Abstract
Chronic exposure of the retina to light and high concentrations of polyunsaturated fatty acid in photoreceptor cells make this tissue susceptible to oxidative damage. As retinal degenerative diseases are associated with photoreceptor degeneration, the antioxidant activity of both hydrogen sulfide (H2S) and glutathione (GSH) may play an important role in ameliorating disease progression. H2S production is driven by cystathionine-γ-lyase (CSE) and cystathionine β–synthase (CBS), the key enzymes that also drive transsulfuration pathway (TSP) necessary for GSH production. As it is currently unclear whether localized production of either H2S or GSH contributes to retinal homeostasis, we undertook a comparative analysis of CBS and CSE expression in canine, non-human primates (NHP) and human retinas to determine if these antioxidants could play a regulatory role in age-related or disease-associated retinal degeneration. Retinas from normal dogs, NHPs and humans were used for the study. Laser capture microdissection (LCM) was performed to isolate individual layers of the canine retina and analyze CBS and CSE gene expression by qRT-PCR. Immunohistochemistry and western blotting were performed for CBS and CSE labeling and protein expression in dog, NHP, and human retina, respectively. Using qRT-PCR, western blot, and immunohistochemistry (IHC), we showed that CBS and CSE are expressed in the canine, NHP, and human retina. IHC results from canine retina demonstrated increased expression levels of CBS but not CSE with post-developmental aging. IHC results also showed non-overlapping localization of both proteins with CBS presenting in rods, amacrine, horizontal, and nerve fiber cell layers while CSE was expressed by RPE, cones and Mϋller cells. Finally, we demonstrated that these enzymes localized to all three layers of canine, NHP and human retina: photoreceptors, outer plexiform layer (OPL) and notably in the ganglion cells layer/nerve fiber layer (GCL/NFL). QRT-PCR performed using RNA extracted from tissues isolated from these cell layers using laser captured microdissection (LCM) confirmed that each of CBS and CSE are expressed equally in these three layers. Together, these findings reveal that CSE and CBS are expressed in the retina, thereby supporting further studies to determine the role of H2S and these proteins in oxidative stress and apoptosis in retinal degenerative diseases.
Keywords: cystathionine-β-synthase (CBS), cystathionine-γ-lyase (CSE), retina, dog, human
Introduction
The unique structural and functional organization of the retina, consisting of an outer nuclear layer (ONL) containing photoreceptors, an inner segment layer (INL) containing neurons required to process visual signals, and ganglion cell layer (GCL), is essential for proper vision. However, the unique structure of the retina also makes it vulnerable to dysfunction and disease (Masland, 2012; Wright et al., 2010). Inherited retinal degenerations are caused by mutations in a substantial number of genes which principally affect photoreceptor cells or retinal pigment epithelium (Baehr and Frederick, 2009; Jacobson et al., 2015; Miyadera et al., 2012). Retinal degeneration is characterized by photoreceptor cells apoptosis, a process which is marked by morphological and biochemical changes and considered a common final pathway in most retinal degenerative diseases (Portera-Cailliau et al., 1994). Apoptosis can be induced by an extrinsic pathway triggered by tumor necrosis factor (TNF) or intrinsic pathway induced by severe cell damage/stress (Locksley et al., 2001). Mitochondria play a key role in initiating apoptosis via the intrinsic pathway (Wang and Youle, 2009) and increased mitochondrial dysfunction as a result of subischemic injury is associated with oxidative stress-induced ganglion cells injury in death (Kong et al., 2012). However, the exact role of oxidative stress in age-and/or disease-dependent retinal degeneration remains unknown.
Hydrogen sulfide (H2S) is a gaseous neurotransmitter with antioxidant, anti-apoptotic, and anti inflammatory effects that can play a protective role in neurodegenerative diseases (Eto et al., 2002; Kamat et al., 2015). H2S decreases apoptosis both in vitro and in vivo, and activates anti apoptotic proteins (Henderson et al., 2010; Minamishima et al., 2009). The role of H2S in retinal diseases has been gaining increasing attention. Reduced levels of H2S are associated with ganglion cell apoptosis in a rat experimental glaucoma model while H2S treatment promotes ganglion cells survival (Huang et al., 2018). The anti-oxidant activity of H2S also plays a protective role against retinal injury in rats in vivo (Sakamoto et al., 2014). The putative mechanisms underlying the neuroprotective role for H2S in the central nervous system (CNS) include preservation of mitochondrial function, reduction of oxidative stress, suppression of glial activation, inhibition of inflammatory pathways and downregulation of autophagy (Hu et al., 2010). The majority of biosynthesized H2S is produced by the activity of two pyridoxal-5′-phosphate-dependent enzymes, CSE and CBS (Bukovska et al., 1994; Erickson et al., 1990; Stipanuk and Beck, 1982). Expression of both enzymes is required for H2S production in some tissues while in other tissues the activity of either enzyme is sufficient (Hosoki et al., 1997; Levonen et al., 2000; Lu et al., 1992; Meier et al., 2001; van der Molen et al., 1997). In addition, the expression of CSE and CBS is tissue specific. While localized production of H2S could potentially impact either age- or disease-related retinal degeneration, it is not yet clear if the enzymes responsible for its production are expressed in the retina or if their expression is restricted to specific retinal layers or cells within retina.
Glutathione (GSH) is one of the main antioxidant sources in the retina and its reduction may cause oxidative stress and increased retinal cell death (Roh et al., 2007). GSH synthesis is dependent on both CBS and CSE, as cysteine, a required substrate for GSH synthesis is generated through CBS conversion of homocysteine to cystathionine which is subsequently converted to cysteine by CSE (Finkelstein, 1990; Jhee and Kruger, 2005; Vitvitsky et al., 2006). In vitro silencing of CBS decreases GSH levels and consequently enhances levels reactive oxygen species (ROS) that can drive oxidative DNA damage (Bhattacharyya et al., 2013). Although CBS expression has been observed in the retina of human, pig (Persa et al., 2006), mouse (Markand et al., 2013) and salamander (Pong et al., 2007), there are no reports in which the retinal expression of both CBS and CSE are simultaneously analyzed at the transcript and protein level in different layers of the same retinas. To determine if these enzymes could potentially contribute to either age- or disease-dependent retinal degenerative diseases, we examined CBS and CSE expression in canine, non-human primate (NHP) and human retinas.
As we observed expression of these proteins in different retina layers, we also used specific markers to identify canine retinal cells capable of expressing CBS or CSE and determine the potential effects of post-developmental aging on CBS and CSE expression. This information will be essential for further examination of the role of these enzymes in retinal degenerative processes.
Material and methods
Sources of canine, NHP, and human samples
Retinas from normal dogs (age: 5, 12, 22, 31 weeks and 3.5 years), NHP (age: 4.6, 4.9 and 5.8 years) and human (age: 78, 79, 80 and 90 years) were used for the study. All dogs were housed under identical conditions (diet, ambient illumination with cyclic 12 hrs ON-12 hrs OFF light) at the Retinal Disease Studies (RDS) facility in Kennett Square, Pennsylvania. The study was approved (protocol number 804956) by the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania and strictly adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Eyes from normal NHP that were being euthanized after being used as control for another study were used. Human samples were processed in Dr. Joshua Dunaief’s laboratory, School of Medicine, University of Pennsylvania and were collected adhering the principle of the Declaration of Helsinki. To rule out variation in expression with time of day all canine tissues were collected between 7:30–9:30 am and NHP tissues were collected mid-morning. Obviously, time of death for human subjects is variable, and variability is also influenced by factors such as time on life support, time between death and tissue collection etc. Because this variability is beyond our control, the emphasis in the studies is placed on the samples for which we have absolute control-dogs and NHP.
Laser capture microdissection
Laser capture microdissection (LCM) was performed to isolate individual layers (outer nuclear layer, ONL; inner nuclear layer, INL; ganglion cell layer, GCL) of three normal canine retinas (age: 22 weeks). Twelve micron thick retinal sections were cut from the fresh frozen retinas that were embedded in OCT medium without fixation under RNase-free conditions. The sections were collected on RNase-free PEN-membrane slides (Zeiss, Gottingen, Germany), stained with hematoxylin for 2 minutes and quickly passed through increasing concentrations of ethanol. The slides were dried, stored in dry ice, and used the same day for microdissection using a Leica LMD 7000 microscope. Each layer was laser-dissected and collected in AdhesiveCap clear tubes (Zeiss, Gottingen, Germany), and the tubes were stored at −80°C until further processing.
RNA extraction and cDNA synthesis and qRT-PCR
RNA was purified from LCM sections using RNeasy Plus Micro Kit (Qiagen, Germantown, MD). First strand cDNA was synthesized using SuperScript III following manufacturer’s guidelines (ThermoFisher Scientific, Waltham, MA). A single round of antisense RNA amplification was performed using the MEGAScript T7 Transcription kit (ThermoFisher Scientific, Waltham, MA). Quantitative real-time PCR was performed using SYBR Green PCR Mix and ViiA 7 Real-Time PCR System (348-well format) (Applied Biosystems, Thermo Fisher Scientific). 100 ng of amplified RNA and 10uM primers were used, and each reaction was performed in triplicate. All PCR reactions were normalized to the Ct value of GAPDH. Fold change was calculated as 2−( ∆∆Ct). The sense and antisense primers for CSE, CBS, and GAPDH were designed using the PrimerQuest Tool from Integrated DNA Technologies (Table 1).
Table 1.
PCR primer sequences, optimal amplification cycles, optimal annealing temperature, and accession number.
| Gene | Accession number |
Primer sequence | Optimal condition |
|---|---|---|---|
| CSE | XM_537115 | F: GCAATGGAATTCTCGTGCCG R: ATGCAAAGGCCAAACTGTGC |
40 cycles Annealing 60°C |
| CBS | XM_014109845 | F: GGCTGGAAAGGTGCGGCCAT R: CTTGCTGGACATGCCATTGCTG |
40 cycles Annealing 60°C |
| GAPDH | NM-001003142 | F: 5-CTGTCGAGTCGCGTCCACCC-3 R: 5-ACATGCCGGAGCCGTTGTCG-3’ |
40 cycles Annealing 60°C |
Protein extraction and western blot analysis
The neuroretina was homogenized in a buffer containing 0.23 M sucrose, 2 mM EDTA, 5 mM Tris HCl, pH 7.4, 1% Triton™ X-100 and a protease and phosphatase inhibitor cocktail (Halt, Thermo Scientific, Waltham, MA) by vortexing with 1.5 mm zirconium beads (Benchmark Scientific Inc., Sayreville, NJ) and then sonicated. The samples were then centrifuged and total protein concentration in the supernatant measured by BCA assay. Aliquots of protein (20µg) were then separated on 10 % Tris-Glycine SDS polyacrylamide gels (Bio-Rad, Hercules, CA) and subsequently transferred onto nitrocellulose membranes electrophoretically. The membranes were blocked for 1 h in 1× Odyssey blocking buffer (LI-COR, Lincoln, NE) and washed with 0.01 % Tween® 20 in PBS and were then incubated with the primary antibody raised against CBS or CSE at a dilution of 1:1000 overnight at 4°C. The following day, membranes were washed three times with PBST (PBS containing 0.1% Tween® 20) and incubated for 1 h at room temperature in goat anti-rabbit IRDye680RD- or goat anti-mouse IRDye800CW-conjugated immunoglobulin G (IgG) secondary antibodies (LI-COR, Lincoln, NE) diluted 1/10,000 each in 1× Odyssey Blocking Buffer containing 0.1% Tween® 20. Table 2 provides details on the antibodies and dilutions used. Immunoblots were washed three times in PBST, once in PBS, and scanned on the Li-COR Odyssey Fc Dual-Mode Imaging System with 700- and 800-nm channels (LI-COR, Lincoln, NE). Normalization to GAPDH and analyses were done using Image Studio Software provided by LI-COR (LI-COR, Lincolc, NE). GAPDH was used as the housekeeping protein to control for loading. Because our ultimate goal is to examine CSE and CBS in degenerating retinas where the loss of actin and tubulin enriched photoreceptors may affect interpretation of expression changes occurring in inner retinal layers with degeneration GAPDH was used a housekeeping gene in this study.
Table 2.
Antibodies used for western blot and immunohistochemistry
| Antigen | Antibody | Host | Type | Immunogen species |
% Identity with immunogen |
Dilution for WB |
Dilution for IHC |
|---|---|---|---|---|---|---|---|
| CBS | Proteintech, 14787–1-AP |
Rabbit | IgG Polyclonal |
Human | 86% Dog 98% NHP |
1:1000 | 1:500 |
| CSE | Proteintech, 60234–1-Ig |
Mouse | IgG1 Monoclonal |
Human | 91% Dog 95% NHP |
1:1000 | 1:500 |
| GAPDH | Proteintech, 10494–1-AP |
Rabbit | IgG Polyclonal |
Human | 95% Dog 99% NHP |
1:10000 | - |
| GAPDH | Proteintech, 60004–1-lg |
Mouse | IgG2b Monoclonal |
Human | 95% Dog 99% NHP |
1:10000 | - |
Blocking peptides corresponding to the CBS (Proteintech Ag6437, stock 0.25µg/µl) or CSE (Proteintech Ag2872, stock 0.4µg/µl) antibody epitopes were used to validate the specificity of the primary antibodies. Briefly, primary antibodies were incubated at 4°C, overnight with a molar excess of their corresponding blocking peptide, spun for 15 minutes to discard antibody blocking peptide complex and subsequently used to incubate with the immunoblot. A parallel experiment without blocking peptide was also performed as a control. For CBS antibody validation, blocking peptide concentration was an excess of 10× the usual amount of antibody typically used for western immunoblot. For CSE antibody validation, blocking peptide concentration was an excess of 4×.
Immunodetection of CBS and CSE in Intact Retinal Tissue
Expression and localization of CBS and CSE was analyzed by immunohistochemistry (IHC) using primary antibodies against CBS and CSE. Additionally, specific retinal cell markers were used for colocalization of CBS and CSE in canine retinal cells. Ten µm-thick retinal cryosections were washed three times with PBS Triton™ X-100 and blocked with blocking buffer (3% normal horse serum, 1% BSA and 0.3% Triton™ X-100 in PBS) for 1h at room temperature. The working dilutions and sources of antibodies used in this study are listed in Tables 2 and 3. Sections were stained with primary antibodies at 4 °C overnight. Antigen antibody complexes were visualized with Alexa Fluor®-conjugated secondary antibodies (Invitrogen, Thermo Fisher Scientific). The counterstain DAPI (1µg/µl) was used to label the nuclei. Specificity of CBS and CSE primary antibodies used in IHC was validated in dogs using isotype controls. For CBS, canine cryosections were incubated with either CBS primary antibody or equimolar amount of rabbit IgG isotype control (Invitrogen 02–6102). Similarly, for CSE, canine cryosections were incubated with either CSE antibody or equimolar amounts of mouse IgG1 isotype control (Invitrogen14–4714-82). IHC was completed as usual. Gelvatol Mounting medium (containing polyvinyl alcohol and glycerol) was used for mounting and then slides were examined with an epifluorescence microscope (Axioplan, Carl Zeiss Meditec, Thornwood, NY). For illustration of IHC findings, images were digitally captured approximately midpoint between the optic disc and ora serrata using a Spot 4.0 camera with SPOT 4.0 software (SPOT Imaging, Sterling, Heights, MI) and prepared for display using ImageJ software (NIH, Bethesda, MD). A TCS-SP5 confocal microscope system (Leica Microsystems, Buffalo Grove, IL) was used to acquire confocal images at 0.25-µm Z-steps. For either conventional or confocal fluorescent microscopy, each set of sections evaluating a specific antibody, the same exposures and the image handling procedures were utilized for IHC. However, because the parvalbumin labeling in amacrine’s dendrites is weaker than CBS in AII amacrine cells, the signal for parvalbumin has been enhanced to illustrate colocalization, and this is noted in the figure legend.
Table 3.
Antibodies used for specific cell markers in immunohistochemistry
| Antigen | Antibody | Host | Type | Localized cells | Dilution | Reference |
|---|---|---|---|---|---|---|
| RPE65 | J.M Nickerson U of Georgia |
Rabbit | IgG polyclonal |
RPE | 1:1000 | (Guziewicz et al., 2018; Vecino et al., 2016) |
| hCAR (Human cone arrestin) |
W, Beltran, U of Pennsylvania |
Goat | IgG Polyclonal |
Cones | 1:50 | (Kondo et al., 2015) |
| Rhodopsin | ||||||
| Millipore MABN15 |
Mouse | IgG2b Monoclonal |
Rods | 1:500 | (Cideciyan et al., 2018) | |
| Ezrin | ||||||
| Abcam ab4069 |
Mouse | IgG1 Monoclonal |
RPE microvilli | 1:500 | (Guziewicz et al., 2018) | |
| PNA (Peanut agglutinin) |
Invitrogen L32458 |
Lectin |
______ |
Cone extracellular matrix |
1:50 |
(Guziewicz et al., 2018) |
| WGA (wheat germ agglutinin) |
Invitrogen W11262 |
______ |
Rod extracellular matrix |
1:100 |
(Hauzman et al., 2017) |
|
| Parvalbumin | Millipore MAB1572 |
Mouse | IgG1 Monoclonal |
Horizontal and AII amacrine cells |
1:1000 | (Roski et al., 2018) |
| Neurofilament 200KDa |
Millipore MAB5266 |
Mouse | IgG1 Monoclonal |
GCL/NFL | 1:200 | (Singh et al., 2014; Vecino et al., 2016) |
| PKCα | Millipore MAB302 |
Mouse | IgG2a Monoclonal |
Rod bipolar cells | 1:1000 | (Singh et al., 2014; Vecino et al., 2016) |
| GAD65 | R&D Systems, AF2247 |
Goat | IgG Polyclonal |
GABAergic amacrine cells |
1:100 | (Schubert et al., 2010) |
| GS (Glutamine Synthetase |
BD Bioscience, 610107 |
Mouse | IgG2b Monoclonal |
Müller cells | 1:100 | (Sudharsan et al., 2017) |
Statistics
Data are expressed as mean ± standard deviation. The unpaired independent t-test, One-way ANOVA, and Tukey’s multiple comparison tests were used for statistical analysis using Graph Pad version 7. The experiments were performed in three samples with technical triplicates for each sample, and the results are expressed as mean ± SD. The p value of <0.05 was regarded as statistically significant.
Results
CBS and CSE gene expression in canine retina layers
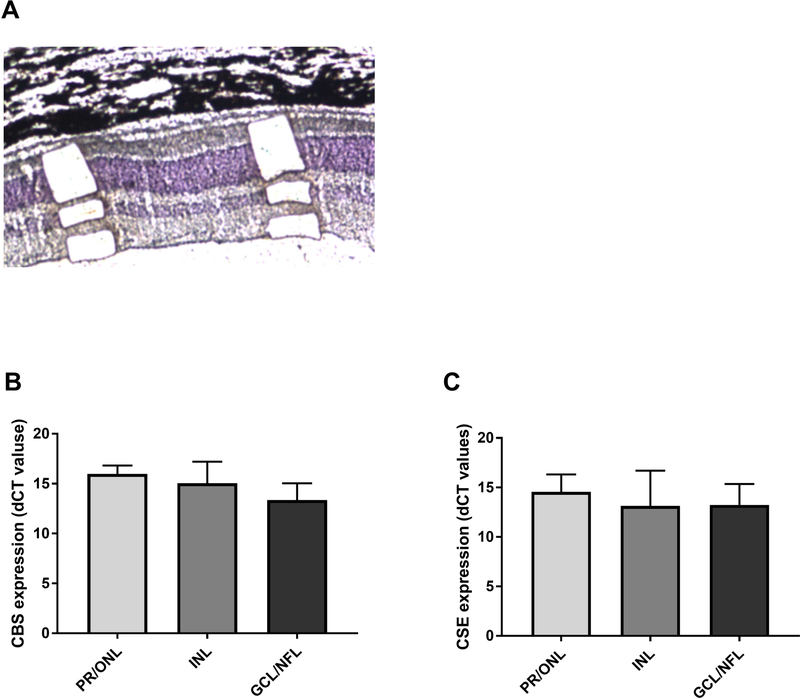
While CBS and CSE are known to be expressed in the retina, it is currently unclear if they are ubiquitously expressed or if their expression is retinal layer-specific. To specifically address this question, we isolated tissue from PR/ONL, INL, and GCL/NFL layers using LCM. We then undertook quantitative PCR analysis on mRNA isolated from these tissues (Figure 1). Our results confirmed that CBS and CSE were expressed in all three layers and that were not significant differences in expression of these genes in the three retinal layers sampled (Figure 1).
Figure 1.
Laser capture microdissection (LCM) and qRT-PCR analysis of CSE and CBS expression in PR/ONL, INL, and GCL/NFL in normal canine retina. LCM was performed to collect the cells from PR/ONL, INL, and GCL/NFL (A). The cells collected from these areas were used for RNA extraction to perform qRT-PCR. Samples from the 3 layers were collected by LCM and total RNA was extracted from cells and converted to cDNA to run qPCR. The results showed that CSE and CBS are equally expressed in PR/ONL, INL, and GCL/NFL in retina. CSE and CBS mRNA expression was normalized with GAPDH gene and expressed as fold change. The results also showed that there was not a significant differences in level of CSE and CBS expression in these layers. Results are shown as the mean ± SD (ANOVA with a post hoc Tukey’s test for multiple comparisons). The samples were collected from three different eyes of 3 normal dogs at 24 weeks.
CBS and CSE protein expression in canine, non-human primate and human retinas
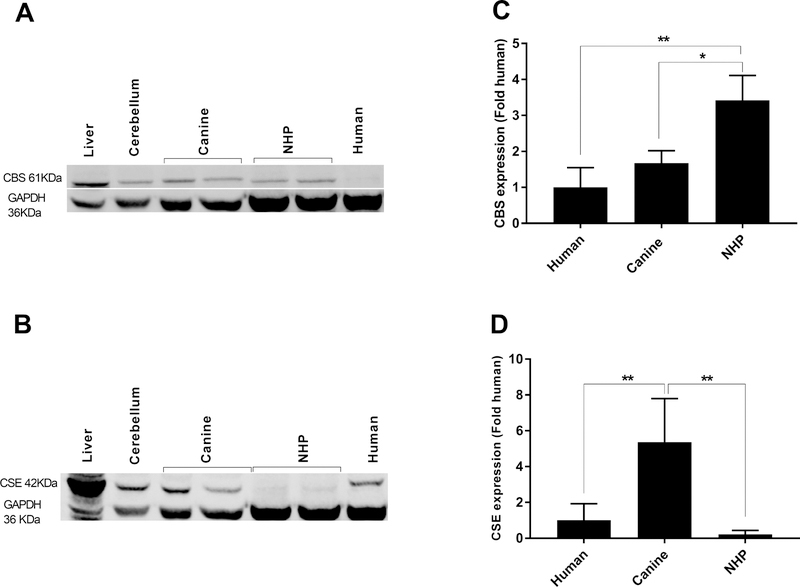
Western blot was next performed to examine CBS and CSE protein expression in the retinas of three different species. As a positive control we used canine liver and cerebellum, two tissues in which these proteins are known to be expressed (Enokido et al., 2005; Markand et al., 2013; Pong et al., 2007). Immunoblot analysis for CBS showed a single protein band of approximately 61 kDa (Figure 2A). A significantly higher level of CBS expression was observed in the NHP retina, with levels 3.4 fold higher than in the human retina (one-way ANOVA, p<0.01) (Figure 2C). CBS expression in canine retina was also significantly higher with levels 1.7 fold higher than the human retina (one-way ANOVA, p<0.01) (Figure 2C). Western blot analysis also confirmed a single protein band of approximately 42 kDa corresponding to the CSE (Figure 2B). Our results indicated a significantly higher level of CSE expression in canine retina with levels 5.4 fold higher than the human retina (one-way ANOVA, p<0.01) (Figure 2D). CSE expression in NHP was 0.2 fold lower than the human retina (one-way ANOVA, p<0.01) (Figure 2D). To verify the specificity of CBS and CSE antibodies, western blotting was performed using epitope-specific blocking peptides. The corresponding bands for CBS and CSE detected by primary antibodies (Figure 3A and C) disappeared when primary antibodies were incubated with specific blocking peptides (Figure 3B and D) confirming their specificity. Together, these results indicate that CBS and CSE are present in the retina, but that the levels vary between species.
Figure 2.
Western blot (A and B) and densitometry analysis (C and D) of CBS and CSE expression in normal canine, NHP and human retinas. CBS and CSE protein expression was normalized with GAPDH (housekeeping protein) and expressed as fold increase over human samples. CBS protein levels in the NHP samples were higher than canines and human. Canine retina also showed higher level of CBS protein expression compared to human samples. CSE protein levels in the canine retina samples were higher than NHP and human. The results are shown as the means ± SD. (One-way ANOVA, **= p< 0.01 and * =p< 0.05). Protein samples were prepared from 3 different animals and human and the experiment was repeated 3 times, representative levels are illustrated.
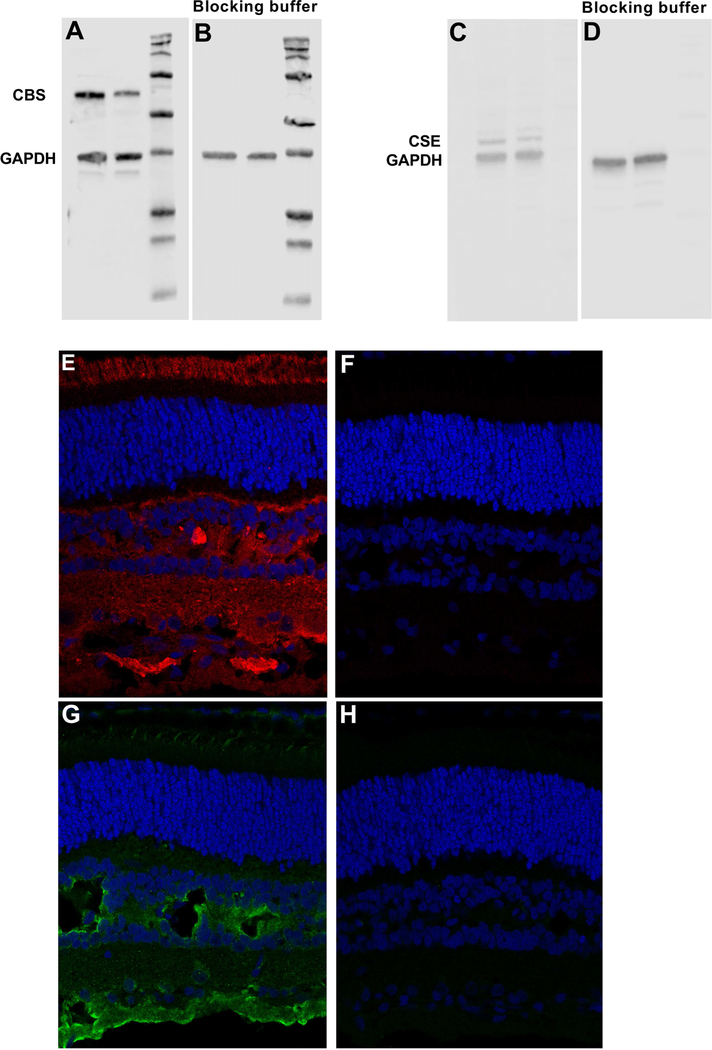
Figure 3.
CBS and CSE antibody validation using blocking peptides kit and isotype match antibody in western blot and IHC. Western blot was perform with and without blocking peptide to determine CBS (A and B) and CSE (C and D) antibodies specificity. Results showed that there wasn’t any band on the blot when blocking peptide was used (B,D) while corresponding bands for CBS or CSE was detected on the blots using primary antibodies without blocking peptides (A, C). IHC results also confirmed specificity of the CBS and CSE antibodies used in this experiment. Results showed that CBS and CSE proteins were not detected when corresponding isotype controls were used for CBS (F) and CSE (H) while CBS (E) and CSE (G) protein were detected using primary antibodies.
Immunohistochemical localization of retinal CBS and CSE
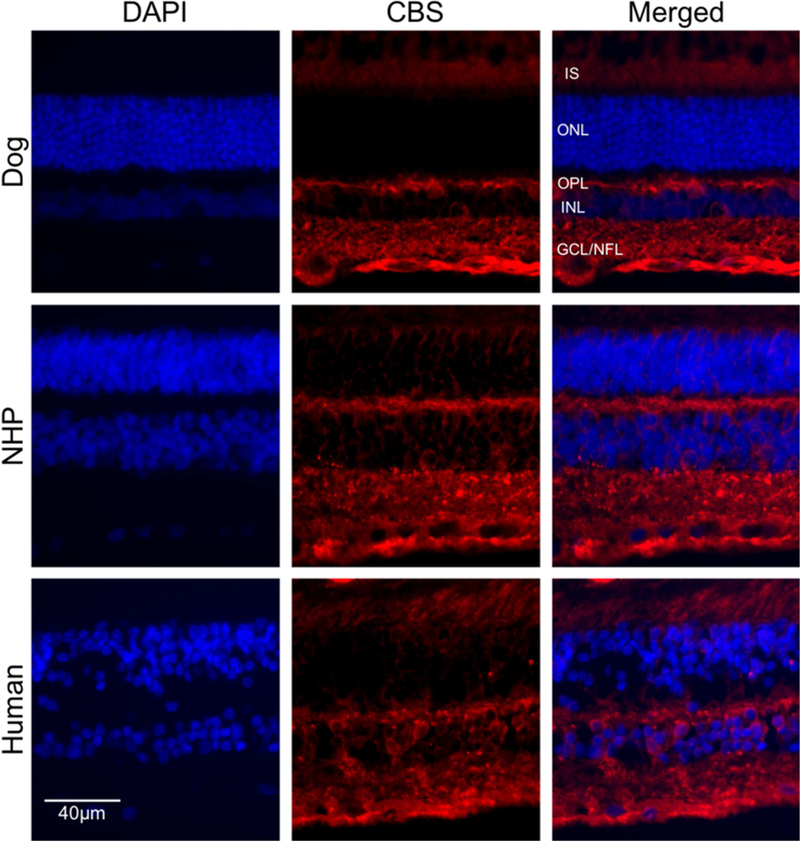
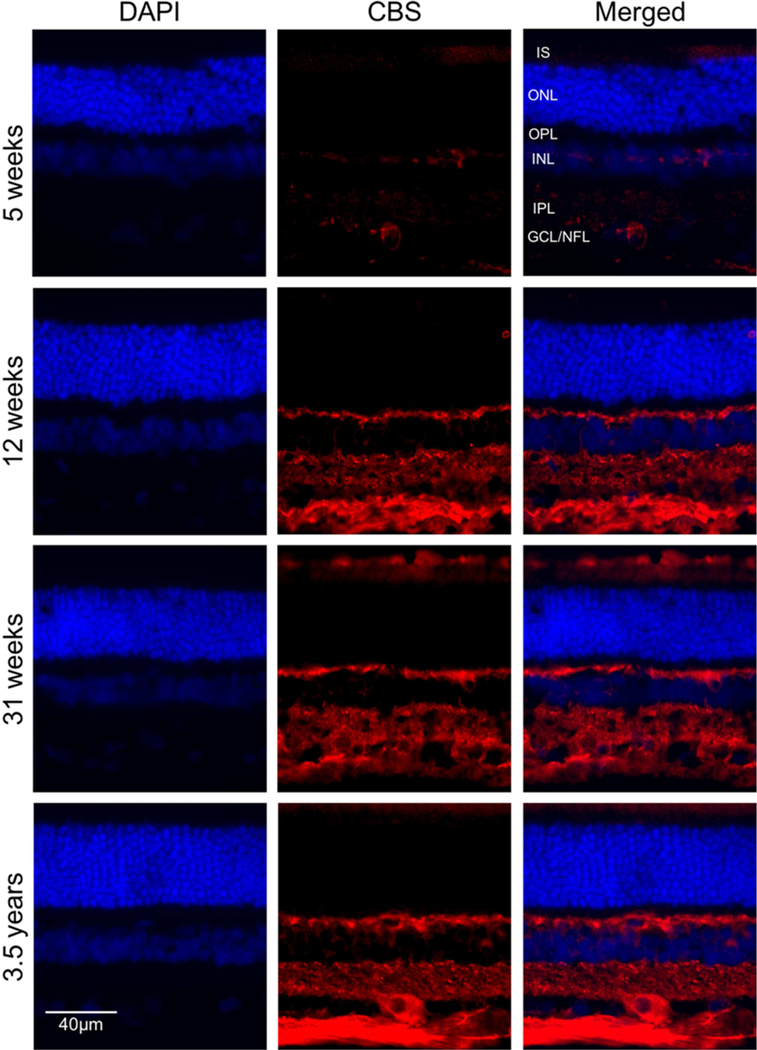
Given that CBS and CSE are expressed at the mRNA level in different retinal layers, we next asked if these proteins were differentially expressed in these layers. Immunohistochemical (IHC) studies were performed on intact canine, NHP and human retinal cryosections. CBS was localized in several retinal layers, with predominant expression observed in the photoreceptors outer segment layer, horizontal cells in the OPL and ganglion cells/ nerve fiber layer (GCL/NFL) (Figure 4). To examine the effect of age on CBS expression, we stained cryosections obtained from retinas of dogs aged 5, 12, 31 weeks and 3.5years with anti-CBS antibody. The results showed an increased level of CBS labeling with post-developmental aging in cells in the OPL, IIPL and GCL/NFL (Figure 5).
Figure 4.
Immunohistochemical analysis of CBS in retinas from canine, NHP and human. Retinal cryosections from (A-C) canine (3.5 years old), NHP (D-F) (4.9 years old) and human (G-I) (79 years old) were labeled with antibody against CBS. CBS (red fluorescence) was detected in photoreceptor cells (Inner segment), OPL and GCL/NFL. DAPI was used to stain nuclei (A, D, G). C, F and I are merged images. (Scale bar: 20 µm; OPL: outer plexiform layer; GCL: ganglion cell layer; NFL: nerve fiber layer; IS: Inner segment layer of photoreceptor cells).
Figure 5.
Immunohistochemical analysis of CBS expression in normal dogs of different ages. Retinal cryosections from (A-C) five weeks, (D-F) twelve weeks, (G-I) thirty-one weeks and (J-L) three and a half year old dogs were labeled with antibody against CBS. CBS (red fluorescence) was detected in photoreceptor cells (Inner segment), OPL and GCL/NFL. DAPI was used to stain nuclei (A, D, G and J). C, F, I and L are merged images. (Scale bar: 20 µm; OPL: outer plexiform layer; GCL: ganglion cell layer; NFL: nerve fiber layer; IS: Inner segment layer of photoreceptor cells).
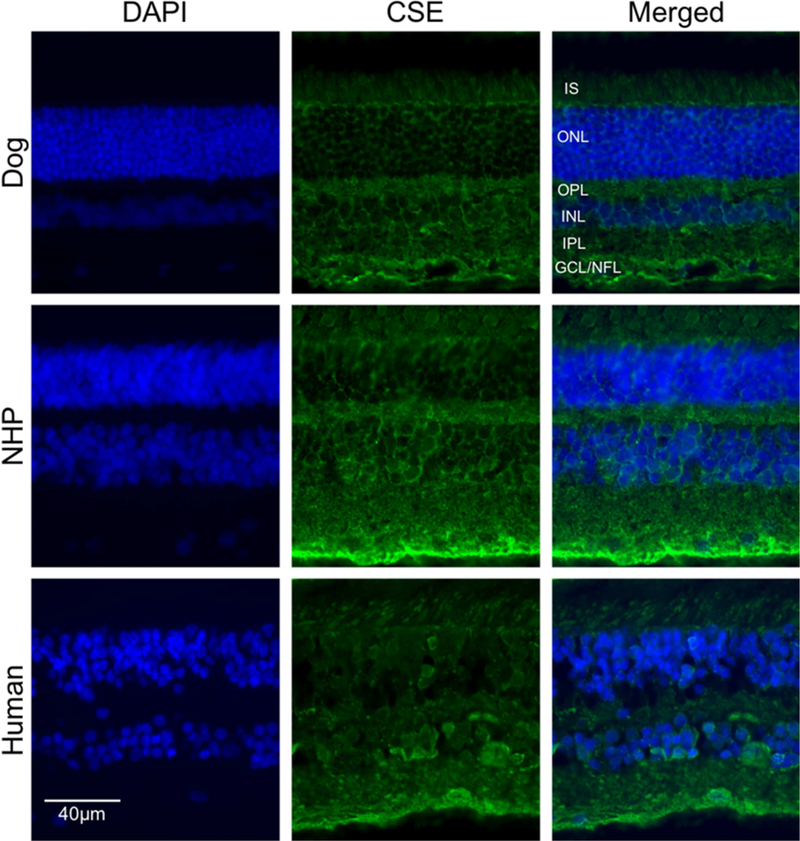
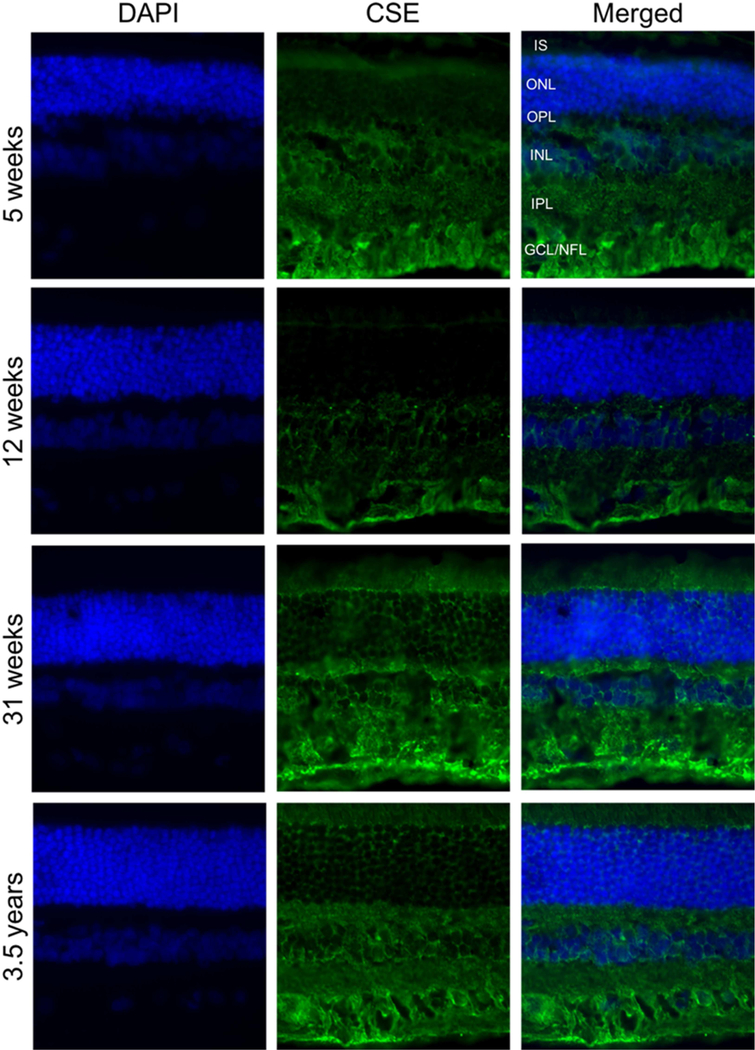
IHC analysis of CSE expression in canine, NHP and human retina (Figure 6) revealed that in the dog, CSE labeling was predominantly in the GCL and OPL with lower levels of immunolabeling in photoreceptor cells. In both NHP and human retinas examined, labeling was predominantly in the GCL and labeling was less intense than in the dog retina. CSE labeling of photoreceptor cells was not strong in any of the species (Figure 6). In contrast to CBS, CSE expression in the dog retina was mainly in GCL/NFL and IPL, and labeling intensity did not change with age (Figure 7). Isotype controls validated primary antibody specificity as no staining was detected (Figure 3F and H) in canine retina. These results confirmed CBS and CSE proteins are localized in canine, NHP and human retina.
Figure 6.
Immunohistochemical analysis of CSE labeling in normal retina from canine, non-human primate and human. Retinal cryosections from (A-C) canine, non-human primate (D-F) and human (G-I). Immunolabeling showed RPE layer, and mainly in GCL. DAPI was used to stain nuclei. (Scale bar: 20 µm; GCL: ganglion cell layer; NFL: nerve fiber layer; IS: Inner segment layer of photoreceptor cells).
Figure 7.
Immunohistochemical analysis of CSE in canine retinas at different ages. Retinal cryosections from (A, B, C) five weeks, (D, E, F) twelve weeks, (G, H, I) thirty-one weeks and (J, K, L) three and a half years old dogs were stained with antibody against CSE. CSE (green fluorescence) was mainly in GCL. DAPI was used to stain nuclei (A, D, G and J). C, F, I and L are merged pictures. (Scale bar: 20 µm; PR: Photoreceptor cells, GCL: ganglion cell layer; NFL: nerve fiber layer; IS: Inner segment layer of photoreceptor cells).
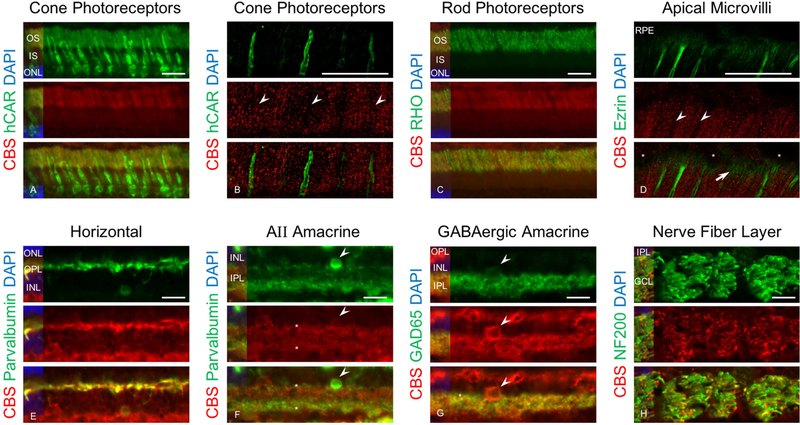
To determine if CBS and CSE expression was cell type specific, we double labeled canine retinal cryosections (age: 5 weeks) using specific retinal cells markers. Double immunolabeling for CBS and hCAR, a cone-specific marker, showed that CBS did not localize in cones (Figure 8A). Confocal microscopy confirmed the localization of CBS in the photoreceptor cell layer but excluded in cones (Figure 8B). Immunolabeling for CBS and rhodopsin, a marker for rod outer segments, confirmed localization of CBS in outer segments; colocalization was present in the great majority of rod outer segments, although not present in all. This finding could indicate that not all rod outer segments express CBS (Figure 8C). Immunolabeling for CBS and ezrin, the latter a marker for RPE, rod and cone microvilli, showed ezrin was present in the RPE microvilli and did not colocalize with CBS (Figure 8D). CBS also localizes in horizontal cells (Figure 8E), both AII and GABAergic amacrine cells (Figure 8F and G) and the NFL (Figure 8H).
Figure 8.
Immunohistochemical analysis of canine retinal cryosections labeled with CBS and retinal cells markers. Retinal cryosections immunolabeled with anti-CBS (red), DAPI (blue), hCAR (green), rhodopsin (green), ezrin ( green), parvalbumin (green), GAD65 (green) and NFH (green). Results showed that CBS was not localized in cones (A) and confocal microscopy analysis of section immunolabeled with hCAR and CBS confirmed that cones did not express CBS (arrow heads shows the locations of cones) (B). Double immunnolabeling with rhodopsin (a marker for rods outer segment) and CBS confirmed localization of CBS in rods outer segment (C). Confocal analysis of section showed that RPE apical microvilli illustrated by ezrin labeling show lack of CBS in cones (arrow heads) but highlight CBS expression associated with the rods (arrows) (asterisks show the nucleus for RPE) (D). The results also confirmed localization of CBS in horizontal (E), AII amacrine (arrow head shows the cell bodies and dendrites are labeled by asterisk) (F) and GABAergic amacrine cells (arrow heads shows the cell body and dendrites are labeled by asterisk) (G) and NFL (H). Because the parvalbumin labeling (green) in amacrine’s dendrites is weaker than CBS (red) in AII amacrine cells, the green signal has been enhanced to illustrate colocalization (Scale bar: 20 µm).
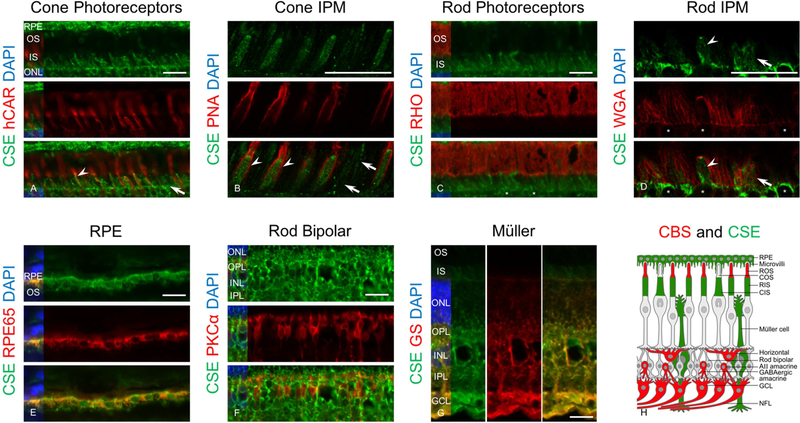
IHC results showed localization of CSE in cones (Figure 9A) and RPE cells (Figure 9E). Confocal microscopy analysis of CSE and PNA, a cone-specific insoluble extracellular matrix sheath label, confirmed localization of CSE in the inner segments of the cones (Figure 9B). CSE and rhodopsin double immunolabeling showed that CSE localized in only the inner segments of the photoreceptor cell layer (Figure 9C). Labeling for rhodopsin and WGA, a marker for rod extracellular matrix, showed CSE labeled rod inner segments, a finding confirmed by confocal microscopy (Figure 9D). In contrast, CSE is not expressed in rod outer segments or rod bipolar cells (Figure 9F). Our results also confirmed localization of CSE in Müller cells (Figure 9G). A schematic figure of retinal cells labeled with CBS or CSE is shown (Figure 9H) and emphasizes on the non-overlapping distribution of these proteins in the retinal cells. Taken together, the data from these experiments confirmed that CBS and CSE are differentially expressed which could potentially contribute to their functional effects in the retina.
Figure 9.
Immunohistochemical analysis of canine retinal cryosections labeled with CSE and specific markers for retinal cells. Retinal cryosections immunolabeled with anti-CSE (green), DAPI (blue), RPE65 (red), hCAR (red), PNA (red), rhodopsin (red), PKCα (red) and GS (red). Results showed that CSE localized in cones (arrow head) but other cells also labeled with CSE that probably are rods (arrow) (A). Confocal microscopy analysis of sections immunolabeled with PNA (a marker for cone extracellular matrix and CSE confirmed localization of CSE in inner segment of the cones (arrow heads) and also showed the cells that are labeled with CSE but not PNA) and these cells are rods (arrows) (B). Double labeling of sections with CSE and rhodopsin (a marker for rods outer segment) showed lack of CSE in rods outer segments (asterisks show the nucleus for rods) (C) but confocal microcopy analysis of sections immunolabeled with CSE and WGA (a marker for rods extracellular matrix) confirmed CSE protein expression in rods inner segment (arrow) (arrow heads show cones labeled with CSE) (asterisks show the nucleus for cones) (D). Immunolabeling with CSE and RPE65 (a marker for RPF cells) or CSE and glutamine synthetase (a marker for Müller cells) confirmed CSE localization in RPE (E) and Müller cells (G). CSE and PKCα (a marker for rod bipolar cells) immunolabeling confirmed lack of CSE in rod bipolar cells (F) (Scale bar: 20 µm). A schematic figure showing CBS and CSE antibody labeling different retinal cells (H).
Discussion
CBS and CSE are the main transsulfuration pathway (TSP) enzymes driving the production of H2S and GSH. Several reports have documented expression of CBS and CSE in brain and treatment with selective CBS and CSE inhibitors and gene knockout experiments confirm that CBS is responsible for H2S production in the brain (Lee et al., 2009; Yang et al., 2008). Although there are some reports on the enzymatic activity and expression of these proteins in the retina (Markand et al., 2013; Pong et al., 2007), this information is more limited than for the CNS, and the localization of these proteins in specific retinal cells in unknown. To determine if increased GSH and H2S production in the retina could potentially impact age- and disease dependent retinal degeneration, we examined the expression of two key enzymes, CBS and CSE that are critically involved in their biosynthetic pathway. Specifically, we used qRT-PCR, immunoblot, and IHC analysis to comprehensively evaluate the expression of these enzymes in canine, NHP, and human retinas. IHC analysis detected these proteins in three different layers of retina: photoreceptors, OPL, and GCL/NFL. A similar pattern of CBS distribution in mouse retina was reported previously (Markand et al., 2013). We also used specific markers to determine the expression of CBS and CSE protein in specific retinal cells by IHC.
To examine the expression of CBS and CSE in different layers of the retina, we first used LCM to obtain cells from each layer of canine retina, and quantified transcription expression by qRT-PCR. We found comparable expression of both genes at the mRNA level in PR/ONL, INL, and GCL/NFL. On the other hand, while IHC analysis identified protein expression in each layer, it revealed differences between mRNA and protein expression. The reasons for this are presently unknown, but could result from secretion of the proteins from site of synthesis; a similar mechanism has been reported in cultured microvascular endothelial cells and hepatocytes (Bearden et al., 2010). Alternatively, there might be differential translational regulation of these proteins in the different cellular compartments (Reid and Nicchitta, 2012). We plan to address these possibilities in future studies.
Finally, immunoblot analysis revealed that CBS and CSE proteins are expressed in canine, NHP, and human retinas, as previously reported for CBS in mouse (Markand et al., 2013), human (Persa et al., 2006) and salamander (Pong et al., 2007) retinas. We too found that both proteins are expressed in canine, NHP and human retinas thus suggesting a key role for GSH and H2S in retina produced by the activity of these enzymes.
IHC results confirmed CBS labeling in outer segments of rods, horizontal cells, both kinds of amacrine cells (AII and GABAergic) and NFL. Localization of CBS in amacrine cells which form a synaptic layer with ganglion and bipolar cells, as well as in horizontal cells that connect to the output of the cones and rods signify a potential role of H2S in synapses. Indeed, H2S can act as a signaling molecule that modulates the synaptic activity (Kamat et al., 2015) and GABAergic neurotransmission in the brain (Puschina, 2011). Localization of CBS in presynaptic and postsynaptic cells in the retina suggest that H2S may act as a signaling molecule and neurotransmitter at retinal synapses. However, as CBS and CSE have promiscuous activities, protecting against oxidative damage via reinstating the GSH level (Kimura et al., 2010), contributing to the biogenesis of H2S, which has anti-apoptotic (Huang et al., 2018; Liu et al., 2017), anti-inflammatory and oxidative stress suppressing activity (Du et al., 2017; Sone et al., 2018) in retina. Our data provides compelling evidence that CBS protein is expressed in synaptic layers in retina and may contribute neurotransmission activity through GSH and H2S production.
We also showed that CSE localizes in the RPE, inner segment of the cones and Müller cells. GSH biosynthesis requires CSE expression and recent reports show GSH induces release of GABA, a neurotransmitter in the retina (Freitas et al., 2016; Freitas and Reis, 2017). IHC results showed that rods are the only cells that express both CSE (IS) and CBS (OS) and that these proteins do not colocalize in the same cellular compartments. In regards to Müller cells, these findings suggest they have a protective role via GSH production and potentially as a communicator in retina by releasing GABA. Both Müller cells and RPE modulate immune and inflammatory responses in retina, thereby protecting retinal nerve cells against oxidative stress and pathological damage. Our finding suggests that the protective influence of RPE and Müller cells utilize CSE could be aided by CSE-dependent generation of GSH and H2S.
Prior studies have shown CBS and CSE enzymes activation in the retina (Markand et al., 2013; Pong et al., 2007), an indication of their possible involvement in H2S production in this tissue. Our IHC results show that CBS and CSE don’t overlap in retinal cells, suggesting that these proteins may act differently, in a cell-specific manner, in regards to aging, metabolism, or diseases. While expression of both CBS and CSE enzymes are required for H2S production, the cell-specificity of these enzymes indicates that only one of these enzymes is involved in H2S production at the cellular level. This differential cellular expression indicates that the specific roles of H2S and GSH in neuroprotection, antioxidant generation, metabolism detoxification and neurotransmitter production may be cell-specific and not uniform throughout the retina.
The increased CBS labeling with aging in canine retinas found in this study suggest that CBS expression is age dependent. Similarly, Persa et al reported a correlation between CBS expression and senescence in human retina (Persa et al., 2006). In contrast to CBS, CSE levels did not increase with aging. The increased levels of CBS are consistent with its role as a key enzyme in homocysteine metabolism, as homocysteine levels also increase with age (reviewed by Ansari (Ansari et al., 2014) and can cause retinal cell death (Ganapathy et al., 2009). As CBS can actively produce H2S in retina (Markand et al., 2013; Persa et al., 2006) and brain (Kamat et al., 2015), the age-dependent increase in CBS may link to the antioxidant activity of H2S in the retina (George et al., 2018; Sakamoto et al., 2014) and highlight an active role for CBS in H2S production compared to CSE. Another explanation for the increased levels of CBS by aging could be because its role in iron hemostasis (Zhou et al., 2018) as increased levels of iron is linked to retinal diseases (Chen et al., 2009) and iron chelation is shown to prevent iron-induced retinal degenerative diseases (Song et al., 2014). We are also interested in studying the role of CBS and CSE in age- and disease-dependent human retinal degeneration. As a first step, we determined the localization and expression of these enzymes in human retina and NHP retinas. Due to limited access to retina samples we were unable to perform CBS and CSE enzyme activity to confirm H2S production in canine retina. However, CBS enzyme activity in mice (Markand et al., 2013), human and pig retina (Persa et al., 2006) shows a close correlation between protein expression and its enzyme activity, an indication of the role of CBS in generating of H2S in the retina. These findings propose a prominent role for CBS in homeostasis of antioxidant levels with aging suggesting a protective role for it against increased oxidative damage with aging.
The retina is extensively exposed to light and high oxygen tension and its photoreceptors are rich in polyunsaturated fatty acids, both of which make the neuroretina vulnerable to oxidative damage leading to mitochondrial dysfunction and mitochondrial DNA damage. These alterations can trigger apoptosis (Bonnel et al., 2003; Schreier et al., 2010). Increased levels of ROS may account for the increase in oxidative damage in cells associated with aging and pathology of retinal diseases (Ansari et al., 2014; Ayala et al., 2014; Cui et al., 2012; Davalli et al., 2016; Ganapathy et al., 2009; George et al., 2018). The expression of the transsulfuration pathway enzymes, CBS and CSE in the retina suggest a potential protective role for these enzymes in retina redox hemostasis alteration (Mikami et al., 2011; Minamishima et al., 2009). H2S may play an anti-oxidative and neuroprotective activity in the retina (Huang et al., 2018; Sakamoto et al., 2014; Schreier et al., 2010). The role of the oxidative damage in retinal cells apoptosis in dogs is not reported yet. Further investigation on the effect of oxidative stress on mitochondrial function could illustrate the role of CBS in neuroprotection in the canine retina. Alternatively, our findings support a potential physiological role for H2S as a neuromodulator in the retina, a possibility which needs to be examined further to determine its therapeutic potential.
In conclusion, our results confirmed the expression of CBS and CSE transcripts in canine retina, and further show protein expression in canine, NHP and human retinas. This study also confirmed expression of the CBS in rods, amacrine, horizontal and NFL and CSE expression in RPE, cones and Müller cells. As such our findings suggest a potential role for CBS and CSE-dependent redox balance in the retina and support further studies to investigate the possible protective roles of CBS, CSE, and H2S in canine/human retinal degenerative diseases.
Highlights.
CBS and CSE protein express in canine, non-human primate and human
Each of CBS and CSE genes are expressed equally in different layers of canine retina
CBS and CSE proteins are localized in different layers of retina, different cellular compartment and not overlapping
CBS is localized in rod outer segment, horizontal, amacrine, and GCL/NFL
CSE is localized in RPE, cone and rod inner segment, and Mϋller cells
Acknowledgment:
The authors thank RDS facility staff for their assistance with providing archived retina samples used in this study. We are also grateful to Dr. William Beltran for NHP retina samples, Dr. Leslie King for editorial advice and helpful suggestions, Lydia Melnyk for research coordination and Gordon Ruthel for technical assistance in Penn Vet Imaging Core. This research is supported by an NEI/NIH EY-06855 and -17549, Foundation Fighting Blindness (FFB), Van Sloun Fund for Canine Genetic Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ansari R, Mahta A, Mallack E, Luo JJ, 2014. Hyperhomocysteinemia and neurologic disorders: a review. J Clin Neurol 10, 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala A, Munoz MF, Arguelles S, 2014. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014, 360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehr W, Frederick JM, 2009. Naturally occurring animal models with outer retina phenotypes. Vision Res 49, 2636–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden SE, Beard RS Jr., Pfau JC, 2010. Extracellular transsulfuration generates hydrogen sulfide from homocysteine and protects endothelium from redox stress. Am J Physiol Heart Circ Physiol 299, H1568–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Saha S, Giri K, Lanza IR, Nair KS, Jennings NB, Rodriguez-Aguayo C, Lopez-Berestein G, Basal E, Weaver AL, Visscher DW, Cliby W, Sood AK, Bhattacharya R, Mukherjee P, 2013. Cystathionine beta-synthase (CBS) contributes to advanced ovarian cancer progression and drug resistance. PLoS One 8, e79167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnel S, Mohand-Said S, Sahel JA, 2003. The aging of the retina. Exp Gerontol 38, 825–831. [DOI] [PubMed] [Google Scholar]
- Bukovska G, Kery V, Kraus JP, 1994. Expression of human cystathionine beta-synthase in Escherichia coli: purification and characterization. Protein expression and purification 5, 442–448. [DOI] [PubMed] [Google Scholar]
- Chen H, Lukas TJ, Du N, Suyeoka G, Neufeld AH, 2009. Dysfunction of the retinal pigment epithelium with age: increased iron decreases phagocytosis and lysosomal activity. Invest Ophthalmol Vis Sci 50, 1895–1902. [DOI] [PubMed] [Google Scholar]
- Cideciyan AV, Sudharsan R, Dufour VL, Massengill MT, Iwabe S, Swider M, Lisi B, Sumaroka A, Marinho LF, Appelbaum T, Rossmiller B, Hauswirth WW, Jacobson SG, Lewin AS, Aguirre GD, Beltran WA, 2018. Mutation-independent rhodopsin gene therapy by knockdown and replacement with a single AAV vector. Proc Natl Acad Sci U S A 115, E8547–E8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Kong Y, Zhang H, 2012. Oxidative stress, mitochondrial dysfunction, and aging. J Signal Transduct 2012, 646354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalli P, Mitic T, Caporali A, Lauriola A, D’Arca D, 2016. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid Med Cell Longev 2016, 3565127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Jin H, Yang L, 2017. Role of Hydrogen Sulfide in Retinal Diseases. Front Pharmacol 8, 588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enokido Y, Suzuki E, Iwasawa K, Namekata K, Okazawa H, Kimura H, 2005. Cystathionine beta-synthase, a key enzyme for homocysteine metabolism, is preferentially expressed in the radial glia/astrocyte lineage of developing mouse CNS. FASEB J 19, 1854–1856. [DOI] [PubMed] [Google Scholar]
- Erickson PF, Maxwell IH, Su LJ, Baumann M, Glode LM, 1990. Sequence of cDNA for rat cystathionine gamma-lyase and comparison of deduced amino acid sequence with related Escherichia coli enzymes. The Biochemical journal 269, 335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto K, Asada T, Arima K, Makifuchi T, Kimura H, 2002. Brain hydrogen sulfide is severely decreased in Alzheimer’s disease. Biochem Biophys Res Commun 293, 1485–1488. [DOI] [PubMed] [Google Scholar]
- Finkelstein JD, 1990. Methionine metabolism in mammals. J Nutr Biochem 1, 228–237. [DOI] [PubMed] [Google Scholar]
- Freitas HR, Ferraz G, Ferreira GC, Ribeiro-Resende VT, Chiarini LB, do Nascimento JL, Matos Oliveira KR, Pereira Tde L, Ferreira LG, Kubrusly RC, Faria RX, Herculano AM, Reis RA, 2016. Glutathione-Induced Calcium Shifts in Chick Retinal Glial Cells. PLoS One 11, e0153677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas HR, Reis RA, 2017. Glutathione induces GABA release through P2X7R activation on Muller glia. Neurogenesis (Austin) 4, e1283188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganapathy PS, Moister B, Roon P, Mysona BA, Duplantier J, Dun Y, Moister TK, Farley MJ, Prasad PD, Liu K, Smith SB, 2009. Endogenous elevation of homocysteine induces retinal neuron death in the cystathionine-beta-synthase mutant mouse. Invest Ophthalmol Vis Sci 50, 4460–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George AK, Singh M, Homme RP, Majumder A, Sandhu HS, Tyagi SC, 2018. A hypothesis for treating inflammation and oxidative stress with hydrogen sulfide during age-related macular degeneration. Int J Ophthalmol 11, 881–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guziewicz KE, McTish E, Dufour VL, Zorych K, Dhingra A, Boesze-Battaglia K, Aguirre GD, 2018. Underdeveloped RPE Apical Domain Underlies Lesion Formation in Canine Bestrophinopathies. Adv Exp Med Biol 1074, 309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauzman E, Bonci DMO, Suarez-Villota EY, Neitz M, Ventura DF, 2017. Daily activity patterns influence retinal morphology, signatures of selection, and spectral tuning of opsin genes in colubrid snakes. BMC Evol Biol 17, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson PW, Weinstein AL, Sung J, Singh SP, Nagineni V, Spector JA, 2010. Hydrogen sulfide attenuates ischemia-reperfusion injury in in vitro and in vivo models of intestine free tissue transfer. Plastic and reconstructive surgery 125, 1670–1678. [DOI] [PubMed] [Google Scholar]
- Hosoki R, Matsuki N, Kimura H, 1997. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochemical and biophysical research communications 237, 527–531. [DOI] [PubMed] [Google Scholar]
- Hu LF, Lu M, Tiong CX, Dawe GS, Hu G, Bian JS, 2010. Neuroprotective effects of hydrogen sulfide on Parkinson’s disease rat models. Aging Cell 9, 135–146. [DOI] [PubMed] [Google Scholar]
- Huang S, Huang P, Lin Z, Liu X, Xu X, Guo L, Shen X, Li C, Zhong Y, 2018. Hydrogen sulfide supplement attenuates the apoptosis of retinal ganglion cells in experimental glaucoma. Exp Eye Res 168, 33–48. [DOI] [PubMed] [Google Scholar]
- Jacobson SG, Cideciyan AV, Aguirre GD, Roman AJ, Sumaroka A, Hauswirth WW, Palczewski K, 2015. Improvement in vision: a new goal for treatment of hereditary retinal degenerations. Expert Opin Orphan Drugs 3, 563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhee KH, Kruger WD, 2005. The role of cystathionine beta-synthase in homocysteine metabolism. Antioxid Redox Signal 7, 813–822. [DOI] [PubMed] [Google Scholar]
- Kamat PK, Kalani A, Tyagi N, 2015. Role of hydrogen sulfide in brain synaptic remodeling. Methods Enzymol 555, 207–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Goto Y, Kimura H, 2010. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal 12, 1–13. [DOI] [PubMed] [Google Scholar]
- Kondo M, Das G, Imai R, Santana E, Nakashita T, Imawaka M, Ueda K, Ohtsuka H, Sakai K, Aihara T, Kato K, Sugimoto M, Ueno S, Nishizawa Y, Aguirre GD, Miyadera K, 2015. A Naturally Occurring Canine Model of Autosomal Recessive Congenital Stationary Night Blindness. PLoS One 10, e0137072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong YX, van Bergen N, Bui BV, Chrysostomou V, Vingrys AJ, Trounce IA, Crowston JG, 2012. Impact of aging and diet restriction on retinal function during and after acute intraocular pressure injury. Neurobiol Aging 33, 1126 e1115–1125. [DOI] [PubMed] [Google Scholar]
- Lee M, Schwab C, Yu S, McGeer E, McGeer PL, 2009. Astrocytes produce the antiinflammatory and neuroprotective agent hydrogen sulfide. Neurobiol Aging 30, 1523–1534. [DOI] [PubMed] [Google Scholar]
- Levonen AL, Lapatto R, Saksela M, Raivio KO, 2000. Human cystathionine gamma-lyase: developmental and in vitro expression of two isoforms. The Biochemical journal 347 Pt 1, 291–295. [PMC free article] [PubMed] [Google Scholar]
- Liu H, Anders F, Thanos S, Mann C, Liu A, Grus FH, Pfeiffer N, Prokosch-Willing V, 2017. Hydrogen Sulfide Protects Retinal Ganglion Cells Against Glaucomatous Injury In Vitro and In Vivo. Invest Ophthalmol Vis Sci 58, 5129–5141. [DOI] [PubMed] [Google Scholar]
- Locksley RM, Killeen N, Lenardo MJ, 2001. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104, 487–501. [DOI] [PubMed] [Google Scholar]
- Lu Y, O’Dowd BF, Orrego H, Israel Y, 1992. Cloning and nucleotide sequence of human liver cDNA encoding for cystathionine gamma-lyase. Biochemical and biophysical research communications 189, 749–758. [DOI] [PubMed] [Google Scholar]
- Markand S, Tawfik A, Ha Y, Gnana-Prakasam J, Sonne S, Ganapathy V, Sen N, Xian M, Smith SB, 2013. Cystathionine beta synthase expression in mouse retina. Curr Eye Res 38, 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland RH, 2012. The neuronal organization of the retina. Neuron 76, 266–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier M, Janosik M, Kery V, Kraus JP, Burkhard P, 2001. Structure of human cystathionine beta-synthase: a unique pyridoxal 5’-phosphate-dependent heme protein. The EMBO journal 20, 3910–3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami Y, Shibuya N, Kimura Y, Nagahara N, Yamada M, Kimura H, 2011. Hydrogen sulfide protects the retina from light-induced degeneration by the modulation of Ca2+ influx. J Biol Chem 286, 39379–39386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamishima S, Bougaki M, Sips PY, Yu JD, Minamishima YA, Elrod JW, Lefer DJ, Bloch KD, Ichinose F, 2009. Hydrogen sulfide improves survival after cardiac arrest and cardiopulmonary resuscitation via a nitric oxide synthase 3-dependent mechanism in mice. Circulation 120, 888–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyadera K, Acland GM, Aguirre GD, 2012. Genetic and phenotypic variations of inherited retinal diseases in dogs: the power of within- and across-breed studies. Mamm Genome 23, 40–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persa C, Osmotherly K, Chao-Wei Chen K, Moon S, Lou MF, 2006. The distribution of cystathionine beta-synthase (CBS) in the eye: implication of the presence of a trans-sulfuration pathway for oxidative stress defense. Exp Eye Res 83, 817–823. [DOI] [PubMed] [Google Scholar]
- Pong WW, Stouracova R, Frank N, Kraus JP, Eldred WD, 2007. Comparative localization of cystathionine beta-synthase and cystathionine gamma-lyase in retina: differences between amphibians and mammals. J Comp Neurol 505, 158–165. [DOI] [PubMed] [Google Scholar]
- Portera-Cailliau C, Sung CH, Nathans J, Adler R, 1994. Apoptotic photoreceptor cell death in mouse models of retinitis pigmentosa. Proc Natl Acad Sci U S A 91, 974–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puschina E.V.a.V., A.A, 2011. Hydrogen Sulfide-, Parvalbumin-, and GABA-Producing Systems in the Masu Salmon Brain. Nuerophysiology 43. [Google Scholar]
- Reid DW, Nicchitta CV, 2012. Primary role for endoplasmic reticulum-bound ribosomes in cellular translation identified by ribosome profiling. J Biol Chem 287, 5518–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh YJ, Moon C, Kim SY, Park MH, Bae YC, Chun MH, Moon JI, 2007. Glutathione depletion induces differential apoptosis in cells of mouse retina, in vivo. Neurosci Lett 417, 266–270. [DOI] [PubMed] [Google Scholar]
- Roski C, Langrock C, Korber N, Habermann G, Buse E, Reichenbach A, Pannicke T, Francke M, 2018. Comparison of cellular localisation of the Ca(2+) -binding proteins calbindin, calretinin and parvalbumin in the retina of four different Macaca species. Anat Histol Embryol [DOI] [PubMed]
- Sakamoto K, Suzuki Y, Kurauchi Y, Mori A, Nakahara T, Ishii K, 2014. Hydrogen sulfide attenuates NMDA-induced neuronal injury via its anti-oxidative activity in the rat retina. Exp Eye Res 120, 90–96. [DOI] [PubMed] [Google Scholar]
- Schreier SM, Muellner MK, Steinkellner H, Hermann M, Esterbauer H, Exner M, Gmeiner BM, Kapiotis S, Laggner H, 2010. Hydrogen sulfide scavenges the cytotoxic lipid oxidation product 4-HNE. Neurotox Res 17, 249–256. [DOI] [PubMed] [Google Scholar]
- Schubert T, Huckfeldt RM, Parker E, Campbell JE, Wong RO, 2010. Assembly of the outer retina in the absence of GABA synthesis in horizontal cells. Neural Dev 5, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RK, Kolandaivelu S, Ramamurthy V, 2014. Early alteration of retinal neurons in Aipl1−/−animals. Invest Ophthalmol Vis Sci 55, 3081–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone K, Mori A, Sakamoto K, Nakahara T, 2018. GYY4137, an Extended-Release Hydrogen Sulfide Donor, Reduces NMDA-Induced Neuronal Injury in the Murine Retina. Biological & pharmaceutical bulletin 41, 657–660. [DOI] [PubMed] [Google Scholar]
- Song D, Zhao L, Li Y, Hadziahmetovic M, Song Y, Connelly J, Spino M, Dunaief JL, 2014. The oral iron chelator deferiprone protects against systemic iron overload-induced retinal degeneration in hepcidin knockout mice. Invest Ophthalmol Vis Sci 55, 4525–4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipanuk MH, Beck PW, 1982. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. The Biochemical journal 206, 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudharsan R, Simone KM, Anderson NP, Aguirre GD, Beltran WA, 2017. Acute and Protracted Cell Death in Light-Induced Retinal Degeneration in the Canine Model of Rhodopsin Autosomal Dominant Retinitis Pigmentosa. Invest Ophthalmol Vis Sci 58, 270–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Molen EF, Hiipakka MJ, van Lith-Zanders H, Boers GH, van den Heuvel LP, Monnens LA, Blom HJ, 1997. Homocysteine metabolism in endothelial cells of a patient homozygous for cystathionine beta-synthase (CS) deficiency. Thrombosis and haemostasis 78, 827–833. [PubMed] [Google Scholar]
- Vecino E, Rodriguez FD, Ruzafa N, Pereiro X, Sharma SC, 2016. Glia-neuron interactions in the mammalian retina. Prog Retin Eye Res 51, 1–40. [DOI] [PubMed] [Google Scholar]
- Vitvitsky V, Thomas M, Ghorpade A, Gendelman HE, Banerjee R, 2006. A functional transsulfuration pathway in the brain links to glutathione homeostasis. J Biol Chem 281, 35785–35793. [DOI] [PubMed] [Google Scholar]
- Wang C, Youle RJ, 2009. The role of mitochondria in apoptosis*. Annu Rev Genet 43, 95–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AF, Chakarova CF, Abd El-Aziz MM, Bhattacharya SS, 2010. Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. Nat Rev Genet 11, 273–284. [DOI] [PubMed] [Google Scholar]
- Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R, 2008. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science 322, 587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YF, Wu XM, Zhou G, Mu MD, Zhang FL, Li FM, Qian C, Du F, Yung WH, Qian ZM, Ke Y, 2018. Cystathionine beta-synthase is required for body iron homeostasis. Hepatology 67, 21–35. [DOI] [PubMed] [Google Scholar]