Abstract
Objective.
Impaired cognition is a hallmark of schizophrenia and spectrum disorders such as schizotypal personality disorder, and is the best predictor of functional outcome. Cognitive remediation therapy has demonstrated efficacy for improving cognition and augmenting other rehabilitation efforts in schizophrenia, with gains in real-world functioning. Pharmacological augmentation of cognitive remediation has been attempted but the effects of augmentation on combined therapies such as cognitive remediation and social skills training has not been studied.
Method.
Twenty-eight participants with schizotypal personality disorder enrolled in an 8-week, randomized, double-blind, placebo-controlled trial of guanfacine plus cognitive remediation + social skills training (15 guanfacine, 13 placebo). Cognition was assessed with the MATRICS Clinical Consensus Battery (MCCB), social cognition with the Movie for the Assessment of Social Cognition (MASC), and functional capacity with the UCSD Performance Based Skills Assessment (UPSA).
Results.
There was a statistically significant time (pre- versus post-treatment) effect for MCCB speed of processing, verbal learning, and visual learning, and UPSA total score. There was a significant time x medication condition (guanfacine, placebo) interaction for MCCB reasoning and problem solving, and UPSA total score; the time x treatment condition interaction approached significance for MASC hypomentalizing errors.
Conclusions.
In this trial, both the agent guanfacine and our psychosocial intervention of cognitive remediation plus social skills training were well-tolerated, with no side effects or drop-outs. Participants treated with cognitive remediation plus social skills training plus guanfacine demonstrated statistically significant improvements in reasoning and problem solving, as well as in functional capacity and possibly social cognition, compared to those treated with cognitive remediation plus social skills training plus placebo. Cognitive remediation plus social skills training may be an appropriate intervention for individuals with schizotypal personality disorder and guanfacine appears to be a promising agent to augment cognitive remediation plus social skills training in this population.
Keywords: Cognition, Schizophrenia, Schizotypal, working memory, COGNITIVE REMEDIATION THERAPY, guanfacine
Impaired cognition is a hallmark feature of schizophrenia [1]: deficits are pervasive, occurring in 75–85% of patients [2], and frequently precede the onset of other symptoms [2, 3]. They are also persistent [4], remaining after psychotic symptoms have been effectively treated [5]. Cognitive deficits are among the best predictors of certain aspects of functional outcome in schizophrenia [6], and their presence predicts reduced adaptive and social skills [7], and increased risk of relapse in first-episode patients [8]. The impacts of cognitive deficits on everyday functioning appear to be mediated by their association with the ability to perform everyday functional skills which in turn predict everyday disability [9]. Impairments are also found in first degree relatives and individuals with prodromal symptoms [4].
Cognitive deficits in domains impaired in schizophrenia, including attention, working memory, episodic memory, and executive functioning, have been identified in a similar pattern in people with other schizophrenia spectrum disorders such as schizotypal personality disorder [10] and attenuated psychosis syndrome. Schizotypal personality disorder patients exhibit deficits in the same domains as people with schizophrenia, with impairments in verbal and spatial episodic memory, vigilance, attention, abstract reasoning, recognition memory, cognitive inhibition, verbal fluency, and verbal and spatial working memory [11]. Importantly, studies of cognition in schizotypal personality disorder obviate confounds associated with antipsychotic medication treatment, and the cognitive deficits of schizotypal personality disorder are less global, providing a unique opportunity to test the new interventions for cognitive enhancement in the schizophrenia spectrum.
Additionally, emerging research suggests that individuals with schizotypal personality disorder demonstrate impaired performance on measures of functional capacity similar to that found in schizophrenia [11], as well as manifesting impairments in everyday functioning in similar domains as patients with schizophrenia [12]. In fact, in a study of cognitive deficits, functional capacity (UCSD Performance-Based Skills Assessment: UPSA), and real world-functioning in schizotypal personality disorder, we found that the correlation between functional capacity and cognition in schizotypal personality disorder was as strong as that found in multiple previous studies of schizophrenia. Patients with schizotypal personality disorder had reduced educational attainment, earned less money per hour, and were less likely to live independently compared to healthy control or avoidant personality disorder participants. Thus, treatment of cognitive deficits in schizotypal personality disorder is not only a model for treatment efforts in schizophrenia, but is also important as there are real-world functional gains relevant to schizotypal personality disorder to be realized in the treatment of this population through the possible improvement in functional capacity.
One paradigm with demonstrated efficacy for improving cognition in schizophrenia is cognitive remediation therapy [13]. These approaches use techniques from clinical neuropsychology and focus on the acquisition of cognitive skills in the areas most impaired in schizophrenia such as attention and concentration, psychomotor speed, learning and memory, and executive functions [14]. These programs generally use computerized strategies that use adaptive titration of difficulty to allow patients to move more quickly through areas in which they have better skills and to spend more time on areas of greater impairment. Cognitive remediation has been shown to have substantial success when psychiatric rehabilitation interventions targeting skills deficits, such as supportive employment services, are also added to cognitive remediation [15]. In addition, there is also evidence for the effectiveness of cognitive remediation in the broader spectrum, for example for attenuated psychosis [16, 17].
Although cognitive remediation has been shown to effectively ameliorate cognitive deficits seen in schizophrenia, trials of pharmacological agents targeting these symptoms have been much less successful. However, recent research suggests that there may be a detectable pharmacological benefit from synergistic approaches combining pharmacological interventions with either cognitive remediation or psychosocial interventions [18]. However, to date no one has examined the triple interaction of pharmacological interventions, computerized cognitive remediation, and a psychosocial intervention such as social skills training, which may offer the most benefit to patients in terms of both improved cognition and functional outcome.
One agent with promising support for augmenting cognitive remediation + social skills training, is guanfacine, a selective agonist for the adrenergic α2a-receptor subtype [19]. Alpha 2A-adrenergic agonists improve cognitive function in a variety of disorders, such as ADHD [20], Tourette’s [21], opioid dependence [22], and pervasive developmental disorder [23]. Treatment with guanfacine increases cerebral blood flow in anterior frontal cortex in regions critical to working memory performance [24]. Furthermore, schizotypal personality disorder participants treated with guanfacine demonstrated improvement on a context processing task, which is heavily dependent on working memory, compared to those given placebo [19], Additionally, schizophrenia patients treated with guanfacine alone have shown some small improvements [25].
The current study sought to extend the literature in two important ways. First, although individuals with schizotypal personality disorder demonstrate similar, albeit attenuated, cognitive deficits compared to schizophrenia, there have been no studies to date of cognitive remediation as a treatment for cognitive deficits found in schizotypal personality disorder. Additionally, although there have been several studies investigating the possible pharmacological facilitation of a cognitive remediation therapy regimen in individuals with schizophrenia [18], all pharmacological studies in schizotypal personality disorder have looked at medications alone. Thus, the current study is the first, to our knowledge, to investigate synergistic effect of pharmacological interventions, cognitive remediation, and psychosocial treatments.
The goal of the present study, therefore, was to evaluate the ability of guanfacine to augment cognitive remediation + social skills training in individuals with schizotypal personality disorder, in a double-blind, placebo controlled trial. We hypothesized that cognitive remediation + social skills training would result in improvements in cognitive performance and functional skills, and that schizotypal personality disorder participants randomized to guanfacine would exhibit even greater improvement compared with those treated with cognitive remediation + social skills training alone.
Methods and Materials
Participants
Participants were recruited from the community in and around Icahn School of Medicine at Mt. Sinai in New York, NY, using newspaper and online advertisements targeting key features of schizotypal personality disorder (magical thinking, odd beliefs, and social isolation). Throughout the enrollment period, 744 individuals responded to these ads by calling the research office. Of these, 104 declined to schedule a screening interview, 345 failed to attend their screening interview, and 136 were excluded following the screening interview as they met exclusionary criteria for the study. An additional 123 participants were excluded after failing to meet diagnostic criteria for schizotypal personality disorder or for a failure of the general medical clearance, and eight participants who met eligibility criteria declined to participate due to scheduling conflicts or disinterest in medication, leaving 28 individuals (20 males, 8 females) with DSM-IV schizotypal personality disorder who enrolled in the trial. Participants ranged in age from 22–62 (mean = 43.78, SD = 11.44). All participants were free of psychotropic medication at the time of enrollment and were medically healthy as determined by a full medical clearance including EKG, medical history, physical and lab work. Participants with a lifetime diagnosis of a psychotic disorder (schizophrenia, schizoaffective disorder, or bipolar disorder I with psychotic features) or current major depressive episode were excluded, as were participants with current substance abuse or lifetime substance dependence. Participants were evaluated using the Structured Interview for DSM Personality Disorders (SID-P) Pfohl, 1997 [26]) and the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) [27]. All interviews were conducted by doctoral-level, clinical psychologists. Consensus diagnoses were reached in a meeting of all raters with an expert diagnostician. None of the participants met diagnostic criteria for any DSM-IV psychotic disorder; however, subclinical psychotic symptoms, consistent with a diagnosis of schizotypal personality disorder, were prevalent in this sample. Seventy-one percent of participants endorsed ideas of reference, 57% reported odd beliefs/magical thinking, 57% endorsed unusual perceptual experiences, 82% demonstrated odd thinking and speech, 79% endorsed paranoid ideation, and 64% exhibited odd appearance.
All participants provided informed consent in accordance with the Institutional Review Board approvals of this study at The Icahn School of Medicine at Mount Sinai.
Materials
MATRICS Consensus Cognitive Battery (MCCB) [28].
The MCCB was developed as a primary outcome measures for clinical trials of cognitive enhancement in schizophrenia and has become the gold standard in this field. For the current trial, five MCCB domains, all of which have been implicated in schizophrenia patients, were included: speed of processing, working memory, verbal learning, visual-spatial learning, and reasoning and problem solving (social cognition was assessed using a supplemental measure in this study). For all MCCB domains, age-corrected T-scores served as the dependent variable.
UCSD Performance-Based Skills Assessment (UPSA) [29].
The UPSA is an office-based test to measure schizophrenia patients’ ability to perform day-to-day tasks [29]. This test was designed for outpatients and measures performance in a number of domains of everyday functioning through the use of props and standardized performance situations. In line with our previous research [30–32], four domains of the UPSA were used. Comprehension/ Planning, Finance, Communication, and the Transportation/ Mobility tasks were administered. Lower scores on the UPSA have been demonstrated to correlate with more severe cognitive deficits and negative symptoms, but to be unassociated with the severity of positive psychotic symptoms, in schizophrenia samples [6], and UPSA scores also correlate with cognitive performance and real-world functioning in schizotypal personality disorder samples [11]. The UPSA total score summed across the four domains. served as the dependent variable for the current study.
Movie for the Assessment of Social Cognition (MASC) [33].
The MASC involves watching a 15-minute movie about 4 characters getting together for a dinner party. The video is paused 45 times and questions concerning the characters’ feelings, thoughts, and intentions are asked. It takes 40 minutes to complete. The multiple-choice version of the MASC allows a qualitative social cognition error analysis. The MASC dependent variables are: 1)Mentalization accuracy: sum score for all correct questions; 2) Hypomentalization errors (hypomentalization or no mentalization); 3) Hypermentalization errors. For the current trial, hypomentalizing errors, which are more closely associated with cognitive and negative symptoms in schizophrenia, served as the dependent variable [34].
Additional Neuropsychological Assessments.
We supplemented the MCCB with four neuropsychological assessments of working memory and context processing. The N-back Working Memory Task (N-back) is a commonly used measure of working memory [35, 36]. Participants observed letters presented on a computer screen one at a time over three conditions: 1) 0-back; 2) 1-back; and 3) 2-back. In the 0-back condition, participants responded to a single pre-specified target letter (e.g., X). In the 1-back condition, the target was any letter identical to the one immediately preceding it (i.e., one trial back). In the 2-back condition, the target was any letter identical to the one presented two trials back. For the current study, the dependent variables were the number of correct responses for the 2-back condition. The Paced Auditory Serial Addition Test (PASAT) is a test of auditory verbal working memory that has been well described and validated in this population [37, 38]. Briefly, subjects listen to a tape-recorded voice presenting a series of numbers (50 numbers at a rate of one digit per two seconds) and are asked to add each adjacent pair of numbers and respond by verbalizing the sum. The total number of correct responses is the dependent variable. The DOT Test [39] is a test of visuospatial working memory in wide research use. Subjects are presented a dot at a specific position on a standard size paper and then asked to reproduce it at the same location on a separate sheet after different periods of delay (no delay, 10, 20, or 30 sec delay). Performance is measured as the distance in cm between the drawn dot and the actual dot (distance error). The distance error at the 30sec delay (longest memory load of all three delays) minus the distance error at the immediate condition is the dependent variable. During the AX-CPT Task, sequences of letters were visually presented one at a time in a continuous fashion on a computer display. Participants were instructed to make an affirmative response on target trials and a negative response otherwise (for a full description, please see [40]). The delay between cue and probe was 5 sec and the inter-trial interval was 1 sec. The task was presented in 2 blocks of 50 trials. Participants were asked to respond as quickly as possible to each stimulus while maintaining accuracy. The d’-context score served as the dependent variable.
Cognitive Remediation Therapy Intervention
The computer-based software is a multimedia (auditory and visual) Windows-based program designed by Psychological Services Inc. [41] The exercises are aimed at improving areas of deficit within the schizophrenia spectrum, and this software was chosen as it has been successfully paired with social skills training and for the sophisticated graphics that would appeal to a community-recruited sample. In each session of every cohort, participants were divided into small groups no greater than two to three members and engaged in the exercises; participants worked independently on an individual laptop but were encouraged to interact with one another in order to stimulate problem solving skills, as well as to provide opportunities to improve social skills. Each group was led by a Bachelors or Master’s level research assistant who had received training to operate audio visual equipment and was familiar with each of the exercises. The primary role of the facilitator was to ensure that computers and software were operating properly, to gently encourage participants to stay on task and to interact when opportunities presented themselves, and to monitor the number of correct responses for each participant during the tasks. Although, not present during computer sessions, a trained doctoral level psychologist knowledgeable of cognitive remediation was available for consultation as needed. For the current study, cognitive remediation therapy consisted of group members first working on exercises to increase attentional functioning and then, after two session, selecting any of the eight memory exercises or six problem solving exercises at a given session. Each exercise provides several permutations of graduated difficulty levels and other modifiable parameters. Participants were encouraged to challenge themselves by the group facilitator, who monitored their performances and manually adjusted the difficulty for each exercise to approach approximately 80% correct responses.
Social Skills Training
The twice-weekly social skills training followed the manualized curriculum outline and materials provided from Cognitive Enhancement Therapy program [42]. This manualized program was specifically designed to augment training with Psychological Services Inc., software. Substantial modifications were made from the full Cognitive Enhancement Therapy program to the current study; however, the basic design, structure and execution of the program was maintained. Similar to the full program, the current study incorporated the core material from each of the three modules. Module one provided participants with an orientation and an overview of basic concepts. The second module consists of social cognitive skills training. The final module focuses on the real-world applications of cognitive and social-cognitive skills acquired during the treatment. All groups were led by a doctoral-level clinical psychologist. Participants received a small monetary compensation for attendance at each session.
Procedure
The baseline cognitive and functional skill assessment battery was completed prior to the initiation of guanfacine or placebo. Following this, participants entered into an 8-week, double-blind, placebo-controlled treatment phase during which they were randomly assigned to receive either guanfacine or placebo. All schizotypal personality disorder participants recruited for this trial performed at least one SD below healthy control means on at least one MCCB domain. Participants on active drug were titrated to 2.0 mg daily over the first two weeks and remained on 2.0 mg for the duration of the study. Participants’ blood pressure and heart rate were monitored weekly, and participants met with the study physician weekly to assess for potential side effects. Fifteen participants were randomized to guanfacine, and 13 were randomized to placebo. At the completion of the trial, medication was discontinued for all participants, although those who reported that they experienced benefit from the medication were encouraged to follow-up with their medical providers.
All participants received the active cognitive remediation + social skills training intervention, which consisted of a combination of computer-based cognitive enhancement exercises (Psychological Services Inc., and Odie Bracy, 2012) and manualized-social skills training modified from Cognitive Enhance Therapy [42]. Specifically, participants met for 60 minutes of computer based cognitive enhancement exercises and 60 minutes of social skills training twice weekly (4 hours total per week) over 8 weeks for a total of 30 sessions (15 computer-based cognitive remediation + 15 small group social skills training).
The cognitive and functional skill assessment battery was re-administered at the completion of the trial.
Data Analysis
Data were analyzed using SPSS 21.0 for Mac. For the current trial, the primary endpoint was cognition as evaluated by the MCCB domains. Secondary endpoints were social cognition, as evaluate by the MASC, and function skills, as evaluated by the UPSA. We computed a series of 2 (time: baseline, post-treatment) by 2 (treatment condition: guanfacine, placebo) repeated measures analyses of variance (ANOVA), one for each MCCB domain, as well as for UPSA total score and MASC hypomentalizing errors.
Results
Adherence to the protocol was excellent. All participants completed the trial with the exception of one participant who was removed from the trial due to an increase in blood pressure noted at the weekly medical check-in, although this participant was later revealed to have been randomized to placebo. Across both the computerized cognitive remediation and group social skills training sessions, the mean attendance was 80.12% (See Table 1). In addition, guanfacine was very well-tolerated by this population, with no side effects being noted in our active treatment group.
Table 1.
Demographic variables by treatment group.
| Guanfacine | Placebo | Group Comparison | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age | 46.5 | 12.2 | 40.7 | 10.1 | t(26)=1.4, p=.2 |
| N | % | N | % | ||
| Gender (Male) | 11 | 73.3 | 10 | 76.9 | χ2(1)=.1, p=.8 |
| Handedness (Right) | 9 | 69.2 | 10 | 76.9 | χ2(2)=0.4, p=.8 |
Results of our repeated measures ANOVAs suggest that participants with schizotypal personality disorder benefited from cognitive remediation + social skills training. See table 2 for the results. We found statistically significant main effects for time (pre- versus post-) on MCCB speed of processing, F(1, 24)=6.86, p=.015, verbal learning, F(1, 25)=5.14, p=.011, and visual learning, F(1,25)=7.50, p=.032. In addition, there was a statistically significant improvement across groups for UPSA total score, F(1, 24)=5.73, p=.025. For all variables, participants’ performances improved following the intervention (see Table 2 for raw scores).
Table 2.
T-scores for the MATRICS Consensus Cognitive Battery (MCCB) Domains, and Raw Scores for the UCSD Performance Based-Skills Assessment (UPSA), and the Movie for the Assessment of Social Cognition (MASC) by group.
| Cognitive Remediation Therapy + Social Skills Training Plus Guanfacine | Cognitive Remediation Therapy + Social Skills Training Plus Placebo | ANOVA Results | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post-Test | Baseline | Post-Test | Time | Time x Medication | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | F | p | F | p | |
| MCCB Domain Scoresa | ||||||||||||
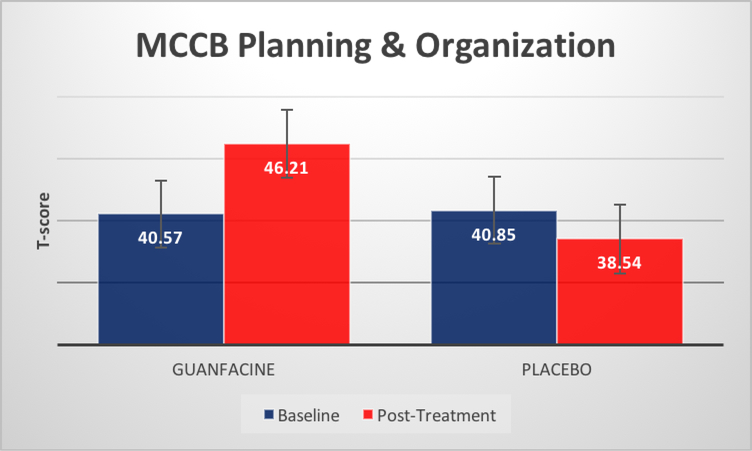
| Reasoning & Problem Solving | 40.6 | 8.7 | 46.2 | 9.0 | 40.8 | 10.9 | 38.5 | 11.6 | 1.5 | 0.24 | 8.36 | 0.01* |
| Speed of Processing | 40.8 | 13.7 | 44.8 | 10.9 | 35.6 | 9.4 | 39.5 | 8.9 | 6.85 | 0.02* | 0.00 | 1.00 |
| Working Memory | 40.9 | 12.7 | 39.8 | 13.0 | 36.2 | 9.7 | 36.5 | 10.2 | 0.06 | 0.81 | 0.13 | 0.72 |
| Verbal Learning | 35.6 | 8.8 | 47.2 | 16.0 | 39.1 | 11.5 | 40.9 | 12.5 | 5.14 | 0.03* | 2.82 | 0.11 |
| Visual Learning | 41.5 | 12.8 | 50.4 | 12.8 | 38.8 | 11.5 | 43.0 | 10.9 | 7.50 | 0.01** | 1.17 | 0.29 |
| UPSA | ||||||||||||
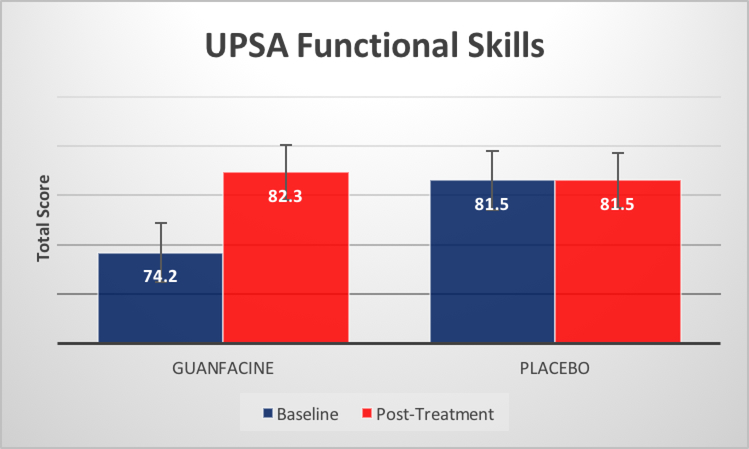
| Total Scoreb | 73.9 | 12.9 | 82.3 | 9.1 | 81.5 | 9.9 | 81.7 | 10.7 | 5.73 | 0.03* | 5.62 | 0.03* |
| MASCc | ||||||||||||
| Hypomentalizing Errors | 8.3 | 3.9 | 6.1 | 3.2 | 6.7 | 2.6 | 7.2 | 2.3 | 1.79 | 0.19 | 3.55 | 0.07 |
Notes:
T-scores: mean = 50 and SD=10
Total range of scores is 0–100
Higher scores reflect more errors
In addition, there was a significant time x medication condition interaction for MCCB reasoning and problem solving domain, F(1,25)=8.36, p=.005 (see Figure 1), and for UPSA total score F(1,24)=5.62, p=.026 (see Figure 2), with individuals in our guanfacine group demonstrating greater improvement over the course of treatment than those in our placebo group for both cognition and functional skills. Finally, the time x medication condition interaction for MASC hypomentalization errors approached statistical significance, F(1,22)=3.55, p=.07. There was no statistically significant change for MCCB working memory or for our supplemental neuropsychological assessments (all ps>.05), in this trial (See Supplemental Materials).
Figure1.
Time x medication interaction, MCCB Planning & Organization F(1,25)=8.36, p=.005
Figure2.
Time x medication interaction, UPSA total score F(1,24)=5.62, p=.026
Discussion
Overall, we found that this intervention was extremely well-tolerated by schizotypal personality disorder participants. We had excellent adherence to the cognitive remediation + social skills training protocol, as well as no noted side effects for the medication, guanfacine. In addition, individuals with schizotypal personality disorder did appear to benefit from cognitive remediation + social skills training. Statistically significant improvements were seen in three domains of cognitive performance: speed of processing, verbal learning, and visual learning, following 8 weeks of cognitive remediation + social skills training. Two of the three domains are also assessed by alternate forms of memory tests, so practice effects would be expected to be minimized. In addition, schizotypal personality disorder participants demonstrated improvements in their scores on the UPSA following cognitive remediation + social skills training.
Most importantly, guanfacine augmentation enhanced the impact of cognitive remediation + social skills training in this sample. Individuals who were given guanfacine in conjunction with cognitive remediation + social skills training demonstrated significantly greater improvement in their reasoning and problem solving and in their functional skills, and they demonstrated improvements in social cognition that approached statistical significance. These results, when taken together, suggest that this agent, which is hypothesized to enhance an individual’s attentional ability, augments the results of cognitive remediation + social skills training alone.
During computer sessions, attendees were encouraged to problem-solve together and support each other. As such, computer training was an additional forum in which to practice skills discussed during social cognition training. Some members were interested in continuing connections with other group members and exchanged contact information for subsequent meetups outside of group. This was neither encouraged nor discouraged by group facilitators. For some, group content and computer training provided a shared experience and common grounds, while others reported less interest in making connections and greater focus on improving task performance. In general, although symptoms of schizotypal personality disorders were still present for most participants at the conclusion of the trial, the majority demonstrated improvements in both cognitive performance, functional skills, and social skills and reported qualitative benefit from the experience.
In addition to these important results, there are several notable innovations to the current study. To our knowledge, the current study is the first to examine the efficacy of a combined psychosocial intervention, cognitive remediation + social skills training, in a sample of individuals with schizotypal personality disorder, a schizophrenia spectrum disorder in which there is considerable functional impairment but no efficacious treatment. Furthermore, it is the first study to examine the synergistic impact of computerized cognitive remediation, a psychosocial intervention, and the pharmacological agent guanfacine. Although cognitive remediation trials in schizophrenia have demonstrated that there is considerable benefit for this intervention, not all participants respond or respond equally well to this intervention. Identification of agents and adjunctive therapies that augment cognitive remediation is therefore an important step in increasing the effectiveness of the intervention.
There are several limitations in this study. There was no completely inactive treatment, in that all participants received the combined psychosocial intervention. Thus, a placebo effect cannot be excluded as an explanation for the changes from baseline in the cognitive remediation + social skills training intervention alone. As noted above, some of the tests that improved were examined with alternate forms. This limitation does not apply to the differences seen between the guanfacine and placebo groups in the study. Our sample size was small and it is very difficult to determine how representative the patients are compared to other studies of schizotypal personality disorder, which were themselves often quite small. Finally, practice effects are always a potential explanation for improvements in performance after retesting. Our recent study addressed practice effects on the MCCB in patients with schizophrenia [43]. We found that, on average, MCCB scores improved by a little more than 1 point per reassessment. As our statistically significant changes on the MCCB were considerably larger than that, we assume that practice effects are not a viable explanation for changes in performance in the cognitive remediation + social skills training alone group.
The robust results of the current study suggest that cognitive remediation + social skills training is an effective intervention for improving both cognitive performance and functional skills in the schizophrenia spectrum, and that guanfacine is a promising agent to enhance the effectiveness of cognitive remediation + social skills training. As cognitive impairments are closely linked to functional outcomes for individuals across the spectrum, this is an important next step in improving the real-world outcomes of individuals with these disorders.
Supplementary Material
Acknowledgements:
This research was supported by a NARSAD Young Investigator Award from the Brain and Behavior Foundation to Dr. McClure, by NIMH Grant Number MH 097799 to Dr. New, by VA Merit Award i01CX000609–01 to Dr. Hazlett, and by the VA Advanced Fellowship in Mental Health, VISN2 Mental Illness Research, Education, and Clinical Center (MIRECC), and by the VA Advanced Fellowship in Mental Health, VISN 2 MIRECC.
This work was presented at the 2017 International Congress on Schizophrenia Research and the 2018 American College of Neuropsychopharmacology Meeting.
Footnotes
Disclosures: Dr. Harvey has received consulting fees or travel reimbursements from Akili, Boehringer Ingelheim, Intra Cellular Therapeutics, Lundbeck Pharma, Minerva Pharma, OtsukaAmeica, Sanofi Pharma, Sunovion Pharma, Takeda Pharma, and Teva during the past year. He has a research grant from Takeda and from the Stanley Medical Research Foundation. Dr. Perez is site-PI of a study funded by Neurocrine Biosciences. Drs. McClure, Graff, Triebwasser, Rosell, Koenigsberg, Hazlett, Siever, & New report no financial relationships with commercial interests.
References
- 1.Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 2006;67 Suppl 9:3–8; discussion 36–42. [PubMed] [Google Scholar]
- 2.Reichenberg A, Weiser M, Rapp MA, Rabinowitz J, Caspi A, Schmeidler J, et al. Elaboration on premorbid intellectual performance in schizophrenia: premorbid intellectual decline and risk for schizophrenia. Arch Gen Psychiatry. 2005;62(12):1297–304. [DOI] [PubMed] [Google Scholar]
- 3.Sorensen HJ, Mortensen EL, Parnas J, Mednick SA. Premorbid neurocognitive functioning in schizophrenia spectrum disorder. Schizophr Bull. 2006;32(3):578–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seidman LJ, Giuliano AJ, Meyer EC, Addington J, Cadenhead KS, Cannon TD, et al. Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Arch Gen Psychiatry. 2010;67(6):578–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heinrichs RW. The primacy of cognition in schizophrenia. Am Psychol. 2005;60(3):229–42. [DOI] [PubMed] [Google Scholar]
- 6.Strassnig MT, Raykov T, O’Gorman C, Bowie CR, Sabbag S, Durand D, et al. Determinants of different aspects of everyday outcome in schizophrenia: The roles of negative symptoms, cognition, and functional capacity. Schizophr Res. 2015;165(1):76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowie CR, Harvey PD. Cognition in schizophrenia: impairments, determinants, and functional importance. Psychiatr Clin North Am. 2005;28(3):613–33, 26. [DOI] [PubMed] [Google Scholar]
- 8.Chen EY, Hui CL, Dunn EL, Miao MY, Yeung WS, Wong CK, et al. A prospective 3-year longitudinal study of cognitive predictors of relapse in first-episode schizophrenic patients. Schizophr Res. 2005;77(1):99–104. [DOI] [PubMed] [Google Scholar]
- 9.Bowie CR, Leung WW, Reichenberg A, McClure MM, Patterson TL, Heaton RK, et al. Predicting schizophrenia patients’ real-world behavior with specific neuropsychological and functional capacity measures. Biol Psychiatry. 2008;63(5):505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitropoulou V, Harvey PD, Zegarelli G, New AS, Silverman JM, Siever LJ. Neuropsychological performance in schizotypal personality disorder: importance of working memory. Am J Psychiatry. 2005;162(10):1896–903. [DOI] [PubMed] [Google Scholar]
- 11.McClure MM, Harvey PD, Bowie CR, Iacoviello B, Siever LJ. Functional outcomes, functional capacity, and cognitive impairment in schizotypal personality disorder. Schizophr Res. 2013;144(1–3):146–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGurk SR, Mueser KT, Mischel R, Adams R, Harvey PD, McClure MM, et al. Vocational functioning in schizotypal and paranoid personality disorders. Psychiatry Res. 2013;210(2):498–504. [DOI] [PubMed] [Google Scholar]
- 13.Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168(5):472–85. [DOI] [PubMed] [Google Scholar]
- 14.McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007;164(12):1791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGurk SR, Mueser KT, Xie H, Welsh J, Kaiser S, Drake RE, et al. Cognitive Enhancement Treatment for People With Mental Illness Who Do Not Respond to Supported Employment: A Randomized Controlled Trial. Am J Psychiatry. 2015;172(9):852–61. [DOI] [PubMed] [Google Scholar]
- 16.Fisher M, Loewy R, Hardy K, Schlosser D, Vinogradov S. Cognitive interventions targeting brain plasticity in the prodromal and early phases of schizophrenia. Annu Rev Clin Psychol. 2013;9:435–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hooker CI, Carol EE, Eisenstein TJ, Yin H, Lincoln SH, Tully LM, et al. A pilot study of cognitive training in clinical high risk for psychosis: initial evidence of cognitive benefit. Schizophr Res. 2014;157(1–3):314–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harvey PD, Sand M. Pharmacological Augmentation of Psychosocial and Remediation Training Efforts in Schizophrenia. Front Psychiatry. 2017;8:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McClure MM, Barch DM, Romero MJ, Minzenberg MJ, Triebwasser J, Harvey PD, et al. The effects of guanfacine on context processing abnormalities in schizotypal personality disorder. Biol Psychiatry. 2007;61(10):1157–60. [DOI] [PubMed] [Google Scholar]
- 20.Chan E, Fogler JM, Hammerness PG. Treatment of Attention-Deficit/Hyperactivity Disorder in Adolescents: A Systematic Review. JAMA. 2016;315(18):1997–2008. [DOI] [PubMed] [Google Scholar]
- 21.Quezada J, Coffman KA. Current Approaches and New Developments in the Pharmacological Management of Tourette Syndrome. CNS Drugs. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gowing L, Farrell M, Ali R, White JM. Alpha(2)-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev. 2016(5):CD002024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nash K, Carter KJ. Treatment options for the management of pervasive developmental disorders. Int J Psychiatry Med. 2016;51(2):201–10. [DOI] [PubMed] [Google Scholar]
- 24.Alamo C, Lopez-Munoz F, Sanchez-Garcia J. Mechanism of action of guanfacine: a postsynaptic differential approach to the treatment of attention deficit hyperactivity disorder (adhd). Actas Esp Psiquiatr. 2016;44(3):107–12. [PubMed] [Google Scholar]
- 25.Friedman JI, Adler DN, Temporini HD, Kemether E, Harvey PD, White L, et al. Guanfacine treatment of cognitive impairment in schizophrenia. Neuropsychopharmacology. 2001;25(3):402–9. [DOI] [PubMed] [Google Scholar]
- 26.Pfohl B BN, Zimmerman M. Structured Interview for DSM-IV Personality: SIDP-IV. Iowa: Department of Psychiatry, University of Iowa; 1995. [Google Scholar]
- 27.First MB SR, Robert L, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 28.Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203–13. [DOI] [PubMed] [Google Scholar]
- 29.Patterson TL, Goldman S, McKibbin CL, Hughs T, Jeste DV. UCSD Performance-Based Skills Assessment: development of a new measure of everyday functioning for severely mentally ill adults. Schizophr Bull. 2001;27:235–45. [DOI] [PubMed] [Google Scholar]
- 30.Bowie CR, Reichenberg A, Patterson TL, Heaton RK, Harvey PD. Determinants of real-world functional performance in schizophrenia subjects: correlations with cognition, functional capacity, and symptoms. Am J Psychiatry. 2006;163:418–25. [DOI] [PubMed] [Google Scholar]
- 31.Bowie CR, Reichenberg A, McClure MM, Leung WL, Harvey PD. Age-associated differences in cognitive performance in older community dwelling schizophrenia patients: differential sensitivity of clinical neuropsychological and experimental information processing tests. Schizophr Res. 2008;106(1):50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McClure MM, Bowie CR, Patterson TL, Heaton RK, Weaver C, Anderson H, et al. Correlations of functional capacity and neuropsychological performance in older patients with schizophrenia: evidence for specificity of relationships? Schizophr Res. 2007;89(1–3):330–8. [DOI] [PubMed] [Google Scholar]
- 33.Dziobek I, Fleck S, Kalbe E, Rogers K, Hassenstab J, Brand M, et al. Introducing MASC: a movie for the assessment of social cognition. J Autism Dev Disord. 2006;36(5):623–36. [DOI] [PubMed] [Google Scholar]
- 34.Fretland RA, Andersson S, Sundet K, Andreassen OA, Melle I, Vaskinn A. Theory of mind in schizophrenia: error types and associations with symptoms. Schizophr Res. 2015;162(1–3):42–6. [DOI] [PubMed] [Google Scholar]
- 35.Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5(1):49–62. [DOI] [PubMed] [Google Scholar]
- 36.Casey BJ, Cohen JD, Jezzard P, Turner R, Noll DC, Trainor RJ, et al. Activation of prefrontal cortex in children during a nonspatial working memory task with functional MRI. Neuroimage. 1995;2(3):221–9. [DOI] [PubMed] [Google Scholar]
- 37.Gronwall DM. Paced auditory serial-addition task: a measure of recovery from concussion. Percept Mot Skills. 1977;44(2):367–73. [DOI] [PubMed] [Google Scholar]
- 38.Stuss DT, Alexander MP, Hamer L, Palumbo C, Dempster R, Binns M, et al. The effects of focal anterior and posterior brain lesions on verbal fluency. J Int Neuropsychol Soc. 1998;4(3):265–78. [PubMed] [Google Scholar]
- 39.Keefe RS, Lees-Roitman SE, Dupre RL. Performance of patients with schizophrenia on a pen and paper visuospatial working memory task with short delay. Schizophr Res. 1997;26(1):9–14. [DOI] [PubMed] [Google Scholar]
- 40.Barch DM, Mitropoulou V, Harvey PD, New AS, Silverman JM, Siever LJ. Context-processing deficits in schizotypal personality disorder. J Abnorm Psychol. 2004;113(4):556–68. [DOI] [PubMed] [Google Scholar]
- 41.Bracy O PSS CogRehab, Version 2003. Indianapolis, IN: Psychological Software Services, Inc; 2003. [Google Scholar]
- 42.Hogarty GE, Greenwald DP, Eack SM. Durability and mechanism of effects of cognitive enhancement therapy. Psychiatr Serv. 2006;57(12):1751–7. [DOI] [PubMed] [Google Scholar]
- 43.Keefe RSE, Davis VG, Harvey PD, Atkins AS, Haig GM, Hagino O, et al. Placebo Response and Practice Effects in Schizophrenia Cognition Trials. JAMA Psychiatry. 2017;74(8):807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.