Abstract
Exacerbation of liver enzymes after the initiation of feeding in malnourished patients is caused by refeeding syndrome or persistent starvation. There are no definite clinical markers for distinguishing between the two conditions. We herein report a 63-year-old woman with starvation-induced liver enzyme elevation. Her body weight was inversely associated with the liver enzyme levels after refeeding, which was a different course from refeeding syndrome. Normalization of liver enzymes ensued as the caloric intake increased and weight gain progressed. Daily changes in body weight can be a useful clinical marker for distinguishing between refeeding syndrome and starvation-induced liver enzyme elevation.
Keywords: starvation, refeeding syndrome, liver enzyme, body weight, resting energy expenditure
Introduction
After the initiation of refeeding therapy in patients with severe malnutrition, the elevation of serum liver enzyme levels is sometimes observed (1-6). Although most cases are caused by refeeding syndrome (1-3), persistent starvation can also cause an exacerbation of liver enzyme levels (4-6). Differentiating between the two conditions is important, as the treatment plans are quite different. Refeeding syndrome is defined as the potentially fatal condition that can occur as a result of a shift in fluids and electrolytes caused by the rapid initiation of refeeding after a period of malnutrition (1). Patients with refeeding syndrome often show hypophosphatemia, hypokalemia, vitamin deficiencies, congestive heart failure, rhabdomyolysis, and seizures. Liver enzyme elevation, which has been associated with hepatic steatosis, also often develops (2). A reduction in the amount of calories and a change in the nutrient composition may be required. However, only a few studies have been conducted on starvation-induced liver enzyme elevation after the initiation of refeeding (4-6). The mechanism underlying starvation-induced liver enzyme elevation has not been clarified. Suggested causes include acute hypoperfusion (7), low glutathione levels with resultant oxidant stress (8), and hepatocyte autophagy (9). In such patients, the caloric intake should be gradually increased.
Differentiating between refeeding syndrome and starvation-induced liver enzyme elevation is often challenging as there are no clear clinical criteria. We herein report a case of starvation-induced liver enzyme elevation that worsened after the initiation of feeding therapy and was successfully managed through focusing on daily changes in body weight.
Case Report
A 63-year-old woman was referred to our hospital because of elevated serum liver enzyme levels. She had been eating small meals for the past six years, and the amount of the calories had markedly decreased for no obvious reason two months earlier. The food she consumed included items such as bread, cheese, raisins, walnuts, and tofu, with a calorie amount of <400 kcal/day. When the patient started feeling numb in her extremities, she consulted her family physician and underwent a blood examination, which showed the following: aspartate aminotransferase (AST), 634 IU/L; alanine aminotransferase (ALT), 462 IU/L; lactate dehydrogenase (LDH), 769 IU/L; alkaline phosphatase, 764 IU/L; and γ-glutamyltransferase, 218 IU/L. She had also been taking over-the-counter vitamin B1, B6, and B12 supplements. The patient reported being a non-smoker, did not consume alcohol, and had no remarkable medical history.
On a physical examination, she was conscious and alert. Her blood pressure was 102/54 mmHg, pulse rate 64 beats/min, respiratory rate 12 breaths/min, and body temperature 36.4°C. Her height was 161 cm, and her body weight was 29.0 kg, with a body mass index of 11.2 kg/m2. The patient's breath and heart sounds were normal, and the abdomen was soft and flat. Neurological findings were unremarkable. She had a white blood cell count of 5,600 /μL with 85% neutrophils, hemoglobin level of 14.1 g/dL, platelet count of 19.8×104/μL, total protein of 5.5 g/dL, albumin of 3.3 g/dL, AST of 267 IU/L, ALT of 338 IU/L, LDH of 557 IU/L, alkaline phosphatase of 797 IU/L, γ-glutamyltransferase of 227 IU/L, transthyretin of 8 mg/dL, transferrin of 1.3 mg/dL, retinol-binding protein of 0.7 mg/dL, vitamin B1 of 3.0 μg/dL, vitamin B12 of >1,500 pg/mL, and C-reactive protein level of 0.08 mg/dL. Serologic tests for hepatitis B and C viruses and anti-mitochondrial antibody were negative (Table). Abdominal ultrasonography showed no abnormal findings. Her resting energy expenditure, as measured by indirect calorimetry, was 654 kcal/day. We suspected that she had severe malnutrition due to an eating disorder and was at high risk of developing refeeding syndrome.
Table.
Laboratory Findings on Admission.
| Complete blood count | Serology | |||||||
| WBC | 5,600 | /μL | HBsAg | (-) | ||||
| Neutrophils | 85.0 | % | HCVAb | (-) | ||||
| Lymphocytes | 11.0 | % | CMV IgG | (+) | ||||
| Hemoglobin | 14.1 | g/dL | CMV IgM | (-) | ||||
| Hematocrit | 39.4 | % | ANA | <40 | ||||
| Platelet | 19.8×104 | /μL | AMA | <20 | ||||
| PT-% | 82.1 | % | ||||||
| Biochemistry | ||||||||
| Albumin | 3.3 | g/dL | TSH | 3.95 | µU/mL | |||
| T-Bil | 0.9 | mg/dL | Free T4 | 1.09 | ng/dL | |||
| AST | 267 | IU/L | IgG | 666 | mg/dL | |||
| ALT | 338 | IU/L | IgM | 135 | mg/dL | |||
| LD | 557 | IU/L | Ferritin | 2,750.0 | ng/mL | |||
| ALP | 797 | IU/L | Fe | 150 | µg/dL | |||
| γ-GTP | 227 | IU/L | UIBC | 5 | µg/dL | |||
| CK | 244 | IU/L | Transthyretin | 8 | mg/dL | |||
| BUN | 11.1 | mg/dL | Transferrin | 113 | mg/dL | |||
| Creatinine | 0.41 | mg/dL | Retionol-binding protein | 0.7 | mg/dL | |||
| Glucose | 66 | mg/mL | ||||||
WBC: white blood cell, PT: prothrombin time, T-Bil: total bilirubin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LD: lactate dehydrogenase, ALP: alkaline phosphatase, γ-GTP: γ-glutamyltransferase, CK: creatine kinase, BUN: blood urea nitrogen, HBsAg: hepatitis B surface antigen, HCVAb: hepatitis C antibody, CMV: cytomegalovirus, ANA: anti-nuclear antibody, AMA: anit-mitochodrial antibody, TSH: thyroid stimulating hormone, T4: thyroxine, UIBC: unsaturated iron binding capacity
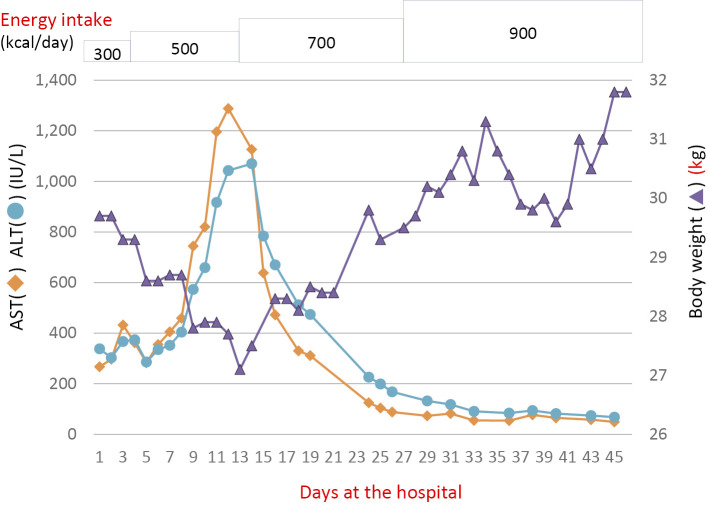
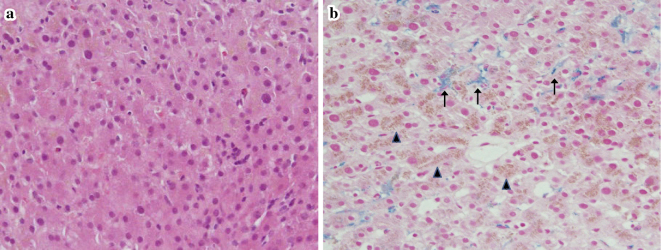
Supplemental enteral nutrition with meals of 300 kcal/day was initiated; the calorie amount was increased to 500 kcal/day on day 4. Vitamin B1 100 mg was also administered intravenously. Although the patient was asymptomatic, her serum AST and ALT levels started to worsen on day 8 (Fig. 1), raising concerns of refeeding syndrome-related exacerbation of liver enzymes. However, there was no clinical evidence of refeeding syndrome, and her serum electrolytes remained stable. On day 12, the AST and ALT levels reached 1,288 IU/L and 1,043 IU/L, respectively, and the patient's body weight decreased to 27.1 kg. Repeat abdominal ultrasonography showed a normal liver. On the same day, a liver biopsy was performed. Histology revealed swelling of the hepatocytes (Fig. 2a) and iron as well as lipofuscin deposition in the hepatocytes (Fig. 2b). However, no steatosis or glucose deposition in the hepatocytes was observed.
Figure 1.
The clinical course of the patient. ALT: alanine aminotransferase, AST: aspartate aminotransferase
Figure 2.
Liver histology. (a) Hematoxylin and Eosin staining of the liver revealed ballooning of the hepatocytes and increased brown pigment. (b) Berlin blue and Periodic acid-Schiff staining showed increased hemosiderin (arrows) and lipofuscin in the hepatocytes (arrowheads) (low-power field, ×100).
We suspected that the elevated serum liver enzyme levels were caused by starvation and not by refeeding syndrome. Therefore, the amount of enteral calories was increased to 700 kcal/day from day 13 onwards for a duration of 14 days, followed by 900 kcal/day for 18 days. Subsequently, the patient's liver enzyme levels exhibited a downward trend and returned to nearly normal levels on day 45 (Fig. 1). Her body weight increased concomitant with the calorie increase and reached 31.8 kg on day 45. She was discharged on day 46 and has since been followed by her family physician.
Discussion
The findings of this case suggest two important clinical issues. First, a change in body weight can be a clinical marker that distinguishes between refeeding syndrome and starvation-induced liver enzyme elevation that develops after the initiation of refeeding. Second, the amount of calories near the resting energy expenditure can be safely administered at the initiation of feeding therapy in patients with starvation-induced liver enzyme elevation.
In the present case, the patient's body weight showed an inverse association with the liver enzyme levels. After the initiation of refeeding therapy, her liver enzyme levels increased, and her body weight decreased. The maximum elevation of the liver enzymes was seen when her body weight was lowest. Continued feeding with controlled enteral supplementation eventually led to a decline in the liver enzyme levels alongside an increase in body weight. Only a few reports have described an association between the body weight and liver enzyme elevation associated with prolonged starvation (3,5,6). These previous cases showed a near normalization of liver enzymes associated with weight gain, which is consistent with the findings of our case. In contrast, in refeeding syndrome, liver enzyme elevation was seen when the patients were gaining body weight after the initiation of refeeding therapy (3). Although the etiological relationship between the body weight and liver enzyme levels is not fully understood, the present case suggests that a change in body weight can be a clinical marker that distinguishes between refeeding syndrome and starvation-induced liver enzyme elevation.
In patients with starvation-induced liver enzyme elevation after initiation of feeding, the ideal nutritional management may be a rapid increase in the caloric intake (3-6). However, how many calories are adequate and can be administered safely at the beginning of feeding therapy in such patients has not yet been clarified. In the present patient, both the body weight and the liver enzyme abnormalities began to improve when the caloric intake slightly exceeded her resting energy expenditure. The resting energy expenditure represents the amount of energy expended by a person at rest, which is measured by indirect calorimetry in a recumbent person (10). The present case suggests that, in patients with starvation-induced liver enzyme elevation, the amount of calories near the resting energy expenditure can be safely administered initially and promote an improvement in their condition. However, when refeeding syndrome is suspected, the calorie intake should be kept low initially, such as around 5 kcal/kg/day (2,11).
Regarding the histological findings of the liver, the hallmark of refeeding syndrome-related liver enzyme elevation is hepatic fat and glucose deposition, known as steatosis (4,12); this was not observed in the present case, thus making refeeding syndrome-related liver enzyme elevation unlikely. Unfortunately, only a few reports on the liver histology in patients with starvation-induced liver enzyme elevation have been published. Increased lipofuscin deposition, as was seen in our case, has been reported in cases of anorexia nervosa (9); however, this may be a common finding in liver biopsies and offers little diagnostic information. Increased iron deposition, as was seen in this case, has also been described in cases of anorexia nervosa (9,13) and other forms of starvation (14). This is probably because protein deficiency causes a decrease in the substrate for hemoglobin synthesis and a reduced demand for iron in the body, leading to the accumulation of unused iron in the liver. In the present case, the histological findings of the liver suggest that the liver enzyme elevation was caused by starvation.
Liver ultrasonography may be helpful for distinguishing refeeding syndrome from starvation-induced liver enzyme elevation. If the liver is large and shows high echogenicity on ultrasonography, refeeding syndrome should be suspected. If the liver is small or normal in size and shows normal echogenicity, starvation should be suspected (15). Furthermore, the liver enzyme elevation seen in patients with refeeding syndrome tends to be lower than that seen in patients with severe malnutrition (3,16). It has been shown that the extent of AST and ALT elevation before effective feeding was greater in individuals with severe malnutrition (AST, ALT>1,000 U/L) than in those with refeeding syndrome (AST, ALT<500). These findings also support the notion that the liver enzyme elevation after the initiation of feeding was caused by persistent starvation in our case.
In the present case, for the detailed differential diagnosis of liver enzyme elevation and nutritional assessment, it might have been better to check more items, such as antibodies for hepatitis A and E viruses, free fatty acid, choline esterase, and carnitine.
In conclusion, when elevated serum liver enzyme levels are observed after feeding in patients presenting with malnutrition, the differential diagnoses should include refeeding syndrome and persistent starvation requiring urgent caloric repletion. A change in body weight after the initiation of feeding therapy can be a clinical marker that can help differentiate between the two conditions.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Solomon SM, Kirby DF. The refeeding syndrome: a review. JPEN J Parenter Enteral Nutr 14: 90-97, 1990. [DOI] [PubMed] [Google Scholar]
- 2. Mehanna HM, Moledina J, Travis J. Refeeding syndrome: what it is, and how to prevent and treat it. BMJ 336: 1495-1498, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ozawa Y, Shimizu T, Shishiba Y. Elevation of serum aminotransferase as a sign of multiorgan-disorders in severely emaciated anorexia nervosa. Intern Med 37: 32-39, 1998. [DOI] [PubMed] [Google Scholar]
- 4. Sakurai-Chin C, Ito N, Taguchi M, Miyakawa M, Takeshita A, Takeuchi Y. Hypoglycemic coma in a patient with anorexia nervosa coincident with acute exacerbation of liver injury induced by oral intake of nutrients. Intern Med 49: 1553-1556, 2010. [DOI] [PubMed] [Google Scholar]
- 5. Narayanan V, Gaudiani JL, Harris RH, Mehler PS. Liver function test abnormalities in anorexia nervosa--cause or effect. Int J Eat Disord 43: 378-381, 2010. [DOI] [PubMed] [Google Scholar]
- 6. Harris RH, Sasson G, Mehler PS. Elevation of liver function tests in severe anorexia nervosa. Int J Eat Disord 46: 369-374, 2013. [DOI] [PubMed] [Google Scholar]
- 7. De Caprio C, Alfano A, Senatore I, Zarrella L, Pasanisi F, Contaldo F. Severe acute liver damage in anorexia nervosa: two case reports. Nutrition 22: 572-575, 2006. [DOI] [PubMed] [Google Scholar]
- 8. Zenger F, Russmann S, Junker E, Wüthrich C, Bui MH, Lauterburg BH. Decreased glutathione in patients with anorexia nervosa. Risk factor for toxic liver injury? Eur J Clin Nutr 58: 238-243, 2004. [DOI] [PubMed] [Google Scholar]
- 9. Rautou PE, Cazals-Hatem D, Moreau R, Francoz C, Feldmann G, Lebrec D, et al. Acute liver cell damage in patients with anorexia nervosa: a possible role of starvation-induced hepatocyte autophagy. Gastroenterology 135: 840-848, 2008. [DOI] [PubMed] [Google Scholar]
- 10. Blasco Redondo R. Resting energy expenditure; assessment methods and applications. Nutr Hosp 31 (Suppl): 245-254, 2015. [DOI] [PubMed] [Google Scholar]
- 11.Nutrition Support for Adults: Oral Nutrition Support, Enteral Tube Feeding and Parenteral Nutrition. CG32, 2006 [Internet]. [cited 2018 June 6]. Available from: http://guidance.nice.org.uk/CG32 [Google Scholar]
- 12. Tajiri K, Shimizu Y, Tsuneyama K, Sugiyama T. A case report of oxidative stress in a patient with anorexia nervosa. Int J Eat Disord 39: 616-618, 2006. [DOI] [PubMed] [Google Scholar]
- 13. Hunt DP, Becker AE, Guimaraes AR, Stemmer-Rachamimov A, Misdraji J. Case records of the Massachusetts General Hospital. Case 21-2012. A 27-year-old man with fatigue, weakness, weight loss, and decreased libido. N Engl J Med 367: 157-169, 2012. [DOI] [PubMed] [Google Scholar]
- 14. Vootla VR, Daniel M. Abnormal liver function tests in an anorexia nervosa patient and an atypical manifestation of refeeding syndrome. Case Rep Gastroenterol 9: 261-265, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mickley D, Greenfeld D, Quinlan DM, Roloff P, Zwas F. Abnormal liver enzymes in outpatients with eating disorders. Int J Eat Disord 20: 325-329, 1996. [DOI] [PubMed] [Google Scholar]
- 16. Korbonits M, Blaine D, Elia M, Powell-Tuck J. Metabolic and hormonal changes during the refeeding period of prolonged fasting. Eur J Endocrinol 157: 157-166, 2007. [DOI] [PubMed] [Google Scholar]