Abstract
Silica nanoparticles (SiNPs) have been used as vehicles for drug delivery, molecular detection, and cellular manipulations in nanoneuromedicine. SiNPs may cause adverse effects in brain including neurotoxicity, neuroinflammation, neurodegeneration and enhancing levels of amyloid beta protein (Aβ); all pathological hallmarks of Alzheimer’s disease. Therefore, the extent to which SiNPs influence Aβ generation and the underlying mechanisms by which this occurs deserves investigation. Our studies were focused on the effects of SiNPs on endolysosomes which uptake, traffic, and mediate the actions of SiNPs. These organelles are also where amyloidogenesis largely originates. We found that SiNPs, in primary cultured hippocampal neurons, accumulated in endolysosomes and caused a rapid and persistent deacidification of endolysosomes. SiNPs significantly reduced endolysosome calcium stores as indicated by a significant reduction in the ability of the lysosomotropic agent GPN to release calcium from endolysosomes. SiNPs increased Aβ1–40 secretion whereas two agents that acidified endolysosomes, ML-SA1 and CGS21680, blocked SiNPs-induced deacidification and increased generation of Aβ1–40. Our findings suggest that SiNP-induced deacidification of and calcium release from endolysosomes might be mechanistically-linked to increased amyloidogenesis. The use of SiNPs might not be the best nanomaterial for therapeutic strategies against AD and other neurological disorders linked to endolysosome dysfunction.
Keywords: Alzheimer’s disease, amyloid beta protein, endolysosomes, nanoparticles, ML-SA1, silica nanoparticles, SiNP
Introduction
The discovery of silica nanoparticles (SiNPs) has led, in part, to the growth of nanotechnology in various fields including electronics, aerospace engineering, health care, and biomedicine (Singh et al. 2009, Bitar et al. 2012). In biomedical applications, appealing physicochemical features of SiNPs (Bae et al. 2012, Li et al. 2012, Slowing et al. 2008, Trewyn et al. 2007) helped promote the promiscuous use of these inorganic nanoparticles as carriers for a diverse series of molecules and pharmaceuticals (Biju 2014). Indeed, SiNPs have been used successfully to enhance the actions of pharmaceuticals (Kwon et al. 2013, Bitar et al. 2012), enzymes (Coll et al. 2011), biosensors and bio-imaging agents (Korzeniowska et al. 2013), and theranostics (Vivero-Escoto et al. 2012). Because SiNPs are able to freely pass the blood-brain barrier and enter into the CNS (Klejbor et al. 2007), their clinical application has extended to the diagnosis of and possible therapeutic intervention against such neurological diseases as spinal cord and traumatic brain injury, and such neurodegenerative disorders as Alzheimer’s and Parkinson’s disease (Cho et al. 2010, Sharma et al. 2009).
Relatively little is known about the biocompatibility, clearance, biodegradation and safety profiles of nanotechnology in the CNS. Nanoparticles including SiNPs have possible adverse effects including the promotion of neuroinflammation and neurotoxicity (Migliore et al. 2015). Relevant to Parkinson’s disease, SiNPs damaged dopaminergic neurons in rodent striatum (Wu et al. 2011) and induced alpha-synuclein formation and aggregation in cultured PC12 cells (Xie & Wu 2016). With relevance to Alzheimer’s disease (AD), SiNPs increased the levels of amyloid beta protein (Aβ) in (Yang et al. 2014). Therefore, greater attention towards understanding underlying mechanisms by which SiNPs might lead to neurological complications appears warranted. Endolysosomes are acidic organelles that play important physiological roles in brain including energy homeostasis, plasma membrane repair, immune regulation and cellular signaling (Xu & Ren 2015). Pathophysiologically, endolysosomes have been implicated in the pathogenesis of lysosomal storage diseases, metabolic disorders and various neurodegenerative disorders including AD (Nixon 2007, Nixon et al. 2008, Nixon & Cataldo 1995, Nixon & Cataldo 2006). Evidence suggests that endolysosome de-acidification can increase amyloidogenesis within and the release of calcium from endolysosomes and may contribute to the pathogenesis of AD and other neurodegenerative disorders (Hui et al. 2012a, Chen et al. 2013, Chen et al. 2010). Clearly, nanoparticles can accumulate in endolysosomes and in doing so can affect significantly many important functions attributed to these organelles (Cupaioli et al. 2014). Very little is known about negative effects of SiNPs in brain, but it has been reported recently that SiNPs can alter autophagy and impair autophagic-lysosome functions in non-neuronal cells (Schutz et al. 2016). Accordingly, we determined here the extent to which SiNPs could affect endolysosomes in neurons including their ability to accumulate in endolysosomes, and their effects on neuronal viability, endolysosome pH, calcium stores and amyloidogenesis.
Materials and Methods
Synthesis of pure SiNPs and Rubpy-doped SiNPs
Pure and Rubpy-doped SiNPs were synthesized using a modified reverse-microemulsion method (Bagwe et al. 2004). An aliquot of 7.5 ml of cyclohexane, 1.8 ml of 1-hexanol, and 1.77 ml of Triton X-100 were mixed and stirred for 20 min to form a homogenous solution. Afterwards, an aliquot of 340 µl H2O or Rubpy solution was added for making pure SiNPs or Rubpy-doped SiNPs, respectively. The mixture was stirred for 20 min and then 100 μl of tetraethylorthosilicate (TEOS, 98%) was added. 60 µl of 29% NH4OH was added to initiate the polymerization of TEOS and the solution was stirred for 24 hours to form SiNPs. The microemulsion was stopped by adding 10 ml of acetone and SiNPs were collected by centrifugation at 8,000 rpm for 20 min and washed three-times with ethanol and three-times with water. SiNPs underwent ultra-sonication (10 min) for re-dispersion prior to their application to primary cultured neurons.
Primary neuronal cultures
As previously described, primary cultures of hippocampal neurons were prepared from Sprague-Dawley rats (Charles River Laboratories) (Buscemi et al. 2007, Hui et al. 2012b). Pregnant dams (embryonic day 18) were sacrificed by asphyxiation with CO2. The fetuses were removed, decapitated, and meninges-free hippocampi were isolated, trypsinized, and plated onto 35-mm poly-D-lysine-coated glass bottom tissue culture dishes (MatTek Corp, Ashland, US). Neurons grown in Neurobasal™ medium plus L-glutamine, antibiotic/antimycotic and B27 supplement (ThermoFisher, Grand Island, US) were maintained at 37°C and 5% CO2 for 10–14 days at which time they were taken for experimentation. Typically, the purity of the neuronal cultures was greater than 95% as determined by immunostaining with neuron markers mouse anti-NeuN and goat anti-MAP2 antibodies (Millipore, Bilerica, US), as well as with the astrocyte marker mouse anti-GFAP antibody (Sigma, St. Louis, US).
MTT assay
We determined cell viability with the colorimetric MTT metabolic activity assay. Primary neurons (1 × 104 cells/well) were cultured in 96-well plates at 37°C, and exposed to varying concentrations of SiNPs for 24 h. Cells treated with DMSO served as vehicle controls. After treatment, 10 μl of MTT solution (5 mg/ml in PBS) was added to each well. After incubation for 4 h, the resultant formazan crystals were dissolved in DMSO (100 μl) and the absorbance intensity was measured using a microplate reader (Molecular Devices, San Jose, US) set at 490 nm with a reference wavelength of 620 nm. Data were expressed as a percentage of viable cells relative to the total number of cells in each well.
Endolysosome accumulation of SiNPs
Neurons were incubated with RuBpy-doped SiNPs for 24 hours at 37°C and then further incubated for 15 min with LysoTracker. After washing with PBS, neurons were examined by confocal microscopy (Zeiss LSM800, Germany).
Endolysosome pH measurements
As previously described (Liu et al. 2008, Hui et al. 2012a), endolysosome pH was measured using a ratio-metric endolysosome pH indicator dye (LysoSensor Yellow/Blue DND-160, ThermoFisher Scientific, Euegene, US); a dual excitation dye that permits pH measurements in acidic organelles independently of dye concentration. Neurons were loaded with 2 µM LysoSensor for 5 minutes at 37°C. Light emitted at 520 nm in response to excitation at 340 nm and 380 nm was measured for 20 msec every 30 seconds using a filter-based imaging system (Zeiss, Germany). The ratios of light excited (340/380 nm) versus light emitted (520 nm) were converted to pH using a calibration curve established using 10 µM of the H+/Na+ ionophore monensin, and 20 µM of the H+/K+ ionophore nigericin; both were dissolved in 20 mM 2-(N-morpholino) ethane sulfonic acid (MES), 110 mM KCl, and 20 mM NaCl adjusted to pH 3.0 to 7.0 with HCl/NaOH.
Endolysosome calcium store measurements
Levels of free intracellular Ca2+ were determined using the Ca2+-specific fluorescent probe Fura-2/AM (Invitrogen, Euegene, US). Neurons were incubated with 2 μM Fura-2/AM for 30 min at 37°C, washed with calcium-free buffer (145 mM NaCl, 5 mM KCl, 1 mM MgCl2, 10 mM glucose, 0.2 mM EGTA and 10 mM HEPES, pH=7.4) to remove extracellular Fura-2/AM, and incubated at 37°C for another 10 min to allow for intraneuronal de-esterification of Fura-2/AM to Fura-2. Neurons were excited at 340 and 380 nm, and light emitted at 510 nm was measured using our filter-based calcium imaging system (Zeiss, Germany). Images were acquired every 2 seconds and at this acquisition rate we were able to measure baseline as well as peak increases in levels of free intracellular calcium. The ratio of 340/380 was used as a measurement of intracellular calcium levels. Endolysosome calcium levels were measured indirectly using the lysosomotropic agent glycyl-L-phenylalanine 2-naphthylamide (GPN) by measuring free intraneuronal calcium levels in the absence or presence of GPN (Penny et al. 2014; Lee et al. 2015). GPN causes brief permeabilization of the endolysosome membrane (Jadot et al. 1984), de-acidifies endolysosomes, and releases calcium from endolysosomes. SiNP-induced reduction in the ability of GPN to release calcium from endolysosomes was interpreted as indicating the ability of SiNPs to stimulate the release of calcium from endolysosomes and thereby decrease readily releasable calcium from the endolysosome pool.
Quantification of Aβ levels by ELISA
Aβ levels were quantified using human/rat Aβ1–40 and Aβ1–42 ELISA kits as per the manufacturer’s protocol (Wako, Osaka, Japan). Media from cultured neurons were collected and diluted (1:2 for Aβ1–42, 1:5 for Aβ1–40) with standard diluent buffer. Aβ levels were measured in duplicate using a calorimetric sandwich ELISA method (Wako, Osaka, Japan). Aβ levels were normalized to total protein content in each sample as determined by a DC protein assay (Bio-Rad, US). For total protein determinations, neurons in each dish were lysed with RIPA buffer (Pierce, Rockford, US) containing 10 mM NaF, 1 mM Na3VO4 and Protease Inhibitor Cocktail (Sigma-Aldrich, USA). After centrifugation (14,000 X g for 10 min at 4°C), supernatants were collected for DC protein assays.
Reagents
Tetraethylorthosilicate (98%), cyclohexane, 1-hexanol, acetone, Triton X-100, MTT reagent, CGS21680, and GPN were purchased from Sigma-Aldrich (US). Ammonium hydroxide (28.0−30.0%), ethanol (99%), LysoTracker DND99, LysoSensor DND160, calcium fluorescent dye Fura-2/AM and DMSO were obtained from Fisher Scientific (USA). Sodium citrate, 1-pentanol, and poly (vinylpyrrolidone) (average molecular weight = 40,000) were purchased from Alfa Aesar. Tris(bipyridine)-ruthenium(II) dichloride (Rubpy) was purchased from ICN Biomedicals. Human/Rat ELISA kits were obtained from Wako (Japan). ML-SA1 was obtained from R&D (US).
Statistics
All data were expressed as means and SEM. Statistical significance was determined by one-way ANOVA plus a Tukey post hoc test, or by Student’s-t test. p < 0.05 was considered to be statistically significant.
Results
Characterization of nanoparticles
The morphology of synthesized nanoparticles was characterized using a scanning electron microscope (Figures 1A and 1B). SiNPs were spherical in shape with diameters of 68 ± 4 nm (Figure 1A). Rubpy-doped SiNPs were spherical with diameters of 71 ± 5 nm (Figure 1B). To further confirm the surface properties of the SiNPs, zeta potentials were measured (Figures 1C and 1D). SiNPs displayed high negative zeta potentials at −38.33 ± 0.55 mV. After doping with Rubpy dye, the zeta potentials of Rubpy-SiNPs were less negatively charged (−7.46 ± 0.25 mV) compared to control SiNPs. In addition, an elemental analysis of SiNPs was conducted using energy-dispersive X-ray spectroscopy (EDS) (Figures 1E and 1F). The results of EDS clearly showed the presence of Si and O, both of which were from the SiNPs. In addition, peaks of C and Cu were detected.
Figure 1.


Characterization of silica nanoparticles (SiNPs). Using a Hitachi SU8010 field scanning-electron microscope, sizes of synthesized SiNPs and Rubpy-doped SiNPs were detected. (A) The diameters of SiNPs were 68 ± 4 nm. (B) The diameters of Rubpy-doped SiNPs were 71 ± 5 nm. Zeta potentials of SiNPs (C) were −38.33 ± 0.55 mV and for Rubpy-doped SiNPs (D) were −7.46 ± 0.25 mV. EDS patterns for SiNPs (E) and Rubpy-doped SiNPs (F) were illustrated.
Effects of SiNPs on neuronal viability
We first determined the extent to which SiNPs affected the viability of primary cultured neurons. Neurons incubated for 24 hours with concentrations of SiNPs ranging from 0.01 to 1 μg/ml were tested for viability using the MTT assay. SiNPs were found to decrease neuronal viability significantly (p<0.05) by about 40% at the highest concentrations tested (1 μg/ml) (Figure 2). Accordingly, for all subsequent studies of endolysosome function we used a non-toxic concentration (0.1 μg/ml) of SiNPs.
Figure 2.

Effects of SiNPs on neuronal viability. Primary neurons were grown in culture for 10–14 days and were then treated with SiNPs (Zhao et al. 2008) at various concentrations as indicated. Cell viability was determined by MTT assay 24 hours after SiNPs were applied. (*p<0.05, vs control, n=6).
Endolysosome accumulation of SiNPs in primary cultured neurons
Because SiNPs were found by others to be endocytosed (Lesniak et al. 2012) and to accumulate in endolysosomes (Shi et al. 2010), we next determined the extent to which SiNPs were endocytosed into and accumulated by neuronal endolysosomes. Primary cultured neurons incubated for 24 hours with SiNP-tagged RuBpy and for 15 min with LysoTracker showed (Figure 3A) colocalization of SiNP-tagged RuBpy with LysoTracker. There results indicated that SiNPs were endocytosed by and accumulated in neuronal endolysosomes. However, no attempt was made to quantify the amount of SiNP accumulation in the endolysosomes.
Figure 3.

Endocytosis of SiNPs in endolysosomes and the effects of SiNPs on endolysosome pH in primary cultured neurons. (A) Cells were treated with RuBpy tagged SiNP (NP-RuBpy) for 24 h followed by incubation with LysoTracker; an endolysosome marker dye. Images shown were neurons labeled with LysoTracker and NP-RuBpy separately as well as a merger of the images. Images shown demonstrated that SiNPs were accumulated in endolysosomes (A). Scale bar = 10 μm. (B-C) Endolysosome pH was measured ratio-metrically using LysoSensor dye within the first 5 minutes of (B) and 24 hours after (C) application of SiNPs (Zhao et al. 2008) at 0.1 μg/ml. Application of SiNPs to neurons produced immediate endolysosome de-acidification (B) (n=13) and the endolysosome de-acidification was sustained over a 24 h treatment period (C) (n>10). (***p<0.001, vs control).
[Ca2+]cyt
Effects of SiNPs on endolysosome pH in primary cultured neurons
Endolysosome pH is an established indicator of endolysosome function because the acidic environment critically controls a variety of cellular functions and homeostatic mechanisms (Appelqvist et al. 2013). After confirming that SiNPs trafficked to the endolysosome system, we determined the extent to which SiNPs affected endolysosome pH acutely (within 5 min) and persistently (24 h post treatment). Using an approach similar to that used by us to show that endolysosome de-acidification contributed to pathological features shared by several neurodegenerative disorders (Chen et al. 2013, Hui et al. 2012a), we found here that non-toxic concentrations of SiNPs (0.1 μg/ml) significantly (p<0.001) de-acidified endolysosomes within 5 min of application (Figure 3B). 24 hours after application, SiNPs at 0.1 μg/ml significantly (p<0.001) de-acidified endolysosomes (Figure 3C). Thus, SiNPs at non-toxic concentrations could significantly de-acidify endolysosome acutely and persistently.
Effects of SiNPs on endolysosome calcium stores in primary cultured neurons
Others and we have shown that neuronal endolysosomes have readily releasable stores of calcium equivalent to those found in endoplasmic reticulum; levels sufficient to affect calcium homeostasis and neuronal function (Brailoiu et al. 2005, Dickinson et al. 2010, Pandey et al. 2009, Hui et al. 2015). Here, we determined the extent to which SiNPs affected endolysosome calcium stores using a lysosomotropic agent GPN that releases calcium from endolysosomes. Under nominal calcium-free conditions, GPN significantly (p<0.001) increased levels of intracellular calcium shortly after its application to neurons (Figure 4A) indicating that GPN induced calcium release from endolysosomes. Next, we treated neurons for 24 hours with non-toxic concentrations of SiNPs (0.01 and 0.1 μg/ml) and measured effects on GPN-induced calcium release from endolysosomes. Compared with controls, SiNPs at 0.1 μg/ml significantly (p<0.001) decreased GPN-induced calcium release from endolysosomes (Figure 4B). This suggested that SiNPs at non-toxic concentrations released calcium from endolysosomes thus decreasing the pool of GPN-releasable calcium.
Figure 4.

Effects of SiNPs on GPN-inducible release of calcium from endolysosomes in primary cultured neurons. (A) GPN increased significantly (***p<0.001, vs control, n>10) levels of free intracellular calcium as a result of increased release of calcium from endolysosomes. (B) Compared to controls, SiNPs (0.01 μg/ml and 0.1 μg/ml) significantly (*p<0.05, ***p<0.001, vs control, n=10) and concentration-dependently reduced GPN-inducible stores of calcium in endolysosomes.
Effects of SiNPs on extra-neuronal levels of Aβ in primary cultured neurons
Endolysosomes are major sites where amyloidogenic processing of AβPP occurs following AβPP internalization, and we have shown that de-acidifying endolysosomes increases the formation of Aβ (Hui et al. 2012a). Because SiNPs were shown (see above) to de-acidify endolysosomes, we next determined the extent to which SiNPs, at non-toxic concentrations, affected extra-neuronal Aβ levels in cultured neurons. 24 hours after the application of SiNPs, we collected the media and determined levels of Aβ1–40 and Aβ1–42. SiNPs at 0.1 μg/ml modestly but statistically significantly (p<0.05) increased levels of extra-neuronal Aβ1–40 compared to controls (Figure 5A). In contrast, SiNPs failed to significantly affect levels of extra-neuronal Aβ1–42 at concentrations of either 0.01 or 0.1 μg/ml (Figure 5B).
Figure 5.
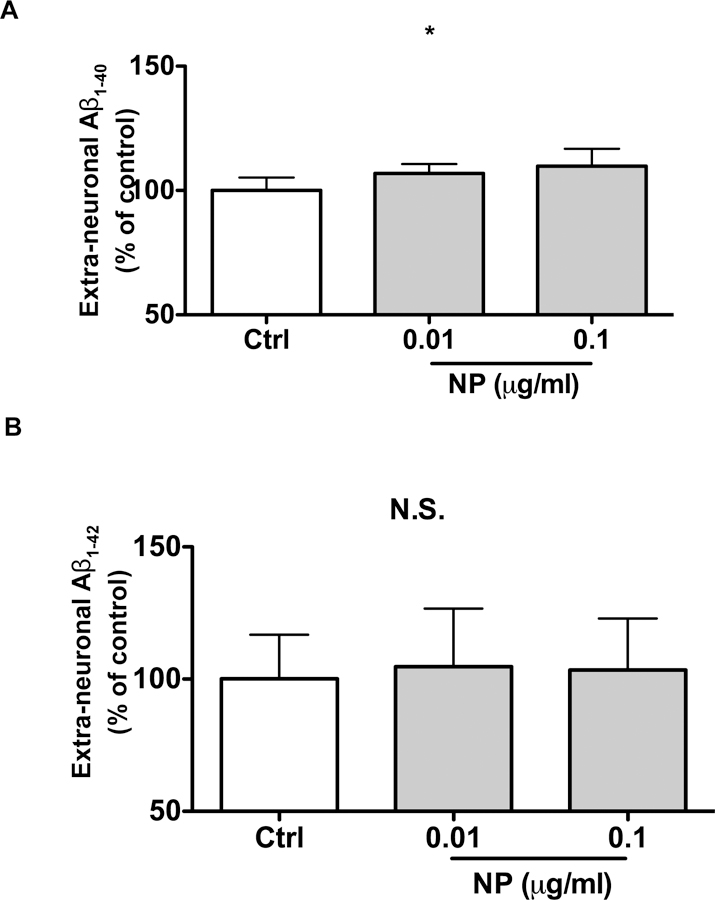
Effects of SiNPs on extra-neuronal levels of Aβ in primary cultured neurons. Primary cultured neurons were treated for 24 hours with SiNPs at 0.01 and 0.1 μg/ml. At the end of the treatment period, media were collected and levels of Aβ1–40 (A) and Aβ1–42 (B) were determined by ELISA assay. (A) SiNPs at 0.1 μg/ml modestly but statistically significantly (*p<0.05, vs control, n=6) increased levels of extra-neuronal Aβ1–40 compared to controls. (B) Neither of the SiNPs at the concentrations tested significantly affected levels of extra-neuronal Aβ1–42 (n=4).
Effects of endolysosome acidifying agents on SiNP-induced endolysosome de-acidification and increases in Aβ1–40 release in primary cultured neurons
Using ML-SA1, a TRPML channel agonist, and CGS21680, an agonist of adenosine A2a receptors, that have been shown to acidify endolysosomes (Bae et al. 2014, Liu et al. 2008), we determined next the extent to which these endolysosome acidifying agents could reverse SiNP-induced endolysosome de-acidification and increases in Aβ1–40 levels. In confirmation of results noted above, SiNPs at 0.1 μg/ml increased significantly endolysosome pH (p<0.001) (Figure 6A) and levels of Aβ1–40 (p<0.05) (Figure 6B). After 24 h treatment, ML-SA1 (20 μM) and CGS21680 (100 μM) did not significantly affect endolysosome pH but both significantly (p<0.001) blocked SiNP-induced endolysosome de-acidification (Figure 6A) and SiNP-induced increases in secreted levels of Aβ1–40 (p<0.01) (Figure 6B).
Figure 6.

Effects of endolysosome acidifying agents on SiNP-induced endolysosome de-acidification and increases in Aβ1–40 release in primary cultured neurons. (A) SiNPs (0.1 μg/ml) induced deacidification of endolysosome pH was significantly (***p<0.001, vs control, n=20) decreased by ML-SA1 (20 μM) and CGS21680 (100 μM). (B) SiNP (0.1 μg/ml) induced increases in extra-neuronal Aβ1–40 levels were reduced significantly (*p<0.05, **p<0.01 vs control, n=5) by ML-SA1 and CGS21680.
Discussion
The rapid development of and enthusiasm for the use of nanotechnology in health and disease has been accompanied by concerns about the potential toxicity of nanomaterials (Stone & Donaldson 2006). Among the variety of nanomaterials used, SiNPs have attracted attention in biomedical fields because of their favorable biocompatibility and their scalable synthetic capability (Liberman et al. 2014). The importance of safety is tantamount to any use of SiNPs in nanomedicine (Elsaesser & Howard 2012, Mohamed et al. 2011, Fadeel & Garcia-Bennett 2010) and the study of their safety profiles is complicated by the variable procedures used to produce SiNPs and their diverse physicochemical properties (Fadeel & Garcia-Bennett 2010, Tang & Cheng 2013). Rather than regarding dosage levels as the only major toxic concern (Moss & Wong 2006, Oberdorster et al. 2007, Wittmaack 2007), many other important factors have been identified to influence safety such as cell types (Nan et al. 2008, Rabolli et al. 2010, Yu et al. 2011), physicochemical properties of SINPs (Yu et al. 2012a, Yu et al. 2011, Yu et al. 2012b), and the apparent high tolerance of some experimental animals for even large doses of SiNPs (Liu et al. 2011, Lu et al. 2010). Here, we focused our studies on endolysosomes because these acidic organelles likely are affected first upon nanoparticle entry into cells and might serve as early indicators of the biological effects of SiNPs. We found that at non-toxic concentrations, SiNPs de-acidified endolysosomes, decreased readily releasable stores of calcium in endolysosomes, and increased amyloidogenesis in primary cultured neurons; findings that provide mechanistic insight into SiNP-induced neurotoxicity and enhanced amyloidogenesis.
Depending on the particle size and surface treatment, SiNPs enter cells via different pathways including phagocytosis, micropinocytosis, and receptor-mediated endocytosis (Zhang et al. 2015). SiNPs without conjugated ligands may also be endocytosed non-specifically. Although we did not explore the invovlement of surface proteins that could mediate the endocytosis of SiNPs, we did observe that SiNPs were accumulated in endolysosomes of primary cultured neurons. Keeping this in mind, we also acknowledge the possibilty that SiNPs may be present in other organelles besides the endolysosomes. SiNPs are often engineered so that the release of guest molecules is affected by acidic environments under physiological conditions in stomach and endolysosomes, and under such pathological conditions as tumors and pro-inflammatory conditions (Song et al. 2017, Yang et al. 2012). However, more attention has been focused on how pH regulates the function of SiNPs, and little is known whether SiNPs themselves can impact pH. Thus, we explored the potential direct effects of SiNPs on functions of endolysosomes. As acidic intracellular organelles, endolysosomes are especially important for regulating neuronal functions because neurons are mainly long-lived post-mitotic cells that require the endolysosome system in turning over cellular components and obsolete organelles (Nixon & Cataldo 1995, Nixon & Cataldo 2006). Because maintaining an optimum acidic environment in endolysosomes is critical to maintaining endolysosome function, we determined the extent to which SiNPs affected endolysosome pH. We demonstrated that SiNPs, at non-neurotoxic concentrations, de-acidified endolysosomes acutely and persistently. Our findings suggest that SiNPs directly affect endolysosome pH, which could lead to neuronal cell death at higher concentrations. Currently, we do not know how SiNPs de-acidify endolysosomes, but their physiochemical properties could play a role.
Endolysosomes are important intracellular calcium stores containing high concentrations (400–600 μM) of readily releasible calcium. Given the dynamic properties of endolysosomes, calcium released from endolysosomes can regulate a variety of fundamental calcium-dependent processes including vesicular trafficking, endolysosome fusion, lysosome-related organelle biogenesis, membrane repair, and exocytosis-endocytosis coupling (Ruas et al. 2010, Lloyd-Evans et al. 2010, Huynh et al. 2004, Lima et al. 2012, Li et al. 2008, Hosoi et al. 2009). Indeed, for neurons, endolysosome calcium has been implicated in neurotransmitter release, neuronal excitability, synaptic plasticity, neurite extension, and neuronal viability (Brailoiu et al. 2005, Dickinson et al. 2010, Pandey et al. 2009, Hui et al. 2015). Moreover, decreased endolysosome calcium has been implicated in neurodegeneration (Feng et al. 2014) and neurological diseases including AD (McBrayer & Nixon 2013). Although the mechanisms responsible for calcium uptake into and release from endolysosomes are not fully understood, others and we have shown that decreased endolysosome calcium can be a direct consequence of endolysosome de-acidification (McBrayer & Nixon 2013, Hui et al. 2015). Here, we found that non-toxic concentrations of SiNPs could decrease the releasable pool of calcium in endolysosomes and increase cytosolic calcium levels, and this might lead to calcium dyshomeostasis and impact neuron function (Gilardino et al. 2015). It is possible that other calcium stores could be contributing to the SiNP-induced increase in cytosolic calcium levels, e.g. calcium released from endolysosomes might trigger the release of calcium from endoplasmic reticulum stores or induce calcium influx across the plasma membrane (Hui et al., 2015). Moreover, further investigations are necessary to understand more fully how SiNPs affect highly coordinated calcium events including endolysosome calcium release, calcium uptake and release from other organelles, calcium levels in cytoplasm, and the influx of extracellular calcium.
Endolysosomes play an important and early role in the pathogenesis of sporadic AD that precede the development of amyloid plaques (Tate & Mathews 2006, Boland et al. 2008). Amyloid plaques are comprised of aggregated Aβ, which have two major isoforms 1–40 and 1–42; Aβ1–42 is more fibrillogenic than Aβ1–40 although the levels of Aβ1–40 are several fold higher than Aβ1–42 (Gu & Gou 2013). Endolysosomes are major sites where Aβ is generated following AβPP internalization, and Aβ is degraded in acidic lysosomes (Edgar et al. 2015, Miners et al. 2011, Vingtdeux et al. 2012). Even modest de-acidification can compromise degradation capabilities of endolysosomes, resulting in increased intraneuronal accumulation and secreted levels of Aβ. Indeed, SiNPs have been shown to promote amyloidogenesis (Yang et al. 2014). Here, we explored the extent to which endolysosome de-acidification played a role in SiNP-induced amyloidogenesis. We demonstrated that SiNPs de-acidified endolysosomes and increased extra-neuronal levels of Aβ1–40 but interestingly did not affect extra-neuronal levels of Aβ1–42. It is possible that SiNP’s might be increasing the intracellular production but also inhibiting the secretion of Aβ1–42. Further studies would need to be conducted to determine the effects of SiNPs on producing, processing, and releasing Aβ. To further explore the causal relationship between SiNP induced de-acidification and amyloidogenesis, we determined the extent to which acidifying endolysosomes prevented SiNP-induced amyloidogenesis, and ML-SA1 (a TRPML agonist) and CGS21680 (an adenosine A2A receptor agonist) that have been shown to acidify endolysosomes were used (Bae et al. 2014; Liu et al. 2008). Acute treatment with ML-SA1 and CGS21680 acidifies endolysosomes, but longer-term 24 h treatment did not change endolysosome pH. One explanation might be because over the 24 h timespan the neuronal endolysosomes are able to homeostatically regulate pH levels back to normal. Similarly, 24 h treatment with ML-SA1 and CGS21680 did not significantly reduce extra-neuronal Aβ1–40 levels, but did block SiNP-induced endolysosome de-acidification and amyloidogenesis in neurons. Thus, endolysosome de-acidification appears to play an important role in SiNP-induced amyloidogenesis. Altogether, our findings have led us to suggest a proposed mechanism by which SiNP might induce neurotoxicity; SiNPs are trafficked into the endolysosome system and once within endolysosomes disrupt the function of these organelles thereby inducing calcium dyshomeostasis and increased levels of extra-neuronal Aβ.
Clearly, there is a great promise that SiNPs may benefit biomedical studies and therapeutic strategies. However, the utilization of SiNPs should proceed advisedly because of findings such as ours that these nanoparticles de-acidify endolysosomes, increase calcium release from endolysosomes and increase levels of beta amyloid protein.
Figure 7.

Following endocytosis of SiNPs into neurons, endolysosome pH is increased (de-acidification), calcium in endolysosomes is released into the cytosol, and Aβ levels are increased extra-cellularly. It appears endolysosome pH is an important starting point for these actions because endolysosome acidification with the TRPML1 agonist ML-SA1 or the adenosine A2A receptor agonist CGS21680 block the effects of SiNPs on calcium and Aβ.
Acknowledgements
This work was supported by the following grants awarded to us by NIH (P30GM103329, R01MH100972, R01MH105329, R21DA040519) and NSF (CHE1709160). We greatly acknowledge the help of Dr. Bryon Grove and Ms. Sarah Abrahamson in using the Edward C. Carlson Imaging and Image Analysis Core Facility.
Abbreviation:
- SiNPs
Silica nanoparticles
- Aβ
amyloid beta protein
- AD
Alzheimer’s disease
- GPN
glycyl-L-phenylalanine 2-naphthylamide
- ML-SA1
a TRPML channel agonist
- CGS21680
an adenosine A2a receptor agonist
- TEOS
tetraethylorthosilicate
- ELISA
enzyme-linked immunosorbent assay
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
Footnotes
Conflict of interest
None declared
References
- Appelqvist H, Waster P, Kagedal K and Ollinger K (2013) The lysosome: from waste bag to potential therapeutic target. J Mol Cell Biol, 5, 214–226. [DOI] [PubMed] [Google Scholar]
- Bae M, Patel N, Xu H et al. (2014) Activation of TRPML1 clears intraneuronal Abeta in preclinical models of HIV infection. J Neurosci, 34, 11485–11503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae SW, Tan W and Hong JI (2012) Fluorescent dye-doped silica nanoparticles: new tools for bioapplications. Chem Commun (Camb), 48, 2270–2282. [DOI] [PubMed] [Google Scholar]
- Bagwe RP, Yang C, Hilliard LR and Tan W (2004) Optimization of dye-doped silica nanoparticles prepared using a reverse microemulsion method. Langmuir, 20, 8336–8342. [DOI] [PubMed] [Google Scholar]
- Biju V (2014) Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem Soc Rev, 43, 744–764. [DOI] [PubMed] [Google Scholar]
- Bitar A, Ahmad NM, Fessi H and Elaissari A (2012) Silica-based nanoparticles for biomedical applications. Drug Discov Today, 17, 1147–1154. [DOI] [PubMed] [Google Scholar]
- Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH and Nixon RA (2008) Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer’s disease. J Neurosci, 28, 6926–6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu E, Hoard JL, Filipeanu CM, Brailoiu GC, Dun SL, Patel S and Dun NJ (2005) Nicotinic acid adenine dinucleotide phosphate potentiates neurite outgrowth. J Biol Chem, 280, 5646–5650. [DOI] [PubMed] [Google Scholar]
- Buscemi L, Ramonet D and Geiger JD (2007) Human immunodeficiency virus type-1 protein Tat induces tumor necrosis factor-alpha-mediated neurotoxicity. Neurobiol Dis, 26, 661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Hui L, Geiger NH, Haughey NJ and Geiger JD (2013) Endolysosome involvement in HIV-1 transactivator protein-induced neuronal amyloid beta production. Neurobiol Aging, 34, 2370–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wagener JF, Morgan DH, Hui L, Ghribi O and Geiger JD (2010) Endolysosome mechanisms associated with Alzheimer’s disease-like pathology in rabbits ingesting cholesterol-enriched diet. J Alzheimers Dis, 22, 1289–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Shi R, Ivanisevic A and Borgens RB (2010) Functional silica nanoparticle-mediated neuronal membrane sealing following traumatic spinal cord injury. J Neurosci Res, 88, 1433–1444. [DOI] [PubMed] [Google Scholar]
- Coll C, Mondragon L, Martinez-Manez R, Sancenon F, Marcos MD, Soto J, Amoros P and Perez-Paya E (2011) Enzyme-mediated controlled release systems by anchoring peptide sequences on mesoporous silica supports. Angew Chem Int Ed Engl, 50, 2138–2140. [DOI] [PubMed] [Google Scholar]
- Cupaioli FA, Zucca FA, Boraschi D and Zecca L (2014) Engineered nanoparticles. How brain friendly is this new guest? Prog Neurobiol, 119–120, 20–38. [DOI] [PubMed] [Google Scholar]
- Dickinson GD, Churchill GC, Brailoiu E and Patel S (2010) Deviant nicotinic acid adenine dinucleotide phosphate (NAADP)-mediated Ca2+ signaling upon lysosome proliferation. J. Biol Chem, 285, 13321–13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JR, Willen K, Gouras GK and Futter CE (2015) ESCRTs regulate amyloid precursor protein sorting in multivesicular bodies and intracellular amyloid-beta accumulation. J Cell Sci, 128, 2520–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsaesser A and Howard CV (2012) Toxicology of nanoparticles. Adv Drug Deliv Rev, 64, 129–137. [DOI] [PubMed] [Google Scholar]
- Fadeel B and Garcia-Bennett AE (2010) Better safe than sorry: Understanding the toxicological properties of inorganic nanoparticles manufactured for biomedical applications. Adv Drug Deliv Rev, 62, 362–374. [DOI] [PubMed] [Google Scholar]
- Feng X, Xiong J, Lu Y, Xia X and Zhu MX (2014) Differential mechanisms of action of the mucolipin synthetic agonist, ML-SA1, on insect TRPML and mammalian TRPML1. Cell Calcium, 56, 446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilardino A, Catalano F, Ruffinatti FA, Alberto G, Nilius B, Antoniotti S, Martra G and Lovisolo D (2015) Interaction of SiO2 nanoparticles with neuronal cells: Ionic mechanisms involved in the perturbation of calcium homeostasis. Int J Biochem Cell Biol, 66, 101–111. [DOI] [PubMed] [Google Scholar]
- Gu L and Guo Z (2013) Alzheimer’s Aβ42 and Aβ40 peptides form interlaced amyloid fibrils. J Neurochem, 126, 305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoi N, Holt M and Sakaba T (2009) Calcium dependence of exo- and endocytotic coupling at a glutamatergic synapse. Neuron, 63, 216–229. [DOI] [PubMed] [Google Scholar]
- Hui L, Chen X and Geiger JD (2012a) Endolysosome involvement in LDL cholesterol-induced Alzheimer’s disease-like pathology in primary cultured neurons. Life Sci, 91, 1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui L, Chen X, Haughey NJ and Geiger JD (2012b) Role of endolysosomes in HIV-1 Tat-induced neurotoxicity. ASN Neuro, 4, 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui L, Geiger NH, Bloor-Young D, Churchill GC, Geiger JD and Chen X (2015) Release of calcium from endolysosomes increases calcium influx through N-type calcium channels: Evidence for acidic store-operated calcium entry in neurons. Cell Calcium, 58, 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh C, Roth D, Ward DM, Kaplan J and Andrews NW (2004) Defective lysosomal exocytosis and plasma membrane repair in Chediak-Higashi/beige cells. Proc Natl Acad Sci U S A, 101, 16795–16800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadot M, Colmant C, Wattiaux-De Coninck S and Wattiaux R (1984) Intralysosomal hydrolysis of glycyl-L-phenylalanine 2-naphthylamide. J. Biochem, 219, 965–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klejbor I, Stachowiak EK, Bharali DJ, Roy I, Spodnik I, Morys J, Bergey EJ, Prasad PN and Stachowiak MK (2007) ORMOSIL nanoparticles as a non-viral gene delivery vector for modeling polyglutamine induced brain pathology. J Neurosci Methods, 165, 230–243. [DOI] [PubMed] [Google Scholar]
- Korzeniowska B, Nooney R, Wencel D and McDonagh C (2013) Silica nanoparticles for cell imaging and intracellular sensing. Nanotechnology, 24, 442002. [DOI] [PubMed] [Google Scholar]
- Kwon S, Singh RK, Perez RA, Abou Neel EA, Kim HW and Chrzanowski W (2013) Silica-based mesoporous nanoparticles for controlled drug delivery. J Tissue Eng, 4, 2041731413503357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, McBrayer MK, Wolfe DM, Haslett LJ, Kumar A, Sato Y, Lie PP, Mohan P, Coffey EE, Kompella U, Mitchell CH, Llyod-Evans E and Nixon RA (2015) Presenillin 1 maintains lysosomal Ca2+ homeostasis via TRPML1 by regulating vATPase-Mediated Lysosome Acidifcation. Cell Rep, 12, 1430–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniak A, Fenaroli F, Monopoli MP, Aberg C, Dawson KA and Salvati A (2012) Effects of the presence or absence of a protein corona on silica nanoparticle uptake and impact on cells. ACS Nano, 6, 5845–5857. [DOI] [PubMed] [Google Scholar]
- Li D, Ropert N, Koulakoff A, Giaume C and Oheim M (2008) Lysosomes are the major vesicular compartment undergoing Ca2+-regulated exocytosis from cortical astrocytes. J Neurosci, 28, 7648–7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Barnes JC, Bosoy A, Stoddart JF and Zink JI (2012) Mesoporous silica nanoparticles in biomedical applications. Chem Soc Rev, 41, 2590–2605. [DOI] [PubMed] [Google Scholar]
- Liberman A, Mendez N, Trogler WC and Kummel AC (2014) Synthesis and surface functionalization of silica nanoparticles for nanomedicine. Surf Sci Rep, 69, 132–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima WC, Leuba F, Soldati T and Cosson P (2012) Mucolipin controls lysosome exocytosis in Dictyostelium. J Cell Sci, 125, 2315–2322. [DOI] [PubMed] [Google Scholar]
- Liu J, Lu W, Reigada D, Nguyen J, Laties AM and Mitchell CH (2008) Restoration of lysosomal pH in RPE cells from cultured human and ABCA4(−/−) mice: pharmacologic approaches and functional recovery. Invest Ophthalmol Vis Sci, 49, 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Li L, Teng X, Huang X, Liu H, Chen D, Ren J, He J and Tang F (2011) Single and repeated dose toxicity of mesoporous hollow silica nanoparticles in intravenously exposed mice. Biomaterials, 32, 1657–1668. [DOI] [PubMed] [Google Scholar]
- Lloyd-Evans E, Waller-Evans H, Peterneva K and Platt FM (2010) Endolysosomal calcium regulation and disease. Biochem Soc Trans, 38, 1458–1464. [DOI] [PubMed] [Google Scholar]
- Lu J, Liong M, Li Z, Zink JI and Tamanoi F (2010) Biocompatibility, biodistribution, and drug-delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Small, 6, 1794–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBrayer M and Nixon RA (2013) Lysosome and calcium dysregulation in Alzheimer’s disease: partners in crime. Biochem Soc Trans, 41, 1495–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliore L, Uboldi C, Di Bucchianico S and Coppede F (2015) Nanomaterials and neurodegeneration. Environ Mol Mutagen, 56, 149–170. [DOI] [PubMed] [Google Scholar]
- Miners JS, Barua N, Kehoe PG, Gill S and Love S (2011) Abeta-degrading enzymes: potential for treatment of Alzheimer disease. J Neuropathol Exp Neurol, 70, 944–959. [DOI] [PubMed] [Google Scholar]
- Mohamed BM, Verma NK, Prina-Mello A et al. (2011) Activation of stress-related signalling pathway in human cells upon SiO2 nanoparticles exposure as an early indicator of cytotoxicity. J Nanobiotechnology, 9, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss OR and Wong VA (2006) When nanoparticles get in the way: impact of projected area on in vivo and in vitro macrophage function. Inhal Toxicol, 18, 711–716. [DOI] [PubMed] [Google Scholar]
- Nan A, Bai X, Son SJ, Lee SB and Ghandehari H (2008) Cellular uptake and cytotoxicity of silica nanotubes. Nano Lett, 8, 2150–2154. [DOI] [PubMed] [Google Scholar]
- Nixon RA (2007) Autophagy, amyloidogenesis and Alzheimer disease. J Cell Sci, 120, 4081–4091. [DOI] [PubMed] [Google Scholar]
- Nixon RA and Cataldo AM (1995) The endosomal-lysosomal system of neurons: new roles. Trends Neurosci, 18, 489–496. [DOI] [PubMed] [Google Scholar]
- Nixon RA and Cataldo AM (2006) Lysosomal system pathways: genes to neurodegeneration in Alzheimer’s disease. J Alzheimers Dis, 9, 277–289. [DOI] [PubMed] [Google Scholar]
- Nixon RA, Yang DS and Lee JH (2008) Neurodegenerative lysosomal disorders: a continuum from development to late age. Autophagy, 4, 590–599. [DOI] [PubMed] [Google Scholar]
- Oberdorster G, Oberdorster E and Oberdorster J (2007) Concepts of nanoparticle dose metric and response metric. Environ Health Perspect, 115, A290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey V, Chuang CC, Lewis AM, Aley PK, Brailoiu E, Dun NJ, Churchill GC and Patel S (2009) Recruitment of NAADP-sensitive acidic Ca2+ stores by glutamate. Biochem J, 422, 503–512. [DOI] [PubMed] [Google Scholar]
- Penny CJ, Kilpatrick BS, Han JM, Sneyd J and Patel S (2014) A computational model of lysosome-ER Ca2+ microdomains. J Cell Sci, 127, 2943–2943. [DOI] [PubMed] [Google Scholar]
- Rabolli V, Thomassen LC, Princen C et al. (2010) Influence of size, surface area and microporosity on the in vitro cytotoxic activity of amorphous silica nanoparticles in different cell types. Nanotoxicology, 4, 307–318. [DOI] [PubMed] [Google Scholar]
- Ruas M, Rietdorf K, Arredouani A et al. (2010) Purified TPC Isoforms Form NAADP Receptors with Distinct Roles for Ca(2+) Signaling and Endolysosomal Trafficking. Curr Biol [DOI] [PMC free article] [PubMed]
- Schutz I, Lopez-Hernandez T, Gao Q et al. (2016) Lysosomal Dysfunction Caused by Cellular Accumulation of Silica Nanoparticles. J Biol Chem, 291, 14170–14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma HS, Muresanu DF, Sharma A, Patnaik R and Lafuente JV (2009) Chapter 9 - Nanoparticles influence pathophysiology of spinal cord injury and repair. Prog Brain Res, 180, 154–180. [DOI] [PubMed] [Google Scholar]
- Shi H, He X, Yuan Y, Wang K and Liu D (2010) Nanoparticle-based biocompatible and long-life marker for lysosome labeling and tracking. Anal Chem, 82, 2213–2220. [DOI] [PubMed] [Google Scholar]
- Singh N, Manshian B, Jenkins GJ, Griffiths SM, Williams PM, Maffeis TG, Wright CJ and Doak SH (2009) NanoGenotoxicology: the DNA damaging potential of engineered nanomaterials. Biomaterials, 30, 3891–3914. [DOI] [PubMed] [Google Scholar]
- Slowing II, Vivero-Escoto JL, Wu CW and Lin VS (2008) Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv Drug Deliv Rev, 60, 1278–1288. [DOI] [PubMed] [Google Scholar]
- Song Y, Li Y, Xu Q and Liu Z (2017) Mesoporous silica nanoparticles for stimuli-responsive controlled drug delivery: advances, challenges, and outlook. Int J Nanomedicine, 12, 87–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone V and Donaldson K (2006) Nanotoxicology: signs of stress. Nat Nanotechnol, 1, 23–24. [DOI] [PubMed] [Google Scholar]
- Tang L and Cheng J (2013) Nonporous Silica Nanoparticles for Nanomedicine Application. Nano Today, 8, 290–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate BA and Mathews PM (2006) Targeting the role of the endosome in the pathophysiology of Alzheimer’s disease: a strategy for treatment. Sci Aging Knowledge Environ, 2006, re2. [DOI] [PubMed]
- Trewyn BG, Giri S, Slowing II and Lin VS (2007) Mesoporous silica nanoparticle based controlled release, drug delivery, and biosensor systems. Chem Commun (Camb), 3236–3245. [DOI] [PubMed]
- Vingtdeux V, Sergeant N and Buee L (2012) Potential contribution of exosomes to the prion-like propagation of lesions in Alzheimer’s disease. Front Physiol, 3, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivero-Escoto JL, Huxford-Phillips RC and Lin W (2012) Silica-based nanoprobes for biomedical imaging and theranostic applications. Chem Soc Rev, 41, 2673–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmaack K (2007) In search of the most relevant parameter for quantifying lung inflammatory response to nanoparticle exposure: particle number, surface area, or what? Environ Health Perspect, 115, 187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Wang C, Sun J and Xue Y (2011) Neurotoxicity of silica nanoparticles: brain localization and dopaminergic neurons damage pathways. ACS Nano, 5, 4476–4489. [DOI] [PubMed] [Google Scholar]
- Xie H and Wu J (2016) Silica nanoparticles induce alpha-synuclein induction and aggregation in PC12-cells. Chem Biol Interact, 258, 197–204. [DOI] [PubMed] [Google Scholar]
- Xu H and Ren D (2015) Lysosomal physiology. Annu Rev Physiol, 77, 57–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Gai S and Lin J (2012) Functionalized mesoporous silica materials for controlled drug delivery. Chem Soc Rev, 41, 3679–3698. [DOI] [PubMed] [Google Scholar]
- Yang X, He C, Li J et al. (2014) Uptake of silica nanoparticles: neurotoxicity and Alzheimer-like pathology in human SK-N-SH and mouse neuro2a neuroblastoma cells. Toxicol Lett, 229, 240–249. [DOI] [PubMed] [Google Scholar]
- Yu T, Greish K, McGill LD, Ray A and Ghandehari H (2012a) Influence of geometry, porosity, and surface characteristics of silica nanoparticles on acute toxicity: their vasculature effect and tolerance threshold. ACS Nano, 6, 2289–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Hubbard D, Ray A and Ghandehari H (2012b) In vivo biodistribution and pharmacokinetics of silica nanoparticles as a function of geometry, porosity and surface characteristics. J Control Release, 163, 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Malugin A and Ghandehari H (2011) Impact of silica nanoparticle design on cellular toxicity and hemolytic activity. ACS Nano, 5, 5717–5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Gao H and Bao G (2015) Physical Principles of Nanoparticle Cellular Endocytosis. ACS Nano, 9, 8655–8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Fux B, Goodwin M et al. (2008) Autophagosome-independent essential function for the autophagy protein Atg5 in cellular immunity to intracellular pathogens. Cell Host Microbe, 4, 458–469. [DOI] [PMC free article] [PubMed] [Google Scholar]


