Abstract
Objective
Rectal lymphoid follicular aphthous (LFA) lesions are related to ulcerative colitis (UC) and can be initial lesions of UC. We investigated the clinical course and prognosis of rectal LFA lesions.
Methods
This is a retrospective analysis of the clinical records at a single center.
Patients
Thirteen consecutive cases with LFA lesions treated at Hiroshima University Hospital between 1998 and 2015 were evaluated. Another 49 consecutive cases with ulcerative proctitis treated in the same period were enrolled as the control group. The clinical course and prognosis of both groups were evaluated.
Results
The group with LFA lesions included 9 women and 4 men with a median age of 39.9 years (range, 21-70 years). A total of 11 cases progressed to typical UC at 5-51 months. Proximal extension of these typical UC lesions was observed in 7 (53.8%) cases, which was significantly higher than in the control group (10 cases, 20.8%). Three cases (5-year accumulation incidence rate, 27.3%) progressed to steroid-intractable UC, a significantly higher incidence than that of the control group (3 cases; 5-year accumulation incidence rate, 6.9%).
Conclusion
Rectal LFA lesions frequently progress to typical UC with proximal extension, some of which become intractable to corticosteroid treatment.
Keywords: aphthous lesions, proctitis, proximal extension, ulcerative colitis
Introduction
Ulcerative colitis (UC) is a chronic inflammatory disorder of the colon characterized by colonic mucosal ulceration (1). In patients with UC, the inflamed mucosa starts at the anorectal verge and extends proximally. Endoscopic images show characteristically granular and erythematous appearance with friability and reduced vascular pattern. In moderate cases, multiple erosions and ulcers occur (1). In recent years, with remarkable progress in endoscopic technology and the popularization of colonoscopy, it has become possible to detect small lesions and some atypical lesions, such as inflamed lesion of the appendiceal orifice, skip lesions, and aphthous lesions (2). Rectal aphthous lesions with a lymphoid follicular elevated hemispherical appearance, so-called lymphoid follicular aphthous (LFA) lesions, are particularly rare, but several reports have shown that they sometimes progress to typical UC (3,4). However, the clinical course and prognosis of these cases remain undefined.
In the present study, we evaluated the clinical course and prognosis of cases with LFA lesions.
Materials and Methods
Study design and patients
This report was a retrospective single-center cohort study covering an 18-year period between January 1, 1998, and December 31, 2015. Thirteen consecutive cases with LFA lesions and 49 consecutive cases with ulcerative proctitis treated in Hiroshima University Hospital were evaluated. The diagnosis of UC was made according to the clinical symptoms (sustained or repeated hematochezia), characteristic endoscopic findings (faded/absent vascular pattern, fine granular appearance, vulnerability of the mucosa, and mucosal erosion/ulcer), and histological features compatible with UC (infiltrates of lymphocytes, plasma cells, and granulocytes; crypt abscesses; goblet cell depletion; and distorted crypt architecture). Ulcerative proctitis data were obtained from the medical and endoscopic records of all UC patients encountered during the period in our hospital. Ulcerative proctitis was diagnosed by colonoscopy with characteristic findings of UC only in the rectum, with no extended findings beyond the rectosigmoid junction, and the presence of histological features compatible with UC in only rectal specimens. Patients who did not undergo total colonoscopy (reached at cecum) during the diagnosis of ulcerative proctitis or follow-up colonoscopy to evaluate proximal extension were excluded.
The clinical activity was evaluated using a partial Mayo score (stool frequency, rectal bleeding, physician's global assessment). Clinical remission was defined as a partial Mayo score ≤2 and rectal bleeding score of 0; clinical efficacy was defined as reduction of the partial Mayo score by ≥2 after treatment; and a clinical response was defined as the achievement of clinical efficacy but not clinical remission.
Steroid-intractable UC was defined as steroid-resistant UC or steroid-dependent UC. Steroid-resistant UC was defined as unresponsiveness to oral or intravenous corticosteroid therapy (daily prednisolone dose of more than 1 mg/kg) over at least 1 week, and steroid-dependent UC was defined as relapse during the tapering of steroids.
Definition of rectal LFA
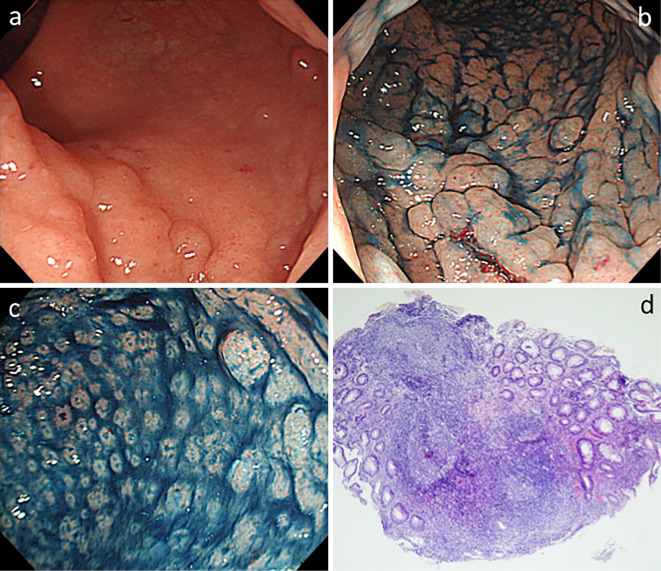
LFA was defined as 1) a dense granular lesion with erosion on its apex, 2) granule size of ≤5 mm, 3) exclusion of chlamydial proctitis (negative of serum IgA for Chlamydia trachomatis and/or polymerase chain reaction of rectal swab for C. trachomatis) and presence of extranodal marginal zone lymphoma of the mucosa-associated lymphoid tissue (MALT) lymphoma type, 4) exclusion of characteristic endoscopic findings of UC, and 5) pathological findings of acute inflammation (Fig. 1). All cases with LFA were diagnosed at Hiroshima University Hospital.
Figure 1.
Endoscopic and histological findings of LFA lesions. a: Endoscopic findings of LFA showing lymphoid follicles with an elevated hemispherical appearance (like salmon caviar) restricted to the rectum. b, c: Chromoendoscopic findings of LFA showing dense granular deposits with erosion on the apex. d: Histological appearance of the biopsy specimen showing acute inflammatory cells in the lamina propria of the mucosa, superficial ulceration, crypt distortion, and large lymphoid follicular hyperplasias. LFA: lymphoid follicular aphtha
The progression to typical UC was defined as flattening of LFA lesions and appearance of characteristic endoscopic findings of UC. Proximal extension was defined as inflammation extending proximally from proctitis to left-sided or extensive colitis.
Statistical analyses
Student's t-test for unpaired data and Fisher's exact test were used to compare the characteristics between the two groups. To evaluate the long-term prognosis, the survival times were evaluated using the Kaplan-Meier method, and statistical analyses were evaluated via the log-rank test. Fisher's exact test was used to evaluate the clinical responses to treatment. p values <0.05 were considered statistically significant.
Results
Clinical characteristics of LFA
The clinical characteristics of patients with LFA lesions are shown in Table 1. There were 9 women and 4 men, with an average age of 39.9 years (range, 21-70 years). All patients underwent total colonoscopy for bloody stool symptoms, and the lesions were only found in the rectum. In total, 12 cases were treated with 5-aminosalicylic acid (5-ASA): oral 5-ASA, 7 cases; oral and topical 5-ASA, 3 cases; and oral 5-ASA with topical steroid, 2 cases.
Table 1.
Clinical Characteristics of Cases with Lymphoid Follicular Aphthous Lesions.
| Case | Age* | Gender | Symptom | First treatment | Response to first treatment | Progress to typical UC |
Proximal extension |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| at 4 weeks | at 8 weeks | |||||||||||||||
| 1 | 24 | M | bloody stool, diarrhea | oral 5-ASA | not effective | remission | (+) | (+) | ||||||||
| 2 | 22 | F | bloody stool, nausea | oral & topical 5-ASA | remission | remission | (+) | (-) | ||||||||
| 3 | 24 | F | bloody stool | oral 5-ASA | not effective | not effective | (+) | (-) | ||||||||
| 4 | 61 | F | bloody stool | oral 5-ASA | remission | remission | (+) | (+) | ||||||||
| 5 | 59 | F | bloody stool | oral 5-ASA | not effective | remission | (+) | (+) | ||||||||
| 6 | 38 | F | bloody stool, diarrhea | oral 5-ASA | remission | remission | (+) | (+) | ||||||||
| 7 | 36 | M | bloody stool | oral 5-ASA & topical steroid | not effective | not effective | (+) | (+) | ||||||||
| 8 | 21 | F | bloody stool | oral & topical 5-ASA | responded | responded | (+) | (+) | ||||||||
| 9 | 31 | F | bloody stool | oral 5-ASA & topical steroid | not effective | responded | (+) | (+) | ||||||||
| 10 | 64 | M | bloody stool (temporal) | none | (-) | (-) | ||||||||||
| 11 | 38 | F | bloody stool | oral & topical 5-ASA | not effective | remission | (+) | (-) | ||||||||
| 12 | 70 | M | bloody stool | oral 5-ASA | remission | remission | (+) | (-) | ||||||||
| 13 | 31 | F | bloody stool | oral 5-ASA | not effective | not effective | (-) | (-) | ||||||||
*Age in years, M: male, F: female, UC: ulcerative colitis, 5-ASA: 5-aminosalicylic acid
LFA frequently progress to typical UC
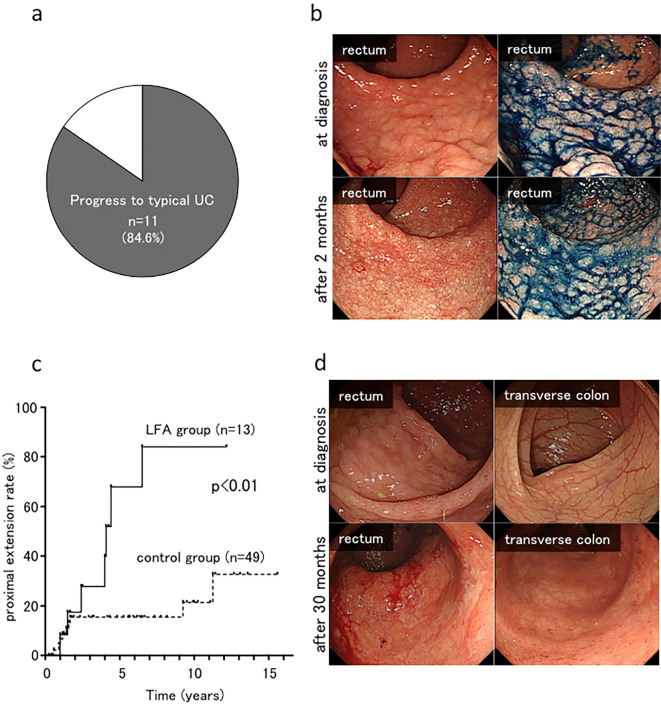
A total of 11 (84.6%) cases progressed to typical UC lesions at 2-51 months [mean±standard deviation (SD), 15.5±14.0 months] (Fig. 2a). In case 5, the LFA lesions became flattened, and typical UC lesions appeared after two months of treatment with oral 5-ASA (Fig. 2b).
Figure 2.
Progress to typical UC and the proximal extension of UC lesion in LFA cases. a, b: Proportion of cases with lesions that progressed to typical UC. In case 5, the lymphoid follicle with an elevated hemispherical appearance became flattened and typical of UC lesions after two months. c, d: The overall cumulative rate of proximal extension in the LFA group and the control (ulcerative proctitis) group. In case 9, proximal extension into the transverse colon was observed at 30 months after the diagnosis. LFA: lymphoid follicular aphtha, UC: ulcerative colitis
Proximal extension of UC lesions in cases with LFA
We compared the clinical characteristics of the proximal extension rate of rectal LFA lesions (LFA group) with those of ulcerative proctitis (control group). The clinical details are shown in Table 2. In the LFA group, 7 (53.8%) cases exhibited proximal extension of the UC lesion at 17-78 months (mean±SD, 42.7±21.3 months). In 5 (71.4%) of 7 cases, typical UC lesions of the rectum appeared before the appearance of proximal extension. In 2 (28.6%) of 7 cases, typical UC lesions of the rectum and proximal extension appeared at the same time as colonoscopy. In the control group, 10 (20.4%) cases had proximal extension at 3-135 months (mean±SD: 34.9±47.0 months). The overall cumulative rate of proximal extension in the LFA group was significantly higher than that of the control group (67.9% vs. 17.1% at 5 years and 84.0% vs. 23.0% at 10 years, respectively; p<0.01) (Fig. 2c, d). In the LFA group, 3 cases progressed to left-sided UC (Montreal classification of inflammatory bowel disease E2) (5), and 4 progressed to extensive UC (Montreal classification E3). In the control group, 5 cases progressed to left-sided UC (E2), and 5 progressed to extensive UC (E3).
Table 2.
Clinical Characteristics of Lymphoid Follicular Aphthous Lesions Group and Control Group.
| LFA group n=13 |
Control group n=49 |
p value | ||||
|---|---|---|---|---|---|---|
| Mean Age | 39.9 (21-70) | 41.7 (16-81) | n.s. | |||
| [average (range), y.o.] | ||||||
| Gender | ||||||
| Male | 4 | 26 | n.s. | |||
| Female | 9 | 23 | ||||
| Surveillance period | 82.2 (13-177) | 86.0 (8-203) | n.s. | |||
| [average (range), months] | ||||||
| Location of disease | ||||||
| (Montreal score) | ||||||
| Rectum (E1) | 13 | 49 | n.s. | |||
| Left-sided (E2) | 0 | 0 | ||||
| Pancolitis (E3) | 0 | 0 | ||||
| Apendiceal orifice inflammation | 2 | 9 | n.s. | |||
| pMayo score (mean±SD) | 3.4±1.6 | 4.7±1.8 | n.s. | |||
| First treatment | ||||||
| oral mesalazine | 12 | 46 | n.s. | |||
| topical mesalazine | 3 | 6 | ||||
| topical steroid | 2 | 10 | ||||
| non-therapy | 1 | 1 |
y.o.: year-old, pMayo score: partial Mayo score, n.s.: not significant, SD: standard deviation
Patients with LFA tended to have steroid-intractable UC
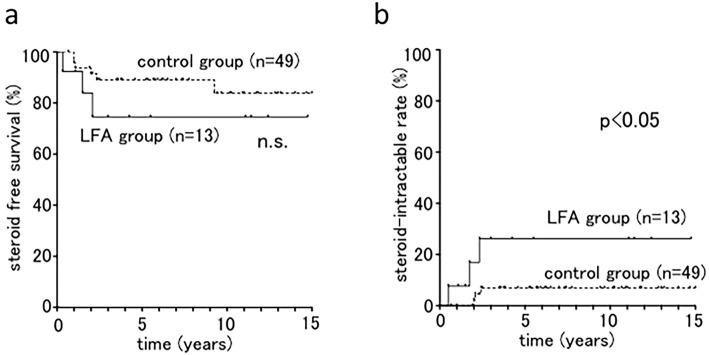
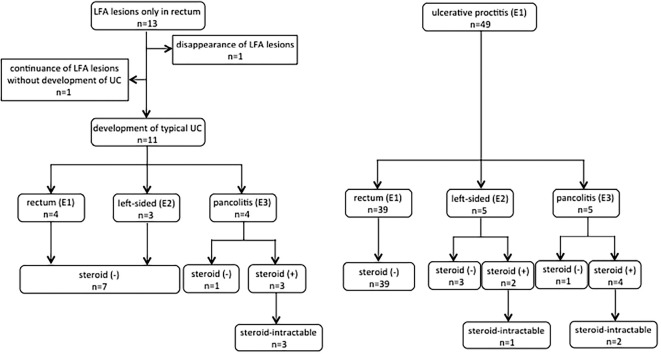
In both groups, the initial treatment for the majority of the cases was oral 5-ASA or oral 5-ASA with topical therapy (5-ASA or steroid). The steroid-free survival rates were 74.6% in the LFA group versus 88.1% in the control group at 5 years (Fig. 3a). In addition, 3 cases (5-year accumulation incidence rate, 26.2%) had steroid-intractable UC in the LFA group, which was a significantly higher frequency than in the control group (3 cases; 5-year accumulation incidence rate, 6.9%) (Fig. 3b). Cases in the LFA group were more likely to be intractable to steroid therapy. The clinical flowchart is shown in Fig. 4.
Figure 3.
Steroid treatment for LFA lesions and ulcerative proctitis. a: The steroid-free survival rate of the LFA and control (ulcerative proctitis) groups. b: The overall cumulative steroid-intractable rate of the LFA and control groups. LFA: lymphoid follicular aphtha
Figure 4.
Clinical flowchart of the LFA and control groups. LFA: lymphoid follicular aphtha
Treatment for steroid-intractable cases
In total, there were 3 cases each of steroid-intractable UC in the LFA and control groups. All cases showed proximal extension before becoming steroid-intractable. In the LFA group, one case was treated with anti-tumor necrosis factor α (TNFα) agents, and two cases were treated with tacrolimus when their lesions became steroid-intractable. Although these 2 cases were treated with tacrolimus and improved temporarily, their symptoms became exacerbated within 60 months after tacrolimus therapy was withdrawn. Subsequently, these two cases were treated with anti-TNFα agents; eventually, all three steroid-intractable cases in the LFA group received anti-TNFα agents. The efficacy rate of anti-TNFα agents for these cases was 33.3% at 8 weeks.
Discussion
Intestinal aphthous lesions are observed in some diseases, such as intestinal Behçet's disease, infectious enterocolitis, drug-induced enterocolitis, Crohn's disease, and UC. The regions and forms of aphthous lesions differ depending on the disease (6). In the rectum, lymphoid follicles with an elevated hemispherical appearance resembling salmon caviar have been observed in chlamydial proctitis, rectal MALT lymphoma, and so-called lymphoid follicular proctitis (LFP), as proposed by Flejou et al. (7). In addition, rectal LFA lesions have recently been reported to have a relationship with UC and may be initial lesions of UC. Indeed, several previous reports have shown that patients with LFA-like lesions progress to typical UC (3,4).
In the present report, we focused on the lymphoid follicular aphthous lesions with an elevated hemispherical appearance that were localized to the rectum. This is the first report to define LFA lesions and evaluate the prognosis of patients with LFA lesions in relation to UC. In addition, as patients with LFA lesions are very rare, we feel that this report will prove very valuable despite the small number of cases.
Although LFA resembles LFP endoscopically, the pathological findings are different. LFP is characterized by congested and granular mucosa with no ulceration, well-defined and large follicles, reactive germinal centers and preserved mantle zones, scanty or absent neutrophils and eosinophils, and no evidence of hyperplastic or atrophic changes in the crypt (7). In contrast, LFA lesions have acute inflammatory cells in the lamina propria of the mucosa, and the endoscopic findings are characterized by granules and erosion on its apex, indicating that LFA is accompanied by acute inflammation. The existence of ulcerative proctitis with lymphoid follicles might indicate the progression to UC through LFA. Case 5 exhibited such progression, with typical UC lesions appearing two months after the diagnosis (Fig. 2b), but some lymphoid follicles also remained. If initial colonoscopy had been performed at that time, this case might have been diagnosed as ulcerative proctitis with lymphoid follicles.
Our results showed that the patients had a high probability of progressing to typical UC from LFA with long-term observation. Notably, one patient progressed to typical UC more than four years after the diagnosis. As Flejou et al. (7) reported, so-called LFP is unrelated to ulcerative proctitis or UC limited to the rectum; LFA may be a phenotypically different disease from LFP.
Previous reports have found that the proximal extension rate of ulcerative proctitis was 20-37% at 5 years and 30-54% at 10 years (8-13). In our case series, the LFA group showed significantly higher proximal extension rates than the control group. Furthermore, this rate was higher than that of ulcerative proctitis in previous reports as well. Although a previous report showed that appendiceal orifice inflammation is associated with proximal extension of UC lesions (14), there were only two cases of LFA accompanied by appendiceal orifice in the present report, and neither showed proximal extension.
The majority of patients in both groups were treated with oral 5-ASA, and some were treated with topical drugs. The patients with LFA lesions tended to become steroid-intractable after proximal extension of the UC lesion. Three cases in the LFA group needed tacrolimus and/or anti-TNFα agents, but the efficacy of these therapies was limited.
Given the high rate of proximal extension and the intractability to medical therapies, LFA might have the potential to progress to a more severe inflammatory disorder than typical ulcerative proctitis. However, this report is limited because of its retrospective design. In addition, the control group might have included LFA lesions, as these lesions might have no longer been detectable when initial colonoscopy was performed. The accumulation of further reports will be required to resolve these points.
The present findings suggest that rectal LFA lesions frequently progress to typical UC with proximal extension, and some become intractable to corticosteroids. Further studies in more cases with careful follow-up will be needed in order to prove this point.
Our study protocol conformed to the ethical standards of the responsible committees on human experimentation (institutional and national) and with the 1964 Declaration of Helsinki and later versions and was approved by the Institutional Review Board. Informed consent was obtained from all patients included in this report.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med 365: 1713-1725, 2011. [DOI] [PubMed] [Google Scholar]
- 2. Okawa K, Aoki T, Sano K, Harihara S, Kitano A, Kuroki T. Ulcerative colitis with skip lesion at the mouth of the appendix. Am J Gatroenterol 93: 2405-2410, 1998. [DOI] [PubMed] [Google Scholar]
- 3. Matsumoto T, Kubokura N, Yada S, et al. Aphthous lesions of the colon as a precursor of ulcerative colitis. I to Cho (Stomach and Intestine) 44: 1514-1521, 2009(in Japanese, Abstract in English). [Google Scholar]
- 4. Yokoyama J, Watanabe K, Ajioka Y, et al. Aphthous lesions of the colorectum-clinical significance of the initial lesion of ulcerative colitis. I to Cho (Stomach and Intestine) 44: 1523-1533, 2009(in Japanese, Abstract in English). [Google Scholar]
- 5. Satsangi J, Silverberg MS, Vermeire S, Columbel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 55: 749-753, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iida M, Hizawa K, Aoyagi K, et al. Radiographic differential diagnosis of aphthoid lesions of the colon: focused on Crohn's disease with aphthoid lesions alone. I to Cho (Stomach and Intestine) 28: 397-410, 1992(in Japanese, Abstract in English). [Google Scholar]
- 7. Flejou JF, Potet F, Bogomoletz WV, et al. Lymphoid follicular proctitis. A condition different from ulcerative proctitis? Dig Dis Sci 33: 314-320, 1988. [DOI] [PubMed] [Google Scholar]
- 8. Hiwatashi N, Yamazaki H, Kimura M, Morimoto T, Watanabe H, Toyota T. Clinical course and long-term prognosis of Japanese patients with ulcerative colitis. Gastroenterol Jpn 26: 312-318, 1991. [DOI] [PubMed] [Google Scholar]
- 9. Siproudhis L, Vilotte J, Bonfils S, Mignon M. Idiopathic ulcerative colitis. Clinical presentation and endoscopic outcome. Gastroenterol Clin Biol 15: 315-321, 1991. [PubMed] [Google Scholar]
- 10. Meucci G, Vecchi M, Astegiano M, et al. The natural history of ulcerative proctitis: a multicenter, retrospective study. Gruppo di Studio per le Malattie Infiammatorie Intestinali (GSMII). Am J Gastroenterol 95: 469-473, 2000. [DOI] [PubMed] [Google Scholar]
- 11. Pica R, Paoluzi OA, Iacopini F, et al. Oral 5-ASA (5-ASA) treatment may protect against proximal extension of mucosal inflammation in ulcerative proctitis. Inflamm Bowel Dis 10: 731-736, 2004. [DOI] [PubMed] [Google Scholar]
- 12. Park SH, Kim YM, Yang SK, et al. Clinical features and natural history of ulcerative colitis in Korea. Inflamm Bowel Dis 13: 278-283, 2007. [DOI] [PubMed] [Google Scholar]
- 13. Alkim C, Alkim H, Dağli U, Parlak E, Ulker A, Sahın B. Extension of ulcerative colitis. Turk J Gastroenterol 22: 382-387, 2011. [DOI] [PubMed] [Google Scholar]
- 14. Anzaki H, Hata K, Kishikawa J, et al. Appendiceal orifice inflammation is associated with proximal extension of disease in patients with ulcerative colitis. Colorectal Dis 18: O278-O282, 2016. [DOI] [PubMed] [Google Scholar]