Abstract
Background:
Alcohol use disorders (AUDs) are highly prevalent in persons living with HIV (PLWH) and are associated with increased HIV risk behaviors, suboptimal treatment adherence, potential interaction with medication pharmacodynamics, and greater risk for disease progression. Preclinical studies show that chronic binge alcohol administration accelerates disease progression and aggravates pathogenesis in the simian immunodeficiency virus (SIV)-infected rhesus macaque model despite viral suppression by antiretroviral therapy.
Methods:
To translate preclinical findings in the rhesus macaque model of chronic binge alcohol administration and SIV infection and to address areas of uncertainty surrounding the biological mechanisms and socioenvironmental modifiers that contribute to the relationship between alcohol use and HIV-associated comorbidities, precocious aging, and disease progression, we designed a translational multi-project, longitudinal, cohort study, the New Orleans Alcohol Use in HIV [NOAH] Study. The NOAH Study is led by a multidisciplinary team of scientists, with a research focus on the interaction of AUD and HIV. The overarching hypothesis is that alcohol use will lead to adverse health outcomes in PLWH. In this report, we describe the study design and baseline descriptive characteristics of our cohort.
Results:
Three hundred sixty-five participants completed the baseline testing. The cohort is predominantly male (69%) and African American (83.5%). The majority of participants report incomes below 200% of the federal poverty level. CD4 counts less than 200 cells/μL were found in 12.8% and viral loads less than 50 copies/mL were found in 73.6%. These HIV status variables did not differ based upon alcohol use.
Conclusions:
The NOAH Study facilitates bidirectional translational investigation of alcohol’s impact on PLWH. Translation of preclinical findings to PLWH permits confirmation of basic biological mechanisms in humans and also allows incorporation of sociobehavioral factors that may affect biology but are challenging to replicate in preclinical models.
Keywords: alcohol, HIV, frailty, immunosenescence, comorbidities
Introduction
Alcohol use disorders (AUDs) are increasingly common, particularly in marginalized populations (Grant BF et al., 2017). Further, people living with Human Immunodeficiency Virus (PLWH) are especially vulnerable to the negative health consequences of alcohol misuse, even in the era of effective combined antiretroviral therapy (cART) (Azar MM et al., 2010). At risk alcohol use is tightly linked to HIV acquisition through high risk behaviors. In addition, alcohol consumption affects every stage of the HIV Care Continuum; (Valdiserri RO, Holtgrave DR, 2018) delays in diagnosis, low engagement and retention in medical care, poor adherence to treatment, and failure to suppress circulating virus are associated with harmful alcohol use.
Effective cART has prolonged survival and shifted the demographics of PLWH. Mean ages of PLWH have steadily risen with presently more than half of PLWH in the developed world older than 50 years of age. These demographic shifts are largely driven by a marked decrease in death due to AIDS-related infections and cancers. An abundance of data suggests that HIV infection leads to precocious aging, geriatric comorbidities, and frailty (Gutierrez AD, Balasubramanyam A, 2012). Increased prevalence of cancers, chronic liver disease, osteoporosis, cardiovascular disease, diabetes, and metabolic dysregulation have been widely reported in PLWH. HIV is also associated with geriatric syndromes including polypharmacy, physical and cognitive impairment, and frailty (Kolter DP, 2003; Guaraldi G et al., 2011; Gutierrez AD, Balasubramanyam A, 2012; Piggott DA et al., 2013; Williams EC et al., 2016; Molina PE et al., 2018).
The factors contributing to the early onset of comorbidity and aging in PLWH are incompletely understood and many mechanisms have been proposed. Social, environmental, and behavioral factors appear to be primary drivers of adverse health effects. However, preclinical and translational evidence indicate that alcohol also dysregulates biological pathways, particularly immune and metabolic systems (Gutierrez AD, Balasubramanyam A, 2012; Klatt NR et al., 2013; Molina PE et al., 2018). Alcohol misuse is well recognized to promote chronic inflammation. Immune system activation and a chronic pro-inflammatory state promote HIV disease progression and comorbidity pathogenesis (Molina PE et al., 2018). These factors are central to biological aging and to increased risk of comorbidities in PLWH.
The impact of alcohol on viral suppression and CD4 T cell counts is predominantly linked to poor cART adherence. However, the detrimental effects of AUD on HIV-associated comorbidities may depend on direct perturbation of biological pathways. Clearly, in the context of circulating virus, HIV antigens overshadow most other immune drivers. The contribution of alcohol use to the pathogenesis of chronic, HIV-linked comorbidities becomes an increasingly important factor as the HIV epidemic shifts from an acute fulminant immunodeficiency illness toward a chronic medical condition.
Studies from our group at the Louisiana State University Health Sciences Center - New Orleans (LSUHSC-NO) National Institute on Alcohol Abuse and Alcoholism (NIAAA)-funded Comprehensive Alcohol-HIV/AIDS Research Center (CARC) have advanced the understanding of the impact of chronic binge alcohol administration on disease progression using the simian immunodeficiency virus (SIV)-infected rhesus macaque model (Molina PE et al., 2008; Katz PS et al., 2015; Molina PE et al., 2018). Our preclinical data show that clinical outcomes are worse in alcohol-administered SIV-infected macaques despite viral suppression by cART. This raises the question whether alcohol-induced inflammation mediates comorbidity pathogenesis and precocious aging in PLWH when viral loads are suppressed.
The New Orleans Alcohol Use in HIV [NOAH] Study, facilitates a bidirectional translational investigation of alcohol’s impact on PLWH. The NOAH Study aims to address areas of uncertainty surrounding the biological mechanisms and socioenvironmental modifiers that contribute to the relationship between alcohol use and HIV-associated comorbidities, precocious aging, and disease progression. This report details the study design and baseline descriptive characteristics of the NOAH cohort. The NOAH cohort data collection was designed with sufficient flexibility to support additional projects over time; however, the initial scheme focused upon three major research themes.
Alcohol & Metabolic Dysregulation in PLWH
Mechanistically, we hypothesize that chronic inflammation, accentuated by alcohol consumption and biological aging, is a significant underlying mechanism disrupting metabolic homeostasis. Thus, the role of alcohol consumption on (a) the incidence of decreased lean body mass and muscle strength, (b) lipodystrophy (fat redistribution, altered lipid profile), and (c) prediabetes (fasting plasma glucose 100 to 125 mg/dl, and glycosylated hemoglobin 5.7% to 6.4%) will be examined in PLWH.
Metabolic alterations, particularly defects related to glucose and lipid metabolism, lead to a higher risk for insulin resistance manifested as an increased frequency of glucose intolerance or diabetes mellitus in PLWH. The mechanisms underlying dysglycemia in PLWH have remained poorly understood (Gutierrez AD, Balasubramanyam A, 2012). However, chronic subclinical inflammation is thought to contribute to adipose tissue dysregulation and metabolic derangements seen in ART-treated PLWH (Kolter DP, 2003). Our preclinical studies in SIV-infected non-human primates have shown that chronic binge alcohol administration accentuates metabolic derangements, including end-stage disease wasting that is associated with localized skeletal muscle inflammation, profound depletion of antioxidant capacity (oxidative stress), increased proteasomal activity, and decreased myoblast differentiation potential (Lecapitaine NJ et al., 2011; Simon L et al., 2014). We predict this translates into decreased metabolic homeostasis in the clinical setting.
Alcohol Use Disorder & Aging in HIV: The Role of the Microbiota & Inflamm-Aging
Alcohol misuse hastens aging in the general population, but its role in aging and frailty in PLWH remains unknown. Some reports have suggested a detrimental effect of substance misuse on frailty in HIV-infected patients (Piggott DA et al., 2013). The term “inflamm-aging” connotes a central role for immune activation caused by chronic antigenic stress as the origin of immune cell senescence and exhaustion characteristic of aging (De Martinis M et al., 2005). In this model, antigenic burden drives naïve T cells toward clonal expansion resulting in a restricted functional capacity. The intestinal tract is a major target for early HIV infection, viral expansion, and CD4+ T cell destruction. It has also become clear that loss of mucosal integrity, accompanied by microbial product translocation into the systemic circulation, is associated with HIV infection, disease progression, and mortality (Klatt NR et al., 2013).
The effects of AUD on the alimentary tract microbiota in PLWH by phylogenetic analysis of ribosomal DNA sequences from oral and stool samples will be investigated. Moreover, the role of alcohol in escalating immune cell activation and senescence in PLWH and the contribution of alimentary tract microbial community structure as an intermediary in this relationship will be examined. Finally, we will examine the association between alcohol use and validated indices of aging and frailty including the deficit index and phenotypic frailty (Fried LP et al., 2001; Searle SD et al., 2008), along with telomere length as a biomarker of cellular aging.
Lifetime and Current Stress and Allostatic Load in PLWH
Among PLWH, higher levels of both psychosocial and physiological stress have been associated with poorer immune status, increased viral load over time, faster disease progression, and higher rates of mortality (Leserman J et al., 2000; Ironson G et al., 2006). Chronic psychological stress has been implicated in precocious aging and allostatic load – the cumulative wear and tear on physiological systems and organs due to chronic stress (Geronimus A et al., 2006).
The overall goal of this component is to examine the impact of both chronic psychological stress and early life adversity on behavioral and biological outcomes among men and women living with HIV. Alcohol use, allostatic load, mental health, medication adherence, retention in care, and viral suppression will be examined in relation to chronic psychological stress and early life adversity overall and how these may differ by sex in PLWH. The mediating role of alcohol use between chronic psychological stress and early life adversity and biological, mental health, and clinical outcomes will be determined using the NOAH cohort.
Materials and Methods
Overall Design
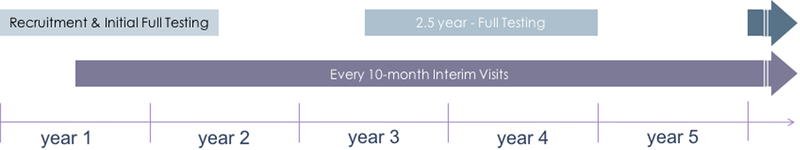
The NOAH Study aims to address areas of uncertainty surrounding the biological mechanisms and socioenvironmental modifiers that contribute to the relationship between alcohol use and HIV-associated comorbidities, precocious aging, and disease progression. Enrolled participants complete a battery of behavioral, demographic, and functional assessments at baseline and at 2.5 years with interim limited assessment visits every 10 months (Figure 1).
Figure 1:
NOAH Study Design Overview
Study Population
A clinic-based sample of 365 HIV-infected patients under care in the Greater New Orleans, Louisiana metropolitan area, aged ≥18 years were enrolled in the full study after screening 655 subjects. Patients with potentially hazardous alcohol use as defined by Alcohol Use Disorders Identification Test (AUDIT) ≥ 8 were oversampled. Exclusion criteria included an acute illness within the last 6 weeks as defined by unscheduled healthcare utilization for a new or exacerbated illness, non-prophylaxis prescription of antibiotics, or pregnancy. Excluded patients were eligible for enrollment after resolution of the acute phase of identified illness.
Data Collection and Analysis
LSUHSC-NO’s Human Research Protection Program and Institutional Review Board approved the study and maintains oversight of research activities. Biospecimen samples collected include a fasting blood sample, gargles, buccal swabs, vaginal swabs, and fecal samples. Anthropometric measurements, body composition estimates by air displacement plethysmography, blood pressure, oral examination, and clinical laboratory tests are also performed. Data from interviewer-administered questionnaires covering alcohol use, socioenvironmental status, psychosocial stress, medical history, physical and cognitive function, and health behaviors are supplemented by a electronic health record abstraction. Additional details are provided in Supplemental Materials.
A strength of the NOAH Study is the collection of data for a multi-dimensional characterization of alcohol exposure for the cohort (Supplemental Table S1). Alcohol exposures and consequences are categorized by drinking status, drinking patterns, quantity-frequency of alcohol consumption, alcohol use disorder, and addiction severity, as well as by a biomarker of recent alcohol use. Other substance use (cannabis, cocaine, opioids, etc.) is assessed, as it is likely to have significant modifying effects on alcohol-related biomedical outcomes.
Sample size (N=350) was estimated to allow testing the hypotheses described under the three main research themes. Our sample size is inflated by 20% to account for dropouts and lost to follow-up.
Results
The baseline enrollment and exam/testing of the NOAH cohort was completed and descriptive statistics characterizing the cohort are provided in Table 1. A total of 655 participants completed the screening visit. Of these, 430 were invited to continue to full testing and a total of 365 participants (85%) attended the baseline testing. Unadjusted, descriptive statistics for the initial testing visits reveal that our cohort is predominantly non-Hispanic black, majority male, and middle age. Sex was not a consideration in our inclusion or exclusion criteria. The frequency of women in our cohort mirrors the clinic’s demographics [32% female] in the low alcohol consumers but is lower in participants with potentially hazardous drinking. The majority also have annual incomes less than $20,000 and a high school degree or less. A substantial proportion are current smokers. Compliance with the study protocol was high as illustrated in Supplemental Table S2.
Table 1. Distribution of Research Participants by Alcohol Use Disorders Identification Test (AUDIT) and Demographic Parameters.
Significant differences are shown for those characteristics that differed between participants with AUDIT <8 and AUDIT ≥8. *1 participant was transgender. Data shown as total numbers and percentage in parenthesis.
| AUDIT | |||
|---|---|---|---|
| All | <8 | ≥8 | |
| (n=365) | (n=218) | (n=147) | |
| % (n) | %(n) | % (n) | |
| Sex | |||
| Female | 31.0 (113) | 36.7 (80) | 23.1 (34) |
| Male | 69.0 (252) | 63.3(138) | 76.9 (113) |
| p-value | 0.0062 | ||
| Race | |||
| African American | 83.6 (305) | 82.6 (180) | 85.0 (125) |
| White | 15.6 (57) | 16.1 (35) | 15.0 (22) |
| Other | 0.8 (3) | 1.4 (3) | 0 |
| p-value | 0.7912 | ||
| Age, years | |||
| 20–29 | 5.2 (19) | 5.5 (12) | 4.8 (7) |
| 30–39 | 16.4 (60) | 16.1 (35) | 17.0 (25) |
| 40–49 | 24.1 (88) | 22.9 (50) | 25.8 (38) |
| 50–59 | 41.6 (152) | 41.3 (90) | 42.2 (62) |
| 60+ | 12.6 (46) | 14.3 (31) | 10.2 (15) |
| p-value | 0.5361 | ||
| Housing | |||
| Single Family Dwelling | 84.1 (307) | 84.4 (184) | 83.7 (123) |
| HIV-Specific Group Facility | 10.7 (39) | 11.0 (24) | 10.2 (15) |
| Homeless/Shelter | 4.4 (16) | 3.2 (7) | 6.1 (9) |
| Group Home | 0.8 (3) | 1.4 (3) | 0 |
| p-value | 0.5219 | ||
| Income | |||
| < $20,000 | 88.7 (323) | 88.1 (192) | 89.1 (131) |
| $20,000-$39,999 | 8.5 (31) | 9.2 (20) | 7.5 (11) |
| $40,000+ | 2.7 (10) | 2.3 (5) | 3.4 (5) |
| p-value | 0.9180 | ||
| Education | |||
| < High School | 40.8 (149) | 35.8 (78) | 48.3 (71) |
| High School Graduate | 31.2 (114) | 33.5 (73) | 27.9 (41) |
|
Some College, Junior College, Community
College, Vocational/Trade School |
22.2 (81) | 24.8 (54) | 18.4 (27) |
| 4-Year College Graduate | 3.8 (14) | 3.7 (8) | 4.1 (6) |
| Graduate/Professional School | 1.9 (7) | 2.3 (5) | 1.4 (2) |
| p-value | 0.0449 | ||
| BMI (n=362) | |||
| < 18.5 | 4.4 (16) | 4.2 (9) | 4.8 (7) |
| 18.5–24.9 | 38.4 (140) | 35.2 (76) | 43.8 (64) |
| 25–29.9 | 29.0 (106) | 29.6 (64) | 28.8 (42) |
| 30–34.9 | 17.0 (62) | 20.4 (44) | 12.3 (18) |
| 35+ | 10.4 (38) | 10.6 (23) | 10.3 (15) |
| p-value | 0.1031 | ||
| Alcohol Measures | |||
| 30-day TLFB (grams) Median (Quartile Range) | 158.2 (728) | 37.1 (186.2) | 770 (1811.3) |
| p-value | <0.0001 | ||
| PEth ≥ 50-mg/dL | 162 (45.9) | 60 (28.8) | 102 (70.3) |
| p-value | <0.0001 | ||
| Smoking Status | |||
| Non-Smoker | 23.0 (84) | 27.5 (60) | 16.3 (24) |
| Current Smoker | 60.8 (222) | 53.2 (116) | 72.1 (106) |
| Former Smoker | 16.2 (59) | 19.3 (42) | 11.6 (17) |
| p-value | 0.5991 | ||
| CD4 Count (n=357) | |||
| < 200 | 13.2 (47) | 12.7 (27) | 13.9 (20) |
| 200–350 | 13.2 (47) | 14.1 (30) | 11.8 (17) |
| > 350 | 73.7 (263) | 73.2 (156) | 74.3 (107) |
| p-value | 0.9847 | ||
| Viral Load (n=357) | |||
| < 50 | 75.4 (269) | 77.5 (165) | 72.2 (104) |
| p-value | 0.2595 | ||
| < 400 | 87.1 (311) | 86.4 (184) | 88.2 (127) |
| 400–5000 | 4.5 (16) | 3.8 (8) | 5.5 (8) |
| > 5000 | 8.4 (30) | 9.9 (21) | 6.3 (9) |
| p-value | 0.3860 | ||
Discussion
The NOAH Study provides data on a cohort of in-care PLWH, with and without AUD. This study provides a valuable resource for resolving emerging questions regarding the consequences of alcohol use and other health behaviors on disease process and risk of comorbidities in PLWH despite use of cART and viral suppression. While the study design establishes a priori hypotheses to be tested, the testing strategy is sufficiently comprehensive and intentionally flexible to allow introduction of additional projects in the future.
Many pivotal findings have been reported in cohorts of PLWH with significant immunosuppression, high levels of viremia, and/or in comparison to the seronegative population (Williams EC et al., 2016; Molina PE et al., 2018). The design of the NOAH Study offers added relevance to the current state of the HIV pandemic in which effective cART is more widely available, viral suppression can be achieved with adherence to treatment, and PLWH increasingly suffer a disproportionate burden of chronic disease and precocious aging. By focusing on a clinic-based sample, the NOAH Study is well positioned to investigate the translational biology, and the influence of behaviors and environment, of PLWH under care.
Challenges and learning opportunities during the initial phase of the study include assessments of alcohol exposure and AUD that rely primarily on validated but self-reported questionnaires. Early in the study we found that AUDIT scores during screening were higher than at retesting during the full baseline visit (data not shown), skewing the initial targeted distribution of alcohol exposure among recruited participants. While the reason(s) for this are not entirely clear, social pressure and hope of secondary gain are probable confounders. This variability of self-reported consumption may reflect true variance in behaviors. We implemented a number of measures to minimize these effects including de-emphasizing the focus on alcohol and substance use, inclusion of a phosphatidylethanol (PEth), a biomarker of alcohol consumption, and frequent longitudinal reassessments.
This observational study cannot confirm mechanisms. However, several approaches partially mitigate this limitation including our longitudinal design, planned ex vivo mechanistic experiments, and intervention studies on subsets of the NOAH cohort. Furthermore, the LSUHSC CARC is operationalized as a bidirectional research program, and we are positioned to conduct mechanistic studies based on observations from the NOAH Study in our SIV-infected rhesus macaque model of alcohol consumption. Thus, the NOAH Study provides valuable data to test the impact of alcohol and other substance use on PLWH engaged in care. Inclusion of biological and sociobehavioral assessments offer the opportunity to test novel hypotheses highly relevant to the ongoing HIV pandemic as it enters its next stage, when the clinical importance of chronic disease outweighs overwhelming immune suppression and opportunistic infection. This knowledge will lead to clinical interventions to improve the health of PLWH.
Supplementary Material
Acknowledgements
We thank the NOAH Study participants for their willingness to participate. We acknowledge the essential contributions by study staff and referring clinicians. They are key to the success of the study. The authors recognize the editorial support received from Rebecca Gonzales and the contributions of study personnel: Chloe Ball, Sadie Beckett, Jonathan Boudin, Leslie Brennan, Kenya Brooks, Jessica Cucinello, Scott Edwards, Kimberly Edwards, Pauline Fink, Virginia Garrison, Betsy Giaimo, Nicholas Gilpin, Paula Gregory, Jasmine Hall, Brittney Harbin, Kasi Islam, Mark Juanet, Stephen Kantrow, Julie Ketchens, Alexandra Kharalampiev, Tracey Knaus, Danielle Levitt-Budnar, KC Madhav, Vincent Maffei, Soham Mahato, Charlotte Marshall, Rhonda Martinez, Heather McGarrah, Erin Meyaski, Mary Meyaski-Schluter, Patricia Mott, Ikenna Nnamani, Lauren O’Rear, Oluwaseun Oguntomole, Christopher Parsons, Francesca Peruzzi, Connie Porretta, Stefany Primeaux, Lauren Richey, Martin Ronis, Erika Rosen, Derrick Samuelson, Wendemi Sawadogo, Jane Schexnayder, Tianjiao Shen, Aneisha Simon, Aubrey Spriggs, Quinette Thomas, Curtis Vande Stouwe, Maeve Wallace, Alice Yeh, Arnold Zea.
Funding Sources: This project is funded by the National Institute of Alcohol and Alcohol Abuse and Alcoholism (NIAAA) of the National Institutes of Health (NIH) under award number P60AA009803. Additional support was provided by U54GM104940 from the National Institute of General Medical Sciences (NIGMS) of the NIH, which funds the Louisiana Clinical and Translational Science Center, and by the Office of the Dean of LSUHSC School of Medicine. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Availability of Data and Materials
The datasets generated during the current study will be available per the LSUHSC-NO Comprehensive Alcohol-HIV/AIDS Research Center’s data sharing policy.
Conflict of Interests
No competing interests to disclose.
References
- Azar MM, Springer SA, Meyer JP, Altice FL (2010) A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug Alcohol Depend 112(3):178–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martinis M, Franceschi C, Monti D, Ginaldi L (2005) Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett 579(10):2035–2039. [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, Mcburnie MA (2001) Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- Geronimus A, Hicken M, Keene D, Bound J (2006) “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health 96(5):826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, Huang B, Jung J, Zhang H, Fan A, Hasin DS (2017) Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001–2002 to 2012–2013: Results from the national epidemiologic survey on alcohol and related conditions. JAMA Psychiatry 74(9):911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guaraldi G, Orlando G, Zona S, Menozzi M, Carli F, Garlassi E, Berti A, Rossi E, Roverato A, Palella F (2011) Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 53(11):1120–1126. [DOI] [PubMed] [Google Scholar]
- Gutierrez AD, Balasubramanyam A (2012) Dysregulation of glucose metabolism in HIV patients: Epidemiology, mechanisms, and management. Endocrine 41(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ironson G, Stuetzle R, Fletcher M (2006) An increase in religiousness/spirituality occurs after HIV diagnosis and predicts slower disease progression over 4 years in people with HIV. J Gen Intern Med 21(Suppl 5):S62–S68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz PS, Siggins RW, Porretta C, Armstrong ML, Zea AH, Mercante DE, Parsons C, Veazey RS, Bagby GJ, Nelson S, Molina PE, Welsh DA (2015) Chronic alcohol increases CD8+ T-cell immunosenescence in simian immunodeficiency virus-infected rhesus macaques. Alcohol 49(8):759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt NR, Funderburg NT, Brenchley JM (2013) Microbial translocation, immune activation, and HIV disease. Trends Microbiol 21(1):6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolter DP (2003) Current concepts of metabolic abnormalities in HIV patients: Focus on lipodystrophy. AIDS Read 13(12 Suppl):S5–13. [PubMed] [Google Scholar]
- Lecapitaine NJ, Wang ZQ, Dufour JP, Potter BJ, Bagby GJ, Nelson S, Cefalu WT, Molina PE (2011) Disrupted anabolic and catabolic processes may contribute to alcohol-accentuated SAIDS-associated wasting. J Infect Dis 204(8):1246–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leserman J, Petitto JM, Golden RN, Gaynes BN, Gu H, Perkins DO, Silva SG, Folds JD, Evans DL (2000) Impact of stressful life events, depression, social support, coping, and cortisol on progression to AIDS. Am J Psychiatry 157(8):1221–1228. [DOI] [PubMed] [Google Scholar]
- Molina PE, Lang CH, Mcnurlan M, Bagby GJ, Nelson S (2008) Chronic alcohol accentuates simian acquired immunodeficiency syndrome-associated wasting. Alcohol Clin Exp Res 32(1):138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina PE, Simon L, Amedee AM, Welsh DA, Ferguson TF (2018) Impact of alcohol on HIV disease pathogenesis, comorbidities and aging: Integrating preclinical and clinical findings. Alcohol Alcohol 53(4):439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggott DA, Muzaale AD, Mehta SH, Brown TT, Patel KV, Leng SX, Kirk GD (2013) Frailty, HIV infection, and mortality in an aging cohort of injection drug users. PLoS One 8(1):e54910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K (2008) A standard procedure for creating a frailty index. BMC Geriatr 8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L, Lecapitaine N, Berner P, Vande Stouwe C, Mussell JC, Allerton T, Primeaux SD, Dufour J, Nelson S, Bagby GJ, Cefalu W, Molina PE (2014) Chronic binge alcohol consumption alters myogenic gene expression and reduces in vitro myogenic differentiation potential of myoblasts from rhesus macaques. Am J Physiol Regul Integr Comp Physiol 306(11):R837–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdiserri RO, Holtgrave DR (2018) Ending america’s HIV epidemic: Why the national HIV/AIDS strategy still matters. AIDS Behav 22(7):2033–2041. [DOI] [PubMed] [Google Scholar]
- Williams EC, Hahn JA, Saitz R, Bryant K, Lira MC, Samet JH (2016) Alcohol use and human immunodeficiency virus (HIV) infection: Current knowledge, implications, and future directions. Alcohol Clin Exp Res 40(10):2056–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.