Abstract
α-Synuclein is strongly implicated in the pathogenesis of Parkinson’s disease as well as in other neurodegenerative diseases. However, its normal function in cells is not understood. The N-termini of α-, β-, and γ-synuclein contains six to seven 11-amino acid repeats that are predicted to form amphipathic helices. Membrane-binding and membrane-curving abilities of synuclein raise the possibility that synuclein could alter cellular processes that involve highly curved structures. In the present study we examined the localization of endogenous synuclein in bovine chromaffin cells by immunocytochemistry and its possible function to control protein discharge upon fusion of the granule with the plasma membrane by regulating the fusion pore. We found with quantitative immunocytochemistry that endogenous β-synuclein associates with secretory granules. Endogenous α-synuclein only rarely co-localized with secretory granules. Overexpression of α-synuclein but not β-synuclein quickened the post-fusion discharge of BDNF-pHluorin by ~30%. However, neither α- nor β-synuclein significantly altered curvature dynamics associated with fusion pore expansion that were measured by the combination of polarization and total internal reflection fluorescence microscopy (pTIRFM). Whatever the mechanism, the physiological significance of the small increased rate of post-fusion protein discharge caused by α-synuclein remains to be demonstrated, especially since endogenous β-, but not α-synuclein is the predominant synuclein isoform associated with chromaffin granules.
Keywords: synuclein, immunocytochemistry, secretion, fusion pore, TIRFM, pTIRFM
INTRODUCTION
α-Synuclein is strongly implicated in the pathogenesis of Parkinson’s disease as well as in other neurodegenerative diseases (see review [1]). However, its normal function in cells is not understood. The presynaptic localization of synuclein raises the possibility that it normally regulates synaptic transmission and secretion. Indeed, α-synuclein modestly reduces the frequency of catecholamine secretion in chromaffin cells [2] and of synaptic transmission in nigrostriatal [3, 4], and hippocampal [5] nerve terminals. These effects could reflect the ability of synuclein to modulate synaptic vesicle recycling [6] and to regulate SNARE proteins that are necessary for exocytosis [7, 8].
There is another potential function for synuclein that is suggested by its structure. α-Synuclein is one of three members of a gene family consisting of α-, β-, and γ-synuclein with 50–60% sequence identity and similar domain organization. The N-termini of the three isoforms are comprised of six to seven 11-amino acid repeats that are predicted to form amphipathic helices. Indeed, α-synuclein binds to negatively charged lipids, especially small vesicles [9] and tubulates and vesiculates lipids [10]. The membrane-binding and membrane-curving abilities raise the possibility that synuclein affects the highly curved fusion pore that arises upon fusion of the granule membrane with the plasma membrane, a possibility that motivated the present study.
In the present study we investigated with several anti-synuclein antibodies the localization of α- and β-synuclein in bovine chromaffin cells. We examined the effects of α- and β-synuclein overexpression on protein discharge upon secretory granule fusion and, importantly, the effects of the synucleins on fusion pore expansion by using a combination of polarization and total internal reflection fluorescent microscopy (pTIRFM).
METHODS
Culture and transfection.
Primary bovine adrenal medullary chromaffin cells were isolated as previously described [11, 12] and transfected with the Neon Transfection System (Invitrogen). BDNF-pHluorin was constructed from BDNF (prBDNF-stop-EGFP-N1), which was obtained from Professor V. Lessman (Otto-Von-Guericke Universitat, Magelburg, Germany). EGFP-labeled human α-synuclein was obtained from Addgene. Unlabeled α-synuclein was constructed by removing the synuclein sequence and inserting it into pcDNA3 using HindIII and EcoRI restriction sites. The construct used to express human β-synuclein was made using synthetic gBlocks (Integrated DNA Technologies, Coralville, IA). The β-synuclein consensus sequence used was from accession number NM_001001502.2. The coding sequence was flanked by HindIII and the Kozak sequence GCCGCCACC on the 5’ end and a stop codon and BamHI on the 3’ end. The gBlock was digested with HindIII and BamHI and ligated into pcDNA3.1(+).
Immunocytochemistry - Confocal Microscopy.
Cells were fixed with 4% paraformaldehyde, permeabilized with methanol, incubated with various antibodies, rinsed, incubated with fluorescent-tagged 2° antibodies and imaged on a confocal microscope with a 60x objective. Images with different excitations were acquired sequentially. Within an experiment, initial settings were adjusted so that the brightest pixels for each color were unsaturated, and these settings were maintained throughout.
Antibodies.
Antibodies were from the following sources: DSHB Hybridoma Product H3C against α/β-synuclein (Developmental Studies Hybridoma Bank, The University of Iowa, Iowa City, IA 52242); antibodies specific for α- or β-synuclein (Abcam 138501 and Abcam 15532, respectively); Alexafluor-labeled secondary antibodies (Life Technologies (Molecular Probes)). Rabbit anti-bovine CgA was a gift from the laboratory of Daniel O’Connor, formerly of the Department of Medicine and Center for Molecular Genetics, University of California, San Diego, California 92093.
Secretion experiments.
All experiments were performed 3–5 days post-transfection in a room equilibrated to 34 ± 1°C. Individual cells were perfused through a pipet (100 μm inner diameter) using positive pressure from a computer-controlled perfusion system DAD-6VM (ALA Scientific Instruments, Westbury, NY). The bath solution and the initial perfusion solution were physiological saline solution: 145 mM NaCl, 5.6 mM KCl, 5 mM CaCl2, 0.5 mM MgCl2, 5.6 mM glucose, and 15 mM HEPES, pH 7.4). Cells were stimulated by perfusion with elevated potassium physiological salt solution: 95 (or 51) mM NaCl, 56 (or 100) mM KCl, 2.2 (or 5) mM CaCl2, 0.5 mM MgCl2, 5.6 mM glucose, and 15 mM HEPES, pH 7.4.
TIRFM and polarized TIRFM were performed as previously described [12, 13]. Time-varying local backgrounds were determined by capturing the intensity of a neighboring ROI without a fusion event and were subtracted frame by frame from the intensities of the discharge events.
Blinded analyses.
All secretion and pTIRFM duration measurements were performed blinded to assure that subjective biases did not distort the analyses. We found that blinding was necessary to be confident in the detection of relatively small changes in the analysis of BDNF-pHl discharge and the assessment of fusion pore expansion measured by pTIRFM.
RESULTS
Endogenous β-synuclein, but not α-synuclein, is bound to ~80% of secretory granules in bovine chromaffin cells.
We examined the localization of α- and β-synuclein in cultured bovine chromaffin cells by immunocytochemistry using a battery of three antibodies: one specific for α-synuclein, the second specific for β-synuclein, and a third which is non-isoform specific and detects both α- and β-isoforms. The sequences of bovine α- and β-synuclein and the epitopes seen by the antibodies are shown in Suppl. Fig. 1. The specificity of each antibody was verified by its ability to recognize the appropriate overexpressed protein(s).
In bovine chromaffin cells, all three anti-synuclein antibodies exhibited punctate rather than diffuse cytoplasmic staining. Puncta labeled by the antibody that recognizes both α- and β-synuclein (Fig. 1B, D) and by antibody specific for β-synuclein (Fig. 1F) were abundant and found in most cells. In contrast, puncta labeled by the α-synuclein antibody were relatively sparse and were often undetectable in some cells (Fig. 1H, Suppl. Figure 2).
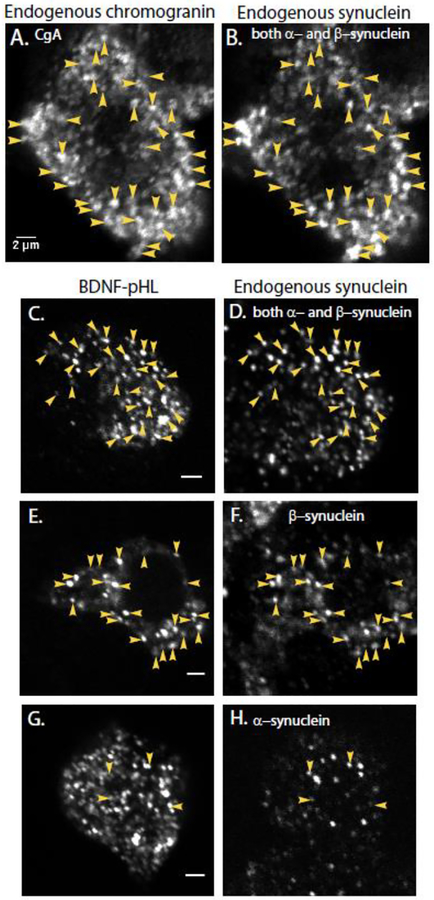
Fig. 1. Endogenous synuclein labels secretory granules in non-transfected cells (A, B) and newly synthesized chromaffin granules expressing exogenous BDNF-pHl in transfected cells (C-H).
Granules were identified with an antibody against chromogranin A (A) and endogenous synuclein with a monoclonal antibody that recognizes both the α- and β-isoforms of bovine synuclein (DSHB H3C, B). Immunocytochemistry was performed on chromaffin cells transiently expressing the chromaffin granule marker BDNF-pHl (C-H). (D) Endogenous synuclein was again detected by DSHB H3C, which recognizes both α- and β-synuclein. Arrowheads indicate some of the co-localized puncta. Synuclein puncta in a neighboring non-transfected cell are visible at the bottom of the image. (F) Endogenous synuclein as detected by an antibody specific for β-synuclein (Abcam 15532); (H) Endogenous synuclein as detected by an antibody specific for α-synuclein (Abcam 138501). Panels A and B, C and D, E and F and G and H are paired images of the same field of view. Note that panels C, E, and G show single transfected cells. Panels D, F, and H show several cells including the transfected cell. Scale bars = 2 μm.
In order to determine whether the synuclein puncta reflect binding of the protein to secretory granules in non-transfected cells, secretory granules (chromaffin granules) were identified by an antibody against the major lumenal protein in chromaffin cell secretory granules, chromogranin A (CgA) (Fig. 1A, B). Ninety percent of CgA puncta co-localized with synuclein (identified by the non-isoform-specific antibody, 500 total granules from 4 cells). Only 6% of the synuclein puncta did not co-localize with CgA. A similar result was obtained when granules were labeled by transfection with a plasmid encoding brain-derived neurotrophic factor (BDNF) fused to pHluorin (pHl). This protein traffics to secretory granules in a wide variety of cell types including neurons [14, 15] and mouse chromaffin cells [16]. Eighty-nine percent of the BDNF-pHl puncta (410 granules in 12 cells) co-localized with synuclein (identified by the non-isoform-specific antibody, Fig. 1C ,D). Synuclein was also present on numerous other puncta, which were likely secretory granules synthesized prior to transfection with BDNF-pHl.
Because of the high intensity and low background imaging of BDNF-pHl-containing granules, the identity of the synuclein isoform on secretory granules was investigated in cells expressing BDNF-pHl. The antibody specific for β-synuclein recognized a majority of BDNF-pHl granules (Fig. 1E and F), labeling 78% (626 total granules in 16 cells). This high frequency of labeling was similar to the labeling with the antibody that recognizes both α- and β-synuclein. In contrast, puncta seen by the α-synuclein antibody rarely coincided with BDNF-pHl granules (Fig. 1G and H), labeling only 5% (828 granules in 8 cells). Together, these data indicate that endogenous synuclein is associated with secretory granules in bovine chromaffin cells with β-synuclein being the major isoform.
The subcellular localization of transiently transfected, unlabeled α- and β synuclein was also determined by immunocytochemistry (Suppl. Fig. 3). Soluble GFP was co-transfected to identify transfected cells. There was considerable cell-to-cell variability in the subcellular localization of the overexpressed synucleins. Overexpressed synuclein (both α and β) was sometimes largely punctate throughout the cytoplasm (Suppl. Fig. 3A and C) but also sometimes concentrated in the cell periphery (Suppl. Fig. 3B and D).
Overexpressed α-synuclein, but not β-synuclein, causes a 30% shortening of the post-fusion discharge time of BDNF-pHluorin from chromaffin granules.
Chromaffin cells were co-transfected with a plasmid encoding BDNF-pHl and either a plasmid encoding unlabeled α-or β-synuclein or control plasmid (pcDNA3). Immunocytochemistry indicated that transfected α-synuclein or β-synuclein was co-expressed with BDNF-pHl in 95–100% of the cells. Live cell experiments were conducted at 34–35°C, close to physiological temperature with imaging at 36 Hz. Cells were stimulated by perfusion of single cells with solution containing elevated K+. PHluorin, whose fluorescence is extremely pH sensitive, is almost undetectable in the acidic interior (pH ~5.5) of the secretory granule in living cells before fusion. Upon fusion there is a profound increase in pHluorin fluorescence as the granule lumen equilibrates with the extracellular pH (7.4) followed by a decrease (falling phase) in intensity as the protein diffuses into the medium (Fig. 2A). The kinetics of labeled protein discharge can be complex, with variable shapes of fluorescence intensity vs time and uncertain baseline plateaus. A custom computer program was used to measure duration (described in [12]) that is robust against changes in event shape and is relatively insensitive to baseline selection.
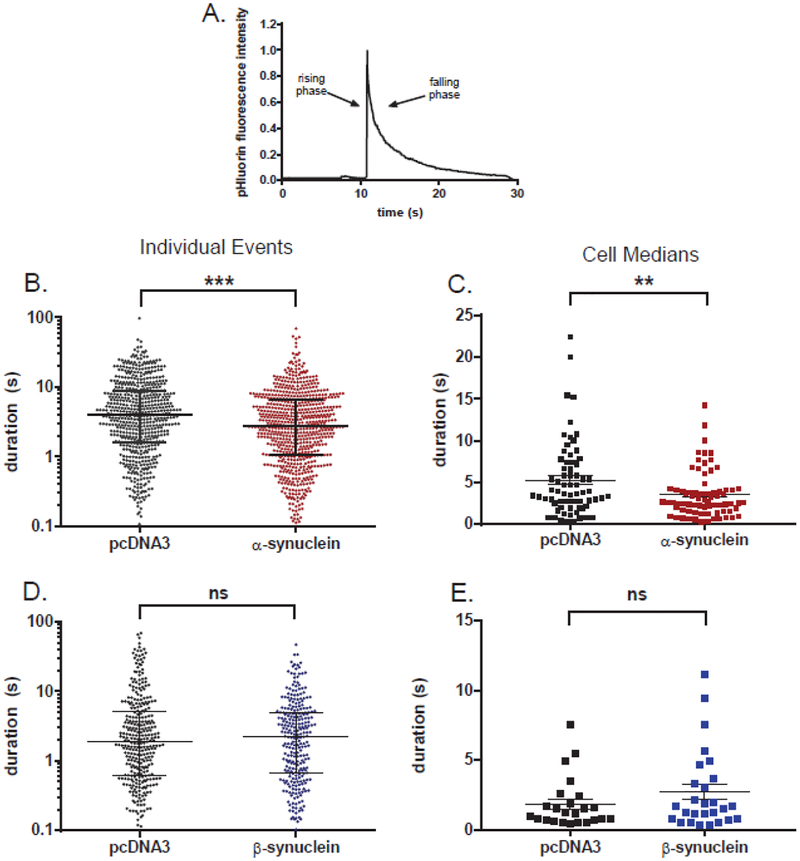
Fig. 2. α-Synuclein but not β-synuclein speeds the post-fusion discharge of BDNF-pHl.
(A) An example of the discharge of BDNF-pHl from a secretory granule upon fusion. (B) Scatter plot of event durations for α-synuclein-transfected cells and control cells (n=685 and 598 events, respectively). Note that the ordinate is log scale. ***, p = 0.0001 (Kolmogorov-Smirnov test). (C) Scatter plot of the median BDNF-pHl duration in each α-synuclein cell and control cell with greater than five events (n=73 and 67 cells, respectively). **, p = 0.006 (Student’s t-test). (D) Scatter plot of all event durations for β-synuclein-transfected and control cells (n=277 and 327 events, respectively). ns, non-significant difference (Kolmogorov-Smirnov test). (E) Scatter plot of the median BDNF-pHl duration in each β-synuclein-transfected and control cell with greater than five events (n=27 and 24 cells, respectively). ns, Student’s t-test. Horizontal bars indicate the median (longest bar) and the upper and lower quartiles in B and D. The mean of the cell medians (longest horizontal bar) and SEM (error bars) are shown in C and E.
α-Synuclein quickened to a small degree the post-fusion discharge of BDNF-pHl. Median duration decreased from 3.9 s (593 events) to 2.8 s (685 events, p=0.0001) (Fig. 2B). The effects of α-synuclein were also evident when the data were analyzed cell-by-cell (Fig. 2C). The average of the median event durations in individual cells decreased from 5.3 s (67 control cells) to 3.5 s (73 α-synuclein transfected cells, p=0.006). In contrast, β-synuclein did not have a significant effect on BDNF-pHl discharge either when the events were grouped together from many cells (Fig. 2D) or when the events were expressed cell-by-cell (Fig. 2E). (The β-synuclein secretion experiments were performed approximately one year after the α-synuclein experiments with somewhat shorter control discharge durations.)
Most BDNF-pHl events reached peak fluorescence within one or two frames (28–56 ms). There was no change in the rise times upon overexpression of either α- or β-synuclein.
Neither α- nor β-synuclein significantly alter fusion pore curvature dynamics associated with the fusion of secretory granules.
The effect of α-synuclein to speed the discharge of labeled BDNF may reflect an increased rate of fusion pore expansion. This possibility was directly investigated by determining the effects of synuclein on the curvature dynamics associated with the expanding fusion pore using a combination of polarization and TIRFM. Chromaffin cells were co-transfected with BDNF-EGFP and α- or β-synuclein or an empty vector control (pcDNA3). Plasma membranes were stained with DiI and stimulated to secrete by perfusion with elevated K+. At sites of BDNF-EGFP discharge, the curvature associated with the fusion pore was measured by monitoring the orientation of DiI, which incorporates into the plasma membrane bilayer with its preferred polarization of light absorption and emission parallel to the local plane of the membrane [17]. The technique used to detect the orientation of DiI relies on a combination of polarization and TIR optics (pTIRF) and has been described in detail in earlier publications [12, 18]. Prior to fusion, DiI is restricted to the flat plasma membrane with its absorption dipole orientated parallel to the coverslip, and is excited preferentially by S-polarization. After fusion, DiI diffuses into the fused granule membrane where it can be orientated with a component perpendicular to the coverslip. It is significantly excited by P-polarization. The key curvature measurement is an increase in ratio of emissions from P-polarization excitation and S-polarization excitation (termed P/S) and is independent of fluorophore concentration.
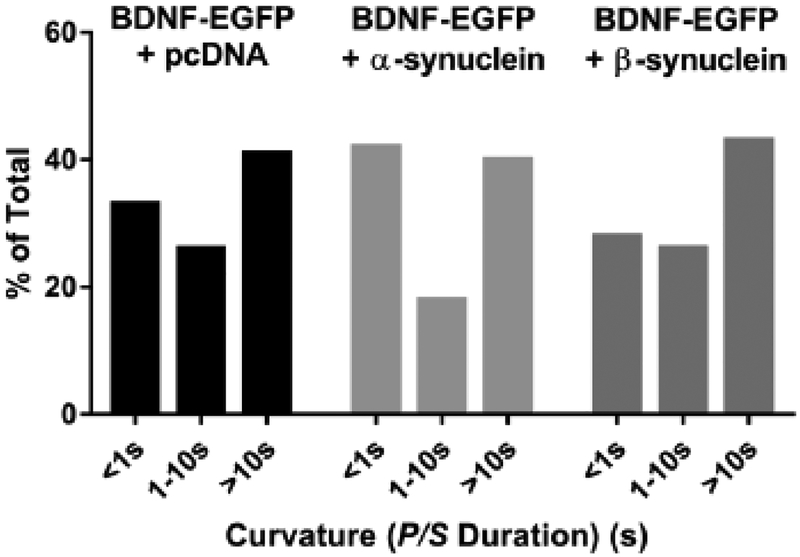
As with the secretion experiments described above, pTIRFM imaging was performed at 34–35 °C. Punctate changes in P/S ratio were detected coincident with BDNF-EGFP discharge. The length of time P/S remained elevated above the pre-fusion baseline was measured and binned into three categories, 1 s or less, greater than 1 s but less than 10 s, and greater than 10 s (Fig. 3). P/S durations were evenly distributed between those with durations less than one second and those with durations greater than 10 s with or without α- or β-synuclein. Co-expression of either α- or β-synuclein did not alter the distribution of fusion pore curvature lifetimes associated with BDNF-EGFP discharge (chi-squared test).
Fig. 3. Neither α-synuclein nor β-synuclein alters fusion pore curvature associated with BDNF discharge.
Fusion pore curvature associated with elevated K+-induced BDNF-EGFP discharge was monitored using pTIRF. The length of time P/S was elevated above the pre-fusion baseline was calculated for 88 BDNF-EGFP + pcDNA events, 88 BDNF + α-synuclein events, and 46 BDNF-EGFP + β-synuclein events and binned into three categories. The distributions were not significantly different.
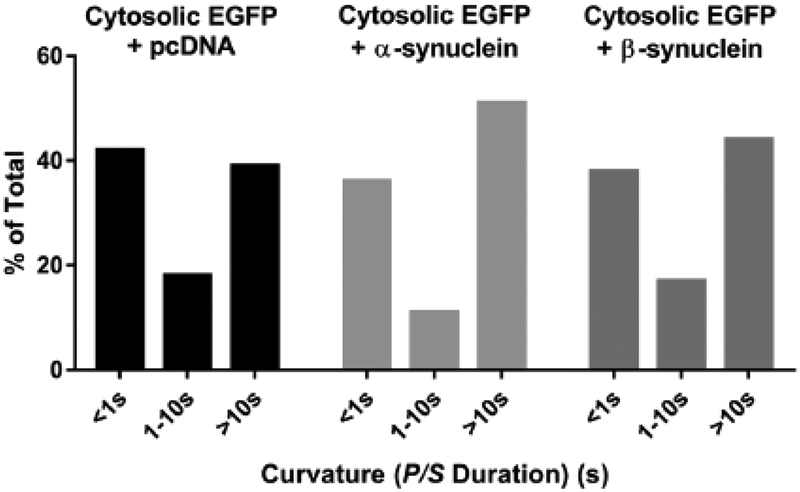
The distribution of fusion pore curvature durations associated with fusion of granules containing exogenous proteins can be different from those containing only endogenous proteins [13]. To investigate the effects of synuclein overexpression on fusion pore dynamics of granules not containing exogenous protein, chromaffin cells were co-transfected with plasmids encoding unlabeled α- or β-synuclein or a control plasmid and EGFP (expressed in the cytosol, to enable the identification of transfected cells). Immunocytochemistry revealed that virtually all cells expressing cytosolic EGFP expressed either co-transfected α- or β-synuclein. Plasma membranes were labeled with DiI, stimulated to secrete with elevated K+, and imaged using pTIRFM. Fusion of secretory granules (without exogenous protein) resulted in discrete, punctate changes in DiI fluorescence with increases in P/S. The events required Ca2+ in the medium (data not shown). The lengths of time that the P/S remained elevated were binned into three categories of durations as described above. P/S was similarly distributed between short and long duration events in the transfected cells (Fig. 4). Neither α- nor β-synuclein altered the distributions (chi-squared test).
Fig. 4. Neither α-synuclein nor β-synuclein alters fusion pore curvature associated with fusion of granules not expressing exogenous protein.
Chromaffin cells were co-transfected with EGFP + pcDNA, EGFP + α-synuclein, or EGFP + β-synuclein. Upon stimulation, fusion of endogenous granules (i.e, not containing exogenous transfected protein) was identified as discrete, punctate changes in DiI fluorescence. At these sites, the length of time P/S remained elevated was measured for 88 EGFP + pcDNA events, 137 EGFP + α-synuclein events, 52 EGFP + β-synuclein events and binned into three categories. The distributions were not significantly different.
DISCUSSION
The purpose of these experiments was to examine the possible role of α- and β-synuclein in regulating fusion pore expansion and post-fusion protein discharge upon exocytosis in adrenal medullary chromaffin cells. These cells are not only important components of the sympathetic nervous system, but are also a powerful, versatile model system for investigating Ca2+-dependent protein secretion. We found that: 1) β-Synuclein but not α-synuclein is the synuclein isoform most associated with secretory granules (chromaffin granules) in chromaffin cells. 2) Overexpression of α-synuclein but not β-synuclein modestly shortens the post-fusion discharge time of transfected BDNF. 3) Neither α- nor β-synuclein alters the dynamics of fusion pore expansion as detected by pTIRFM.
Mechanism for the α-synuclein-induced quickening of post-fusion protein discharge.
Because the speed of protein discharge after fusion has been associated with fusion pore expansion [19, 20], one explanation for the more rapid protein discharge with α-synuclein overexpression is an increased rate of fusion pore expansion. However, direct investigation of the fusion pore using pTIRFM did not detect an alteration in curvature dynamics upon overexpression of either α- or β-synuclein. The absence of changes in curvature dynamics is consistent with amperometric studies that failed to detect differences in the kinetics of catecholamine discharge upon granule fusion in chromaffin cells derived from wild type mice, mice without α-synuclein and mice overexpressing either wild type or A30P α-synuclein [2]. We, of course, cannot rule out a subtle change in fusion pore dynamics that is undetectable by amperometry or pTIRFM.
It should be noted that based upon the ability of α-synuclein to confer increased curvature to lipid vesicles in vitro because of repeated amphipathic helices in its N-terminal domain [9, 10], one predicts that α-synuclein would stabilize the high-curvature neck of the early fusion pore, thereby slowing fusion pore expansion, just the opposite of what would explain the more rapid BDNF discharge. Indeed, the absence of effect of overexpressed β-synuclein on BDNF-pHl discharge suggests that the N-terminal amphipathic helices common to both α- and β-synuclein are not responsible for the α-synuclein effect on protein discharge.
We have recently found that exogenous BDNF in chromaffin cell secretory granules is virtually immobile (Abbineni, Holz and Axelrod, in preparation). Hence, the discharge rate may depend upon the dynamics of dissolution of the protein following fusion (rather than upon fusion pore expansion). For example, the state of the lumenal protein could be influenced by granule-bound synuclein through unknown effects on granule maturation following exit of the immature granule from trans Golgi network (TGN) or on ion transport across the granule membrane.
Significance of α-synuclein effect to speed post-fusion protein discharge.
The effect of α-synuclein was at most modest, a 30% quickening in post-fusion discharge time that required analysis of many hundreds of events from tens of cells in order to attain statistical significance. The analyses of BDNF-pHl discharge (and curvature changes detected by pTIRF) were performed blinded so that subjective biases did not influence the results. The speedier discharge did not reflect the appearance of a new subpopulation of fusion events. Whatever the mechanism, the physiological significance of the effect of α-synuclein on protein discharge remains to be demonstrated. This is especially true in chromaffin cells, since endogenous β-, but not α-synuclein is the predominant synuclein isoform associated with chromaffin granules.
β-Synuclein exclusively binds to chromaffin granules.
Perhaps the most significant finding of this study is that β-synuclein was bound almost exclusively to secretory granules (identified by endogenous chromogranin A) in chromaffin cells. At least 80% of the secretory granules were labeled; only 6% of the synuclein puncta did not co-localize with chromogranin A puncta. Endogenous α-synuclein rarely labeled secretory granules. Identification of the ligand to which β-synuclein binds on the granule membrane and the dynamics of binding are important avenues of future work that may illuminate its function in neuroendocrine cells.
Relationship to previous work.
A similar effect of α-synuclein to modestly speed post-fusion BDNF-pHl discharge was observed in mouse chromaffin cells [16]. There were no direct measurements of fusion pore expansion. However, in contrast to our study, the previous study found that β-synuclein, as well as α-synuclein, increased the rate of BDNF-pHl discharge. We do not have an explanation for the discrepancy, except for the different animal source (bovine vs. mouse), the different temperature of the experiments (34°C in current experiments vs. room temperature in the mouse study) and the different method of expression of exogenous synuclein and BDNF-pHl (electroporation vs. lentivirus).
Supplementary Material
Highlights.
α-Synuclein is strongly implicated in the pathogenesis of Parkinson’s disease as well as in other neurodegenerative diseases. However, its normal function in cells is not understood. The N-termini of α-, β-, and γ-synuclein are comprised of multiple 11-amino acid repeats that are predicted to form amphipathic helices. α-Synuclein binds to negatively charged lipids, especially small vesicles, and tubulates and vesiculates lipids, raising the possibility that the α-synuclein normally functions to control fusion pore expansion and lumenal granule protein discharge after fusion. In the current paper we find that β-synuclein is the major synuclein isoform on secretory granules in bovine chromaffin cells and labels almost every secretory granule. Overexpressed α-synuclein, but not β-synuclein, increases by approximately 30% the post-fusion discharge rate of co-expressed BDNF-pHl without altering fusion pore dynamics measured by a combination of polarization and TIRFM. The mechanism by which α-synuclein speeds protein discharge from fused granules and the physiological significance of the small effect remains to be determined. Identification of the ligand to which β-synuclein binds on the granule membrane, the dynamics of binding and its function are important avenues of future work.
Acknowledgements.
We are grateful to William T. Dauer for providing synuclein constructs and timely advice. We thank Robert H. Edwards and Todd Logan (UCSF) for engaging discussions. Our work was supported by the Rapid Response Innovation Award from the Michael J. Fox Foundation for Parkinson’s Research to RWH, by NIH Grant RO1–170553 to RWH and DA, and by 5T32HL7853–17 to KPB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Bendor JT, Logan TP, Edwards RH, The Function of alpha-Synuclein, Neuron 79 (2013) 1044–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Larsen KE, Schmitz Y, Troyer MD, Mosharov E, Dietrich P, Quazi AZ, Savalle M, Nemani V, Chaudhry FA, Edwards RH, Stefanis L, Sulzer D, {alpha}-Synuclein Overexpression in PC12 and Chromaffin Cells Impairs Catecholamine Release by Interfering with a Late Step in Exocytosis, Journal of Neuroscience 26 (2006) 11915–11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A, Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system, Neuron 25 (2000) 239–252. [DOI] [PubMed] [Google Scholar]
- [4].Yavich L, Tanila H, Vepsalainen S, Jakala P, Role of alpha-synuclein in presynaptic dopamine recruitment, J Neurosci 24 (2004) 11165–11170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nemani VM, Lu W, Berge V, Nakamura K, Onoa B, Lee MK, Chaudhry FA, Nicoll RA, Edwards RH, Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis, Neuron 65 (2010) 66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang L, Das U, Scott DA, Tang Y, McLean PJ, Roy S, alpha-synuclein multimers cluster synaptic vesicles and attenuate recycling, Curr Biol 24 (2014) 2319–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC, Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro, Science 329 (2010) 1663–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Sudhof TC, Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration, Cell 123 (2005) 383–396. [DOI] [PubMed] [Google Scholar]
- [9].Davidson WS, Jonas A, Clayton DF, George JM, Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes, J Biol Chem 273 (1998) 9443–9449. [DOI] [PubMed] [Google Scholar]
- [10].Westphal CH, Chandra SS, Monomeric synucleins generate membrane curvature, J Biol Chem 288 (2013) 1829–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wick PW, Senter RA, Parsels LA, Holz RW, Transient transfection studies of secretion in bovine chromaffin cells and PC12 cells: generation of kainate-sensitive chromaffin cells, Journal of Biological Chemistry 268 (1993) 10983–10989. [PubMed] [Google Scholar]
- [12].Bohannon KP, Bittner MA, Lawrence DA, Axelrod D, Holz RW, Slow fusion pore expansion creates a unique reaction chamber for co-packaged cargo, J Gen Physiol 149 (2017) 921–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Abbineni PS, Bittner MA, Axelrod D, Holz RW, Chromogranin A, the major lumenal protein in chromaffin granules, controls fusion pore expansion, J Gen Physiol (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Matsuda N, Lu H, Fukata Y, Noritake J, Gao H, Mukherjee S, Nemoto T, Fukata M, Poo MM, Differential activity-dependent secretion of brain-derived neurotrophic factor from axon and dendrite, J Neurosci 29 (2009) 14185–14198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Xia X, Lessmann V, Martin TF, Imaging of evoked dense-core-vesicle exocytosis in hippocampal neurons reveals long latencies and kiss-and-run fusion events, J Cell Sci 122 (2009) 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Logan T, Bendor J, Toupin C, Thorn K, Edwards RH, alpha-Synuclein promotes dilation of the exocytotic fusion pore, Nat Neurosci 20 (2017) 681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Axelrod D, Carbocyanine dye orientation in red cell membrane studied by microscopic fluorescence polarization, Biophys.J. 26 (1979) 557–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Anantharam A, Onoa B, Edwards RH, Holz RW, Axelrod D, Localized topological changes of the plasma membrane upon exocytosis visualized by polarized TIRFM, The Journal of Cell Biology 188 (2010) 415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Weiss AN, Anantharam A, Bittner MA, Axelrod D, Holz RW, Lumenal Protein within Secretory Granules Affects Fusion Pore Expansion, Biophys J 107 (2014) 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rao TC, Passmore DR, Peleman AR, Das M, Chapman ER, Anantharam A, Distinct fusion properties of synaptotagmin-1 and synaptotagmin-7 bearing dense core granules, Mol Biol Cell 25 (2014) 2416–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.