Abstract
Prevention of mother-to-child transmission of HIV (PMTCT) is a foundational component of a comprehensive HIV treatment program. In addition to preventing vertical transmission to children, PMTCT is an important catch-point for universal test-and-treat strategies that can reduce community viral load and slow the epidemic. However, systematic reviews suggest that care engagement in PMTCT programs is sub-optimal. This study enrolled a cohort of 200 women initiating PMTCT in Kilimanjaro, Tanzania, and followed them to assess HIV care engagement and associated factors. Six months after delivery, 42/200 (21%) of participants were identified as having poor care engagement, defined as HIV RNA >200 copies/mL or, if viral load was unavailable, being lost-to-follow-up in the clinical records or self-reporting being out of care. In a multivariable risk factor analysis, younger women were more likely to have poor postpartum care engagement; with each year of age, women were 7% less likely to have poor care engagement (aRR: 0.93; 95% CI: 0.89, 0.98). Additionally, women who had told at least one person about their HIV status were 47% less likely to have poor care engagement (aRR: .53; 95% CI: 0.29, 0.97). Among women who entered antenatal care with an established HIV diagnosis, those who were pregnant for the first time had increased risk of poor care engagement (aRR 4.16; 95% CI 1.53, 11.28). The findings suggest that care engagement remains a concern in PMTCT programs, and must be addressed to realize the goals of PMTCT. Comprehensive counseling on HIV disclosure, along with community-based stigma reduction programs to provide a supportive environment for people living with HIV, are crucial to address barriers to care engagement and support long-term treatment. Women presenting to antenatal care with an established HIV status require support for care engagement during the crucial period surrounding childbirth, particularly those pregnant for the first time.
Keywords: Tanzania, HIV, prevention of mother-to-child transmission of HIV (PMTCT), pregnancy, care engagement
Introduction
Over the past 20 years, the scale-up and integration of services for the prevention of mother-to-child transmission of HIV (PMTCT) have been at the forefront of HIV treatment and prevention efforts in low- and middle-income countries (LMICs) (World Health Organization, 2010). PMTCT programs reduce new HIV infections among children, improve long-term health outcomes for women living with HIV, and prevent the forward transmission of HIV to sexual partners (Nachega et al., 2012; UNAIDS, 2016). The antenatal care setting is a key entry point for HIV testing (Chandisarewa et al., 2007) and initiation of lifetime antiretroviral therapy (ART) under the recommended Option B+ guidelines (World Health Organization, 2016).
Although delivery of ART to HIV-infected pregnant and breastfeeding women has improved dramatically, suboptimal retention in HIV care and adherence to ART among pregnant and postpartum women threaten to weaken the potential impact of PMTCT. Retention rates among women enrolled in PMTCT under Option B+ are lower than those among the general adult population receiving HIV care (Knettel et al., 2018). Loss-to-follow up (LTFU) in PMTCT programs may be explained by the added complexity and vulnerability of women’s lives during pregnancy, including the demands of new motherhood, pressure to begin treatment before one feels ready, poor coordination between PMTCT and other HIV services, and inadequate counseling and support (Colvin et al., 2014; Gourlay, Birdthistle, Mburu, Iorpenda, & Wringe, 2013; Matheson et al., 2015; Ngarina, Popenoe, Kilewo, Biberfeld, & Ekstrom, 2013).
To address shortcomings in PMTCT retention and implement systems to improve care engagement, it is imperative to put effective monitoring and quality improvement processes in place. Previous studies assessing HIV care engagement demonstrate a substantial degree of heterogeneity in the measurement of care engagement, and have typically relied only on one method of measurement, most commonly retrospective medical chart review of HIV clinic visit compliance or patient self-report of care engagement (Knettel et al., 2018; Mugavero et al., 2012; Mugavero, Davila, Nevin, & Giordano, 2010). Such methods have notable flaws that may lead to misclassifications; for example, studies commonly fail to track patients who silently transfer to another clinic, miss patients who were diagnosed with HIV but never initiated ART, and fail to recognize the role of medication adherence along with clinic attendance (Mugavero et al., 2012; Risher et al., 2017; Rollins et al., 2014; Yehia et al., 2012). In addition, medical records, particularly in under-resourced settings, may contain inaccurate or missing information, leading to unreliable results (Pirkle, Dumont, & Zunzunegui, 2012). Few studies on PMTCT in sub-Saharan Africa have triangulated care engagement data using multiple methods, or used a clinical biomarker to support care engagement outcomes (Knettel et al., 2018; Mugavero et al., 2012; Risher et al., 2017; Rollins et al., 2014). Triangulation of data can strengthen the accuracy and reliability of the reported care engagement measures used to inform PMTCT policy and intervention strategies.
In order to support the implementation of PMTCT programs, it is necessary to assemble robust data on care engagement among pregnant and postpartum women. The goal of this prospective study of women initiating PMTCT care in Tanzania was to: 1) describe HIV care engagement outcomes at 6-months post-partum through triangulation of data via viral load measurements, retrospective medical chart review and self-report, and 2) assess the intrapersonal, interpersonal, and facility-level risk factors that are associated with poor care engagement outcomes.
Methods
Study setting and participants
This prospective cohort study enrolled 200 HIV-infected women from nine PMTCT clinics in the Kilimanjaro Region of Tanzania. The study sites included two referral hospitals and seven local clinics, including three clinics from the major city in Kilimanjaro (Moshi) and four clinics from surrounding peri-urban and rural areas. At the time of the study, all facilities were following the national protocol for PMTCT care (The United Republic of Tanzania Ministry of Health and Social Welfare, 2013), which is based on the Option B+ guidelines and the integration of HIV services within antenatal and postpartum care for the mother and child up until 18 months after delivery.
Women were eligible to enroll if they were enrolled in PMTCT care from one of the nine study clinics, had been in PMTCT care for at least one month, were at least 16 weeks or greater gestational age, were at least 18 years of age, and were able to provide informed consent. Clinic staff introduced the study to patients and referred them to a member of the research staff for additional information. If women were referred to the study before meeting eligibility criteria and were interested in participating, their contact information was recorded and they were scheduled for enrollment at a later date. The cohort was restricted to women who had been in care for one month in order to not overburden women on the day of an HIV diagnosis and to allow for baseline assessment of factors such as HIV disclosure and perceptions of care.
At the conclusion of the enrollment period, the ANC registries of the nine study clinics were reviewed to capture all HIV-infected women who attended ANC during the enrollment period, in order to compare the cohort to HIV-infected pregnant women who were not enrolled in the cohort. A medical record review was conducted of patient records who were over 18 and had either reached gestational age of 24 weeks or been on ART for at least one month by the close of enrollment. Information was extracted on whether they initiated ART at the clinic (and would have been eligible for study enrollment), whether they returned to PMTCT after the first visit, and whether they remained in care at 6 months postpartum.
Study procedures
Following informed consent, participants completed the baseline assessment, an interviewer-administered structured survey that took approximately 60 minutes. Participants provided contact information to facilitate study retention. Three additional follow-up assessments were conducted: within one month of the birth of the child, three months postpartum, and six months postpartum. Participants were reminded of study visits via phone call, text message, or in person when they returned to the clinic; home visits were made to participants who were otherwise unreachable, if permission for home visits had been provided at enrollment. In the event that a participant was unable or unwilling to return to the clinic but still wanted to participate, an abbreviated version of the follow-up survey was conducted by phone. At each study visit, participants were reimbursed for their travel expenses to and from the study appointment, including those participants who had moved out of the Kilimanjaro Region.
At the six-month assessment, participants provided a blood sample to measure HIV RNA. Samples were processed using an Abbott Real Time HIV-1 PCR Machine at the Kilimanjaro Clinical Research Institute biotechnology laboratory, which has Virology Quality Assurance certification. If participants did not complete a six-month assessment, postpartum HIV RNA data was abstracted from the medical record, if available. Participants were considered to have an elevated viral load if the HIV RNA was >200 copies/mL. A threshold of >200 copies/mL is defined as virologic failure by the AIDS Clinical Trials Group (ACTG) and the U.S. Department of Health and Human Services guidelines, and accounts for low level viremia due to isolated virologic blips or assay variability (Ribaudo, Lennox, & Currier, 2009). Following the six-month visit, medical records were reviewed at the site of enrollment to assess for participants’ PMTCT care engagement. Data were extracted on dates of PMTCT visits, ART medication received at each visit, and notations of transfer to another clinic.
Primary outcome
The primary outcome was HIV care engagement at six months postpartum. Care engagement was characterized based upon the composition of three types of data: HIV RNA or viral load data, self-reported survey data, and medical record abstraction. Participants were defined to have poor care engagement, if they met one of two criteria: (1) HIV RNA was >200 copies/mL; or, if HIV RNA data was unavailable, (2) the participant self-reported to be out of care or not taking ARVs or the participant was lost to follow up (LTFU) by medical record review (i.e. at six months postpartum the participant had not attended clinic for >90 days and was not noted to have transferred to another clinic).
Covariates
The survey included participant self-report measures reflecting individual, inter-personal and facility constructs, and informed by prior research (Knettel et al., 2018). All measures were translated from English to Swahili, back-translated to English, and discussed to reach consensus on appropriate translations. The covariates used in this study were all measured at the baseline (pregnancy) time point.
Intrapersonal
At the intrapersonal level, demographic, pregnancy, HIV and mental health risk factors, and knowledge of PMTCT were assessed. Demographics included age, education level, and relationship status. Women reported history of prior pregnancy and the gestational age at which they first presented to antenatal care. Attitude toward the pregnancy was measured using eight items (e.g., “I feel happy about being pregnant”; α=0.91) (Speizer, Santelli, Afable-Munsuz, & Kendall, 2004). The timing of women’s HIV diagnosis was dichotomized as either being a new diagnosis (i.e., the participant learned her HIV status during the index pregnancy) or being an established diagnosis (i.e., the participant knew her HIV status prior to presenting to ANC). Acceptance of one’s HIV status was measured using the acceptance subscale of the Illness Cognition Questionnaire (ICQ; α=835) (Evers et al., 2001), and attitude toward ART was measured using the Beliefs About Medicines Questionnaire (BMQ; α=0.68) (Horne, Weinman, & Hankins, 1999). Depressive symptoms were measured with the Edinburgh Postnatal Depression Scale (EPDS; α=0.88) (Cox, Holden, & Sagovsky, 1987), and internalized feelings of shame and stigma regarding one’s HIV status were measured using Scale A of the HIV and Abuse Related Shame Inventory (HARSI; α=0.86) (Neufeld, Sikkema, Lee, Kochman, & Hansen, 2012). To assess knowledge of PMTCT, women were asked seven questions that focused on: the length of time women should be on ART, the use and duration of prophylactic Nevirapine for the exposed child, and recommended breastfeeding practices.
Interpersonal level factors
Interpersonal factors included the partner’s HIV status, HIV disclosure, two measures of perceived support, and experienced violence. Women self-reported their partner’s HIV status and whether or not they had ever disclosed their HIV status to a person outside of the heath care workers directly involved in their antenatal and PMTCT care. Social support was measured using the Perceived Availability of Support Scale (PAS; α=0.82) (O’Brien, Wortman, Kessler, & Joseph, 1993), and support specifically from the father of the child was measured with the Norbeck Social Support Questionnaire (NSSQ; α=0.96) (Norbeck, Lindsey, & Carrieri, 1981). Additionally, women were asked whether or not they had ever experienced verbal, physical or sexual violence as an adult.
Facility related factors
Women reported where they initiated antenatal care (local clinic or hospital), the amount of time it typically took to travel to the clinic, and perceptions of quality of care. Perceived quality of care was measured using a version of the Patient-Provider Relationship Scale (PPRS; α=0.87), adapted for length and cultural context (Barry et al., 2012).
Data analysis
Descriptions of each risk factor of interest were presented using frequencies and proportions for binary variables or medians and interquartile ranges for continuous variables. Scaled measures were summed and analyzed as continuous variables; missing data were imputed with the mean of completed questions when the measure was at least 75% completed. The relative risk of the primary outcome was assessed for each risk factor using a log-binomial regression. In instances where this regression failed to converge, a Poisson regression with robust variance was used to approximate a log-binomial regression and the relative risk. A multivariable model was constructed using any variable that had a p-value ≤ .10 in univariate analysis; additionally, several variables were selected a priori based upon previous studies, including age, education, relationships status, and timing of HIV diagnosis (i.e., new vs. established diagnosis). As a secondary analysis, we examined stratified models, separating women who were diagnosed with HIV during the index pregnancy from those who were known to be living with HIV when they presented for ANC.
Results
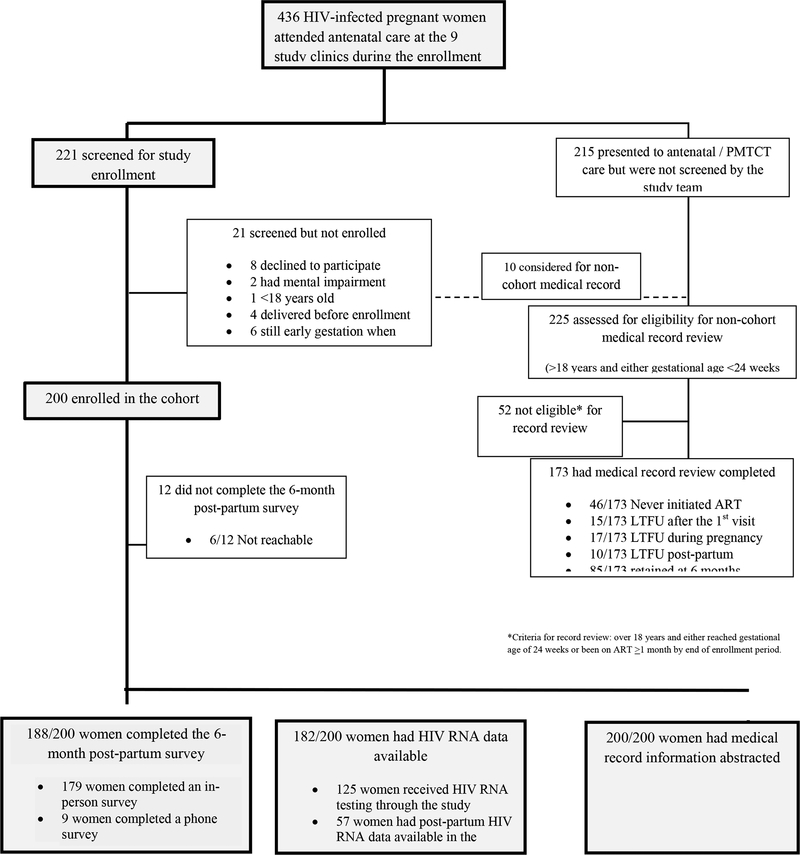
Figure 1 illustrates the flowchart of study enrollment and follow-up. During the period of enrollment, 436 HIV-infected pregnant women attended ANC at the nine study clinics. Of note, of the 173 women who were not included in the cohort, 46 (26.6%) did not initiate ART and would not have been eligible for the study, and 42 (24.2%) became lost to follow-up (LTFU) from the clinic prior to 6 months postpartum.
Figure 1:
Study enrollment
For the 200 women enrolled in the cohort, six-month follow up was completed for 188 (94.0%), with 179 of these surveys completed in person and 9 done via an abbreviated phone survey. Viral load results were available from 181 participants, with most (n=124) taken by the study staff and 57 abstracted from the medical records. Medical record review was completed for all 200 participants.
Participants had a median age of 30 years, and the majority (58%) did not have education beyond primary school (Table 1). Most participants (69%) lived with a partner, and most (78%) reported that this was their first pregnancy. The sample was fairly evenly split between women who were newly diagnosed during the index pregnancy (n=94) and women who entered ANC already knowing their HIV status (n=106).
Table 1:
Baseline Participant characteristics
| Good care engagement (n = 157) |
Poor care engagement (n = 43) |
Total (n = 200) | |
|---|---|---|---|
| Intrapersonal factors | Median (Q1, Q3) or # (%) | ||
| Demographics | |||
| Age | 31.0 (26.0, 36.0) | 28.0 (23.0, 32.0) | 30.0 (25.0, 35.0) |
| Education - started secondary or higher | 65 (41%) | 18 (43%) | 83 (42%) |
| Relationship - lives alone or no partner | 46 (29%) | 16 (38%) | 62 (31%) |
| Pregnancy | |||
| First pregnancy | 30 (19%) | 14 (33%) | 44 (22%) |
| Gestational age > 24 weeks at initiation of ANC | 29 (18%) | 8 (19%) | 37 (19%) |
| Attitude toward pregnancy scale [range: 0–24; high score ~ better attitudes] |
16.0 (11.0, 19.0) | 16.0 (12.0, 19.0) | 16.0 (11.7, 19.0) |
| HIV | |||
| Diagnosed with HIV during index pregnancy | 71 (45%) | 23 (55%) | 94 (47%) |
| Acceptance of HIV - ICQ scale [range: 6–24; high score ~ more acceptance] |
18.0 (15.0, 20.0) | 18.0 (15.0, 20.0) | 18.0 (15.0, 20.0) |
| Attitude toward ART - BMQ scale [range: 10 – 50; high score ~ better attitude] |
34.0 (32.0, 36.0) | 36.0 (33.0, 37.0) | 34.0 (32.0, 37.0) |
| Knowledge of Option B+ [range 0–5; high score ~ less knowledge] |
2.0 (0.0, 2.0) | 1.0 (0.0, 2.0) | 2.0 (0.0, 2.0) |
| Mental Health | |||
| Depression - EPDS scale [range 0–30; high score ~ more depression] |
5.0 (2.0, 9.0) | 5.5 (2.0, 10.0) | 5.0 (2.0, 9.5) |
| Shame – HARSI [range: 0–52; high score ~ more shame] |
16.0 (12.0, 24.0) | 16.5 (15.0, 30.0) | 16.0 (12.0, 25.0) |
| Interpersonal factors | |||
| Partner is HIV-infected | 53 (34%) | 8 (19%) | 61 (30%) |
| HIV Disclosure - has told someone | 131 (83%) | 28 (67%) | 159 (80%) |
| Experienced interpersonal violence as an adult | 32 (20%) | 10 (24%) | 42 (21%) |
| Perceived social support - PAS Scale [range: 8–40; high score ~ more support] |
29.0 (24.0, 34.0) | 28.0 (24.0, 34.0) | 29.0 (24.0, 34.0) |
| Partner support - NSSQ Scale [range: 7–35; high score ~ more support] |
25.0 (18.0, 30.0) | 21.0 (10.0, 27.0) | 24.0 (16.0, 30.0) |
| Facility related factors | |||
| Received care at a local clinic (vs. hospital) | 100 (63%) | 28 (67%) | 128 (64%) |
| Travel time to clinic (minutes) | 30.0 (30.0, 60.0) | 30.0 (15.0, 60.0) | 30.0 (30.0, 60.0) |
| Perceptions of quality of care – PPRS Scale [range: 12–48; high score ~ better quality] |
37.0 (33.0, 41.0) | 37.5 (33.0, 42.0) | 37.0 (33.0, 41.0) |
NOTE: Full data was available for all measures except: gestational age (1 response missing), NSSQ Scale (1 response missing), and PPRS Scale (2 responses missing).
Six months after delivery, 42/200 (21%) of participants were considered to have poor care engagement. Of the 182 participants with measured viral loads, 107 (59%) had undetectable viral load, 47 (26%) had HIV RNA levels < 200, 6 (3%) had HIV RNA levels between 200 and 1000, and 22 (12%) had HIV RNA levels > 1,000. For the 18 participants without HIV RNA data, 14 (78%) were considered LTFU in the medical record review and/or reported to the study team that they had stopped attending their HIV appointments or stopped taking their ART. Disaggregated outcomes for HIV RNA, medical record review, and self-reported care engagement at 6 months are presented in Supplemental Table 1.
In a multivariable risk factor analysis for the full cohort of 200 women, age and disclosure of HIV status were independently associated with postpartum care engagement (Table 2). When controlling for other factors, younger women were more likely to have poor postpartum care engagement; with each year of increased age, women were 7% less likely to have poor care engagement (aRR: 0.93; 95% CI: 0.89, 0.97). Additionally, women who had told at least one person about their HIV status were 47% less likely to have poor care engagement (aRR: .53; 95% CI: 0.29, 0.97). Reporting higher levels of social support from a partner was associated with less likelihood of poor care engagement in univariate analysis (cRR: 0.96; 95% CI: 0.93, 0.99), but was no longer a significant predictor in multivariate analysis (aRR: 0.98; 95% CI: 0.94, 1.02).
Table 2:
Factors associated with poor PMTCT care engagement at six months postpartum (n = 198)
| Unadjusted relative risk (95% CI) |
Adjusted relative risk (95% CI) |
|
|---|---|---|
| Intrapersonal | ||
| Demographics | ||
| Age | 0.93 (0.89, 0.97)** | 0.93 (0.89, 0.98)** |
| Education - started secondary or higher [ref: completed primary or less] |
1.06 (0.61, 1.82) | 0.76 (0.42, 1.38) |
| Relationship - lives alone or no partner [ref: lives with a partner] |
1.37 (0.79, 2.36) | 0.68 (0.34, 1.35) |
| Pregnancy | ||
| First pregnancy [ref: prior pregnancy] |
1.77 (1.03, 3.06)* | 1.16 (0.55, 2.44) |
| Gestational age > 24 weeks at initiation of ANC [ref: <= 24 weeks] |
1.03 (0.52, 2.04) | 0.90 (0.45, 1.82) |
| Attitude toward pregnancy [range: 0–24; high score ~ better attitudes] |
1.01 (0.96, 1.06) | |
| HIV | ||
| Diagnosed with HIV index pregnancy [ref: established diagnosis] |
1.37 (0.80, 2.34) | 0.91 (0.50, 1.68) |
| Acceptance of HIV - ICQ scale [range: 6–24; high score ~ more acceptance] |
1.03 (0.96, 1.12) | |
| Attitude toward ART - BMQ scale [range: 10 – 50; high score ~ better attitude] |
1.05 (0.98, 1.12) | |
| Knowledge of Option B+ [range 0–5; high score ~ less knowledge] |
1.03 (0.84, 1.27) | |
| Mental Health | ||
| Depression - EPDS scale [range 0–30; high score ~ more depression] |
1.00 (0.96, 1.05) | |
| Shame - HARSI [range: 0–52; high score ~ more shame] |
1.02 (0.99, 1.04) | |
| Interpersonal | ||
| Partner is HIV-infected [ref: partner negative or unknown] |
0.54 (0.26, 1.09)‡ | 0.80 (0.38, 1.71) |
| HIV Disclosure - has told someone [ref: has not told anyone] |
0.52 (0.30, 0.89)* | 0.53 (0.29, 0.97)* |
| Experienced any violence as an adult [ref: no violence experienced] |
1.18 (0.63, 2.19) | |
| Perceived social support - PAS Scale [range: 8–40; high score ~ more support] |
1.00 (0.96, 1.04) | |
| Partner support - NSSQ Scale [range: 7–35; high score ~ more support] |
0.96 (0.93, 0.99)* | 0.98 (0.94, 1.02) |
| Facility Related | ||
| Local clinic [ref: hospital] |
1.12 (0.63, 2.00) | |
| Travel time to clinic (minutes) | 1.00 (1.00, 1.01) | |
| Perceptions of quality of care – PPRS Scale [range: 12–48; high score ~ better quality] |
1.03 (0.97, 1.09) |
p<.10
p<.05
p<.01
p<.001
For women who were known to be living with HIV at the time they became pregnant, those who were pregnant for the first time had 4.16 times the risk of poor care engagement (aRR: 4.16; 95% CI: 1.53, 11.28) (Table 3). For women with a new diagnosis of HIV, age was the only independent predictor of care engagement. Similar to the full cohort, women who were younger were more likely to have poor care engagement; women were 8% less likely to have poor care engagement with each year of increased age (aRR: 0.92; 95% CI: 0.86, 0.98).
Table 3:
Risk factors for poor care engagement, stratified by the timing of the HIV diagnosis
| Established diagnosis (n = 105) | ||
|---|---|---|
| Unadjusted relative risk (95% CI) |
Adjusted relative risk (95% CI) |
|
| Intrapersonal | ||
| Demographics | ||
| Age | 0.92 (0.86, 0.98)* | 0.98 (0.91, 1.05) |
| Education - started secondary or higher [ref: completed primary or less] |
1.75 (0.78, 3.92) | 1.12 (0.44, 2.84) |
| Relationship - lives alone or no partner [ref: lives with a partner] |
1.76 (0.76, 4.11) | 0.51 (0.16, 1.70) |
| Pregnancy | ||
| First pregnancy [ref: prior pregnancy] |
4.57 (2.24, 9.32)*** | 4.16 (1.53, 11.28)** |
| Gestational age > 24 weeks at initiation of ANC [ref: <= 24 weeks] |
0.91 (0.29, 2.79) | |
| Attitude toward pregnancy [range: 0–24; high score ~ better attitudes] |
0.99 (0.92, 1.06) | |
| HIV | ||
| Acceptance of HIV - ICQ scale [range: 6–24; high score ~ more acceptance] |
1.01 (0.88, 1.16) | |
| Attitude toward ART - BMQ scale [range: 10 – 50; high score ~ better attitude] |
1.06 (0.97, 1.17) | |
| Knowledge of Option B+ [range 0–5; high score ~ less knowledge] |
1.15 (0.86, 1.55) | |
| Mental Health | ||
| Depression - EPDS scale [range 0–30; high score ~ more depression] |
1.01 (0.94, 1.07) | |
| Shame - HARSI [range: 0–52; high score ~ more shame] |
1.01 (0.96, 1.05) | |
| Interpersonal | ||
| Partner is HIV-infected [ref: partner negative or unknown] |
0.48 (0.19, 1.25) | |
| HIV Disclosure - has told someone [ref: has not told anyone] |
0.38 (0.14, 0.99)* | 0.46 (0.17, 1.26) |
| Experienced any violence as an adult [ref: no violence experienced] |
1.29 (0.52, 3.20) | |
| Perceived social support - PAS Scale [range: 8–40; high score ~ more support] |
1.02 (0.96, 1.09) | |
| Partner support - NSSQ Scale [range: 7–35; high score ~ more support] |
0.96 (0.91, 1.00)‡ | 0.98 (0.93, 1.03) |
| Facility Level | ||
| Local clinic [ref: hospital] |
1.12 (0.48, 2.62) | |
| Travel time to clinic (minutes) | 1.00 (0.98, 1.01) | |
| Perceptions of quality of care – PPRS Scale [range: 12–48; high score ~ better quality] |
1.01 (0.92, 1.11) | |
| Diagnosed with HIV during index pregnancy (n = 93) | ||
|---|---|---|
| Unadjusted relative risk (95% CI) |
Adjusted relative risk (95% CI) |
|
| Intrapersonal | ||
| Demographics | ||
| Age | 0.95 (0.88, 1.01) | 0.92 (0.86, 0.98)* |
| Education - started secondary or higher [ref: completed primary or less] |
0.64 (0.31, 1.34) | 0.64 (0.31, 1.35) |
| Relationship - lives alone or no partner [ref: lives with a partner] |
1.04 (0.51, 2.12) | 1.11 (0.54, 2.30) |
| Pregnancy | ||
| First pregnancy [ref: prior pregnancy] |
0.85 (0.39, 1.85) | 0.63 (0.26, 1.49) |
| Gestational age > 24 weeks at initiation of ANC [ref: <= 24 weeks] |
1.10 (0.47, 2.57) | 0.93 (0.39, 2.23) |
| Attitude toward pregnancy [range: 0–24; high score ~ better attitudes] |
1.01 (0.94, 1.09) | |
| HIV | ||
| Acceptance of HIV - ICQ scale [range: 6–24; high score ~ more acceptance] |
1.06 (0.96, 1.17) | |
| Attitude toward ART - BMQ scale [range: 10 – 50; high score ~ better attitude] |
1.04 (0.96, 1.13) | |
| Knowledge of Option B+ [range 0–5; high score ~ less knowledge] |
0.94 (0.70, 1.25) | |
| Mental Health | ||
| Depression - EPDS scale [range 0–30; high score ~ more depression] |
1.00 (0.95, 1.06) | |
| Shame - HARSI [range: 0–52; high score ~ more shame] |
1.02 (0.99, 1.06) | |
| Interpersonal | ||
| Partner is HIV-infected [ref: partner negative or unknown] |
0.73 (0.25, 2.17) | |
| HIV Disclosure - has told someone [ref: has not told anyone] |
0.62 (0.31, 1.25) | |
| Experienced any violence as an adult [ref: no violence experienced] |
1.10 (0.47, 2.57) | |
| Perceived social support - PAS Scale [range: 8–40; high score ~ more support] |
0.99 (0.94, 1.03) | |
| Partner support - NSSQ Scale [range: 7–35; high score ~ more support] |
0.97 (0.93, 1.01) | |
| Facility Level | ||
| Local clinic [ref: hospital] |
1.07 (0.49, 2.33) | |
| Travel time to clinic (minutes) | 1.00 (1.00, 1.01) | |
| Perceptions of quality of care - PPRS Scale [range: 12–48; high score ~ better quality] |
1.04 (0.96, 1.11) | |
p<.10
p<.05
p<.01
p<.001
Discussion
This prospective cohort study of 200 HIV-infected pregnant women receiving PMTCT services in the Kilimanjaro region of Tanzania found that about one-fifth of patients had indicators of poor HIV care engagement by six months after delivery. Women who were younger and had not disclosed their HIV status were more likely to have poor care engagement. Among women entering PMTCT with established HIV diagnoses, those presenting during their first pregnancy were at higher risk for poor care engagement than those who had been pregnant previously.
The findings of postpartum care engagement in this study are consistent with previous findings from a meta-analysis of PMTCT studies during the Option B+ era, which found that among women who attended PMTCT beyond the first appointment, 20.6% of participants were LTFU by six months (Knettel et al., 2018). While most previous studies relied on medical record review alone, and were thus limited by the quality of the record keeping, this study used a biological marker of viral load combined with self-report and medical record data. The use of a biomarker also provides an indicator of adherence to medication. Future studies should consider adding biological markers of medication adherence using dried blood spot or hair samples (Alcaide et al., 2017); currently, this technology is not available in most LMICs, but its successful use in research may lead to future uptake in health systems to monitor adherence.
The levels of care engagement found in this study should be considered in context of the UNAIDS 90-90-90 goal, which sets a target that 90% of individuals on ART be virally suppressed (UNAIDS, 2014). In this cohort, among women who had a viral load measurement, 85% were virally suppressed (HIV RNA ≤ 200). While pregnant and postpartum women face unique challenges to HIV care engagement, they also share characteristics with individuals who initiate ART in a test-and-treat framework; namely, they are typically tested and enrolled in lifelong HIV treatment soon after diagnosis, without experiencing symptoms, and with varying degrees of autonomy and voice about whether they can refuse treatment. As a result, women may have little time to accept the weight of their HIV diagnosis and make a personal, values-driven commitment to long-term treatment. As Tanzania rolls out its new policy for universal test-and-treat for all individuals diagnosed with HIV (National AIDS Control Programme, 2017), consideration should be given for sharing lessons and implementation strategies from the PMTCT setting to support ART initiation and retention in the general population, and vice versa. Supporting long-term care engagement requires differentiated models of care that identify individuals at elevated risk of poor care engagement and target services and support to those individuals (Grimsrud et al., 2016).
Consistent with previous research, younger age was a significant risk factor for poor care engagement (Erlwanger et al., 2017; Mitiku, Arefayne, Mesfin, & Gizaw, 2016; Musomba et al., 2017; Tweya et al., 2014). Contextually, in Tanzania and the Kilimanjaro region specifically, young pregnant women who are new mothers typically have significant involvement from their partner’s family (Urassa, Pembe, & Sunguya, 2009). This level of oversight by in-laws may provide additional support to a woman, but at the same time may make it difficult for her to discretely engage with PMTCT services and receive HIV care without disclosing her serostatus. Qualitative research has identified additional barriers to PMTCT care engagement that may be more common with younger age, including instability in romantic relationships (Buregyeya et al., 2017; Cataldo et al., 2017; Napúa et al., 2016), difficulty in accepting one’s HIV status (Atanga et al., 2017; Clouse et al., 2014; Flax et al., 2017; Katirayi et al., 2016; McLean et al., 2017), and fear of beginning a lifelong treatment regimen (Buregyeya et al., 2017; Gill et al., 2017; Katirayi et al., 2016). This may be explained in part by greater feelings of shame and perceived stigma among younger individuals living with HIV (Enane, Vreeman, & Foster, 2018; Hosek, Harper, & Domanico, 2005). Efforts to support younger women in PMTCT care engagement should consider ways to strength their social support networks, either by engaging their existing supporters in HIV care or by adding external peer supporters, such as other young women with HIV who are living healthy lives and fulfilling their social and familial roles.
HIV disclosure has been identified in the literature as a persistent determinant of long-term HIV care engagement, which was confirmed by our data. Non-disclosure of HIV to partners, family members, and close friends can create or perpetuate substantial barriers to attending the clinic, and can make it difficult to take pills consistently and on time (Bernier et al., 2018; King et al., 2008). Decisions not to disclose are often driven by legitimate fears of violence, abandonment, and stigma, especially from an intimate partner (Lugalla, Yoder, Sigalla, & Madihi, 2012; Shamu, Zarowsky, Shefer, Temmerman, & Abrahams, 2014). However, women who do disclose their status typically report improved care engagement and a variety of other positive outcomes, including improvements in mental health and reductions in sexual risk behaviors. To support HIV disclosures, there is a need for evidence-based and culturally-informed interventions that assist pregnant women in making decisions about HIV disclosures (Naigino et al., 2017; Spangler, Onono, Bukusi, Cohen, & Turan, 2014). These may include models that utilize peer support or couples HIV testing and counseling to improve communication about HIV and facilitate healthy disclosures (Turan et al., 2018). Additionally, interventions should seek to address HIV stigma, both in the form of improving HIV acceptance among infected women and reducing broader community misunderstanding and mistreatment of people living with HIV.
As test-and-treat programs become the standard of care in HIV, women are increasingly entering antenatal care knowing their HIV status and already initiated on ART. Pregnancy, and the entry into PMTCT, can provide an opportunity to reassess and support HIV care engagement. Among women entering care with an established HIV diagnosis, being pregnant for the first time was a risk factor for poor care engagement. Pregnancy, childbirth, and the experience of being a new mother all introduce new and unique challenges to HIV care engagement which must be reconciled, even with a known HIV status (Cichowitz, Watt, & Mmbaga, 2018; Psaros, Remmert, Bangsberg, Safren, & Smit, 2015).
The strengths of this study include the prospective design, triangulation of multiple sources of data, and excellent follow-up with study participants, with all 200 cohort women retained in the final analysis. At the same time, the close tracking methods used to retain our cohort, including reminder calls and home visits if necessary, likely contributed to improved retention in care over time. Further, the study was limited in that it enrolled women only after they had been prescribed ART for over one month. Women who did not initiate PMTCT were excluded from participation, and the women who did not return to the clinic after being prescribed ART were unlikely to be enrolled. This recruitment strategy over-estimated the true postpartum care engagement at the study clinics and was not able to identify factors associated with failure to initiate PMTCT. Additionally, the study may have been under-powered to detect differences in outcomes, particularly in the stratified analysis, and may have led to overfitting of the final models. Finally, it is possible that using a viral load measure as a marker of care engagement may have misclassified participants, as the presence of resistant strains of HIV (either through acquired or transmitted drug resistance) may lead to a suboptimal response to ART, even when taken as prescribed, and subsequent virologic failure. Despite these limitations, HIV viral load is considered the most important indicator of response to ART, and is the preferred monitoring approach to diagnose and confirm treatment failure. Given the limitations of self-report and medical record data, using viral load as a proxy for treatment adherence and retention in care is considered standard practice (HIV Surrogate Marker Collaborative Group, 2000).
A comprehensive PMTCT program is an essential component of achieving the goal of an AIDS-free generation (Fauci & Folkers, 2012). PMTCT programs should have effective monitoring, including regular viral load measurement, and support for long-term care engagement with appropriate clinic support. Differentiated models of care that recognize and respond to the unique needs of women with new and established diagnoses, first time mothers, and young women, are essential components of a PMTCT program. Quality counseling that is based on a patient-centered care framework (Epstein & Street, 2011) is essential to help women make decisions about HIV disclosure and identify and address the barriers to long-term care engagement that they may face during pregnancy, breastfeeding, and beyond the postpartum period.
Supplementary Material
Acknowledgements
This study was funded by a grant from the National Institutes of Health (NIH; R21 AI124344). Contributors to this work were supported by training grants from NIH (T32 AI007392; D43 TW009595; D43 TW010138) and from the Duke Center for AIDS Research (P30 AI064518).
Footnotes
Declaration of interest statement: No potential conflict of interest
References
- Alcaide ML, Ramlagan S, Rodriguez VJ, Cook R, Peltzer K, Weiss SM, … Jones DL (2017). Self-Report and Dry Blood Spot Measurement of Antiretroviral Medications as Markers of Adherence in Pregnant Women in Rural South Africa. AIDS and Behavior, 21(7), 2135–2140. 10.1007/s10461-017-1760-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanga PN, Ndetan HT, Achidi EA, Meriki HD, Hoelscher M, & Kroidl A (2017). Retention in care and reasons for discontinuation of lifelong antiretroviral therapy in a cohort of Cameroonian pregnant and breastfeeding HIV-positive women initiating “Option B+” in the South West Region. Tropical Medicine & International Health, 22(2), 161–170. 10.1111/tmi.12816 [DOI] [PubMed] [Google Scholar]
- Barry OM, Bergh A-M, Makin JD, Etsane E, Kershaw TS, & Forsyth BWC (2012). Development of a measure of the patient-provider relationship in antenatal care and its importance in PMTCT. AIDS Care, 24(6), 680–686. 10.1080/09540121.2011.630369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier A, Yattassaye A, Beaulieu-Prévost D, Otis J, Henry E, Flores-Aranda J, … Keita BD (2018). Empowering Malian women living with HIV regarding serostatus disclosure management: Short-term effects of a community-based intervention. Patient Education and Counseling, 101(2), 248–255. 10.1016/j.pec.2017.07.030 [DOI] [PubMed] [Google Scholar]
- Buregyeya E, Naigino R, Mukose A, Makumbi F, Esiru G, Arinaitwe J, … Wanyenze RK (2017). Facilitators and barriers to uptake and adherence to lifelong antiretroviral therapy among HIV infected pregnant women in Uganda: a qualitative study. BMC Pregnancy and Childbirth, 17(1):94 10.1186/s12884-017-1276-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo F, Chiwaula L, Nkhata M, van Lettow M, Kasende F, Rosenberg NE, … Phiri S (2017). Exploring the Experiences of Women and Health Care Workers in the Context of PMTCT Option B Plus in Malawi: Journal of Acquired Immune Deficiency Syndromes, 74(5), 517–522. 10.1097/QAI.0000000000001273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandisarewa W, Stranix-Chibanda L, Chirapa E, Miller A, Simoyi M, Mahomva A, … Shetty AK (2007). Routine offer of antenatal HIV testing (“opt-out” approach) to prevent mother-to-child transmission of HIV in urban Zimbabwe. Bulletin of the World Health Organization, 85(11), 843–850. 10.2471/BLT.06.035188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichowitz C, Watt MH, & Mmbaga BT (2018). Childbirth experiences of women living with HIV: a neglected event in the prevention of mother-to-child transmission care continuum. AIDS, 1 10.1097/QAD.0000000000001860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse K, Schwartz S, Van Rie A, Bassett J, Yende N, & Pettifor A (2014). “What they wanted was to give birth, nothing else”: Barriers to retention in Option B+ HIV care among postpartum women in South Africa. Journal of Acquired Immune Deficiency Syndromes, 67(1), e12–8. 10.1097/QAI.0000000000000263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin CJ, Konopka S, Chalker JC, Jonas E, Albertini J, Amzel A, & Fogg K (2014). A systematic review of health system barriers and enablers for antiretroviral therapy (ART) for HIV-infected pregnant and postpartum women. PLOS ONE, 9(10), e108150 10.1371/journal.pone.0108150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JL, Holden JM, & Sagovsky R (1987). Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. British Journal of Psychiatry, 150, 782–786. [DOI] [PubMed] [Google Scholar]
- Enane LA, Vreeman RC, & Foster C (2018). Retention and adherence: global challenges for the long-term care of adolescents and young adults living with HIV. Current Opinion in HIV and AIDS, 13(3), 212–219. 10.1097/COH.0000000000000459 [DOI] [PubMed] [Google Scholar]
- Epstein RM, & Street RL (2011). The Values and Value of Patient-Centered Care. The Annals of Family Medicine, 9(2), 100–103. 10.1370/afm.1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlwanger AS, Joseph J, Gotora T, Muzunze B, Orne-Gliemann J, Mukungunugwa S, … Mangwiro A-Z (2017). Patterns of HIV Care Clinic Attendance and Adherence to Antiretroviral Therapy Among Pregnant and Breastfeeding Women Living With HIV in the Context of Option B+ in Zimbabwe. Journal of Acquired Immune Deficiency Syndromes, 75(Suppl.2), S198–S206. 10.1097/QAI.0000000000001347 [DOI] [PubMed] [Google Scholar]
- Evers AW, Kraaimaat FW, van Lankveld W, Jongen PJ, Jacobs JW, & Bijlsma JW (2001). Beyond unfavorable thinking: the illness cognition questionnaire for chronic diseases. Journal of Consulting and Clinical Psychology, 69(6), 1026–1036. [PubMed] [Google Scholar]
- Fauci AS, & Folkers GK (2012). Toward an AIDS-Free Generation. JAMA, 308(4), 343 10.1001/jama.2012.8142 [DOI] [PubMed] [Google Scholar]
- Flax VL, Hamela G, Mofolo I, Hosseinipour MC, Hoffman IF, & Maman S (2017). Factors influencing postnatal Option B+ participation and breastfeeding duration among HIV-positive women in Lilongwe District, Malawi: A qualitative study. PLOS ONE, 12(4), e0175590 10.1371/journal.pone.0175590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill MM, Umutoni A, Hoffman HJ, Ndatimana D, Ndayisaba GF, Kibitenga S, … Bobrow EA (2017). Understanding Antiretroviral Treatment Adherence Among HIV-Positive Women at Four Postpartum Time Intervals: Qualitative Results from the Kabeho Study in Rwanda. AIDS Patient Care and STDs, 31(4), 153–166. 10.1089/apc.2016.0234 [DOI] [PubMed] [Google Scholar]
- Gourlay A, Birdthistle I, Mburu G, Iorpenda K, & Wringe A (2013). Barriers and facilitating factors to the uptake of antiretroviral drugs for prevention of mother-to-child transmission of HIV in sub-Saharan Africa: a systematic review. Journal of the International AIDS Society, 16(1), 18588 10.7448/IAS.16.1.18588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsrud A, Bygrave H, Doherty M, Ehrenkranz P, Ellman T, Ferris R, … Bekker L-G (2016). Reimagining HIV service delivery: the role of differentiated care from prevention to suppression. Journal of the International AIDS Society, 19(1), 21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIV Surrogate Marker Collaborative Group. (2000). Human immunodeficiency virus type 1 RNA level and CD4 count as prognostic markers and surrogate end points: a meta-analysis. AIDS Research and Human Retroviruses, 16(12), 1123–1133. 10.1089/088922200414965 [DOI] [PubMed] [Google Scholar]
- Horne R, Weinman J, & Hankins M (1999). The beliefs about medicines questionnaire: The development and evaluation of a new method for assessing the cognitive representation of medication. Psychology & Health, 14(1), 1–24. 10.1080/08870449908407311 [DOI] [Google Scholar]
- Hosek SG, Harper GW, & Domanico R (2005). Predictors of medication adherence among HIV-infected youth. Psychology, Health & Medicine, 10(2), 166–179. 10.1080/1354350042000326584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katirayi L, Namadingo H, Phiri M, Bobrow EA, Ahimbisibwe A, Berhan AY, … Tylleskär T (2016). HIV-positive pregnant and postpartum women’s perspectives about Option B+ in Malawi: a qualitative study. Journal of the International AIDS Society, 19(1), 20919 10.7448/IAS.19.1.20919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R, Katuntu D, Lifshay J, Packel L, Batamwita R, Nakayiwa S, … Bunnell R (2008). Processes and outcomes of HIV serostatus disclosure to sexual partners among people living with HIV in Uganda. AIDS and Behavior, 12(2), 232–243. 10.1007/s10461-007-9307-7 [DOI] [PubMed] [Google Scholar]
- Knettel BA, Cichowitz C, Ngocho JS, Knippler ET, Chumba LN, Mmbaga BT, & Watt MH (2018). Retention in HIV Care During Pregnancy and the Postpartum Period in the Option B+ Era: A Systematic Review and Meta-Analysis of Studies in Africa. Journal of Acquired Immune Deficiency Syndromes, 77(5), 427–438. 10.1097/QAI.0000000000001616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugalla J, Yoder S, Sigalla H, & Madihi C (2012). Social context of disclosing HIV test results in Tanzania. Culture, Health & Sexuality, 14(Suppl.1), S53–S66. 10.1080/13691058.2011.615413 [DOI] [PubMed] [Google Scholar]
- Matheson R, Moses-Burton S, Hsieh A, Dilmitis S, Happy M, Sinyemu E, … Sharma A (2015). Fundamental concerns of women living with HIV around the implementation of Option B+. Journal of the International AIDS Society, 18(6(Suppl 5)). 10.7448/IAS.18.6.20286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean E, Renju J, Wamoyi J, Bukenya D, Ddaaki W, Church K, … Wringe A (2017). “I wanted to safeguard the baby”: a qualitative study to understand the experiences of Option B+ for pregnant women and the potential implications for “test-and-treat” in four sub-Saharan African settings. Sexually Transmitted Infections, 93(Suppl. 3). 10.1136/sextrans-2016-052972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitiku I, Arefayne M, Mesfin Y, & Gizaw M (2016). Factors associated with loss to follow-up among women in Option B+ PMTCT programme in northeast Ethiopia: a retrospective cohort study. Journal of the International AIDS Society, 19(1), 20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugavero MJ, Davila JA, Nevin CR, & Giordano TP (2010). From access to engagement: measuring retention in outpatient HIV clinical care. AIDS Patient Care and STDs, 24(10), 607–613. 10.1089/apc.2010.0086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugavero MJ, Westfall AO, Zinski A, Davila J, Drainoni M-L, Gardner LI, … Retention in Care (RIC) Study Group. (2012). Measuring retention in HIV care: the elusive gold standard. Journal of Acquired Immune Deficiency Syndromes, 61(5), 574–580. 10.1097/QAI.0b013e318273762f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musomba R, Mubiru F, Nakalema S, Mackline H, Kalule I, Kiragga AN, … Castelnuovo B (2017). Describing Point of Entry into Care and Being Lost to Program in a Cohort of HIV Positive Pregnant Women in a Large Urban Centre in Uganda. AIDS Research and Treatment, 2017, 1–6. 10.1155/2017/3527563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachega JB, Uthman OA, Anderson J, Peltzer K, Wampold S, Cotton MF, … Mofenson LM (2012). Adherence to antiretroviral therapy during and after pregnancy in low-income, middle-income, and high-income countries: a systematic review and meta-analysis. AIDS, 26(16), 2039–2052. 10.1097/QAD.0b013e328359590f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naigino R, Makumbi F, Mukose A, Buregyeya E, Arinaitwe J, Musinguzi J, & Wanyenze RK (2017). HIV status disclosure and associated outcomes among pregnant women enrolled in antiretroviral therapy in Uganda: a mixed methods study. Reproductive Health, 14(1):107 10.1186/s12978-017-0367-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napúa M, Pfeiffer JT, Chale F, Hoek R, Manuel J, Michel C, … Chapman RR (2016). Option B+ in Mozambique: Formative Research Findings for the Design of a Facility-Level Clustered Randomized Controlled Trial to Improve ART Retention in Antenatal Care. Journal of Acquired Immune Deficiency Syndromes, 72, S181–S188. 10.1097/QAI.0000000000001061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National AIDS Control Programme. (2017). National Guidelines for the Management of HIV and AIDS. The United Republic of Tanzania; Retrieved from www.nacp.go.tz/site/download/NATIONAL_DECEMBER_2017.pdf [Google Scholar]
- Neufeld SAS, Sikkema KJ, Lee RS, Kochman A, & Hansen NB (2012). The development and psychometric properties of the HIV and Abuse Related Shame Inventory (HARSI). AIDS and Behavior, 16(4), 1063–1074. 10.1007/s10461-011-0086-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngarina M, Popenoe R, Kilewo C, Biberfeld G, & Ekstrom AM (2013). Reasons for poor adherence to antiretroviral therapy postnatally in HIV-1 infected women treated for their own health: experiences from the Mitra Plus study in Tanzania. BMC Public Health, 13, 450 10.1186/1471-2458-13-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbeck JS, Lindsey AM, & Carrieri VL (1981). The development of an instrument to measure social support. Nursing Research, 30(5), 264–269. [PubMed] [Google Scholar]
- O’Brien K, Wortman CB, Kessler RC, & Joseph JG (1993). Social relationships of men at risk for AIDS. Social Science & Medicine (1982), 36(9), 1161–1167. [DOI] [PubMed] [Google Scholar]
- Pirkle CM, Dumont A, & Zunzunegui M-V (2012). Medical recordkeeping, essential but overlooked aspect of quality of care in resource-limited settings. International Journal for Quality in Health Care, 24(6), 564–567. 10.1093/intqhc/mzs034 [DOI] [PubMed] [Google Scholar]
- Psaros C, Remmert JE, Bangsberg DR, Safren SA, & Smit JA (2015). Adherence to HIV care after pregnancy among women in sub-Saharan Africa: falling off the cliff of the treatment cascade. Current HIV/AIDS Reports, 12(1), 1–5. 10.1007/s11904-014-0252-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribaudo H, Lennox J, & Currier J (2009). Virological failure endpoint definition in clinical trials: Is using HIV-1RNA threshold <200 copies/mL better than 50 copies/mL? An analysis of the ACTG studies. Paper presented at the Conference on Retroviruses and Opportunistic Infections, Montreal. [Google Scholar]
- Risher KA, Kapoor S, Daramola AM, Paz-Bailey G, Skarbinski J, Doyle K, … Shah M (2017). Challenges in the Evaluation of Interventions to Improve Engagement Along the HIV Care Continuum in the United States: A Systematic Review. AIDS and Behavior, 21(7), 2101–2123. 10.1007/s10461-017-1687-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins NC, Becquet R, Orne-Gliemann J, Phiri S, Hayashi C, Baller A, & Shaffer N (2014). Defining and Analyzing Retention-in-Care Among Pregnant and Breastfeeding HIV-Infected Women: Unpacking the Data to Interpret and Improve PMTCT Outcomes. Journal of Acquired Immune Deficiency Syndromes, 67, S150–S156. 10.1097/QAI.0000000000000355 [DOI] [PubMed] [Google Scholar]
- Shamu S, Zarowsky C, Shefer T, Temmerman M, & Abrahams N (2014). Intimate Partner Violence after Disclosure of HIV Test Results among Pregnant Women in Harare, Zimbabwe. PLOS ONE, 9(10), e109447 10.1371/journal.pone.0109447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler SA, Onono M, Bukusi EA, Cohen CR, & Turan JM (2014). HIV-Positive Status Disclosure and Use of Essential PMTCT and Maternal Health Services in Rural Kenya. Journal of Acquired Immune Deficiency Syndromes, 67(Suppl 4), S235–S242. 10.1097/QAI.0000000000000376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speizer IS, Santelli JS, Afable-Munsuz A, & Kendall C (2004). Measuring factors underlying intendedness of women’s first and later pregnancies. Perspectives on Sexual and Reproductive Health, 36(5), 198–205. 10.1363/psrh.36.198.04 [DOI] [PubMed] [Google Scholar]
- The United Republic of Tanzania Ministry of Health and Social Welfare. (2013). National guidelines for comprehensive care services for prevention of mother-to-child transmission of HIV and keeping mothers alive. Retrieved from http://ihi.eprints.org/3335/
- Turan JM, Darbes LA, Musoke PL, Kwena Z, Rogers AJ, Hatcher AM, … Bukusi EA (2018). Development and Piloting of a Home-Based Couples Intervention During Pregnancy and Postpartum in Southwestern Kenya. AIDS Patient Care and STDs, 32(3), 92–103. 10.1089/apc.2017.0285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweya H, Gugsa S, Hosseinipour M, Speight C, Ng’ambi W, Bokosi M, … Phiri S (2014). Understanding factors, outcomes and reasons for loss to follow-up among women in Option B+ PMTCT programme in Lilongwe, Malawi. Tropical Medicine & International Health, 19(11), 1360–1366. 10.1111/tmi.12369 [DOI] [PubMed] [Google Scholar]
- UNAIDS. (2014). 90-90-90: An ambitious treatment target to help end the AIDS epidemic Geneva: UNAIDS; Retrieved from www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf [Google Scholar]
- UNAIDS. (2016). On the Fast-Track to an AIDS-free generation. Geneva: UNAIDS; Retrieved from http://www.unaids.org/sites/default/files/media_asset/GlobalPlan2016_en.pdf [Google Scholar]
- Urassa D, Pembe A, & Sunguya B (2009). Childbirth in Tanzania: Individual, Family, Community In Childbirth Across Cultures: Ideas and Practices of Pregnancy, Childbirth and the Postpartum. New York: Springer Publishing Company. [Google Scholar]
- World Health Organization. (2010). PMTCT Strategic Vision 2010–2015: Preventing mother-to-child transmission of HIV to reach the UNGASS and Millennium Development Goals. Geneva: WHO; Retrieved from http://apps.who.int/iris/handle/10665/44268 [Google Scholar]
- World Health Organization. (2016). Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach (Second edition). Geneva: WHO; Retrieved from http://apps.who.int/iris/bitstream/10665/208825/1/9789241549684_eng.pdf?ua=1 [PubMed] [Google Scholar]
- Yehia BR, Fleishman JA, Metlay JP, Korthuis PT, Agwu AL, Berry SA, … HIV Research Network. (2012). Comparing different measures of retention in outpatient HIV care. AIDS, 26(9), 1131–1139. 10.1097/QAD.0b013e3283528afa [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.