Abstract
Developmental exposure to endocrine disruptor bisphenol A (BPA) is associated with metabolic defects during adulthood. In sheep, prenatal BPA treatment causes insulin resistance (IR) and adipocyte hypertrophy in the female offspring. To determine if changes in insulin sensitivity mediators (increase in inflammation, oxidative stress, and lipotoxicity and/or decrease in adiponectin) and the intracrine steroidal milieu contributes to these metabolic perturbations, metabolic tissues collected from 21-month-old female offspring born to mothers treated with 0, 0.05, 0.5, or 5 mg/kg/day of BPA were studied. Findings showed prenatal BPA in non-monotonic manner (1) increased oxidative stress; (2) induced lipotoxicity in liver and muscle; and (3) increased aromatase and estrogen receptor expression in visceral adipose tissues. These changes are generally associated with the development of peripheral and tissue level IR and may explain the IR status and adipocyte hypertrophy observed in prenatal BPA-treated female sheep.
Keywords: Endocrine disruptor, bisphenol A, insulin resistance, oxidative stress, inflammation, lipotoxicity
INTRODUCTION
While communicable diseases were the major causes of mortality during the 18th and 19th centuries, currently non-communicable diseases (NCD), such as cancer and cardio-metabolic diseases, are the leading cause of death worldwide [1, 2]. One of the causes attributed for the rise in NCD is developmental exposure to man-made environmental chemicals resulting from increased industrialization. This is supported by the data from the United States (US) Centers for Disease Control and Prevention national biomonitoring program which determined that almost all Americans have detectable levels of a wide variety of environmental chemicals including endocrine-disrupting chemicals (EDCs) in their bodies [3]. Both Centers for Disease Control and Prevention and the National Health and Nutrition Examination Survey (NHANES) have found that bisphenol A (BPA), one such EDC, is detectable in >90% of the US population [4, 5].
BPA is used in the production of epoxy and polycarbonate resins that are used in the manufacture of common household plastics. The main endocrine disrupting function attributed to BPA is its estrogenic activity [6–8] but other properties including antiandrogen [9], thyroid modifying [10] and metabolic functions [7, 8] are described. Epidemiological surveys in the humans have linked BPA exposure to metabolic defects including insulin resistance (IR), diabetes, obesity and metabolic syndrome [11–13]. Although associations between prenatal exposure to BPA and adverse anthropometric birth outcomes have been documented from human cohort studies [14, 15], studies linking prenatal exposure to BPA to adult onset metabolic disorders are limiting. However, presence of high levels of BPA in human maternal circulation during first trimester have been linked to reduced birth weight [15], a major risk factor for adult onset cardio-metabolic disorders [16]. Studies in various animal models provide direct evidence that developmental exposure to BPA leads to metabolic defects [17–19]. Gestational BPA exposure in sheep, a precocial species like humans [20] and hence of translational value, induces metabolic defects in the female offspring at adult age characterized by peripheral IR with compensatory hyperinsulinemia, adipocyte hypertrophy in visceral adipose tissue (VAT), and elevated markers of oxidative stress in the circulation and VAT [19, 21]. However, the mechanisms that lead to the development of these insulin sensitivity and adipocyte defects are not completely understood.
Various factors are implicated in the development of IR including increase in factors that negatively affect insulin sensitivity such as chronic low-grade inflammation, dyslipidemia, oxidative stress, and lipotoxicity in metabolic tissues [22–24] with or without decrease in factors that promote insulin sensitivity such as antioxidants and adiponectin [25, 26]. Inflammation is characterized by activation of resident macrophages leading to production of proinflammatory cytokines that attract infiltration of additional macrophages setting up a vicious cycle of proinflammatory cytokine production that deactivate members of the insulin signaling thereby interfering with tissue insulin sensitivity [23, 27]. Infiltration of macrophages also increases oxidative stress [28] and promotes lipolysis leading to dyslipidemic state [24], which can cause ectopic accumulation of lipids and lipotoxicity in liver and muscle. Lipids negatively affect glucose metabolism and interfere with insulin action and contribute to development of IR [29]. Similarly, development of oxidative stress can oxidize or nitrosylate proteins involved in insulin signaling, reduce gene expression and damage cellular components leading to reduced insulin sensitivity [30]. On the contrary the decrease in positive mediator of insulin sensitivity such as adiponectin (ADIPOQ), a adipokine produced predominantly by adipose tissue [31], is also strongly associated with metabolic disorders [26]. It is present in the circulation in oligomeric low- (LMW), middle- (MMW), and high-molecular weight (HMW) forms, of which the HMW form is the metabolically active form [32]. Antioxidants such as glutathione reductase (GSR) and superoxide dismutases (SOD) are cellular defenses against reactive species and promote insulin sensitivity by reducing oxidative stress [25].
Steroids also have a role in regulating adipocyte proliferation, differentiation [33], insulin sensitivity and inflammation [34]. Both direct and developmental exposures to native steroids or EDC with steroid potential have been shown to influence adipocyte size [33, 35]. For instance, prenatal BPA exposed rats [36] and sheep [19] manifest large adipocytes. Furthermore, because adipose tissue is a site of steroid production and action [37], local changes in steroid biosynthetic machinery and steroid receptor expression can contribute to adipocyte differentiation and size.
As such insulin sensitivity of the metabolic tissues may be impacted by plethora of interactive regulators and adipocyte hypertrophy by the intracrine steroidal machinery. Utilizing female sheep exposed prenatally to BPA at levels spanning environmental to occupational exposure levels, the goal of this study was to understand the contributions of adiponectin, inflammatory / oxidative stress status, and lipotoxicity in metabolic tissues and steroidal machinery in the VAT in the development of IR and adipocyte hypertrophy.
METHODS
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Michigan and are consistent with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals. These studies were conducted at the University of Michigan Sheep Research Facility (Ann Arbor, MI) using Suffolk sheep breed. Breeder sheep (2–5-year-old) were acquired from local farmers, group housed and kept outdoors with access to shelter. Their maintenance, breeding, prenatal treatments and lambing were performed as described previously [38]. All animals including the control group were maintained together and any exposures to potential sources of phytoestrogens via diet were similar across treatment groups.
Prenatal BPA treatments
Pregnant sheep (n=8 to 13 per group) were randomly assigned to the different treatment groups (Figure 1). Treatment groups involved controls mothers that received only the vehicle (corn oil) and animals receiving 0.05, 0.5, or 5 mg/kg/·day (BPAlow, BPAmed, and BPAhigh, respectively) of BPA (purity ≥99%, cat. no. 239658; Aldrich Chemical, Milwaukee, WI). BPA was dissolved in corn oil and administered daily through subcutaneous (sc) injections from days 30 through 90 of gestation (term: ~147 days). Daily sc administration of 0.5mg/kg/day dose of BPA from days 30–90 of gestation produced umbilical arterial levels of 2.62±0.52ng/ml of free BPA [39] on day 90 of fetal life, when measured in samples collected within an hour after the last injection of BPA. These levels are well within the range found in mid-gestation umbilical cord blood concentrations of free BPA (<LOD - 52.26 ng/mL) in a California based study [40] or of total BPA in term cord blood level (<LOD - 51.5 ng/mL) in Korean population [41]. The concentration of free BPA achieved in cord blood in our study is consistent with levels achieved following maternal sc injection of 5mg/kg/day of BPA in sheep (10-fold higher dose than used in our study) from gestational days 28 to term in an earlier study (29.7±4.6 ng/ml) [42]. Although comparable levels of free BPA were detected, considering BPA was administered as single daily subcutaneous injections, it needs to be recognized that the levels in fetal circulation will likely fluctuate. Only female lambs were used for the study and these were weaned at ~8 week of age and provided with a maintenance diet consisting of 0.64 kg of corn, 0.64 kg hay·lamb−1·day−1, and 0.014 kg of supplement (36% crude protein) to prevent development of obesity. Wooden feeders with crossover metallic bars were used. Only one female offspring from each dam was utilized if twin pregnancies were involved. The number of female offspring available to study from each treatment group (one per mom) were control: 5; low BPA: 8; medium BPA: 10; high BPA: 5. The impact of prenatal BPA exposure on ovarian follicular dynamics, LH surge, insulin sensitivity, and adiposity utilizing the animals from this cohort have been previously reported [21, 43, 44].
Figure 1:
Schematic showing the study design with the time and duration of treatment with different doses of BPA and time of tissue collection.
Tissue Collection
Metabolic tissues were collected during the second breeding season at ~21 months of age following a 48h fast. Estrous was synchronized with two injections of prostaglandin F2α (PGF2α, 10 mg, i.m.; Lutalyse, Pfizer Animal Health, Florham Park, NJ) administered 11 days apart. Tissues were harvested 24 hours after the second PGF2α injection during the follicular phase much before the preovulatory increase in estradiol and the LH surge. The timing of tissue harvest was determined based on timing relationships from the preceding cycle where preovulatory increase in estradiol rise The timing of tissue harvest was determined based on and LH surge occurred namely ~40h and ~47h after second PGF2alpha injection in controls (prenatal BPA treatment had no effect on these timings) [43]. Blood samples from the jugular vein were collected in a heparinized tube and all animals euthanized by barbiturate overdose (Fatal Plus; Vortech Pharmaceuticals, Dearborn, MI). Circulating LH levels at the time of collection were well within the range of pulsatile LH secretion (data not shown). Liver was obtained from the tip of the left lobe, skeletal muscle from the vastus lateralis, and visceral adipose tissue (VAT) from the mesenteric fat surrounding the ventral sac of the rumen. Tissues were flash-frozen and stored at −80 °C until processed.
Inflammatory cytokines and antioxidant measures
Proinflammatory cytokines and antioxidant gene expression was assessed using SYBRgreen based real time RT-PCR on a BioRad myiQ iCycler instrument utilizing total RNA that was isolated, purified and reverse transcribed as described before [45]. Oligonucleotide primers for the genes under study were designed using Primer Express software (Life Technologies, Carlsbad, CA) and the sequences for the primers used are shown in Table 1. The relative amount of each transcript was calculated using the ΔΔCT method and normalized to the endogenous reference gene ribosomal protein L19 (RPL19).
Table 1:
Sequence of the primers used for real time RT-PCR
| Gene ID | Forward Primer | Reverse Primer | Accession Number |
|---|---|---|---|
| IL1B | CGAACATGTCTTCCGTGATG | GAAGCTCATGCAGAACACCA | NM_001009465.2 |
| IL6 | ACATCGTCGACAAAATCTCTGCAA | GCCAGTGTCTCCTTGCTGTTT | NM_001009392.1 |
| TNF | ACACCATGAGCACCAAAAGC | AGGCACAAGCAACTTCTGGA | XM_005223596.3 |
| CCL2 | CCAGCAGCAAGTGTCCTAAAG | GGCTTTGGAGTTTGGTTTTTC | XM_004012471.2 |
| CD68 | CAGGGGACAGGGAATGACT | CCAAGTGGTGGTTCTGTGG | NM_001045902.1 |
| GSR | CTGGAAGAGTTGCCTCGCC | TCATTATTGATGTCTTAGAACCCAGG | XM_015104590.1 |
| SOD1 | ATCATGGGTTCCACGTCCA | CATGCCTCTCTTCATCCTTTGG | NM_001145185.1 |
| SOD2 | CGCTGGAGAAGGGTGATGTC | CAGATTTGTCCAGAAGATGCTGTG | NM_001280703.1 |
| CYP17 | AGACATATTCCCTGCGCTGA | GCAGCTTTGAATCCTGCTCT | XM_012102863.2 |
| CYP19 | TGGCCTGGTGCGCATGGT | TGCGCCGCATGAGGGTCAAC | NM_001123000 |
| ESR1 | TCGTCTCGGTTCCGTATGATG | GACAGAAATGTGTACACCCCAGAAT | AY033393.1 |
| ESR2 | GCCGACAAGGAACTGGTACAC | CCACGAAGCCCGGAATCT | NM_001009737.1 |
| AR | GCCCATCTTTCTGAATGTCC | CAAACACCATAAGCCCCATC | XM_001253942 |
| RPL19 | CCTTGGCTCGCCGGA | CATGTGGCGGTCAATCTTCTTA | XM_012186026.1 |
The activity of antioxidant GSR was also determined in liver, muscle and VAT using a commercially available activity assay kit (Cayman chemicals, Ann Arbor, MI) as per manufacturer’s recommendations.
Adiponectin
Plasma adiponectin was assessed using a previously published method [45, 46] using the bovine anti-adiponectin antibody kindly provided by Dr. Helga Sauerwein from University of Bonn, Germany. Plasma proteins were separated under reducing conditions to detect LMW forms and under non-reducing conditions to detect HMW forms and transferred to nitrocellulose membranes. Membranes were first stained with Ponceau to visualize the protein loading and incubated with primary antibody (Table 2) overnight at 4°C, washed and probed with HRP-tagged secondary antibody raised against the species in which primary antibody was generated. Adiponectin protein was detected by electrochemiluminescent (ECL) reaction using ProteinSimple FluorChem E system (San Jose, CA). Band densities for adiponectin from ECL reaction and albumin in the Ponceau stained membrane were determined using ImageJ software.
Table 2:
Antibodies used for immunoblot analysis
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised in; Monoclona l or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| Bovine Adiponectin | Bovine anti-adiponectin | Dr. Helga Sauerwein (University of Bonn, Germany) | Rabbit; polyclonal | 1:1000 | AB_2650604 | |
| CYP17 | Rabbit anti-17α-hydroxylase | Dr. Walter L Miller (University of California, San Francisco) | Rabbit; polyclonal | 1:1000 | AB_2732802 | |
| CYP19 | Aromatase Polyclonal Antibody | Thermo Fisher Scientific, PA1–21398 | Rabbit; polyclonal | 1:1000 | AB_2088676 | |
| AR | AR (N-20) | Santa Cruz Biotechnology, SC-816 | Rabbit; polyclonal | 1:1000 | AB 1563391 | |
| ESR1 | ERα (clone 1D5) | Thermo Scientific, MS-354 | Mouse; monoclonal | 1:500 | AB 61341 | |
| ESR2 | Estrogen receptor beta (ER 2) antibody | Dr. Benita S Katzenellenbog en (University of Illinois) | Mouse; monoclonal | 1:1000 | AB_2722105 | |
| GAPDH | GAPDH (14C10) | Cell Signaling, 3683 | Rabbit; monoclonal | 1:1000 | AB 1642205 |
Steroidogenic enzymes and steroid receptors
Steroidogenic enzymes and steroid receptors were assessed in VAT by both real time RT-PCR and immunoblotting. Real time RT-PCR was performed as described above. For immunoblotting, whole-tissue protein extracts were prepared from tissue homogenates and equal amounts of protein (~40 μg) were resolved on SDS-PAGE, transferred onto a nitrocellulose membrane and protein content visualized and quantified as described for adiponectin with detection of GAPDH used as the loading control.
Oxidative stress measures
As markers of oxidative stress, levels of protein-bound oxidized tyrosine moieties, 3-nitrotyrosine (NY) and o, o’-dityrosine (DY) were quantified by isotope dilution liquid chromatography electrospray ionization tandem mass spectrometry (HPLC-ESI-MS/MS) as described previously [47] in tissue extracts from muscle and liver and lipidperoxidation marker Thiobarbituric Acid Reactive Substances (TBARS) utilizing commercially available colorimetric assay kit (Cayman Chemicals, Ann Arbor, MI) in tissue extracts from VAT, muscle and liver. For oxidized tyrosine quantification, tissue extracts were delipidated, precipitated and proteins hydrolyzed along with known amounts of isotopically labeled internal standards13C6-Y and 13C6-NY, 13C6-ClY, and 13C12-o, o’-DY. The extracts were then subjected to solid-phase extraction and the amount of oxidized amino acids was quantified by HPLC-ESI-MS/MS. The levels of the oxidized amino acids in each sample were then normalized to the amino acid tyrosine content in the respective samples and expressed as the ratio of the oxidized product over the total tyrosine. Intraassay coefficients of variation for NY and DY were 8.47% and 8.16%, respectively. For TBARS assay, total protein of the tissue was isolated and assay carried out as per manufacturer’s recommendation and Intra- and inter-assay coefficients were 5.6% and 10.2%, respectively.
Ectopic lipid accumulation
Lipid accumulation in liver were assessed by Oil Red O staining of cryosections and in both liver and muscle by measuring triglyceride content utilizing a commercially available assay kit as described before [45]. For oil red O staining, 3 slides containing sections chosen 50 microns apart were used and five regions corresponding to the 4 corners and center of each section were imaged for determining the oil red O stain positive area. The area of oil red O positive stain to the total area of the imaged section was calculated using the Image Pro-Plus 3.0.1 system (Media Cybernetics, Rockville, MD) using a well-validated densitometrical methodology [48] as described previously [49]. Tissue triglyceride content was assessed in 100mg of tissue subject to lipid extraction as per the Bligh and Dyer method [50] with slight modifications as described before [45] using the Wako L-Type TG M kit (Wako Diagnostics, Mountain View, CA) as per manufacturer’s recommendations. Each sample was assayed in duplicate and the intra- and inter-assay coefficients of variance were 3.32 ± 0.57 and 5.2 ± 0.73%, respectively. The measurable range for this assay is 1.1 to 2000 mg/dL. Plasma triglyceride content was assessed using a colorimetric assay kit (Cayman Chemicals, Ann Arbor, MI) as per manufacturer’s recommendation. The intra- and inter-assay coefficients of variance were 2.14 and 6.71%, respectively with the detection limit of 0.5mg/dL
Statistical analysis
Heterogeneity of variance was tested with Bartlett’s χ2 test and when required data were log-transformed. Changes in gene expression, markers of oxidative stress, and triglyceride content were analyzed using one-way ANOVA followed by Tukey’s post hoc test using the Prism 6 software (GraphPad, La Jolla, CA) and the threshold for significance was set as p ≤ 0.05. Data were also subjected to Cohen’s effect size analysis [51–53]. ANOVA outcomes when significant (P<0.05) and Cohen’s d value of >0.8 are presented in Figures with specifics in Figure legends.
RESULTS
Inflammatory markers
Prenatal BPA treatment did not lead to statistically significant changes in any of the inflammatory markers at all three sites studied. Results from Cohen’s effect size analysis are presented below.
Liver:
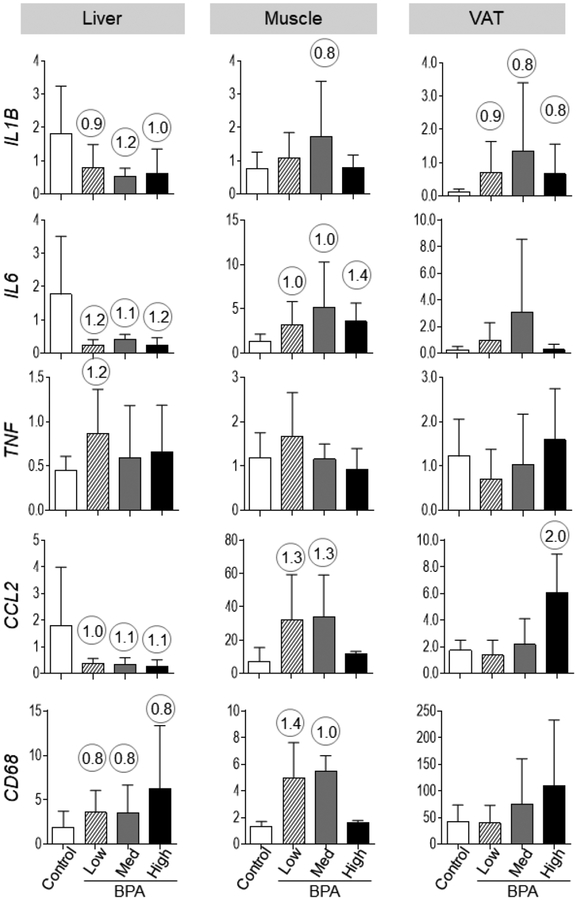
Effect size analysis lead to mixed outcomes depending on the inflammatory marker. This was reflected as 1) a reduction in the expression of several of the pro-inflammatory markers (interleukin 1 beta [IL1B; d = 0.90, 1.23 and 1.04], IL6 [d = 1.22, 1.23 and 1.26] and chemokine (C-C) ligand 2 [CCL2; d = 0.97, 1.12 and 1.18]) for BPAlow, BPAmed, and BPAhigh doses respectively, 2) an increase (d = 1.16) with tumor necrosis factor alpha (TNF) with only BPAlow dose, and 3) an increase in the macrophage marker CD68 (d = 0.90, 1.23 and 1.04, respectively for BPAlow, BPAmed, and BPAhigh doses) (Figure 2).
Figure 2:
Mean ±SEM of proinflammatory cytokines (mRNA expression of IL1B, IL6, TNF, CCL2) and macrophage marker (CD68) expression in control, BPAlow, BPAmed and BPAhigh dose prenatal BPA-treated groups in the liver (left), muscle (middle) and VAT (right panel). Numerical superscripts are Cohen’s d values ≥ 0.8 comparing control and different dose groups of BPA. None of these changes achieved statistical significance.
Skeletal muscle:
Cohen’s effect size analysis also pointed to an increase in 1) IL6 (d = 0.97, 1.04 and 1.40 for the BPAlow, BPAmed, and BPAhigh doses, respectively), 2) IL1B (d = 0.81, only with BPAmed dose), and 3) CCL2 (d = 1.28 and 1.26) and the macrophage marker CD68 (d = 1.36 and 1.00) expression with BPAlow and BPAmed doses (Figure 2).
VAT:
Effect size analysis suggested increases in IL1B with all 3 doses (d = 0.90, 0.85 and 0.85 for the BPAlow, BPAmed, and BPAhigh doses, respectively) and for CCL2 with only the BPAhigh dose (d = 2.02) (Figure 2).
Oxidative stress markers
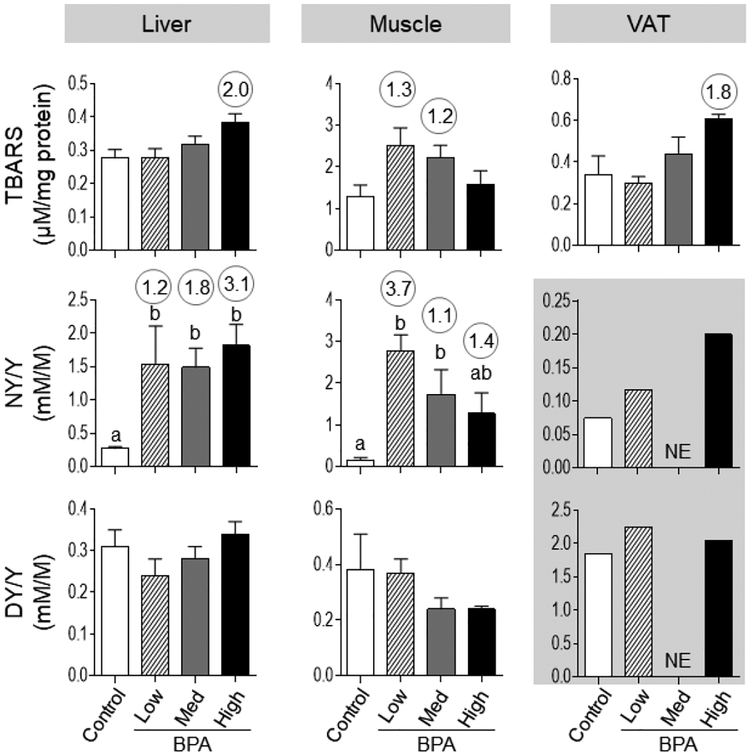
Prenatal BPA treatment induced a significant increase in oxidized tyrosine moieties (NY but not DY content) at all doses in the liver and in BPAlow and BPAmed groups in the muscle (Figure 3). We have previously shown that both in circulation and VAT, prenatal exposure to BPAhigh induces a significant increase in oxidative stress marker NY but not DY (Figure 3; mean levels from previous publication [21] shown in the grey boxed area for comparison). Effect size analysis pointed to an increase in lipid peroxidation marker, TBARS with the BPAhigh dose in the liver (d = 2.05) and VAT (d = 1.83) and in the BPAlow and BPAmed doses in the muscle (d = 1.31 and 1.24, respectively) (Figure 3).
Figure 3:
Mean ± SEM tissue TBARS and oxidized tyrosine concentrations in control, BPAlow, BPAmed and BPAhigh dose prenatal BPA-treated groups in the liver (left), muscle (middle) and VAT (right panel). Numerical superscripts are Cohen’s d values ≥ 0.8 comparing control and different dose groups of BPA. Mean values of oxidized tyrosine concentrations in VAT that is previously published [21] are shown in the grey area (NE = Not Examined). Differing letters in histograms (NY/Y in liver and muscle) indicate statistically significant differences; a vs. b: p<0.05; ab not different from a or b.
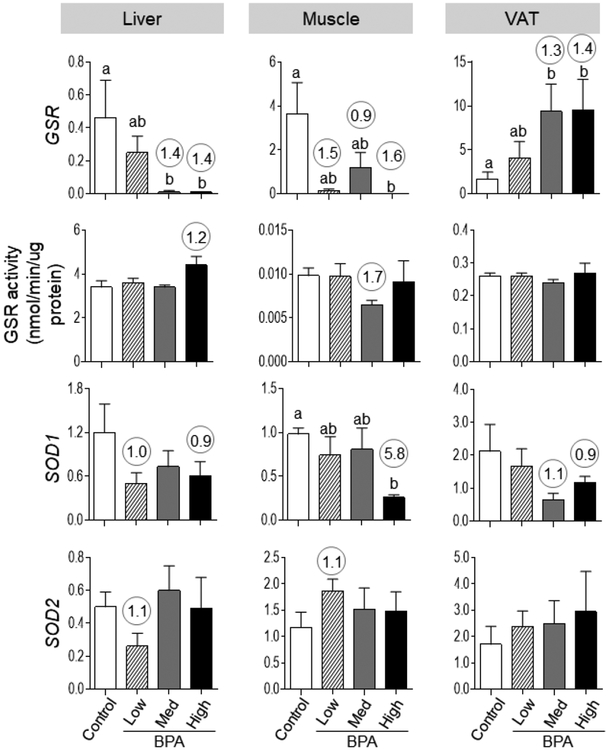
The expression of antioxidants in the liver and muscle was generally reduced with significant decrease in GSR mRNA expression with BPAmed and BPAhigh doses in liver and both GSR and SOD1 mRNA expression with BPAhigh dose in the muscle (Figure 4). In contrast, in the VAT only antioxidant GSR showed a significant increase at BPAmed and BPAhigh dose.
Figure 4:
Mean ± SEM antioxidant [GSR (top row), SOD1 (middle-lower row) and SOD2 (bottom row)] expression in the liver (left), muscle (middle) and VAT (right panel) tissues of control, BPAlow, BPAmed and BPAhigh dose prenatal BPA-treated groups. Mean ± SEM of enzymatic activity of GSR are also shown for these different tissues and treatment groups in middle-top panel. Differing alphabets above the histogram (GSR in liver, muscle and VAT, SOD1 in muscle) indicate statistically significant differences by ANOVA (a vs. b: p<0.05; ab not different from a or b) and numerical superscripts are Cohen’s d values ≥ 0.8 comparing control and different dose groups of BPA.
Effect size analysis pointed to decreases in expression of antioxidants 1) GSR mRNA with BPAmed (d = 1.38) and BPAhigh (d = 1.39) doses in the liver, all doses (d = 1.53, 0.93 and 1.60 for the BPAlow, BPAmed, and BPAhigh doses, respectively) in the muscle and BPAmed (d = 1.30) and BPAhigh (d = 1.40) in the VAT, 2) SOD1 mRNA with BPAlow (d = 1.02) and BPAhigh (d = 0.85) in the liver, BPAhigh (d = 5.77) in the muscle, and BPAmed (d = 1.10) and BPAhigh (d = 0.91) in the VAT, and 3) SOD2 mRNA with BPAlow (d = 1.13) in the liver and muscle (1.12). The GSR activity appeared as an increase (d = 1.24) with BPAhigh dose in contrast to decreased mRNA expression in the liver. The GSR activity appeared as a decrease at BPAmed dose (d = 1.70) in the muscle consistent with its mRNA expression (Figure 4).
Lipotoxicity
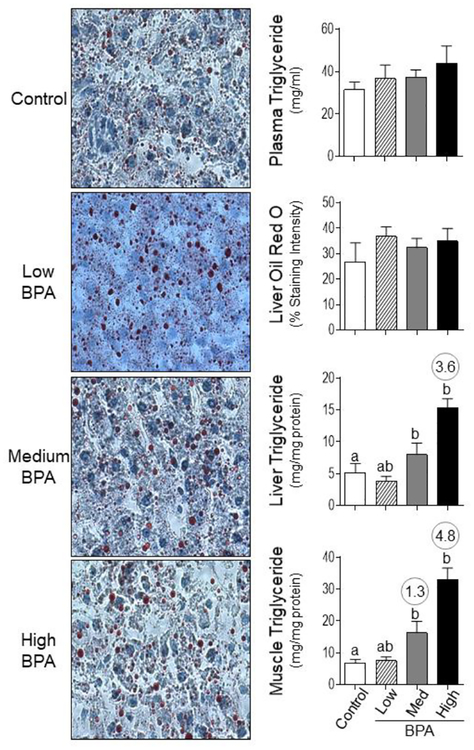
Plasma triglyceride content did not change between the different BPA groups (Figure 5), however, a significant increase in both hepatic and muscular triglyceride content was evident at BPAmed and BPAhigh dose groups (Figure 5) by ANOVA. Effect size analysis also showed an increase (d = 3.64) in hepatic triglyceride content in BPAhigh dose group and BPAmed (d = 1.32) and BPAhigh (d = 4.79) dose groups in the muscle. No significant changes in hepatic lipid content was evident when assessed by oil red O staining with any of the BPA treatment (Figure 5).
Figure 5:
Examination of lipid accumulation in plasma, liver and muscle from control, BPAlow, BPAmed and BPAhigh dose prenatal BPA-treated groups. The cryosections of liver stained with oil red O and imaged for assessment of staining intensity as described in the methods are shown in the left panels (Bar = 25μm). On the right are shown mean ± SEM of the percent staining of oil red O in liver (right middle-top) and triglyceride content in plasma (right top), liver (right middle-bottom) and muscle (right bottom). Differing letters in histograms (liver and muscle triglyceride) indicate statistically significant differences by ANOVA (a vs. b: p<0.05; ab not different from a or b) and numerical superscripts are Cohen’s d values ≥ 0.8 comparing control and different dose groups of BPA.
Adiponectin
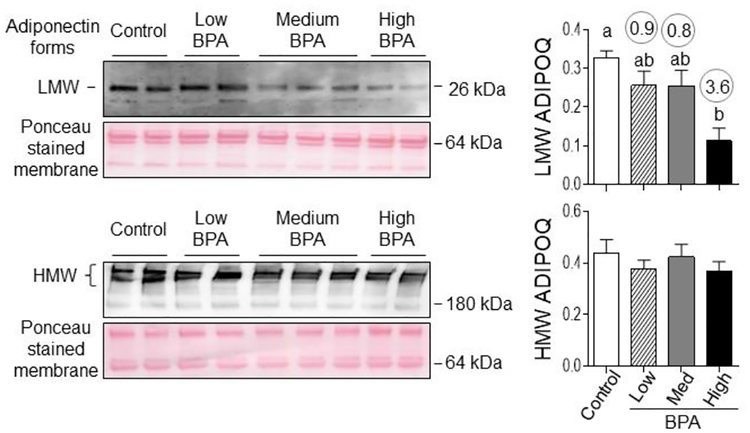
The plasma LMW adiponectin levels were significantly reduced only in the BPAhigh dose group (Figure 6). Effect size analysis pointed to a decrease (d = 0.87, 0.83 and 3.55 with BPAlow, BPAmed, and BPAhigh doses, respectively) for LMW but not HMW adiponectin forms (Figure 6).
Figure 6:
Plasma levels of low (LMW) and high (HMW) molecular weight forms of adiponectin in control, BPAlow, BPAmed and BPAhigh dose prenatal BPA-treated groups. Top panels show representative blots under reducing conditions (left, LMW) and non-reducing conditions (right, HMW forms) along with Ponceau staining for protein loading. Right panels show mean ± SEM of density ratios between LMW (top) and HMW (bottom) forms of adiponectin with the 64 kDa protein from the Ponceau stained membrane. Numerical superscripts are Cohen’s d values ≥ 0.8 comparing control and different dose groups of BPA. Differing letters in histograms (LMW ADIPOQ) indicate statistically significant differences by ANOVA (a vs. b: p<0.05; ab not different from a or b).
VAT steroidal milieu
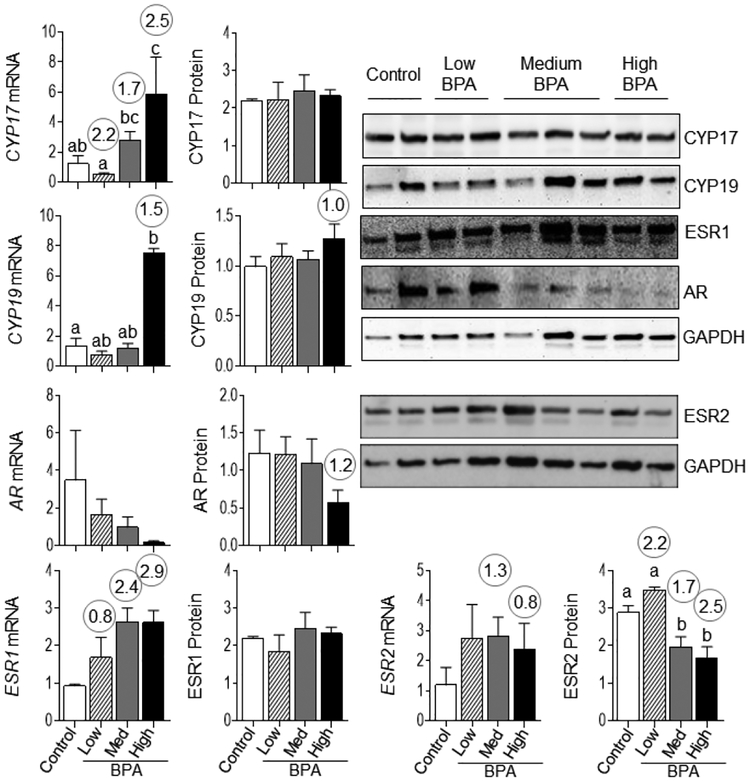
Relative to androgens, prenatal BPA induced significant increase in CYP17 mRNA with BPAhigh dose. No change in CYP17 protein and AR mRNA expression was evident with any of doses tested. Effect size analysis indicated a decrease (d = 1.18) in AR protein with the high BPA dose (Figure 7). Relative to estrogen, a significant increase in CYP19 mRNA and a significant decrease in ESR2 protein at BPAmed and BPAhigh doses were observed. Effect size analysis revealed an increase (d = 1.02) in CYP19 protein only with the BPAhigh dose, ESR1 mRNA at all doses (d = 0.76, 2.35, 2.91 for BPAlow, BPAmed, and BPAhigh doses, respectively) and ESR2 mRNA at BPAmed (d = 1.3) and BPAhigh (d = 0.8) doses (Figure 7). No change in ESR1 protein was evident at any of the doses.
Figure 7:
Changes in steroidogenic enzymes CYP19 and CYP17, and steroid receptors ESR1, ESR2 and AR mRNA and protein expression in VAT from control, BPAlow, BPAmed and BPAhigh dose prenatal BPA-treated groups are shown. Gene expression was quantified by RT-PCR and presented as mean ± SEM of fold changes of treated vs. control samples. Protein content was determined by immunoblotting and mean ± SEM of ratio of densitometric values for the respective protein to GAPDH are plotted. Representative immunoblots are shown in the right side of the panel. Differing alphabets above the histogram indicate significant changes by ANOVA and numerical superscript are Cohen’s d values ≥ 0.8 comparing control and different dose groups of BPA. Differing letters in histograms (CYP17 and CYP19 mRNA) indicate statistically significant differences by ANOVA (a vs. b: p<0.05; ab not different from a or b).
DISCUSSION
The findings from this study indicate that an increase in oxidative stress in the metabolic tissues, lipotoxicity in the liver and muscle, and systemic changes such as reduced adiponectin are all potential contributors of the IR status [19] of the prenatal BPA-exposed female sheep. Additionally, the increased estrogenic milieu with increased expression of estrogen biosynthetic enzymes and receptors in the VAT may have contributed to the adipocyte hypertrophy [19] observed in the prenatal BPA-exposed female sheep. Furthermore, many of the observed changes resulting from prenatal exposure to environmental to occupational levels of BPA-exposure followed a non-monotonic response curve, a finding consistent with other studies of BPA exposure [54–56]. The significance of these findings and their contribution to the IR status of these animals are discussed below.
Systemic Changes:
The observation of reduced LMW adiponectin in prenatal BPA-exposed female sheep is suggestive of hypoadiponectinemic state that is typical of metabolic conditions characterized with IR [23, 26]. Findings from this study and our previous findings of increased oxidative stress state [21] are therefore consistent with the IR state observed in prenatal BPA-exposed female sheep [19]. Low adiponectin levels such as that evidenced in this study are also a feature of perinatal BPA-exposed female mice [57] and male rats [58]. This contrasts with the lack of decrease in HMW adiponectin the form that promotes the insulin sensitizing action, low levels of which are reported in other conditions associated with IR [26]. The lack of change in plasma triglyceride content is surprising considered it is associated with IR status [23, 26]. In contrast, changes in TG were observed in perinatal and postnatal BPA exposed mice [59] and perinatal BPA exposed rats [60].
Liver:
Increase in oxidative state (characterized by increase in oxidative stress marker NY and downregulation of antioxidant gene expression) and triglyceride content in the liver from prenatal BPA-treated female sheep indicates that the negative mediators of insulin sensitivity are increased in the liver of prenatal BPA-treated sheep. This is consistent with the increases in hepatic lipid accumulation and oxidative stress observed in the male but not female Sprague-Dawley rats born to mothers exposed to BPA during gestation and lactation [61]. Hepatic lipid accumulation was manifested as increase in triglyceride content but not oil red O staining and this may be a function of oil red O stain only accumulating in neutral lipids [62]. A potential mechanism for hepatic lipid accumulation may involve altered expression of hepatic lipid metabolism related genes (adipose triglyceride, hormone sensitive and lipoprotein lipases) as observed in perinatal BPA exposed mice [63]. The changes in inflammatory cytokines were evident as either increase in TNF (low dose only) and macrophage marker CD68 or decrease for IL1B, IL6 and CCL2, by effect size analysis only. Although these findings did not achieve statistical significance, the directionality of the changes by effect size analysis suggests that additional studies involving larger sample sizes are required to establish the potential involvement of liver inflammatory status to its IR status.
The hepatic oxidative stress and lipid accumulation in prenatal BPA-treated sheep parallel what is seen with the pathological condition, non-alcoholic fatty liver disease (NAFLD) [64, 65]. In this condition, a strong correlation has been reported between development of systemic and hepatic IR and accumulation of lipids in the liver [66]. Other studies pointing to amelioration of IR state with reversal of hepatic steatosis point to causal relationship between the two [67]. Additional support for the link between the two comes from studies in sheep treated during the same susceptibility window with native steroid, testosterone; these sheep not only manifested hepatic lipid accumulation, inflammation and oxidative stress that are characteristic of NAFLD [45] but also reduced phospho-AKT response to insulin stimulation [68] consistent with hepatic IR. Considering testosterone can be aromatized to estrogen and co-treatment with androgen antagonist partially reversed the hepatic lipid accumulation in this model [45], the programming of this defect is likely estrogen-mediated. These findings raise the possibility that changes in mediators of hepatic insulin sensitivity are likely accompanied by compromised hepatic insulin sensitivity also in the prenatal BPA (estrogen mimic)-treated sheep, a premise that remains to be tested.
Skeletal muscle:
The effects of prenatal BPA treatment on oxidative stress markers and lipid accumulation followed similar directionality as in the liver. This was reflected as increased muscle NY and a trend for TBARS content (p = 0.08 for low and medium dose, T test), reduced antioxidant gene expression and increased triglyceride accumulation. These findings indicate that negative mediators of insulin signaling are also increased in the skeletal muscle of prenatal BPA-treated female sheep. Although the muscle insulin sensitivity remains to be tested in this model, finding of reduced phospho-AKT response to insulin stimulation that accompanied increases in negative mediators of insulin sensitivity [68] in prenatal testosterone treated sheep raises the possibility that the increase in negative mediators of insulin sensitivity in the prenatal BPA-treated model may also be accompanied by compromised muscle insulin sensitivity. Such decreased AKT and glycogen synthase kinase 3 beta have been observed in the skeletal muscle from orally BPA administered mice [69]. Cohen’s effect size analysis pointed to an increase in multiple inflammatory cytokines in the skeletal muscle. As these data did not reach statistical significance the relative contribution of inflammation in IR status of skeletal muscle needs to be examined with larger sample size.
VAT:
Adipocyte hypertrophy, inflammation and oxidative stress in the VAT are characteristic features of conditions associated with IR [70–72]. Findings from the present study of a trend towards increased oxidative stress marker TBARS (p = 0.06, high dose only) and previously reported increased oxidative stress marker NY [21], negative mediators of insulin sensitivity, are consistent with the adipocyte hypertrophy [19] and IR [19], reported in this model. The increase in oxidative stress state manifested as lack of increase in GSR activity in BPAlow and BPAhigh dose groups coupled with increases in oxidative stress markers (22) is also a feature consistent with conditions associated with IR [70]. Considering that the inflammatory status of the adipose tissue was evident as increase in expression of cytokines IL1B and CCL2 (high dose) by effect size analysis only, confirmation of their proinflammatory status needs further investigation. The reduced circulating level of adiponectin, a major adipokine with anti-inflammatory role secreted by the white adipose tissue [26], is supportive of potential proinflammatory state.
Findings from this study of increase in the mRNA expression of CYP19 in the VAT suggests that the steroidogenic biosynthetic machinery favors increase in estrogen biosynthesis. This coupled with increased ESR1 (p < 0.05 for medium and high dose, by t test) and the trend for decreased AR mRNA expression (p = 0.09 for high dose only by t test) indicates a predominant tendency for intracrine estrogenic milieu in the VAT of prenatal BPA-treated sheep. An estrogenic intracrine milieu is not consistent with this adipocyte hypertrophy phenotype [19] and contrasts with the observations in prenatal testosterone-treated female sheep where an increase in estrogenic milieu in the VAT accompanies smaller adipocytes [73, 74]. Estrogens have been shown to reduce adipocyte cell size by decreasing adipocyte lipid storage and increasing lipolysis [33, 75]. The increase in liver and muscle lipid accumulation in prenatal BPA-treated sheep coupled with the intracrine estrogenic milieu is suggestive of increased lipolysis. Therefore, the increase in adipocyte size in the prenatal BPA-treated sheep may be independent of estrogen’s effect at the level of lipid storage and may occur at the level of adipocyte differentiation; enhanced differentiation of preadipocytes have been observed in sheep exposed to BPA during gestation [76].
Conclusions
In summary, prenatal BPA treatment increases the negative mediators of insulin sensitivity such as oxidative stress and lipotoxicity and reduces positive mediators of insulin sensitivity such as adiponectin and antioxidants in the circulation and the metabolic tissues of the prenatal BPA-treated female sheep (Table 3). While there is the possibility of involvement of inflammatory pathways in the muscle and VAT, these findings need to interpreted with caution considering that such changes were only based on effect size analysis. Although the findings relative to the inflammatory status of tissues studied are inconclusive and a limitation of this study, the directionality of changes in inflammatory cytokines suggested by effect size analysis is in line with other significant changes in negative mediators of insulin sensitivity (oxidative stress and lipotoxicity) that are generally associated with development of IR at the peripheral as well as tissue level [77]. Additional studies are warranted to conclusively prove the involvement of inflammatory markers in prenatal BPA induction of tissue-specific IR. Changes in negative mediators of insulin sensitivity in muscle, liver and adipose tissues is postulated to be the common cause of IR associated with metabolic conditions such as type 2 diabetes, obesity, cardiovascular diseases, NAFLD, etc. [78, 79], a feature also seen in tissue-specific conditional knockouts of insulin receptor (an animal model with loss of insulin sensitivity) [80]. Whether these changes in negative mediators of insulin sensitivity accompanies reduced insulin signaling at the tissue level, such as that observed in prenatal testosterone-treated sheep [77] needs to be determined. Additional studies are also warranted to understand the mechanisms via which gestational exposure to BPA program these tissue-specific changes. The finding of increased hepatic DNA methylation and development of IR during adulthood [81] in perinatal BPA treated rats suggests that epigenetic modifications could be the mediaries. While this study focused on the changes in mediators of insulin sensitivity, recent finding that gestational BPA alters fetal thyroid function [82], important regulators of metabolic functions including hepatic steatosis [83], suggests disruptions in thyroid function may also be a contributor to the prenatal BPA-induced metabolic disruptions.
Table 3:
Summary of tissue-specific changes in the mediators of insulin sensitivity in prenatal BPA-treated female sheep
| Tissue | Adiponectin | Inflammatory State | Oxidative Stress | Lipotoxicity | Steroidal Milieu | Predicted Effect on Insulin Sensitivity |
|---|---|---|---|---|---|---|
| Systemic | ↓ | NE | ↑# | - | NE | Resistant |
| Liver | - | ? | ↑ | ↑ | NE | Resistant |
| Skeletal Muscle | - | Inflammatory* | ↑ | ↑ | NE | Resistant |
| VAT | NE | Inflammatory* | ↑# | NE | Estrogenic* | Resistant |
NE= not examined; Arrows indicate significant outcomes.
Effect size analysis point to this outcome- requires validation with larger sample size.
Previously reported in Veiga-Lopez et al 2015 (21)
Although these studies were carried out in a polygastric species, findings have the potential to be of translational relevance to human, a monogastric species. There are several similarities between the two species relative to the metabolic focus of the study namely low blood glucose levels due to fasting, postprandial changes in blood glucose / insulin, tissue-level insulin action, and development of insulin resistance with increased adiposity, [84–87]. Additionally, fetal developmental timeline, precociality at birth, diurnal behavioral pattern, and pathways that regulate appetite, energy balance, and adipogenesis, and distribution of brown/beige fat in white adipose tissues are also similar between sheep and humans [20, 88–91] thus making them valuable translational models. However, as these polygastric animals predominantly use non-esterified free fatty acids (NEFFA) as a source of energy, further studies are required to evaluate if disruptions in homeostasis of NEFFA and their associated regulatory pathways have any role in the manifestation of metabolic disruptions.
In summary, findings from this study provide evidence in support of tissue-specific developmental impact of prenatal BPA treatment, at environmentally relevant levels, on metabolic mediators of insulin sensitivity.
Highlights.
Prenatal BPA disrupts mediators of insulin sensitivity in female sheep
Prenatal BPA induces oxidative stress status in metabolic tissues
Prenatal BPA induces dyslipidemia and lipotoxicity in the liver and muscle
Elevated negative mediators of insulin sensitivity underlie prenatal BPA effects
ACKNOWLEDGMENTS
We thank Douglas Doop and Gary McCalla of the Department of Pediatrics at the University of Michigan for their valuable assistance in breeding, lambing, and careful animal care and Dr. Almudena Veiga-Lopez, Dr. Bachir Abi Salloum, Jacob Moeller, Evan M. Beckett, Carol Herkimer, Alexandria Williams, and Aishwarya Navalpakam of the Department of Pediatrics at the University of Michigan for providing help with prenatal treatment and tissue harvest.
Funding: This work was supported by National Institute of Environmental Health Sciences Grant R01-ES-016541 (VP) and Ruth L. Kirschstein Institutional Training Grant from the National Institutes of Health/National Institute for Environmental Health Sciences T32 ES007062 to MP
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: The authors have nothing to disclose.
REFERENCES
- [1].Landrigan PJ, Sly JL, Ruchirawat M, Silva ER, Huo X, Diaz-Barriga F, Zar HJ, King M, Ha EH, Asante KA, Ahanchian H, Sly PD, Health Consequences of Environmental Exposures: Changing Global Patterns of Exposure and Disease, Ann Glob Health 82(1) (2016) 10–9. [DOI] [PubMed] [Google Scholar]
- [2].Zimmet PZ, Alberti KG, Introduction: Globalization and the non-communicable disease epidemic, Obesity (Silver Spring) 14(1) (2006) 1–3. [DOI] [PubMed] [Google Scholar]
- [3].Fourth National Report on Human Exposure to Environmental Chemicals, US Department of Health and Human Services Centres for Disease Control and Prevention, Atlanta, GA: 2014. [Google Scholar]
- [4].Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL, Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population, Environmental health perspectives 113(4) (2005) 391–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL, Exposure of the US population to bisphenol A and 4-tertiary-octylphenol: 2003–2004, Environmental health perspectives 116(1) (2008) 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vogel SA, The politics of plastics: the making and unmaking of bisphenol a “safety”, Am J Public Health 99 Suppl 3 (2009) S559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vom Saal FS, TRIENNIAL REPRODUCTION SYMPOSIUM: Environmental programming of reproduction during fetal life: Effects of intrauterine position and the endocrine disrupting chemical bisphenol A, J Anim Sci 94(7) (2016) 2722–36. [DOI] [PubMed] [Google Scholar]
- [8].Vom Saal FS, Nagel SC, Coe BL, Angle BM, Taylor JA, The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity, Molecular and cellular endocrinology 354(1–2) (2012) 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lee HJ, Chattopadhyay S, Gong EY, Ahn RS, Lee K, Antiandrogenic effects of bisphenol A and nonylphenol on the function of androgen receptor, Toxicol Sci 75(1) (2003) 40–6. [DOI] [PubMed] [Google Scholar]
- [10].Ahmed RG, Maternal bisphenol A alters fetal endocrine system: Thyroid adipokine dysfunction, Food Chem Toxicol 95 (2016) 168–74. [DOI] [PubMed] [Google Scholar]
- [11].Newbold RR, Impact of environmental endocrine disrupting chemicals on the development of obesity, Hormones (Athens) 9(3) (2010) 206–17. [DOI] [PubMed] [Google Scholar]
- [12].Teppala S, Madhavan S, Shankar A, Bisphenol A and Metabolic Syndrome: Results from NHANES, Int J Endocrinol 2012 (2012) 598180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang T, Li M, Chen B, Xu M, Xu Y, Huang Y, Lu J, Chen Y, Wang W, Li X, Liu Y, Bi Y, Lai S, Ning G, Urinary bisphenol A (BPA) concentration associates with obesity and insulin resistance, J Clin Endocrinol Metab 97(2) (2012) E223–7. [DOI] [PubMed] [Google Scholar]
- [14].Lee BE, Park H, Hong YC, Ha M, Kim Y, Chang N, Kim BN, Kim YJ, Yu SD, Ha EH, Prenatal bisphenol A and birth outcomes: MOCEH (Mothers and Children’s Environmental Health) study, Int J Hyg Environ Health 217(2–3) (2014) 328–34. [DOI] [PubMed] [Google Scholar]
- [15].Veiga-Lopez A, Kannan K, Liao C, Ye W, Domino SE, Padmanabhan V, Gender-Specific Effects on Gestational Length and Birth Weight by Early Pregnancy BPA Exposure, J Clin Endocrinol Metab 100(11) (2015) E1394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Barker DJ, The developmental origins of insulin resistance, Horm Res 64 Suppl 3 (2005) 2–7. [DOI] [PubMed] [Google Scholar]
- [17].Alonso-Magdalena P, Vieira E, Soriano S, Menes L, Burks D, Quesada I, Nadal A, Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring, Environmental health perspectives 118(9) (2010) 1243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].vom Saal FS, Akingbemi BT, Belcher SM, Birnbaum LS, Crain DA, Eriksen M, Farabollini F, Guillette LJ Jr., Hauser R, Heindel JJ, Ho SM, Hunt PA, Iguchi T, Jobling S, Kanno J, Keri RA, Knudsen KE, Laufer H, LeBlanc GA, Marcus M, McLachlan JA, Myers JP, Nadal A, Newbold RR, Olea N, Prins GS, Richter CA, Rubin BS, Sonnenschein C, Soto AM, Talsness CE, Vandenbergh JG, Vandenberg LN, Walser-Kuntz DR, Watson CS, Welshons WV, Wetherill Y, Zoeller RT, Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure, Reprod Toxicol 24(2) (2007) 131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Veiga-Lopez A, Moeller J, Sreedharan R, Singer K, Lumeng C, Ye W, Pease A, Padmanabhan V, Developmental programming: interaction between prenatal BPA exposure and postnatal adiposity on metabolic variables in female sheep, Am J Physiol Endocrinol Metab 310(3) (2016) E238–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Padmanabhan V, Veiga-Lopez A, Reproduction Symposium: developmental programming of reproductive and metabolic health, J Anim Sci 92(8) (2014) 3199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Veiga-Lopez A, Pennathur S, Kannan K, Patisaul HB, Dolinoy DC, Zeng L, Padmanabhan V, Impact of gestational bisphenol A on oxidative stress and free fatty acids: Human association and interspecies animal testing studies, Endocrinology 156(3) (2015) 911–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liu W, Baker RD, Bhatia T, Zhu L, Baker SS, Pathogenesis of nonalcoholic steatohepatitis, Cell Mol Life Sci 73(10) (2016) 1969–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N, Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes, Diabetes research and clinical practice 105(2) (2014) 141–50. [DOI] [PubMed] [Google Scholar]
- [24].Verdile G, Keane KN, Cruzat VF, Medic S, Sabale M, Rowles J, Wijesekara N, Martins RN, Fraser PE, Newsholme P, Inflammation and Oxidative Stress: The Molecular Connectivity between Insulin Resistance, Obesity, and Alzheimer’s Disease, Mediators of inflammation 2015 (2015) 105828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Styskal J, Van Remmen H, Richardson A, Salmon AB, Oxidative stress and diabetes: what can we learn about insulin resistance from antioxidant mutant mouse models?, Free Radic Biol Med 52(1) (2012) 46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Caselli C, Role of adiponectin system in insulin resistance, Mol Genet Metab 113(3) (2014) 155–60. [DOI] [PubMed] [Google Scholar]
- [27].Lee BC, Lee J, Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance, Biochimica et biophysica acta 1842(3) (2014) 446–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rani V, Deep G, Singh RK, Palle K, Yadav UC, Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies, Life sciences 148 (2016) 183–93. [DOI] [PubMed] [Google Scholar]
- [29].Shulman GI, Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease, N Engl J Med 371(12) (2014) 1131–41. [DOI] [PubMed] [Google Scholar]
- [30].Bashan N, Kovsan J, Kachko I, Ovadia H, Rudich A, Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species, Physiol Rev 89(1) (2009) 27–71. [DOI] [PubMed] [Google Scholar]
- [31].Kadowaki T, Yamauchi T, Adiponectin and adiponectin receptors, Endocr Rev 26(3) (2005) 439–51. [DOI] [PubMed] [Google Scholar]
- [32].Fu Y, Adiponectin signaling and metabolic syndrome, Prog Mol Biol Transl Sci 121 (2014) 293–319. [DOI] [PubMed] [Google Scholar]
- [33].Newell-Fugate AE, The role of sex steroids in white adipose tissue adipocyte function, Reproduction 153(4) (2017) R133–R149. [DOI] [PubMed] [Google Scholar]
- [34].Turgeon JL, Carr MC, Maki PM, Mendelsohn ME, Wise PM, Complex actions of sex steroids in adipose tissue, the cardiovascular system, and brain: Insights from basic science and clinical studies, Endocr Rev 27(6) (2006) 575–605. [DOI] [PubMed] [Google Scholar]
- [35].Janesick AS, Blumberg B, Obesogens: an emerging threat to public health, Am J Obstet Gynecol 214(5) (2016) 559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Somm E, Schwitzgebel VM, Toulotte A, Cederroth CR, Combescure C, Nef S, Aubert ML, Huppi PS, Perinatal exposure to bisphenol a alters early adipogenesis in the rat, Environmental health perspectives 117(10) (2009) 1549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Li J, Papadopoulos V, Vihma V, Steroid biosynthesis in adipose tissue, Steroids 103 (2015) 89–104. [DOI] [PubMed] [Google Scholar]
- [38].Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, Brown MB, Foster DL, Padmanabhan V, Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep, Endocrinology 145(2) (2004) 790–8. [DOI] [PubMed] [Google Scholar]
- [39].Veiga-Lopez A, Luense LJ, Christenson LK, Padmanabhan V, Developmental programming: gestational bisphenol-A treatment alters trajectory of fetal ovarian gene expression, Endocrinology 154(5) (2013) 1873–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gerona RR, Woodruff TJ, Dickenson CA, Pan J, Schwartz JM, Sen S, Friesen MW, Fujimoto VY, Hunt PA, Bisphenol-A (BPA), BPA glucuronide, and BPA sulfate in midgestation umbilical cord serum in a northern and central California population, Environ Sci Technol 47(21) (2013) 12477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lee J, Choi K, Park J, Moon HB, Choi G, Lee JJ, Suh E, Kim HJ, Eun SH, Kim GH, Cho GJ, Kim SK, Kim S, Kim SY, Kim S, Eom S, Choi S, Kim YD, Kim S, Bisphenol A distribution in serum, urine, placenta, breast milk, and umbilical cord serum in a birth panel of mother-neonate pairs, Sci Total Environ 626 (2018) 1494–1501. [DOI] [PubMed] [Google Scholar]
- [42].Viguie C, Collet SH, Gayrard V, Picard-Hagen N, Puel S, Roques BB, Toutain PL, Lacroix MZ, Maternal and fetal exposure to bisphenol a is associated with alterations of thyroid function in pregnant ewes and their newborn lambs, Endocrinology 154(1) (2013) 521–8. [DOI] [PubMed] [Google Scholar]
- [43].Veiga-Lopez A, Beckett EM, Abi Salloum B, Ye W, Padmanabhan V, Developmental programming: prenatal BPA treatment disrupts timing of LH surge and ovarian follicular wave dynamics in adult sheep, Toxicol Appl Pharmacol 279(2) (2014) 119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Veiga-Lopez A, Moeller J, Patel D, Ye W, Pease A, Kinns J, Padmanabhan V, Developmental programming: impact of prenatal testosterone excess on insulin sensitivity, adiposity, and free fatty acid profile in postpubertal female sheep, Endocrinology 154(5) (2013) 1731–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Puttabyatappa M, Andriessen V, Mesquitta M, Zeng L, Pennathur S, Padmanabhan V, Developmental Programming: Impact of Gestational Steroid and Metabolic Milieus on Mediators of Insulin Sensitivity in Prenatal Testosterone-Treated Female Sheep, Endocrinology (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Mielenz M, Mielenz B, Singh SP, Kopp C, Heinz J, Haussler S, Sauerwein H, Development, validation, and pilot application of a semiquantitative Western blot analysis and an ELISA for bovine adiponectin, Domest Anim Endocrinol 44(3) (2013) 121–30. [DOI] [PubMed] [Google Scholar]
- [47].Vivekanandan-Giri A, Byun J, Pennathur S, Quantitative analysis of amino Acid oxidation markers by tandem mass spectrometry, Methods in enzymology 491 (2011) 73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lejeune M, Jaen J, Pons L, Lopez C, Salvado MT, Bosch R, Garcia M, Escriva P, Baucells J, Cugat X, Alvaro T, Quantification of diverse subcellular immunohistochemical markers with clinicobiological relevancies: validation of a new computer-assisted image analysis procedure, J Anat 212(6) (2008) 868–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ortega HH, Salvetti NR, Padmanabhan V, Developmental programming: prenatal androgen excess disrupts ovarian steroid receptor balance, Reproduction 137(5) (2009) 865–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bligh EG, Dyer WJ, A rapid method of total lipid extraction and purification, Can J Biochem Physiol 37(8) (1959) 911–7. [DOI] [PubMed] [Google Scholar]
- [51].Cohen J, A power primer, Psychol Bull 112(1) (1992) 155–9. [DOI] [PubMed] [Google Scholar]
- [52].Nakagawa S, Cuthill IC, Effect size, confidence interval and statistical significance: a practical guide for biologists, Biol Rev Camb Philos Soc 82(4) (2007) 591–605. [DOI] [PubMed] [Google Scholar]
- [53].Padmanabhan V, Veiga-Lopez A, Herkimer C, Abi Salloum B, Moeller J, Beckett E, Sreedharan R, Developmental Programming: Prenatal and Postnatal Androgen Antagonist and Insulin Sensitizer Interventions Prevent Advancement of Puberty and Improve LH Surge Dynamics in Prenatal Testosterone-Treated Sheep, Endocrinology 156(7) (2015) 2678–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lagarde F, Beausoleil C, Belcher SM, Belzunces LP, Emond C, Guerbet M, Rousselle C, Non-monotonic dose-response relationships and endocrine disruptors: a qualitative method of assessment, Environ Health 14 (2015) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Faulk C, Kim JH, Jones TR, McEachin RC, Nahar MS, Dolinoy DC, Sartor MA, Bisphenol A-associated alterations in genome-wide DNA methylation and gene expression patterns reveal sequence-dependent and non-monotonic effects in human fetal liver, Environ Epigenet 1(1) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Vandenberg LN, Non-monotonic dose responses in studies of endocrine disrupting chemicals: bisphenol a as a case study, Dose Response 12(2) (2014) 259–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].van Esterik JC, Dolle ME, Lamoree MH, van Leeuwen SP, Hamers T, Legler J, van der Ven LT, Programming of metabolic effects in C57BL/6JxFVB mice by exposure to bisphenol A during gestation and lactation, Toxicology 321 (2014) 40–52. [DOI] [PubMed] [Google Scholar]
- [58].Song S, Zhang L, Zhang H, Wei W, Jia L, Perinatal BPA exposure induces hyperglycemia, oxidative stress and decreased adiponectin production in later life of male rat offspring, Int J Environ Res Public Health 11(4) (2014) 3728–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Miyawaki J, Sakayama K, Kato H, Yamamoto H, Masuno H, Perinatal and postnatal exposure to bisphenol a increases adipose tissue mass and serum cholesterol level in mice, J Atheroscler Thromb 14(5) (2007) 245–52. [DOI] [PubMed] [Google Scholar]
- [60].Wei J, Lin Y, Li Y, Ying C, Chen J, Song L, Zhou Z, Lv Z, Xia W, Chen X, Xu S, Perinatal exposure to bisphenol A at reference dose predisposes offspring to metabolic syndrome in adult rats on a high-fat diet, Endocrinology 152(8) (2011) 3049–61. [DOI] [PubMed] [Google Scholar]
- [61].Strakovsky RS, Wang H, Engeseth NJ, Flaws JA, Helferich WG, Pan YX, Lezmi S, Developmental bisphenol A (BPA) exposure leads to sex-specific modification of hepatic gene expression and epigenome at birth that may exacerbate high-fat diet-induced hepatic steatosis, Toxicol Appl Pharmacol 284(2) (2015) 101–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Mehlem A, Hagberg CE, Muhl L, Eriksson U, Falkevall A, Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease, Nat Protoc 8(6) (2013) 1149–54. [DOI] [PubMed] [Google Scholar]
- [63].Biasiotto G, Zanella I, Masserdotti A, Pedrazzani R, Papa M, Caimi L, Di Lorenzo D, Municipal wastewater affects adipose deposition in male mice and increases 3T3-L1 cell differentiation, Toxicol Appl Pharmacol 297 (2016) 32–40. [DOI] [PubMed] [Google Scholar]
- [64].Foulds CE, Trevino LS, York B, Walker CL, Endocrine-disrupting chemicals and fatty liver disease, Nat Rev Endocrinol 13(8) (2017) 445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Lonardo A, Nascimbeni F, Maurantonio M, Marrazzo A, Rinaldi L, Adinolfi LE, Nonalcoholic fatty liver disease: Evolving paradigms, World J Gastroenterol 23(36) (2017) 6571–6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Perry RJ, Samuel VT, Petersen KF, Shulman GI, The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes, Nature 510(7503) (2014) 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Lassailly G, Caiazzo R, Pattou F, Mathurin P, Perspectives on Treatment for Nonalcoholic Steatohepatitis, Gastroenterology 150(8) (2016) 1835–48. [DOI] [PubMed] [Google Scholar]
- [68].Lu C, Cardoso RC, Puttabyatappa M, Padmanabhan V, Developmental Programming: Prenatal Testosterone Excess and Insulin Signaling Disruptions in Female Sheep, Biology of reproduction 94(5) (2016) 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Moon MK, Jeong IK, Jung Oh T, Ahn HY, Kim HH, Park YJ, Jang HC, Park KS, Long-term oral exposure to bisphenol A induces glucose intolerance and insulin resistance, J Endocrinol 226(1) (2015) 35–42. [DOI] [PubMed] [Google Scholar]
- [70].Evans JL, Maddux BA, Goldfine ID, The molecular basis for oxidative stress-induced insulin resistance, Antioxid Redox Signal 7(7–8) (2005) 1040–52. [DOI] [PubMed] [Google Scholar]
- [71].Gustafson B, Hedjazifar S, Gogg S, Hammarstedt A, Smith U, Insulin resistance and impaired adipogenesis, Trends Endocrinol Metab 26(4) (2015) 193–200. [DOI] [PubMed] [Google Scholar]
- [72].Kloting N, Bluher M, Adipocyte dysfunction, inflammation and metabolic syndrome, Reviews in endocrine & metabolic disorders 15(4) (2014) 277–87. [DOI] [PubMed] [Google Scholar]
- [73].Cardoso RC, Veiga-Lopez A, Moeller J, Beckett E, Pease A, Keller E, Madrigal V, Chazenbalk G, Dumesic D, Padmanabhan V, Developmental Programming: Impact of Gestational Steroid and Metabolic Milieus on Adiposity and Insulin Sensitivity in Prenatal Testosterone-Treated Female Sheep, Endocrinology 157(2) (2016) 522–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Puttabyatappa M, Lu C, Martin JD, Chazenbalk G, Dumesic D, Padmanabhan V, Developmental Programming: Impact of Prenatal Testosterone Excess on Steroidal Machinery and Cell Differentiation Markers in Visceral Adipocytes of Female Sheep, Reprod Sci (2017) 1933719117746767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Tchernof A, Calles-Escandon J, Sites CK, Poehlman ET, Menopause, central body fatness, and insulin resistance: effects of hormone-replacement therapy, Coron Artery Dis 9(8) (1998) 503–11. [DOI] [PubMed] [Google Scholar]
- [76].Pu Y, Gingrich JD, Steibel JP, Veiga-Lopez A, Sex-Specific Modulation of Fetal Adipogenesis by Gestational Bisphenol A and Bisphenol S Exposure, Endocrinology 158(11) (2017) 3844–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Puttabyatappa M, Padmanabhan V, Prenatal Testosterone Programming of Insulin Resistance in the Female Sheep, Adv Exp Med Biol 1043 (2017) 575–596. [DOI] [PubMed] [Google Scholar]
- [78].Withers DJ, White M, Perspective: The insulin signaling system--a common link in the pathogenesis of type 2 diabetes, Endocrinology 141(6) (2000) 1917–21. [DOI] [PubMed] [Google Scholar]
- [79].Kaur J, A comprehensive review on metabolic syndrome, Cardiol Res Pract 2014 (2014) 943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [80].Biddinger SB, Kahn CR, From mice to men: insights into the insulin resistance syndromes, Annu Rev Physiol 68 (2006) 123–58. [DOI] [PubMed] [Google Scholar]
- [81].Ma Y, Xia W, Wang DQ, Wan YJ, Xu B, Chen X, Li YY, Xu SQ, Hepatic DNA methylation modifications in early development of rats resulting from perinatal BPA exposure contribute to insulin resistance in adulthood, Diabetologia 56(9) (2013) 2059–67. [DOI] [PubMed] [Google Scholar]
- [82].Guignard D, Gayrard V, Lacroix MZ, Puel S, Picard-Hagen N, Viguie C, Evidence for bisphenol A-induced disruption of maternal thyroid homeostasis in the pregnant ewe at low level representative of human exposure, Chemosphere 182 (2017) 458–467. [DOI] [PubMed] [Google Scholar]
- [83].Mullur R, Liu YY, Brent GA, Thyroid hormone regulation of metabolism, Physiol Rev 94(2) (2014) 355–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Sternbauer K, Luthman J, Insulin sensitivity of heifers on different diets, Acta Vet Scand 43(2) (2002) 107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Janes AN, Weekes TE, Armstrong DG, Insulin action and glucose metabolism in sheep fed on dried-grass or ground, maize-based diets, Br J Nutr 54(2) (1985) 459–71. [DOI] [PubMed] [Google Scholar]
- [86].Sano H, Matsunobu S, Nakagawa M, Terashima Y, Insulin responsiveness to glucose and tissue responsiveness to insulin over the feeding cycle in sheep, J Anim Sci 68(11) (1990) 3736–41. [DOI] [PubMed] [Google Scholar]
- [87].Clarke IJ, Models of ‘obesity’ in large animals and birds, Front Horm Res 36 (2008) 107–17. [DOI] [PubMed] [Google Scholar]
- [88].Bassett JM, Diurnal patterns of plasma insulin, growth hormone, corticosteroid and metabolite concentrations in fed and fasted sheep, Aust J Biol Sci 27(2) (1974) 167–81. [DOI] [PubMed] [Google Scholar]
- [89].McMillen IC, Adam CL, Muhlhausler BS, Early origins of obesity: programming the appetite regulatory system, J Physiol 565(Pt 1) (2005) 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Gonzalez-Bulnes A, Chavatte-Palmer P, Contribution of Large Animals to Translational Research on Prenatal Programming of Obesity and Associated Diseases, Curr Pharm Biotechnol 18(7) (2017) 541–551. [DOI] [PubMed] [Google Scholar]
- [91].Fuller-Jackson JP, Henry BA, Adipose and skeletal muscle thermogenesis: studies from large animals, J Endocrinol 237(3) (2018) R99–R115. [DOI] [PubMed] [Google Scholar]