Abstract
Epithelial wound healing is essential for maintaining the function and clarity of the cornea. Successful repair after injury involves the coordinated movements of cell sheets over the wounded region. While collective migration has been the focus of studies, the effects that environmental changes have on this form of movement are poorly understood. To examine the role of substrate compliancy on multi-layered epithelial sheet migration, we performed traction force and confocal microscopy to determine differences in traction forces and to examine focal adhesions on synthetic and biological substrates. The leading edges of corneal epithelial sheets undergo retraction or contraction prior to migration, and alterations in the sheet’s stiffness are affected by the amount of force exerted by cells at the leading edge. On substrates of 30 kPa, cells exhibited greater and more rapid movement than on substrates of 8 kPa, which are similar to that of the corneal basement membrane. Vinculin and its phosphorylated residue Y1065 were prominent along the basal surface of migrating cells, while Y822 was prominent between neighboring cells along the leading edge. Vinculin localization was diffuse on a substrate where the basement membrane was removed. Furthermore, when cells were cultured on fibronectin-coated acrylamide substrates of 8 and 50 kPa and then wounded, there was an injury-induced phosphorylation of Y1065 and substrate dependent changes in the number and size of vinculin containing focal adhesions. These results demonstrate that changes in substrate stiffness affected traction forces and vinculin dynamics, which potentially could contribute to the delayed healing response associated with certain corneal pathologies.
Keywords: Extracellular matrix, cornea, organ culture, confocal fluorescence microscopy, vinculin, traction force microscopy
1. Introduction
The migration of an epithelial sheet over an injury requires a complex series of events to occur in order to heal the wound. First, the epithelial sheet must move as a unit. To do this, the sheet must generate traction forces with the extracellular matrix (ECM) or basement membrane and maintain cell-cell junctions of neighboring cells. Then, the epithelial barrier integrity must be repaired and restored (Brugués et al., 2014; Crosby and Waters, 2010; Anon et al., 2012; Gardel et al., 2010; Minns et al., 2016). The traction forces, which cause motility of the epithelial sheet, occur through the presence of lamellipodial protrusions at the leading edge of the wound. These protrusions are associated with the assembly and disassembly of focal adhesions, which occur through the following process: retraction of cells, enlargement and maturation of nascent adhesions, and formation of focal adhesion complexes (Petrie and Yamada, 2012). Therefore, the interactions that occur between epithelial cells and their underlying ECM are critical for optimal migration.
The composition and the rigidity of the basement membrane plays a critical role in the signaling that underlies cell adhesion, migration, and differentiation of the epithelial sheet (Discher et al., 2005; Mammoto and Ingber, 2010; Sazonova et al., 2011). For example, the composition of the corneal basement membrane changes with age and during certain pathologies, (ie. diabetes), ultimately resulting in less adhesion (Torricelli et al., 2013). It is hypothesized that the degree of force generated by cells on their substrate during migration is due to the rigidity of the substrate (Peyton et al., 2007; Roca-Cusachs et al., 2012). While there are numerous studies examining how single cells or clusters of cells change shape and exert force (Chen et al., 2015; Gardel et al., 2010; Mak et al., 2016), it is not understood how multi-layered epithelial sheets exert a force and migrate; however, there have been a few studies using monolayer cultures of Madin-Darby Canine Kidney Epithelial (MDCK) cells. In one study, MDCK cells were seeded onto 1 kPa polyacrylamide substrates and several rows away from the leading edge, stresses of approximately 8 kPa were measured, indicating that a monolayer of cells could potentially drive collective migration (Tambe et al., 2011). Another study used confluent MDCK cells that were seeded onto collagen-coated 3 kPa substrates and the cells were subsequently ablated. The cells at the edge exhibited forces that pointed away from the edge and towards the wound (Brugués et al., 2014), and as the wound closed, these forces decreased in strength. These studies demonstrate that a number of factors are involved in successful sheet migration. Our work aims to investigate the complexities of this process using multi-layered intact corneal epithelial sheets.
The corneal epithelium is an excellent tissue model to study wound healing, as it is avascular and oxygenated via diffusion. The epithelium is multi-layered with 5–7 layers of basal, wing, and apical cells. Under unwounded control conditions, the corneal epithelium adheres to a basement membrane through hemidesmosomes that are comprised of a complex of proteins. The integrity of the epithelial sheet depends on a complex of cell-cell junctions and is impaired by a decrease in the desmosomal protein, plakoglobin (Kokado et al., 2018). After injury, fibronectin is released and is present transiently along the basement membrane, or substrate (Blanco-Mezquita et al., 2011; Lee et al., 2014). Studies have shown that mechanical stretching causes fibronectin to partially unfold, leading to the exposure of cryptic binding sites necessary for fibronectin self-association (Foolen et al., 2016); therefore, we speculate that as epithelial cells migrate over the underlying substrate, they pull and stretch the fibronectin fibers.
Vinculin, a focal adhesion protein, has been shown to have a role in the formation of cell-matrix and cell-cell adherens junctions (Bays et al., 2014; Carisey and Ballestrem, 2011; Goldmann et al.; Grashoff et al., 2010). Moreover, in corneal epithelium, vinculin was found to increase 22-fold after injury compared to stationary epithelium (Zieske et al., 1989). After a wound, there is a release of ATP that is followed by specific changes in phosphorylation of a number of growth factor receptors, focal adhesion proteins, and adaptor proteins, such as epidermal growth factor receptor, paxillin, and Src, respectively. These changes in phosphorylation can be abrogated by knockdown of the purinoreceptor, P2Y2R (Kehasse et al., 2013). It is believed that the phosphorylation of c-Src is required for the conformational change in vinculin and its phosphorylation at pY1065 (Auernheimer et al., 2015; Bois et al., 2006).
The goal of this study was to determine how alterations in substrate stiffness affect collective epithelial cell migration, cellular traction forces, and focal adhesion dynamics after corneal injury. Three model systems (multi-layered epithelial sheets, cell monolayers, and 3-D organ cultures) were used to study whether alterations in substrate stiffness affect collective cell migration. Traction forces and directionality of cell motility were analyzed after epithelial sheets were cultured on fibronectin-coated 8 and 30 kPa polyacrylamide substrates. The results demonstrate differences in the localization of forces generated and a distinct retraction of the epithelial sheet prior to forward motility. After a scratch wound, focal adhesions containing vinculin increased on the more rigid substrates. In addition, the localization of pY1065 vinculin changed over time in corneal organ cultures after injury. Examination of cell behavior will help us understand why cell migration and wound healing is compromised under pathologic conditions where there are changes in the stiffness of the basement membrane or underlying substrate.
2. Materials and Methods
2.1. Reagents and antibodies:
Anti-Vinculin monoclonal mouse antibodies (clone hVIN-1), anti-phospho-Vinculin (pTyr822) polyclonal rabbit antibodies, and anti-β-actin monoclonal mouse antibodies (clone AC-15) were purchased from Sigma Aldrich (St. Louis, MO). Anti-Vinculin (pTyr1065) phosphospecific unconjugated polyclonal rabbit antibodies, Rhodamine Phalloidin, Alexa Fluor 488 goat anti-rabbit IgG (H+L), and Alexa Fluor 633 goat anti-mouse IgG (H+L) were obtained from Thermo Fisher Scientific (Cambridge, MA). Anti-E-cadherin was obtained from ABCAM (Cambridge, MA). Human plasma Fibronectin purified proteins and hydrochloric acid were purchased from EMD Millipore Corporation (Temecula, CA). Type IV collagen from human placenta (Bornstein and Traub Type IV), Temed (N,N,N’,N’ Tetramethylethylenediamine), HEPES 1M solution, and Ponceau S were obtained from Sigma Aldrich (St. Louis, MO). The following were acquired from Thermo Fisher Scientific: Opti-MEM I (1X), Amphotericin B (Fungizone), Lipofectamine 2000 Reagent, and FluoSpheres sulfate 1.0 µm fluorescent microspheres, yellow-green (505/515 excitation). Keratinocyte serum-free medium (K-SFM) 1X, human recombinant Epithelial Growth Factor (EGF), and Bovine Pituitary Extract were purchased from GIBCO (Grand Island, NY). Penicillin-streptomycin and Dulbecco’s Modified Eagle’s medium (DMEM) with 1g/L glucose were purchased from Cellgro (Herndon, VA). VectaSHIELD Antifade Mounting Medium with DAPI was obtained from Vector Laboratories (Burlingame, CA). Sulfo-SANPAH crosslinker (sulfosuccinimidyl 6-[4′-azido-2′-nitrophenylamino] hexanoate) was purchased from ProteoChem (Hurricane, UT). Bicinchoninic acid assay (BCA), 40% w/v Acrylamide solution, and 2% Bis solution were acquired from Bio-Rad Laboratories (Hercules, CA). Dispase II neutral protease was purchased from Roche (Indianapolis, IN).
2.2. Cell Culture:
Human corneal limbal epithelial cells were cultured in Keratinocyte-serum free media (K-SFM) supplemented with 0.5% amphotericin B, 0.02 nM Epidermal growth factor, 25 µg/mL bovine pituitary extract, 0.03 mM CaCl2, 100 U/mL penicillin, and 100 µg/mL streptomycin. Cells were passaged when 80% confluent and plated at a density of 50–75 cells/mm2 on fibronectin-coated polyacrylamide gels (Matrigen, Brea, CA). For scratch wounding and live cell-imaging experiments, growth media was removed 16 hours prior to experimentation and replaced with supplement-free K-SFM.
2.3. Organ Culture:
The research protocol conformed to the standards of the Association for Research in Vision and Ophthalmology for the Use of Animals in Ophthalmic Care and Vision Research and the Boston University IACUC. Rat organ cultures were used as described (Lee et al., 2014). Briefly, 3 mm abrasion wounds were performed on rat corneas (Charles River Labs, Wilmington, MA) (Payne et al., 2000; Spurr and Gipson 1985). The corneas and a rim of sclera were removed and placed on agar domes to maintain the curvature of the cornea. Medium was added until the apical surface of the scleral rim was covered to allow the epithelium to remain exposed to air.
2.4. Immunofluorescence and Confocal Microscopy:
Organ cultures or epithelial cells were fixed with 4% paraformaldehyde and corneas were sectioned radially. The tissue or cells were permeabilized with 0.1% v/v Triton X-100 in phosphate buffered saline (PBS) and blocked with 4% bovine serum albumin (BSA) in PBS for indirect immunofluorescence. Samples were incubated in a specific primary antibody solution overnight at 4°C, washed, and incubated in Alexa Fluor-conjugated secondary antibodies (1:100; Thermo Fisher Scientific) in 1% BSA in PBS for 1 hour at room temperature. Samples were counterstained with rhodamine phalloidin (1:50; Thermo Fisher Scientific) to visualize F-actin and mounted using VectaSHIELD with DAPI to stain nuclei (Vector Labs). Images were taken on a Zeiss LSM 700 confocal microscope (Zeiss, Thornwood, NY) using either the 40× oil or 63× oil objective. Secondary antibody controls were used to set the gain of the laser at a negative or black and all experimental samples were imaged at that gain. Pinhole size was kept at 1 Airy Unit and images were obtained using Zen Black Edition software (Zeiss, Thornwood, NY). Analysis was performed using FIJI/ImageJ (NIH, Bethesda, MD; http://imagej.nih.gov/ij/).
2.5. Western Blot Analysis:
Cells were cultured to confluence on 0.2 mg/mL fibronectin-coated polyacrylamide gels and scratch wounds were performed. Cells were washed with ice-cold PBS and lysates harvested in 10 mM Tris-HCl buffer containing 1% Triton X-100, 0.1% SDS, 150 mM NaCl, 0.5% sodium deoxycholate, 1mM phenylmethylsulfonylfluoride, and 1mM sodium orthovanadate (pH 7.5). Lysates were homogenized, centrifuged for 10 minutes at 11,000 g, and supernatants were collected. Protein concentration was determined using the bicinchoninic acid assay (Thermo Fisher Scientific) and 10% SDS-PAGE gels were run with equivalent protein. Immunoblots were probed with a primary antibody overnight at 4°C, probed with specific horseradish peroxidase-conjugated secondary antibodies, and imaged using a chemiluminescent agent (Denville Scientific, Metuchen, NJ). Band intensity was analyzed using FIJI/ImageJ (NIH, Bethesda, MD; http://imagej.nih.gov/ij/).
2.6. Traction Force Microscopy:
Round 30 mm glass coverslips #1.5 (Warner Instruments, Hamden, CT) were cleaned with a plasma cleaner (Harrick Plasma, Ithaca, NY) and the surface coated with 5% 3-aminopropyl triethoxysilane in ethanol (3-APTES: Sigma Aldrich). Coverslips were washed with distilled water, cross-linked with 0.5% glutaraldehyde in PBS, and stored in PBS containing 2% penicillin-streptomycin at 4ºC. Polyacrylamide gels with a Young’s modulus of 8 kPa or 30 kPa were made as described (Kraning-Rush et al., 2012). The gels were dialyzed against PBS containing 2% penicillin-streptomycin and treated with Sulfo-SANPAH (ProteoChem, Hurricane, UT) in a 0.1 M HEPES solution for linkage of microspheres. The gels were dialyzed against PBS containing 2% penicillin-streptomycin and treated with Sulfo-SANPAH (The dish was exposed to ultraviolet light for 5 min using a tissue culture hood, and the Sulfo-SANPAH solution was removed. Gels were incubated in sterile water containing 1.0 µm fluorescent microspheres (505 nm/515 nm excitation: Thermo Fisher Scientific). Gels were coated with 0.2 mg/mL of fibronectin overnight at 4ºC and excess protein was removed with washing. Gels were kept in sterile PBS with 2% Fungizone at 4ºC.
2.7. Traction Stress Calculations:
Fourier transform traction microscopy was used to compute cellular traction forces using a widely utilized technique (Butler et al., 2002; Krishnan et al., 2012). Each microbead image was compared with its reference image to obtain the displacement field in the substrate plane. From the displacement field, the elastic properties of the gel, and a manual trace of the cell contour, we calculated the traction field. From the traction field, we calculated the contractile moment matrix (M) as a first-order moment of the traction field. By constructing an ellipse whose semiaxes are equal to the eigenvalues and directions determined by the corresponding eigenvectors of M, we tracked traction field realignment, following the angle () of the major axis of the ellipse relative to the axis perpendicular to stretch. By calculating components of M in the direction parallel with the stretch axis (M) and in the direction perpendicular to the stretch axis (M), we could track changes of the traction field along these two directions. For comparison between different cells, we normalized the semiaxes of the ellipses with the prestretch baseline value of the net contractile moment; the net contractile moment is defined as the trace of M (Butler et al., 2002).
2.8. Adhesion and Imaging of Epithelial Sheets:
Epithelial sheets were removed from the basement membrane of corneas as described (Trinkaus-Randall and Gipson, 1984). Briefly, rat corneas were incubated for 35 minutes in a solution of DMEM and 1.2 U/mL of Dispase II (Roche, Indianapolis, IN). Epithelial sheets were removed and placed onto the fibronectin-coated beaded gels in the presence of growth media. Gels were placed on the stage of a Zeiss LSM 880 confocal microscope within an environmental chamber (35ºC, 5% CO2) and imaged every 10 minutes for 12 hours. Phase contrast and fluorescent images were collected for every frame. A minimum of five sheets were imaged per experiment and z-stacks were taken for each region. A minimum of 5 independent experiments were performed. To determine the directionality of the forces exerted during epithelial sheet movement, fluorescent bead displacement was tracked using FIJI/ImageJ (NIH, Bethesda, MD; http://imagej.nih.gov/ij/). The X and Y coordinates of individual beads were obtained at specific time points for the duration of the experiment. The leading edge (assigned as the first 10 rows of cells) and area back from the leading edge (BFLE: designated as 20 cell rows away from the leading edge) were analyzed. Fourier transform traction cytometry (FTTC) analysis was employed to determine the forces exerted between epithelial sheets and the underlying substrates.
2.9. Statistical Analysis:
Data shown as mean ± SEM of at least three independent experiments. Statistical significance was determined by two-way analysis of variance (ANOVA) or unpaired, one-tailed t-test using GraphPad Prism 7 (GraphPad Software, San Diego, CA).
3. RESULTS and DISCUSSION
3.1. Forces employed by multi-layered epithelial sheets to migrate
To determine how intact sheets of epithelium move along the underlying substrate, multi-layered epithelial sheets were removed (Trinkaus-Randall and Gipson 1984) and placed onto fibronectin-coated substrates, which were produced by covalently linking fibronectin to 1.0-µm fluorescent beads conjugated to a polyacrylamide surface. Two substrates of different physiologically relevant rigidities were examined: an 8 kPa substrate, which has a stiffness similar to a basement membrane, and a 30 kPa substrate, which is similar to that of a stromal collagen proteoglycan matrix (Last et al., 2012). Fibronectin was used as it is present transiently in the basement membrane of corneal wounds for the first 18 – 48 hours (Lee et al., 2014). A minimum of 5 experiments were performed for each substrate, and fluorescent and phase images were collected every 10 minutes for 12 hours on 5 sheets for each experiment. Imaging analysis demonstrated that the epithelial sheets on both substrates retracted prior to forward motility. On the 8 kPa substrates, epithelial sheets retracted for 6 to 7 hours (Fig. 1A.a,b), while on the 30 kPa substrates retraction was completed after 2 to 3 hours (Fig. 1A.d,e), indicating that the duration of maximal retraction was substrate dependent (Fig. 1A). In addition to retraction time, the morphology of the leading edge during retraction was substrate dependent. On the 8 kPa substrates, the leading edge was raised and rolled at its most retracted point and individual apical cells were observed (Fig. 1A.b,B.a), whereas on the 30 kPa substrates, the leading edge was not distinct (Fig. 1A.e,B.b).
Figure 1. Substrate stiffness affects leading edge dynamics and the localization of cellular traction stresses during collective epithelial sheet migration.
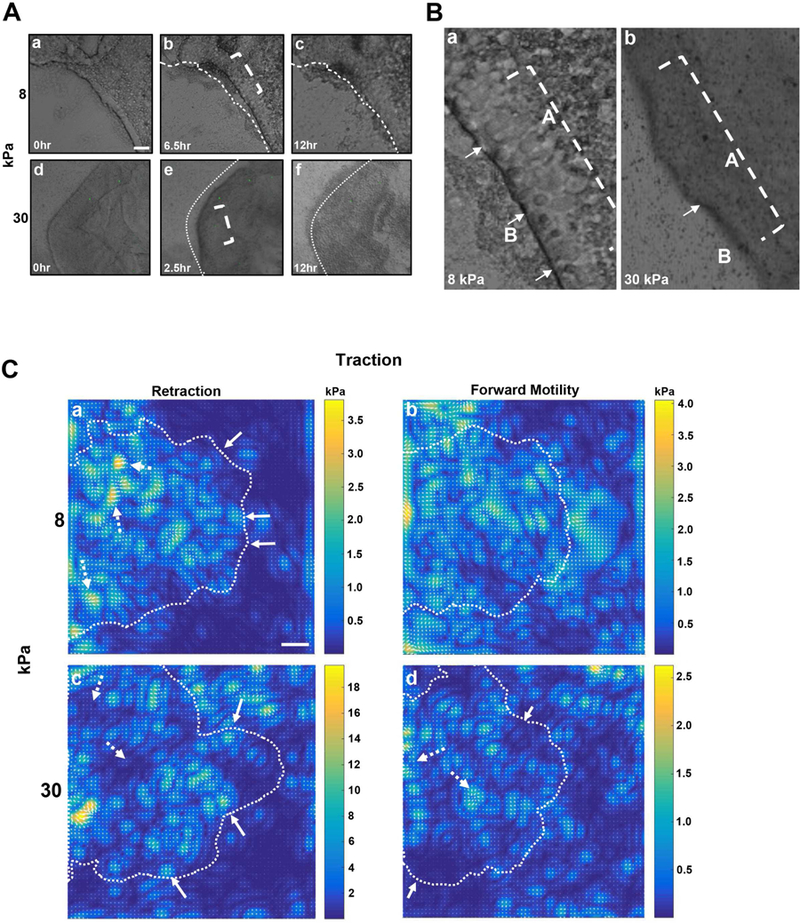
Epithelial sheets were removed from rat corneas and placed onto fibronectin-coated 8 and 30 kPa polyacrylamide gels with 1.0 µm fluorescent beads conjugated to the surfaces. Phase contrast and fluorescent images were taken every 10 minutes for 12 hours using a 10x objective on a Zeiss LSM 880 confocal microscope. A minimum of 5 epithelial sheets were imaged per experiment with N=5 experiments. Representative images are shown. Supplemental movies 1 and 2 demonstrate movement of epithelium. A. The initial phase of sheet migration involves retraction of the leading edge on 8 and 30 kPa substrates. Images of the initial (t=0 hours), furthest retracted (6.5 and 2.5 hrs), and final positions (t=12 hrs) of epithelial sheets are shown. Dotted lines mark the location of the leading edge at t=0. Scale bar is 200 µm. B. Close up of the leading edge of an epithelial sheet on an 8 kPa and 30 kPa substrate. The dotted bracket indicates the apical (A) epithelial cells involved in forward sheet migration and white arrows denote basal epithelial cells (B). C. Traction force analysis was performed using fourier transform traction cytometry. Images provide color-coded intensities of traction forces applied to gels. Dotted lines mark epithelial sheet boundaries. a,c. Retraction. a. Epithelial sheets on 8 kPa substrates exhibit greatest stress away from the leading edge (dotted white arrow). c. Epithelial sheets on 30 kPa substrates exert greatest stress at the leading edge (solid white arrow). b,d. Forward motility. b. Stresses are applied more uniformly beneath epithelial sheets on 8 kPa substrates. d. Epithelial sheet on 30 kPa substrates has greatest stress back from the leading edge (dotted white arrow). At the leading edge low stress was exerted (solid arrow).
At the most retracted point and at the onset of forward motility, apical cells appeared to “stream” forward to initiate the migratory process. In Figure 1B, the area from within brackets (Fig. 1A.b,e) was enlarged to show a magnified view of the apical cells (within dotted bracket) and basal cells (arrows) at the leading edge on both substrates. These results suggest that two phases of motility are present during corneal epithelial migration and indicate that different cell populations are involved. During sheet retraction, basal cells may have the primary role in dictating movement at the leading edge, while the apical cells move over the basal cells and may play an important role in forward sheet movement. The epithelial sheets moved more rapidly on the stiffer substrate as indicated in Figure 1. The sheets had an average motility during the retraction phase of 0.7 and 3.14 µm/hour at the leading edge on the 8 and 30 kPa substrates respectively. In addition during the forward phase the sheets had a motility of 0.36 and 0.7 µm/hour on the 8 and 30 kPa substrates, respectively. The rate of motility of the sheets suggests that they are under extensive strain.
The contraction that was detected in these epithelial sheets also was detected in other tissues after injury. For example, after refractive surgical procedures to the cornea there was a contraction due to the ingrowth of fibroblastic cells (Jester et al., 1992). In contrast, after an abrasion injury, where the epithelium was removed, there was a transient expression of fibronectin that was associated with an increase in the integrin,avb6 (Stepp 2006). Likewise in psoriasis, the epidermis expressed higher levels of fibronectin after injury and was associated with differential regulation of integrins and proposed change in cell integrity (Gubán et a l., 2016). The retraction that was detected in the epithelium after injury may be regulated by ion channel proteins, as indicated by the reduction in actin bundling and focal adhesion turnover (Minns et al., 2016).
To analyze the forces exerted under the epithelial sheet, fourier transform traction cytometry (FTTC) analysis was performed after each experiment on the fluorescent beads conjugated to the polyacrylamide surfaces. The images generated data on the change in bead position over time as the sheets migrated. The distance that a bead was displaced from its original position was correlated to the amount of force exerted on the beads by the cells. Control experiments were performed to demonstrate that the beads only exhibited localized displacement when epithelial sheets were present (Supplemental figure 1). As intact multi-layered epithelial sheets migrated in 2 phases, traction stresses were determined as sheets retracted and then moved forward on the 8 and 30 kPa fibronectin-coated substrates (Fig. 1C.a-d).
During retraction, traction stress was measured from t=0 to 6.5 hours (or most retracted) on the 8 kPa substrates (Fig. 1C.a) and t=0 to 2.5 hours on the 30 kPa substrates (Fig. 1C.c). For the forward motility phase, traction stress was measured from the time the epithelial sheet was at its most retracted point (t=6.5 or 2.5 hours) to its final position at t=12 hours (Fig. 1C.b,d). In Figure 1C (a-d), FTTC images show the traction stresses applied to the entire substrate, regardless of the presence of the epithelial sheet. The dotted boundary line in each image indicates the edge of the original epithelial sheet. For the retraction phase, the stress applied on 8 kPa substrates ranged from 0.2 – 3.5 kPa (Fig. 1C.a). The strongest stresses (dotted white arrows) were a distance from the leading edge, indicating that these cells exerted greater stress as sheets retracted. This suggests that cells away from the leading edge may behave as tethers and enable the sheet to generate enough stress to move over the 8 kPa substrates. Traction stress was detected underneath basal cells along the leading edge (solid white arrows), but the forces were smaller than those away from the edge (Fig. 1C.a). On the 30 kPa substrates during retraction (Fig. 1C.c), the range of values was greater (2 – 18 kPa) and the localization of the stresses differed compared to those on the 8 kPa substrates (Fig. 1C.a). On the stiffer substrate the larger stresses were detected at the leading edge (solid white arrows) and several rows back. Together these results indicate that on stiffer surfaces, cells away from the leading edge do not adhere as tightly to the substrate. As these are multi-layered sheets of epithelium, we speculate that the stresses exerted by the basal cells at the leading edge might be enough to influence the direction of sheet movement.
The traction stress associated with the second phase, or the forward migration of the epithelial sheets, was also examined (Fig. 1C.b, d). On the 8 kPa substrates (Fig. 1C.b), the stress applied by the epithelial sheet during forward migration was similar to that exerted as the sheets retracted (Fig. 1C.a). In addition, the stress exerted by the basal cells was relatively uniform (2 – 4 kPa) beneath the multi-layered sheet (Fig. 1C.b). In contrast, on the 30 kPa substrates (Fig. 1C.d), the stress applied by cells at the leading edge (solid arrows) was about 5-fold less than that exerted during the retraction phase (Fig. 1C.c). In some regions along the leading edge, the stress was less than 0.5 kPa (Fig. 1C.d); however, further away from the leading edge the traction stress increased (dotted white arrows). The pattern of the stresses and the observation that the cells consistently exert forces above zero indicate that the values are not due to random force fluctuations (Tambe et al., 2011). The data illustrated in Figure 1C reflect the traction forces applied by the basal cells to the underlying substrate. While we were unable to distinguish between the contribution of the forces exerted by the apical and the basal cells, we were able to examine forces on substrates of different stiffnesses by intact multi-layered sheets of epithelium. These data suggest that the stresses exerted by multi-layered sheets from a tissue are distinct from those cultured on substrates.
The majority of traction force microscopy experiments to date have been performed on single cells or small cell clusters. Some studies examined the relationship between traction forces and the localization of focal adhesions, with the largest forces measured at paxillin-positive focal adhesions in migrating cells (Beningo et al., 2001; Choi et al., 2008; Gardel et al., 2008). Other investigators examined the forces exerted during collective cell migration of cell monolayers (Brugués et al., 2014). The hypothesis that only ‘leader’cells along the leading edge generated enough force to pull the entire sheet forward was tested, and it was found that their traction forces pointed away from the leading edge (Gov, 2007; Khalil and Friedl, 2010). In comparison, the stress exerted by the cells at the leading edge of the multi-layered epithelial sheet upon retraction on the 8 kPa substrates (Fig. 1C.a) was low (< 1 kPa) compared to that detected back from the leading edge (3.5 kPa; blue and yellow on intensity scale, respectively). In addition, in the second phase when epithelial sheets migrated forward on the 30 kPa substrates (Fig. 1C.d), the traction stress was less than 0.25 kPa at the leading edge (blue on intensity wedge) and 1.5 – 2.5 kPa away from the leading edge, indicating that stress was exerted primarily by cells further back from the leading edge (Fig. 1C.d).
Several investigators have focused on force dynamics along the leading edge of migrating cells. In confluent cells, Brugues et al. (2014) demonstrated that not only were traction forces directed away from the leading edge, but forces also were directed towards the wound. Furthermore, it was determined that forces decreased away from the leading edge and pointed away from the wound. Tambe et al. (2011) used several epithelial cell lines as they have a round cell shape and are oriented in the direction of migration. For both of these studies, the cells were maintained as a single layer (Brugués et al., 2014; Tambe et al., 2011), and thus did not provide information about the forces generated during the movement of multi-layered cell sheets, which are present in numerous tissues, including the corneal epithelium. For example, during forward migration on 8 kPa substrates, epithelial sheets applied strong forces to the underlying substrate both at and away from the leading edge. Interestingly, cell sheets on 30 kPa substrates exerted strong forces back from the leading edge during forward motility, while forces remained low and less than 0.5 kPa at the leading edge. In summary, our data indicates that the collective migration of corneal epithelial cells involves not only the basal cells that are attached directly to the substrate, but also the apical cells. We speculate that that there are local interactions that are the underlying basis for molecular interactions such as cadherins or integrins. Localized changes in environment might result in changes in charge or divalent cations causing alterations in the molecular interactions that are calcium dependent. Additionally, the localization of the forces exerted during collective movement differs between substrates of different stiffness.
3.2. Cellular movement within sheets is greater on stiffer substrates
To examine the dynamics of epithelial sheet migration, the movement of localized displacements within the sheets was examined and differences in the directionality of cell movement on 8 and 30 kPa determined. Traction force microscopy generated displacement vectors that indicated the direction of exerted traction force and individual cell movement (Fig. 2A,B). The displacement values under the epithelial sheets were small (as shown in a representative experiment; Table I); therefore, bead displacement was determined by calculating ∆Z or the hypotenuse from changes in X and Y coordinates over time. Changes in bead displacement were analyzed for both retraction and forward movement. As described, phase one (retraction) included bead displacement from the initial (t=0 hour) position to its furthest point of retraction (t=6.5 hours for 8 kPa and t=2.5 hours for 30 kPa substrates). For phase two or forward motility, bead displacement of the epithelial sheet was measured from the furthest retracted point to the final location at t=12 hours. Displacement of beads was only determined when sheets were in contact with beads as determined by video analysis after experiments were performed.
Figure 2. Traction force analysis was performed and displacement vectors indicate epithelial sheet movement.
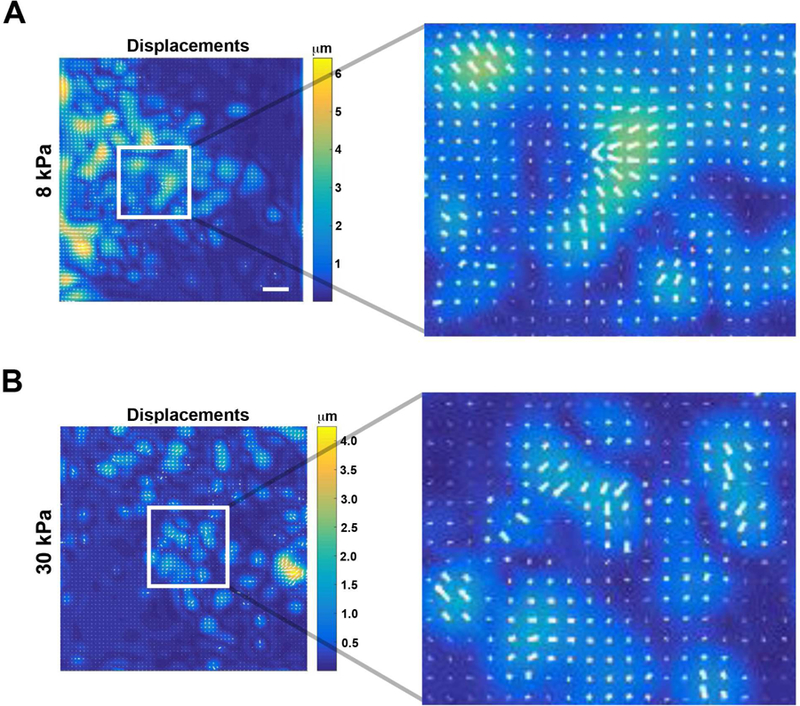
Traction force analysis was performed on epithelial sheets placed on 8 and 30 kPa gels using FTTC. Enlargements of insets show vectors (white) within a region of an 8 and 30 kPa gel. Scale bar is 400 µm.
Table I.
Cells within corneal epithelial sheets exhibit differences in movement during retraction and forward motility on 8 kPa and 30 kPa substrates.
| 8 kPa | 30 kPa | |||||
|---|---|---|---|---|---|---|
| Retraction (μm) | X | Y | Z | X | Y | Z |
| LE | 2.1+/.15 | 2.5+/−.16 | 3.3 | 4.6+/−.14 | 2.6+/.3 | 5.3 |
| BFLE | 10.1+/−.94 | 3.1+/−.19 | 10.6 | 6.8+/−.2 | 15+/−3.3 | 16.5 |
| Forward (μm) | X | Y | Z | X | Y | Z |
| LE | 1.6+/−.3 | 1.5+/−.3 | 2.2 | 3+/−.3 | 7.1+/−.2 | 7.7 |
| BFLE | 1.6+/−.3 | 1.3+/−.2 | 2.1 | 4.9+/−.1 | 4.7+/−.3 | 6.8 |
Epithelial sheets were removed from rat corneas and placed onto fibronectin-coated 8 kPa and 30 kPa polyacrylamide gels. Images were obtained every 10 minutes for 12 hours using the 10x objective on a Zeiss LSM 880 confocal microscope. A minimum of 5 epithelial sheets were imaged per experiment with a minimum of 5 experiments. Magnitude of bead displacement or hypotenuse (∆Z) was obtained by measuring the change in X (∆X) and Y (∆Y) coordinates of individual beads throughout the course of experimentation. SEM is calculated for the change in x and y. Analysis was performed using FIJI/ImageJ (NIH, Bethesda, MD; http://imagej.nih.gov/ij/).
On the 8 kPa substrates (Fig. 2A), displacement of individual beads during the retraction phase was less at the leading edge (∆Z=3.3 µm) than back from the leading edge (∆Z=10.6 µm). Similarly, on the 30 kPa substrates (Fig. 2B), average displacement of beads was less at the leading edge (∆Z=5.3 µm) than away from the leading edge (∆Z=16.5 µm). During the second phase when the epithelial sheets moved forward, there was greater displacement on the stiffer substrate. However, there was minimal difference in displacement of beads between the 2 regions for a given substrate (8 kPa: ∆Z=2.2 µm at the leading edge and ∆Z=2.1 µm away from it; 30 kPa: ∆Z=7.7 µm at the leading edge and ∆Z=6.8 µm away from the edge). These data indicate that as epithelial sheets retract there is a difference in the mode and morphology of the sheet, as well as in the streaming forward of apical cells (Fig. 1).
3.3. Changes in phosphorylation of vinculin on corneal epithelial sheets after removal of epithelium or epithelium and basement membrane
A 3-D organ culture model was used to examine changes in vinculin localization after corneal injury (Gordon et al., 2010; Lee et al., 2014). Organ culture is an ideal method to examine epithelial sheet migration as it has the correct conformation and rigidity of a normal cornea. To perform the experiments, a 3mm diameter area of the epithelium or the epithelium and basement membrane were removed from the central region and the corneas were placed onto agar molds and incubated for 4 or 18 hours. These time points were chosen to represent both early (4 hours) and late (18 hours) stages of wound healing. Corneas were fixed and stained for total vinculin, vinculin pY822, and vinculin pY1065. The phosphorylated forms of vinculin, pY822 and pY1065, have been demonstrated to indicate cell-cell and cell-matrix signaling, respectively (Bays et al., 2014).
In the unwounded tissue, total vinculin was diffuse throughout the epithelium with concentrated localization along the basal and basolateral surface (Fig. 3A.a). After an epithelial wound, total vinculin was prominent along the basal surface of the migrating epithelium (arrows) at both time points (Fig. 3A.b,c). The diffuse staining in the unwounded and focal staining in the wounded tissue was similar to that observed in vivo (Zieske et al., 1989).
Figure 3. The localization of total vinculin, vinculin pY1065, and vinculin pY822 before and after injury.
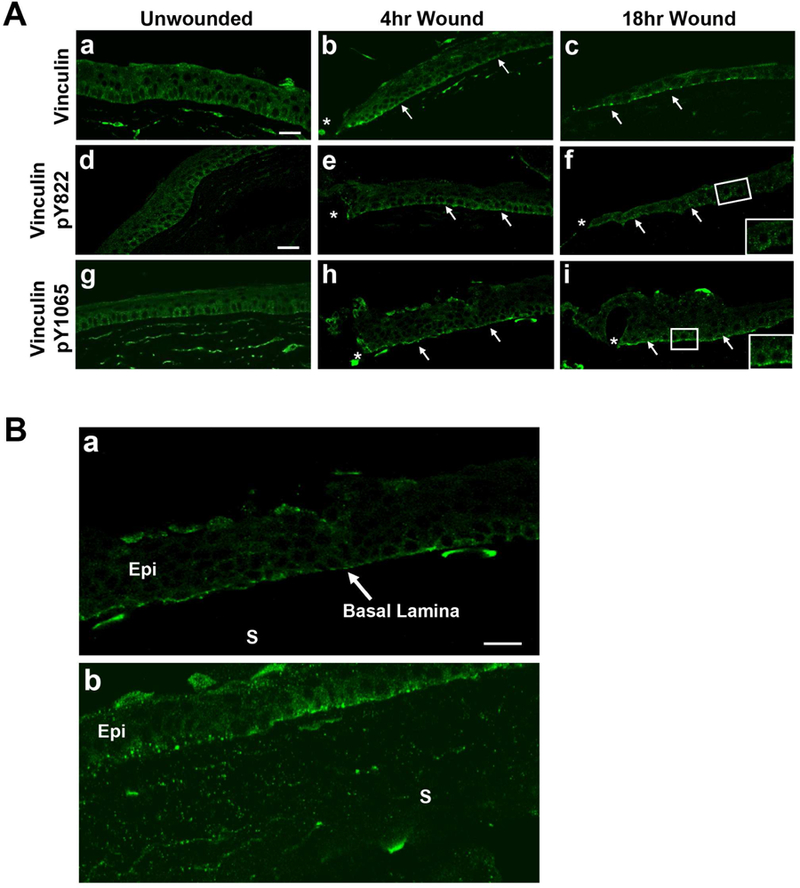
A-C. Epithelium was removed leaving basement membrane, and corneas were incubated for 4 or 18 hours in organ culture. Corneas were fixed and immunohistochemistry was performed. Images were obtained using a 40x objective on a Zeiss Axiovert LSM 700 confocal microscope. Arrows denote localization along the basal surface of epithelium. * indicates wound edge. Images represent a minimum of 3 independent experiments. Scale bar is 50 µm. A. a-c. Vinculin localizes to cell borders, along the basal surface of the migrating epithelium and the anterior stroma (s).d-f Vinculin pY822 is punctate throughout the epithelium 18 hours after injury. (f. Corner box is a higher magnification of inset.) g-i. Vinculin pY1065 is localized along the basal surface after injury. (i. Corner box is a higher magnification of inset.) B. Comparison of vinculin pY1065 in migrating epithelium after removal of epithelium alone (a) or removal of epithelium and basement membrane (b). Vinculin pY1065 is diffuse in stroma (s) and epithelium (Epi) after basement membrane removal. Figure 3B is a higher magnification of 3A.h.
To determine if there was a change in cell-cell communication, we examined the localization of vinculin pY822 before and after an epithelial wound. In the unwounded corneal epithelium, a punctate pattern of vinculin pY822 was present at sites of contact between cells (Fig. 3A.d). Four hours after injury, vinculin pY822 localized to both the basal surface (arrow) and between cells (Fig. 3A.e). By 18 hours, vinculin pY822 was localized along cell contacts (Fig. 3A.f; arrows and inset).
Likewise, we examined for changes in vinculin pY1065 localization before and after an epithelial wound. Similar to total vinculin, vinculin pY1065 was localized diffusely in the epithelium of unwounded corneas (Fig. 3A.g), with concentrated localization in the basal epithelial cells and the anterior stroma. After injury, vinculin pY1065 was present along the basal epithelial surface at 4 hours (Fig. 3Ah; arrows), and by 18 hours after injury, the staining became punctate (Fig. 3A.i; arrows and inset). This was in contrast to the localization of pY1065, which was prominent along the basal surface of the migrating epithelium (Figure 3A. f; arrows and inset). These results indicate that injury to the epithelium leads to changes in vinculin pY1065 localization as cells migrate over the wounded region (Fig. 3A.h,i).
As the cornea is the outermost layer of the eye, it is constantly exposed to environmental insults and injury. Changes in integrins can occur and induce a change in the contraction or fibrosis of the cornea (Wu et al 2018). In the cornea, superficial wounds expose the basement membrane, while deeper wounds that penetrate the basement membrane expose the underlying collagen-proteoglycan stromal matrix (Ljubimov and Saghizadeh 2015; Torricelli et al., 2016). When this occurs, epithelial cells migrate over a stiffer (30 – 50 kPa) matrix (Last et al., 2012). During healing, the migrating epithelium gradually repairs the wounded matrix and basement membrane. In Figure 3B, the localization of vinculin pY1065 in two types of wounds was compared at 4 hours. As mentioned earlier, when only the epithelium was removed, vinculin pY1065 was present along the basal epithelial surface (Fig. 3B.a; higher magnification image of Fig. 3A,h). When both the epithelium and basement membrane were removed, vinculin pY1065 was still detected along the basal epithelial cell surface (Fig. 3B.b); however, the staining was more diffuse than when the basement membrane was left intact (Fig. 3B.a). Another striking difference in the tissue lacking a basement membrane was the presence of punctate staining of vinculin pY1065 throughout the stroma (Fig. 3B.b). These changes in vinculin pY1065 localization detected along corneal substrates of different stiffness may be explained by alterations in the tension exerted by the epithelial cells.
Investigators have shown that after injury, changes in matrix stiffness led to alterations in vinculin activation and localization within cell-matrix and/or cell-cell adhesions (Bays et al., 2014; Carisey and Ballestrem, 2011). While vinculin pY1065 localized to the basal surface after injury to the epithelium (Fig. 3A) and epithelium/basement membrane (Fig. 3B.b), increased staining was observed during migration on the stromal surface (Fig. 3B.b). In addition, the punctate staining of vinculin pY1065 observed within stromal fibroblasts (Fig. 3B.b) was not detected after injury with an intact basement membrane (Fig. 3B.a). The results indicate that the stiffer substrate increased the activation of vinculin at Y1065. These observations were consistent with work that focused on the activation of focal adhesion proteins on rigid surfaces compared to soft surfaces (Discher et al., 2005; Grashoff et al., 2010). It is possible that changes in topography itself adds an additional directionality cue to the cells; for example cells might sense a durotaxis directionality due to stromal lamellae. The transient fibronectin and its fibrillogenesis should enhance the directionality of the substrate.
3.4. Increased substrate stiffness reduces focal adhesion number along the wound edge of an epithelial sheet
To determine the signaling involved as cells migrate on the two substrates, vinculin-positive focal adhesions were examined. Epithelial cells were cultured to confluency on fibronectin-coated 8 and 50 kPa substrates and scratch wounds were made (Minns et al., 2016). Cells were examined prior to the wound (unwounded), and 10 minutes and 4 hours after, with wound closure occurring by 8 hours. In the unwounded cells, total vinculin localization differed on the 2 substrates, with diffuse localization throughout the cells on 8 kPa substrates and concentrated localization along the cell borders on 50 kPa substrates (Fig. 4A.a,d,g,j). After injury, vinculin was detected in focal adhesions (FAs) along the leading edge (arrows) and in several cells back from the leading edge after 4 hours (Fig. 4A.b,c,e,f,h,I,k,l).
Figure 4. Increased surface stiffness reduces focal adhesion number and actin bundle thickness along the leading edge.
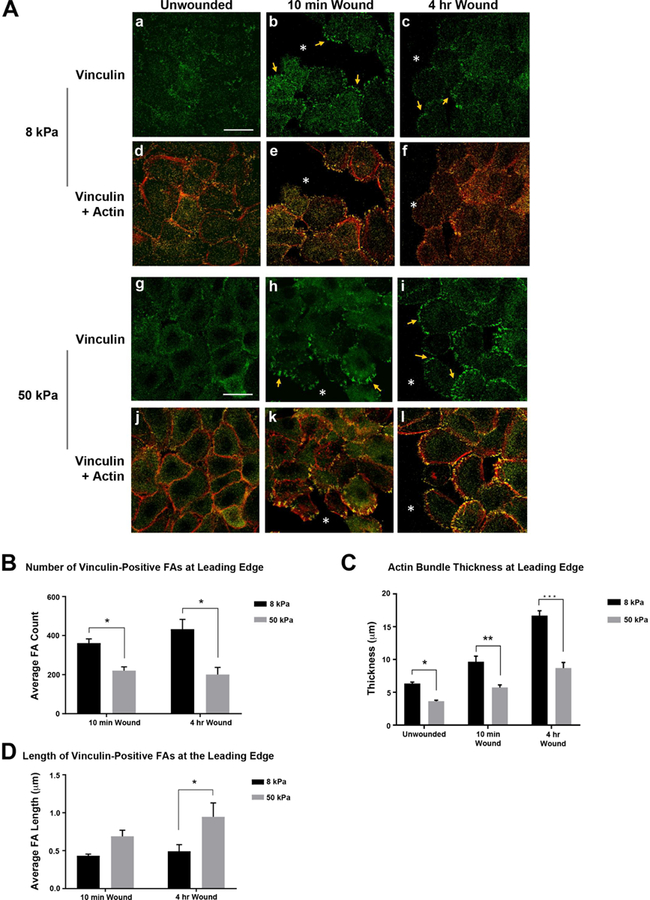
Human corneal limbal epithelial cells were cultured on fibronectin-coated 8 kPa and 50 kPa substrates until confluent. Scratch wounds were made and cultures were fixed 10 minutes and 4 hours after injury. Cells were stained for vinculin and counter stained with rhodamine phalloidin to detect F-actin. Images were obtained using a 63x objective on a Zeiss Axiovert LSM 700 confocal microscope. Staining with the secondary antibody was performed to set the negative level and all experimental conditions were imaged at the same setting as was used for secondary antibody alone. Images represent a minimum of 4 independent experiments. A.a,g. Localization of vinculin in unwounded cells is affected by substrate stiffness 8 or 50 kPa. A.d,j. Vinculin and F-actin (red). A. b-c,g-i. Vinculin is localized within focal adhesions after injury (arrows). A. e-f,k-l. Vinculin and F-actin (red). *wound edge. Scale bar is 25 µm. B-D. Leading edges were analyzed in 500 µm intervals. Actin bundle thickness along with focal adhesion number and length were determined using FIJI/ImageJ (NIH, Bethesda, MD; http://imagej.nih.gov/ij/). Statistical analysis of each time point and condition was conducted (Two way ANOVA). B. Decreased number of vinculin-positive focal adhesions on stiffer substrates. C. Actin bundle thickness at the leading edge is reduced in cells on stiffer substrates for all conditions. Thickest regions of actin were measured in cells along the wound edge. D. Vinculin focal adhesion length increases with increased substrate stiffness 4 hr after injury. Standard error bars are ± S.E.M. *P < 0.05, **P < 0.01, ***P < 0.005.
To evaluate whether stiffness affected the recruitment of vinculin to FA sites, the number and size of vinculin-positive FAs at the leading edge was determined. Increased substrate stiffness led to a significant reduction in the number of vinculin-positive FAs along the leading edge (Fig. 4A.b,c,h,i; B: *P < 0.05). In contrast, FA length increased on the stiffer substrates (Fig. 4A.b,c,h,i; D: *P < 0.05). These results indicate that corneal epithelial cells respond to substrate stiffness similar to that of other cell types (Discher et al., 2005). On rigid substrates, many cell types (i.e. fibroblasts, myocytes) form large FAs due to increases in external force (Engler et al., 2004; Tan et al., 2003).
Actin bundle thickness at the leading edge was also measured (Fig. 4A,C). Baseline values were determined on unwounded cultures. After injury, actin thickness increased in cells along the leading edge (Minns et al., 2016). On both 8 and 50 kPa substrates, actin bundle thickness was greatest 4 hours after injury (Fig. 4A.f,l; C). Furthermore, in the unwounded and wounded state, the actin bundle was thicker in cells on 8 kPa substrates (Fig. 4A.d-f; C: *P < 0.05, **P < 0.01, ***P < 0.005; ANOVA). These observations may explain the differences in morphology at the leading edge of epithelial sheets on the two different substrates (Fig. 1B).
3.5. Vinculin pY1065 localization is substrate dependent in unwounded cells
Vinculin is phosphorylated at tyrosine Y1065 by c-Src. Then, vinculin pY1065 is recruited to integrins along the cell membrane, initiating the formation of FA complexes (Carisey and Ballestrem, 2011; Johnson and Craig, 1995). It is unknown if changes in substrate stiffness affect this phosphorylation event and the subsequent recruitment to the corneal epithelial cell membrane; therefore, to evaluate for this, the localization of vinculin pY1065 was examined before and after injury in epithelial cells on the 2 substrates (Fig. 5A). Localization and intensity were depicted using an intensity scale (Fig. 5A.c, inset). In the unwounded cells cultured on the 8 kPa substrates, vinculin pY1065 was present intensely along the cell borders (Fig. 5A.a). In contrast, on the stiffer 50 kPa substrate, vinculin pY1065 was diffuse (Fig. 5A.d). After injury, vinculin pY1065-positive FAs were detected (arrows) on both 8 and 50 kPa substrates (Fig. 5A.b,c,e,f).
Figure 5. Vinculin pY1065 localization and phosphorylation are affected by increased substrate stiffness.
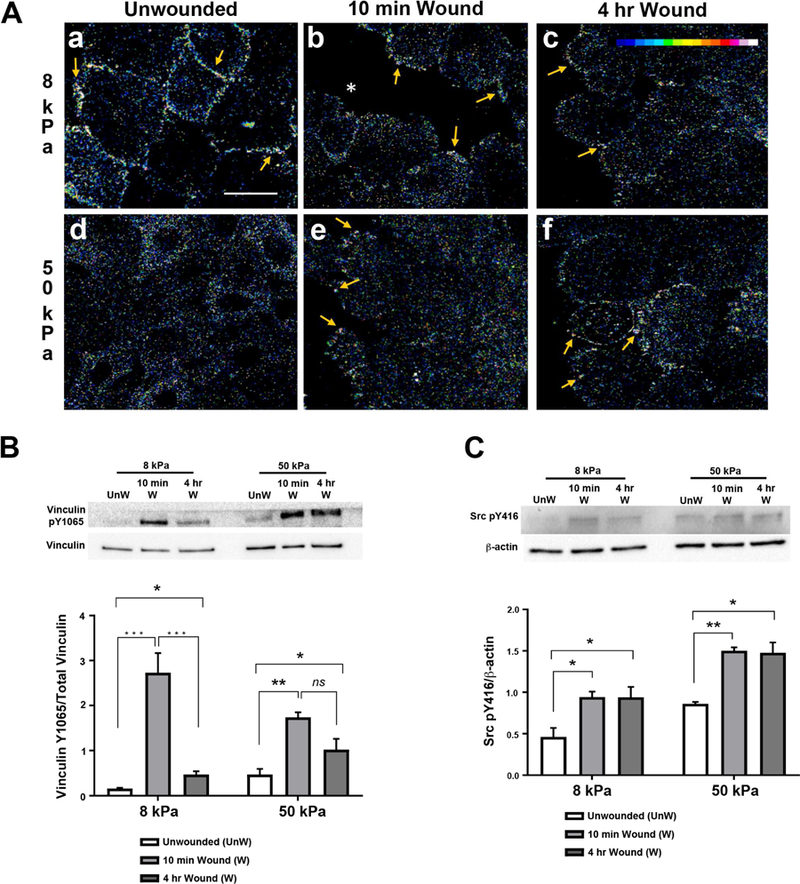
HCLE cells were cultured and scratch wounds made. Immunostaining is shown as changes in fluorescence intensity. Images were obtained using a 63x objective on a Zeiss Axiovert LSM 700 confocal microscope. Secondary antibody controls were set to negligible levels and all experimental cell conditions were imaged at same setting that was used for secondary antibody alone. A. In unwounded cultures, vinculin pY1065 localizes to membranes on 8 kPa substrates but remains diffuse throughout cells on 50 kPa substrates. Arrows indicate vinculin pY1065 at the leading edge and on cell membranes. *, wound edge. Scale bar is 25 µm. B-C. Western blot analysis was conducted on samples 10 minutes and 4 hours after injury. Protein was extracted, resolved by 10% SDS-PAGE, and immunoblotted for vinculin pY1065 and Src pY416. Relative expression of vinculin pY1065/total vinculin and Src pY416/ β-actin was determined by quantifying band intensity. Representative blots are shown. Unw, unwounded. B. Phosphorylation of tyrosine 1065 on vinculin remains elevated after injury in cells on stiffer substrates. C. Src phosphorylation remains elevated after injury on 8 kPa and 50 kPa substrates. Data represent a minimum of 4 independent experiments. Standard error bars are ± S.E.M. Statistical analysis conducted using Two way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.005.
To determine the overall phosphorylation of vinculin at tyrosine Y1065, western blot analysis was performed at equivalent time points. On both 8 and 50 kPa substrates, vinculin pY1065 increased significantly 10 minutes after injury (Fig. 5B: **P < 0.01, ***P < 0.005). After 4 hours, vinculin pY1065 decreased on the 8 kPa substrate (***P < 0.005) as compared to 10 minutes, but remained significantly greater than the baseline (unwounded: P < 0.05). On the more rigid 50 kPa substrate, even though there was a significant increase of pY1065 at 10 minutes after wounding (**P < 0.01), it was less than that on the 8 kPa substrate, and phosphorylation did not decrease significantly over time. As phosphorylation of vinculin at tyrosine Y1065 requires Src pY416 (Carisey and Ballestrem, 2011; Johnson and Craig, 1995), we examined the phosphorylation of Src at the same time points. Increased Src phosphorylation was detected after injury and remained elevated while cells migrated to close the wound (Fig. 5C: *P < 0.05, **P < 0.01; Two way ANOVA). Together these data indicate that substrate stiffness alone was not responsible for the initial phosphorylation but did lead to a difference in the sustained elevation of vinculin pY1065 four hours after injury.
3.6. Phosphorylation of vinculin at tyrosine 822 is substrate independent
To determine whether changes in substrate stiffness alter cell-cell signaling mechanisms during corneal wound healing, the localization and activation of vinculin pY822 was examined. The images were depicted using an intensity scale (Fig. 6A.c; inset). In comparison to vinculin pY1065, vinculin pY822 was diffuse in unwounded cultures on both 8 and 50 kPa substrates (Fig. 6A.a,d). After the scratch wound was made, vinculin pY822 was prominent along the cell-cell contacts (Fig. 6A.b,c,e,f; arrows). The staining along the cell-cell contacts on the 50 kPa substrate was more intense than on the 8 kPa substrate. A similar localization of vinculin pY822 within adherens junctions along the leading edge has been observed in MDCK epithelial cells (Miyake et al., 2006).
Figure 6. Alterations in substrate stiffness does not affect localization or phosphorylation of vinculin at tyrosine 822 after injury.
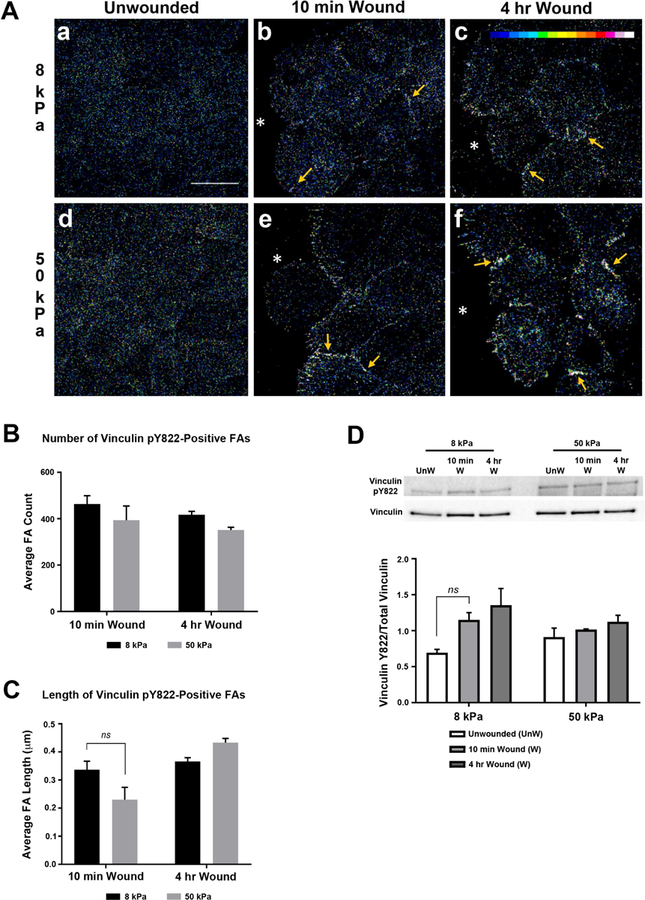
HCLE cells were cultured and scratch wounds made. Images were obtained using a 63x objective on a Zeiss Axiovert LSM 700 confocal microscope. Secondary antibody controls were set to dark and experimental cell conditions were imaged at the same setting that was used for secondary antibody alone. A.a-f. Localization of vinculin pY822 is not affected by changes in substrate stiffness. Arrows indicate vinculin pY822 at cell membranes. *, wound edge. Scale bar is 25 µm. B. Increased stiffness does not significantly affect the number or length of vinculin pY822-positive adhesions. FA, focal adhesion. C. Phosphorylation at tyrosine 822 is not affected by changes in substrate stiffness. Western blot analysis was conducted. Densitometry analysis was performed using FIJI/ImageJ (NIH, Bethesda, MD; http://imagej.nih.gov/ij/). Phosphorylation of vinculin at tyrosine 822 was normalized to total vinculin. Representative blots are shown. Unw, unwounded. Data represent a minimum of 3 independent experiments. Statistical analysis conducted using Two way-ANOVA. Standard error bars are ± S.E.M. ns, not significant.
The number and size of vinculin pY822-positive FAs were similar at both stiffness values and time points after injury (Fig. 6B,C). The level of vinculin phosphorylation at tyrosine Y822 did not change after wounding or with increased stiffness (Fig. 6D). The localization of E-cadherin was also examined, as it is part of the cell-cell scaffolding apparatus. There were no major differences in E-cadherin on either substrate (Fig. 7). These results indicate that phosphorylation of vinculin at tyrosine Y822 is present constitutively in corneal epithelial cells and does not appear to depend on substrate compliancy. The data correlates with studies performed by le Duc (2010) when cells were cultured under conditions of both low and high external tension, vinculin pY822 continuously localized to cell-cell contacts in MDCK cell monolayers (le Duc et al., 2010). Vinculin pY822 recruitment to cell-cell junctions was inhibited after treatment with blebistatin, indicating that myosin II has a role in the recruitment process. However, as vinculin pY822 also localizes to cell-cell contacts when the external force is low, additional myosin II-independent mechanisms may be involved in vinculin pY822 recruitment (Brugues et al., 2014; Pasapera et al.,2010).
Figure 7. Presence of e-cadherin before and after cadherin is substrate independent.
HCLE cells were cultured and scratch wounds made. Images were obtained using a 63x objective on a Zeiss Axiovert LSM 700 confocal microscope. Secondary antibody controls were set to dark and experimental cell conditions were imaged at the same setting that was used for secondary antibody alone. Images represent a minimum of 3 independent experiments at each time point.
4. Conclusion
In summary, 3 model systems were used to demonstrate that changes in substrate stiffness alter the localization of traction force of multi-layered intact epithelial sheets. In addition, the leading edge morphology of the epithelial sheets was substrate dependent, and increased substrate stiffness resulted in differences in vinculin pY1065 localization after injury along both the stromal matrix and on cells cultured on 50 kPA substrates. This study provides comprehensive data demonstrating that increased substrate stiffness mediates collective migration and signaling after injury.
Supplementary Material
Supplemental figure 1. Control for traction force analysis. Traction force analysis was performed on an image using FTTC where the epithelial sheet was placed on a 30 kPa gel in the far left corner (indicated by an open rectangle). The remainder of the field lacks cells (asterisk) and lack any displacement vectors.
Epithelial cells exert differential traction stress in response to substrate stiffness.
Motility of an epithelial sheet has two distinct phases; retraction and forward migration.
The motility of epithelial sheets exert distinct traction stresses on substrates of different stiffness.
Increased surface stiffness reduces vinculin-focal adhesion number along the leading edge of migrating epithelial cells.
Vinculin pY1065 localization and phosphorylation are affected by increased substrate stiffness.
Alterations in substrate stiffness does not affect localization or phosphorylation of vinculin at pY822 after injury.
Acknowledgements:
We gratefully acknowledge the critical comments and editorial assistance of Dr. Karen Symes and Audrey Hutcheon
Funding: This work was supported by the National Institutes of Health [EY06000 and EY06000S] (V.T-R), Massachusetts Lions Eye Research Fund and the New England Corneal Transplant Fund to Boston University and National Science Foundation [Grant CEBET-1150467] (M. L. S).
Abbreviations:
- F-actin
filamentous actin
- DMEM
Dulbecco’s modified eagles medium
- wk
week
- FTTC
Fourier transform traction cytometry
- unw
unwound
- BSA
bovine serum albumin
- PBS
phosphate buffered saline
- K-SFM
Keratinocyte-serum free media
- MDCK
Madin-Darby Canine Kidney Epithelial
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no competing or financial interests.
References
- Anon E, Serra-Picamal X, Hersen P, Gauthier NC, Sheetz MP, Trepat X, and Ladoux B (2012). Cell crawling mediates collective cell migration to close undamaged epithelial gaps. Proc. Natl. Acad. Sci. U. S. A 109, 10891–10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auernheimer V, Lautscham LA, Leidenberger M, Friedrich O, Kappes B, Fabry B, and Goldmann WH (2015). Vinculin phosphorylation at residues Y100 and Y1065 is required for cellular force transmission. J. Cell Sci 128, 3435–3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bays JL, Peng X, Tolbert CE, Guilluy C, Angell AE, Pan Y, Superfine R, Burridge K, and DeMali KA (2014). Vinculin phosphorylation differentially regulates mechanotransduction at cell–cell and cell–matrix adhesions. J. Cell Biol 205, 251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Mezquita JT, Hutcheon AEK, Stepp MA, and Zieske JD (2011). αVβ6 Integrin Promotes Corneal Wound Healing. Invest. Ophthalmol. Vis. Sci 52, 8505–8513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bois PRJ, O’Hara BP, Nietlispach D, Kirkpatrick J, and Izard T (2006). The vinculin binding sites of talin and alpha-actinin are sufficient to activate vinculin. J. Biol. Chem 281, 7228–7236. [DOI] [PubMed] [Google Scholar]
- Brugués A, Anon E, Conte V, Veldhuis JH, Gupta M, Colombelli J, Muñoz JJ, Brodland GW, Ladoux B, and Trepat X (2014). Forces driving epithelial wound healing. Nat. Phys 10, 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JP, Toli´c-Nørrelykke IM, Fabry B, Fredberg JJ. Estimating traction fields, moments, and strain energy that cells exert on their surroundings. Am J Physiol Cell Physiol 282: C595–C605, 2002. [DOI] [PubMed] [Google Scholar]
- Carisey A, and Ballestrem C (2011). Vinculin, an adapter protein in control of cell adhesion signalling. Eur. J. Cell Biol 90, 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-C, Allen SG, Ingram PN, Buckanovich R, Merajver SD, and Yoon E (2015). Single-cell Migration Chip for Chemotaxis-based Microfluidic Selection of Heterogeneous Cell Populations. Sci. Rep 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby LM, and Waters CM (2010). Epithelial repair mechanisms in the lung. Am. J. Physiol. - Lung Cell. Mol. Physiol 298, L715–L731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discher DE, Janmey P, and Wang Y (2005). Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science 310, 1139–1143. [DOI] [PubMed] [Google Scholar]
- le Duc Q, Shi Q, Blonk I, Sonnenberg A, Wang N, Leckband D, and de Rooij J (2010). Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II–dependent manner. J. Cell Biol 189, 1107–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Griffin MA, Sen S, Bönnemann CG, Sweeney HL, and Discher DE (2004). Myotubes differentiate optimally on substrates with tissue-like stiffness. J. Cell Biol 166, 877–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foolen J, Shiu JY, Mitsi M, Zhang Y, Chen CS, and Vogel V (2016). Full-Length Fibronectin Drives Fibroblast Accumulation at the Surface of Collagen Microtissues during Cell-Induced Tissue Morphogenesis. PloS One 11, e0160369–e0160369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardel ML, Sabass B, Ji L, Danuser G, Schwarz US, and Waterman CM (2008). Traction stress in focal adhesions correlates biphasically with actin retrograde flow speed. J. Cell Biol 183, 999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardel ML, Schneider IC, Aratyn-Schaus Y, and Waterman CM (2010). Mechanical Integration of Actin and Adhesion Dynamics in Cell Migration. Annu. Rev. Cell Dev. Biol 26, 315–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann WH, Auernheimer V, Thievessen I, and Fabry B Vinculin, cell mechanics and tumour cell invasion. Cell Biol. Int 37, 397–405. [DOI] [PubMed] [Google Scholar]
- Gordon MK, DeSantis A, Deshmukh M, Lacey CJ, Hahn RA, Beloni J, Anumolu SS, Schlager JJ, Gallo MA, Gerecke DR, et al. (2010). Doxycycline Hydrogels as a Potential Therapy for Ocular Vesicant Injury. J. Ocul. Pharmacol. Ther 26, 407–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubán B, Vas K, Balog Z, Manczinger M, Bebes A, Gro ma G, Széll M, Kemény L, Bata-Csörgő Z. (2016) Abnormal regulation of fibronectin production by fibroblasts in psoriasis. Br J Dermatol 2016 March;174(3):533–41. [DOI] [PubMed] [Google Scholar]
- Gov NS (2007). Collective cell migration patterns: Follow the leader. Proc. Natl. Acad. Sci. U. S. A 104, 15970–15971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, Yang MT, McLean MA, Sligar SG, Chen CS, Ha T, et al. (2010). Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466, 263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Petroll WM, Feng W, Essepian J, and Cavanagh HD (1992). Radial keratotomy. 1. The wound healing process and measurement of incisional gape in two animal models using in vivo confocal microscopy. Invest. Ophthalmol. Vis. Sci 33, 3255–3270. [PubMed] [Google Scholar]
- Johnson RP, and Craig SW (1995). F-actin binding site masked by the intramolecular association of vinculin head and tail domains. Nature 373, 261–264. [DOI] [PubMed] [Google Scholar]
- Kehasse A, Rich CB, Lee A, McComb ME, Costello CE, and Trinkaus-Randall V (2013). Epithelial Wounds Induce Differential Phosphorylation Changes in Response to Purinergic and EGF Receptor Activation. Am. J. Pathol 183, 1841–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil AA, and Friedl P (2010). Determinants of leader cells in collective cell migration. Integr. Biol 2, 568–574. [DOI] [PubMed] [Google Scholar]
- Kokado M, Miyajima M, Okada Y, Ichikawa K, Yamanaka O, Liu CY, Kao WW, Shou W, Saika S.Lab Invest. 2018. Lack of plakoglobin impairs integrity and wound healing in corneal epithelium in mice 98(11):1375–1383. doi: 10.1038/s41374-018-0082-z Epub 2018 May 25.PMID:29802338 [DOI] [PubMed] [Google Scholar]
- Kraning-Rush CM, Carey SP, Califano JP, and Reinhart-King CA (2012). Chapter 6 - Quantifying Traction Stresses in Adherent Cells. In Methods in Cell Biology, Asthagiri AR, and Arkin AP, eds. (Academic Press; ), pp. 139–178. [DOI] [PubMed] [Google Scholar]
- Krishnan R, Canović EP, Iordan AL, Rajendran K, Manomohan G, Pirentis AP, Smith ML, Butler JP, Fredberg JJ, and Stamenović D (2012). Fluidization, resolidification, and reorientation of the endothelial cell in response to slow tidal stretches. Am. J. Physiol. - Cell Physiol 303, C368–C375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Last JA, Thomasy SM, Croasdale CR, Russell P, and Murphy CJ (2012). Compliance profile of the human cornea as measured by atomic force microscopy. Micron Oxf. Engl 1993 43, 1293–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Derricks K, Minns M, Ji S, Chi C, Nugent MA, and Trinkaus-Randall V (2014). Hypoxia-induced changes in Ca2+ mobilization and protein phosphorylation implicated in impaired wound healing. Am. J. Physiol. - Cell Physiol 306, C972–C985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljubimov AV, Saghizadeh M (2015) Progress in corneal wound healing. Progress in Retinal and Eye Research 49:17–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak M, Spill F, Kamm RD, and Zaman MH (2016). Single-Cell Migration in Complex Microenvironments: Mechanics and Signaling Dynamics. J. Biomech. Eng 138, 0210041–0210048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto T, and Ingber DE (2010). Mechanical control of tissue and organ development. Dev. Camb. Engl 137, 1407–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minns MS, Teicher G, Rich CB, and Trinkaus-Randall V (2016). Purinoreceptor P2X7 Regulation of Ca2+ Mobilization and Cytoskeletal Rearrangement Is Required for Corneal Reepithelialization after Injury. Am. J. Pathol 186, 285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake Y, Inoue N, Nishimura K, Kinoshita N, Hosoya H, and Yonemura S (2006). Actomyosin tension is required for correct recruitment of adherens junction components and zonula occludens formation. Exp. Cell Res 312, 1637–1650. [DOI] [PubMed] [Google Scholar]
- Pasapera AM, Schenieder IC, Rericha E, Schlaepfer DD, and Waterman CM (2010). Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J Cell Biol 188(6):877–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne J, Gong H, Trinkaus-Randall V. (2000). Tyrosine phosphorylation: a critical component in the formation of hemidesmosomes. Cell Tissue Res June;300(3):401–11. [DOI] [PubMed] [Google Scholar]
- Petrie RJ, and Yamada KM (2012). At the leading edge of three-dimensional cell migration. J. Cell Sci 125, 5917–5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyton SR, Ghajar CM, Khatiwala CB, and Putnam AJ (2007). The emergence of ECM mechanics and cytoskeletal tension as important regulators of cell function. Cell Biochem. Biophys 47, 300–320. [DOI] [PubMed] [Google Scholar]
- Roca-Cusachs P, Iskratsch T, and Sheetz MP (2012). Finding the weakest link – exploring integrin-mediated mechanical molecular pathways. J Cell Sci 125, 3025–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazonova OV, Lee KL, Isenberg BC, Rich CB, Nugent MA, and Wong JY (2011). Cell-Cell Interactions Mediate the Response of Vascular Smooth Muscle Cells to Substrate Stiffness. Biophys. J 101, 622–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr SJ Gipson IK (1985). Isolation of corneal epithelium with Dispase II or EDTA. Effects on the basement membrane zone. Invest Ophthalmol Vis Sci June;26(6):818–27. [PubMed] [Google Scholar]
- Stepp MA. (2006). Corneal integrins and their functions. Exp Eye Res 2006 July;83(1):3–15. Epub 2006 Mar 31. [DOI] [PubMed] [Google Scholar]
- Tambe DT, Hardin CC, Angelini TE, Rajendran K, Park CY, Serra-Picamal X, Zhou EH, Zaman MH, Butler JP, Weitz DA, et al. (2011). Collective cell guidance by cooperative intercellular forces. Nat. Mater 10, 469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, and Chen CS (2003). Cells lying on a bed of microneedles: An approach to isolate mechanical force. Proc. Natl. Acad. Sci. U. S. A 100, 1484–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torricelli AAM, Singh V, Santhiago MR, and Wilson SE (2013). The Corneal Epithelial Basement Membrane: Structure, Function, and Disease. Invest. Ophthalmol. Vis. Sci 54, 6390–6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torricelli AAM, Santhanam A, Wu J, Singh V, Wilson SE (2016). The corneal fibrosis response to epithelial-stromal injury. Experimental Eye Research 142:110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinkaus-Randall V, and Gipson IK (1984). Role of calcium and calmodulin in hemidesmosome formation in vitro. J. Cell Biol 98, 1565–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Hutcheon AEK, Sriram S, Tran JA, Zieske JD. 2018. Initiation of fibrosis in the integrin Αvβ6 knockout mice. Exp Eye Res 180:23–28. doi: 10.1016/j.exer.2018.11.027 [Epub ahead of print]PMID:30500364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieske JD, Bukusoglu G, and Gipson IK (1989). Enhancement of vinculin synthesis by migrating stratified squamous epithelium. J. Cell Biol 109, 571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1. Control for traction force analysis. Traction force analysis was performed on an image using FTTC where the epithelial sheet was placed on a 30 kPa gel in the far left corner (indicated by an open rectangle). The remainder of the field lacks cells (asterisk) and lack any displacement vectors.


