Abstract
Cancer-causing genome instability is a major concern during space travel due to exposure of astronauts to potent sources of high-linear energy transfer (LET) ionizing radiation. Hematopoietic stem cells (HSCs) are particularly susceptible to genotoxic stress, and accumulation of damage can lead to HSC dysfunction and oncogenesis. Our group recently demonstrated that aging human HSCs accumulate microsatellite instability coincident with loss of MLH1, a DNA Mismatch Repair (MMR) protein, which could reasonably predispose to radiation-induced HSC malignancies. Therefore, in an effort to reduce risk uncertainty for cancer development during deep space travel, we employed an Mlh1+/− mouse model to study the effects high-LET 56Fe ion space-like radiation. Irradiated Mlh1+/− mice showed a significantly higher incidence of lymphomagenesis with 56Fe ions compared to γ-rays and unirradiated mice, and malignancy correlated with increased MSI in the tumors. In addition, whole exome sequencing analysis revealed high SNVs and INDELs in lymphomas being driven by loss of Mlh1 and frequently mutated genes had a strong correlation with human leukemias. Therefore, the data suggest that age-related MMR deficiencies could lead to HSC malignancies after space radiation, and that countermeasure strategies will be required to adequately protect the astronaut population on the journey to Mars.
Introduction
The success of manned missions to outer space depends on many factors, including overcoming health risks such as space radiation. Space radiation is composed of protons and high (H) atomic number (Z) and energy (E) (HZE) charged ions that arise from Solar Particle Events (SPEs), Galactic Cosmic Radiation (GCR), and the Van Allen radiation belts 1–3. In particular, GCR is composed of 90% of protons, 9% of alpha particles (4He nuclei), and ~1% nuclei of HZE particles such as 12C, 16O, 20Ne, 24Mg, 26Al, 28Si, and 56Fe ions 4, 5. These particles have a broad range of LET characteristics (densities of induced ionization events along particle tracks). The extent to which differences in LET relate to different types of health risks remains largely unknown, and current mitigation strategies and shielding materials are ineffective to protect astronauts from HZE radiation due to the penetrance of the particles. In addition, there is incomplete understanding of the radiobiology of HZE particles and a lack of accurate risk assessment models, which puts future human-based space missions in question.
A major space radiation-induced health risk to astronauts is tumorigenesis. Cancer fatality risk prediction is an important consideration for deep space missions for government agencies including the National Aeronautics and Space Administration (NASA). Data for low-LET radiation-induced cancer risk in humans come from epidemiological studies of Japanese A-bomb survivors, radiotherapy patients, and occupational radiation workers 6; while data for high-LET radiation rely mostly animal modeling. Various studies performed using mice have identified cancers such as mammary tumors, hepatocellular carcinoma, colorectal cancer, and leukemia as being HZE-induced 7–9. The data show clear differences between high- and low-LET radiation, both in tumor type and incidence. Radiation-induced lymphomas and leukemia represent a significant concern for astronauts during space travel due to the efficiency of radiation induced hematopoietic malignancies.
Ionizing radiation (IR) produces a variety of DNA damage products that are repaired by multiple DNA damage response (DDR) processes. The DNA mismatch repair (MMR) pathway is part of the DDR that fixes mismatches generated by DNA polymerase during replication, but also repairs base damage from a variety of stresses including radiation 10, 11. In particular, MMR eliminates IR-induced buildup of 8-oxoguanine lesions to prevent adenine misincorporation during DNA replication 12–14. MMR defects in tumors are associated with microsatellite instability (MSI) – gain or loss of nucleotides from microsatellite tracks in DNA. MSI is classically associated with colorectal cancers where loss of functional MMR components is frequently found, and tumor cells are said to display a mutator phenotype indicating the lack of a key caretaker pathway 15, 16. MMR could thus play a radioprotective tumor suppressor role, a concept supported by studies that have shown enhanced induction of intestinal carcinogenesis in MMR defective mice exposed to oxidative stress 17, and others that have found induction of a preleukemic state in HSCs 18. MMR consists of seven different proteins, including MLH1, which is crucial for bringing repair machinery to mismatch repair sites. Studies have found epigenetic silencing of MLH1 in cancers such as glioblastoma multiforme, endometrial, lung, and head and neck squamous carcinomas 19–22. Therefore, loss of MLH1 may predispose cells to become cancerous, particularly if exposed to high-LET ionizing radiation.
A recent study by our group demonstrated that MSI accumulates in human HSCs as a function of age, with loss of MLH1 by promoter hypermethylation 23, 24. Given that upper end of astronauts are ~46 years old, HSCs with deficient MMR function will likely be exposed to space radiation. We thus sought to characterize the interaction between loss of MLH1 and exposure to high-LET radiation in the induction of hematopoietic malignancies. Using Mlh1+/− mice, that exhibit MSI 25, exposed to 100 or 250 cGy of low-LET γ-rays and 10 or 100 cGy of high-LET 56Fe ion particles, we find that Mlh1 status does not have an impact on long-term HSC function. However, Mlh1 allelic deficiency significantly increases the risk of hematopoietic malignancy after γ-ray or 56Fe ion radiation with associated loss of Mlh1 function determined by high levels of single nucleotide variants (SNVs)/insertions and deletions (INDELs) in resulting tumors.
Materials and Methods
Animals
Institutional Animal Care and Use Committee approved protocols were followed at Case Western Reserve University (CWRU) and Brookhaven National Laboratory (BNL). The Mlh1+/− strain B6.129-Mlh1tm1Rak/NCI was acquired from the National Cancer Institute at Frederick 25. All animals were bred and maintained at the CWRU Animal Research Core. All mice had ad libitum access to food (Laboratory Rodent Diet 5LOD, Lab Diet, St. Louis, MO) and water. The animal housing room was maintained on a 12:12h light:dark cycle and constant temperature (72 ± 2° F).
Particle irradiation
Adult B6.129-Mlh1tm1Rak male and female mice (~12 weeks) were shipped to BNL roughly one week prior to irradiation. The animals were divided into 10 groups of ~ 40 animals to obtain statistical power, including sham-irradiated Mlh1+/+ and Mlh1+/−. On the day of exposure, animals were arranged into an animal pie-shaped holder and placed perpendicular to a 20×20 cm beam line to expose with 10 or 100 cGy of 600 MeV/n 56Fe ions at a dose rate of 5–50 cGy/minute. Additional animals were exposed to 100 or 250 cGy of γ-rays in a Shepherd Mark I irradiator-containing 137Cs at BNL. Bone marrow (BM) cells were irradiated at NSRL for clonogenic survival assays and competitive repopulation assays. Mlh1+/+ and Mlh1+/− mice (5 animals per genotype) were sacrificed on site, and bone marrow cells were harvested and irradiated with 0, 10, 50, 100, or 250 cGy of 600 MeV/n 56Fe ions. Additional BM cells were irradiated with 0, 10, 50, 100, or 250 cGy of γ-rays.
Clonogenic survival assay
Irradiated Mlh1+/+ and Mlh1+/− BM cells were plated with complete methylcellulose media (MethoCult™ GF M3434 or MethoCult™ M3630, STEMCELL Technologies) to measure survival by colony forming unit (CFU) assay. M3434 media was used for myeloid colony formation, and M3630 media for pre-B lymphoid assays. All assays were performed twice with three replicates (50,000 cells/plate for myeloid CFU and 250,000 cells/plate for lymphoid CFU), and counted between 7–14 days post-plating.
Competitive repopulation assay
Three million irradiated or sham-irradiated whole BM cells from Mlh1+/+ or Mlh1+/− mice (CD45.2) were mixed with whole BM cells of age matched wild type mice (CD45.1) at a 1:1 ratio and injected via tail vein into lethally irradiated (1100 cGy) CD45.1 recipient mice. Blood was collected via the submandibular vein at 4-week and at 10-week time points post bone marrow transplant (BMT) and analyzed by flow cytometry to measure CD45.2 positive cells in the peripheral blood.
Histology and Immunohistochemistry
Animals were euthanized at first signs of morbidity and tumors were collected. All tumors were fixed in 10% formaldehyde for 24 hours followed by immersion into 70% ethanol until processed and sectioned. Hematoxylin and eosin (H&E) stains were performed and then analyzed at the In Vivo Animal Core facility at the University of Michigan. Selected lymphomas were further analyzed by immunohistochemistry (IHC) with B220 (BD Pharmingen # 550286), CD3 (Thermo Fisher # RM9107), or F4/80 (Abd Serotec # MCA497RT) antibodies.
Microsatellite instability
Tumors were assessed for four mononucleotide repeats (mBat-26, mBat-37, mBat-59, and mBat-64) 26. Amplification of each mononucleotide repeat was performed separately by PCR. Detection of amplified PCR fragments was performed on an Agilent TapeStation and analyzed by TapeStation Analysis Software A.02.01 SR1. Each marker length (deletion or addition of nucleotides) measured by the software was compared to marker length of a normal tissue to identify each marker as being stable or unstable. The classification of microsatellite instability was accomplished by calculating the number of unstable markers for each tumor sample. We classified tumors as MSI stable, low, or high based on numbers of these markers with instabilities being 0/4, 1/4, or >1/4, respectively.
Whole-exome sequencing
Whole-exome sequencing (WES) was carried out by using a Truseq Exome library prep kit according to manufacturer’s protocol, and a 2×75bp HS run was performed using an Illumina HiSeq2500. Sequencing quality was assessed using FastQC (ver.11.5). Trimmomatic (ver.0.32) was used to remove sequence adapters and low quality leading and trailing bases from reads 27. Filtered and trimmed reads were aligned to reference genome mm10 using the Burrows-Wheeler Aligner (ver.0.7.12) algorithm 28. Refinement of reads alignment was performed using GATK (ver.3.4.0) analysis toolkit, including PCR duplicated removal, local INDEL realignment, and base recalibration 29. For variant calling, we performed individual tumor sample calling using Mutect2, against the sample from normal mouse tissue as normal reference 30. Final SNVs and INDELs were selected with stringent criteria and final variants were annotated using VariantAnnotation (ver. 1.20.3) R package 31. Data are deposited in SRA at NCBI (accession #PRJNA487630)
Results
Mlh1 heterozygosity significantly increases high-LET radiation induced malignancy.
Knockout animals are known to be tumor prone, and thus do not phenocopy aged people; in contrast, Mlh1+/− animals exhibit relatively low spontaneous tumorigenesis in spite of partial loss of MMR function 32. During follow-up, mice were euthanized at the onset of signs of morbidity or the appearance of visible tumors (figure 1A). We found a significant reduction in tumor-free survival of Mlh1+/− mice irradiated with 100 or 250 cGy of γ-ray vs. sham-irradiated Mlh1+/− mice or irradiated Mlh1+/+ mice (p<0.0001, figure 1B) Interestingly, we observed significantly increased mortalities in Mlh1+/− mice exposed to 10 or 100 cGy 56Fe ions vs. sham-irradiated Mlh1+/− or irradiated Mlh1+/+ mice (p<0.0001, figure 1C). Indeed, the biological impact of 100 cGy of 56Fe ions exceeded 100 cGy of γ-rays (p=0.0470). In addition, Mlh1+/− mice exposed to 100 cGy 56Fe ion IR showed a significant lower tumor free survival compared to mice exposed to 10 cGy 56Fe ion IR (p=0.0456). In contrast, we observed no significant increases in tumorigenesis of Mlh1+/+ mice regardless of the type of radiation used. To gain insight into the time of onset of disease, the same data with exposed animals grouped by genotype were used to estimate 70% survival times: sham-irradiated Mlh1+/− mice, 513 days; Mlh1+/− mice irradiated with 100 or 250 cGy of γ-rays, 446 and 444 days; Mlh1+/− mice irradiated with 10 or 100 cGy of 56Fe ion irradiation, 424 and 385 days; and all Mlh1+/+ mice, regardless of being irradiated, time not reached (figures 1D–1F, supplementary table 1). Thus, loss of Mlh1 enhances radiation-induced tumorigenesis that heavily depends on radiation quality.
Figure 1: Long-term tumorigenesis assay.
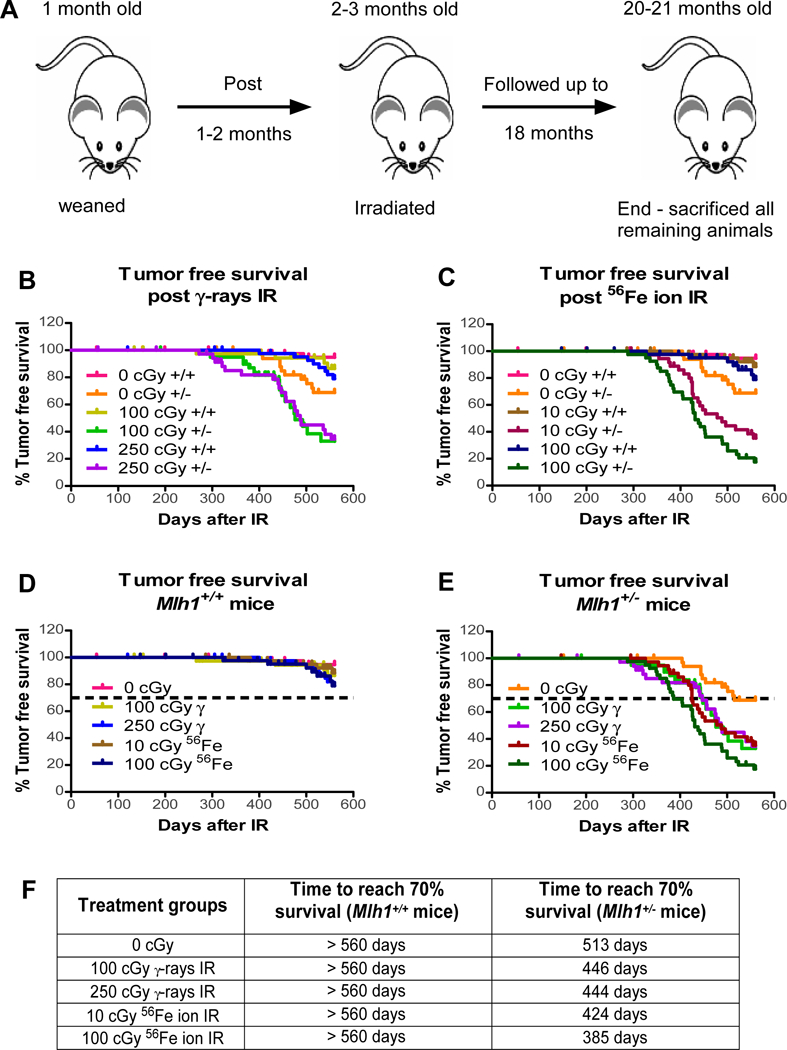
(A) Schematic representation of long-term tumorigenesis assay design. Tumor free survival of Mlh1+/+ and Mlh1+/− mice post (B) 100 or 250 cGy γ-rays, or (C) 10 or 100 cGy 56Fe ions (n=36–44, number of Mlh1+/+ or Mlh1−/− mice used for each radiation exposure). (D) Tumor free survival of Mlh1+/+ mice post 0, 100 or 250 cGy γ-rays, or 10 or 100 cGy 56Fe ions. (E) Tumor free survival of Mlh1+/− mice post 0, 100 or 250 cGy γ-rays, or 10 or 100 cGy 56Fe ions. (F) Days post-irradiation to reach 70% survival. Variance between groups is not significantly different.
Mlh1 deficiency increases the incidence of lymphomagenesis after low and high LET radiation.
Loss of Mlh1 is associated with a higher incidence of lymphomas and gastrointestinal tumors in animal models 33, 34. Therefore, we next examined tumors collected from Mlh1+/+ and Mlh1+/− mice by hematoxylin and eosin staining to determine tumor types and if radiation exposure altered the distribution of tumor types formed. Histopathology analysis revealed different tumor types, but lymphoma was found to be the most common tumor (figure 2A). Sporadic age-related tumors such as hepatocellular adenomas (HCA), hepatocellular carcinomas (HCC), histiocytic sarcoma (HS), and other rare tumors (figure 2B–2F; supplementary table 2) were also observed. The analysis determined that ~40% of total tumors found in Mlh1+/+ cohorts were lymphomas (figure 2G). Interestingly, we observed a significant difference in tumor type distribution between Mlh1+/+ mice treated or not with low- or high-LET radiation (p=0.0447). In contrast, ~80% of tumors of Mlh1+/− cohorts were lymphomas (figure 2H). Further, Mlh1+/− cohorts revealed significantly higher incidence of multiple tumors per mouse compared to Mlh1+/+ cohorts (p=0.0288, figure 2I). These data argue that Mlh1 deficiency increases incidence mostly of hematopoietic malignancies after IR, independent of radiation quality (i.e. LET).
Figure 2: Histopathology of tumors from Mlh1+/+ and Mlh1+/− mice.
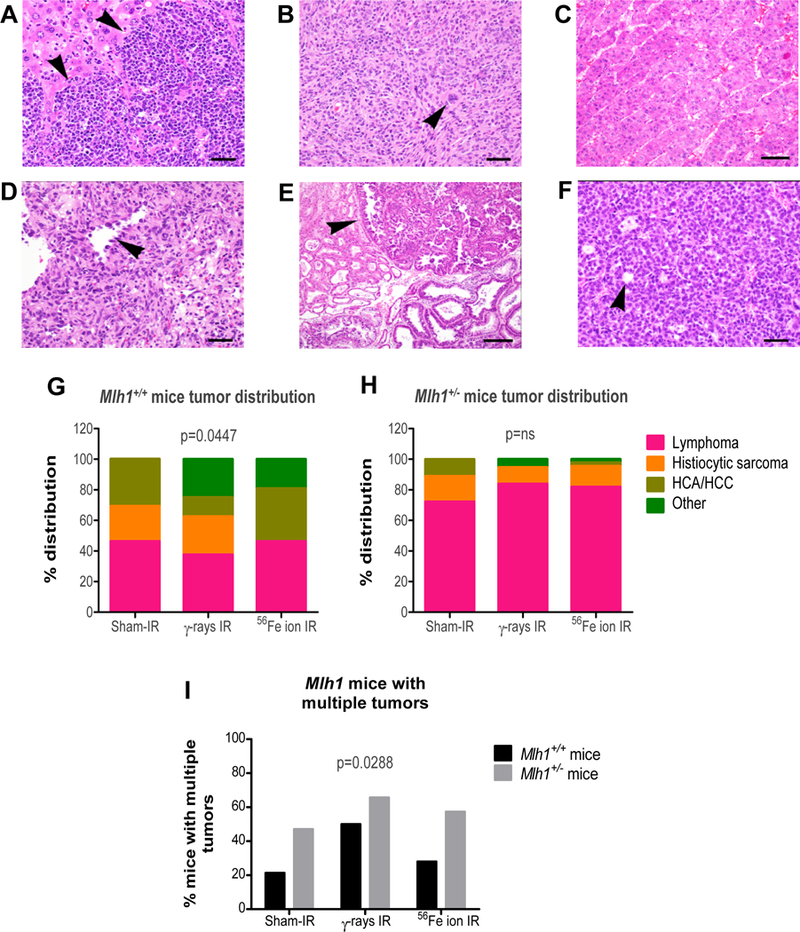
(A) Lymphoma in sections of liver, characterized by sheets of neoplastic lymphocytes infiltrating and effacing normal hepatic parenchyma (arrowheads) (40X, bar = 20um). (B) Histiocytic sarcoma composed of round to spindyloid neoplastic cells with occasional multinucleate giant cells (arrowhead) (20X, bar = 50um). (C) Hepatocellular carcinoma composed of lobules, cords, and trabeculae of atypical hepatocytes replacing normal parenchyma (bar = 50um). (D) Hemangiosarcoma composed of sheets and bundles of spindle-shaped cells forming haphazard vascular channels (arrowhead) lined by neoplastic endothelial cells (40X, bar = 20um). (E) Harderian gland adenoma characterized by an expansile proliferation (arrowhead) of tubules and acini of fairly well differentiated glandular epithelial cells (bar = 100um). (F) Ovarian granulosa cell tumor composed of solid lobules and nests of neoplastic cells often forming rudimentary follicular structures (arrowhead) (40X, bar = 20um). (G) Percentage tumor distribution based on histology of tumors collected from Mlh1+/+ mice treated with sham-, γ-, or 56Fe ion irradiation. (H) Percentage tumor distribution based on histology of tumors collected from Mlh1+/− mice treated with sham-, γ-, or 56Fe ion irradiation. (I) Aggressive cancer measured by percentage of mice with multiple tumor types or same tumor type in multiple organs. Histopathology was performed on 13–27 tumors of Mlh1+/+ origin and 18–44 tumors of Mlh1+/− origin. Tumor distribution was analyzed by Chi-square and multiple tumor incidence was analyzed by two-way ANOVA; ns = non-significant.
Mlh1+/− cohorts have higher incidence of T-cell rich B-cell lymphomas.
Lymphomas are classified by immunophenotype. The majority of lymphomas show immune cell infiltrates in the tumor microenvironment, which is associated with profound influence on disease pathology 35. Therefore, we decided to further explore the lymphomas based on IHC analysis. We used CD3, B220, and F4/80 to discern T cell, B cell, and macrophage/histiocytes in the tumors, respectively. Staining patterns revealed six different types of lymphoma that include T-cell rich B-cell (TRB) lymphoma, B-cell lymphoma, T-cell lymphoma, histiocytic sarcoma, B/T mixed lymphoma, and T-cell/histiocyte rich B-cell lymphoma (figure 3A–3F; supplementary table 3). We found 40–60% of lymphomas were TRB lymphomas in the Mlh1+/+ mice (figure 3G). Interesting, we observed roughly 30% of lymphomas were histiocytic sarcoma in sham-irradiated and γ-irradiated Mlh1+/+ mice, whereas no histiocytic sarcomas were found in 56Fe particle irradiated Mlh1+/+ mice. Similarly, we observed that the majority of lymphomas were TRB lymphomas in all treatment groups of Mlh1+/− mice (figure 3H). Collectively, the data show that TRB lymphomas were common in Mlh1+/+ and Mlh1+/− mice regardless of radiation type and that infiltrating T-cells might play a role in the process of lymphomagenesis.
Figure 3: Immunohistochemistry of lymphomas from Mlh1+/+ and Mlh1+/− mice.
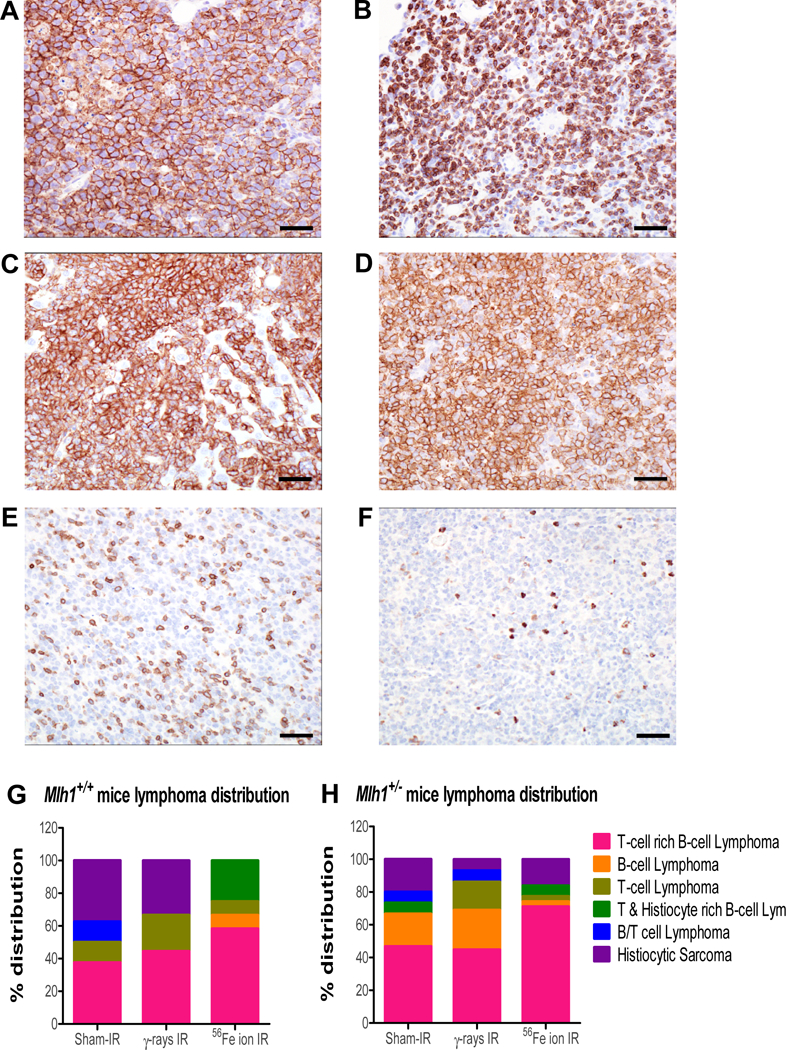
(A) B-cell lymphoma in a mesenteric lymph node shows diffuse and strong positive membrane immunoreactivity for B220 antibody. (B) T-cell lymphoma in mesenteric lymph node shows diffuse membrane and cytoplasmic immunoreactivity to CD3 antibody. (C) Histiocytic sarcoma in the liver shows strong and diffuse membrane immunoreactivity to F4/80 antibody. (D-F) The majority of neoplasms had an immunophenotype of T-cell rich, B-cell lymphomas, characterized by a dominant population of neoplastic B cells immunoreactive to B220 antibody (D), with a minority population of well-differentiated T-cells immunoreactive to CD3 antibody (E), and only a few resident macrophages illustrated by F4/80 immunoreactivity (F). (A-F) 40X, bar = 20um. (G) Distribution, based on immunohistochemistry, of lymphomas collected from Mlh1+/+ mice treated with sham-, γ-, or 56Fe ion irradiation. (H) Distribution, based on immunohistochemistry, of lymphomas collected from Mlh1+/− mice treated with sham-, γ-rays, or 56Fe ion irradiation. IHC was performed on 8–12 lymphomas of Mlh1+/+ origin and 15–31 lymphomas of Mlh1+/− origin.
Mlh1+/− tumors exhibit elevated levels of microsatellite instability.
Loss of MMR strongly correlates with MSI in many human cancers 36, and we anticipated that mononucleotide repeats would be highly susceptible to MSI in Mlh1+/− tumors compared to Mlh1+/+ tumors, particularly because heterozygosity of Mlh1 has been shown to associate with decreased DNA repair 23, 25. The average sizes of four mononucleotide markers were measured from MMR-proficient control samples and shown as peak values in figures 4A–4D. MSI analysis showed that a majority of markers had a deletion of one or more nucleotides in the stretch of mononucleotide repeats in Mlh1+/− tumors (figure 4E–4H). We found that roughly 80% of Mlh1+/− tumors showed high MSI, while only 2% of these tumors showed stable MSI. In contrast, we found that roughly 45% of Mlh1+/+ tumors were high MSI, and that 55% were either stable or low MSI. Thus, MSI differed in Mlh1+/+ vs. Mlh1+/− tumors (p=0.0048, figure 4I). Interestingly, we observed no change in high MSI of Mlh1+/− tumors by different radiation types, including sham-irradiated (figure 4J). Collectively, the data show that MSI associates with tumorigenesis in both the Mlh1 wild type and heterozygous mice, and hence MMR status could be a potential risk stratification marker for individuals exposed to high LET ionizing radiation.
Figure 4: Microsatellite instability found in Mlh1+/+ and Mlh1+/− tumors.
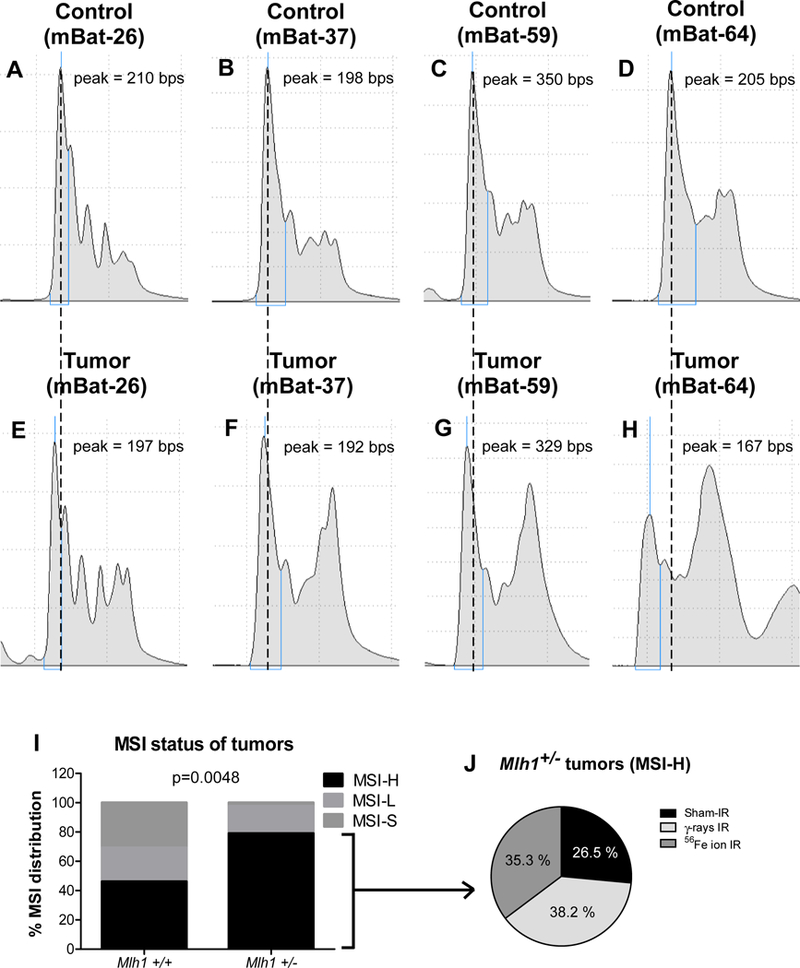
Stable MSI (MSI-S) was found in control tissue (Mlh1+/+) via the markers mBat-26 (A), mBat-37 (B), mBat-59 (C), and mBat-64 (D). Similarly, high MSI (MSI-H) was observed in Mlh1+/− tumor sample also via mBat-26 (E), mBat-37 (F), mBat-59 (G), and mBat-64 (H). MSI distribution in Mlh1+/+ vs Mlh1+/− tumors (I). MSI-H distribution found in tumors of irradiated Mlh1+/− mice (J). Number of Mlh1+/+ and Mlh1+/− tumors used for the analysis were 15 and 43, respectively. Distributions were tested using Chi-square tests.
Significantly elevated levels of SNVs and INDELs appear in Mlh1+/− lymphomas.
MMR deficiency is associated with a mutator phenotype. In particular, loss of Msh2 and Mlh1, key components of MMR, have been shown to increase mutational frequency in newborn mice and during different stages of embryogenesis 37, 38. After verifying high MSI in Mlh1 heterozygous tumors, we decided to further analyze TRB lymphomas by WES to study SNV/INDEL patterns in wildtype vs heterozygous lymphomas. The WES analysis revealed a significant increase in mutation rate of Mlh1+/− compared to Mlh1+/+ TRB lymphomas arising from sham-, γ-rays, or 56Fe ion IR (p<0.0001, figure 5A, 5B). Surprisingly, radiation exposure showed no further increase in number of SNVs of irradiated cohorts compared to sham-irradiated cohorts, regardless of Mlh1 status (p=0.9225, figure 5A,5B). In addition, WES analysis showed significantly higher INDELs in the Mlh1+/− irradiated cohorts compared to Mlh1+/+ irradiated cohorts (p=0.0314, figure 5A, 5C). The data suggest that Mlh1 heterozygosity was associated with higher SNVs, while INDELs were correlated with irradiation plus loss of Mlh1.
Figure 5: WES analysis of Mlh1+/+ and Mlh1+/− TRB lymphomas.
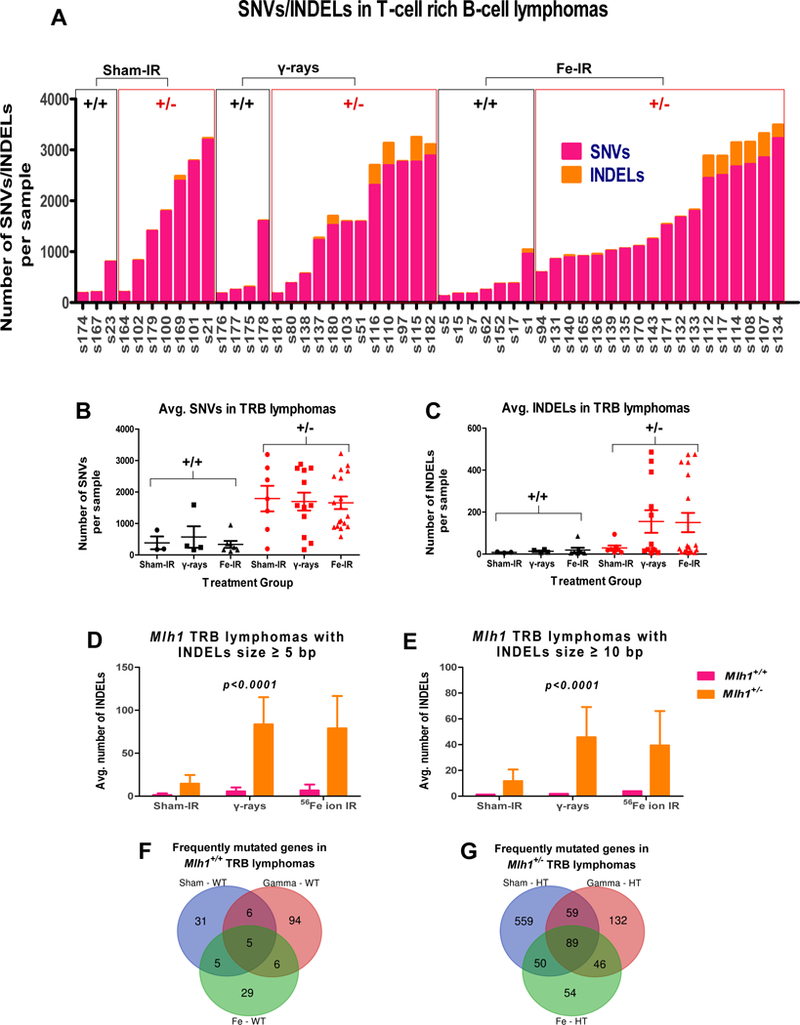
(A) Number of SNVs and INDELs found in each TRB lymphoma arising from sham- (n = 3 and 7 for Mlh1+/+ and Mlh1+/−, respectively), γ-rays (n = 4 and 12 for Mlh1+/+ and Mlh1+/−, respectively), or 56Fe ion irradiation (n = 7 and 12 for Mlh1+/+ and Mlh1+/−, respectively). (B) Average number of SNVs per Mlh1+/+ and Mlh1+/− cohorts. (C) Average number of INDELs per Mlh1+/+ and Mlh1+/− cohorts. (D) Size of INDELs ≥ 5 bp in each cohort of Mlh1+/+ and Mlh1+/− TRB lymphomas. (E) Size of INDELs ≥ 10 bp in each cohort of Mlh1+/+ and Mlh1+/− TRB lymphomas. Venn Diagram shows number of frequently mutated genes found in (F) Mlh1+/+, and (G) Mlh1+/− cohorts. P values were determined by a two-way ANOVA model. Data plotted are means ± SEM.
For further analysis, we identified frequently mutated genes in Mlh1+/+ and Mlh1+/− cohorts based on type of radiation exposure. To examine the role of recurring mutations occurred at specific loci, we defined a gene as frequently mutated if it was found to be mutated in ≥ 40% of at least one cohort. The analysis revealed that a significantly higher number of frequently mutated genes were found in Mlh1+/− cohorts compared to Mlh1+/+ cohorts of TRB lymphomas (p<0.0001, figure 5F, 5G). Mlh1 heterozygosity not only increased the mutation rate, but the repeated nature of mutations occurring at the same loci suggests importance of these genes in tumorigenesis. In fact, we compared frequently mutated genes to well-defined cancer causing genes and discovered that ~13% of the genes in each cohort of Mlh1+/− TRB lymphomas were associated with cancer (supplementary table 4). Collectively, WES analysis not only revealed higher SNVs and INDELs in Mlh1+/− TRB lymphomas, but also that mutations occurred frequently in genes responsible for tumorigenesis.
High LET radiation induces a unique spectrum of genetic alterations in genes associated with human leukemia.
The C57BL/6 mouse model is a useful resource for studying radiation-induced cancers if parallels can be drawn between the mechanisms of radiation-induced tumorigenesis of mouse lymphomas and human leukemias. Many studies have shown that expression changes in genes such as Ikaros, Bcl11b, and Epha7 occur in both mouse lymphomas and various types of human leukemias 39–42. Therefore, we asked whether genes frequently mutated in TRB lymphomas are relevant to human leukemia, and whether 56Fe ions produced unique mutations compared to γ-rays. We identified 8 and 39 recurrently altered human leukemia genes in Mlh1+/+ and Mlh1+/− TRBs, respectively (figure 6A, 6B). Interestingly, irradiated cohorts showed different gene mutational patterns compared to sham-IR tumors, suggesting a distinct pathway leading to lymphomagenesis. For instance, high rates of somatic mutations in Cbl, Huwe1, Runx1, and Ttn genes were found in all Mlh1+/− cohorts. In contrast, some genes were found mutated in specific treatment groups: Jag1, Kit, Nup214, and Pik3cd were prominently found mutated in the sham-IR cohort; Dnmt3a and Myb were prominently found in γ-ray cohort; and Myc was only found in 56Fe ion IR cohort. In addition, the majority of the mutations were nonsynonymous in nature (figure 6C). Thus sequence analyses of TRB lymphomas suggests that common mechanisms underlie these mouse lymphomas and radiation induced human leukemias, and strengthens the position that MLH1 defects will predispose space radiation-exposed astronauts to disease development.
Figure 6: Correlation between frequently mutated mouse TRB lymphoma genes vs human leukemia genes.
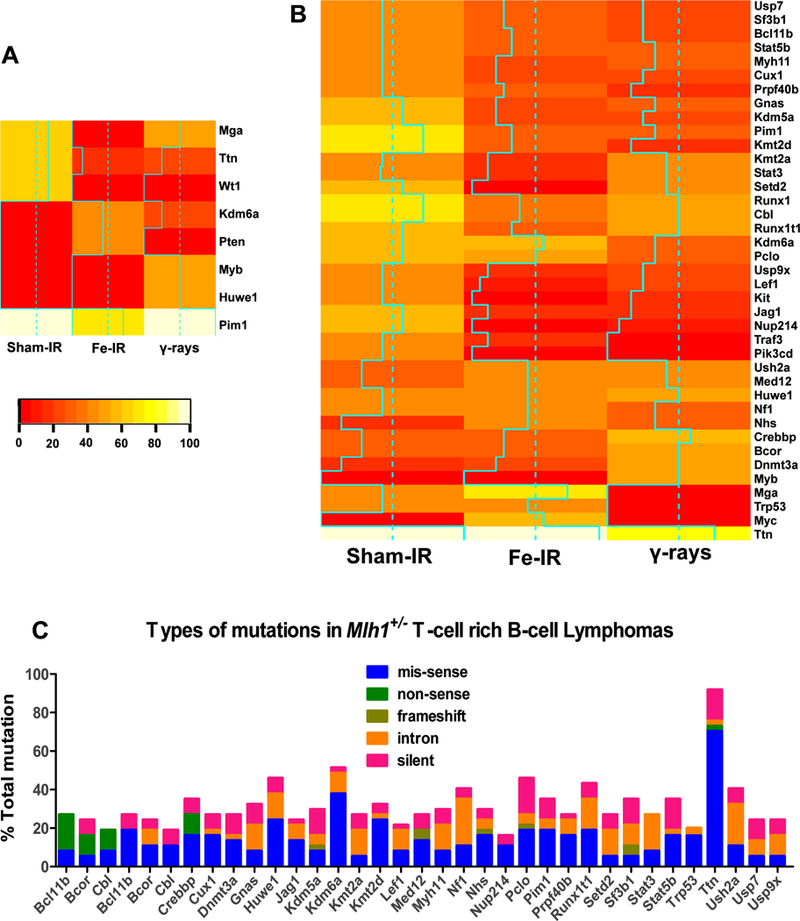
Heatmap represents human leukemia genes also found to be frequently mutated in (A) Mlh1+/+, and (B) Mlh1+/− mouse TRB lymphoma cohorts. Solid aqua lines in each Heatmap represent actual mutational frequency of a gene in that particular cohort. (C) Different types of mutations (mis-sense, non-sense, frameshift, intron, and silent) found in each gene of Mlh1+/− TRB lymphomas.
Discussion
The impact of age-associated MMR defects to the risk of space radiation-induced malignancies has not been previously assessed. The current study provides evidence that loss of Mlh1 in HSCs, which occurs as a function of age in normal healthy individuals 23 leads to a significantly higher incidence of tumorigenesis after exposure to high LET radiation, and that the incidence is dependent on the type of radiation exposure. At the same time, we observed no significant changes in acute hematopoietic functions of Mlh1+/− vs Mlh1+/+ BM cells measured by CFU and competitive repopulation assays (supplementary figure 1). Further, long-term differentiation potential of HSCs was also unaffected by Mlh1 status (supplementary figure 2). Thus, the critical observation described here is that MMR defective animals are cancer prone when exposed to cosmic radiation. Mlh1+/− mice show increased incidence of lymphomagenesis compared to Mlh1+/+ mice, and MSI is coincident with tumorigenesis in all cohorts. WES analysis of the tumors revealed a significantly higher rate of SNVs/INDELs in Mlh1 haploinsufficient TRB lymphomas along with strong evidence of recurrent gene mutations occurring in carcinogenic and leukemogenic genes. The data are in agreement with the observation that MMR deficiency due to Msh2 loss has been shown to promote a preleukemic state without affecting HSC repopulation function 18. Together, our studies demonstrate that low- and high-LET radiation induce elevated tumorigenesis in Mlh1 deficient contexts that could alter the risk paradigm for astronauts on deep space missions.
After high-LET iron particle exposure, nearly all energy deposition occurs in confined regions of the cell near the particle track and associated δ-ray penumbras, causing dense local ionization and clustered DNA lesions 43, 44. Thus, the likelihood of repair of DNA damage and survival of cells is significantly reduced for most cell types following the same doses of high-LET compared to low-LET irradiation. In our studies, we observed a significantly higher impact of high-LET 56Fe particles on HSC acute functions compared to low-LET γ-rays, regardless of Mlh1 status. Similarly, we found that radiation exposure significantly accelerated tumorigenesis in Mlh1+/− mice compared to wild type mice, and that high LET radiation was markedly more effective. The findings are in agreement with work from the Weil group and others which showed higher incidence of tumorigenesis in animals exposed to high-LET IR compared to low-LET γ-rays exposure 8, 45–47. Mlh1+/− mice exposed to 100 cGy 56Fe ion IR were reduced to 70% survival ~130 and ~60 days earlier compared to sham-IR and 100 cGy γ-rays exposed Mlh1+/− mice, respectively. Collectively, these findings suggest that loss of Mlh1 and high-LET radiation exposure together are responsible for not only higher frequency but early incidence of tumorigenesis.
Radiation induced damage by high-LET sources may have an indirect role in leading to tumorigenesis. Late-occurring chromosomal aberrations and global DNA methylation in hematopoietic stem/progenitor cells have been shown after 28Si ion irradiation 48. Kennedy, et al, have also observed altered methylation in bronchial epithelial cells after 56Fe and 28Si exposure, contributing to lung cancer, which in theory could also contribute to the mechanism of loss of MLH1 expression in HSCs49. Mice exposed to high-LET 16O (600 MeV/n) ions showed significantly higher level of ROS in HSCs three months after irradiation, suggesting that cells experience continuous damage stress 50, 51. Continuous ROS levels in HSCs post irradiation could lead to mutation accumulation in absence of functional MMR and may explain our observation of significantly higher SNVs in all cohorts of Mlh1+/− TRB lymphomas. However, we did not detect differences in SNVs between sham and irradiated cohorts, which may be due to the longer time taken by the sham-IR cohort to reach to 70% survival hence allowing extra time to accumulate SNVs. In addition, we discovered significantly higher mean INDEL size (≥5 and ≥10 base pairs) in all Mlh1+/− cohorts compared to Mlh1+/+ cohorts, implying Mlh1 plays a role not only in MMR but also in double strand break repair (figure 5D, 5E), which has been suggested in other models 52, 53. Collectively, the WES analysis suggests that Mlh1 loss is strongly associated with high mutational burden in lymphomas, and high mean INDELs size could be due to Mlh1 involvement in repair mechanism other than MMR.
We observed not only a high mutation rate in Mlh1 haploinsufficient lymphomas, but also frequent mutations occurring in carcinogenic loci. Mlh1 loss is associated with frequent mutations occurring in the loci of NF1 and ATR 54, 55. Similarly, we found frequent mutations in Nf1 and Atr along with 12 other carcinogenic genes (Met, Cacna1d, Ptprd, Nbea, Gnaq, Cntnap2, Csmd3, Pabpc1, Lrp1b, Zfhx3, Dcc, and Ctnna2) across all cohorts of Mlh1+/− TRB lymphomas. In addition, each cohort of Mlh1+/− TRB lymphoma showed radiation-specific gene mutational profiles. For instance, well-defined carcinogenic genes such as Cdh1, Eps15, Was, Atp2b3, Cdh11, and Myc were predominantly mutated in 56Fe ion IR Mlh1+/− cohort while the γ-ray Mlh1+/− cohort revealed frequent mutations in genes such as Ddx6, Tsc2, Raf1, Nt5c2, Crebbp, Tfe3, Stat3, Map2k1, Dnmt3a, Bcor, Map3k1, and Arid2. Critically, we observed an enrichment of mutations in the Mlh1+/− lymphomas that also occur in human leukemias. The data also revealed radiation quality specific effects, such as the observation of Myc mutation exclusively in 56Fe, Myb mutation exclusively in γ-rays, and Nup214 mutation predominately in sham irradiated Mlh1+/− lymphomas. It is unclear at this point what mechanism would lead to gene-specific mutations, but the observation is similar to one recently published by Porada and colleagues when human HSCs were exposed to HZE radiation that showed enrichment in mutations in leukemia-associated genes within 24 hours of exposure 56. Therefore, our study suggests that age-related MLH1 loss in astronaut HSCs results in a preleukemic state that can be exacerbated by high-LET radiation exposures received during space travel.
Increased use of high-LET radiotherapy also raises concern for therapy-related malignancies in patients with MMR defects, both in the hematopoietic system and beyond. Although further studies will be required to better characterize the molecular nature of tumors formed in our studies, and what types of doses and LET are sufficient for enhancing tumor development, the results should be interpreted carefully, as astronauts in outer space may be exposed to several types of HZE particles with different fluences and energies. Future studies will be required to assess the effects of medium LET species and subsequently mixed ion beam fields and lower dose-rates to better mimic space radiation. In summary, the data suggest that loss of Mlh1 in HSCs, either genetically or as a function of age, could play a critical role in sensitizing humans to space-radiation induced HSC malignancies. Further studies will be required to more accurately calculate risks, both for missions into outer space and for patients undergoing current proton or future carbon-ion radiotherapy.
Supplementary Material
Acknowledgements
This research was funded by NASA grant NNX14AC95G. The authors are grateful to all members of NASA Space Radiation Laboratory and support staff at Brookhaven National Laboratory, in particular to Adam Rusek, Chiara La Tessa, and Peter Guida, for their assistance. The authors are also thankful to shared resources of the Case Comprehensive Cancer Center including Radiation Resources, Integrated Genomics, Cytometry & Microscopy, and Hematopoietic Biorepository & Cellular Therapy. We also thank the generosity of Thomas F. Peterson, Jr.
Footnotes
Competing Interests
The authors declare there are no competing financial interests.
This research was funded by NASA grant NNX14AC95G. Authors also declare no conflicts of interest.
References
- 1.Cucinotta FA, Schimmerling W, Wilson JW, Peterson LE, Badhwar GD, Saganti PB, et al. Space radiation cancer risks and uncertainties for Mars missions. Radiation research 2001. November; 156(5 Pt 2): 682–688. [DOI] [PubMed] [Google Scholar]
- 2.Edwards AA. RBE of radiations in space and the implications for space travel. Physica medica : PM : an international journal devoted to the applications of physics to medicine and biology : official journal of the Italian Association of Biomedical Physics 2001; 17 Suppl 1: 147–152. [PubMed] [Google Scholar]
- 3.Schimmerling W, Cucinotta FA, Wilson JW. Radiation risk and human space exploration. Advances in space research : the official journal of the Committee on Space Research 2003; 31(1): 27–34. [DOI] [PubMed] [Google Scholar]
- 4.Heinrich W, Roesler S, Schraube H. Physics of cosmic radiation fields. Radiation protection dosimetry 1999; 86(4): 253–258. [DOI] [PubMed] [Google Scholar]
- 5.Chancellor JC, Scott GB, Sutton JP. Space Radiation: The Number One Risk to Astronaut Health beyond Low Earth Orbit. Life 2014. September 11; 4(3): 491–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.V B. Health Effects of Exposure to Low Levels of Ionizing Radiation. 1990 20140718 ISBN- 0309039959 ISBN- 0309039975.
- 7.Bielefeldt-Ohmann H, Genik PC, Fallgren CM, Ullrich RL, Weil MM. Animal studies of charged particle-induced carcinogenesis. Health physics 2012. November; 103(5): 568–576. [DOI] [PubMed] [Google Scholar]
- 8.Weil MM, Bedford JS, Bielefeldt-Ohmann H, Ray FA, Genik PC, Ehrhart EJ, et al. Incidence of acute myeloid leukemia and hepatocellular carcinoma in mice irradiated with 1 GeV/nucleon (56)Fe ions. Radiation research 2009. August; 172(2): 213–219. [DOI] [PubMed] [Google Scholar]
- 9.Datta K, Suman S, Kallakury BV, Fornace AJ Jr. Exposure to heavy ion radiation induces persistent oxidative stress in mouse intestine. PloS one 2012; 7(8): e42224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei K, Clark AB, Wong E, Kane MF, Mazur DJ, Parris T, et al. Inactivation of Exonuclease 1 in mice results in DNA mismatch repair defects, increased cancer susceptibility, and male and female sterility. Genes & development 2003. March 01; 17(5): 603–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iyer RR, Pluciennik A, Burdett V, Modrich PL. DNA mismatch repair: functions and mechanisms. Chemical reviews 2006. February; 106(2): 302–323. [DOI] [PubMed] [Google Scholar]
- 12.Fritzell JA, Narayanan L, Baker SM, Bronner CE, Andrew SE, Prolla TA, et al. Role of DNA mismatch repair in the cytotoxicity of ionizing radiation. Cancer research 1997. November 15; 57(22): 5143–5147. [PubMed] [Google Scholar]
- 13.Mazurek A, Berardini M, Fishel R. Activation of human MutS homologs by 8-oxo-guanine DNA damage. The Journal of biological chemistry 2002. March 08; 277(10): 8260–8266. [DOI] [PubMed] [Google Scholar]
- 14.Macpherson P, Barone F, Maga G, Mazzei F, Karran P, Bignami M. 8-oxoguanine incorporation into DNA repeats in vitro and mismatch recognition by MutSalpha. Nucleic acids research 2005; 33(16): 5094–5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vilar E, Gruber SB. Microsatellite instability in colorectal cancer-the stable evidence. Nature reviews Clinical oncology 2010. March; 7(3): 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinzler KW, Vogelstein B. Cancer-susceptibility genes. Gatekeepers and caretakers. Nature 1997. April 24; 386(6627): 761, 763. [DOI] [PubMed] [Google Scholar]
- 17.Piao JS, Nakatsu Y, Ohno M, Taguchi K, Tsuzuki T. Mismatch Repair Deficient Mice Show Susceptibility to Oxidative Stress-Induced Intestinal Carcinogenesis. Int J Biol Sci 2014; 10(1): 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qing Y, Gerson SL. Mismatch repair deficient hematopoietic stem cells are preleukemic stem cells. PloS one 2017; 12(8): e0182175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma Y, Chen Y, Petersen I. Expression and promoter DNA methylation of MLH1 in colorectal cancer and lung cancer. Pathology, research and practice 2017. April; 213(4): 333–338. [DOI] [PubMed] [Google Scholar]
- 20.Gutierrez VF, Marcos CA, Llorente JL, Guervos MA, Iglesias FD, Tamargo LA, et al. Genetic profile of second primary tumors and recurrences in head and neck squamous cell carcinomas. Head & neck 2012. June; 34(6): 830–839. [DOI] [PubMed] [Google Scholar]
- 21.Stark AM, Doukas A, Hugo HH, Hedderich J, Hattermann K, Maximilian Mehdorn H, et al. Expression of DNA mismatch repair proteins MLH1, MSH2, and MSH6 in recurrent glioblastoma. Neurological research 2015. February; 37(2): 95–105. [DOI] [PubMed] [Google Scholar]
- 22.Cosgrove CM, Cohn DE, Hampel H, Frankel WL, Jones D, McElroy JP, et al. Epigenetic silencing of MLH1 in endometrial cancers is associated with larger tumor volume, increased rate of lymph node positivity and reduced recurrence-free survival. Gynecologic oncology 2017. September; 146(3): 588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kenyon J, Fu P, Lingas K, Thomas E, Saurastri A, Santos Guasch G, et al. Humans accumulate microsatellite instability with acquired loss of MLH1 protein in hematopoietic stem and progenitor cells as a function of age. Blood 2012. October 18; 120(16): 3229–3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kenyon J, Nickel-Meester G, Qing Y, Santos-Guasch G, Drake E, PingfuFu, et al. Epigenetic Loss of MLH1 Expression in Normal Human Hematopoietic Stem Cell Clones is Defined by the Promoter CpG Methylation Pattern Observed by High-Throughput Methylation Specific Sequencing. International journal of stem cell research and therapy 2016; 3(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edelmann W, Cohen PE, Kane M, Lau K, Morrow B, Bennett S, et al. Meiotic pachytene arrest in MLH1-deficient mice. Cell 1996. June 28; 85(7): 1125–1134. [DOI] [PubMed] [Google Scholar]
- 26.Bacher JW, Abdel Megid WM, Kent-First MG, Halberg RB. Use of mononucleotide repeat markers for detection of microsatellite instability in mouse tumors. Molecular carcinogenesis 2005. December; 44(4): 285–292. [DOI] [PubMed] [Google Scholar]
- 27.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 2014. August 1; 30(15): 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009. July 15; 25(14): 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nature genetics 2011. May; 43(5): 491-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, Sougnez C, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol 2013. March; 31(3): 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Obenchain V, Lawrence M, Carey V, Gogarten S, Shannon P, Morgan M. VariantAnnotation: a Bioconductor package for exploration and annotation of genetic variants. Bioinformatics 2014. July 15; 30(14): 2076–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tokairin Y, Kakinuma S, Arai M, Nishimura M, Okamoto M, Ito E, et al. Accelerated growth of intestinal tumours after radiation exposure in Mlh1-knockout mice: evaluation of the late effect of radiation on a mouse model of HNPCC. International journal of experimental pathology 2006. April; 87(2): 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edelmann W, Yang K, Kuraguchi M, Heyer J, Lia M, Kneitz B, et al. Tumorigenesis in Mlh1 and Mlh1/Apc1638N mutant mice. Cancer research 1999. March 15; 59(6): 1301–1307. [PubMed] [Google Scholar]
- 34.Yao X, Buermeyer AB, Narayanan L, Tran D, Baker SM, Prolla TA, et al. Different mutator phenotypes in Mlh1- versus Pms2-deficient mice. Proceedings of the National Academy of Sciences of the United States of America 1999. June 8; 96(12): 6850–6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scott DW, Gascoyne RD. The tumour microenvironment in B cell lymphomas. Nature reviews Cancer 2014. August; 14(8): 517–534. [DOI] [PubMed] [Google Scholar]
- 36.Hause RJ, Pritchard CC, Shendure J, Salipante SJ. Classification and characterization of microsatellite instability across 18 cancer types. Nature medicine 2016. November; 22(11): 1342–1350. [DOI] [PubMed] [Google Scholar]
- 37.Fan X, Li Y, Zhang Y, Sang M, Cai J, Li Q, et al. High Mutation Levels are Compatible with Normal Embryonic Development in Mlh1-Deficient Mice. Radiation research 2016. October; 186(4): 377–384. [DOI] [PubMed] [Google Scholar]
- 38.He D, Chen Y, Li H, Furuya M, Ikehata H, Uehara Y, et al. Role of the Msh2 gene in genome maintenance and development in mouse fetuses. Mutation research 2012. June 1; 734(1–2): 50–55. [DOI] [PubMed] [Google Scholar]
- 39.Dovat S, Song C, Payne KJ, Li Z. Ikaros, CK2 kinase, and the road to leukemia. Molecular and cellular biochemistry 2011. October; 356(1–2): 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Payne KJ, Dovat S. Ikaros and tumor suppression in acute lymphoblastic leukemia. Critical reviews in oncogenesis 2011; 16(1–2): 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gutierrez A, Kentsis A, Sanda T, Holmfeldt L, Chen SC, Zhang J, et al. The BCL11B tumor suppressor is mutated across the major molecular subtypes of T-cell acute lymphoblastic leukemia. Blood 2011. October 13; 118(15): 4169–4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopez-Nieva P, Vaquero C, Fernandez-Navarro P, Gonzalez-Sanchez L, Villa-Morales M, Santos J, et al. EPHA7, a new target gene for 6q deletion in T-cell lymphoblastic lymphomas. Carcinogenesis 2012. February; 33(2): 452–458. [DOI] [PubMed] [Google Scholar]
- 43.Cucinotta FA, Nikjoo H, Goodhead DT. Model for radial dependence of frequency distributions for energy imparted in nanometer volumes from HZE particles. Radiation research 2000. April; 153(4): 459–468. [DOI] [PubMed] [Google Scholar]
- 44.Mirsch J, Tommasino F, Frohns A, Conrad S, Durante M, Scholz M, et al. Direct measurement of the 3-dimensional DNA lesion distribution induced by energetic charged particles in a mouse model tissue. Proceedings of the National Academy of Sciences of the United States of America 2015. October 06; 112(40): 12396–12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weil MM, Ray FA, Genik PC, Yu Y, McCarthy M, Fallgren CM, et al. Effects of 28Si ions, 56Fe ions, and protons on the induction of murine acute myeloid leukemia and hepatocellular carcinoma. PloS one 2014; 9(7): e104819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suman S, Kumar S, Moon BH, Strawn SJ, Thakor H, Fan Z, et al. Relative Biological Effectiveness of Energetic Heavy Ions for Intestinal Tumorigenesis Shows Male Preponderance and Radiation Type and Energy Dependence in APC(1638N/+) Mice. International journal of radiation oncology, biology, physics 2016. May 1; 95(1): 131–138. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Farris Iii AB, Wang P, Zhang X, Wang H, Wang Y. Relative effectiveness at 1 gy after acute and fractionated exposures of heavy ions with different linear energy transfer for lung tumorigenesis. Radiation research 2015. February; 183(2): 233–239. [DOI] [PubMed] [Google Scholar]
- 48.Rithidech KN, Honikel LM, Reungpathanaphong P, Tungjai M, Jangiam W, Whorton EB. Late-occurring chromosome aberrations and global DNA methylation in hematopoietic stem/progenitor cells of CBA/CaJ mice exposed to silicon ((28)Si) ions. Mutation research 2015. November; 781: 22–31. [DOI] [PubMed] [Google Scholar]
- 49.Kennedy EM, Powell DR, Li Z, Bell JSK, Barwick BG, Feng H, et al. Galactic Cosmic Radiation Induces Persistent Epigenome Alterations Relevant to Human Lung Cancer. Scientific reports 2018. April 30; 8(1): 6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sridharan DM, Asaithamby A, Bailey SM, Costes SV, Doetsch PW, Dynan WS, et al. Understanding cancer development processes after HZE-particle exposure: roles of ROS, DNA damage repair and inflammation. Radiation research 2015. January; 183(1): 1–26. [DOI] [PubMed] [Google Scholar]
- 51.Chang J, Luo Y, Wang Y, Pathak R, Sridharan V, Jones T, et al. Low Doses of Oxygen Ion Irradiation Cause Acute Damage to Hematopoietic Cells in Mice. PloS one 2016; 11(7): e0158097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eccleston J, Yan C, Yuan K, Alt FW, Selsing E. Mismatch repair proteins MSH2, MLH1, and EXO1 are important for class-switch recombination events occurring in B cells that lack nonhomologous end joining. Journal of immunology 2011. February 15; 186(4): 2336–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chahwan R, van Oers JM, Avdievich E, Zhao C, Edelmann W, Scharff MD, et al. The ATPase activity of MLH1 is required to orchestrate DNA double-strand breaks and end processing during class switch recombination. The Journal of experimental medicine 2012. April 9; 209(4): 671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gutmann DH, Winkeler E, Kabbarah O, Hedrick N, Dudley S, Goodfellow PJ, et al. Mlh1 deficiency accelerates myeloid leukemogenesis in neurofibromatosis 1 (Nf1) heterozygous mice. Oncogene 2003. July 17; 22(29): 4581–4585. [DOI] [PubMed] [Google Scholar]
- 55.Fang Y, Tsao CC, Goodman BK, Furumai R, Tirado CA, Abraham RT, et al. ATR functions as a gene dosage-dependent tumor suppressor on a mismatch repair-deficient background. The EMBO journal 2004. August 04; 23(15): 3164–3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodman C, Almeida-Porada G, George SK, Moon J, Soker S, Pardee T, et al. In vitro and in vivo assessment of direct effects of simulated solar and galactic cosmic radiation on human hematopoietic stem/progenitor cells. Leukemia 2017. June; 31(6): 1398–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


