the facility. Informed consent was obtained from all participants or the durable power of attorney. Patients were followed for a maximum of 180 days or until discharge from the facility, death, or requesting withdrawal. Patients were excluded from the study if they did not provide informed consent, did not speak English, or were receiving end-of-life care.
Clinical Data Collection
To determine bacterial colonization status, patient cultures were obtained using Culturette® swabs at multiple anatomic sites including the nares, oropharynx, groin, perianal area, and hands at each visit. Cultures of open wounds and indwelling device insertion sites were also collected if accessible. Indwelling devices included suprapubic catheters and enteral feeding tubes. Samples were collected upon enrollment, 14 days following enrollment, and every 30 days thereafter for up to 180 days.
Demographic and relevant clinical information including gender, age, indwelling device use, recent antibiotic usage, the presence of open wounds, and underlying comorbidities were obtained through patient chart review for inclusion in risk factor analysis. Comorbidities were assessed at baseline using the Charlson Comorbidity Index (CCI) with higher scores indicating increased comorbidity.25 Wounds with any discharge noted, vascular ulcers, diabetic ulcers, and pressure ulcers greater than stage 1 were all considered as ‘open wounds’. Functional status was assessed for each patient at baseline and each follow-up visit using the Lawton and Brody Physical Self Maintenance Scale (PSMS) and given a score ranging from 6-30 with increasing severity of functional decline.26 For patients with ≥30 days of follow-up, data were also collected on repeat hospitalizations since admission to their facility.
Microbiologic Methods
Swab samples were streaked onto tryptic soy agar containing 5% sheep blood, mannitol salt agar, and bile esculin agar containing 6μg/mL vancomycin, and incubated at 35°C for 24 hours. Patient hand swabs were first placed in brain heart infusion broth and incubated at 35°C overnight before being streaked onto plates. Bright yellow colonies grown on mannitol salt agar were isolated and S. aureus identification was confirmed by positive catalase and coagulase (Staphaurex, Remel, Lenexa, KS) tests. Methicillin resistance was tested using cefoxitin disk diffusion. Colonies causing a black coloration on bile esculin-vancomycin plates were isolated and VRE identification was confirmed by a positive pyrrolidonyl arylamidase test. VRE isolate speciation was achieved by following a modified PCR assay protocol developed and validated by Tan TY et al.27
Colonization Definitions
For analysis of anatomic-site level incidence, co-colonization was defined as the isolation of both MRSA and VRE from a single anatomic site on the same visit. For all other analyses, patients were considered co-colonized with MRSA and VRE if they had positive cultures for both species at any combination of anatomic sites on the same visit. This definition was previously used by Flannery et al when investigating MRSA and VRE co-colonization among long-stay NF populations with indwelling devices,20 and by requiring same day isolation of organisms it is more conservative than those used in other studies investigating co-colonization in acute care settings.9, 11-13, 20
Statistical Analysis
Significant differences between baseline characteristics of patients who were co-colonized at some point during the study and those who were never co-colonized were identified using χ2 and two-sample t-tests where appropriate. The prevalence of MRSA and VRE co-colonization, MRSA colonization alone, and VRE colonization alone was calculated as the percentage of total visits during which patients were positive for colonization to reflect the dynamic nature of bacterial colonization over time. To calculate overall incidence rates of colonization, dates of bacterial acquisition were set at the midpoint between the date of the most recent negative swab culture, and the date of the first positive swab culture. For example, if a patient was not colonized on day 30 and co-colonized on day 60, then the date of bacterial acquisition was set at day 45. Calculation of anatomic-site specific incidence rates were conducted using only patients with ≥1 follow-up visit and no missing anatomic site swabs to more accurately reflect true incidence rates. Because of limited access to patient wounds and indwelling device insertion points for culturing, incidence rates were not calculated at these sites. Cox regression analysis was performed with a primary endpoint of co-colonization to identify patient risk factors for decreased time to co-colonization with MRSA and VRE. Additionally, we used a generalized linear mixed effect model to determine the effect of clinical and demographic characteristics on colonization at any point via multinomial logistic regression. Colonization at any point was defined as: no-colonization, MRSA only, VRE only, and co-colonization. This model allowed for adjustments based on random error and potential longitudinal correlation effects within individual patients as their colonization statuses changed from one visit to the next. All statistical analyses were performed using STATA 13 (Stata Corporation, College Station, TX).
Results
Study Group Characteristics
In total there were 1384 eligible newly admitted NF patients across all 6 facilities of which 508 (36.7%) were enrolled in this study within 7 days of their admission. The main reasons for non-enrollment were patient refusal (n=458, 33.1%) or family or legal guardian refusal (n=172, 12.4%). An average of 85 patients were enrolled per facility (range 43-128), and were followed for a total of 1266 visits. The average length of stay was 28.5 days. The number of cultures obtained from each anatomic site varied due to differences in site accessibility among patients resulting in a total of 1103 hand cultures, 1096 nares cultures, 1062 oropharynx cultures, 1102 groin cultures, and 727 perianal cultures. 13.2% of patients were co-colonized with both MRSA and VRE at any point during the study. Co-colonized patients were more likely to have used antibiotics in the previous 30 days, had increased severity of underlying comorbidities, decreased functional status, and were more likely to have open wounds upon enrollment compared to patients who were not colonized at all or colonized only with MRSA or VRE alone (Table 1).
Table 1.
Baseline Demographic and Clinical Characteristics for 508 Nursing Facility Patients
| Characteristic | Overall n=508 |
Co-colonized n=38 |
Not Co-colonized n=470 |
P-value |
|---|---|---|---|---|
| Mean ± SD | ||||
| Follow-up Days | 28.5 (44.2) | 40.9 (51.0) | 27.5 (43.5) | 0.03 |
| Years of Age | 73.8 (12.1) | 74.5 (12.4) | 73.8 (12.1) | 0.36 |
| CCI | 2.6 (2.1) | 3.5 (2.0) | 2.5 (2.1) | 0.003 |
| PSMS | 14.0 (4.4) | 15.4 (4.7) | 13.9 (4.3) | 0.02 |
| No. (%) of Patients | ||||
| Male | 219 (43.1) | 19 (50) | 200 (42.6) | 0.37 |
| Diabetes | 204 (40.2) | 19 (50) | 185 (39.4) | 0.20 |
| Recent Antibiotic Use* | 305 (61.7) | 29 (78.4) | 276 (60.4) | 0.03 |
| Device Use at Baseline | 48 (9.5) | 6 (15.8) | 42 (8.9) | 0.17 |
| Open Wound at Baseline** | 84 (17.8) | 10 (31.3) | 74 (16.9) | 0.04 |
Abbreviations: CCI, Charlson Comorbidity Index score (proportional to severity of underlying comorbidities); PSMS, Lawton and Brody Physical Self Maintenance Score (6-30 with increasing severity of functional decline)
n=494
n=471
MRSA and VRE Prevalence and Incidence
At baseline 7.5% of patients were co-colonized with MRSA and VRE, 8.1% were colonized with MRSA alone, and 26.2% were colonized with VRE alone. In total, 414 MRSA isolates and 686 VRE isolates were obtained. Of the VRE isolates, 306 (44.6%) were E. faecium and 267 (38.9%) were E. faecalis. Overall prevalence of MRSA and VRE co-colonization was 8.7%, 8.9% for MRSA alone, and 23.4% for VRE alone. Incidence rates were calculated to further describe new colonization acquisition in patients who were not colonized upon enrollment (Figure 1). Overall, co-colonization occurred at a rate of 3.0 per 1000 patient-days (95% CI: 2.1-4.3), MRSA colonization alone at a rate of 4.0 per 1000 patient-days (95% CI: 2.9-5.5), and VRE colonization alone at a rate of 9.0 per 1000 patient-days (95% CI: 6.9-11.6). Univariate cox regression analysis demonstrated that indwelling device use, recent antibiotics use within the previous 30 days, the presence of open wounds, and being previously colonized with either MRSA or VRE were all risk factors for becoming co-colonized during patient stays (Table 2). In the multivariate analysis, however, only indwelling device use and being previously colonized with MRSA or VRE were significant predictors of becoming co-colonized with hazard ratios of 4.2, 17.0, and 6.3 respectively (95% CI: 1.4-12.7, 5.3-54.6, 2.2-18.2) (Table 2). Co-colonization was most frequent on patient hands (Figure 1).
Figure 1.
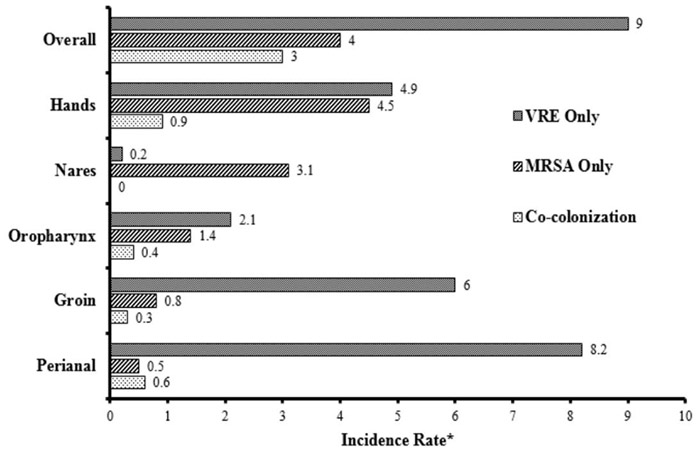
Incidence of MRSA and VRE Colonization
*Incident rates reported per 1000 patient-days
Table 2.
Risk Factors for time to New Acquisition of MRSA/VRE Co-colonization
| Univariate Analysis |
Multivariate Analysis |
|||||
|---|---|---|---|---|---|---|
| Characteristic | HR | 95% CI | P-Value | HR | 95% CI | P-Value |
| Age | 1.0 | .97-1.0 | .97 | 1.0 | .97-1.0 | .75 |
| PSMS | 1.0 | .93-1.1 | .92 | .96 | .88-1.1 | .4 |
| CCI | .95 | .79-1.1 | .58 | .93 | .73-1.2 | .55 |
| Device Use | 3.3 | 1.4-7.8 | .006 | 4.2 | 1.4-12.7 | .01 |
| Antibiotic Use | 2.1 | 1.0-4.4 | .05 | 1.0 | .42-2.6 | .94 |
| Diabetes | .82 | .38-1.8 | .61 | 1.1 | .42-2.8 | .86 |
| Open Wounds | 3.0 | 1.4-6.6 | .005 | 2.2 | .95-5.1 | .07 |
| Hospitalization | 1.8 | .62-5.2 | .28 | 1.3 | .43-4.1 | .62 |
| Previous MRSA* | 6.3 | 2.8-14.1 | <.001 | 17.0 | 5.3-54.6 | <.001 |
| Previous VRE** | 3.0 | 1.4-6.2 | .004 | 6.3 | 2.2-18.2 | .001 |
Abbreviations: PSMS, Lawton and Brody Physical Self Maintenance Score (6-30 with increasing severity of functional decline); CCI, Charlson Comorbidity Index (proportional to severity of underlying comorbidities)
Colonized with MRSA alone at previous visit
Colonized with VRE alone at previous visit
Association of Colonization Status with Demographic and Clinical Characteristics
Multivariable analysis using a generalized linear mixed model found indwelling device use, recent antibiotic use within the previous 30 days, having diabetes, and the presence of open wounds to be significant independent predictors of patient co-colonization with MRSA and VRE (Table 3). Patients with indwelling devices in place were 5.5 times as likely to be co-colonized compared to those without devices (95% CI: 2.2-13.7) Those with recent antibiotics use were 2.5 times as likely to be co-colonized (95% CI: 1.4-4.2). Patients with diabetes were 1.9 times as likely to be co-colonized (95% CI: 1.0-3.8). Those with open wounds were 1.9 times as likely to be co-colonized (95% CI: 1.0-3.6).
Table 3.
Effect of Covariates on Colonization from Multinomial Logistic Regression (n=508 Patients)
| Co-Colonization versus no colonization | MRSA Only versus no colonization | VRE Only versus no colonization | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | OR | 95% CI | P-Value | OR | 95% CI | P-Value | OR | 95% CI | P-Value | |||
| Age | 1.0 | .98-1.0 | .88 | 1.0 | .97-1.0 | .84 | 1.0 | .96-1.0 | .82 | |||
| Follow-up Days | 1.0 | .99-1.0 | .61 | 1.0 | .99-1.0 | .54 | .99 | .99-1.0 | .01 | |||
| PSMS | 1.0 | .91-1.1 | .27 | 1.1 | .99-1.1 | .10 | 1.0 | .96-1.1 | .64 | |||
| CCI | 1.1 | .94-1.3 | .21 | 1.1 | .90-1.2 | .50 | 1.2 | 1.1-1.4 | .007 | |||
| Device | 5.5 | 2.2-13.7 | <.001 | 1.6 | .56-4.4 | .39 | 2.1 | .90-5.0 | .09 | |||
| Antibiotic Use | 2.5 | 1.4-4.2 | .001 | .73 | .41-1.3 | .28 | 3.3 | 2.2-5.1 | ≤.001 | |||
| Diabetes | 1.9 | 1.0-3.8 | .05 | 1.1 | .58-2.3 | .70 | 1.9 | 1.0-3.3 | .04 | |||
| Open Wounds | 1.9 | 1.0-3.6 | .04 | 2.0 | 1.1-3.8 | .03 | 1.5 | .89-2.6 | .13 | |||
| Hospitalized | .81 | .46-1.4 | .45 | 1.1 | .62-1.9 | .78 | .83 | .55-1.3 | .39 | |||
Abbreviations: PSMS, Lawton and Brody Physical Self Maintenance Score (6-30 with increasing severity of functional decline); CCI, Charlson Comorbidity Index (proportional to severity of underlying comorbidities)
Discussion
In this prospective cohort study we conducted active surveillance of 508 newly admitted patients at 6 different NFs for the presence of MRSA and VRE co-colonization in order to further characterize the epidemiology of these organisms in this unique setting that is a well-recognized reservoir for MDROs. Patients enrolled in our study consisted primarily of a post-acute short-stay population. Co-colonization with MRSA and VRE was frequent and perhaps driven by a high prevalence of VRE in our study population. At the anatomic site level, and unique to our study, we show that patient hands are the most common site of concurrent co-colonization with MRSA and VRE. This finding emphasizes the role of patient hand contamination in transmission of MDROs and may have implications for future targeted infection prevention measures. Lastly, indwelling device use, recent antibiotics use, diabetes, and open wounds were all found to be independent predictors of being co-colonized at any point during the study.
Overall, patients enrolled in this study comprised a relatively short-stay population with an average follow-up time of 28.5 days. All patients were admitted to a NF following hospitalization, and nearly 42% of patients were colonized with MRSA and/or VRE upon admission. Co-colonization was common among our study population with an overall prevalence of 8.7%. While MRSA and VRE colonization alone in NFs has been investigated by multiple studies, limited data exists investigating the prevalence of co-colonization in these settings. One study investigating co-colonization in nursing homes found an overall prevalence of 7.9%, but this study differed from ours in that it focused specifically on a high-risk subgroup of long-stay residents with indwelling devices that may not be comparable to our patient population.20 MRSA/VRE co-colonization in hospitalized patients is better studied with prevalence estimates that varying widely, ranging from 2.7-28.6%.9, 11-13 Such discrepancy across studies can be explained by differences in study populations, whether surveillance or clinical cultures were collected, and variability in co-colonization definitions. There is a need for consistency across future studies in order to appropriately track and compare MRSA and VRE co-colonization in different settings.
Our study furthermore describes an evolving MDRO epidemiology in PAC facilities with a rising prevalence of VRE colonization of 23.4% compared to previously reported prevalence estimates ranging from 5-18%.3, 14, 28 Furthermore, 38.9% were VR E. Faecalis which has been associated with transferring glycopeptide resistance in the majority of VRSA cases.9,10 There is a known increased prevalence of VRE bacterial populations in Southeast Michigan compared to the rest of the country which may explain this finding. We believe that the transmission of VRE among facility patients is a key driving force of co-colonization with MRSA.29 This is evidenced by the high incidence of VRE colonization occurring at over 2 times the rate of MRSA colonization. Our regression analysis demonstrated that being previously colonized with MRSA alone placed patients at nearly 3 times the risk for acquiring VRE and becoming co-colonized. Whether this co-colonization increases infection rates with either MRSA or VRE was beyond the scope of this study and needs to be investigated further in order to evaluate effectiveness of an intervention.
When determining what targeted intervention practices to implement it is important to understand anatomic patterns of bacterial colonization on a patient’s body. Anatomic site-level analysis revealed that same-site MRSA and VRE co-colonization and MRSA colonization alone occurred most frequently on patient hands, while VRE colonization alone occurred most frequently in the perianal region. VRE, a common colonizer of the gastrointestinal tract, may easily translocate to other anatomic sites leading to co-colonization and was also commonly present on patient hands and groins. Methods of translocation are varied and may include patient self-inoculation and healthcare workers when they provide more contact-intensive care such as toileting, bathing, and dressing.14, 20, 21 This can be addressed through a variety of interventions including patient and healthcare worker hand hygiene and the enforcement of standard precautions including face masks, gloves, and gowns whenever there is a risk of exposure to body fluid or mucosae. In addition, education of healthcare workers and providing feedback are critical as several studies have shown that the healthcare personnel in these settings are often sub-optimally trained in the fundamentals of infection prevention and control resulting in decreased adherence to infection prevention measures.30,31
In addition to targeted intervention practices, identifying patients at high risk for bacterial colonization also guides the implementation of screening measures that may lead to reduced rates of MRSA and VRE co-colonization in PAC settings. In this study we identified the use of indwelling devices, antibiotics use within the previous 30 days, diabetes, and open wounds as significant independent predictors of being co-colonized at any point during a patient’s stay. The use of indwelling devices are a well-known risk factor for MDRO colonization as they compromise host defenses and mandate more contact-intense care placing patients at increased risk for the acquisition of new bacteria as well as transmitting bacteria to healthcare workers and the environment.14, 22, 32, 33 Antibiotics use and open wounds have been shown in other studies to be predictors of co-colonization, and diabetes has been demonstrated to increase the risk for MRSA and VRE colonization in other healthcare settings.3, 11-12, 24, 34-37 Further studies are needed to investigate the benefits of identifying these high risk individuals and implementing specific infection prevention measures such as collecting surveillance cultures, utilizing proper contact and isolation precautions, and hand hygiene on patient co-colonization rates.
Our study has several limitations. First, the majority of our patients were short-stay and the epidemiology of co-colonization may be different in long-stay populations, therefore these findings are not generalizable to all NFs. Second, at some visits participants refused perianal and wound cultures which could underestimate co-colonization rates. Additional studies are needed to investigate the ability of wounds to facilitate MRSA and VRE co-colonization, as well as the effect adequate wound care including scheduled dressing changes, wound cleaning, and the use of barrier precautions have on the incidence of co-colonization.
We note several strengths. This is one of the largest microbial studies in post-acute care settings with active surveillance of multiple anatomic sites for MRSA and VRE that extends findings from other studies based on less extensive culturing practices. In addition, since this study collected cultures from all newly admitted patients, we were able to define epidemiology of MRSA and VRE co-colonization at a population level.
MRSA and VRE are prevalent in these settings, and future studies are needed to further investigate the role VRE transmission, the presence of wounds, and targeted screening and prevention measures have on patient co-colonization.
Highlights:
Device use, antibiotic use, diabetes, and wounds predicted MRSA/VRE co-colonization
Same-site co-colonization occurred most frequently on patient hands
Rates of VRE were higher than expected which may be driving co-colonization
Acknowledgements
We thank the patients and their families who participated in this study.
Funding. This work was supported by the National Institutes of Health [RO1 AG041780 and K24 AG050685].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential conflicts of interest. The study authors declare no conflicts of interest.
References
- 1.Griggs C, Palms D, Stone ND, et al. Burden of Invasive Methicillin-Resistant Staphylococcus aureus Infections in Nursing Homes. J Am Geriatr Soc 2018; doi: 10.1111/jgs.15451. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopez-Alcalde J, Mateos-Mazon M, Guevara M, et al. Gloves, gowns and masks for reducing the transmission of methicillin-resistant Staphylococcus aureus (MRSA) in the hospital setting. The Cochrane Database of Systematic Reviews 2015; 7: CD007087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cassone M, Mody L. Colonization with Multi-Drug Resistant Organisms in Nursing Homes: Scope, Importance, and Management. Current Geriatrics Reports 2015; 4(1): 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Claeys KC, Lagnf AM, Hallesy JA, et al. Pneumonia Caused by Methicillin-Resistant Staphylococcus aureus: Does Vancomycin Heteroresistance Matter? Antimicrob Agents Chemother 2016; 60(3): 1708–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGuinness WA, Malachowa N, DeLeo FR. Vancomycin Resistance in Staphylococcus aureus. Yale J Biol Med 2017; 90(2): 269–81. [PMC free article] [PubMed] [Google Scholar]
- 6.Walters MLD, Rasheed K, Albrecht V, McAllister S, Limbago B, Kallen A. Investigation and Control of Vancomycin-Resistant Staphylococcus aureus (VRSA): 2015 Update In: Prevention CfDCa. Atlanta, GA, 2015. [Google Scholar]
- 7.Sievert DM, Rudrik JT, Patel JB, McDonald LC, Wilkins MJ, Hageman JC. Vancomycin-resistant Staphylococcus aureus in the United States, 2002-2006. Clinical Infectious Diseases 2008; 46(5): 668–74. [DOI] [PubMed] [Google Scholar]
- 8.Albrecht VS, Zervos MJ, Kaye KS, et al. Prevalence of and risk factors for vancomycin-resistant Staphylococcus aureus precursor organisms in Southeastern Michigan. Infect Control Hosp Epidemiol 2014; 35(12): 1531–4. [DOI] [PubMed] [Google Scholar]
- 9.Hayakawa K, Marchaim D, Bathina P, et al. Independent risk factors for the co-colonization of vancomycin-resistant Enterococcus faecalis and methicillin-resistant Staphylococcus aureus in the region most endemic for vancomycin-resistant Staphylococcus aureus isolation. Eur J Clin Microbiol Infect Dis 2013; 32(6): 815–20. [DOI] [PubMed] [Google Scholar]
- 10.Zhu W, Clark N, Patel JB. pSK41-like plasmid is necessary for Inc18-like vanA plasmid transfer from Enterococcus faecalis to Staphylococcus aureus in vitro. Antimicrob Agents Chemother 2013; 57(1): 212–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furuno JP, Perencevich EN, Johnson JA, et al. Methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococci co-colonization. Emerging Infectious Diseases 2005; 11(10): 1539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reyes K, Malik R, Moore C, et al. Evaluation of risk factors for coinfection or cocolonization with vancomycin-resistant enterococcus and methicillin-resistant Staphylococcus aureus. J Clin Microbiol 2010; 48(2): 628–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKinley L, Becerra B, Moriarty H, et al. Vancomycin-resistant Enterococcus co-colonization rates with methicillin-resistant Staphylococcus aureus and Clostridium difficile in critically ill veterans. Am J Infect Control 2016; 44(9): 1047–9 [DOI] [PubMed] [Google Scholar]
- 14.Fisch J, Lansing B, Wang L, et al. New acquisition of antibiotic-resistant organisms in skilled nursing facilities. J Clin Microbiol 2012; 50(5): 1698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mody L, Bradley SF, Galecki A, et al. Conceptual model for reducing infections and antimicrobial resistance in skilled nursing facilities: focusing on residents with indwelling devices. Clin Infect Dis 2011; 52(5): 654–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montoya A, Mody L. Common infections in nursing homes: a review of current issues and challenges. Aging Health 2011; 7(6): 889–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKinnell JA, Miller LG, Singh R, et al. Prevalence of and factors associated with multidrug resistant organism (MDRO) colonization in 3 nursing homes. Infect Control Hosp Epidemiol 2016; 37:1485–8 [DOI] [PubMed] [Google Scholar]
- 18.Jones AL, Dwyer LL, Bercovitz AR, Strahan GW. The National Nursing Home Survey: 2004 overview. Vital and health statistics Series 13, Data from the National Health Survey 2009; (167): 1–155. [PubMed] [Google Scholar]
- 19.Mathei C, Niclaes L, Suetens C, Jans B, Buntinx F. Infections in residents of nursing homes. Infect Dis Clin North Am 2007; 21(3): 761–72, ix. [DOI] [PubMed] [Google Scholar]
- 20.Flannery EL, Wang L, Zollner S, Foxman B, Mobley HL, Mody L. Wounds, functional disability, and indwelling devices are associated with cocolonization by methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci in southeast Michigan. Clin Infect Dis 2011; 53(12): 1215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mody L, Krein SL, Saint S, et al. A targeted infection prevention intervention in nursing home residents with indwelling devices: a randomized clinical trial. JAMA 2015; 175(5): 714–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mody L, Maheshwari S, Galecki A, Kauffman CA, Bradley SF. Indwelling device use and antibiotic resistance in nursing homes: identifying a high-risk group. J Am Geriatr Soc 2007; 55(12): 1921–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers MA, Mody L, Kaufman SR, Fries BE, McMahon LF Jr., Saint S. Use of urinary collection devices in skilled nursing facilities in five states. J Am Geriatr Soc 2008; 56(5): 854–61. [DOI] [PubMed] [Google Scholar]
- 24.Mody L, Foxman B, Bradley S, et al. Longitudinal Assessment of Multidrug-Resistant Organisms in Newly Admitted Nursing Facility Patients: Implications for an Evolving Population. Clin Infect Dis 2018; ciy194, https://org/10.1093/cid/ciy194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Diseases 1987; 40(5): 373–83. [DOI] [PubMed] [Google Scholar]
- 26.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969; 9(3): 179–86. [PubMed] [Google Scholar]
- 27.Tan TY, Jiang B, Ng LSY. Faster and economical screening for vancomycin-resistant enterococci by sequential use of chromogenic agar and real-time polymerase chain reaction. J Microbiol Immunol Infect 2017; 50(4): 448–53. [DOI] [PubMed] [Google Scholar]
- 28.Hayakawa K, Marchaim D, Vidaillac C, et al. Growing prevalence of vancomycin-resistant Enterococcus faecalis in the region with the highest prevalence of vancomycin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol 2011; 32(9): 922–4. [DOI] [PubMed] [Google Scholar]
- 29.Omotola AM, Li Y, Martin ET, et al. Risk factors for and epidemiology of community-onset vancomycin-resistant Enterococcus faecalis in southeast Michigan. American journal of infection control 2013; 41(12): 1244–8. [DOI] [PubMed] [Google Scholar]
- 30.Koo E, McNamara S, Lansing B, et al. Making infection prevention education interactive can enhance knowledge and improve outcomes: Results from the Targeted Infection Prevention (TIP) Study. Am J Infect Control 2016; 44(11): 1241–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mody L, Saint S, Galecki A, Chen S, Krein SL. Knowledge of evidence-based urinary catheter care practice recommendations among healthcare workers in nursing homes. J Am Geriatr Soc 2010; 58(8): 1532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cassone M, Mantey J, Perri MB, et al. Environmental Panels as a Proxy for Nursing Facility Patients With Methicillin-Resistant Staphylococcus aureus and Vancomycin-Resistant Enterococcus Colonization. Clin Infect Dis 2018; 67(6): 861–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Min L, Galecki A, Mody L. Functional disability and nursing resource use are predictive of antimicrobial resistance in nursing homes. J Am Geriatr Soc 2015; 63(4): 659–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKinnell JA, Miller LG, Eells SJ, Cui E, Huang SS. A systematic literature review and meta-analysis of factors associated with methicillin-resistant Staphylococcus aureus colonization at time of hospital or intensive care unit admission. Infect Control Hosp Epidemiol 2013; 34(10): 1077–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy CR, Quan V, Kim D, et al. Nursing home characteristics associated with methicillin-resistant Staphylococcus aureus (MRSA) Burden and Transmission. BMC Infectious Diseases 2012; 12: 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papadimitriou-Olivgeris M, Drougka E, Fligou F, et al. Risk factors for enterococcal infection and colonization by vancomycin-resistant enterococci in critically ill patients. Infection 2014; 42(6): 1013–22. [DOI] [PubMed] [Google Scholar]
- 37.Rabinowitz RP, Kufera JA, Makley MJ. A hidden reservoir of methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus in patients newly admitted to an acute rehabilitation hospital. Phys Med Rehabil 2012; 4(1): 18–22. [DOI] [PubMed] [Google Scholar]


