Abstract
Conventional manufacturing of protein biopharmaceuticals in centralized, large-scale single-product facilities is not well-suited to the agile production of drugs for small patient populations or individuals. Solutions for small-scale manufacturing are potentially more nimble, though previous systems are limited in both process reproducibility and product quality, owing to complicated means of protein expression and purification1–4. We describe an automated bench-top multi-product manufacturing system, called Integrated Scalable Cyto-Technology (InSCyT), for the end-to-end production of hundreds to thousands of doses of clinical-quality protein biologics in about three days. We also demonstrate that InSCyT can accelerate process development from sequence to purified drug in 12 weeks. We produced hGH, IFNα-2b, and G-CSF using highly similar processes on InSCyT and found that the purity and potency of these products is comparable to that of marketedreference products.
Biologic medicines, such as recombinantly expressed cytokines, hormones, replacement enzymes, blood factors, or antibodies, are routinely used to treat cancer, autoimmune disorders and rare diseases. Increasingly, protein biologics are tailored to small groups of patients based on an understanding of the underlying biology of their disease5. The need for only a few doses of many products poses a challenge to conventional manufacturers who produce drugs in large volumes to achieve economies of scale6. Furthermore, different classes of biopharmaceuticals (e.g., enzymes, hormones, vaccines) generally require unique customized processes for each molecule from expression to purification, constraining commercial facilities to a single class of product.
New technologies to manufacture many different pharmaceutical-quality biologics in small quantities with efficiency and agility are needed to make precision biologic medicines both available and economically feasible7. Technologies such as automated lab-scale batch processes, in vitro transcription and translation, and microfluidics can rapidly produce limited quantities of different biomolecules on-demand1–4. Whilst some of the products expressed using these technologies have biological activity, they lack sufficient quality attributes for clinical use, including identity, purity, safety, and potency as required by regulatory agencies. To address this need, we developed an automated multi-product manufacturing system capable of rapidly producing clinical-grade recombinant proteins and requiring only minimal reconfiguration to make different biopharmaceuticals.
We selected Pichia pastoris as our expression host because it can grow quickly to high cell densities and efficiently secrete recombinant proteins8. Other advantages of P. pastoris include low levels of secreted host-cell proteins, little to no risk of viral contamination, validated expression of myriad proteins including FDA/EMA-approved therapeutics, and the capability for human-like post-translational modifications in engineered strains9,10.
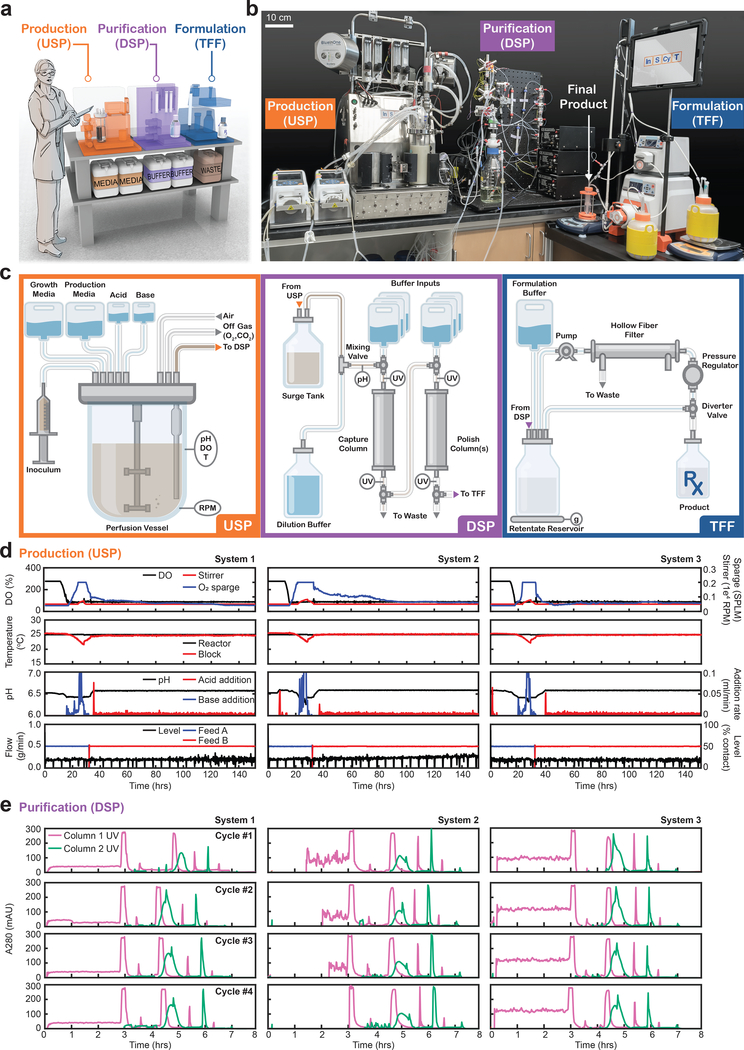
InSCyT uses fluidically-connected modules for fermentation, multi-stage chromatography, and ultrafiltration/diafiltration, as well as integrated sensors and system controllers for system-wide programmed operations (Fig. 1a,b and Supplementary Fig. 1). We implemented continuous fermentation by perfusion to reduce the volume of the bioreactor and enable high space-time yields11. To this end, we adapted a sub-liter benchtop bioreactor for in-tank perfusion and equipped it with sensors to control input and output flows, pH, temperature, impeller speed, and dissolved oxygen (DO) (Fig. 1c). The bioreactor was connected to a module for in-line pH adjustment of the cell culture fluid prior to chromatographic separations; this module allowed for balancing flow rates between those for production and purification. An integrated module for purification was designed to enable either two or three stages of chromatographic separation (Fig. 1c). This module allowed straight-through processing with no intermediate holding tanks or adjustments between purification steps. Our design simplifies the operation of the module relative to traditional purifications, where multiple intermediate procedures are often required to adjust pH, conductivity, concentration, and composition of fluids between steps of purification. The final module in the system comprised a tangential flow-filtration system for buffer exchange and formulation to a final liquid dosage-ready form of the product (Fig. 1c). A custom integrated software architecture unified operation of all three modules with appropriate controls as a fully-automated single system.
Figure 1.
Schematic of the InSCyT system for on-demand biomanufacturing and demonstration of consistent operation across three distinct InSCyT systems. (a) To-scale rendering of the InSCyT system. Human figure is approximately 5’7”. (b) Photograph of an operational InSCyT system. (c) Detailed schematic of the InSCyT system including interactions between modules and key control points for the production (upstream processing, USP), purification (downstream processing, DSP) and formulation (tangential flow filtration, TFF) modules. Process parameter profiles collected by the control software from (d) the production (USP) module and (e) the purification (DSP) module of three separate InSCyT systems during hGH fermentation.
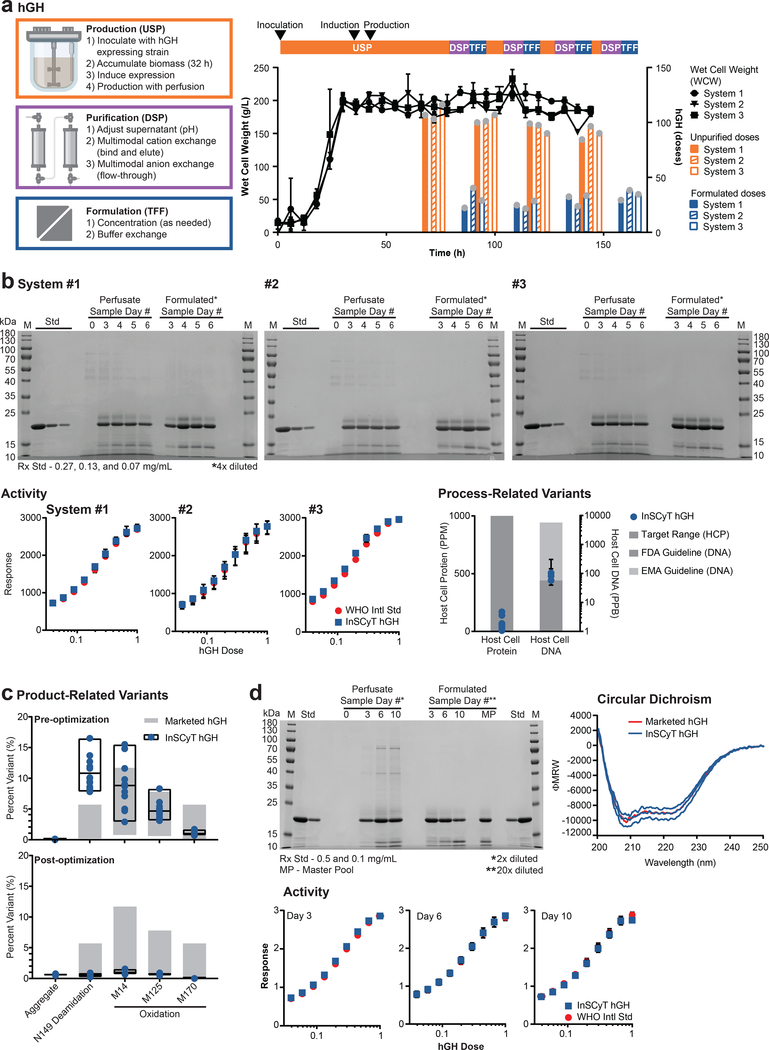
We built and used three independent InSCyT systems to demonstrate consistent operational performance for both production and purification processes (Fig. 1d,e). We first produced and purified the common biologic medicine human growth hormone (hGH), used to treat growth deficiencies12. The extensive knowledge available about this drug’s quality, safety, and potency aided our assessment of InSCyT and the novel process we developed for production of hGH. Our production process uses two straight-through stages of chromatographic purification, and requires more than 50% fewer operations than the innovator process (Supplementary Fig. 2). We implemented our process on three separate InSCyT systems. After inoculation, each process ran “hands-free” and produced > 100 doses of formulated product in less than one week, with initial yields for the process ranging from 27%−31% on each system (Fig. 2a).
Figure 2.
Production of hGH on the InSCyT system. Dose size used was 1.75 mg12. Center values and error bars represent the mean and range, respectively, of technical triplicates unless otherwise indicated. (a) Process flow chart (left), timeline and yields (right) for production of hGH using InSCyT. Wet cell weight (WCW) (black), unpurified (orange) and formulated (blue) doses of hGH produced are shown. Grey circles represent individual data points. (b) Product quality analyses for InSCyT-produced hGH pre-optimization alongside a reference drug substance from a licensed hGH product produced in E. coli. SDS-PAGE (12% tris-glycine) analysis of samples from the USP during biomass accumulation and production (perfusate samples), final, formulated samples (formulated samples) and the reference (Std). Activity of InSCyT hGH alongside the WHO international standard (NIBSC 98/574). The final formulated sample (day 6) was analyzed from each system. Quantification of host-cell protein and host-cell DNA impurities in formulated InSCyT hGH. Host-cell protein limits are shown as a target range14,15. Host-cell DNA guidelines are based on 100 pg/dose (FDA) and 10 ng/dose (EMA)16,30. For host-cell protein data, each point represents a unique sample (12 points total; 4 time points from each of three InSCyT systems). For host-cell DNA, data each point represents a single pooled sample from each system comprising equal volumes of samples from each time point (3 points total; 1 per system). (c) Analysis of product-related variants in formulated InSCyT hGH pre-optimization (top) and post-optimization (bottom) alongside levels typically found in marketed products (Supplementary Fig. 5). Each data point represents a unique sample; there are 12 data points for pre-optimization runs (four time points from each of three InSCyT systems) and 3 data points for post-optimization runs (three time points from a single InSCyT system). Black boxes represent the range of InSCyT hGH samples with an additional line at the mean. (d) Product quality analyses for InSCyT produced hGH post-optimization alongside reference drug substance from a licensed hGH product. SDS-PAGE (12% tris-glycine) analysis of samples from the USP during biomass accumulation and production (perfusate samples), final, formulated samples (formulated samples) and the reference (Std). Activity of InSCyT hGH alongside the WHO international standard (NIBSC 98/574). Secondary structure analysis of InSCyT hGH (individual formulated samples from days 3, 6, and 10) and the reference hGH standard using circular dichroism (CD).
We compared the biophysical and biochemical attributes of our purified product from multiple time points on each system to a marketed drug substance using multiple analytics commonly used to establish identity, potency, safety, and purity of therapeutic proteins13 (Fig. 2b,c and Supplementary Fig. 3). We confirmed the protein sequence (100% coverage) by mass spectrometry (Supplementary Fig. 4). Potency of InSCyT hGH was comparable to the NIBSC WHO 98/574 reference standard (96%−104%) using a cell-based proliferation assay. Key contributors to product safety include the levels of potentially immunogenic product-related impurities, such as aggregates, and the presence of process-related impurities, including host-cell proteins (HCPs) and host-cell DNA. Minimal high molecular weight species were present in the InSCyT hGH product (<0.5%), and levels of process-related impurities were each below typical values for clinical-stage development (1000 PPM for HCPs and 10 ng/dose for DNA)14–16. Regulatory agencies typically consider limits for host-cell proteins on a case-by-case basis, although in vitro studies using PBMCs from both healthy and diseased individuals have shown that HCP levels up to 4000 PPM from Chinese hamster ovary (CHO) cells do not pose a higher immunogenicity risk than a highly purified mAb (<50 PPM)15. A small percentage (average: 6%) of a proteolytically cleaved form of hGH was also observed (two-chain variant); this natural form is both highly potent and previously has been determined as clinically irrelvant17. Further assessment for product-specific impurities by LCMS, however, showed that InSCyT hGH was not comparable to marketed products due to increased levels of deamidation and oxidation (Fig 2c and Supplementary Fig. 5).
Given the consistency of both the operation of individual InSCyT systems and the hGH products produced by each system, we attributed the deamidation and oxidation observed in our product to process parameters, rather than the InSCyT system specifically. We, therefore, modified our process for hGH without any significant hardware changes by changing to a defined medium, adjusting the setpoint for dissolved oxygen and eliminating agitation in the surge tank. Using this adjusted production process, a single InSCyT system produced nearly 50 maximum weight-based doses of hGH in 75 h (Supplementary Fig. 6). Oxidation and deamidation were reduced to below 1% at each residue, while all other quality attributes were maintained (Fig. 2c,d and Supplementary Fig. 6). Process yield also increased to nearly 80%. Together, these data demonstrate the capability of our manufacturing system to rapidly and reproducibly produce tens of doses of a potent and pure form of a biologic drug in an automated, short production cycle.
We next sought to demonstrate on-demand production of hGH with InSCyT. The upstream perfusion process was operated fully automated for 240 hours. In addition to the initial purification and formulation cycle on day 3 (described above), two additional cycles of purification and formulation were performed on-demand during days 6 and 10 (Supplementary Fig. 6). Each on-demand cycle produced between 50 and 75 doses of hGH within 12 hours, with product quality and yields similar to the batch produced on day 3 (Fig. 2c,d and Supplementary Fig. 6). (We attributed the reduced yield (50%) and increased host-cell protein levels observed during the day 6 cycle to overloading the capture column, which we adjusted prior to the day 10 cycle.) Further optimization of the process and column sizing could improve the consistency from batch to batch. Nonetheless, the ability to produce small lots of this product on-demand shows the potential for manufacturing medicines as needed, and highlights the stability of yeast-based bioprocesses in continuous operations.
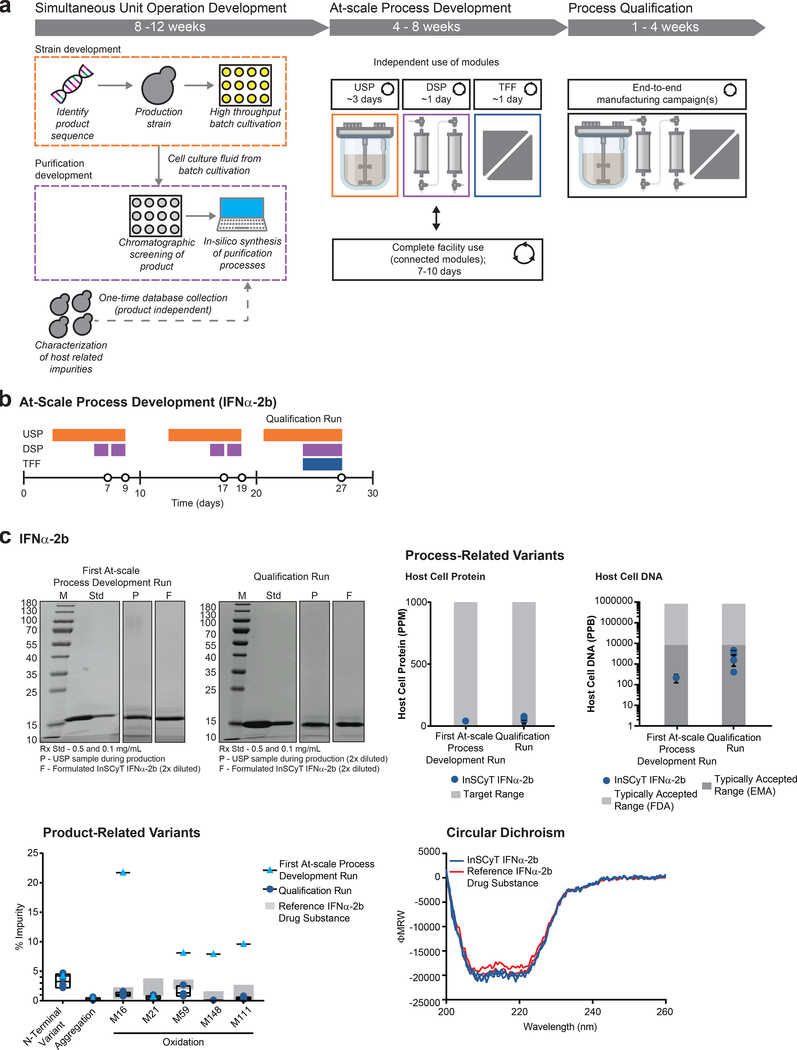
Biopharmaceuticals typically require bespoke manufacturing processes that vary widely, especially for non-mAbs, and require unique facility designs11. This constraint limits facility flexibility toward additional products, which would be essential for on-demand production. We therefore assessed whether our modular manufacturing system and choice of host could readily produce other molecules with no substantial hardware alterations. We selected IFNα-2b as a second example. This potent 19.2 kDa cytokine is used in both monotherapies and drug combinations to treat cancer and hepatitis, and is produced commercially in a unique 13-step process using E. coli18 (Supplementary Fig. 2). Due to the ease of targeted transgene insertion and simplicity of upstream process development in P. pastoris, we developed a draft fermentation process for producing secreted IFNα-2b less than 4 weeks after identifying the product sequence (Fig. 3a). We have found that P. pastoris routinely secretes a consistent set of host-cell proteins along with the heterologous product during fermentation to yield a high level of initially pure product (>80%)19. This feature made it possible to develop an in silico tool to predict draft multistage purification processes20. With this in silico tool, we selected a process to purify IFNα-2b within an additional 4 weeks. The procedures developed for both production and purification did not require modifications to the InSCyT system itself.
Figure 3.
Accelerated process development using the InSCyT system and production of IFNα-2b. Dose size was 12 μg18. Center values and error bars represent the mean and range, respectively, of technical triplicates unless otherwise noted. (a) Process development timeline for new manufacturing processes using the InSCyT system, including simultaneous unit operation development (comprised of strain development and purification development), at-scale process development (comprised of simultaneous experiments on individual modules and on the fully integrated system), and process qualification. (b) Timeline for at-scale process development for IFNα-2b. Horizontal colored bars represent the modules that were used in each experiment (USP – orange, DSP – purple, TFF – blue). Each new bar represents a new set of experimental conditions on that module. (c) Product quality for InSCyT-produced IFNα-2b from the first at-scale run after initial unit operation development (first at-scale process development run) and the final qualification run alongside a reference drug substance produced in E. coli. SDS-PAGE (12% tris-glycine) analysis of samples from the USP during production (P), a final, formulated sample (F) and a reference drug substance (Std). Analysis of process-related variants in formulated InSCyT IFNα-2b (per Fig. 2b). Each data point represents a unique sample, there is 1 data point from the first at-scale process development run and 4 data points from the qualification run (four time points from a single InSCyT system). Product-related variants detected in formulated InSCyT IFNα-2b alongside levels typically found in a reference drug substance (Supplementary Fig. 5). Black boxes represent the range of InSCyT IFNα-2b samples with an additional line at the mean. Secondary structure analysis of InSCyT IFNα-2b (triplicate analyses of an individual sample from the qualification run) and reference drug substance (duplicate analyses of an individual sample) using circular dichroism (CD).
Initial biophysical analyses of IFNα-2b produced in our first run on the InSCyT system indicated minimal high molecular weight species (0.34%), and process-related impurities below typical values for clinical development (Fig. 3c). A cell-based viral replication assay demonstrated that the potency of our IFNα-2b was the same or greater than a reference drug substance. InSCyT-generated IFNα-2b was highly potent (134%) in part owing to a naturally-occurring C-terminal truncation known to increase potency21. Assessment for purity by LCMS and RPLC, however, showed that our product quality was not sufficient due to the presence of oxidized forms that could potentially promote aggregation and immunogenicity22 (Fig. 3c). These data highlighted that rapid production of biomolecules with acceptable bioactivity is necessary, but not sufficient to define a clinical-quality biologic product.
To address these attributes, we optimized process conditions on InSCyT, performing experiments on individual modules simultaneously with fully-integrated experiments (comprising connected modules) (Fig. 3a and Supplementary Fig. 7). After 27 days of process development, a final run showed that oxidation was reduced to < 1.5% at all residues (Fig. 3b,c). During this run the system produced nearly 8,000 formulated doses of IFNα-2b in less than one week (Supplementary Fig. 8). The identity and purity of the product was confirmed by multiple analytical methods at four time points during the campaign (Fig. 3c and Supplementary Fig. 8). RPLC, SEC, and LCMS showed levels of purity within targeted specifications; other analytics showed the specifications for safety and potency of our product were also achieved. Differences in chromatographic behavior of our product were confirmed by MALDI as the naturally-occurring C-terminal truncation mentioned previously21 (Supplementary Fig. 8). The low overall process yield (~11%) is attributed to removal of an N-terminal product variant, which arises due to incomplete cleavage of the secretion leader sequence during expression by our host. Further engineering of the expression vector with alternative signal sequences could alleviate this variant in expression23.
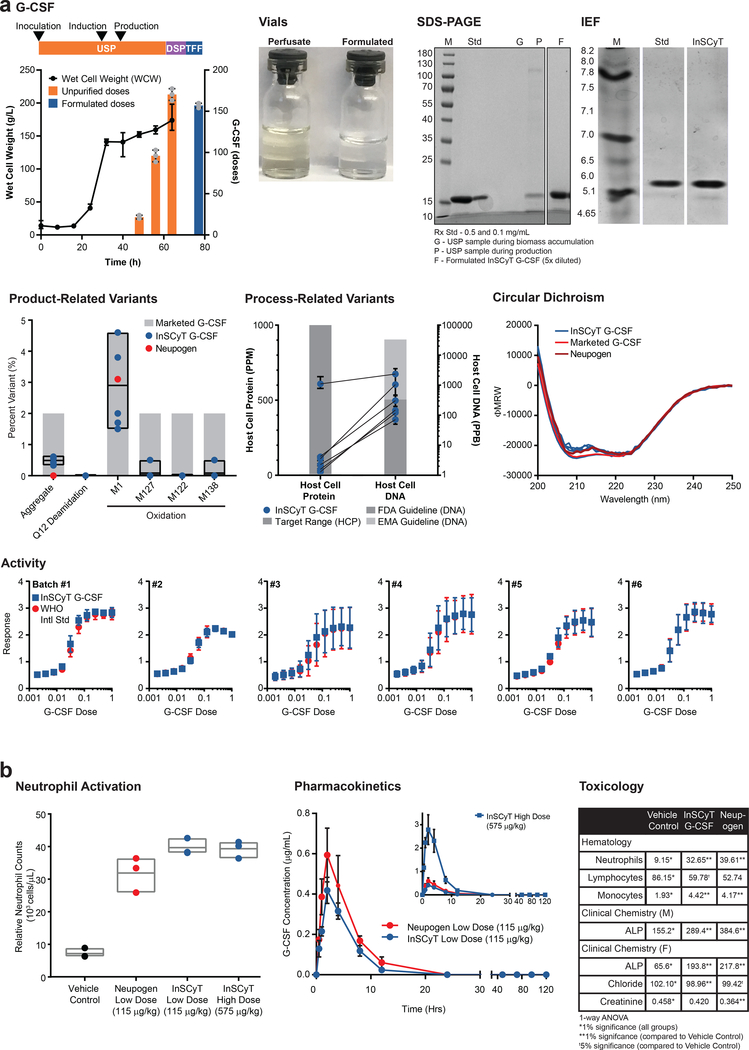
Next, we produced granulocyte colony stimulating factor (G-CSF), used to stimulate blood cell proliferation and reduce infections in cancer patients treated with myelosuppressive chemotherapy24. This drug has manufacturing challenges due to complex folding and a propensity for aggregation and oxidation at specific amino acid residues25,26. We designed a new process for production of G-CSF and implemented it on three InSCyT systems (Supplementary Fig. 2). After inoculation, each automated process yielded more than 165 doses of formulated drug in 100 h (Fig. 4a and Supplementary Fig. 9). Typical process yields for each cycle ranged from 70–90% (average 77%). We assessed biophysical and biochemical attributes of the product using multiple analytics to establish its identity, purity, safety, and potency (Fig. 4a and Supplementary Fig. 9). InSCyT G-CSF was comparable to a drug substance from a licensed product. We confirmed the protein sequence (100% coverage) by mass spectrometry (Supplementary Fig. 10). Minimal high molecular weight (HMW) species were present in the product (0.33%−0.65%). Levels of process-related contaminants in our formulated G-CSF were each below values typical for early-stage clinical development (1000 PPM for HCPs and 100 pg/dose for DNA)14–16. Potency of the InSCyT G-CSF was comparable to the NIBSC WHO 09/136 reference standard (89.6%−141.1%) in a cell-based proliferation assay. Our product contained a minor variant comprising an N-terminal truncation and was a mixture of aglycosylated and glycosylated forms (Supplementary Fig. 9). Neither of these variants are likely to be clinically meaningful, as both truncations and glycosylation have been observed in licensed products without impact on product activity or safety27,28. Overall, these data demonstrate that InSCyT can rapidly and consistently produce therapeutic proteins that are comparable to currently marketed products.
Figure 4.
Production of G-CSF on three identical InSCyT systems. Dose size 300 μg24. Center values and error bars represent the mean and range, respectively, of technical triplicates unless otherwise noted. (a) Timeline and yields for production of G-CSF using the InSCyT system for a single representative sample (Batch #1). Wet cell weight (WCW) (black circles) and cumulative unpurified (orange) and formulated (blue) doses of G-CSF are shown. Grey circles represent individual data points. Product quality for InSCyT-produced G-CSF alongside drug substance from a licensed product produced in E. coli and Neupogen® (produced by Amgen in E. coli). A photo of vials comparing material sampled from the USP (perfusate) to final formulated material (formulated). SDS-PAGE (12% tris-glycine) analysis of Batch #1 from the USP during biomass accumulation (G) and production (P), and a final, formulated InSCyT sample (F) alongside drug substance from a licensed product (Std). Analysis of product purity by isoelectric focusing (IEF) for formulated Batch #1. Gel analyses of Batch #1 are representative of all six batches (Supplementary Fig. 9). Analysis of product-related variants and process-related variants. Each data point represents a unique batch (2 time points from each of 3 distinct systems). Paired data points indicate analyses from a single batch. Product-related variants are shown alongside levels typically found in marketed products (Supplementary Fig. 5). Black boxes represent the range of InSCyT G-CSF samples with an additional line at the mean. Process-related variants are shown alongside common guidelines (per Fig. 2b). Analysis of the secondary structures of InSCyT G-CSF (Batches 1–6) and a reference drug substance from a licensed product using circular dichroism (CD). Activity of InSCyT G-CSF alongside that of the WHO International standard (NIBSC 09/136). (b) Analysis of pharmacokinetics (PK), pharmacodynamics (PD), and toxicology of InSCyT-produced G-CSF and a licensed product (Neupogen®) in a rat model. Neutrophil activation and pharmacokinetic profile of low dose (115 μg/kg, n=3 animals, t1/2 = 2.1 h) and high dose (575 μg/kg, n=3 animals, t1/2 = 4.6 h) InSCyT G-CSF in rats compared to Neupogen (115 μg/kg, n=3 animals, t1/2 = 1.4 h) (PK: p=0.9963, Kolmogorov-Smirnov test). For neutrophil activation, grey boxes represent the range of three individual animals with an additional line at the mean. For PK center points and error bars represent the mean and range, respectively, of three individual animals. Summary of statistically significant results comparing the toxicology of InSCyT G-CSF and Neupogen® to a vehicle control. Values represent the mean; standard deviation and sample size can be found in Supplementary Fig. 11. Statistical significance was determined by one-way ANOVA. ALP – alkaline phosphatase
We performed additional non-clinical studies with InSCyT-produced G-CSF to provide a framework for future clinical development. We assessed the pharmacokinetics (PK), pharmacodynamics (PD), and toxicology of the InSCyT-produced G-CSF by comparing our product to a licensed product (Neupogen®) in a rat model. We found InSCyT-produced G-CSF was comparable to Neupogen® in neutrophil activation during a single-dose administration study (Fig. 4b). InSCyT G-CSF and Neupogen® showed no statistically significant difference in pharmacokinetic profile when administered at the same dose (Kolmogorov-Smirnov test, p=0.9963) (Fig. 4b). A 5-day repeat dosing study also showed our product was comparable to Neupogen® in toxicity based on survival, clinical signs, body weight, quantitative food consumption, hematology, serum chemistry, organ weights, and macroscopic findings (Fig. 4b and Supplementary Fig. 11). Importantly, in all of these studies, no abnormal clinical signs of toxicity, including injection site inflammation, were observed in any animals dosed with InSCyT G-CSF (58 in total). Together, these data suggest that the InSCyT-produced G-CSF has potency in vivo and that potentially immunogenic process-related impurities are appropriately minimized.
InSCyT can produce a variety of clinical-quality recombinant therapeutic proteins in a liquid dosage form through integrated production, purification and formulation under a single control architecture. The efficient secretion of proteins by P. pastoris, combined with a holistic design of purification sequences, enabled new and intensified processes for hGH, IFNα-2b, and G-CSF, which reduced the total number of processing steps by 45% or more, and did not require refolding, excursions in pH or other substantial changes to the protein itself during processing (Supplementary Fig. 2). We demonstrated fast cycles of process development to reach clinically relevant target specifications in 12 weeks, aided by testing production at-scale in a modular and integrated manner on InSCyT. The combination of the manufacturing system with the demonstrated strategy for process development could facilitate the rapid transition of lead molecules into the clinic for translational studies, and reduce subsequent iterations in process development and technology transfer for late-stage and commercial manufacturing.
Further engineering InSCyT to comply with current good-manufacturing practices (cGMP) and concurrent development of an appropriate control strategy would enable its use for CMC manufacturing of new drugs. A fill/finish module would enable product vialing for simple administration to patients. Several relevant solutions have emerged, including systems from MedInstill and Vanrx. Modular facilities for housing manufacturing equipment, such as G-Con PODs® and Germfree BioGO™ Modules, also are becoming widely available for aseptic containment of small-scale manufacturing facilities.
InSCyT could be used in its current form to produce many other products, such as monoclonal antibodies, vaccine components, nanobodies and other antibody-like proteins (e.g., bispecific T-cell engagers, Fabs), blood products (erythropoietin), and therapeutic enzymes (e.g., β-glucocerebrosidase). Other products, such as insulin or modified products such as antibody-drug conjugates or pegylated versions of products, would require additional modules for enzymatic processing, chemical ligation or crystallization; such systems could include de novo synthesis of the key starting materials or APIs as well29. Further integration of multiple units may also facilitate blended products of multi-component vaccines, or unique drug combinations tailored for applications for regional use or precision medicine.
Online Methods
Generation of product secreting strains
Wildtype Komagataella phaffii (NRRL Y-11430 was modified to express human growth hormone (rhGH), interferon alfa-2b (rIFNα-2b), or granulocyte-colony stimulating factor (rG-CSF) using sequences provided below (Table 1) codon optimized for P. pastoris ( GeneOptimizer service; Thermo Fisher Scientific, Waltham, MA) and cloned into a pPICZα-family vector (Thermo Fisher Scientific, Waltham, MA) flush with a truncated form of the Saccharomyces cerevisiae α-factor secretion signal under the immediate control of the methanol-inducible AOX1 promoter.
Table 1.
Nucleotide and amino acid sequences
| Product | Codon optimized DNA sequence | Amino acid sequence |
|---|---|---|
| hGH | TTCCCAACTATCCCATTGTCCAGATTGTTCGACAACGCTA TGTTGAGAGCTCACAGATTGCACCAGTTGGCTTTCGACA CTTACCAAGAGTTCGAAGAGGCTTACATCCCAAAAGAGC AGAAGTACTCCTTCTTGCAAAACCCTCAGACTTCCTTGTG TTTCTCCGAGTCCATTCCAACTCCATCCAACAGAGAAGAG ACTCAGCAGAAGTCCAACTTGGAGTTGTTGAGAATCTCCT TGTTGTTGATCCAGTCCTGGTTGGAGCCAGTTCAGTTCTT GAGATCCGTTTTCGCTAACTCCTTGGTTTACGGTGCTTCC GACTCTAACGTTTACGACTTGTTGAAGGACTTGGAAGAG GGTATCCAGACTTTGATGGGTAGATTGGAAGATGGTTCC CCAAGAACTGGTCAGATCTTCAAGCAGACTTACTCTAAGT TCGACACTAACTCCCACAACGACGACGCTTTGTTGAAGAA CTACGGTTTGTTGTACTGTTTCAGAAAGGACATGGACAAG GTTGAGACTTTCTTGAGAATCGTTCAGTGTAGATCCGTTG AGGGTTCCTGTGGTTTCTAA |
FPTIPLSRLFDNAMLRAHRLHQLAFDTYQEFEEA YIPKEQKYSFLQNPQTSLCFSESIPTPSNREETQ QKSNLELLRISLLLIQSWLEPVQFLRSVFANSLVY GASDSNVYDLLKDLEEGIQTLMGRLEDGSPRTG QIFKQTYSKFDTNSHNDDALLKNYGLLYCFRKDM DKVETFLRIVQCRSVEGSCGF |
| IFNα-2b | TGTGACTTGCCTCAAACTCACTCCCTGGGTTCTAGAAGAA CCTTGATGTTGTTGGCCCAGATGAGAAGAATCTCCTTGTT CTCCTGCCTGAAGGACAGACACGATTTCGGTTTCCCACA AGAAGAGTTCGGTAACCAGTTCCAGAAGGCTGAGACTAT TCCAGTCTTGCACGAGATGATCCAGCAGATCTTCAACCTG TTCTCCACTAAGGATTCTTCCGCTGCTTGGGACGAAACCT TGTTGGACAAGTTCTACACCGAGTTGTACCAGCAGTTGAA CGACTTGGAGGCCTGTGTTATTCAAGGTGTTGGTGTTACC GAGACTCCACTGATGAACGAGGACTCCATTTTGGCCGTC AGAAAGTACTTCCAGAGAATCACCCTGTACCTGAAAGAGA AGAAGTACTCTCCTTGCGCCTGGGAAGTTGTTAGAGCTG AGATTATGAGATCCTTCTCCTTGTCCACCAACCTGCAAGA GTCCTTGAGATCCAAAGAGTAA |
CDLPQTHSLGSRRTLMLLAQMRRISLFSCLKDRH DFGFPQEEFGNQFQKAETIPVLHEMIQQIFNLFS TKDSSAAWDETLLDKFYTELYQQLNDLEACVIQG VGVTETPLMNEDSILAVRKYFQRITLYLKEKKYS PCAWEVVRAEIMRSFSLSTNLQESLRSKE |
| G-CSF | ATGACTCCTTTGGGTCCAGCTTCTTCCTTGCCTCAATCCT TCTTGTTGAAGTGTTTGGAGCAGGTTAGAAAGATCCAGG GTGATGGTGCTGCTTTGCAAGAGAAGTTGTGTGCTACTTA CAAGTTGTGTCACCCAGAAGAGTTGGTTTTGTTGGGTCAC TCCTTGGGTATTCCTTGGGCTCCATTGTCCTCTTGTCCAT CCCAAGCTTTGCAATTGGCTGGTTGTTTGTCCCAATTGC ACTCCGGTTTGTTCTTGTACCAGGGTTTGTTGCAAGCTTT GGAGGGTATTTCTCCAGAGTTGGGTCCAACTTTGGACACA TTGCAGTTGGACGTTGCTGACTTCGCTACTACTATCTGGCAA CAGATGGAAGAATTGGGTATGGCTCCAGCTTTGCAGCCAACTC AAGGTGCTATGCCAGCTTTTGCTTCTGCTTTCCAGAGAA GAGCTGGTGGTGTTTTGGTTGCTTCTCACTTGCAGTCTTTC TTGGAGGTTTCCTACAGAGTTTTGAGACACTTGGCTCAACCA |
MTPLGPASSLPQSFLLKCLEQVRKIQGDGAALQEKLCATYK LCHPEELVLLGHSLGIPWAPLSSCPSQALQLAGCLSQLHSGLFL YQGLLQALEGISPELGPTLDTLQLDVADFATTIW QQMEELGMAPALQPTQGAMPAFASAFQRRAGG VLVASHLQSFLEVSYRVLRHLAQP* |
InSCyT system design and operation control
Production module design and operation control
Cultivation was performed in custom-modified Multifors 2 NW70 bench-top bioreactors equipped with 0.75 L flat-bottomed glass vessels (ID=70 mm, H=195 mm) (Infors USA, Annapolis Junction, MD). Magnetically coupled impellers enabled mixing (two six-blade Rushton or one Rushton and one three-blade marine).
Filtered (0.2 μm PTFE; Tisch Scientific, North Bend, OH) medical grade oxygen (Airgas, Radnor, PA) was delivered through a metal sparge ring at the bottom of the vessel. Dissolved oxygen (DO) tension was measured using a 225 mm VisiFerm™ DO probe (Hamilton, Reno, NV) and controlled using a split-range proportional-integral (PI) controller, manipulating oxygen sparge rates (at low oxygen uptake rates (OUR)) or stirrer speed (high OUR).
A two-level cascade controller was used for temperature control. In the outer loop, vessel temperature was measured using the DO sensor’s built in thermocouple and a PI controller was used to set the jacket setpoint. In the inner loop, the jacket temperature was measured using an embedded thermocouple and used to determine the duty cycle of the resistive heater or chilled 1:1 ethylene glycol/water loop (Julabo USA, Allentown, PA).
pH was measured with a Model F-635 FermProbe (Broadley-James, Irvine, CA) and output isolated pH transmitters (Hanna Instruments, Woonsocket, RI) and controlled using a deadband controller. 5.0 M potassium hydroxide or phosphoric acid (Sigma-Aldrich, St. Louis, MO) were dosed into the bioreactor as needed by a 4-channel/12-roller Ismatec Reglo ICC peristaltic pump (Cole-Parmer, Vernon Hills, IL) through Ismatec 1.52 mm ID 3-stop PharMed BPT tubing cassettes (Cole-Parmer, Vernon Hills, IL).
Disposable gamma-irradiated tubing assemblies were custom-built for the InSCyT system (High Purity New England, Smithfield, RI) to deliver media and collect perfusate. These assemblies were constructed of 1.52 mm ID 3-stop PharMed BPT peristaltic cassettes, platinum-cured silicone tubing, and HDPE carboys, along with appropriate PVDF barbed fittings. Flow into and out of the bioreactor was driven by Ismatec Reglo ICC peristaltic pumps with Asco Scientific 3-way pinch valves (Cole-Parmer, Vernon Hills, IL). Clarified perfusate was withdrawn from the bioreactors using two custom modified probes with each holding two porous 0.2 μm ceramic membranes (FISP, Flownamics, Madison, WI). Feed bottles were suspended on scales (Mettler Toledo, Columbus, OH) and closed-loop control was used to ensure constant flow. A conducting 3/16” grade 2 titanium probe (McMaster-Carr, Robbinsville, NJ), coupled to an Omron Automation 24V AC/DC Monitoring relay (Allied Electronics, Fort Worth, TX) was used for level sensing and transmitting, activating the perfusion pump upon liquid contact. Level was maintained through equal media addition and perfusion rates. A periodic backwash was used to prevent membrane fouling.
Bioreactors were sampled automatically using a Seg-Flow 4800 Sampling System, a FlowFraction 400, and Seg-Mod modules (Flownamics, Inc., Madison, WI), and held at 4°C until further analysis.
Perfusate adjustment module design and operation control
A pH adjustment module (pHAM) was used to adjust the pH of perfusate prior to loading onto the first chromatography column. Supernatant was collected in a 1L surge tank to balance flowrates between the bioreactor and the first column. Custom conductivity-based level sensors enabled automated startup of the downstream process at sufficient volume. Addition of adjustment solution in an in-line mixer (Stamixco, Wollerau, Switzerland) was used to adjust the perfusate pH prior to the first column. The pH was measured using a custom in-line pH probe (Van London Co., Houston, TX), and a PI controller determined the adjustment rate.
Purification module design and operation control
Up to three product-specific chromatography columns, operated in either bind and step gradient elution or flow-through mode, were used for purification. Flow was provided by a micro-annular gear pump (mzr-2905; HNP Mikrosysteme, Schwerin, Germany) and a flow sensor (SLI-2000; Sensiron, Zurich, Switzerland) in closed-loop PID control and passed through a debubbler/degasser (9000–1545, Idex Health and Science, Oak Harbor, WA). The columns were operated either independently or in series using multi-port (C65–3180IA; VICI Valco, Houston, TX) and solenoid (100T3MP24–62-5; BioChem Fluidics, Boonton, NJ) valves. Purification processes were operated using a predetermined sequence of steps, controlled either by time or by A280 measurements (Model 280; Spectrum Labs, CA). Purified drug substance eluted from the final chromatography column was directed to the retentate reservoir within the formulation module.
Formulation module design and operation control
A tangential flow filtration system (TFF) (KRIIi; Spectrum Labs, CA) was used to concentrate and/or buffer exchange the eluted drug substance. The system was equipped with WaterSep Discover24 (or Discover12) membranes (5 kDa MWCO, 1.0mm id) (Marlborough, MA). Automated processing was enabled through custom scripting.
Module integration and automation
Modicon M221 PLCs (Schneider Electric, Andover, MA) were used for the connection of the thermocouple, motors, solenoids, level transmitters, UV transmitters, and pH transmitters with the process local area network (LAN). The DO probes and oxygen mass flow controllers were connected to the process LAN via a MODBUS to Ethernet endpoint (Sealevel, Liberty, SC), while the peristaltic pump and multi-port valve drives were connected to the process LAN via a RS-232 to Ethernet endpoint (Sealevel, Liberty, SC).
Wonderware (Lake Forest, CA) was used as a human-machine interface (HMI) to the integrated system. Custom scripts were written using Intouch QuickScript (Wonderware, Lake Forest, CA) to implement the USP, pHAM, and DSP control loops, recipes, and operating sequences. These scripts were written in house or with assistance from Superior Controls (Superior Controls, Seabrook, NH). Wonderware was also used as a data historian, with local download and processing performed using custom scripts in MATLAB (Mathworks, Natick, MA).
Production of biologics using the InSCyT system
All numbered buffers referenced below are listed in table 2. All chemical reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Table 2.
List of buffers used within the InSCyT system
| Buffer number | Buffer component | Concentration (mM unless indicated) | pH | Conductivity (mS/cm) |
|---|---|---|---|---|
| 1 | Sodium citrate | 20 | 5.5 | 4 |
| 2 | Sodium phosphate | 20 | 6 | 4 |
| 3 | Sodium phosphate | 20 | 8 | 4 |
| 4 | Sodium hydroxide | 1000 | - | - |
| 5 | Sodium citrate | 10 | 6 | - |
| Sodium chloride | 8.7 g/L | |||
| 6 | Sodium citrate | 10 | 6 | - |
| Sodium chloride | 8.7 g/L | |||
| Tween 20 | 0.2% v/v | |||
| 7 | Sodium citrate | 20 | 5 | - |
| 8 | Sodium phosphate | 20 | 6.6 | - |
| 9 | Trisodium citrate | 200 | 7.6 | - |
| Sodium chloride | 100 | |||
| Sodium phosphate | 20 | |||
| 10 | Sodium citrate | 20 | 5.6 | - |
| 11 | Sodium citrate | 20 | 4 | - |
| 12 | Sodium chloride | 240 | 4 | - |
| Sodium citrate | 10 | |||
| 13 | Sodium chloride | 410 | 4 | - |
| Sodium citrate | 10 | |||
| 14 | Sodium chloride | 7.5 g/L | 6.75 | - |
| Sodium phosphate dibasic | 1.8 g/L | |||
| Sodium phosphate monobasic | 1.3 g/L | |||
| 15 | Sodium chloride | 400 | 4 | - |
| Sodium citrate | 10 | |||
| 16 | Sodium phosphate | 40 | 7.6 | - |
| Sodium chloride | 200 | |||
| 17 | Sodium citrate | 20 | 4.2 | - |
| 18 | Sodium citrate | 20 | 3.5 | - |
| 19 | Sodium chloride | 7.5 g/L | 6.75 | - |
| Sodium phosphate dibasic | 1.8 g/L | |||
| Sodium phosphate monobasic | 1.3 g/L | |||
| EDTA | 0.1 g/L | |||
| Tween 80 | 0.1 g/L | |||
| 20 | Sodium chloride | 150 | 5.8 | - |
| Sodium phosphate | 20 | |||
| 21 | Sodium chloride | 150 | 7 | - |
| Sodium phosphate | 20 | |||
| 22 | Sodium citrate | 20 | 5.5 | - |
| 23 | Glutamic acid | 10 | 4.4 | - |
| Sobitol | 5% w/v |
Production of hGH using the InSCyT system
First Generation Production
Bioreactors were filled with buffered glycerol-complex medium (BMGY) through pleated polyethersulfone (PES) 0.2 μm filters (Polycap 36 TC; GE Healthcare, Boston, MA). Buffered glycerol-complex medium (BMGY) and buffered methanol-complex medium (BMMY) were sequentially fed to the reactor for outgrowth and induction respectively31. Sigma A204 anti-foam (A8311; Sigma-Aldrich, St. Louis, MO) was added to BMMY at a concentration of 0.02% v/v. The working volume for all fermentations was 420 mL. Temperature and pH were maintained at 25 °C and 6.5, respectively.
BMGY was fed at 0.5 mL/min for 32 hours to accumulate biomass. DO was maintained at 100%. Mixing was achieved using two Rushton impellers. Perfusate was directed to waste. After 32 hours BMGY was automatically substituted for BMMY and perfusate was directed waste for 8 hours. Forty hours after inoculation, perfusate was automatically diverted to the pHAM.
A first-generation pHAM was used to adjust the perfusate to pH 5.5 prior to loading onto the first chromatography column. pH was measured using an in-line pH probe (InLab Reach 425; Mettler Toledo, Columbus, OH) under a recirculation loop. A feedback controller was used to continuously vary the rate of phosphoric acid (500 mM) addition. A magnetic stir-bar was used for mixing.
For each cycle of the purification module, 100 column volumes (CV) of adjusted perfusate was loaded onto a 5 mL pre-packed multimodal cation exchange (MMCEX) column (CMM HyperCel™; Pall Corporation, Port Washington, NY), equilibrated with buffer 1, washed with buffer 2, and eluted with buffer 3. Eluate from column 1 above 15 mAU was flowed through a 1 mL pre-packed anion exchange (AEX) column (HyperCel™ STAR AX; Pall Corporation, Port Washington, NY). Flow-through from column 2 above 15 mAU was collected for formulation. The columns were stripped with buffer 4 and re-equilibrated with buffers 1 and 3, respectively.
Eluate from the final column was dialyzed against buffer 5 using 3.5K MWCO Slide-A-Lyzer G2 Dialysis Cassettes (ThermoFisher Scientific, Waltham, MA) according to the manufacturer’s recommended protocol.
Second Generation Extended Production
Second generation extended production of hGH was conducted the same as the first-generation production of hGH except for the following changes. BMGY was substituted for rich defined media31 containing 4% glycerol and BMMY was substituted for rich defined media31 containing 3% methanol. DO was maintained at 25%. The pHAM described in the “Perfusate adjustment module design and operation control” section above was used. The adjustment fluid was 100mM phosphoric acid. 80 CVs of adjusted perfusate (60 CVs for the purification cycle on day 10) was loaded onto the first column. Flow-through from the second column above 15 mAU was directed to the TFF module for formulation.
The formulation module was automatically triggered by the attached process computer at a fixed, regular interval, processing any eluate that had collected in the retentate reservoir. The feed pump was operated at a sufficient rate to maintain 50 mL/min crossflow velocity. Permeate rate was controlled though the use of a backpressure regulator valve set to maintain 30 psi transmembrane pressure (TMP). Concentration was performed on the TFF module (concentration factor 2.75x) and then diafiltration was performed with buffer 6 (8 diavolumes).
Production of IFNα-2b using the InSCyT system
IFNα-2b Process Development Experiment 1 (PDE 1)
Production of IFNα-2b during PDE 1 was conducted the same as the first-generation production of hGH except for the following changes. Reactors were inoculated with a rIFNα-2b secreting strain. 100mM citric acid was used to adjust the pH to 5.0 in the pHAM. No recirculation loop was used in the pHAM; the pH was measured in tank by a Model F-635 FermProbes (Broadley- James, Irvine, CA).
60 CV of supernatant was loaded onto a 5 mL pre-packed MMCEX column (Capto MMC ImpRes; GE Healthcare Bio-Sciences, Pittsburgh, PA), equilibrated with buffer 7, washed with buffer 8, and eluted with buffer 9. Eluate from column 1 above 15 mAU was loaded onto a 5 mL hydrophobic charge induction chromatography (HCIC) column (MEP HyperCel™; Pall Corporation, Port Washington, NY), equilibrated with buffer 9, washed with buffer 10, and eluted with buffer 11. Eluate from column 2 above 15 mAU was loaded onto a 5 mL pre-packed cation exchange (CEX) column (SP Sepharose High Performance; GE Healthcare Bio-Sciences, Pittsburgh, PA), equilibrated with buffer 11, washed with buffer 12 and eluted with buffer 13. Eluate from column 3 above 10 mAU was collected for formulation. Columns were then stripped with buffer 4 and re-equilibrated at the equilibration conditions given above.
Eluate from the final column was dialyzed against buffer 14 using 3.5K MWCO Slide-A-Lyzer G2 Dialysis Cassettes (ThermoFisher Scientific, Waltham, MA) according to the manufacturer’s recommended protocol.
IFNα-2b Process Development Experiment 2 (PDE 2)
Production of IFNα-2b during PDE 2 was conducted the same as production of IFNα-2b during PDE 1 except the elution buffer for the third column was buffer 15.
IFNα-2b Process Development Experiment 3 (PDE 3)
Production of IFNα-2b during PDE 3 was conducted the same as the second-generation production of hGH except for the following changes. Reactors were inoculated with a rIFNα-2b secreting strain. The methanol-containing medium had 1% methanol. DO was maintained at 40%. Mixing was achieved using one Rushton impeller (top) and one marine impeller (bottom). Perfusate pH was adjusted to 5.0 using 100mM citric acid in the pHAM.
Purification was the same as in IFNα-2b PDE 2 except all columns were 1mL and 110 CV of supernatant was loaded onto the first column.
IFNα-2b Process Development Experiment 4 (PDE 4)
Production of IFNα-2b using the InSCyT system during PDE 4 was conducted the same as PDE 3 except for the following changes. Column 1 was eluted with buffer 16. Eluate from column 1 above 15 mAU was flowed through a 1 mL AEX column (HyperCel™ STAR AX; Pall Corporation, Port Washington, NY). Flow-through from column 2 above 15 mAU was loaded onto a 1 mL pre-packed multimodal column (HEA HyperCel™; Pall Corporation, Port Washington, NY), equilibrated with buffer 16, washed with buffer 17, and eluted with buffer 18. Eluate from column 3 above 10 mAU was collected for formulation and dialyzed as described in IFNα-2b PDE 1. Columns were then stripped with buffer 4 and re-equilibrated at the equilibration conditions given above.
IFNα-2b Process Qualification Run
Production of IFNα-2b using the InSCyT system during the Process Qualification Run was conducted the same as the second-generation production of hGH except for the following changes. Reactors were inoculated with a rIFNα-2b secreting strain. Upstream samples were not taken between hours 80 and 112 due to failure of the automated sampling system. For yield calculations, the titer during those hours is assumed to be the same as the titer of the previous sample pool (hr 56 – 78). Perfusate was adjusted to pH 5.0 in the pHAM using 100mM citric acid.
Purification was the same as in IFNα-2b PDE 2 except 80 CVs of supernatant was loaded onto the first column. Eluate from column 3 above 10 mAU was directed to the TFF for formulation. No concentration was performed. Diafiltration was performed with buffer 19.
Production of G-CSF using the InSCyT system
Production of G-CSF was conducted the same as the second-generation production of hGH except for the following changes. Reactors were inoculated with a rG-CSF secreting strain. 0.1% CHAPs (3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate hydrate) was added to the media. Mixing was achieved using one Rushton impeller (top) and one marine impeller (bottom). Perfusate was adjusted to pH 5.0 in the pHAM using 100mM citric acid.
100 column volumes (CV) of adjusted perfusate was loaded onto a 5 mL pre-packed MMCEX column (Capto MMC ImpRes; GE Healthcare Bio-Sciences, PA), equilibrated with buffer 7, washed with buffer 20, and eluted with buffer 21. Eluate from column 1 above 15 mAU was flowed through a 1 mL pre-packed AEX column (HyperCel™ STAR AX; Pall Corporation, Port Washington, NY). Flow-through from column 2 above 12 mAU was loaded onto a 5 mL HCIC column (MEP HyperCel™; Pall Corporation, Port Washington, NY), equilibrated with buffer 21, washed with buffer 22 and eluted with buffer 11. Eluate from column 3 above 12 mAU was directed to the TFF for formulation. Each of the columns were then stripped with buffer 4 and re-equilibrated with the equilibration conditions mentioned above for the first and third column, and buffer 21 for the second column. No concentration was performed. Diafiltration was performed with buffer 23.
Analytical methods
Wet cell weight (WCW)
To determine wet cell weight (g/L), 300 μL of re-suspended bioreactor sample was dispensed into a pre-weighed Spin-X centrifuge tube (Corning, NY) and centrifuged at 15000 g for 10 minutes in technical triplicate. The mass of the pellet was determined by weight.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)
SDS-PAGE was carried out under reducing conditions using Novex 12% Tris-Glycine Midi Gels (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s recommended protocol and stained using Instant Blue Protein Stain (Thermo Fisher Scientific, Waltham, MA).
Product-specific enzyme-linked immunosorbent assay (ELISA)
Protein concentrations were determined using sandwich ELISA for G-CSF and direct ELISA for IFNα-2b as described elsewhere except for the following changes32. Table 3 shows the antibodies used. All reagents were added at 100μL/ well instead of 50μL/well. Wash buffer was PBS, 0.05% Tween. Blocking buffer was PBS, 0.05% Tween, 0.25% BSA, and blocking steps were incubated at 37°C for 1 hour. Detection enzyme was incubated for 30 minutes. Substrate solution was ABTS (Thermo Fisher Scientific, Waltham, MA) and was incubated for 20 minutes. Stop solution was 0.1 M citric acid, 0.01% sodium azide. Plates were analyzed for absorbance at 410 nm / 540 nm using a Tecan Infinite M200 Pro plate reader.
Table 3.
Antibodies used in product-specific ELISA
| Protein | Capture | Secondary/detection | Detection | |||
|---|---|---|---|---|---|---|
| G-CSF | Biolegend BVD13–3A5 | 2 μg/mL | Biolegend BVD11–37G10 | 0.4 μg/mL | Abcam Streptavidin-HRP ab7403 | 0.2 μg/mL |
| IFNα-2b | None | AssayPro 31168–05121 | 0.4 μg/mL | Abcam Streptavidin-HRP ab7403 | 0.2 μg/mL | |
Process-related impurity analysis
Samples were analyzed for host-cell protein content using the Pichia pastoris 2nd generation HCP ELISA kit from Cygnus Technologies (Southport, NC) according to the manufacturer’s recommended protocol. Samples were analyzed for residual host-cell DNA using the resDNASEQ™ Quantitative Pichia pastoris DNA kit (Applied Biosystems, Foster City, CA) according to the manufacturer’s recommended protocol. DNA was first extracted from each sample using the PrepSEQ™ Residual DNA Sample Preparation kit (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s recommended protocol. qPCR reactions were performed using a Roche LightCycler 480II equipped with LightCycler software release 1.5.0SP4 (Roche Molecular Systems, Inc., Indianapolis, IN). Analysis was performed using the built in software as recommended in the manufacturer’s protocol.
Isoelectric focusing (IEF)
IEF was run using Novex pH 3–10 IEF Gels (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s recommended protocol and stained with SimpleBlue SafeStain,.
Chromatographic analyses
Reversed phase liquid chromatography (RPLC) (for sample quantification and purity analysis) and size exclusion chromatography (SEC) (for quantification of high molecular weight species) were performed for hGH and G-CSF as described previously20. RPLC and SEC were carried out the same for IFNα-2b except RPLC operating conditions for IFNα-2b can be found in Table 4, where Buffer A and B are as described previously20 and SEC running buffer for IFNα-2b was 50 mM ammonium bicarbonate, 200 mM arginine HCl, 0.02% sodium azide at pH 7.0. All chemical reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Table 4.b:
RPLC operating conditions for IFNα-2b
| Time (min) | Flow (mL/min) | %A | %B |
|---|---|---|---|
| Initial | 0.5 | 100 | 0 |
| 0.1 | 0.5 | 100 | 0 |
| 0.5 | 0.5 | 61 | 39 |
| 5.5 | 0.5 | 54.5 | 45.5 |
| 5.75 | 0.5 | 0 | 100 |
| 6 | 0.5 | 0 | 100 |
| 7.5 | 0.5 | 100 | 0 |
Column Temperature: 60°C
Liquid chromatography-mass spectrometry (LCMS)
100 μg were used from each hGH and G-CSF sample for analysis. 25 μg were analyzed for IFNα-2b. hGH and IFNα-2b samples were dialyzed against 50 mM ammonium bicarbonate (ABC) at pH 7.0 to a final concentration of about 1 mg/mL. G-CSF samples were dialyzed against 50 mM ABC at pH 8.0 to a final concentration of about 1 mg/mL. Reference materials were treated the same as the samples. Samples were then transferred to an Amicon filter and spun at 13,000 rpm for 15 min. hGH and IFNα-2b samples were digested with trypsin, and G-CSF samples were digested with GluC/LysC using 1 μg (protein to enzyme 50:1) of the respective enzyme and incubated overnight at 37°C.
LCMS equipment was used as described previously33, except a microspray ion source was used. Mobile phase A and B were as described previously33 and the flow rate was 200 μL/min. The gradient was as follows: 0–2 min 2% B with curve level 5, 2–30 min to 40% B, 30–39 min to 60% B, 39–42 min to 85% B until 47 min, 48–52 min to 2% B again. The gradient curve level was 6 from 2 to 52 min.
For peptide identification, raw data were searched against the product sequence using Thermo BioPharmaFinder 2.0 (Thermo Fisher Scientific). Peptide mass accuracy was set to 20 PPM. Oxidation of methionine (Met) residues and deamidation of asparigine (Asn) residues were set as potential dynamic modifications. Final confirmation of the peptide identification was performed by manual inspection, extracting the base peak from the chromatogram and matching the MS/MS fragmentation data with the theoretical prediction.
Cell-based potency assays
Potency assays for G-CSF and hGH were conducted by Bioassay GmbH (Heidelberg, Germany). Cell-based proliferation assays for bioactivity determination of G-CSF samples were conducted according to Pharm. Eur. 01/2009:2206. Cell-based proliferation assays for bioactivity determination of hGH samples were conducted using NB2–11 cells and were compared to a standard (WHO NIBSC 98/574). Potency assays for IFNα-2b were conducted by Charles River Biopharmaceutical Services GmbH (Erkrath, Germany). In vitro cell-based assays for bioactivity determination of IFNα-2b samples were conducted according to Pharm. Eur. monograph 1110.
Circular dichroism (CD)
A Jasco 815 spectrometer was used for CD. Spectra were recorded at a scanning speed of 200 nm/min, a band width of 1 nm, and average of 4 scans. Near UV CD spectra were recorded from 240 to 350 nm in a 10 mm path length cuvette, and far UV CD spectra were recorded from 200 to 250 nm in a 1 mm path length cuvette. For hGH and IFNα-2b, samples were prepared by dialysis into a 10 mM sodium phosphate buffer at pH 6.75 with 0.1 g/L Tween 80 for IFNα-2b and pH 6.0 with 2.0 g/L Tween 20 for hGH using a 7K MWCO Slide-A-Lyzer G2 Dialysis Cassette. For G-CSF, samples were diluted with 10 mM sodium phosphate buffer at pH 4.4. Samples for near and far UV spectra were collected at approximate concentrations of 1.0 mg/ml and 0.1 mg/ml, respectively. Normalization was performed using concentration of the samples determined with a Hitachi U2910 UV-Vis Spectrophotometer.
Non-Clinical Studies
Material Preparation
InSCyT G-CSF Batch #1 was used for all non-clinical studies. Endotoxin removal was performed using Pierce High-Capacity Endotoxin Removal Resin Spin Columns (Thermo Fisher Scientific, Waltham, MA) following the manufacturer’s protocol, except incubation was performed at 4°C with gentle end-over-end mixing approximately once every 30 minutes for 3 hours. Endotoxin removal was confirmed using the Pierce LAL Chromogenic Endotoxin Quantitation Kit (Thermo Fisher Scientific, Waltham, MA). Samples were filtered using a 0.2μm filter (Thermo Fisher Scientific, Waltham, MA). G-CSF concentration was determined using A280 measurements (DeNovix DS-11, Denovix, Wilmington, DE) after filtration. Samples were diluted in sterile, endotoxin-free, 10mM glutamic acid, 5% (w/v) sorbitol, at pH 4.4 to obtain a final concentration of 35 μg/mL (low dose) or 150 μg/mL (high dose) and sterilely alliquoted into single doses. Aliquots were stored at −80°C and thawed immediately before administration.
Neupogen® was purchased from Myoderm (Norristown, PA) and stored at 4°C. Neupogen® was diluted in sterile 10mM sodium acetate, 5% (w/v) sorbitol, at pH 4.0 to 35 μg/mL immediately prior to administration. Sterile 10mM sodium acetate, 5% (w/v) sorbitol, at pH 4.0 was used as a vehicle control.
Single dose Pharmacokinetics and Pharmacodynamic (PK/PD) study in Sprague Dawley rats
Pharmacokinetic profile and pharmacodynamics effect (neutrophil stimulation) of InSCyT G-CSF was evaluated by Toxikon Corporation (Bedford, MA) at two concentrations relative to a predicate control (Neupogen®). Thirty-nine male Sprague Dawley rats made up four groups (randomized using Research Randomizer version 4.0 (Middletown, CT)), with 3 animals in untreated control group 1 and 12 animals in each of groups 2–4. Groups 2 and 3 received InSCyT G-CSF (115 μg/kg and 575 μg/kg, respectively) and group 4 received Neupogen (115 μg/kg). The InSCyT test articles and Neupogen control were administered once subcutaneously dorsally between the shoulders at the start of the study (Day 1). Clinical observations were conducted beginning prior to administration and throughout the study. Clinical observations included, but were not limited to, changes in the skin, fur, eyes and mucous membranes, respiratory system, circulatory system, autonomic central nervous system, somatomotor activity, locomotor activity, and behavioral pattern. Particular attention was paid to changes at the injection site. All animals survived for the duration of the study and were humanely euthanized via carbon dioxide inhalation at the end of their in-life portion (Day 6).
Blood samples (approximately 0.5 mL) were collected for pharmacokinetic analysis at pre-dose, 0.5, 1, 2, 4, 8, 12, 24, 48, 72, 96, and 120 hours post dose from 3 animals / time point / test article group and predicate control group into tubes containing K3-EDTA. Tubes were placed on wet ice immediately following collection and centrifuged at 3400 rpm for 10 minutes. The processed plasma samples from all pharmacokinetic animals were analyzed for test article and predicate control article concentration using an ELISA method. Briefly, the assay was developed based on a commercial kit (Quantikine ELISA) specific for recombinant human G-CSF in solution. The assay employed the quantitative sandwich enzyme immunoassay technique. A monoclonal antibody specific for G-CSF was pre-coated onto a micro plate. G-CSF standards and samples were allowed to bind to the immobilized antibody. After washing, an enzyme-linked polyclonal antibody specific for G-CSF was added for detection of the bound G-CSF. Following a wash to remove any unbound antibody-enzyme reagent, enzyme substrate solution was added to the wells. The color produced was directly proportional to the concentration of G-CSF. The color intensities were measured using a micro plate reader. The concentration of the test articles was determined from a standard curve. Data analysis of the resulting plasma concentrations of InSCyT G-CSF test article and Neupogen predicate control was performed using WinNonlin™ software v6.3. A non-compartmental analysis was performed using NCA model 202 for pharmacokinetic parameter determination. AUC values were calculated using the trapezoidal linear interpolation method. Concentration values below the lower limit of quantitation (<LLOQ) of 39 or 78 pg/mL, as applicable, were set to zero for analysis. T1/2 was calculated based on the slope of the curve for 4,8, and 12 hours for InSCyT G-CSF and 8, 12, and 24 hours for Neupogen®.
Blood samples (approximately 2.0 mL) were separately collected 24 hours post-dose into tubes containing K2-EDTA for neutrophil analysis from three rats from each group. Whole blood was analyzed for neutrophil count.
5-day repeat dose study in Sprague Dawley rats
Toxicology of InSCyT G-CSF was evaluated as compared to a predicate control (Neupogen®) in Sprague Dawley rats when administered at 115 μg/kg subcutaneously dorsally once per day for 5 days. Thirty Sprague Dawley rats made up three groups (randomized using Research Randomizer version 4.0 (Middletown, CT)), with five animals per sex in each group. Group 1 received a vehicle control, group 2 received InSCyT G-CSF and group 3 received Neupogen®. Toxicity was evaluated based on survival, clinical signs, body weight, quantitative food consumption, hematology, serum chemistry, organ weights, and macroscopic findings.
Body weights and food consumption were measured daily. Clinical observations were performed twice daily and included, but were not limited to, changes in the skin, fur, eyes and mucous membranes, respiratory system, circulatory system, autonomic central nervous system, somatomotor activity, locomotor activity, and behavioral pattern. Particular attention was paid to changes at the injection site. All animals survived the study without observed toxicity of any kind and were humanely euthanized via carbon dioxide inhalation at the end of their in-life portion (Day 6) of the study.
Clinical pathology analysis was performed on blood samples obtained prior to necropsy on day 6 from all animals (approximately 24 hours after the last dose). Hematology parameters assessed included red blood cell count, hemoglobin, mean corpuscular volume, mean corpuscular hemoglobin concentration, differential white blood cell count, white blood cell count, hematocril, mean corpuscular hemoglobin, platelet count, and reticulocytes. Clinical chemistry parameters assessed included alanine aminotransferase, albumin, albumin / globulin ratio, alkaline phosphatase, aspartate aminotransferase, blood urea nitrogen, calcium, chloride, cholesterol, gamma glutamyltransferase, creatinine, globulin, glucose, phosphorus, potassium, sodium, total bilirubin, total protein, and triglycerides.
Quantitative, continuous data from the study were analyzed using one-way ANOVA using (Provantis 9.3.1). Differences between dose groups was considered statistically significant only if the probability of the differences being due to chance is equal to or less than 5% (p < 0.05).
Statistics
Sample sizes, error bars, and types and number of replicates are defined in the figure legends or in the corresponding method sections above. Kolmogorov-Smirnov test was used to determine significance for PK studies (p=0.9963). For the toxicology studies, significance was determined using one-way ANOVA as described above. Differences were considered statistically significant only if p < 0.05.
Life Sciences Reporting Summary
Further information on experimental design and reagents is available in the Life Sciences Reporting Summary.
Code Availability
Custom code used in this study is available from the corresponding author upon reasonable request.
Data Availability
The data sets generated and analyzed in this study are available from the corresponding author upon reasonable request.
Animal Welfare Statement
To the best of our knowledge, the non-clinical studies described here did not unnecessarily duplicate previous testing and there were no non-animal alternatives acceptable for the evaluation of the test article as defined by the protocol. No evidence of pain and distress was reported to the Veterinarian and/or Study Director. Protocols for each study were approved by Toxikon’s institutional animal care and use committee (IACUC). Toxikon strictly adhered to common standards in maintaining the animal care and use program34–38.
Supplementary Material
Acknowledgements
This work was supported by the Defense Advanced Research Projects Agency (DARPA) and SPAWAR System Center Pacific (SSC Pacific) under contract no. N66001–13-C-4025. This work was also supported in part by the Koch Institute Support (core) grant P30-CA14051 from the National Cancer Institute. J.R.B., N.C.D., and N.J.M. were supported by a NIGMS/MIT Biotechnology Training Program Fellowship under NIH contract no. 2T32GM008334–26. K.A.S. was supported by a Mazumdar Shaw International Fellowship. J.C.L. is a Camille Dreyfus Teacher-Scholar. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute, NIH, DARPA, or SSC Pacific. The authors have filed patents related to this work.
Footnotes
Competing Financial Interests
The authors have filed patents related to this work.
References
- 1.Adiga R et al. Point-of-care production of therapeutic proteins of good-manufacturing-practice quality. Nat. Biomed. Eng (2018). doi: 10.1038/s41551-018-0259-1 [DOI] [PubMed] [Google Scholar]
- 2.Boles KS et al. Digital-to-biological converter for on-demand production of biologics. Nat. Biotechnol 35, 672–675 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Pardee K et al. Portable, on-demand biomolecular manufacturing. Cell 167, 248–259 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Perez-Pinera P et al. Synthetic biology and microbioreactor platforms for programmable production of biologics at the point-of-care. Nat. Commun 7, 12211 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dolsten M & Søgaard M Precision medicine: an approach to R&D for delivering superior medicines to patients. Clin. Transl. Med 1, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patient-centered drug manufacture. Nat. Biotechnol 35, 485 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Schellekens H, Aldosari M, Talsma H & Mastrobattista E Making individualized drugs a reality. Nat. Biotechnol 35, 507–513 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Love KR et al. Comparative genomics and transcriptomics of Pichia pastoris. BMC Genomics 17, 550 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmad M, Hirz M, Pichler H & Schwab H Protein expression in Pichia pastoris: recent achievements and perspectives for heterologous protein production. Appl. Microbiol. Biotechnol 98, 5301–5317 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamilton SR Production of Complex Human Glycoproteins in Yeast. Science. 313, 1441–1443 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Konstantinov KB & Cooney CL White paper on continuous bioprocessing May 20–21 2014 Continuous Manufacturing Symposium. J. Pharm. Sci 104, 813–820 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Genentech Inc. Nutropin Prescribing Information. (2016).
- 13.Center for Biologics Evaluation and Research & Center for Drug Evaluation and Research. Guidance for industry: for the submission of chemistry, manufacturing, and controls information for a therapeutic recombinant DNA-derived product or a monoclonal antibody product for in vivo use. (1996).
- 14.The European Agency for the Evaluation of Medicinal Products. CPMP Position statement on DNA and host cell proteins (HCP) impurities, routine testing versus validation studies. (1997).
- 15.Jawa V et al. Evaluating Immunogenicity Risk Due to Host Cell Protein Impurities in Antibody-Based Biotherapeutics. AAPS J. 18, 1439–1452 (2016). [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Guidelines on the quality, safety, and efficacy of biotherapeutic protein products prepared by recombinant DNA technology. (2013). [Google Scholar]
- 17.Canova-Davis E et al. Properties of a cleaved two-chain form of recombinant human growth hormone. Chem. Biol. Drug Des 35, 17–24 (1990). [DOI] [PubMed] [Google Scholar]
- 18.Merck & Co. Inc. Intron A product information. 1–39 (1986). [Google Scholar]
- 19.Matthews CB et al. Reexamining opportunities for therapeutic protein production in eukaryotic microorganisms. Biotechnol. Bioeng 114, 2432–2444 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Timmick SM et al. An impurity characterization based approach for the rapid development of integrated downstream purification processes. Biotechnol. Bioeng 1–13 (2018). doi: 10.1002/bit.26718 [DOI] [PubMed] [Google Scholar]
- 21.Reinl SJ & Pogue GP C-Terminally truncated interferon. (2011). [Google Scholar]
- 22.Hermeling S et al. Structural characterization and immunogenicity in wild-type and immune tolerant mice of degraded recombinant human interferon alpha2b. Pharm. Res 22, 1997–2006 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Gibson SJ et al. N-terminal or signal peptide sequence engineering prevents truncation of human monoclonal antibody light chains. Biotechnol. Bioeng 114, 1970–1977 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Amgen Inc. Neupogen prescribing information. (2015).
- 25.Krishnan S et al. Aggregation of granulocyte colony stimulating factor under physiological conditions: characterization and thermodynamic inhibition. Biochemistry 41, 6422–6431 (2002). [DOI] [PubMed] [Google Scholar]
- 26.Lu HS et al. Chemical modification and site-directed mutagenesis of methionine residues in recombinant human granulocyte colony-stimulating factor: effect on stability and biological activity. Arch. Biochem. Biophys 362, 1–11 (1999). [DOI] [PubMed] [Google Scholar]
- 27.Bönig H et al. Glycosylated vs non-glycosylated granulocyte colony-stimulating factor (G-CSF)-results of a prospective randomised monocentre study. Bone Marrow Transplant. 28, 259–264 (2001). [DOI] [PubMed] [Google Scholar]
- 28.Sörgel F et al. Comparability of Biosimilar Filgrastim with Originator Filgrastim: Protein Characterization, Pharmacodynamics, and Pharmacokinetics. BioDrugs 29, 123–131 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adamo A et al. On-demand continuous-flow production of pharmaceuticals in a compact, reconfigurable system. Science 352, 61–7 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Food and Drug Administration. Points to consider in the manufacture and testing of monoclonal antibody products for human use. (1997). [DOI] [PubMed]
- 31.Matthews CB, Kuo A, Love KR & Love JC Development of a general defined medium for Pichia pastoris. Biotechnol. Bioeng (2017). doi: 10.1002/bit.26440 [DOI] [PubMed] [Google Scholar]
- 32.Hornbeck P, Winston SE & Fuller SA Enzyme-Linked Immunosorbent Assays (ELISA). in Current Protocols in Molecular Biology 11.2.1–11.2.22 (John Wiley & Sons, Inc, 1991). [DOI] [PubMed] [Google Scholar]
- 33.Wang YA et al. Integrated bottom-up and top-down liquid chromatography-mass spectrometry (LC-MS) for characterization of recombinant human growth hormone degradation products. Anal. Chem acs.analchem.7b03026 (2017). doi: 10.1021/acs.analchem.7b03026 [DOI] [PubMed] [Google Scholar]
- 34.United States Department of Agriculture (USDA) Animal and Plant Health Inspection Service. 9 CFR Ch. 1, Subchapter A - Animal Welfare. (2013). [Google Scholar]
- 35.Office for Laboratory Animal Welfare (OLAW). Public Health Service Policy on Humane Care and Use of Laboratory Animals. (Health Research Extension Act of 1985).
- 36.National Resource Council. Guide for the Care and Use of Laboratory Animals. (2011).
- 37.Biologial Evaluation of Medical Devices - Part 2: Animal Welfare Requirements. (2006).
- 38.Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC) International.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated and analyzed in this study are available from the corresponding author upon reasonable request.