Abstract
Wet age-related macular degeneration (AMD) with choroidal neovascularization (CNV) is a leading cause of vision loss in the elderly. The advent of anti-vascular endothelial growth factor (VEGF) drugs represents a major breakthrough in wet AMD therapy but with limited efficacy to improve visual acuity. Secretogranin III (Scg3, SgIII) was recently discovered as a novel angiogenic factor with VEGF-independent mechanisms. Scg3-neutralizing monoclonal antibody (mAb) was reported to alleviate pathological retinal neovascularization in oxygen-induced retinopathy mice and retinal vascular leakage in diabetic mice with high efficacy and disease selectivity. Herein we investigated whether Scg3 is a novel angiogenic target for CNV therapy in mouse models. We found that anti-Scg3 ML49.3 mAb inhibited Scg3-induced proliferation and Src phosphorylation in human retinal microvascular endothelial cells. Intravitreal injection of Scg3-neutralizing polyclonal antibodies (pAb) or mAb significantly attenuated laser-induced CNV leakage, CNV 3D volume, lesion area and vessel density. Furthermore, subcutaneous administration of Scg3-neutralizing pAb or mAb significantly prevented Matrigel-induced CNV. The efficacy of anti-Scg3 pAb or mAb was comparable to VEGF inhibitor aflibercept. These findings suggest that Scg3 plays an important role in CNV pathogenesis and that anti-Scg3 mAb efficiently ameliorates laser- or Matrigel-induced CNV.
Keywords: Secretogranin III, Scg3, angiogenic factor, anti-angiogenic therapy, choroidal neovascularization, CNV, AMD
1. Introduction
Age-related macular degeneration (AMD) is a major cause of vision impairment and blindness in the elderly in developed countries. It is projected that 196 million people worldwide will be affected by AMD in 2020, increasing to 288 million in 2040 (Wong et al., 2014). AMD has two clinical forms: dry (atrophic) and wet (neovascular or exudative). Wet AMD with choroidal neovascularization (CNV) afflicts 10–20% of individuals with the disease but accounts for ~90% of all cases with severe vision loss from the disease (Votruba and Gregor, 2001). The approval of vascular endothelial growth factor (VEGF) inhibitors, including ranibizumab and aflibercept, represents a major advance in wet AMD therapy (Kim and D’Amore, 2012). However, anti-VEGF therapies have limited efficacies to improve vision (Brown et al., 2009; Rosenfeld et al., 2006), implicating that other angiogenic factors may be involved in the disease pathogenesis. Therapies against other angiogenic factors, such as PDGF, Ang2, integrin αvβ3, erythropoietin and endoglin, are currently under intense investigation (Cabral et al., 2017). Owing to few options, AMD patients with a poor response to one anti-VEGF drug are often switched to another VEGF inhibitor (Pinheiro-Costa et al., 2014), despite their similar mechanisms of action (MOAs). Developing new anti-angiogenic therapies against VEGF-independent angiogenic factors and pathways may help improve the efficacy through alternative or combination therapy.
We recently discovered secretogranin III (Scg3, SgIII) not only as a novel angiogenic factor but also as a highly disease-restricted ligand, which selectively bound to diabetic but not normal retinal vessels in mice (LeBlanc et al., 2017). Indeed, Scg3 preferentially stimulated angiogenesis of diabetic but not normal vasculature through VEGF-independent MOAs. In contrast, VEGF bound to and induced angiogenesis of both diabetic and control vessels. We further developed Scg3-neutralizing ML49.3 mAb and demonstrated its high efficacy to ameliorate retinal vascular leakage in diabetic mice (LeBlanc et al., 2017). Interestingly, Anti-Scg3 mAb also showed high efficacy to inhibit pathological retinal neovascularization in oxygen-induced retinopathy (OIR) mice, suggesting that Scg3 may play an important pathological role in neovascular diseases besides diabetic vascular leakage.
Based on these findings, we hypothesize that Scg3 may also involve in the pathogenesis of wet AMD and could be a potential target for anti-angiogenic therapy of CNV. Here, we investigated the pathogenic role of Scg3 in CNV by characterizing the therapeutic activity of anti-Scg3 mAb. We demonstrated that anti-Scg3 mAb via either intravitreal or subcutaneous administration efficiently alleviated laser- or Matrigel-induced CNV in mice. The implication of these findings to potential anti-Scg3 therapy of wet AMD is discussed.
2. Material and Methods
2.1. Animals
C57BL/6J mice (6 weeks old, male or female) were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were maintained and handled in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Miami.
2.2. Materials
Antigen affinity-purified anti-Scg3 polyclonal antibody (pAb) was purchased from Proteintech (Rosement, IL). Anti-Scg3 ML49.3 mAb was purified from serum-free conditioned medium of ML49.3 hybridoma as described (LeBlanc et al., 2017). All antibodies were washed three times with phosphate-buffered saline (PBS) in Amicon centrifugal filter spin units (10 kDa cutoff, Millipore, Billerica, MA). Human retinal microvascular endothelial cells (HRMVECs) and complete classic medium kit with serum and CultureBoost were obtained from Cell Systems (Kirkland, WA) (LeBlanc et al., 2017; LeBlanc et al., 2016). Human Scg3 were from Sino Biological (Beijing, China). Aflibercept is a drug from Regeneron Pharmaceuticals (Tarrytown, NY).
2.3. Cell proliferation
HRMVECs at 4–8 passages were cultured with Scg3 or medium control in the presence or absence of anti-Scg3 mAb in 96-well plates (LeBlanc et al., 2015). Cells in each well were collected by trypsin digestion at 48 h and counted.
2.4. Src activation
Src kinase activation was detected as described (LeBlanc et al., 2017). HRMVECs were incubated overnight in EBM-2 medium (Lonza, Allendale, NJ) supplemented with 0.2% FBS to reduce the effect of other growth factors. Cells were incubated with Scg3 or PBS in EBM-2 medium with or without anti-Scg3 mAb for 10 min in 37°C, lysed and analyzed by Western blot using antibody against phosphorylated Src (P-Src), Src or β-actin (Santa Cruz Biotechnology, Dallas, TX). Western blot signals were digitalized and normalized against total Src.
2.5. Therapy of laser-induced CNV
C57BL/6 mice were subjected to laser photocoagulation (Argon laser, 532 nm, 100 mW, 100 ms, 100 μm, 4 spots/retina) to induce CNV on Day 0, as described (Wang et al., 2017). Lesions with choroidal hemorrhage and linear or fused lesions were excluded (Poor et al., 2014). However, hemorrhagic spots occurred rarely, and exclusion was mostly for linear spots or fusion spots. On Day 3, anti-Scg3 pAb, control IgG, anti-Scg3 ML49.3 mAb (0.36 μg/1 μl/eye) or aflibercept (2 μg/1 μl/eye) was intravitreally injected. Aflibercept at 2 μg/eye in mice is equivalent to 2 mg/eye in humans for wet AMD therapy based on their relative vitreous volumes. To avoid human bias, reagents were coded for blinded study. On Day 7, mice received intraperitoneal injection of fluorescein sodium (0.1 ml, 2.5%) were analyzed for CNV leakage by fluorescein angiography 6 min post injection. Instrument settings for detection sensitivity were kept at the same levels for all quantification. The intensity of laser spots was quantified using ImageJ software (NIH) and normalized against the total intensity of the entire viewing field. On day 8, eyecups of the retinal pigment epithelium (RPE)-choroid-sclera complex were isolated from mice euthanized by CO2 inhalation, stained with Alexa Fluor 488-isolectin B4, flat-mounted and analyzed by confocal microscopy (Wang et al., 2017). We harvested RPE eyecups 24 h after fluorescein angiography to allow the clearance of fluorescein and minimize its interference with subsequent confocal analysis. CNV 3D volume was deconvoluted from z-stack images and quantified using Volocity software. The area size of the largest CNV z-stack image of each lesion and its fluorescence intensity were quantified to determine the CNV size and vessel density using ImageJ. Vessel density signals were normalized against background control.
2.6. Therapy of Matrigel-induced CNV
Growth factor-reduced Matrigel (Corning, Corning, NY, USA; Cat. #354263) was diluted 1:4 with PBS and injected into the subretinal space of C57BL/6 mice (0.8 μl/retina) in one eye of mice on Day 0 with PBS for the fellow eye (Cao et al., 2010). Anti-Scg3 pAb, rabbit control IgG, anti-Scg3 mAb, mouse control IgG1 (Clone 9E10) (LeBlanc et al., 2017), or aflibercept was subcutaneously injected on Day 0, 2 and 4. All reagents were coded for blinded study. On Day 7, fluorescein angiography was performed to analyze CNV leakage as above.
2.7. Statistical analysis
Data were expressed as mean ± SEM and analyzed by one-way ANOVA test.
3. Results
3.1. Neutralizing activity of anti-Scg3 mAb
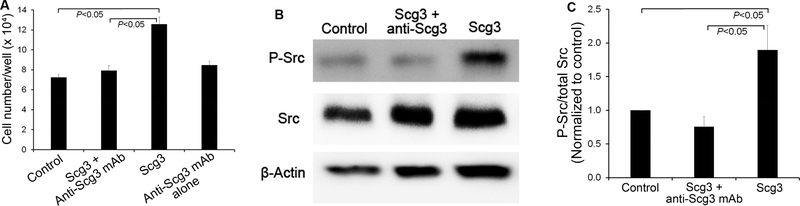
We demonstrated that Scg3 significantly induced the proliferation of HRMVECs (P<0.05) and that ML49.3 mAb inhibited Scg3-induced proliferation (P<0.05, Fig. 1A). Furthermore, signaling studies revealed that Scg3 significantly stimulated the phosphorylation of Src kinase in HRMVECs (P<0.05) and that ML49.3 mAb blocked Scg3-induced Src activation (P<0.05, Fig. 1B,C).
Fig. 1. Anti-Scg3 mAb neutralizes Scg3 functional activity.
(A) Anti-Scg3 mAb inhibits Scg3-induced proliferation of HRMVECs. Cells were incubated with Scg3 (1 μg/ml) in the presence or absence of anti-Scg3 ML49.3 mAb (2 μg/ml) in 96-well plates for 48 h. Cell number per well was quantified. n=6 wells/group. (B) Anti-Scg3 mAb blocks Scg3-induced activation of Src kinase. HRMVECs were incubated with Scg3 (1 μg/ml) in the presence or absence of anti-Scg3 ML49.3 mAb (2 μg/ml) for 10 min. Cells were analyzed by Western blot. (C) Quantification of phosphorylated Src signal intensity (P-Src/total Src) in (B). n=5. ± SEM, one-way ANOVA test.
3.2. Anti-Scg3 pAb inhibits laser-induced CNV
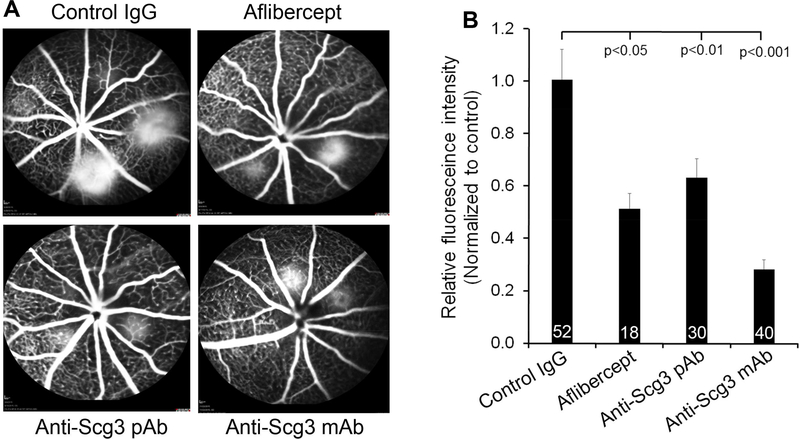
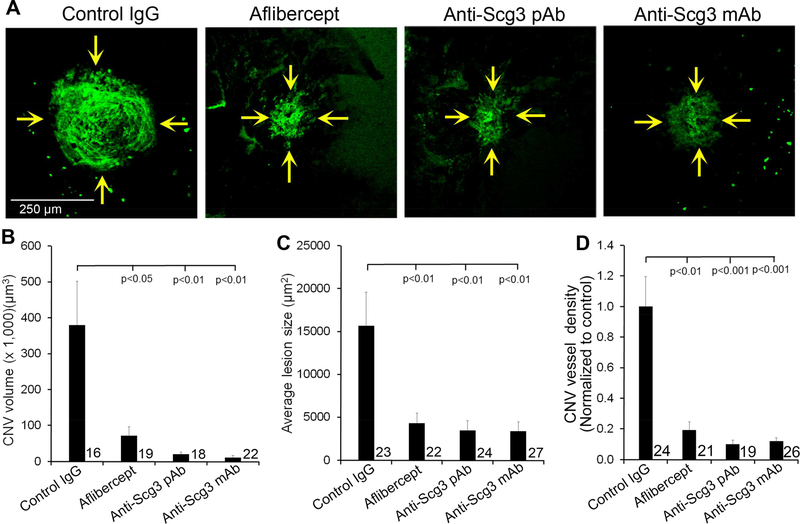
To investigate possible involvement of Scg3 in CNV pathogenesis, we analyzed the capacity of anti-Scg3 pAb (0.36 μg/eye) to alleviate CNV in mice. Fluorescein angiography on Day 7 showed that anti-Scg3 pAb significantly reduced CNV vessel leakage with a similar efficacy to aflibercept (P<0.05, Fig. 2). CNV vessels in eyecups were labeled with Alexa Fluor 488-isolectin B4 and analyzed by confocal microscopy. Anti-Scg3 pAb markedly reduced the size of CNV (Fig. 3A). Quantification of CNV 3D volume, lesion area and vessel density revealed that anti-Scg3 pAb significantly inhibited CNV with high efficacy (Fig. 3B-D). As a positive control, aflibercept (2 μg/eye) also markedly suppressed laser-induced CNV.
Fig. 2. Anti-Scg3 therapy of laser-induced CNV leakage.
Mice were treated with laser photocoagulation on Day 0. Scg3-neutralizing pAb, ML49.3 mAb, control IgG (0.36 μg/1 μg/eye), or aflibercept (2 μg/1 μg/eye) was intravitreally injected on Day 3. (A) Representative images of fluorescein angiography on Day 7. (B) Quantification of CNV fluorescence intensity in (A). ± SEM, n (# of laser spots) is indicated at the bottom of the bars, versus control IgG, one-way ANOVA test.
Fig. 3. Anti-Scg3 therapy of laser-induced CNV.
Mice with laser-induced CNV were treated, as described in Fig. 2. After fluorescein angiography on Day 7, mice were sacrificed on Day 8. Eyecups of the RPE-choroid-sclera complex were stained with Alexa Fluor 488-isolectin B4 and analyzed by confocal microscopy. (A) Representative images of CNV. (B) Quantification of CNV 3D volume in (A). (C) Quantification of CNV lesion size in (A). (D) Quantification of CNV vessel density (i.e., fluorescence intensity) in (A). ± SEM, n (# of laser spots) is indicated in the graphs, one-way ANOVA test.
3.3. Anti-Scg3 mAb suppresses laser-induced CNV
Despite affinity purification, anti-Scg3 pAb recognizing multiple epitopes may nonspecifically bind to other proteins with off-target effects. mAbs minimally cross-react with other proteins and therefore are well recognized as selective reagents for target validation as well as therapy.
Intravitreal injection of Scg3-neutralizing mAb (0.36 μg/eye) significantly ameliorated laser-induced CNV leakage (P<0.0001, Fig. 2), CNV 3D volume (P<0.01), lesion size (P<0.01) and vessel density (P<0.001) with a similar efficacy to aflibercept (Fig. 3).
3.4. Anti-Scg3 therapy of Matrigel-induced CNV
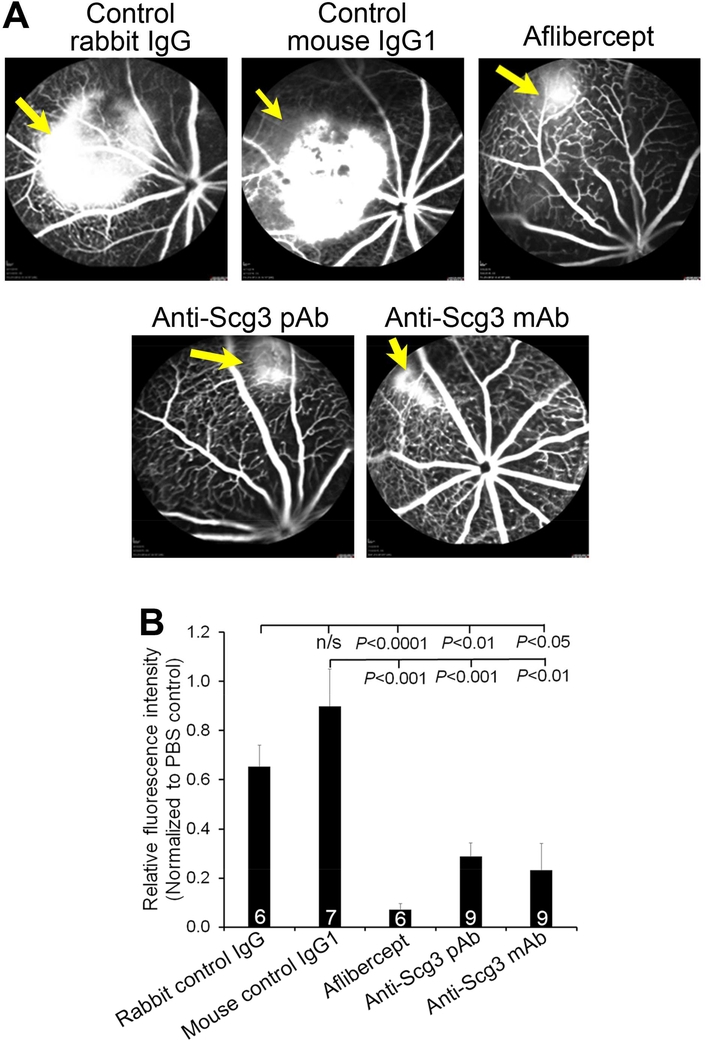
To independently validate the pathogenic role of Scg3, we generated an alternative mouse model of CNV by injecting Matrigel into the subretinal space to induce CNV. To circumvent the side effects of intravitreal injection, we investigated systemic anti-Scg3 therapy in this CNV model. Anti-Scg3 pAb, ML49.3 mAb, control rabbit IgG, mouse IgG1 (25 μg/Kg body weight), aflibercept (250 μg/Kg) or PBS was subcutaneously injected on Day 0, 2 and 4. Fluorescein angiography on Day 7 showed that anti-Scg3 pAb significantly prevented Matrigel-induced CNV (P<0.01, versus rabbit control IgG, Fig. 4). The results were independently verified with ML49.3 mAb (P<0.01, versus control mouse IgG1). As a positive control, aflibercept also significantly inhibited Matrigel-induced CNV (Fig. 4).
Fig. 4. Anti-Scg3 therapy of Matrigel-induced CNV.
Matrigel was injected subretinally on Day 0. Anti-Scg3 pAb, rabbit control IgG, anti-Scg3 mAb, mouse control IgG1 (25 μg/Kg body weight) or aflibercept (250 μg/Kg) was subcutaneously injected on Day 0, 2, and 4. Fluorescein angiography was performed on Day 7 to analyze CNV leakage. (A) Representative images of fluorescein angiography. (B) Quantification of CNV leakage in (A). Blinded study. ± SEM, n (# of mice) is indicated in the graph, one-way ANOVA test.
4. Discussion
Scg3 was initially discovered as a neural protein in 1990 and subsequently found to be predominantly expressed in endocrine, neuroendocrine cells and neurons, including retinal neurons (Hosaka et al., 2002; LeBlanc et al., 2017; Ottiger et al., 1990; Sakai et al., 2003; Sakai et al., 2004). Scg3 belongs to the granin family that is composed of chromogranin A (CgA), chromogranin B (CgB) and secretogranin II-VII (Scg2–7) (Li et al., 2018b). For more than a quarter of a century after its discovery, Scg3 was exclusively investigated as a vesicular protein to regulate the biogenesis of secretory granules (Hosaka and Watanabe, 2010). Scg3 binds to CgA, membrane-associated carboxypeptidase E (CPE) and cholesterol-rich membrane domain in the trans-Golgi network. It was proposed that Scg3, CgA and other granins jointly regulate secretory granule biogenesis and the secretion of hormones, neuropeptides and neurotransmitters (Hosaka and Watanabe, 2010). Although Scg3-null mice fed with a high-fat diet developed pronounced obesity with a decrease in stimulated secretion of active insulin (Maeda et al., 2018), the knockout mice were viable and fertile and exhibited no overt abnormalities under ordinary rearing conditions (Kingsley et al., 1990). These findings suggest that secretion of most vital hormones and neurotransmitters is not impaired in Scg3−/− mice.
We recently developed a new technology of ligandomics to globally profile cell-wide ligands with simultaneous binding activity quantification (Li et al., 2018a). We applied ligandomics to a mouse model of diabetic retinopathy (DR). Quantitative comparison of the entire ligandome profiles for diabetic vs. healthy retina systematically mapped hundreds ligands with altered binding activity to DR endothelium (LeBlanc et al., 2017). Among thousands of identified endothelial ligands, Scg3 was discovered with the highest binding activity ratio to diabetic vs. control vessels (1,731:0) and lowest binding to control vasculature. These results imply that Scg3 receptor(s) (Scg3R) may be markedly induced on diabetic retinal vessels. Indeed, we found that Scg3 selectively induced angiogenesis of diabetic but not normal vessels. To our knowledge, Scg3 represents the first diabetes-selective angiogenic factor. Interestingly, CgA, Scg2 and their processed peptides in the same family are also capable of regulating angiogenesis (Helle and Corti, 2015; Kirchmair et al., 2004). Perhaps the reason that Scg3 was overlooked for so long as an angiogenic factor is its inability to induce angiogenesis in normal vessels. This also highlights the important value of our comparative ligandomics to systematically discover disease-selective ligands that are functionally active only in diseased but not normal vessels or cells.
Similar to VEGF, Scg3 possesses the activity of both angiogenic and vascular leakage activity. Anti-Scg3 ML49.3 mAb was demonstrated with high efficacy to alleviate not only retinal vascular leakage in diabetic mice but also pathological retinal neovascularization in OIR mice (LeBlanc et al., 2017). This study further revealed the high efficacy of anti-Scg3 mAb to ameliorate laser- or Matrigel-induced CNV. Taken together, these results suggest that similar to VEGF, Scg3 may be involved in the pathogenesis of different vascular diseases and that anti-Scg3 mAb could be developed for clinical translation with multiple indications.
To date most angiogenic factors were discovered based on their functional activity to stimulate angiogenesis of normal vessels and subsequently characterized for their roles in disease pathogenesis. Therapies targeting these conventional angiogenic factors may have not only therapeutic activity but also adverse side effects on normal vasculature. Narrow therapeutic windows could restrict clinical doses with limited efficacy and high drug attrition rates, as highlighted by the recent failure of platelet-derived growth factor (PDGF) inhibitor Fovista to treat wet AMD (Dunn et al., 2017). To circumvent this problem, a new trend in drug development is targeted therapy, such as antibody-drug conjugates (ADCs) for cancer therapy (Sau et al., 2017). For anti-angiogenesis, inhibitors targeting disease-selective angiogenic receptors or their cognate ligands may have minimal adverse effects. It is worth noting that CCR3 was reported as an angiogenic receptor specifically expressed on CNV endothelium with undetectable expression in normal choroidal vasculature (Takeda et al., 2009). Anti-CCR3 antibodies and small-molecule antagonists ameliorated laser-induced CNV in mice (Nagai et al., 2015; Takeda et al., 2009). However, CCR3 upregulation in Matrigel-induced CNV was not detected, and CCR3 antagonists showed minimal efficacy in this CNV model (Li et al., 2011). Given the predominant expression of CCR3 on eosinophils (Murphy, 2017), CCR3-null mice developed dysregulated trafficking of eosinophils (Humbles et al., 2002). Phase I clinical trial of a CCR3 antagonist was terminated (ClinicalTrial.gov), presumably due to safety reasons. As a result, targeted anti-angiogenic therapy against CCR3 has never been fully investigated for its efficacy and safety advantages in clinical trials. Owing to its high disease selectivity, anti-Scg3 mAb has the potential to become the first “selective angiogenesis blocker” for ligand-guided, targeted therapy of CNV with minimal side effects on normal vessels.
Our recent study showed that Scg3 and VEGF have distinct receptor pathways (LeBlanc et al., 2017), providing a molecular basis for potential alternative or combination therapy of AMD to improve therapeutic efficacy. The disease selectivity of Scg3 implies marked upregulation of its receptor on diabetic vessels. Given the minimal expression of Scg3R on normal vessels, the therapeutic activity of anti-Scg3 mAb in this study implicate that Scg3R may also be induced in CNV. Because of the unknown identity of Scg3R, the only approach to confirm the increased binding of Scg3 to CNV vasculature is our comparative ligandomics (LeBlanc et al., 2017). An interesting question is why Scg3R is minimally expressed on normal vasculature but markedly induced in disease conditions. We speculate that Scg3R may be similar to the receptor for advanced glycation end products (RAGE), which is minimally expressed on normal vasculature but markedly upregulated on diabetic endothelium to exacerbate diabetic vascular complications (DVCs) (Manigrasso et al., 2014). However, such speculation for Scg3R in CNV is yet to be experimentally corroborated.
Intravenous infusion of bevacizumab for cancer therapy may trigger severe or fatal systemic adverse effects (Falk et al., 2015). To circumvent the problem, all anti-VEGF drugs were approved for wet AMD therapy only via intravitreal administration. However, repetitive intravitreal injections may cause detrimental effects, including endophthalmitis, retinal detachment, increased intraocular pressure and cataract, albeit at a relatively low rate (Dedania and Bakri, 2015; Drug_Information, 2011). This study independently confirmed the therapeutic activity of anti-Scg3 mAb, suggesting that Scg3 may contribute to CNV pathogenesis and is a promising target for anti-angiogenic therapy of CNV. Given that Scg3 is a disease-selective angiogenic factor with undetectable binding to normal vessels (LeBlanc et al., 2017), anti-Scg3 mAb via systemic administration may have minimal adverse effects. Anti-Scg3 mAb could be delivered through sub-tenon injection to circumvent the requirement for intravitreal injection. This notion is supported by high efficacy of subcutaneous anti-Scg3 mAb to attenuate Matrigel-induced CNV (Fig. 4). However, systemic safety of anti-Scg3 mAb is yet to be fully characterized.
VEGF is an important growth factor not only for endothelial cells but also for RPE and neurons (Byeon et al., 2010; Rosenstein et al., 2003). Long-term anti-VEGF therapy for wet AMD may increase the risk of geographic atrophy and retinal fibrosis (Daniel et al., 2014; Enslow et al., 2016; Grunwald et al., 2017). Anti-Scg3 therapy with disease selectivity may offer an alternative solution to these safety concerns. However, such long-term safety is difficult to be investigated in animal models.
In summary, this study characterized the therapeutic activity of anti-Scg3 mAb to alleviate CNV in two mouse models, implicating that Scg3 may play an important role in CNV pathogenesis. Given that Scg3 is a VEGF-independent angiogenic factor (LeBlanc et al., 2017), Scg3-neutralizing mAb will be humanized to facilitate alternative or combination therapy of wet AMD.
Highlights.
Scg3 is a highly disease-selective angiogenic factor.
Scg3 is a novel target for choroidal neovascularization (CNV).
Scg3-neutralizing monoclonal antibody (mAb) alleviates laser-induced CNV.
Scg3-neutralizing mAb ameliorates Matrigel-induced CNV.
Acknowledgements
We thank Keith Webster and Philip Rosenfeld for scientific advice and discussion; Gabriel Gaidosh for confocal service. Supported by NIH R01GM094449 (W.L.), R21HD075372 (W.L.), R21EY027065 (W.L.), R41EY027665 (W.L. and H.T.), American Diabetes Association 1–18IBS-172 (W.L.), Special Scholar Award from Research to Prevent Blindness (RPB) (W.L.), American Heart Association Predoctoral Fellowship 14PRE18310014 and 16PRE27250308 (M.E.L), NIH P30-EY014801 and an institutional grant from RPB.
Footnotes
Disclosure of potential conflicts of interest
M.E.L, H.T. and W.L. are shareholders of Everglades Biopharma, LLC. M.E.L., W.W. and W.L. are inventors of pending patents.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T, and Group AS. 2009. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology 116:57–65 e55. [DOI] [PubMed] [Google Scholar]
- Byeon SH, Lee SC, Choi SH, Lee HK, Lee JH, Chu YK, and Kwon OW. 2010. Vascular endothelial growth factor as an autocrine survival factor for retinal pigment epithelial cells under oxidative stress via the VEGF-R2/PI3K/Akt. Invest Ophthalmol Vis Sci 51:1190–1197. [DOI] [PubMed] [Google Scholar]
- Cabral T, Mello LGM, Lima LH, Polido J, Regatieri CV, Belfort R Jr., and Mahajan VB. 2017. Retinal and choroidal angiogenesis: a review of new targets. Int J Retina Vitreous 3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Zhao L, Li Y, Liu Y, Xiao W, Song Y, Luo L, Huang D, Yancopoulos GD, Wiegand SJ, and Wen R. 2010. A subretinal matrigel rat choroidal neovascularization (CNV) model and inhibition of CNV and associated inflammation and fibrosis by VEGF trap. Invest Ophthalmol Vis Sci 51:6009–6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ClinicalTrial.gov. GW824575 First Time in Human (ClinicalTrials.gov Identifier: NCT01551771), at https://clinicaltrials.gov/ct2/show/NCT01551771?term=age-related+macular+degeneration+AND+CCR3&rank=1.
- Daniel E, Toth CA, Grunwald JE, Jaffe GJ, Martin DF, Fine SL, Huang J, Ying GS, Hagstrom SA, Winter K, Maguire MG, and G. Comparison of Age-related Macular Degeneration Treatments Trials Research. 2014. Risk of scar in the comparison of age-related macular degeneration treatments trials. Ophthalmology 121:656–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedania VS, and Bakri SJ. 2015. Current perspectives on ranibizumab. Clin Ophthalmol 9:533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drug_Information. 2011. Eylea prescribing information by U.S. Food and Drug Administration at http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125387lbl.pdf.
- Dunn EN, Hariprasad SM, and Sheth VS. 2017. An Overview of the Fovista and Rinucumab Trials and the Fate of Anti-PDGF Medications. Ophthalmic Surg Lasers Imaging Retina 48:100–104. [DOI] [PubMed] [Google Scholar]
- Enslow R, Bhuvanagiri S, Vegunta S, Cutler B, Neff M, and Stagg B. 2016. Association of Anti-VEGF Injections with Progression of Geographic Atrophy. Ophthalmol Eye Dis 8:31–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk AT, Barriere J, Francois E, and Follana P. 2015. Bevacizumab: A dose review. Crit Rev Oncol Hematol 94:311–322. [DOI] [PubMed] [Google Scholar]
- Grunwald JE, Pistilli M, Daniel E, Ying GS, Pan W, Jaffe GJ, Toth CA, Hagstrom SA, Maguire MG, Martin DF, and G. Comparison of Age-Related Macular Degeneration Treatments Trials Research. 2017. Incidence and Growth of Geographic Atrophy during 5 Years of Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 124:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helle KB, and Corti A. 2015. Chromogranin A: a paradoxical player in angiogenesis and vascular biology. Cell Mol Life Sci 72:339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka M, and Watanabe T. 2010. Secretogranin III: a bridge between core hormone aggregates and the secretory granule membrane. Endocrine journal 57:275–286. [DOI] [PubMed] [Google Scholar]
- Hosaka M, Watanabe T, Sakai Y, Uchiyama Y, and Takeuchi T. 2002. Identification of a chromogranin A domain that mediates binding to secretogranin III and targeting to secretory granules in pituitary cells and pancreatic beta-cells. Molecular biology of the cell 13:3388–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbles AA, Lu B, Friend DS, Okinaga S, Lora J, Al-Garawi A, Martin TR, Gerard NP, and Gerard C. 2002. The murine CCR3 receptor regulates both the role of eosinophils and mast cells in allergen-induced airway inflammation and hyperresponsiveness. Proc Natl Acad Sci U S A 99:1479–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim LA, and D’Amore PA. 2012. A brief history of anti-VEGF for the treatment of ocular angiogenesis. Am J Pathol 181:376–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley DM, Rinchik EM, Russell LB, Ottiger HP, Sutcliffe JG, Copeland NG, and Jenkins NA. 1990. Genetic ablation of a mouse gene expressed specifically in brain. EMBO J 9:395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchmair R, Gander R, Egger M, Hanley A, Silver M, Ritsch A, Murayama T, Kaneider N, Sturm W, Kearny M, Fischer-Colbrie R, Kircher B, Gaenzer H, Wiedermann CJ, Ropper AH, Losordo DW, Patsch JR, and Schratzberger P. 2004. The neuropeptide secretoneurin acts as a direct angiogenic cytokine in vitro and in vivo. Circulation 109:777–783. [DOI] [PubMed] [Google Scholar]
- LeBlanc ME, Wang W, Caberoy NB, Chen X, Guo F, Alvarado G, Shen C, Wang F, Wang H, Chen R, Liu ZJ, Webster K, and Li W. 2015. Hepatoma-derived growth factorrelated protein-3 is a novel angiogenic factor. PLoS One 10:e0127904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc ME, Wang W, Chen X, Caberoy NB, Guo F, Shen C, Ji Y, Tian H, Wang H, Chen R, and Li W. 2017. Secretogranin III as a disease-associated ligand for antiangiogenic therapy of diabetic retinopathy. J Exp Med 214:1029–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc ME, Wang W, Chen X, Ji Y, Shakya A, Shen C, Zhang C, Gonzalez V, Brewer M, Ma JX, Wen R, Zhang F, and Li W. 2016. The regulatory role of hepatoma-derived growth factor as an angiogenic factor in the eye. Mol Vis 22:374–386. [PMC free article] [PubMed] [Google Scholar]
- Li W, Pang IH, Pacheco MTF, and Tian H. 2018a. Ligandomics: a paradigm shift in biological drug discovery. Drug Discov Today 23:636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Webster KA, LeBlanc ME, and Tian H. 2018b. Secretogranin III: A Diabetic Retinopathy-Selective Angiogenic Factor. Cell Mol Life Sci 75:635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Huang D, Xia X, Wang Z, Luo L, and Wen R. 2011. CCR3 and choroidal neovascularization. PLoS One 6:e17106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y, Kudo S, Tsushima K, Sato E, Kubota C, Kayamori A, Bochimoto H, Koga D, Torii S, Gomi H, Watanabe T, and Hosaka M. 2018. Impaired Processing of Prohormones in Secretogranin III-Null Mice Causes Maladaptation to an Inadequate Diet and Stress. Endocrinology 159:1213–1227. [DOI] [PubMed] [Google Scholar]
- Manigrasso MB, Juranek J, Ramasamy R, and Schmidt AM. 2014. Unlocking the biology of RAGE in diabetic microvascular complications. Trends in endocrinology and metabolism: TEM 25:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K 2017. Janeway’s Immunobiology. 9th Edition, GS Garland Science, Taylor & Francis Group, New York. : [Google Scholar]
- Nagai N, Ju M, Izumi-Nagai K, Robbie SJ, Bainbridge JW, Gale DC, Pierre E, Krauss AH, Adamson P, Shima DT, and Ng YS. 2015. Novel CCR3 Antagonists Are Effective Mono- and Combination Inhibitors of Choroidal Neovascular Growth and Vascular Permeability. Am J Pathol 185:2534–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottiger HP, Battenberg EF, Tsou AP, Bloom FE, and Sutcliffe JG. 1990. 1B1075: a brain-and pituitary-specific mRNA that encodes a novel chromogranin/secretogranin-like component of intracellular vesicles. J Neurosci 10:3135–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro-Costa J, Freitas-da-Costa P, Falcao MS, Brandao EM, Falcao-Reis F, and Carneiro AM. 2014. Switch from intravitreal ranibizumab to bevacizumab for the treatment of neovascular age-related macular degeneration: clinical comparison. Ophthalmologica 232:149–155. [DOI] [PubMed] [Google Scholar]
- Poor SH, Qiu Y, Fassbender ES, Shen S, Woolfenden A, Delpero A, Kim Y, Buchanan N, Gebuhr TC, Hanks SM, Meredith EL, Jaffee BD, and Dryja TP. 2014. Reliability of the mouse model of choroidal neovascularization induced by laser photocoagulation. Invest Ophthalmol Vis Sci 55:6525–6534. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, and Kim RY. 2006. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 355:1419–1431. [DOI] [PubMed] [Google Scholar]
- Rosenstein JM, Mani N, Khaibullina A, and Krum JM. 2003. Neurotrophic effects of vascular endothelial growth factor on organotypic cortical explants and primary cortical neurons. J Neurosci 23:11036–11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai Y, Hosaka M, Hira Y, Harumi T, Ohsawa Y, Wang H, Takeuchi T, Uchiyama Y, and Watanabe T. 2003. Immunocytochemical localization of secretogranin III in the anterior lobe of male rat pituitary glands. J Histochem Cytochem 51:227–238. [DOI] [PubMed] [Google Scholar]
- Sakai Y, Hosaka M, Yoshinaga A, Hira Y, Harumi T, and Watanabe T. 2004. Immunocytochemical localization of secretogranin III in the endocrine pancreas of male rats. Arch Histol Cytol 67:57–64. [DOI] [PubMed] [Google Scholar]
- Sau S, Alsaab HO, Kashaw SK, Tatiparti K, and Iyer AK. 2017. Advances in antibody-drug conjugates: A new era of targeted cancer therapy. Drug Discov Today 22:1547–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A, Baffi JZ, Kleinman ME, Cho WG, Nozaki M, Yamada K, Kaneko H, Albuquerque RJ, Dridi S, Saito K, Raisler BJ, Budd SJ, Geisen P, Munitz A, Ambati BK, Green MG, Ishibashi T, Wright JD, Humbles AA, Gerard CJ, Ogura Y, Pan Y, Smith JR, Grisanti S, Hartnett ME, Rothenberg ME, and Ambati J. 2009. CCR3 is a target for age-related macular degeneration diagnosis and therapy. Nature 460:225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votruba M, and Gregor Z. 2001. Neovascular age-related macular degeneration: present and future treatment options. Eye (Lond) 15:424–429. [DOI] [PubMed] [Google Scholar]
- Wang W, LeBlanc ME, Chen X, Chen P, Ji Y, Brewer M, Tian H, Spring SR, Webster KA, and Li W. 2017. Pathogenic role and therapeutic potential of pleiotrophin in mouse models of ocular vascular disease. Angiogenesis 20:479–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, and Wong TY. 2014. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2:e106–116. [DOI] [PubMed] [Google Scholar]