Abstract
Background:
Angiogenic placental growth factor (PlGF) concentrations rise during pregnancy, peaking at the end of mid-pregnancy. Low PlGF concentrations during pregnancy are associated with pregnancy complications with recognized later life cardiovascular risk. We hypothesized that low PlGF concentrations, especially in mid-pregnancy identify not only a subset of women at risk for pregnancy complications, but also women with greater cardiovascular risk factor burden after pregnancy regardless of pregnancy outcome.
Methods:
In a population-based prospective cohort study of 5529 women, we computed gestational-age-adjusted multiple of the medians of early pregnancy and mid-pregnancy PlGF concentrations. Information on pregnancy complications (pre-eclampsia, small for gestational age and spontaneous preterm birth) was obtained from hospital registries. Six years after pregnancy we measured maternal systolic and diastolic blood pressure (SBP and DBP), cardiac structure (aortic root diameter [AOD], left atrial diameter [LAD], left ventricular mass [LV mass] and fractional shortening ), carotid-femoral pulse wave velocity and central retinal arteriolar and venular calibers. Blood pressure was also measured nine years after pregnancy.
Results:
Women were on average 29.8 (SD5.2) years of age in pregnancy, mostly European (55.2%) and 14.8% developed a pregnancy complication. Quartile analysis showed that especially women with mid-pregnancy PlGF in the lowest quartile (the low PlGF subset) had a larger AOD (0.40mm [95%CI; 0.08, 0.73]), LAD (0.34mm [95%CI; −0.09, 0.78]), LV mass (4.6g [95%CI; 1.1, 8.1]) and SBP (2.3mmHg [95%CI; 0.93, 3.6]) six years after pregnancy than women with the highest PlGF. Linear regression analysis showed that higher mid-pregnancy PlGF concentrations were associated with a smaller AOD (−0.24mm [95%CI; −0.39, −0.10]), LAD (−0.75mm [95%CI; −0.95, −0.56]), lower LV mass (−3.9g [95%CI; −5.5, −2.3]) and SBP (−1.1mmHg [95%CI; −1.7, −0.46]). These differences persisted after exclusion of women with complicated pregnancies.
Conclusions:
Women with low PlGF in mid-pregnancy have a greater AOD, LAD, and LV mass, and higher SBP six and nine years after pregnancy compared to women with higher PlGF, including women with uncomplicated pregnancies. The pathophysiological implications of lower PlGF concentrations in mid-pregnancy might provide insight towards identifying pathways contributing to greater cardiovascular risk factor burden.
Keywords: Placenta Growth Factor, pregnancy outcome, cardiovascular disease, risk factors, pre-eclampsia
INTRODUCTION
Pregnancy is accompanied by extensive maternal hemodynamic changes that allow for proper placental implantation, growth, perfusion and fetal development. This process requires a tight balance between pro-angiogenic (e.g. placental growth factor [PlGF]) and anti-angiogenic (e.g. soluble fms-like tyrosine kinase-1 [sFlt-1]) factors. Dysregulation of PlGF and sFlt-1 is proposed to reflect stress to the syncytiotrophoblast (STB) of the placenta, which produces these factors.1 In response to stress, the STB will decrease the production of PlGF and release more sFlt-1 into the maternal circulation. Consequently, sFlt-1 will bind to circulating PlGF and further decrease PlGF availability. Women with reduced PlGF and increased sFlt-1 are more at risk of a complicated pregnancy (pre-eclampsia [PE], spontaneous preterm birth [sPTB] and children born small for gestational age [SGA]).2 Furthermore, findings from an animal study demonstrate that low PlGF is associated with abnormal cardiovascular remodeling during pregnancy (e.g. excessive increase in left ventricular mass and systolic blood pressure).3 The pregnancy complications associated with reduced circulating PlGF are also associated with an increased risk of CVD in later life.4 The largest difference in PlGF concentration between women with a complicated pregnancy and those with an uncomplicated pregnancy can be observed during mid-pregnancy. Longitudinal studies examining PlGF across pregnancy showed that low mid-pregnancy PlGF was associated with earlier onset of preeclampsia, which is the form of preeclampsia with the highest risk for later life CVD.4, 5 Soluble Flt-1 levels differ less profoundly throughout pregnancy between both groups.1 Low PlGF and high sFlt-1 can lead to vascular inflammation and endothelial dysfunction.6 Endothelial dysfunction can persist for at least 15 years after pregnancy and plays an important role in the development of cardiovascular disease (CVD).7, 8
We propose that lower PlGF concentrations, most evident in mid-pregnancy when PlGF rapidly increases, might identify women at increased risk for developing not only complicated pregnancies but also for greater cardiovascular risk factor burden years before the onset of CVD.9 The aim of this study was to determine whether lower PlGF concentrations during mid-pregnancy were associated with a disadvantageous cardiovascular risk factor profile six years after pregnancy as evaluated by measurement of pulse wave velocity [PWV], fractional shortening [FS], aortic root diameter [AOD], left atrial diameter [LAD], left ventricular mass [LV mass], blood pressure and central retinal arteriolar and venular calibers, and nine years after pregnancy (blood pressure). We also examined whether these associations were valid not only for women with complicated pregnancies but also women with uncomplicated pregnancies.
MATERIALS AND METHODS
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Design and study population
This study was embedded in the Generation R Study, a population-based prospective cohort from early pregnancy onwards.10, 11 The Medical Ethics Committee of the Erasmus Medical Center Rotterdam, the Netherlands approved the study. Written informed consent was obtained from all participants. We included women with a live born singleton, available data on mid-pregnancy PlGF and at least one available cardiovascular measurement of pregnancy or follow-up. We excluded women with: chronic hypertension before pregnancy, pre-existing disease (e.g. cardiovascular disease), and women who were pregnant during the six or nine year follow-up measurements (n = 190 and 51 respectively) or were receiving antihypertensive treatment at that time (n = 46 and 67 respectively). To attempt to exclude the effect of increased blood pressure, women with only gestational hypertension during the index pregnancy (gestational hypertension without sPTB or SGA) were excluded. Women with self-reported gestational hypertension or preeclampsia after an uncomplicated index pregnancy were included in the total analyses but excluded from the analyses in the uncomplicated pregnancy group. The final population for analysis comprised 5475 women (Figure 1) of whom 4664 women had an uncomplicated index pregnancy and 811 women a complicated index pregnancy.
Figure 1.
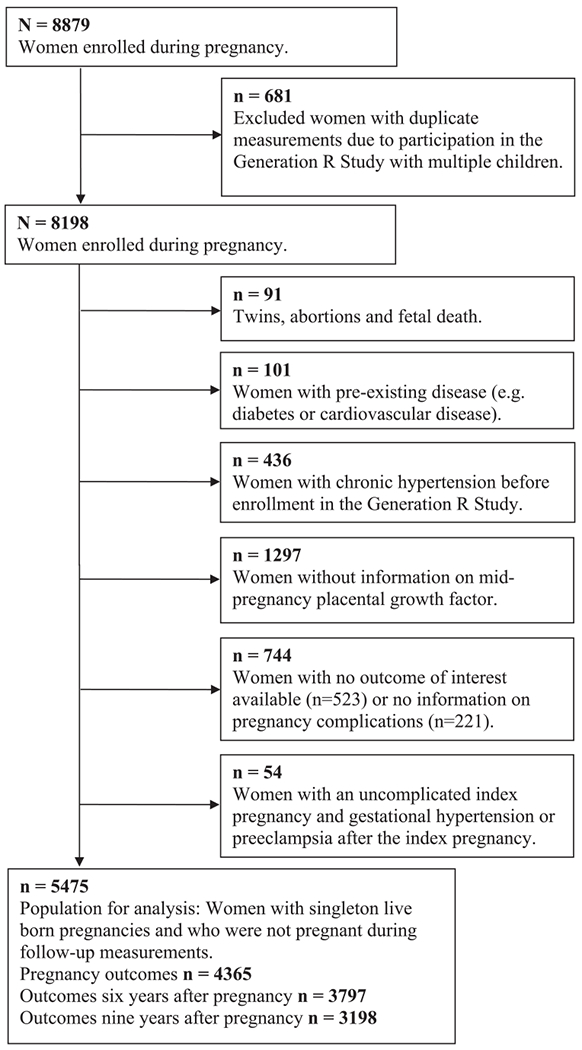
Flowchart
In addition to cardiovascular risk factors after pregnancy, we also studied cardiovascular adaptation during pregnancy to support our hypothesis. Cardiovascular adaptation to pregnancy has been examined previously in The Generation R Study.2 Coolman et al. examined the association of placental biomarkers in early and mid-pregnancy (PlGF and sFlt-1) with placental function (uterine artery resistance index), placental weight, birth weight and pregnancy complications (sPTB, fetal growth restriction and PE).2
Complicated pregnancy
Complicated pregnancies studied included PE, SGA or sPTB pregnancies. We obtained information on clinically diagnosed PE from medical records that were cross-checked with the original hospital charts.12 PE was defined, using the ISSHP criteria that were in effect at the time of the study, as new onset systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg after 20 weeks of gestation and the presence of proteinuria with no evidence of urinary tract infection in a random urine sample.13 Midwife and hospital registries provided information on gestational age at birth, birth weight and child’s sex. We defined sPTB as the spontaneous onset of labor before 37 weeks of gestation. SGA was used as a surrogate for fetal growth restriction and was defined as a birth weight below the 10th percentile adjusted for gestational age and sex of the child. Information on gravidity, parity and the occurrence of gestational hypertension and preeclampsia in pregnancies other than the index pregnancy was obtained in an interview nine years after pregnancy.
Placental growth factor
Our primary analysis was PlGF measured at mid-pregnancy (mean 20.6 weeks of gestation [SD 1.1]) We also measured PlGF in early pregnancy (mean 13.5 weeks of gestation [SD 2.0]). Analyses were performed in non-fasting venous blood samples. Details of processing procedures have been described previously.2, 11 The Department of Clinical Chemistry of the Erasmus Medical Center analyzed PlGF concentrations using an immuno electrochemoluminence assay on the Architect System (Abbott Diagnostics B.V., Hoofddorp, the Netherlands). The between-run coefficients of variation were 4.7% at 24 pg./mL and 3.8% at 113 pg./mL for PlGF.2 PlGF varies with gestational age and is not normally distributed. Therefore, we constructed PlGF gestational-age-adjusted standardized Multiple of the Median (MoM) scores, which we used in all regression models.14 Table 1 presents absolute PlGF concentrations.
Table 1.
Baseline and follow-up characteristics n = 5475
| Pregnancy | |
| Age at enrollment (years), mean (SD) | 29.8 (5.2) |
| Non-European ethnicity (%) | 44.8 |
| No education/primary school (%) | 13.0 |
| Pre-pregnancy BMI (kg/m2), median (90% range) | 22.6 (18.6, 31.6) |
| Lean and normal (%) | 72.8 |
| Overweight (%) | 19.9 |
| Obese and morbidly obese (%) | 7.3 |
| SBP mid-pregnancy (mmHg), mean (SD) | 116.1 (11.6) |
| DBP mid-pregnancy (mmHg), mean (SD) | 66.5 (8.9) |
| PlGF early pregnancy (pg./mL), median (90% range) | 47.5 (17.8, 151.3) |
| PlGF mid-pregnancy (pg./mL), median (90% range) | 146.8 (91.4, 524.4) |
| Smoking (%) | 28.2 |
| Nulliparous (%) | 59.5 |
| Pregnancy outcomes | |
| Spontaneous preterm birth, (%) | 3.8 |
| Small for gestational age (below the 10th percentile), (%) | 10.3 |
| Pre-eclampsia (%) | 1.5 |
| Gestational hypertension (%) | 0.3 |
| Boys (%) | 50.4 |
| Follow-up six years after pregnancy (n = 3797) | |
| Follow-up interval (years), median (90% range) | 6.0 (5.7, 7.3) |
| BMI (kg/m2), median (90% range) | 24.5 (19.7, 35.1) |
| SBP (mmHg), mean (SD) | 118.2 (11.7) |
| DBP (mmHg), mean (SD) | 70.2 (9.2) |
| Smoking (%) | 21.2 |
| More than once pregnant, (%) | 91.1 |
| Gravidity, median (90% range) | 3.0 (1.0, 9.0) |
| Cardiovascular medication (%) | 0.2 |
| Follow-up nine years after pregnancy (n = 3198) | |
| Maternal age (years), mean (SD) | 41.0 (4.8) |
| BMI (kg/m2), median (90% range) | 24.6 (20.0, 35.0) |
| SBP (mmHg), mean (SD) | 113.4 (11.6) |
| DBP (mmHg), mean (SD) | 67.9 (7.7) |
| CVD (%) | 31 (1.0) |
| More than once pregnant, (%) | 92.0 |
| Parity, median (90% range) | 3.0 (1.0, 6.0) |
| Preeclampsia after the index pregnancy (%) | 0.7 |
| Ever preeclampsia (%) | 2.5 |
| Gestational hypertension after the index pregnancy (%) | 1.0 |
| Ever gestational hypertension (%) | 3.5 |
Abbreviations: Body mass index, BMI; Cardiovascular disease, CVD; Diastolic blood pressure, DBP; Systolic blood pressure, SBP; Standard Deviation, SD. Values are percentages for categorical variables, means (SD) for continuous variables with a normal distribution, or medians (90% range) for continuous variables with a skewed distribution. Imputed values are shown for confounders.
Hemodynamic, demographic and anthropometric measurements during pregnancy
We obtained information on maternal age, ethnicity, educational level, gravidity, parity, pre-pregnancy weight, smoking, chronic disease during pregnancy, chronic hypertension before pregnancy and medication use through questionnaires repeatedly applied during index pregnancy.
In the second (mean 20.6 weeks of gestational [SD 1.1]) and third (mean 30.4 weeks of gestational [SD 1.0]) trimesters of pregnancy we performed Doppler velocimetry of the uterine arteries. The uterine artery pulsatility index (UtA-PI) (peak systolic velocity - end diastolic velocity) / time averaged) of the left and right uterine artery was measured near the crossover with the external iliac artery. We recorded three consecutive uniform waveforms by pulsed Doppler ultrasound for each measurement. For further analyses we used the mean of these three measurements and the mean of the left and right uterine artery.15 In addition to the UtA-PI measurement in the third trimester, we assessed the presence of notching (either left or right sided) in flow velocity waveforms. The sonographer was blinded to previous measurements and pregnancy outcomes. Doppler measurements showed a high intraclass correlation coefficient value (>0.80) with corresponding low coefficient of variation value (<10%), which indicates adequate reproducibility.16
Trained research assistants wearing usual clothing (i.e. no white coats) measured systolic and diastolic blood pressures in early, mid- and late pregnancy in the right upper arm with the validated Omron 907® automated digital oscillometric sphygmomanometer (OMRON Healthcare Europe B.V., Hoofddorp, the Netherlands). Before the measurement, women sat in an upright position with back support and relaxed for five minutes. The mean value of two blood pressure readings over a 60-second interval was documented.
At study enrollment (during pregnancy) and in mid-pregnancy, we measured maternal height (cm) and weight (kg) without shoes. Thereafter, body mass index (BMI) (kg/m2) was calculated and categorized in: lean and normal BMI (< 25.0), overweight BMI (≥ 25.0 and < 30.0) and, obese and morbidly obese BMI (≥ 30.0). We obtained information on pre-pregnancy weight with a questionnaire at study enrollment. Pre-pregnancy weight was highly correlated with measured early pregnancy weight (Pearson’s correlation coefficient r 0.95 [P<0.001]).
Maternal hemodynamic and anthropometric measurements six years after pregnancy
Six years after pregnancy (90% range 5.7, 7.3 years) women returned for assessment. Information was obtained by questionnaires on the number of pregnancies after the index pregnancy and medication use. We measured maternal height (cm) and weight (kg) without shoes, and calculated BMI (kg/m2). Blood pressure was measured twice in sitting position with the validated automatic sphygmomanometer Datascope Accutorr Plus (Paramus, NJ, USA). The average of both blood pressure measurements was used for further analyses.
Carotid-femoral PWV was measured with an automatic non-invasive, validated device (Complior®; Artech Medical, Pantin, France) to assess arterial wall stiffness. The device measures the distance between the recording sites at the carotid (proximal) and femoral (distal) artery. We performed two-dimensional M-mode echocardiographic measurements using the ATL-Philips Model HDI 5000 (Seattle, WA, USA) or the Logiq E9 (GE Medical Systems, Wauwatosa, WI, USA) devices. FS, AOD and LAD were measured. LV mass was calculated using the equation derived by Devereux.17 Intraobserver and interobserver intraclass correlation coefficients were described previously and demonstrated good repeatability and reproducibility.18
We took unilateral digital retinal photographs of maternal retinal vascular calibers of the left eye using a Topcon digital retinal camera (model TRC, NW300) with images resolution set to 4096 and 3072 pixels. A semi-automatic computer-imaging program measured the six largest retinal arteriolar and venular calibers of these photographs, located one half to one disc diameter from the optic disc margin. The average of the six largest retinal arteriolar and retinal venular calibers was used as central retinal arteriolar and central retinal venular equivalents.19 Two graders who were blinded to participants’ characteristics operated the semi-automatic computer-imaging program. Grader specific standard deviation scores are used for both central retinal arteriolar and central retinal venular equivalents. Interclass correlation coefficients between graders is excellent for both retinal arteriolar calibers (0.77) and retinal venular calibers (0.87). This suggests adequate reproducibility.
Blood pressure nine years after pregnancy
Blood pressure was measured 9.7 years after pregnancy (90% range 9.4, 10.4 years) with the validated automatic sphygmomanometer Datascope Accutorr Plus (Paramus, NJ, USA). Women were supine while blood pressure was measured four times in the right upper arm. Unlike six years after pregnancy where we used the average of two blood pressure measurements while the subject was sitting, the average of the last three blood pressure measurements with the subject supine was used for our analyses at nine years.
Statistical analyses
We examined baseline and follow-up characteristics within the total population (Table 1). To reduce potential bias due to missing data, we imputed missing values in covariates used as confounders in the regression analyses through multiple imputation procedures. Data were imputed according to the Markov Chain Monte Carlo method, assuming no monotone missing pattern. Data were analyzed in each set separately, and pooled estimates from the five imputed datasets were used to report the effect estimates and their 95% confidence intervals (95% CI).1 For the multiple imputation procedure we performed 10 iterations.20 Statistical Package of Social Sciences version 24.0 for Windows (IBM Corp., Armonk, NY, USA) was used. In the total population for analysis 4.1% had missing information on ethnicity, 7.9% on educational level, 0.8% on gravidity during pregnancy, 0.4% on diastolic blood pressure at study enrollment, 18.2% on pre-pregnancy BMI, 11.1% on smoking during pregnancy, 28.1% on gravidity six years after pregnancy, 2.4% on systolic blood pressure six years after pregnancy, 4.2% on BMI six years after pregnancy and 9.0% on parity nine years after pregnancy. We performed linear and logistic regression analyses to relate mid-pregnancy and early pregnancy PlGF to cardiovascular adaptation to pregnancy and cardiovascular risk factors six and nine years after pregnancy (Table 2 and 3 and Supplemental Table 1 and 2 respectively). The quartile analysis (specifically the lowest quartile) was determined and analyzed to allow comparisons from previous studies using similar strategies and to address the subset of subjects with low PlGF (Table 3 and Supplemental Table 2).5, 21, 22 The association between PlGF and cardiovascular adaptation to pregnancy has been studied previously in The Generation R Study through the uterine artery resistance index.2 In this study, we examine different measurements of cardiovascular adaptation to pregnancy, namely UtA-PI and the presence of notching to characterize pregnancy in the subset of women with low PlGF (Supplemental Table 3 and 4). However, we realize these measurements do not describe a novel finding, we therefore included them in the supplements to support our main findings. The regression models comparing PlGF values with later life cardiovascular markers include covariates selected based on their associations with the outcome of interest from previous studies or a change in effect estimate of >10%. The confounder models included the following variables, depending on the outcome of interest: maternal age at enrollment, gravidity during pregnancy, gestational age at blood sampling in pregnancy, ethnicity, educational level, smoking during pregnancy, change in systolic blood pressure between early pregnancy and mid-pregnancy, systolic blood pressure at the time of PlGF measurement, time interval between pregnancy and follow-up, pulse at the time of PWV measurement and central retinal vascular caliber six years after pregnancy. The BMI models included change in BMI between pre-pregnancy and mid-pregnancy or pre-pregnancy BMI, in addition to the confounder model. We repeated all analyses after stratification into the presence or absence of complicated pregnancies during the index pregnancy (PE, SGA or sPTB). We applied a Benjamin-Hochberg procedure controlling for false discovery rate at 0.05 level.23 We examined a possible trend over the quartiles presented in Table 3 through a linear contrast analysis. We also tested whether there was interaction between PlGF and gravidity or parity for any of our outcomes; this was not the case. Finally, a non-response analysis was carried out to test for differences in baseline characteristics between women included in this study and 1) those with a mid-pregnancy PlGF measurement but no outcome of interest and 2) those without a mid-pregnancy PlGF measurement or any outcome of interest (Supplemental Table 5).
Table 2.
Association of mid-pregnancy placental growth factor with cardiovascular risk factors six and nine years after pregnancy
| Confounder model Beta (95% CI) | P-value | BMI model Beta (95% CI) | P-value | Adjusted P-value | |
|---|---|---|---|---|---|
| Total population | |||||
| Six years after pregnancy (n=3797) | |||||
| ‡Aortic root diameter (mm) | −0.24 (−0.39, −0.10) | 0.001 | −0.24 (−0.39, −0.10) | 0.001 | 0.003 |
| ‡Left atrial diameter (mm) | −0.75 (−0.95, −0.56) | <0.001 | −0.75 (−0.95, −0.56) | <0.001 | <0.001 |
| ‡Left ventricular mass (g) | −3.9 (−5.5, −2.3) | <0.001 | −3.9 (−5.5, −2.3) | <0.001 | <0.001 |
| *Pulse wave velocity (m/s) | 0.05 (−0.01, 0.11) | 0.13 | 0.05 (−0.01, 0.12) | 0.12 | 0.19 |
| ‡Fractional shortening | −0.18 (−0.43, 0.08) | 0.18 | −0.18 (−0.44, 0.08) | 0.17 | 0.21 |
| †Central retinal arteriolar caliber (SDS) | −0.03 (−0.08, 0.03) | 0.30 | −0.03 (−0.08, 0.03) | 0.30 | 0.33 |
| †Central retinal venular caliber (SDS) | −0.01 (−0.06, 0.05) | 0.77 | −0.01 (−0.06, 0.05) | 0.77 | 0.77 |
| ‡SBP (mmHg) | −0.88 (−1.5, −0.28) | 0.004 | −0.88 (−1.5, −0.28) | 0.004 | 0.009 |
| ‡DBP (mmHg) | −0.33 (−0.80, 0.14) | 0.17 | −0.33 (−0.81, 0.14) | 0.17 | 0.21 |
| Nine years after pregnancy (n=3198) | |||||
| ‡SBP (mmHg) | −1.1 (−1.7, −0.45) | 0.001 | −1.1 (−1.7, −0.46) | 0.001 | 0.004 |
| ‡DBP (mmHg) | −0.48 (−0.91, −0.05) | 0.03 | −0.47 (−0.90, −0.05) | 0.03 | 0.06 |
| Uncomplicated pregnancies | |||||
| Six years after pregnancy (n=3270) | |||||
| ‡Aortic root diameter (mm) | −0.28 (−0.44, −0.12) | 0.001 | −0.28 (−0.44, −0.12) | 0.001 | 0.004 |
| ‡Left atrial diameter (mm) | −0.81 (−1.0, −0.60) | <0.001 | −0.81 (−1.0, −0.60) | <0.001 | <0.001 |
| ‡Left ventricular mass (g) | −4.4 (−6.2, −2.7) | <0.001 | −4.4 (−6.2, −2.7) | <0.001 | <0.001 |
| *Pulse wave velocity (m/s) | 0.06 (−0.01, 0.13) | 0.08 | 0.06 (−0.01, 0.13) | 0.08 | 0.15 |
| ‡Fractional shortening | −0.20 (−0.47, 0.09) | 0.17 | −0.20 (−0.47, 0.08) | 0.17 | 0.23 |
| †Central retinal arteriolar caliber (SDS) | −0.03 (−0.08, 0.03) | 0.37 | −0.03 (−0.08, 0.03) | 0.37 | 0.41 |
| †Central retinal venular caliber (SDS) | −0.01 (−0.07, 0.05) | 0.74 | −0.01 (−0.07, 0.05) | 0.74 | 0.74 |
| ‡SBP (mmHg) | −0.95 (−1.6, −0.30) | 0.004 | −0.95 (−1.6, −0.30) | 0.004 | 0.009 |
| ‡DBP (mmHg) | −0.29 (−0.80, 0.22) | 0.27 | −0.29 (−0.80, 0.22) | 0.27 | 0.33 |
| Nine years after pregnancy (n=2748) | |||||
| ‡SBP (mmHg) | −1.1 (−1.8, −0.36) | 0.003 | −1.1 (−1.8, −0.36) | 0.003 | 0.008 |
| ‡DBP (mmHg) | −0.35 (−0.81, 0.11) | 0.14 | −0.34 (−0.80, 0.12) | 0.15 | 0.23 |
Abbreviations: Body mass index, BMI; Confidence Interval, CI; Diastolic blood pressure, DBP; Multiple of the median, MoM; Placental growth factor, PlGF; Pulse wave velocity, PWV; Systolic blood pressure, SBP; Standard deviation score, SDS. Values are regression coefficients with β and 95% confidence interval, based on linear regression models. Estimates represent the unit increase in the outcome per 1 multiple of the median increase in PlGF.
Adjusted for maternal age at enrollment, gravidity during pregnancy, time interval between pregnancy and follow-up, ethnicity, educational level, smoking during pregnancy, change in systolic blood pressure between early pregnancy and mid-pregnancy (and change in BMI between pre-pregnancy and mid-pregnancy in the BMI model).
In addition to the ‡ model adjusted for pulse at the time of PWV assessment.
In addition to the ‡ model adjusted for other retinal vessel.
The adjusted P-value adjusts for multiple comparisons via the Benjamini-Hochberg method.
Table 3.
Association of mid-pregnancy placental growth factor in quartiles with cardiovascular risk factors six and nine years after pregnancy
| Outcome | Low PlGF MoM ≤ 0.72 116.4 pg./mL (62.6, 171.4) Beta (95% CI) |
Medium low PlGF MoM 0.73 - 1.0 176.7 pg./mL (127.6, 247.9) Beta (95% CI) |
Medium high PlGF MoM 1.01 - 1.40 243.9 pg./mL (176.5, 342.5) Beta (95% CI) |
High PlGF MoM ≥ 1.41 422.2 pg./mL (260.2, 714.8) |
P for Trend | Adjusted P-value |
|---|---|---|---|---|---|---|
| Total population (n = 3797) | ||||||
| Six years after pregnancy | ||||||
| ‡Aortic root diameter (mm) | 0.40 (0.08, 0.73) | 0.22 (−0.10, 0.54) | 0.20 (−0.13, 0.52) | ref | <0.001 | <0.001 |
| ‡Left atrial diameter (mm) | 0.34 (−0.09, 0.78) | 0.26 (−0.17, 0.69) | 0.40 (−0.03, 0.83) | ref | <0.001 | <0.001 |
| ‡Left ventricular mass (g) | 4.6 (1.1, 8.1) | 1.2 (−2.3, 4.6) | 0.74 (−2.8, 4.2) | ref | <0.001 | <0.001 |
| ‡Pulse wave velocity (m/s) | 0.01 (−0.13, 0.15) | −0.09 (−0.23, 0.05) | 0.02 (−0.12, 0.15) | ref | 0.33 | 0.61 |
| ‡Fractional shortening | −0.01 (−0.58, 0.55) | −0.05 (−0.60, 0.50) | 0.10 (−0.46, 0.66) | ref | 0.42 | 0.62 |
| †Central retinal arteriolar caliber (SDS) | −0.09 (−0.20, 0.03) | −0.10 (−0.22, 0.01) | −0.04 (−0.16, 0.08) | ref | 0.62 | 0.68 |
| †Central retinal venular caliber (SDS) | 0.04 (−0.08, 0.16) | 0.04 (−0.07, 0.16) | 0.02 (−0.10, 0.14) | ref | 0.86 | 0.86 |
| ‡SBP (mmHg) | 2.3 (0.93, 3.6) | 1.0 (−0.31, 2.3) | 1.3 (0.04, 2.7) | ref | 0.005 | 0.02 |
| ‡DBP (mmHg) | 0.89 (−0.15, 1.9) | 0.63 (−0.40, 1.7) | 1.2 (0.17, 2.2) | ref | 0.51 | 0.62 |
| Nine years after pregnancy (n=3198) | ||||||
| ‡SBP (mmHg) | 2.6 (1.2, 4.0) | 1.2 (−0.21, 2.6) | 2.2 (0.82, 3.6) | ref | 0.04 | 0.09 |
| ‡DBP (mmHg) | 1.8 (0.81, 2.7) | 0.88 (−0.05, 1.8) | 1.4 (0.48, 2.3) | ref | 0.46 | 0.62 |
| Uncomplicated pregnancies (n=3270) | ||||||
| Six years after pregnancy | ||||||
| ‡Aortic root diameter (mm) | 0.45 (0.09, 0.80) | 0.33 (−0.02, 0.67) | 0.30 (−0.05, 0.64) | ref | <0.001 | <0.001 |
| ‡Left atrial diameter (mm) | 0.28 (−0.20, 0.75) | 0.16 (−0.30, 0.62) | 0.30 (−0.16, 0.76) | ref | <0.001 | <0.001 |
| ‡Left ventricular mass (g) | 5.1 (1.3, 8.9) | 2.2 (−1.5, 5.9) | 1.2 (−2.5, 4.8) | ref | <0.001 | <0.001 |
| ‡Pulse wave velocity (m/s) | 0.04 (−0.11, 0.19) | −0.09 (−0.24, 0.06) | 0.01 (−0.13, 0.16) | ref | 0.27 | 0.50 |
| ‡Fractional shortening | −0.12 (−0.72, 0.48) | −0.15 (−0.73, 0.44) | 0.21 (−0.38, 0.79) | ref | 0.41 | 0.56 |
| †Central retinal arteriolar caliber (SDS) | −0.08 (−0.21, 0.04) | −0.09 (−0.21, 0.03) | −0.04 (−0.16, 0.08) | ref | 0.57 | 0.68 |
| †Central retinal venular caliber (SDS) | 0.07 (−0.06, 0.20) | 0.06 (−0.07, 0.19) | 0.02 (−0.11, 0.15) | ref | 0.70 | 0.70 |
| ‡SBP (mmHg) | 1.8 (0.35,3.2) | 0.67 (−0.71, 2.1) | 0.78 (−0.59, 2.2) | ref | 0.003 | 0.008 |
| ‡DBP (mmHg) | 0.82 (−0.29, 1.9) | 0.39 (−0.70, 1.5) | 0.83 (−0.25, 1.9) | ref | 0.40 | 0.56 |
| Nine years after pregnancy (n=2748) | ||||||
| ‡SBP (mmHg) | 2.3 (0.84, 3.8) | 0.78 (−0.70, 2.3) | 1.9 (0.40, 3.3) | ref | 0.047 | 0.10 |
| ‡DBP (mmHg) | 1.6 (0.58, 2.6) | 0.70 (−0.29, 1.7) | 1.1 (0.17, 2.1) | ref | 0.62 | 0.68 |
Abbreviations: Body mass index, BMI ;Confidence Interval, CI; Diastolic blood pressure, DBP; Multiple of the median, MoM; Placental growth factor, PlGF; Systolic blood pressure, SBP; Standard deviation score, SDS. Values are regression coefficients with β and 95% confidence interval, based on linear regression models, and are compared to women with high PlGF. Estimates represent the unit increase in the outcome per 1 multiple of the median increase in PlGF, compared to the reference category.
Adjusted for maternal age at enrollment, gravidity during pregnancy, time interval between pregnancy and follow-up, ethnicity, educational level, smoking during pregnancy, change in systolic blood pressure between early pregnancy and mid-pregnancy (and change in BMI between pre-pregnancy and mid-pregnancy in the BMI model).
In addition to the ‡ model adjusted for pulse at the time of PWV assessment.
In addition to the ‡ model adjusted for other retinal vessel.
The P for trend is the result of univariate ANOVA analysis through linear contrast analysis. The adjusted P-value adjusts for multiple comparisons via the Benjamini-Hochberg method.
RESULTS
Subject characteristics
Table 1 shows baseline characteristics during pregnancy, and six and nine years after pregnancy. The majority of women were: European, highly educated, of lean or normal pre-pregnancy BMI, non-smokers and nulliparous during the index pregnancy. Six years after pregnancy BMI was higher compared to pre-pregnancy and 91% of women had been pregnant more than once. Nine years after pregnancy BMI remained similar to BMI six years after pregnancy.
Results six years after pregnancy
Table 2 shows that the lower mid-pregnancy PlGF concentrations were, the higher were AOD, LAD, LV mass and SBP. Additionally, when PlGF was divided into quartiles women with the lowest mid-pregnancy PlGF concentrations had a higher AOD, LAD,LV mass and SBP six years after pregnancy compared to women with the highest PlGF concentrations (Table 3). Women with medium high PlGF six years after pregnancy also had a higher SBP and DBP than women with high PlGF. These differences persisted after exclusion of women with complicated pregnancies in the index pregnancy from the analyses. We observed no association between mid-pregnancy PlGF concentrations and FS and central retinal arteriolar and venular calibers six years after pregnancy for women in the total population or women with uncomplicated pregnancies (Table 2 and 3).
Lastly, lower early pregnancy PlGF concentrations were associated with a higher systolic blood pressure and AOD after pregnancy (Supplemental Table 1 and 2).
Blood pressure nine years after pregnancy
Lower mid-pregnancy PlGF levels were associated with a higher SBP nine years after pregnancy (Table 2 and 3). Quartile analysis showed that women with low PlGF or medium high PlGF in mid-pregnancy had a higher systolic and diastolic blood pressure nine years after pregnancy than women with high PlGF (Table 3). Early pregnancy PlGF was not associated with blood pressure nine years after pregnancy (Supplemental Table 1 and 2).
Nine years after pregnancy, mean blood pressure values were lower compared to six years after pregnancy (Table 1), whereas we would expect blood pressure to increase over time. Results are most likely explained by differences in measurement technique: blood pressure was measured twice in sitting position six years after pregnancy, whereas it was measured four times in supine position nine years after pregnancy.24 We nonetheless tested whether these results could be explained by selection of a relatively healthy group nine years after pregnancy. However, they could not. Restricting the analyses to: 1) women who participated at both moments or 2) women who never used antihypertensive medication or never had a hypertension diagnosis, did not change the ratio of our results. Neither did stratifying for parity, gravidity, smoking, BMI, ethnicity, education or having had preeclampsia or gestational hypertension more than once.
Results during index pregnancy
The association between PlGF and cardiovascular adaptation to pregnancy has been studied previously in The Generation R Study through the UtA resistance index.2 Our current study showed that higher mid-pregnancy PlGF concentrations were associated with a lower risk of notching in late pregnancy (Supplemental Table 3). Also, systolic blood pressure in early, mid- and late pregnancy, and diastolic blood pressure in late pregnancy, were lower with increasing concentrations of PlGF. After excluding women with complicated pregnancies from the analyses, results remained similar.
Higher early pregnancy PlGF was associated with lower UtA-PI in mid- and late pregnancy, a lower risk of UtA notching in late pregnancy, a lower systolic blood pressure in early pregnancy and lower diastolic blood pressure throughout pregnancy (Supplemental Table 4).
Non-responders
We compared baseline characteristics of women included and not included in the analyses (Supplemental Table 5). Women with information available on mid-pregnancy PlGF and at least one outcome of interest (group 1) were compared to: women with a mid-pregnancy PlGF measurement and no outcome of interest (group 2) and women without a mid-pregnancy PlGF measurement or any outcome of interest (group 3). Women not included in the analyses (group 2 and 3) were on average: one to two years younger, more often of non-European descent, lower educated, more often multiparous and more often affected by sPTB, SGA and PE (Supplemental Table 5).
DISCUSSION
This large prospective study demonstrates that PlGF concentrations in mid-pregnancy were inversely associated with subsequent AOD, LAD, LV mass and SBP. Moreover, women with low mid-pregnancy PlGF had a greater AOD, LAD, and LV mass, and higher SBP at follow-up than women with high PlGF. These findings were not driven by pregnancy complications. The effect estimates in our study are modest and of minimal clinical impact at the time of measurement. However, these measures are in young women and it is likely that in the long term, the magnitude of these findings may increase, making these women more susceptible to CVD. The positive association between AOD, LAD, LV mass, SBP and the risk of CVD has been shown in multiple studies. In a population-based study of middle-aged individuals a 1 unit increase in AOD (indexed by height) was associated with a 2.62 fold increased risk of fatal or non-fatal CVD.25 Another study found that 1cm increase in LAD was associated with a 1.25-fold increased risk of ischemic stroke in women.26 In the Framingham Heart Study there was a 1.57-fold increased risk of CVD for every 50g increase in LV mass in 40 year old women.27 Higher systolic blood pressure was associated with increased risk of heart failure.28 The risk of heart failure increased 1.75 fold for each 20 mm Hg increase in SBP in 30-59 year old men and women.28
We posit that low mid-pregnancy PlGF concentrations, or an unknown factor closely associated with low PlGF concentrations, reflect a suboptimal cardiovascular status in pregnancy and a higher risk of cardiovascular impairment after pregnancy. We propose two pathophysiological mechanisms to explain these associations. Firstly, stress to the STB can reduce the production of PlGF and also lead to an increase in sFlt-1 levels which in turn can bind to circulating PlGF levels and further decrease PlGF availability, thereby leading to endothelial cell dysfunction.21, 29 The association between low PlGF levels in pregnancy and endothelial dysfunction has been well established. Endothelial dysfunction can persist for many years after pregnancy and can lead to cardiovascular impairment.7, 8 Secondly, low PlGF levels in pregnancy could result in suboptimal cardiovascular adaptation and remodelling as demonstrated in cardiac studies of PlGF null pregnant mice.3 PlGF null mice manifest cardiac findings similar to those of hearts exposed to pressure overload (e.g. increased left ventricular mass).3, 30 This abnormal remodelling initiated during pregnancy might persist after pregnancy. The cardiac findings in our study also resemble those found in hearts with pressure overload, which can lead to CVD (e.g. heart failure).31
PWV, a measurement of the velocity at which the arterial pulse propagates through the arteries, is a measure of arterial stiffness. Six years after pregnancy, mid-pregnancy PlGF concentrations were positively, though not significantly, associated with PWV, whereas we expected a negative association. Women with high PlGF smoked more often compared to women with low PlGF (30.3% vs. 16.1% respectively). Smoking has previously been reported to be associated with increased PlGF measured at an average of 17 weeks gestation.32 Smoking might explain the tendency to a larger PWV in women with high PlGF. Previous studies showed that under the age of 40, a rise in PWV precedes a rise in blood pressure, which occurs later in life.33 Therefore, we expect that higher PlGF will be associated with higher blood pressure in smokers at a later age.
We observed no association between mid-pregnancy PlGF concentrations and FS. FS measures the degree of shortening of the left ventricular diameter between end-diastole and end-systole. Low FS is therefore a measure of impaired left ventricular performance.34 A substantial degree of long-axis systolic dysfunction is necessary in order to reduce FS, which is impaired below 25%.35 The women included in our study were young and relatively healthy at the time FS was measured (36.7 [SD 5.0] years). We hypothesize that the onset of FS in women with low mid-pregnancy PlGF will start at a later age when systolic function is more impaired.
The microvasculature measurements (central retinal arteriolar and venular calibers) six years after pregnancy were not associated with mid-pregnancy PlGF in pregnancy. However, low maternal mid-pregnancy PlGF concentrations have been associated with narrower central retinal arteriolar calibers in their offspring, as was shown in a previous report from the Generation R Study.36 These findings may be due to 1) interference of normal vascular growth by low PlGF concentrations or 2) endothelial dysfunction induced by hypoxia in the placental environment due to low PlGF concentrations. Perhaps this is not pertinent to adult vessels.
Women with low or medium high mid-pregnancy PlGF concentrations had a higher blood pressure six and nine years after pregnancy compared to women with high mid-pregnancy PlGF. Conditions that are associated with low PlGF concentrations in pregnancy (e.g. endothelial dysfunction) can also induce an isolated rise in systolic blood pressure.37 Possibly, women with low PlGF in pregnancy have more endothelial dysfunction after pregnancy than women with high PlGF concentrations. This might explain why especially systolic blood pressure and to a lesser extent diastolic blood pressure were associated with low PlGF. The positive association between low mid-pregnancy PlGF and SBP, but not DBP, is consistent with the positive association between low mid-pregnancy PlGF and LVM. Previous studies showed that SBP was more strongly associated with LV mass than was DBP.38 This could be due to LV wall stress, which has been associated with a higher SBP and LV hypertrophy.
During normal pregnancy, the cardiovascular system adapts to expanded plasma volume with increased preload and decreased afterload.39 Cardiac remodeling is initiated consisting of left ventricular dilatation, geometric changes, increased myocardial contractility and therefore increased stroke volume, heart rate, and cardiac output. In normal physiologic pregnancy, all geometric and hemodynamic changes usually return to baseline after approximately one year.39 However in women with previous early-onset PE, asymptomatic left ventricular impairment and diastolic dysfunction can persist at least four years after pregnancy.40, 41 Increased wall stress due to hypertension during PE, might result in persistent cardiac remodeling in these women. In our study, the association of mid-pregnancy PlGF concentrations with cardiac remodeling as indicated by a larger left atrial diameter and left ventricular mass was independent of PE, SGA and sPTB.
Interestingly, similar cardiac changes observed in women with previous PE are described in women with previous fetal growth restriction and in some women with apparently previous uncomplicated pregnancies.41, 42 In a small proportion of women with uncomplicated pregnancies these changes can be identified one year postpartum.41, 43 It is tempting to speculate that these are women with low PlGF concentrations during pregnancy who do not manifest PE. This is in line with our hypothesis that mid-pregnancy PlGF concentration is a distinctive factor associated with cardiovascular function after pregnancy, independent of pregnancy complications.
Our findings regarding mid-pregnancy PlGF concentrations and pregnancy outcomes were consistent with previous studies examining PlGF and the uterine vascular bed with Doppler velocimetry.44 These studies showed that vascular resistance of the uterine arteries was substantially increased in pregnancies affected by PE or SGA, which is proposed to be evidence for a failed vascular adaptation. We suggest that a high resistance in the uterine vascular bed may be a response to low PlGF as vascular maladaptation during pregnancy with low PlGF was also present in uncomplicated pregnancies. Alternatively, in mid- and later pregnancy the hemodynamic demands of the STB grow beyond the capacity of the utero-placental circulation leading to STB stress. The latter will result in a decreased production of PlGF and increased production of sFlt-1 by the STB. A dysregulation between PlGF and sFlt-1 can lead to endothelial dysfunction.
During pregnancy, the placenta is the main source of a dramatic increase in circulating PlGF of approximately 50 times non-pregnant concentration. Outside of pregnancy, PlGF is mainly expressed by endothelial cells.45 Contrary to our findings of lower PlGF concentration in pregnancy being associated with later markers of future CVD, a doubling of circulating PlGF outside of pregnancy is associated with several pathologic conditions including tumor growth, plaque formation and CVD.46, 47 These processes are partly regulated by the effect of PlGF to initiate angiogenesis, vasculogenesis and inflammation. However, exact mechanistic details are unclear.48 PlGF also has a cardioprotective function; During cardiac stress, such as a myocardial infarction, cardiomyocytes upregulate PlGF which is proposed to initiate cardiac remodeling to maintain cardiac function.49 The explanation for the contrasting association of PlGF during and after pregnancy with CVD is not clear. It may reflect the differing impact of the strikingly dissimilar concentrations of PlGF in the two settings or might reflect the mechanism responsible for the alteration of PlGF. Outside of pregnancy, high levels of PlGF most likely result from endothelial inflammation as seen in individuals with cardiac stress.50 During pregnancy, low circulating PlGF results from a stressed STB and placental pathophysiology.1
Our results indicate that mid-pregnancy PlGF concentrations are associated with cardiovascular risk factors six and nine years after pregnancy. Mid-pregnancy PlGF concentrations might identify women at risk of cardiovascular impairment after pregnancy. Though our effect estimates are small, they indicate clear differences between women with lower and high PlGF in mid-pregnancy. We have to take into account that these women are still young and overall healthy. In the long term, the magnitude of these findings will most likely increase and make these women more susceptible to CVD.
Strengths and limitations
This study has some limitations. First, placental hemodynamic measurements were carried out in only two out of three research centers due to lack of equipment. Second, retinal vascular imaging was not obtained from 33.6% of women six years after pregnancy due to introduction of this measurement into the Generation R Study after recruitment for follow-up had already started. Both the first and second limitation were independent of subject characteristics and selection bias therefore seems unlikely. Nevertheless, this might have resulted in loss of power and perhaps an underestimation of our results. Third, a relatively healthy population was selected that might have affected the generalizability of our results. Lastly, since pre-pregnancy cardiovascular risk measurements are not available the observational nature of this study does not allow for inference of causality. Future studies should examine pre-pregnancy cardiovascular risk measurements in relation to PlGF levels. Additionally, future research should examine the consistency of PlGF levels from one pregnancy to another to identify whether the association between mid-pregnancy PlGF levels and cardiovascular measurements differs between a first or subsequent pregnancy.
Our study has several strengths: First, the large sample size and prospective data collection from early pregnancy onwards. Second, all outcomes were obtained following standardized protocols. Third, this is a multi-ethnic study, which we believe will give a good representation of the general population.
Conclusion
Lower PlGF concentrations in mid-pregnancy are associated with a greater AOD, LAD, and LV mass, and higher SBP six and nine years after pregnancy, regardless of whether pregnancy was uncomplicated or complicated. PlGF concentrations in mid-pregnancy might provide insight in the pathways contributing to a worse cardiovascular risk profile after pregnancy.
Supplementary Material
Clinical Perspective.
What is new?
This study identifies low maternal placental growth factor (PlGF) levels in mid-pregnancy to be associated with a greater aortic root diameter, left atrial diameter, and left ventricular mass, and higher systolic blood pressure six and nine years after pregnancy compared to women with high mid-pregnancy PlGF levels.
These associations were observed in women with and without a complicated pregnancy (i.e. affected by pre-eclampsia, small for gestational age and spontaneous preterm birth).
What are the clinical implications?
These results suggest that a woman’s response to the cardiovascular challenges of pregnancy, measured by mid-pregnancy PlGF, could provide insight into the pathophysiological mechanisms leading to future CVD in parous women.
Acknowledgements
The Generation R Study is conducted by the Erasmus Medical Center in close collaboration with the School of Law and the Faculty of Social Sciences of the Erasmus University, Rotterdam; the Municipal Health Service, Rotterdam area; the Rotterdam Homecare Foundation; and the Stichting Trombosedienst and Artsenlaboratorium Rijnmond, Rotterdam, the Netherlands. We gratefully acknowledge the contributions of the general practitioners, hospitals, midwives and the pharmacies in Rotterdam. The Global Pregnancy Collaboration, part of the Pre-eclampsia-Eclampsia Monitoring & Treatment (PRE-EMPT) initiative which is funded by the University of British Columbia, a grantee of the Bill & Melinda Gates Foundation.
Funding
The Generation R Study was made possible by financial support from the Erasmus Medical Center, Erasmus University Rotterdam and the Netherlands Organization for Health Research and Development, the Netherlands organization for Scientific Research, the Ministry of Health, Welfare and Sport, and the Ministry of Youth and Families. Professor Vincent Jaddoe received additional grants from the Netherlands Organization for Health Research and Development (grant nos. 90700303, 916.10159 and VIDI 016.136.361) and a Consolidator Grant from the European Research Council (ERC-2014-CoG-64916). Dr. Ikram received funding from the Netherlands Organization for Health Research and Development (ZonMW; VENI project number: 91612163). This study was made possible by additional funding of the Dutch Heart Foundation (grant number 2013T083). Dr. Gandley received funding from the National Institutes of Health (grants P01-HD-30367, R21 HD083659), and the American Heart Association (16SFRN27810001). Dr. Roberts received funding from the American Heart Association (16SFRN27810001) and the Bill and Melinda Gates foundation (Global Pregnancy Collaboration)
Footnotes
Details of ethics approval
The study was approved by the Medical Ethics Committee of the Erasmus Medical Center, Rotterdam, the Netherlands (MEC 198.782/2001/31).
Disclosures
The authors have nothing to disclose.
REFERENCES
- 1.Redman CW and Staff AC. Preeclampsia, biomarkers, syncytiotrophoblast stress, and placental capacity. Am J Obstet Gynecol. 2015;213:S9 e1–4. [DOI] [PubMed] [Google Scholar]
- 2.Coolman M, Timmermans S, de Groot CJ, Russcher H, Lindemans J, Hofman A, Geurts-Moespot AJ, Sweep FC, Jaddoe VV and Steegers EA. Angiogenic and fibrinolytic factors in blood during the first half of pregnancy and adverse pregnancy outcomes. Obstet Gynecol. 2012;119:1190–1200. [DOI] [PubMed] [Google Scholar]
- 3.Aasa KL, Zavan B, Luna RL, Wong PG, Ventura NM, Tse MY, Carmeliet P, Adams MA, Pang SC and Croy BA. Placental growth factor influences maternal cardiovascular adaptation to pregnancy in mice. Biol Reprod. 2015;92:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellamy L, Casas JP, Hingorani AD and Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powers RW, Roberts JM, Plymire DA, Pucci D, Datwyler SA, Laird DM, Sogin DC, Jeyabalan A, Hubel CA and Gandley RE. Low placental growth factor across pregnancy identifies a subset of women with preterm preeclampsia: type 1 versus type 2 preeclampsia? Hypertension. 2012;60:239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujita K, Tatsumi K, Kondoh E, Chigusa Y, Mogami H, Fujita M and Konishi I. Endothelial function progressively deteriorates during normal pregnancy. Hypertens Pregnancy. 2013;32:129–138. [DOI] [PubMed] [Google Scholar]
- 7.Matsuzawa Y, Kwon TG, Lennon RJ, Lerman LO and Lerman A. Prognostic Value of Flow-Mediated Vasodilation in Brachial Artery and Fingertip Artery for Cardiovascular Events: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2015;4: e002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chambers JC, Fusi L, Malik IS, Haskard DO, De Swiet M and Kooner JS. Association of maternal endothelial dysfunction with preeclampsia. JAMA. 2001;285:1607–1612. [DOI] [PubMed] [Google Scholar]
- 9.Staff AC, Redman CW, Williams D, Leeson P, Moe K, Thilaganathan B, Magnus P, Steegers EA, Tsigas EZ, Ness RB, Myatt L, Poston L, Roberts JM and Global Pregnancy C. Pregnancy and Long-Term Maternal Cardiovascular Health: Progress Through Harmonization of Research Cohorts and Biobanks. Hypertension. 2016;67:251–260. [DOI] [PubMed] [Google Scholar]
- 10.Kooijman MN, Kruithof CJ, van Duijn CM, Duijts L, Franco OH, van IMH, de Jongste JC, Klaver CC, van der Lugt A, Mackenbach JP, Moll HA, Peeters RP, Raat H, Rings EH, Rivadeneira F, van der Schroeff MP, Steegers EA, Tiemeier H, Uitterlinden AG, Verhulst FC, Wolvius E, Felix JF and Jaddoe VW. The Generation R Study: design and cohort update 2017. Eur J Epidemiol. 2016;31:1243–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kruithof CJ, Kooijman MN, van Duijn CM, Franco OH, de Jongste JC, Klaver CC, Mackenbach JP, Moll HA, Raat H, Rings EH, Rivadeneira F, Steegers EA, Tiemeier H, Uitterlinden AG, Verhulst FC, Wolvius EB, Hofman A and Jaddoe VW. The Generation R Study: Biobank update 2015. Eur J Epidemiol. 2014;29:911–927. [DOI] [PubMed] [Google Scholar]
- 12.Coolman M, de Groot CJ, Jaddoe VW, Hofman A, Raat H and Steegers EA. Medical record validation of maternally reported history of preeclampsia. J Clin Epidemiol. 2010;63:932–937. [DOI] [PubMed] [Google Scholar]
- 13.Brown MA, Lindheimer MD, de Swiet M, Van Assche A and Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens Pregnancy. 2001;20:IX–XIV. [DOI] [PubMed] [Google Scholar]
- 14.Tsiakkas A, Duvdevani N, Wright A, Wright D and Nicolaides KH. Serum placental growth factor in the three trimesters of pregnancy: effects of maternal characteristics and medical history. Ultrasound Obstet Gynecol. 2015;45:591–598. [DOI] [PubMed] [Google Scholar]
- 15.Gaillard R, Steegers EA, Tiemeier H, Hofman A and Jaddoe VW. Placental vascular dysfunction, fetal and childhood growth, and cardiovascular development: the generation R study. Circulation. 2013;128:2202–2210. [DOI] [PubMed] [Google Scholar]
- 16.Verburg BO, Steegers EA, De Ridder M, Snijders RJ, Smith E, Hofman A, Moll HA, Jaddoe VW and Witteman JC. New charts for ultrasound dating of pregnancy and assessment of fetal growth: longitudinal data from a population-based cohort study. Ultrasound Obstet Gynecol. 2008;31:388–396. [DOI] [PubMed] [Google Scholar]
- 17.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I and Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. [DOI] [PubMed] [Google Scholar]
- 18.Geelhoed MJ, Snijders SP, Kleyburg-Linkers VE, Steegers EA, van Osch-Gevers L and Jaddoe VW. Reliability of echocardiographic measurements of left cardiac structures in healthy children. Cardiol Young. 2009;19:494–500. [DOI] [PubMed] [Google Scholar]
- 19.Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R and Klein BE. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27:143–149. [DOI] [PubMed] [Google Scholar]
- 20.Graham JW, Olchowski AE and Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci. 2007;8:206–213. [DOI] [PubMed] [Google Scholar]
- 21.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP and Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. [DOI] [PubMed] [Google Scholar]
- 22.Erez O, Romero R, Espinoza J, Fu W, Todem D, Kusanovic JP, Gotsch F, Edwin S, Nien JK, Chaiworapongsa T, Mittal P, Mazaki-Tovi S, Than NG, Gomez R and Hassan SS. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern Fetal Neonatal Med. 2008;21:279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benjamini Y and Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological). 1995;57:289–300. [Google Scholar]
- 24.Cicolini G, Gagliardi G and Ballone E. Effect of Fowler’s body position on blood pressure measurement. J Clin Nurs. 2010;19:3581–3583. [DOI] [PubMed] [Google Scholar]
- 25.Cuspidi C, Facchetti R, Bombelli M, Re A, Cairoa M, Sala C, Tadic M, Grassi G and Mancia G. Aortic root diameter and risk of cardiovascular events in a general population: data from the PAMELA study. J Hypertens. 2014;32:1879–1887. [DOI] [PubMed] [Google Scholar]
- 26.Bouzas-Mosquera A, Broullon FJ, Alvarez-Garcia N, Mendez E, Peteiro J, Gandara-Sambade T, Prada O, Mosquera VX and Castro-Beiras A. Left atrial size and risk for all-cause mortality and ischemic stroke. CMAJ. 2011;183:E657–E664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy D, Garrison RJ, Savage DD, Kannel WB and Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. [DOI] [PubMed] [Google Scholar]
- 28.Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, White IR, Caulfield MJ, Deanfield JE, Smeeth L, Williams B, Hingorani A and Hemingway H. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet. 2014;383:1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP and Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Souders CA, Borg TK, Banerjee I and Baudino TA. Pressure overload induces early morphological changes in the heart. Am J Pathol. 2012;181:1226–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frohlich ED and Susic D. Pressure overload. Heart Fail Clin. 2012;8:21–32. [DOI] [PubMed] [Google Scholar]
- 32.Bersinger NA and Odegard RA. Second-trimester serum levels of placenta growth factor (PLGF) and inhibin A are increased in smokers. Implications for pre-eclampsia risk assessment. Immuno-Anal Biol Spe. 2007;22:19–23. [Google Scholar]
- 33.Mitchell GF. Arterial stiffness and hypertension: chicken or egg? Hypertension. 2014;64:210–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dusch MN, Thadani SR, Dhillon GS and Hope MD. Diastolic function assessed by cardiac MRI using longitudinal left ventricular fractional shortening. Clin Imaging. 2014;38:666–668. [DOI] [PubMed] [Google Scholar]
- 35.Kurita A, Itoh H, Sato F, Ichibori Y and Yoshida A. Longitudinal fractional shortening and its relation to diastolic cardiac function. J Med Ultrason (2001). 2008;35:113–118. [DOI] [PubMed] [Google Scholar]
- 36.Gishti O, Jaddoe VW, Felix JF, Reiss I, Hofman A, Ikram MK, Steegers EA and Gaillard R. Influence of maternal angiogenic factors during pregnancy on microvascular structure in school-age children. Hypertension. 2015;65:722–728. [DOI] [PubMed] [Google Scholar]
- 37.Spradley FT, Tan AY, Joo WS, Daniels G, Kussie P, Karumanchi SA and Granger JP. Placental Growth Factor Administration Abolishes Placental Ischemia-Induced Hypertension. Hypertension. 2016;67:740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Missault LH, De Buyzere ML, De Bacquer DD, Duprez DD and Clement DL. Relationship between left ventricular mass and blood pressure in treated hypertension. J Hum Hypertens. 2002;16:61–66. [DOI] [PubMed] [Google Scholar]
- 39.Melchiorre K, Sharma R, Khalil A and Thilaganathan B. Maternal Cardiovascular Function in Normal Pregnancy: Evidence of Maladaptation to Chronic Volume Overload. Hypertension. 2016;67:754–762. [DOI] [PubMed] [Google Scholar]
- 40.Orabona R, Vizzardi E, Sciatti E, Bonadei I, Valcamonico A, Metra M and Frusca T. Insights into cardiac alterations after pre-eclampsia: an echocardiographic study. Ultrasound Obstet Gynecol. 2017;49:124–133. [DOI] [PubMed] [Google Scholar]
- 41.Melchiorre K, Sutherland GR, Liberati M and Thilaganathan B. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension. 2011;58:709–715. [DOI] [PubMed] [Google Scholar]
- 42.Melchiorre K, Sutherland GR, Liberati M and Thilaganathan B. Maternal cardiovascular impairment in pregnancies complicated by severe fetal growth restriction. Hypertension. 2012;60:437–443. [DOI] [PubMed] [Google Scholar]
- 43.Thilaganathan B Placental syndromes: getting to the heart of the matter. Ultrasound Obstet Gynecol. 2017;49:7–9. [DOI] [PubMed] [Google Scholar]
- 44.Espinoza J, Romero R, Nien JK, Gomez R, Kusanovic JP, Goncalves LF, Medina L, Edwin S, Hassan S, Carstens M and Gonzalez R. Identification of patients at risk for early onset and/or severe preeclampsia with the use of uterine artery Doppler velocimetry and placental growth factor. Am J Obstet Gynecol. 2007;196:326 e1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yonekura H, Sakurai S, Liu X, Migita H, Wang H, Yamagishi S, Nomura M, Abedin MJ, Unoki H, Yamamoto Y and Yamamoto H. Placenta growth factor and vascular endothelial growth factor B and C expression in microvascular endothelial cells and pericytes. Implication in autocrine and paracrine regulation of angiogenesis. J Biol Chem. 1999;274:35172–35178. [DOI] [PubMed] [Google Scholar]
- 46.De Falco S The discovery of placenta growth factor and its biological activity. Exp Mol Med. 2012;44:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsui M, Uemura S, Takeda Y, Samejima K, Matsumoto T, Hasegawa A, Tsushima H, Hoshino E, Ueda T, Morimoto K, Okamoto K, Okada S, Onoue K, Okayama S, Kawata H, Kawakami R, Maruyama N, Akai Y, Iwano M, Shiiki H, Saito Y and Investigators N-C. Placental Growth Factor as a Predictor of Cardiovascular Events in Patients with CKD from the NARA-CKD Study. J Am Soc Nephrol. 2015;26:2871–2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cassidy A, Chiuve SE, Manson JE, Rexrode KM, Girman CJ and Rimm EB. Potential role for plasma placental growth factor in predicting coronary heart disease risk in women. Arterioscler Thromb Vasc Biol. 2009;29:134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Accornero F and Molkentin JD. Placental growth factor as a protective paracrine effector in the heart. Trends Cardiovasc Med. 2011;21:220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsumoto T, Uemura S, Takeda Y, Matsui M, Okada S, Nishida T, Soeda T, Okayama S, Somekawa S, Ishigami K, Onoue K, Kawata H, Kawakami R, Horii M and Saito Y. An elevated ratio of placental growth factor to soluble fms-like tyrosine kinase-1 predicts adverse outcomes in patients with stable coronary artery disease. Intern Med. 2013;52:1019–1027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


