Abstract
Background:
The noradrenergic system has been implicated in AUD and PTSD, with adrenergic agents reducing drinking in individuals with AUD and improving sleep disturbances in individuals with PTSD. In a recent clinical trial (Petrakis et al., 2016), prazosin, an α1-adrenergic antagonist, was not superior to placebo in reducing PTSD symptoms, sleep problems, or alcohol consumption in a comorbid population; however, patients in both treatment conditions improved in all symptom domains over the course of treatment. It remains unknown whether alcohol abstinence is related to changes in PTSD symptoms and medication effects in individuals with this comorbidity.
Methods:
Veterans with comorbid alcohol dependence and PTSD (n=96) were randomized to prazosin (16mg) or placebo in a 12-week outpatient, double-blind clinical trial (Petrakis et al., 2016). In this secondary data analysis, we examined main effects of alcohol abstainer status (abstainer vs. non-abstainer), treatment, and their interaction on changes in PTSD symptoms (CAPS) over time using linear mixed models.
Results:
There was a main effect of alcohol abstainer status on symptoms of PTSD (p=0.03), such that non-abstainers had lower total CAPS scores than abstainers. There was a significant treatment by alcohol abstainer status interaction (p=0.01); specifically, among placebo-treated individuals, those who did not abstain from alcohol had lower total CAPS scores compared to alcohol abstainers. Within the prazosin-treated group, abstainers and non-abstainers did not differ on total CAPS scores. Results were similar for the avoidance (p=0.02), reexperiencing (p=0.01), and hyperarousal (p=0.04) subscales, such that placebo-treated non-abstainers had lower scores overall.
Conclusions:
Overall, prazosin treatment was not significantly related to changes in PTSD symptoms over the course of the 12-week clinical trial in a comorbid population. Interestingly, placebo-treated alcohol non-abstainers had a significant reduction in PTSD symptoms. Whether placebo-treated individuals continued to use alcohol because of ongoing symptoms of PTSD is not known.
Keywords: AUD, PTSD, CAPS, comorbid AUD and PTSD, prazosin
Introduction
Prazosin, an alpha1-adrenergic antagonist, has been shown to reduce alcohol-seeking and self-administration in rodents with and without a genetic predisposition for high alcohol drinking (Froehlich et al., 2013a, Froehlich et al., 2013b, Froehlich et al., 2015, Funk et al., 2016, Le et al., 2011, Rasmussen et al., 2009, Rasmussen et al., 2014, Skelly and Weiner, 2014, Verplaetse et al., 2012, Verplaetse and Czachowski, 2015) and to decrease alcohol craving and drinking in individuals with alcohol use disorder (AUD) (Fox et al., 2012, Simpson et al., 2009, Wilcox et al., 2018). Prazosin has also demonstrated efficacy in decreasing nightmares and sleep disturbances in individuals with posttraumatic stress disorder (PTSD) (Peskind et al., 2003, Raskind et al., 2000, Raskind et al., 2003, Raskind et al., 2007, Raskind et al., 2002, Raskind et al., 2013), although results are mixed (Raskind et al., 2018). AUD and PTSD are highly comorbid in the general population and in veterans (Smith et al., 2016, Pietrzak et al., 2011, Grant et al., 2015), although only two studies have examined prazosin for AUD with comorbid PTSD (Simpson et al., 2015, Petrakis et al., 2016). In a 6-week pilot trial, Simpson and colleagues demonstrated that prazosin was effective in reducing the percentage of drinking days per week and heavy drinking days per week relative to placebo; however, prazosin did not reduce PTSD symptoms (Simpson et al., 2015). In a subsequent clinical trial, our group demonstrated that the same dose of prazosin (16 mg/d) was not superior to placebo in reducing drinking or PTSD symptoms; however, drinking and symptoms of PTSD improved over time (Petrakis et al., 2016).
Understanding the relationship between drinking and symptoms of PTSD has important treatment implications. AUD and PTSD are highly comorbid (Grant et al., 2015), with rates as high as 55% of veterans (Smith et al., 2016). Individuals with this comorbidity exhibit more severe problems related to alcohol use and PTSD and have worse treatment outcomes (Blanco et al., 2013, McCarthy and Petrakis, 2010, Norman et al., 2012). It has been proposed that individuals may drink to manage stress (i.e., the self-medication hypothesis; Baker et al., 2014, Khantzian, 2003); thus, drinking to medicate PTSD symptoms may serve as a maintaining factor for this comorbid condition, particularly as an avoidance strategy. Others have found a reduction in PTSD symptoms during acute protracted abstinence from alcohol (Coffey et al., 2007). In our primary clinical trial, 41% of subjects abstained from drinking throughout the 12-week treatment period (Petrakis et al., 2016). This secondary analysis sought to examine whether alcohol abstainer status was associated with changes in PTSD symptom severity. Further, we aimed to examine whether alcohol abstainer status and drug treatment interacted to effect changes in PTSD symptoms over the course of the 12-week clinical trial. We hypothesized that individuals abstaining from alcohol would exhibit lower PTSD symptoms over the course of the clinical trial compared to non-abstainers, and that this effect of alcohol abstinence on PTSD symptoms would be enhanced in individuals receiving prazosin vs. placebo.
Materials and Methods
This was a secondary data analysis from a clinical trial of prazosin for veterans with alcohol dependence and comorbid PTSD (Petrakis et al., 2016).
Participants.
The participants were veterans (n=96) recruited from West Haven, CT and Bedford, MA VAs (for consort table see Petrakis et al., 2016). Participants were recruited from clinicians in the substance abuse and PTSD treatment programs at both sites and by advertisements at the VA facilities and in the communities. After signing written informed consent, volunteers were evaluated and included if they were men or women, ages 21 to 65, met DSM-IV (American Psychiatric Association, 2000) criteria for current alcohol dependence and PTSD, determined by the Structured Clinical Interview for DSM-IV (First et al., 1996), and reported at least 1 episode of heavy drinking (defined as >5 for men and >4 for women on 1 occasion) over the past 14 days. Participants were screened for medical problems by physical and laboratory examination. Females were not pregnant and were using adequate birth control. Exclusion criteria included unstable or current serious psychotic symptoms, suicidal or homicidal ideation, or medical problems that would contraindicate prazosin treatment. Participants could not be taking medications thought to influence alcohol consumption (e.g., naltrexone, disulfiram, acamprosate), but other psychiatric medications were allowed. Participants were required to be abstinent for 2 days prior to randomization. Abstinence was determined by self-report and a negative breathalyzer reading.
Treatment.
Following completion of baseline assessments, 96 subjects were randomized to either prazosin (16 mg/d) or placebo for 12 weeks. Randomization was conducted by the pharmacy using a 1:1 randomization in blocks of 4. Randomization to treatment condition (i.e., 16 mg/d prazosin or placebo) was stratified by site, gender, and psychiatric medication status. Prazosin was titrated upward during the first 2 weeks, starting at 2 mg/d and increasing to 16 mg/d in divided doses. Study medications were dispensed in identical capsules and blister packs. Medication compliance was monitored at every visit for each blister pack. All subjects also received medical management therapy (Pettinati et al., 2004) administered by a training research nurse. Abstinence from drinking was encouraged.
Assessments.
For this secondary data analysis, Timeline Follow Back (TLFB; (Sobell and Sobell, 1992)) and the Clinician Administered PTSD Scale (CAPS) for DSM-IV (Blake et al., 1995) were used to assess measures of alcohol use and PTSD symptoms.
Outcome Measures.
Primary outcome measures were alcohol abstainer status (abstainer vs. non-abstainer) and PTSD severity. TLFB was administered weekly to collect a detailed self-report of daily alcohol use throughout the 84-day treatment period as well as for the 90-day period prior to randomization. Alcohol intake was confirmed using serum gamma-glutamyl transferase (GGT), collected 4 times during the study (baseline, weeks 4, 8, and 12). Participants were categorized as alcohol abstainers if they did not consume any alcohol over the course of the 12-week clinical trial and alcohol non-abstainers if they consumed any alcohol over the course of the 12-week clinical trial. PTSD symptom severity was assessed every 4 weeks by the CAPS. Side effects and common adverse symptoms were evaluated by the research nurse weekly using a modified version of the Systematic Assessment for Treatment Emergent Events (SAFTEE; (Levine and Schooler, 1986)) and are reported elsewhere (Petrakis et al., 2016).
Data Analysis.
Data analyses were conducted using a similar approach as the parent publication (Petrakis et al., 2016). All analyses were performed on the intent-to-treat sample with a 2-tailed alpha level of 0.05 using the 24.0 version of SPSS (IBM Corporation, Armonk, NY). The outcome variable was PTSD symptoms (CAPS total scores and CAPS subscales). Mixed effects models were used to assess changes in PTSD symptoms. We selected the Toeplitz covariance structure based on Schwartz-Bayesian information criterion. Alcohol abstainer status (abstainer vs. non-abstainer; coded as abstainer=0, non-abstainer=1) and medication (prazosin vs. placebo; coded as placebo=0, prazosin=1) were between-subject factors in the models, and time (baseline [before medication administration], weeks 1, 4, 8, and 12) was used as a within-subject factor.
Results
Baseline Characteristics.
Demographic variables are reported in detail in the parent study (Petrakis et al., 2016). Briefly, the sample was primarily male (93.7%), Caucasian (82.1%), separated/divorced (45.3%), and 43.98 (SD=12.96) years old. Participants exhibited severe PTSD symptoms (CAPS mean=73.71, SD=17.86) and were heavy drinkers (mean=19.47 drinks per drinking day, SD=12.13). There were no significant differences between prazosin vs. placebo on any demographic variable (Petrakis et al., 2016). Additionally, there were no significant differences between alcohol abstainers vs. non-abstainers on any demographic variable (see Table 1). There were n=23 and n=16 alcohol abstainers and n=27 and n=30 alcohol non-abstainers in the prazosin- and placebo-treated groups, respectively. A chi-square comparison across group (abstainers vs. non-abstainers) revealed no significant differences in the number of individuals assigned to prazosin vs. placebo (p=0.26). Among non-abstainers, the range of the number of drinking days during treatment was 1 – 80 (mean=17.85, SD=20.48). The number of drinking days was not significantly correlated with mean total CAPS score at baseline or weeks 1, 4, 8, and 12.
Table 1.
Baseline demographic, PTSD#, and drinking characteristics of alcohol abstainers vs. non-abstainers over the course of a 12-week clinical trial of prazosin for veterans with PTSD# and comorbid alcohol dependence.
| Variables | Abstainers | Non-abstainers | χ2 | p |
|---|---|---|---|---|
| Demographic (n, %) | ||||
| Age | 0.95 | 0.62 | ||
| 21-29 | 11 (50.0) | 11 (50.0) | ||
| 30-44 | 8 (38.1) | 13 (61.9) | ||
| 45+ | 20 (38.5) | 32 (61.5) | ||
| Gender | 0.21 | 0.65 | ||
| Male | 36 (40.4) | 53 (59.6) | ||
| Female | 3 (50.0) | 3 (50.0) | ||
| Ethnicity | 5.43 | 0.07 | ||
| Caucasian | 35 (44.9) | 43 (55.1) | ||
| African American | 2 (14.3) | 12 (85.7) | ||
| Other | 2 (66.7) | 1 (33.3) | ||
| Marital status | 0.76 | 0.86 | ||
| Single | 12 (41.4) | 17 (58.6) | ||
| Married/cohabitating/partner | 7 (33.3) | 14 (66.7) | ||
| Separated/divorced | 19 (44.2) | 24 (55.8) | ||
| Widowed | 1 (50.0) | 1 (50.0) | ||
| Medication condition | 1.25 | 0.26 | ||
| Prazosin | 23 (46.0) | 27 (54.0) | ||
| Placebo | 16 (34.8) | 30 (65.2) | ||
| PTSD# and drinking (mean, SD#) | ||||
| CAPS# | ||||
| Severity of PTSD# symptoms | 74.28 (20.64) | 73.30 (15.75) | 47.55 | 0.65 |
| Avoidance | 30.44 (8.62) | 30.44 (8.04) | 29.99 | 0.36 |
| Reexperience | 22.44 (8.42) | 18.84 (6.97) | 32.41 | 0.30 |
| Hyperarousal | 21.41 (7.21) | 23.69 (6.38) | 23.67 | 0.65 |
| Alcohol consumption | ||||
| Number of drinking days (over 90 days) | 42.38 (30.17) | 47.11 (27.95) | 54.34 | 0.42 |
| Number of heavy drinking days (over 90 days) | 39.05 (29.67) | 41.43 (28.19) | 55.55 | 0.45 |
| Number of drinks per drinking day | 22.54 (12.25) | 17.30 (11.66) | 94.00 | 0.42 |
| Percent drinking days | 43.39 (32.97) | 46.03 (31.32) | 55.55 | 0.45 |
Note:
PTSD=posttraumatic stress disorder, SD=standard deviation, CAPS=clinician administered PTSD scale; chi-square comparisons across group (abstainers vs. non-abstainers) revealed no significant differences in baseline demographic, PTSD, or drinking characteristics.
PTSD Symptoms.
Analyses were performed using time, medication, and alcohol abstainer status in the model. The analysis of CAPS total scores demonstrated a significant main effect of time on symptoms of PTSD (F(4, 146.17)=43.29, p<0.0001), such that PTSD symptoms decreased over the course of the clinical trial (baseline mean=74.49, SE=2.40; week 12 mean=40.79, SE=2.80). There was a significant main effect of alcohol abstainer status on symptoms of PTSD (F(1, 91.79)=5.21, p=0.03), such that non-abstainers had lower total CAPS scores than abstainers overall (non-abstainer mean=47.16, SE=2.45; abstainer mean=55.86, SE=2.92). There was also a significant treatment by alcohol abstainer status interaction (F(1, 91.79)=6.32, p=0.01); specifically, among placebo-treated individuals, those who did not abstain from alcohol had lower CAPS scores overall compared to alcohol abstainers (see Figure 1). Within the prazosin-treated group, abstainers vs. non-abstainers did not differ on total CAPS scores. A time by treatment by alcohol abstainer status three-way interaction was not significant (F(4, 146.17)=0.32, p=0.87; see Figure 2). Results were similar for the avoidance (F(1, 97.69)=5.33, p=0.02), reexperiencing (F(1, 86.80)=6.78, p=0.01), and hyperarousal (F(1, 91.63)=4.33, p=0.04) subscales, such that placebo-treated non-abstainers had lower CAPS scores overall (see Figure 3).
Figure 1.
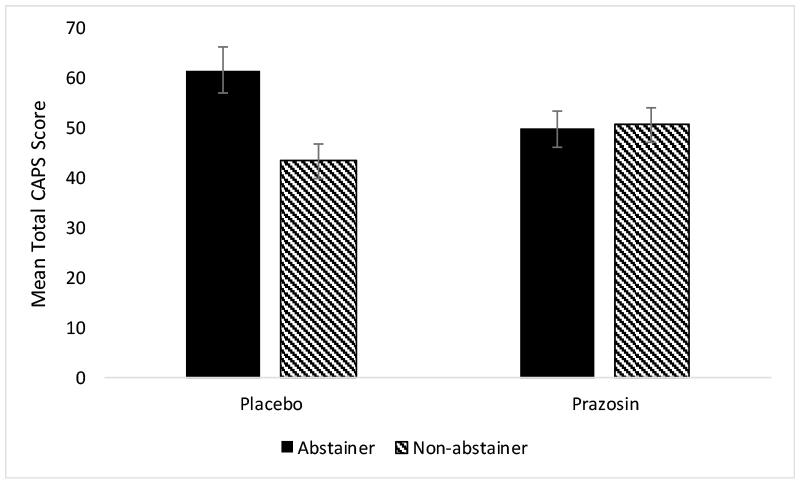
Mean (± SE) total CAPS scores by treatment by alcohol abstainer status.
Figure 2.
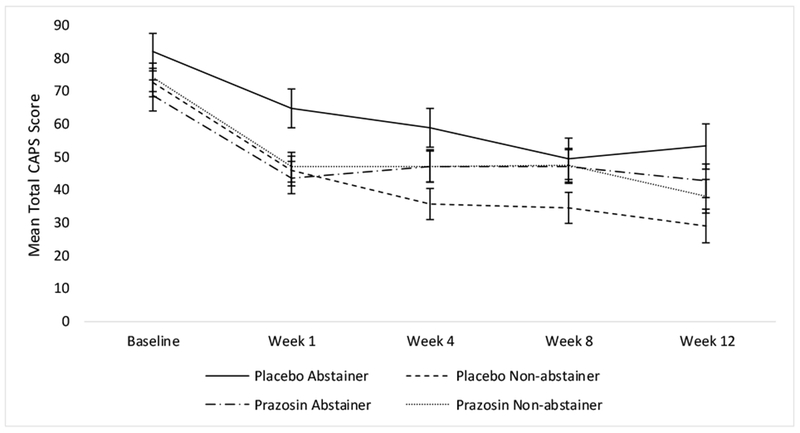
Mean (± SE) total CAPS scores by time by treatment by alcohol abstainer status.
Figure 3.
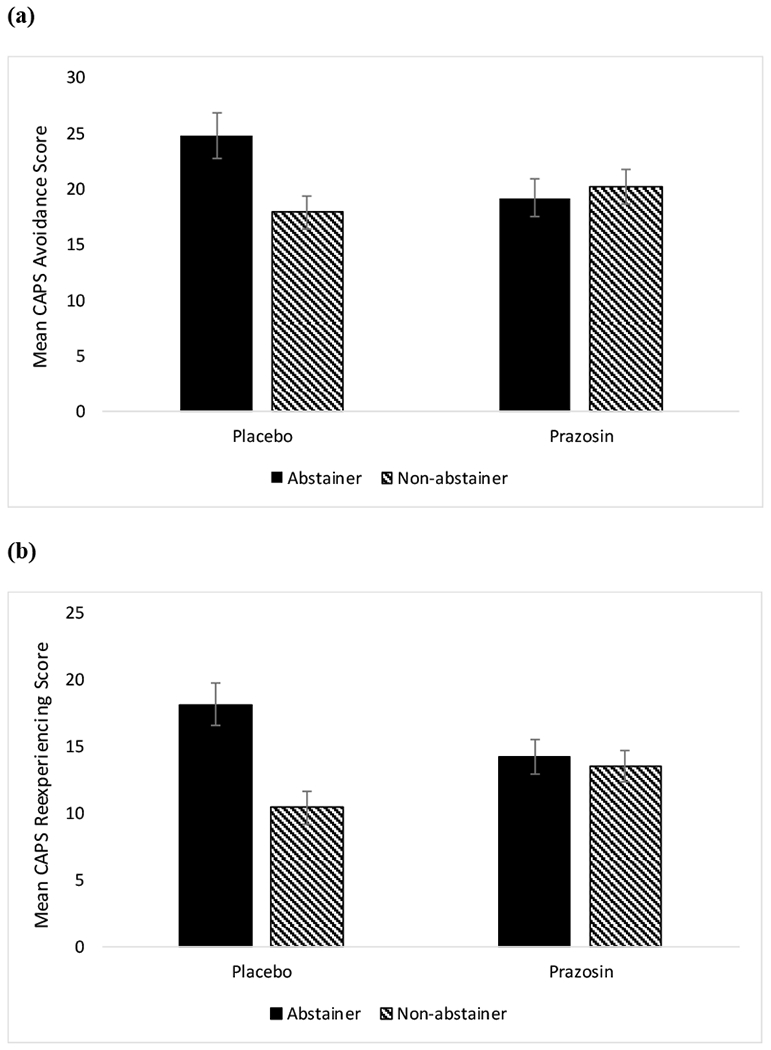
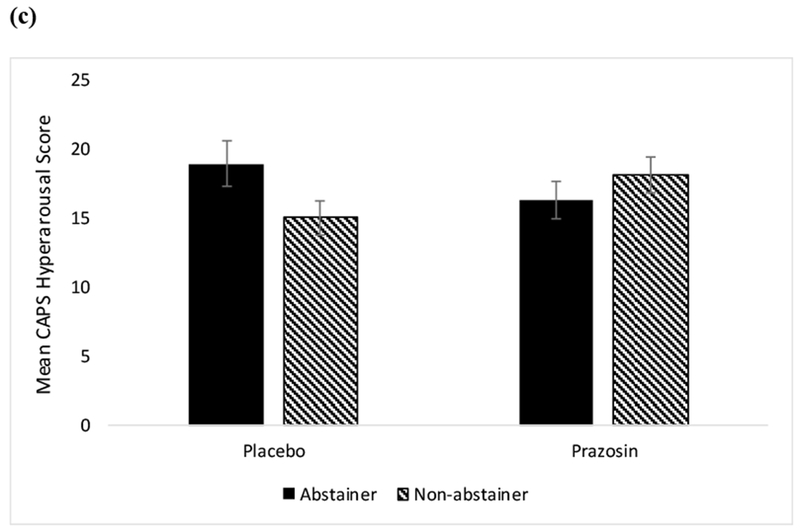
(a) Mean (± SE) CAPS scores on the avoidance subscale by alcohol abstainer status by treatment; (b) Mean (± SE) CAPS scores on the reexperiencing subscale by alcohol abstainer status by treatment; (c) Mean (± SE) CAPS scores on the hyperarousal subscale by alcohol abstainer status by treatment.
Discussion
Findings from this secondary data analysis suggest that (1) while there was an overall effect of abstinence on symptoms of PTSD such that subjects with complete abstinence had greater symptoms of PTSD compared to non-abstainers and (2) a medication by abstainer status interaction showed that placebo-treated alcohol non-abstainers had significantly lower PTSD symptoms. Taken together, these results suggest that those who continued to use alcohol during the clinical trial had attenuated PTSD symptoms. This result was consistent across the avoidance, reexperiencing, and hyperarousal subscales.
Several hypotheses have been put forth to understand the relationship between PTSD and AUD; these have implications for both the development of comorbid conditions and can affect treatment planning. For example, there has been some support for the self-medication hypothesis regarding AUD with comorbid PTSD (Baker et al., 2004, Khantzian, 2003); if individuals drink to medicate symptoms of PTSD, addressing PTSD symptoms early in recovery may improve the ability to abstain from alcohol. A recent study found that elevated PTSD symptoms predicted greater alcohol use on the same day and following day but drinking did not predict PTSD symptoms on the following day (Simpson et al., 2014). Similarly, PTSD symptom fluctuations were associated with the presence of alcohol and cocaine dependence symptoms in individuals with comorbid substance use disorder and PTSD (Ouimette et al., 2010). Our results were unexpected and demonstrated that alcohol non-abstainers had better PTSD outcomes than alcohol-abstainers. Further, although there was no overall effect of prazosin in our study, there was a medication by abstinence interaction. It is possible that among placebo-treated individuals, alcohol was used to manage PTSD symptoms, while among those receiving prazosin, alcohol consumption did not further alleviate symptoms as prazosin might have protected against stress or other PTSD symptoms. It is unlikely that this finding is due to pre-treatment differences as there was no significant difference in PTSD symptoms amongst abstainers and non-abstainers in the placebo group at baseline. Conversely, those who abstained from alcohol may have had an increase in alcohol withdrawal symptoms, which may mimic and/or exacerbate symptoms of PTSD; albeit, PTSD symptoms declined over the course of the clinical trial in both alcohol abstainers and non-abstainers.
It should also be noted that while all subjects were encouraged to decrease drinking, we did not assess individual treatment goals about drinking at the initiation of this clinical trial. There has been some evidence that individuals whose goal is abstinence from alcohol during prazosin treatment exhibit reductions in alcohol craving and negative affect beyond that of individuals who continued to use alcohol during treatment (Hallgren et al., 2018). Thus, it is possible that treatment goals and expectancies in this clinical trial may have influenced our primary outcome in this sample of alcohol abstainers and non-abstainers.
In the parent study to the present investigation, prazosin did not differ from placebo regarding alcohol consumption or PTSD symptoms in veterans with alcohol dependence and comorbid PTSD; although both outcomes decreased over the course of the clinical trial (Petrakis et al., 2016). This contrasts with a pilot trial demonstrating that prazosin reduced percent drinking days per week and percent heavy drinking days per week in individuals with comorbid alcohol dependence and PTSD but did not attenuate PTSD symptoms (Simpson et al., 2015). Our results are also inconsistent with studies finding prazosin to be effective for a singular diagnosis of either AUD or PTSD alone (Fox et al., 2012, Simpson et al., 2009, Peskind et al., 2003, Raskind et al., 2000, Raskind et al., 2007, Raskind et al., 2003, Raskind et al., 2013, Raskind et al., 2002). Individuals with comorbid AUD and PTSD often experience poorer treatment outcomes (McCarthy and Petrakis, 2010); thus, it is also possible that prazosin may not be efficacious in the presence of this comorbidity.
This secondary data analysis is not without limitations. First, our sample size was relatively small, primarily male, and consisted only of veterans with alcohol dependence and comorbid PTSD. These findings may not generalize to females or individuals with this comorbidity in the general population. Second, we collected data on PTSD symptoms at intervals, so we did not capture the interaction between PTSD symptoms and drinking in real time. Third, while individuals enrolled in this study could not be taking medications thought to influence drinking, they could take other psychiatric medications, which may have influenced symptoms of PTSD and possibly confounded our results. Finally, as addressed by Petrakis and colleagues, a high number of participants were in sober housing and those individuals exhibited better outcomes, and there were significant site differences on drinking outcomes in the parent study (Petrakis et al., 2016). These may have obscured or confounded results.
Nevertheless, understanding the relationship between PTSD symptoms is of great clinical importance. In the future, studies which evaluate this relationship in real time, perhaps using techniques such as Ecological Momentary Assessment (EMA), has potential to be of great value. Findings from studies such as this and others have implications for how clinicians treat comorbid AUD and PTSD, especially if individuals may be drinking to alleviate their PTSD symptoms.
Acknowledgments
Funding: This work was supported by NIH grants R01AA010107 (Petrakis) and K01AA025670 (Verplaetse), and the MIRECC (VISN I Mental Illness Research Education and Clinical Center).
Footnotes
Declaration of Conflicting Interests
Ismene L. Petrakis has consulted with Alkermes. All other authors declare that they have no conflicts of interest.
References
- American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders: DSM-IV-TR, American Psychiatric Association, Washington, DC. [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC (2004) Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychological review 111:33. [DOI] [PubMed] [Google Scholar]
- Blake D, Weathers F, Nagy L, Kaloupek D, Gusman F, Charney D, Keane T (1995) The development of a Clinician-Administered PTSD Scale. J Trauma Stress 8:75–90. [DOI] [PubMed] [Google Scholar]
- Blanco C, Xu Y, Brady K, Pérez-Fuentes G, Okuda M, Wang S (2013) Comorbidity of posttraumatic stress disorder with alcohol dependence among US adults: results from National Epidemiological Survey on Alcohol and Related Conditions. Drug and alcohol dependence 132:630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SF, Schumacher JA, Brady KT, Cotton BD (2007) Changes in PTSD symptomatology during acute and protracted alcohol and cocaine abstinence. Drug and alcohol dependence 87:241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Gibbon M, Spitzer R, Williams J (1996) User’s guide for the structured clinical interview for DSM-IV axis I Disorders—Research version. New York: Biometrics Research Department, New York State Psychiatric Institute. [Google Scholar]
- Fox HC, Anderson GM, Tuit K, Hansen J, Kimmerling A, Siedlarz KM, Morgan PT, Sinha R (2012) Prazosin effects on stress-and cue-induced craving and stress response in alcohol-dependent individuals: preliminary findings. Alcoholism: Clinical and Experimental Research 36:351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JC, Hausauer B, Fischer S, Wise B, Rasmussen DD (2015) Prazosin reduces alcohol intake in an animal model of alcohol relapse. Alcoholism: Clinical and Experimental Research 39:1538–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JC, Hausauer BJ, Federoff DL, Fischer SM, Rasmussen DD (2013a) Prazosin reduces alcohol drinking throughout prolonged treatment and blocks the initiation of drinking in rats selectively bred for high alcohol intake. Alcoholism: Clinical and Experimental Research 37:1552–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JC, Hausauer BJ, Rasmussen DD (2013b) Combining naltrexone and prazosin in a single oral medication decreases alcohol drinking more effectively than does either drug alone. Alcoholism: Clinical and Experimental Research 37:1763–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Coen K, Tamadon S, Li Z, Loughlin A, Lê A (2016) Effects of prazosin and doxazosin on yohimbine-induced reinstatement of alcohol seeking in rats. Psychopharmacology 233:2197–2207. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS (2015) Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 72:757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren KA, Delker BC, Simpson TL (2018) Effects of initiating abstinence from alcohol on daily craving and negative affect: results from a pharmacotherapy clinical trial. Alcoholism: Clinical and Experimental Research 42:634–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khantzian EJ (2003) The self-medication hypothesis revisited: The dually diagnosed patient. Primary Psychiatry 10:47–54. [Google Scholar]
- Le A, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C, Shaham Y (2011) Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology 218:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J, Schooler N (1986) SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacology bulletin 22:343. [PubMed] [Google Scholar]
- McCarthy E, Petrakis I (2010) Epidemiology and management of alcohol dependence in individuals with post-traumatic stress disorder. CNS drugs 24:997–1007. [DOI] [PubMed] [Google Scholar]
- Norman SB, Myers US, Wilkins KC, Goldsmith AA, Hristova V, Huang Z, McCullough KC, Robinson SK (2012) Review of biological mechanisms and pharmacological treatments of comorbid PTSD and substance use disorder. Neuropharmacology 62:542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimette P, Read JP, Wade M, Tirone V (2010) Modeling associations between posttraumatic stress symptoms and substance use. Addict Behav 35:64–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskind ER, Bonner LT, Hoff DJ, Raskind MA (2003) Prazosin reduces trauma-related nightmares in older men with chronic posttraumatic stress disorder. Journal of geriatric psychiatry and neurology 16:165–171. [DOI] [PubMed] [Google Scholar]
- Petrakis I, Desai N, Gueorguieva R, Arias A, O’Brien E, Jane J, Sevarino K, Southwick S, Ralevski E (2016) Prazosin for Veterans with Posttraumatic Stress Disorder and Comorbid Alcohol Dependence: A Clinical Trial. Alcohol Clin Exp Res 40:178–186. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Weiss RD, Miller WR, Donovan D, Ernst DB, Rounsaville BJ, Series CM, Mattson ME (2004) Medical Management Treatment Manual A Clinical Research Guide for Medically Trained Clinicians Providing Pharmacotherapy as Part of the Treatment for Alcohol Dependence. COMBINE Monograph Series 2. [Google Scholar]
- Pietrzak RH, Goldstein RB, Southwick SM, Grant BF (2011) Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of anxiety disorders 25:456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskind MA, Dobie DJ, Kanter ED, Petrie EC, Thompson CE, Peskind ER (2000) The alpha1-adrenergic antagonist prazosin ameliorates combat trauma nightmares in veterans with posttraumatic stress disorder: a report of 4 cases. The Journal of clinical psychiatry 61:129–133. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Chow B, Harris C, Davis-Karim A, Holmes HA, Hart KL, McFall M, Mellman TA, Reist C (2018) Trial of prazosin for post-traumatic stress disorder in military veterans. New England Journal of Medicine 378:507–517. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Hoff DJ, Hart KL, Holmes HA, Warren D, Shofer J, O’Connell J, Taylor F, Gross C (2007) A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biological psychiatry 61:928–934. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Kanter ED, Petrie EC, Radant A, Thompson CE, Dobie DJ, Hoff D, Rein RJ, Straits-Tröster K (2003) Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. American Journal of Psychiatry 160:371–373. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peterson K, Williams T, Hoff DJ, Hart K, Holmes H, Homas D, Hill J, Daniels C, Calohan J (2013) A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. American Journal of Psychiatry 170:1003–1010. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Thompson C, Petrie EC, Dobie DJ, Rein RJ, Hoff DJ, McFall ME, Peskind ER (2002) Prazosin reduces nightmares in combat veterans with posttraumatic stress disorder. The Journal of clinical psychiatry 63:565–568. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Alexander LL, Raskind MA, Froehlich JC (2009) The α1-Adrenergic Receptor Antagonist, Prazosin, Reduces Alcohol Drinking in Alcohol-Preferring (P) Rats. Alcoholism: Clinical and Experimental Research 33:264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Beckwith LE, Kincaid CL, Froehlich JC (2014) Combining the α1-Adrenergic Receptor Antagonist, Prazosin, with the β-Adrenergic Receptor Antagonist, Propranolol, Reduces Alcohol Drinking More Effectively Than Either Drug Alone. Alcoholism: Clinical and Experimental Research 38:1532–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson TL, Malte CA, Dietel B, Tell D, Pocock I, Lyons R, Varon D, Raskind M, Saxon AJ (2015) A pilot trial of prazosin, an alpha-1 adrenergic antagonist, for comorbid alcohol dependence and posttraumatic stress disorder. Alcoholism: Clinical and Experimental Research 39:808–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson TL, Saxon AJ, Meredith CW, Malte CA, McBride B, Ferguson LC, Gross CA, Hart KL, Raskind M (2009) A pilot trial of the alpha-1 adrenergic antagonist, prazosin, for alcohol dependence. Alcoholism: Clinical and Experimental Research 33:255–263. [DOI] [PubMed] [Google Scholar]
- Simpson TL, Stappenbeck CA, Luterek JA, Lehavot K, Kaysen DL (2014) Drinking motives moderate daily relationships between PTSD symptoms and alcohol use. Journal of Abnormal Psychology 123:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelly MJ, Weiner JL (2014) Chronic treatment with prazosin or duloxetine lessens concurrent anxiety-like behavior and alcohol intake: evidence of disrupted noradrenergic signaling in anxiety-related alcohol use. Brain and behavior 4:468–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Goldstein RB, Grant BF (2016) The association between post-traumatic stress disorder and lifetime DSM-5 psychiatric disorders among veterans: Data from the National Epidemiologic Survey on Alcohol and Related Conditions-III (NESARC-III). Journal of psychiatric research 82:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB (1992) Timeline follow-back, in Measuring alcohol consumption, Measuring alcohol consumption, pp 41–72, Springer. [Google Scholar]
- Verplaetse TL, Czachowski CL (2015) Low-dose prazosin alone and in combination with propranolol or naltrexone: effects on ethanol and sucrose seeking and self-administration in the P rat. Psychopharmacology 232:2647–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse TL, Rasmussen DD, Froehlich JC, Czachowski CL (2012) Effects of Prazosin, an α1-Adrenergic Receptor Antagonist, on the Seeking and Intake of Alcohol and Sucrose in Alcohol-Preferring (P) Rats. Alcoholism: Clinical and Experimental Research 36:881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CE, Tonigan JS, Bogenschutz MP, Clifford J, Bigelow R, Simpson T (2018) A Randomized, Placebo-controlled, Clinical Trial of Prazosin for the Treatment of Alcohol Use Disorder. J Addict Med. [DOI] [PMC free article] [PubMed] [Google Scholar]


